Abstract
Tissue Engineering (TE) bears potential to overcome the persistent shortage of donor organs in transplantation medicine. Additionally, TE products are applied as human test systems in pharmaceutical research to close the gap between animal testing and the administration of drugs to human subjects in clinical trials. However, generating a tissue requires complex culture conditions provided by bioreactors. Currently, the translation of TE technologies into clinical and industrial applications is limited due to a wide range of different tissue‐specific, non‐disposable bioreactor systems. To ensure a high level of standardization, a suitable cost‐effectiveness, and a safe graft production, a generic modular bioreactor platform was developed. Functional modules provide robust control of culture processes, e.g. medium transport, gas exchange, heating, or trapping of floating air bubbles. Characterization revealed improved performance of the modules in comparison to traditional cell culture equipment such as incubators, or peristaltic pumps. By combining the modules, a broad range of culture conditions can be achieved. The novel bioreactor platform allows using disposable components and facilitates tissue culture in closed fluidic systems. By sustaining native carotid arteries, engineering a blood vessel, and generating intestinal tissue models according to a previously published protocol the feasibility and performance of the bioreactor platform was demonstrated.
Keywords: Clinical application, Industrial application, Modular bioreactor platform, Regenerative Medicine, Tissue Engineering
Abbreviations
- acLDL
acetylated low‐density lipoprotein
- αSMA
alpha smooth muscle actin
- CAD
computer‐aided design
- CD31
cluster of differentiation 31
- H&E
hematoxylin and eosin
- MEM
minimum essential medium
- PCK
pan cytokeratin
- PID
proportional/integral/differential
- SIS
small intestinal submucosa
- TE
tissue engineering
- USB
universal serial bus
1. Introduction
Technologies to culture mammalian cells have already been developed in the early 20th century 1. They have facilitated various achievements in cell biology and paved the way for modern tools in pharmaceutical research. Many cell culture techniques are based on two‐dimensional static culture systems that do not resemble the situation in the microenvironment of native tissue 2. Thus, most cells lack or lose specific functions when cultured in vitro 3. To overcome limitations of standard cell culture methods, Tissue Engineering (TE) has been developed 4. In TE, cells are seeded in scaffolds to generate three‐dimensional (3D) tissues constituting an alternative to donor organs in transplantation medicine 5, 6. Furthermore, TE products are applied in pharmaceutical research as test systems offering new possibilities for closing the gap between animal testing and the administration of drugs to human subjects in clinical trials 7. Since tissues are engineered from human cells, they allow a more accurate assessment of effects that a test substance will exhibit on humans. However, the biological complexity of 3D tissues entails high requirements regarding applicable culture techniques. For instance, ensuring a suitable nutrients supply throughout a 3D construct is challenging in comparison to two‐dimensional cell cultures 3. Additionally, tissue‐specific cues should mimic the in vivo situation to allow generating tissue constructs with required characteristics and functions 8, 9. Therefore, tissues are exposed to mechanical, biochemical or electrical stimuli in bioreactor systems. A bioreactor usually comprises a tissue chamber harboring the tissue construct, cell culture medium reservoirs, and peripheral devices such as pumps or sensors 9, 10, 11. Moreover, incubators are employed for temperature and atmosphere control. The combination of traditional bioreactor equipment with incubators provides flexibility, however, operating a TE bioreactor in an incubator optimized for standard cell culture results in occupying a lot of space, and laborious handling. In addition, many tissue‐specific and tailored setups lack a totally‐integrated character. Components are not connected and controlled from a central user interface.
For TE bioreactors, generic functions can be identified as: (i) transport of cell culture medium, (ii) control of temperature, and (iii) sufficient gas exchange. These functions are adapted to basic physiological processes. Perfusion increases the exchange of nutrients and improves the removal of waste products. Thermoregulation is a vital aspect for homeostasis. In addition to nutrients, the medium also transports dissolved oxygen for cell respiration. An appropriate carbon dioxide concentration is required for the stabilization of the pH value in bicarbonate buffered cell culture medium. While these bioreactor functions facilitate a physiological metabolism, for the practical implementation of a TE process further functions are important: (iv) air bubbles must be removed from the fluidic system and (v) exchange of cell culture medium must be enabled. In addition to functional requirements, several design criteria should be considered to ensure usability of a bioreactor system in basic research as well as clinical and industrial application 12, 13, 14. For instance, a system should provide flexibility to address different TE processes with application‐specific culture conditions. All functions should be integrated into a compact setup allowing lab‐space‐efficient parallelization. A bioreactor system should encapsulate technical complexity to support easy handling and minimal failure rates, in particular when operating multiple bioreactors in parallel. Bioreactors should offer a certain degree of programmable process control and monitoring. Finally, disposable materials should be used for components that are in contact with the tissue or cell culture medium to minimize the risk of contamination and to facilitate the development of good‐manufacturing‐practice‐compliant processes.
The aim of the study was to demonstrate the feasibility of a bioreactor platform concept. Therefore, a bag pump, a heat exchanger, a gas exchanger, a bubble trap, and a control unit with a user interface were developed, characterized, and tested in TE applications. The platform should serve as a versatile tool in TE, supporting the development of different TE applications by operating the device with tissue‐specific process parameters.
2. Materials and methods
2.1. Design and construction
Tailor‐made components were designed using a CAD software package (SolidWorks™, Dassault Systemes Deutschland GmbH, Stuttgart, Germany). Design schematics were sent to a machinery shop (GT‐Labortechnik, Arnstein, Germany) for manufacturing. The heat exchanger was made from aluminum bulk material while the bag pump units and the gas exchanger are made of polyvinylidene fluoride or polysulfone. Housings were constructed from aluminum profile systems (item Industrietechnik GmbH, Solingen, Germany).
2.2. Computational modeling of heat and gas exchangers
To identify an optimal heat exchanger design, computational modeling was employed. Different device geometries were generated by computer‐aided design (CAD, SolidWorks™, Dassault Systemes Deutschland GmbH), and imported into the COMSOL Multiphysics™ simulation software (Comsol Multiphysics GmbH, Göttingen, Germany). In addition to solid parts, a fluid domain was generated representing cell culture medium inside the heat exchanger. For this domain, the Navier‐Stokes equation was applied. In the aluminum, heat conduction in solid material was parameterized. Heat transfer between fluid and solid domain was assumed proportional to the respective temperature difference. Heat transfer to ambient air (21°C) was implemented by heat radiation. As heat source, surface domains were defined in the shape of heat foils (thermo Flächenheizungs GmbH, Rohrbach, Germany) and parameterized with total power of 10, 20, 30, or 40 W. By varying the inlet velocity, power characteristics of a specific heat exchanger design were investigated. Additionally, temperature distributions were analyzed to prevent temperatures exceeding 38°C.
In analogy to the heat exchanger, a computational model for the oxygen transfer in the gas exchanger was developed. To optimize the gas exchanger geometry, designs were generated via CAD and compared in COMSOL Multiphysics™ (Comsol Multiphysics GmbH). Therefore, the fluid domain was perfused at different flow rates and oxygen transfer was calculated. The gas domain was either parameterized as air (21°C) or as nitrogen (21°C). Average oxygen concentration at the outlet was monitored as readout and compared to experimental data.
2.3. Assembly of single‐use fluid circuit
A closed bioreactor fluid circuit was composed of disposable components, i.e. 50 mL Easyflex bags (macropharma, Mouvaux, France), tubing (ESSKA.de GmbH , Hamburg, Germany), connectors (MedNet GmbH, Münster, Germany), and check valves (Qosina, Edgewood, USA). Prior to use, the system was assembled and sterilized by gamma irradiation with 25 kGray. For carotid artery culture, a disposable cartridge was built using silicone tubes (ESSKA.de GmbH) and tube‐reducing adapters (MedNet GmbH). For TE applications, tissue‐specific bioreactor chambers were used.
2.4. Control unit
The control unit was based on an ET200S microcontroller (Siemens AG, Munich, Germany) that was programmed with the TIA‐Portal software from Siemens AG. Modules for analog‐to‐digital (2AIxU ST1, Siemens AG) and digital‐to‐digital (1SI 3964/ASCII, Siemens AG) communication allowed to connect external devices such as sensors or valves. Programming of process parameters was done in a human machine interface TPP700 Comfort (Siemens AG) that also visualized measured values. Data were stored on a hard drive connected to the bioreactor by a universal serial bus (USB) port.
2.5. Pump module characterization
To characterize the pump, pressure in air‐filled bags (Easyflex, macopharma) of the pump and in the fluidic system of the bioreactor system was monitored. For measuring the air bag pressure, a transducer for relative pressures (B&B Thermo‐Technik GmbH, Donaueschingen, Germany) was used, whereas absolute pressure in the medium was recorded by a pressure sensor (HJK Sensoren + Systeme GmbH & Co. KG, Merching, Germany). Volume flow was monitored by a SONOFLOW CO.55 flow sensor (SONOTEC Ultraschallsensorik Halle GmbH, Halle (Saale), Germany).
2.6. Heat exchanger module characterization
A fluid circuit of 200 mL water at 21°C was used for the heat exchanger characterization. Set point of the device temperature was 37.5°C. Temperatures were monitored in the tissue chamber and at the heat exchanger outlet. The measurements were performed under ambient conditions with active bag pump at 75 mL min−1. As a control, a bag filled with 200 mL water was stored in a standard incubator under temperature monitoring.
2.7. Characterization of the gas exchanger module's carbon dioxide transport capacity
The module was assembled from silicone tubes with a wall thickness of 1.0 and 3.0 mm and a total length of 100 mm (ESSKA.de GmbH). To test carbon dioxide transfer, the fluid circulation system of the bioreactor was filled with 100 mL Minimum Essential Medium (MEM, Life Technologies GmbH, Darmstadt, Germany), which was pumped through the gas exchanger at 75 mL min−1. Furthermore, a gas mixture composed of ambient air and 5% carbon dioxide was supplied from a tailor‐made gas blender to the gas exchanger with a total volume flow of 75 mL min−1. Standard cell culture T‐flasks (TPP Techno Plastic Products AG, Trasadingen, Switzerland) filled with 100 mL MEM were used as controls. One T‐flask and the bioreactor were exposed to ambient conditions. In addition, a T‐flask was incubated in a standard incubator (Thermo Scientific, Braunschweig, Germany) at 5% carbon dioxide and 37°C. Following an initial phase of 4 h, 10 µL of 5% acetic acid were added every 2 min and pH values were recorded.
2.8. Characterization of the gas exchanger module's oxygen transport capacity
To investigate oxygen transfer, oxygen concentration in the bioreactor's fluidic system was decreased to 1% absolute oxygen saturation by introducing nitrogen into the cell culture medium. The gas exchanger module was assembled as described in 2.7. Oxygen recovery was detected by an oxygen sensor system Fibox 4 (PreSens – Precision Sensing GmbH, Regensburg, Germany). The sensor was installed inside the tissue chamber. From the oxygen recovery curve, the slope s (% min−1) was derived, and the oxygen flux q ox (mol s−1) was calculated according to:
| (1) |
Hereby, 1% absolute oxygen saturation equals 0.01045 mol m−3, and V (mL) denotes the total sample volume. As a control, a glass bottle equipped with a gas filter (Sartorius Stedim Biotech GmbH, Göttingen, Germany) and filled with 100 mL MEM and under ambient conditions was used. Oxygen depletion was induced by feeding the exchanger with nitrogen at a pressure of 40 kN m−2, to test whether the bioreactor can be operated in hypoxia mode. For the oxygen inflow and oxygen depletion measurement, the fluidic circulation system was loaded with 100 mL MEM and perfused at 75 mL min−1.
2.9. Preparation and culture of carotid arteries
Carotid arteries were obtained from 15 to 25 kg, six to eight weeks old German Landrace pigs. Animals received personal care in compliance with the Guide for Care and Use of Laboratory Animals published by the National Institutes of Health (NIH publication No. 85‐23, revised 1996) after approval from the institutional animal protection board. Surgeries were conducted in compliance with the German Animal Protection Laws (§4 Abs. 3) and the institute's animal protection officer regularly informed the responsible authorities. Following euthanasia, carotid arteries were explanted and transferred into a container occupied with VascuLife® cell culture medium (CellSystems Biotechnologie Vertrieb GmbH, Troisdorf, Germany) supplemented with 1% penicillin/streptomycin (Life Technologies GmbH). Then, three washing steps with phosphate buffered saline without calcium and magnesium were performed, and samples were cultured in the bioreactor platform at a flow rate of 90/45 mL min−1 at 120/80 mmHg. Therefore, 250 mL bicarbonate‐buffered VascuLife® (CellSystems Biotechnologie Vertrieb GmbH) was used. The heat exchanger was operated at 37.5°C. Ambient air supplemented with 5% carbon dioxide in the gas exchanger ensured a stable pH value.
2.10. Tissue Engineering of the blood‐tissue interface
To demonstrate the feasibility of the bioreactor platform, a TE process for the generation of the blood‐tissue was conducted. The process represented the transition between artery sustainment and in vitro tissue generation. A 3D tissue model of the blood‐tissue interface was generated based on primary endothelial cells, a small intestinal submucosa (SIS) without mucosa and mesentery 15 as scaffold, and a tailored compartmented tissue chamber. Primary cells were isolated from foreskin biopsies from juvenile donors aged between one and three years under informed consent according to ethical approval granted by the institutional ethics committee of the Julius‐Maximilians‐University Würzburg (vote 182/10). Additionally, informed and written consent from the next of kin, caretakers, or guardians on behalf of the children was obtained. Cell isolation was performed according to a previously published protocol 16. Scaffolds were seeded with 5 × 105 cells cm−2 and inserted into the tissue chamber. A compartmented tissue chamber allowed culturing the tissue construct composed of scaffold and cells at the blood‐tissue interface under shear stress conditions. Therefore, the tissue chamber for artery sustainment in the fluid circuit was replaced. For tissue culture, 150 mL VascuLife® was used. Cell culture medium was perfused through the tissue chamber at a flow rate of 5 mL min−1. For this flow rate, computational modeling revealed a total shear stress of 4.5 × 10−3 N m−2. During tissue culture for seven days, no medium exchange was performed.
2.11. Tissue Engineering of intestinal tissues
A second process that has already been published using traditional bioreactor equipment 17 allowed the engineering of intestinal tissue models and served as reference to assess the efficacy of the system. Briefly, on a SIS scaffold, Caco‐2 cells were seeded in a density of 3 × 105 cm−2 and cultured for 14 days under constant flow conditions (3.0 mL min−1) in the novel bioreactor platform. In analogy to the blood‐tissue interface, a specific tissue was introduced into the fluidic system of the bioreactor platform.
2.12. Histology
Paraformaldehyde‐fixed (4%), paraffin‐embedded samples were stained with hematoxylin and eosin (H&E). For immunofluorescent analyses, samples were incubated for 1 h with CD31 antibody solution, dilution 1:200 (#ABIN 726140, antibodies‐online, Aachen, Germany), alpha smooth muscle actin (αSMA) antibody solution, dilution 1:200 (#ab7817, Abcam, Cambridge, United Kingdom), or Villin antibody solution, dilution 1:100 (#V1616C01, DCS, Hamburg, Germany), and anti‐pan cytokeratin (PCK) antibody solution, dilution 1:100 (C2562, Sigma‐Aldrich, Munich, Germany). For detection, Alexa Fluor® 647 donkey anti‐mouse, dilution 1:400 (#A‐31571, Invitrogen, Pailey, United Kingdom), Alexa Fluor® 647 donkey anti‐rabbit, dilution 1:400 (#A‐31573, Invitrogen, Pailey, United Kingdom), Alexa Fluor® 555 donkey anti‐mouse, dilution 1:400 (#A‐31570, Invitrogen), and Alexa Fluor® 555 donkey anti‐rabbit, dilution 1:400 (#A‐31572, Invitrogen) were used.
2.13. Blood cleaning functionality assay
To demonstrate functionality of the endothelial layer, an acetylated low‐density lipoprotein (acLDL) uptake assay was performed. Therefore, samples were incubated for 4 h in 2 mL VascuLife® containing 2 µL Alexa‐Fluor®‐488‐labeled acLDL and 20 µL NucBlue® Live Cell Stain ReadyProbes™ reagent (#L23380 and #R37605, Life Technologies GmbH). acLDL was detected at 519 nm and microscopically documented (Laser‐Scan‐Microscope Leica TCS SP8, Wetzlar, Germany).
3. Results
3.1. Optimized Tissue‐Engineering‐specific incubator
To overcome the drawbacks of current incubator systems when used in TE, we developed a TE‐specific incubator with integrated bioreactor components (Fig. 1). Beyond standard parameters, culture conditions such as pH value, fluid pressure and flow as well as oxygen concentration are controlled. The incubator provides high flexibility during process development, however the parallelization of multiple bioreactors for clinical/industrial application was still challenging. The incubator constituted a non‐modular platform for TE applications and served as a first step towards the modular platform concept. Based on the TE‐specific incubator, requirements of a generic TE process were derived, and respective modules were established.
Figure 1.
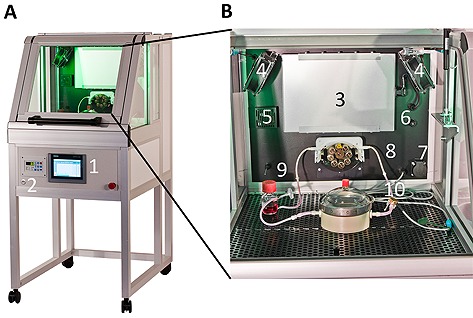
Tissue‐Engineering‐specific incubator. (A) Sensors and active components are installed inside the incubation chamber. Experimental conditions, i.e. oxygen concentration, dynamic flow rates, or dynamic pressures, can be accessed via a user interface. Feedback‐controlled process parameters are documented on universal serial bus (USB) storages. (B) Peristaltic pumps support operation of perfusion bioreactors. Cell culture medium is delivered from a reservoir to a tissue chamber under pressure control. 1, user interface; 2, USB port; 3, heating unit; 4, fan; 5, gas sensor; 6, gas inlet; 7, power outlet; 8, pump; 9, temperature sensor; 10, pressure sensor.
3.2. Pump module
The bag pump consisted of a housing adapted to the geometry of a filled 50 mL infusion bag. Two infusion bags were stacked in the bag pump housing, whereby one bag was connected to a pressure‐controlled air system and one bag was part of the fluid circuit of the bioreactor. The pump concept allowed fluid transport without interfering with the sterile boundary by separating flow regime and air system (Fig. 2A and 2B). To ensure a continuous medium circulation, two complementary‐working pump units were required. While one unit was actively pressurized, the second bag pump was passively filled with cell culture medium from the fluid circuit. Check valves maintained unidirectional flow. At the beginning of a pressure cycle, air pressure in a pump was increased to a user‐defined set point by a proportional valve. Subsequently, air was released through a proportional valve at the bag outlet until a minimum pressure value was reached. Valves were controlled by a proportional/integral/differential (PID) controller. After a specific holding period, a pump cycle ended, and the complementary second unit initiated a new cycle. For culture of carotid arteries, maximum/minimum air pressure was set to 220/60 mm Hg and no hold period was defined (Fig. 3A). Flexible bag material transferred the pressure into the bags of the fluid circuit. Pump dynamics allowed maintaining pressure profiles comparable to physiological pressure regarding frequency and shape (Fig. 3B). Characterization of the pump dynamics revealed that the pump facilitated a broad pressure range from 1 to 300 mm Hg, frequencies up to 1.3 Hz, and different ejection fractions from 1 mL per beat to 30 mL per beat. As depicted in Fig. 3B, constant pressure and flow can be achieved in addition to dynamic flow conditions.
Figure 2.
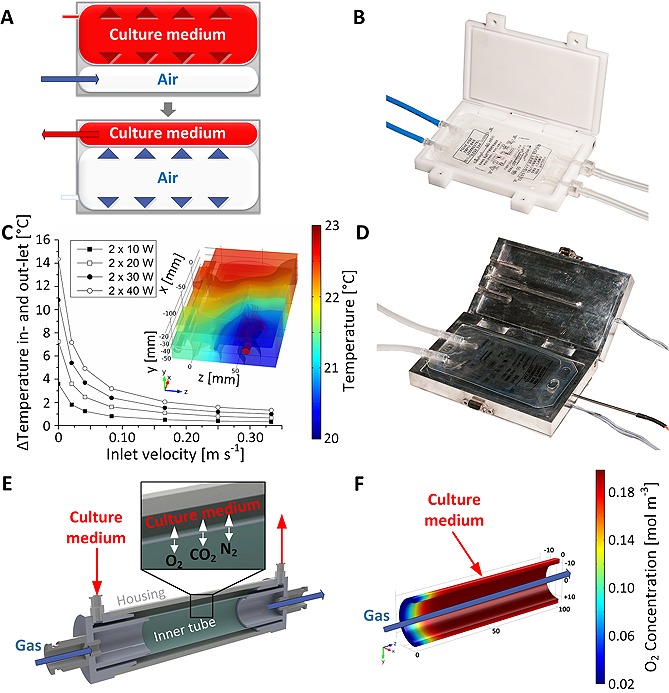
Modules for a bioreactor platform. (A) Concept of air‐driven pump module allowing pulsatile flow. Inside a confinement, two standard 50 mL infusion bags were installed. One bag was connected to an air pressure system; the second bag was part of bioreactor fluid circuit. To establish pulsatile flow conditions, a valve at the inlet of the bag introduced pressurized air. When reaching a preset pressure value, a microcontroller closed the inlet valve, halted the pressure inside the bag for a programmable timeframe, and released the pressure by opening the bags outlet valve. Due to bag expansion and fix total volume, cell culture medium from the complementary bag in the fluidic system was ejected. (B) A custom‐made housing of the bag pump was manufactured of polyvinylidine fluoride. (C) The heat exchanger module was designed employing computational modeling. Simulation of the power characteristic allowed predicting the temperature increase between in‐ and out‐let at specific flow rates and total heating powers. By visualizing the temperature distribution in the aluminum jaws, the warmest area was identified to locate a temperature sensor. (D) Heat exchanger geometry allowed permanent clamping of a 50 mL infusion bag. Cell culture medium was guided from the in‐ to the out‐let of the bag by bar structures ensuring maximum heat exchange. (E) In the gas exchanger, a gaseous and a medium compartment were generated by two silicone tubes. Defined gas mixture was introduced into the inner tube under pressure control. Via diffusion, gas molecules crossed the silicone membrane. (F) Oxygen transport was investigated employing computational modeling.
Figure 3.
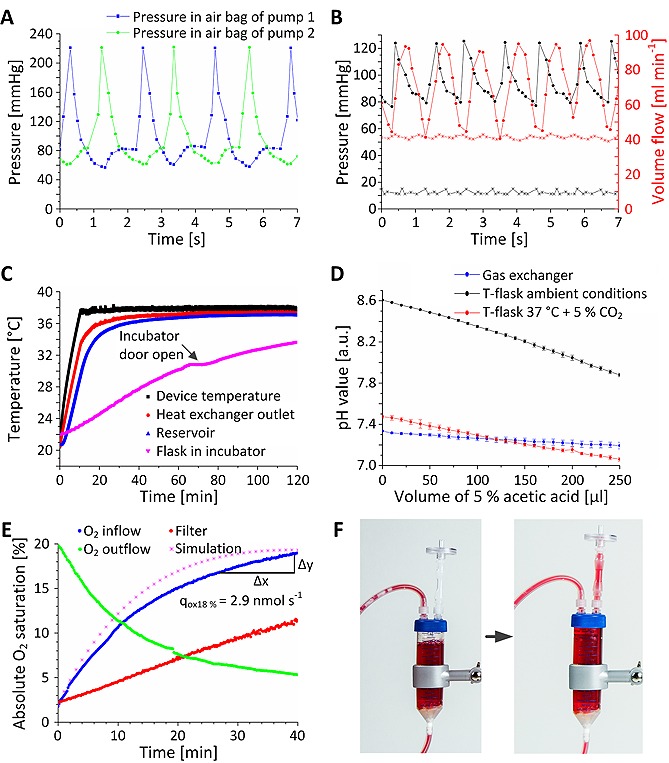
Characterization of bioreactor modules. (A) Dynamic pressure was measured in the 50 mL infusion bags connected to the air pressure system. Two pump units operated complementary resulting in an apparent frequency of approximately 0.96 ± 0.17 Hz. (B) Air pressures of 230/70 mmHg induced physiological pressures of 120/80 mmHg and flow rates with peak values of 95 mL min−1 in the fluid circuit. (C) The heat exchanger achieved a device temperature of 37.5°C after 15 min. 40 min were required to reach a temperature of 37°C at the outlet of the device. Generated heat input increased the temperature of the medium in tissue chamber from initially 21°C to 36°C within 60 min. In comparison, a bag occupied with the same volume in a standard incubator showed a lower temperature increase within the measured timeframe. (D) To demonstrate functionality of the gas exchanger, capacity of carbon‐dioxide‐dependent bicarbonate buffer system of medium was analyzed by adding acetic acid to the medium. Increasing acetic acid concentration resulted in decreasing pH values for the medium incubated at room atmosphere or in a standard incubator, whereas, medium circulating in the gas exchanger exhibited a stable pH value of approximately 7.3. (E) To assess the oxygen transport capacity, oxygen concentration in MEM was lowered to 1% absolute oxygen saturation. By measuring oxygen concentration over time, oxygen flux into the cell culture medium was derived. A standard gas filter exhibited a lower oxygen transport capacity with an almost linear oxygen concentration increase. Furthermore, the in silico model resembled similar oxygen transport characteristics. When feeding the gas exchanger with nitrogen, hypoxia conditions can be achieved in 40 min. (F) A disposable bubble trap minimized floating air bubbles in the closed bioreactor system. Incoming air was trapped underneath the lid of the centrifugation tube and discarded through the hydrophobic filter. Error bars depict standard deviation (n = 4).
3.3. Heat exchanger module
The heat exchanger geometry was optimized employing computational modeling (Fig. 2C). The final design supported rapid heating of cell culture medium without exceeding a device temperature of 38°C. Heat was transferred to the cell culture medium in a perfused 50 mL infusion bag placed in a cavity inside the module (Fig. 2D). A temperature sensor measured the device temperature at the position that was identified as the hottest point in the in silico analysis to prevent impairment of cells and medium. When measuring a temperature < 37.5°C, a relay switched on two 25 W heating foils. For temperatures ≥ 37.5°C, heating foils were switched off. By changing the temperature set point, the outlet temperature can be adjusted between ambient temperature (here 21°C) and 60°C. The two‐point control mechanism allowed robust temperature conditions and reached the required device temperature within approximately 10 min (Fig. 3C). Inside the heat exchanger, cell culture medium was heated to a stable temperature of 37°C without interfering with the sterile boundary. For 200 mL cell culture medium, the required outlet temperature of 37°C was achieved after approximately 40 min. In comparison, an infusion bag filled with 200 mL cell culture medium inside a standard incubator required more than 120 min to reach 37°C. Additionally, the temperature slope exhibited strong dependency on the storage conditions. Opening the incubator door for 10 minutes transiently decreased the slope of the temperature curve.
3.4. Gas exchanger module
Controlled carbon dioxide and oxygen transfer was established through an in‐house constructed gas exchanger (Fig. 2E). Computational modeling ensured an optimal gas exchanger design by calculating gas transfer across a silicone membrane (Fig. 2F). In the gas exchanger module, the silicone membrane separated the bioreactor fluidic system from a compartment continuously perfused with a gas mixture of defined partial pressures. Gas molecules penetrated the membrane by diffusion. To test whether the gas exchanger module was capable of maintaining stable pH values of bicarbonate‐buffered medium, 100 mL MEM was titrated with acetic acid (Fig. 3D). Therefore, 10 µL 5% acetic acid were added every 2 min to mimic increasing hydrogen ion activity. MEM in standard cell culture flasks in an incubator or exposed to room conditions served as controls. Both samples incubated in 5% carbon dioxide atmosphere showed an initial pH value of approximately 7.4. In contrast, MEM exposed to ambient atmosphere revealed a pH value of 8.6. When disturbing the pH value by adding acetic acid, sufficient gas exchange must be ensured for pH stabilization. Up to an acetic acid volume of approximately 100 µL, MEM in the bioreactor and in the standard incubator showed similar values. At higher acetic acid concentrations, pH values of MEM in the standard incubator declined below pH values of MEM in the bioreactor demonstrating improved bidirectional carbon dioxide transport capacity of the gas exchanger compared to the filter of a standard cell culture flask.
To characterize oxygen transport capacity, oxygen concentration of MEM in the bioreactor was measured after introducing low oxygen conditions (Fig. 3E). From the measured concentration curve, transport capacity of oxygen was calculated at 18% absolute oxygen saturation (90% relative oxygen saturation). The standard filter that served as reference exhibited a lower transport capacity. Comparison of the simulated and measured oxygen recovery curve revealed a high prediction power of the computational gas exchanger model. Moreover, measurements demonstrated that also hypoxia conditions can be generated by the gas exchanger.
3.5. Bubble trap module
A tailor‐made disposable bubble trap prevented air bubbles in the fluid circuit (Fig. 3F). The device was composed of commercially‐available standard components such as a centrifuge tube, connectors, and hydrophobic air filters. Air bubbles were trapped in the system and accumulated gas was released through a hydrophobic filter.
3.6. System integration
As proposed in the design criteria, the bioreactor fluid system solely comprised disposable components (Fig. 4A). A sterile fluid circuit was loaded into respective modules without transferring the system into a safety cabinet (Fig. 4B). Independently operating functional modules were connected to a microcontroller according to application requirements, enabling high flexibility of the platform. Medium was filled through a port in the fluid circuit. For both TE applications, specific tissue chambers were used (Fig. 4C and 4D). The bioreactor modules were arranged on a surface area of 300 × 300 mm allowing a compact setup and facilitating lab‐space‐efficient parallelization. Process parameters were programmed and monitored in the control unit via a graphical user interface. The user interface and the possibility to start and operate a bioreactor under ambient conditions supported easy handling. Power characteristics and different operating modes of the system are summarized in Table 1.
Figure 4.
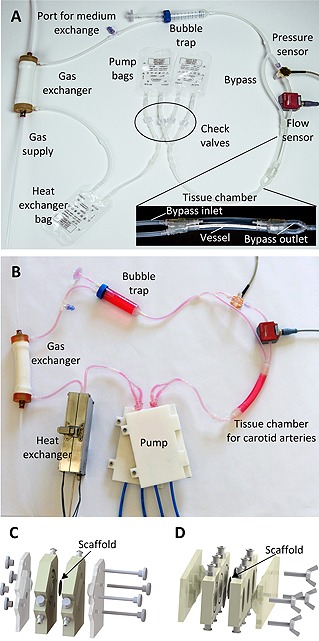
Tissue culture system. (A) For the pump, two parallel‐connected 50 mL infusion bags were installed. At the in‐ and out‐lets, check valves ensured unidirectional flow. From the two bags, medium was delivered in an additional 50 mL infusion bag for the heat exchanger. The outlet of the bag in the heat exchanger was connected to the gas exchanger, which was supplied from a tailored gas blender system. The gas exchanger was connected to the bubble trap to capture floating bubbles from the cell culture medium before entering the disposable tissue chamber supporting culture of blood vessels. Four independent in‐ and out‐lets allowed intramural and extramural perfusion. A tube from the tissue chamber to the pump closed the fluid circuit. (B) The disposable fluid circuit was loaded in the components of the modular bioreactor system. (C) For culture of tissue‐engineered human blood‐tissue interfaces, a chamber harboring a bio logical scaffold was used instead of the blood vessel tissue chamber. (D) Intestinal tissues were generated employing a tissue chamber that ensured a laminar flow regime.
Table 1.
Bioreactor platform specifications
| Module | Parameter | Range |
|---|---|---|
| Pump | Pressure range pulsatile | 1–300 mmHg |
| Pressure range constant pressure | 1–70 mmHg | |
| Ejaction fraction pulsatile pressure | 1–30 mL per beat | |
| Flow rate constant pressure | 1–50 mL min−1 | |
| Frequency range pulsatile pressure | 0.1–1.3 Hz | |
| Heat exchanger | Temperature range at specific flow rate |
Ambient temperatur (21°C)–63°C @25 mL min−1
Ambient temperatur (21°C)–61.5°C @50 mL min−1 Ambient temperatur (21°C)–60°C @75 mL min−1 Ambient temperatur (21°C)–60°C @100 mL min−1 |
| Gas exchanger |
Oxygen transport per 100 mm gas exchanger length and 21% oxygen in gas mixture Oxygen concentration range |
approx. 3 nmol s‐1 5% (hypoxia)–21% (normox) absolute oxygen concentration |
3.7. Tissue sustainment
To assess the feasibility of the bioreactor platform independently from a specific TE application, the system was used to sustain native tissue. Therefore, porcine carotid arteries were cultured for seven days ex vivo in the bioreactor. A tissue chamber was assembled from silicone tubes and commercially available connectors. The bypass loop allowed continuous exchange of cell culture medium in extravasal compartment of the tissue chamber. Thereby, cell culture medium surrounding the artery was replaced with medium from the fluid circuit. Flow through the artery was controlled by the flow sensor at the artery inlet. After seven days of culture, tissues were analyzed by H&E staining, immunofluorescent staining of αSMA and CD31, as well as by a blood cleaning functionality assay (Fig. 5A–5C). Carotid arteries exhibited a typical histological architecture, a closed layer of endothelial cells, and a strong expression of αSMA. Moreover, a high density of acLDL was visible after bioreactor culture.
Figure 5.
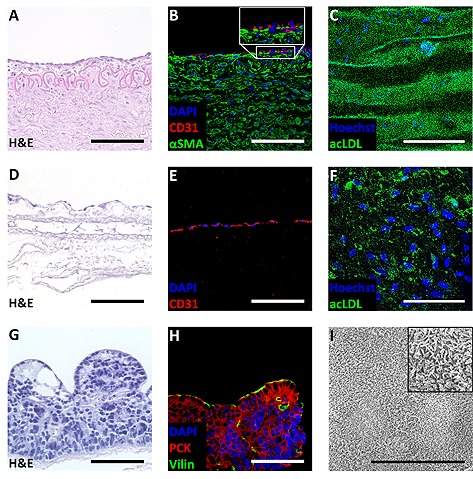
Application of modular bioreactor system. After seven days of culture, porcine carotid arteries were investigated. (A) Hematoxylin and eosin (H&E) staining revealed an artery‐specific tissue architecture of dynamically‐cultured samples. (B) Immunofluorescent staining of CD 31 demonstrated a closed endothelial cell layer. Immunofluorescent staining of alpha smooth muscle actin (αSMA) showed strong expression of αSMA. (C) High intensities of acetylated low‐density lipoprotein (acLDL) confirmed uptake and indicated functionality of the endothelial cells. (n = 4). After seven days of culture in the bioreactor platform, engineered blood‐tissue interfaces were characterized. (D) H&E staining revealed an almost confluent endothelial layer. (E) Immunofluorescent staining of CD 31 confirmed endothelial cell identity. (F) Functional analysis of the endothelial cells was performed by acLDL uptake. (n = 3). Dynamic culture conditions allowed generating intestinal tissue models. (G) In H&E staining, high‐prismatic cells were detected. (H) Cells exhibited positive staining for tissue‐specific villin and pan cytokeratin (PCK). (I) Scanning electron microscopy displayed the presence of microvilli on the cell surface. (n = 3). Scale bar depicts 100 µm, and 10 µm in the scanning electron microscopy image in I.
3.8. Tissue Engineering applications
To transfer the bioreactor platform to a TE process, an equivalent of the human blood‐tissue interface was generated (Fig. 5D–5F). The process was rendering all components of TE, in particular cells, a scaffold, and controlled culture conditions. Based on a biological scaffold, a functional blood‐tissue interface represented by an almost confluent layer of endothelial cells was obtained after seven days of bioreactor culture. Cells exhibited a positive staining for the endothelial marker CD31, and were able to internalize acLDL.
Comparison of the platform with traditional bioreactor equipment was performed by conducting an adapted TE process according to a previously published protocol 17. Analysis of cultured tissues revealed that intestinal tissue models exhibit a high correlation with the results of the original publication (Fig. 5G–5I). The intestinal epithelial cells showed a high‐prismatic morphology and an apical cell surface covered by a dense layer of microvilli.
4. Discussion
To ensure a high level of standardization, a suitable cost‐effectiveness, and a safe graft production, a modular bioreactor platform was developed, characterized, and tested in three TE processes. For this platform, generic bioreactor functions of TE applications were implemented as modular units and design criteria were considered. Although bioreactors have a long history in biotechnology, the versatile bioreactor platform represents a novel approach for bioreactors in TE. Most biotechnological bioreactors, such as the ReadyToProcess™ bioreactors from GE Healthcare or the BIOSTAT® family from Sartorius, were developed for the production of cells or proteins. For cellular manufacturing, these systems render standardized tools, whereas for tissue culture, such standardized platforms are lacking and a wide range of different bioreactors are used 9, 18. Instead of covering a broad application domain, TE bioreactors platforms address a specific application. In basic research, systems were described for investigating oxygen distribution in tissues 19, studying hepatic sinusoids 20, stimulating fetal liver cells 21, generating heart valves 22, culturing whole hearts 23, or for metabolism studies 24. Moreover, bioreactor platforms were used to culture cells and tissues during spaceflights 25. The majority of these systems is not modular and if modularity is provided it is interpreted in terms of bioreactor experiments that can be performed in parallel. Alternatively, modularity is considered regarding the process flow. A patent for an ”Advanced Tissue Engineering System“ presents a system designed for a clinical application 26. This bioreactor comprises a digestion, a proliferation, and a product chamber, thereby demonstrating that modularity relates to process steps such as seeding or harvesting instead to freely combinable bioreactor functions. With the exception of this bioreactor for clinical use, TE bioreactors moreover harness traditional lab equipment. Partial substitution of standard laboratory devices was published by Orr and Burg 10. Nevertheless, the presented bioreactor system still requires an incubator for temperature and gas control, entailing corresponding space requirements and control of all culture parameters through a central user interface is not possible. The novel modular bioreactor platform proposed here is fully integrated and can be operated without additional equipment allowing an efficient integration into a laboratory.
In contrast to respective traditional laboratory equipment, the functional modules displayed a higher performance. Oxygen recovery of the gas exchanger was faster than the oxygen inflow measured for a standard filter system, and a physiological temperature was reached in a shorter timeframe by the heat exchanger compared to an incubator. The bag pump represents a nature‐inspired concept as its functional principle is adapted from a ventricle. The pump module facilitated controlled physiological pressure profiles that are exceeding the dynamic of peristaltic pumps. Pressure regimes with a frequency of 1 Hz are also achieved by peristaltic pumps 16, 22, nevertheless the pressure profile of the bag pump resembles a physiological pressure curve. In addition to a systolic pressure peak, the pulse pressure increase is mimicked. Due to the cell‐preserving pumping mechanism, it can also be used for suspension cultures of highly‐sensitive cells. Moreover, in contrast to peristaltic pumps that continuously exert a mechanical loading on a pump tubing, the bag pump works abrasion‐free minimizing the risk of leakage.
Basic feasibility of the platform for tissue culture was demonstrated by sustaining native carotid arteries. Tissue sustainment served as a model process to investigate the device independently from a specific TE application. In dynamic culture, endothelial as well as smooth muscle layers were preserved for seven days. Functionality of the endothelial cells was investigated by an acLDL uptake. In contrast to smooth muscle cells, endothelial cells internalize seven‐ to 15‐fold more acLDL 27, allowing to identify the signal source. For acLDL internalization, endothelial cells utilize the scavenger cell pathway that is exhibiting a pressure‐dependent blood cleaning functionality 28, 29. Although shear stress generally results in lower acLDL uptake, measured acLDL intensities sampled from dynamically cultured tissues indicate maintained cleaning functionality. When performing a TE process to generate an equivalent of the blood‐tissue interface, findings for the endothelial layer obtained from tissue sustainment were confirmed. The comparison of the modular bioreactor platform with traditional bioreactor equipment was performed by using an adapted TE protocol published by Pusch et al. 17. The intestinal models generated by the novel bioreactor platform displayed comparable characteristics as the tissue models presented in the previous publication.
Characterization of the single modules and performed TE applications demonstrate the performance of the novel bioreactor concept. In addition to the required bioreactor functions (i‐v), proposed design criteria were met. For instance, the novel bioreactor platform provides a high degree of flexibility allowing address different TE applications. Therefore, developed modules facilitate a broad range of possible culture parameter (see also Table 1) and can be application‐specifically combined by connecting the modules to the control unit. To culture a specific tissue, optimal set points for the process parameters must be programmed and a suitable tissue chamber must be installed in the fluidic system. Both can be done without constructional changes as shown in this study. Moreover, in accordance to an open platform concept, modules for new functions can be introduced – either by our group or other researchers – due to standardized connectors and a microcontroller supporting various communication protocols, i.e. RS232, or Profinet. Such modules could facilitate complementary features, e.g. mechanical deformation, or sampling, to enhance the application domain of the platform. The open communication concept furthermore ensures advanced monitoring possibilities. Sensors, e.g. for pH value, oxygen concentration, volume flow, or temperature, were connected and inserted into the fluid circuit via standard connectors. An additional advantage of the bioreactor platform is its comparably small footprint, enabling lab‐space‐efficient parallelization, e.g. by implementation of a rack system to stack several bioreactors. To start an experiment, a ready‐to‐use fluid circuit is inserted into the functional modules comparable to a cartridge system. All parts that are in direct contact with cell culture medium or the tissue can be assembled, sterilized, and stored as a closed system upon usage. In analogy to pharmaceutical production, where disposable approaches already pave the way towards safe and economic processing 30, the fluid circuit is composed of single‐use components. During the experiments, no manual processing in a safety cabinet was required. Ports enable access into the fluid circuit, e.g. for seeding of a scaffold or for introducing a test substance. Additionally, culture processes are performed automatically employing feedback control. This feature reduces laborious handling, and increases the maximum number of bioreactors operated per user.
Conclusion
The aim of the study was to demonstrate the value of a generic modular bioreactor platform in TE. The successful implementation of three independent tissue culture processes proved the applicability of the modular design and the functionality of the system. Additional characteristics such as flexibility or easy handling ensure usability. Thus, the patented bioreactor platform 31 renders a versatile tool for basic research as well as a future production device for clinical and industrial applications.
Acknowledgement
We thank Dr. Jan Saam from the OSPIN GmbH for his interlectual contribution within the project.
Our work was funded by the German Federal Ministry of Education and Research; program NanoMatFutur; grant agreement number 13N12971 – ETface.
The authors declare no conflicts of interest.
References
- 1. Nema, R. , Khare, S. , An animal cell culture: Advance technology for modern research. Adv. Biosci. Biotechnol. 2012, 3, 219–226. [Google Scholar]
- 2. Grainger, D. W. , Cell‐based drug testing; this world is not flat. Adv. Drug Delivery Rev. 2014, 69‐70, vii‐xi. [DOI] [PubMed] [Google Scholar]
- 3. Alepee, N. , Bahinski, T. , Daneshian, M. , De Wever, B. et al., State‐of‐the‐art of 3D cultures (organs‐on‐a‐chip) in safety testing and pathophysiology. ALTEX 2014, 31, 441–477.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Pampaloni, F. , Reynaud, E. G. , Stelzer, E. H. , The third dimension bridges the gap between cell culture and live tissue. Nat. Rev. 2007, 8, 839–845. [DOI] [PubMed] [Google Scholar]
- 5. Sabetkish, S. , Kajbafzadeh, A. M. , Sabetkish, N. , Khorramirouz, R. et al., Whole‐organ tissue engineering: Decellularization and recellularization of three‐dimensional matrix liver scaffolds. J. Biomed. Mater. Res. Part A 2015, 103, 1498–1508. [DOI] [PubMed] [Google Scholar]
- 6. Caralt, M. , Uzarski, J. S. , Iacob, S. , Obergfell, K. P. et al., Optimization and critical evaluation of decellularization strategies to develop renal extracellular matrix scaffolds as biological templates for organ engineering and transplantation. Am. J. Transplant. 2015, 15, 64–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hartung, T. , Look back in anger – what clinical studies tell us about preclinical work. ALTEX 2013, 30, 275–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Jin, G. , Yang, G. H. , Kim, G. , Tissue engineering bioreactor systems for applying physical and electrical stimulations to cells. J. Biomed. Mater. Res. Part B 2014, 103, 935–948. [DOI] [PubMed] [Google Scholar]
- 9. Hansmann, J. , Groeber, F. , Kahlig, A. , Kleinhans, C. , Walles, H. , Bioreactors in tissue engineering – principles, applications and commercial constraints. Biotechnol. J. 2013, 8, 298–307. [DOI] [PubMed] [Google Scholar]
- 10. Orr, D. E. , Burg, K. J. , Design of a modular bioreactor to incorporate both perfusion flow and hydrostatic compression for tissue engineering applications. Ann. Biomed. Eng. 2008, 36, 1228–1241. [DOI] [PubMed] [Google Scholar]
- 11. Vinci, B. , Duret, C. , Klieber, S. , Gerbal‐Chaloin, S. et al., Modular bioreactor for primary human hepatocyte culture: Medium flow stimulates expression and activity of detoxification genes. Biotechnol. J. 2011, 6, 554–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Martin, I. , Smith, T. , Wendt, D. , Bioreactor‐based roadmap for the translation of tissue engineering strategies into clinical products. Trends Biotechnol. 2009, 27, 495–502. [DOI] [PubMed] [Google Scholar]
- 13. Portner, R. , Nagel‐Heyer, S. , Goepfert, C. , Adamietz, P. , Meenen, N. M. , Bioreactor design for tissue engineering. J. Biosci. Bioeng. 2005, 100, 235–245. [DOI] [PubMed] [Google Scholar]
- 14. Haycock, J. W. , 3D cell culture: A review of current approaches and techniques. Methods Mol. Biol. 2011, 695, 1–15. [DOI] [PubMed] [Google Scholar]
- 15. Schanz, J. , Pusch, J. , Hansmann, J. , Walles, H. , Vascularised human tissue models: A new approach for the refinement of biomedical research. J. Biotechnol. 2010, 148, 56–63. [DOI] [PubMed] [Google Scholar]
- 16. Groeber, F. , Kahlig, A. , Loff, S. , Walles, H. , Hansmann, J. , A bioreactor system for interfacial culture and physiological perfusion of vascularized tissue equivalents. Biotechnol. J. 2013, 8, 308–316. [DOI] [PubMed] [Google Scholar]
- 17. Pusch, J. , Votteler, M. , Gohler, S. , Engl, J. et al., The physiological performance of a three‐dimensional model that mimics the microenvironment of the small intestine. Biomaterials 2011, 32, 7469–7478. [DOI] [PubMed] [Google Scholar]
- 18. Plunkett, N. , O'Brien, F. J. , Bioreactors in tissue engineering. Technol. Health Care 2011, 19, 55–69. [DOI] [PubMed] [Google Scholar]
- 19. Lovett, M. , Rockwood, D. , Baryshyan, A. , Kaplan, D. L. , Simple modular bioreactors for tissue engineering: A system for characterization of oxygen gradients, human mesenchymal stem cell differentiation, and prevascularization. Tissue Eng. Part C 2010, 16, 1565–1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Illa, X. , Vila, S. , Yeste, J. , Peralta, C. et al., A novel modular bioreactor to in vitro study the hepatic sinusoid. PLoS One 2014, 9, e111864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Schmelzer, E. , Triolo, F. , Turner, M. E. , Thompson, R. L. et al., Three‐dimensional perfusion bioreactor culture supports differentiation of human fetal liver cells. Tissue Eng. Part A 2010, 16, 2007–2016. [DOI] [PubMed] [Google Scholar]
- 22. Vismara, R. , Soncini, M. , Talo, G. , Dainese, L. et al., A Bioreactor with Compliance Monitoring for Heart Valve Grafts. Ann. Biomed. Eng. 2010, 38, 100–108. [DOI] [PubMed] [Google Scholar]
- 23. Hulsmann, J. , Aubin, H. , Kranz, A. , Godehardt, E. et al., A novel customizable modular bioreactor system for whole‐heart cultivation under controlled 3D biomechanical stimulation. J. Artif. Organs 2013, 16, 294–304. [DOI] [PubMed] [Google Scholar]
- 24. Vinci, B. , Murphy, E. , Iori, E. , Marescotti, M. C. et al., Flow‐regulated glucose and lipid metabolism in adipose tissue, endothelial cell and hepatocyte cultures in a modular bioreactor. Biotechnol. J. 2010, 5, 618–626. [DOI] [PubMed] [Google Scholar]
- 25. Freed, L. E. , Vunjak‐Novakovic, G. , Spaceflight bioreactor studies of cells and tissues. Adv. Space Biol. Med. 2002, 8, 177–195. [DOI] [PubMed] [Google Scholar]
- 26. Smith, T. J. N. , Pugh, S. M. , Misener, L. , Oram, G. , Hagg, R. , Tommasini, R. , Larcher, Y. , Advanced tissue engineering system, Patent WO 2005116186 A1 2005.
- 27. Voyta, J. C. , Via, D. P. , Butterfield, C. E. , Zetter, B. R. , Identification and isolation of endothelial cells based on their increased uptake of acetylated‐low density lipoprotein. J. Cell Biol. 1984, 99, 2034–2040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Shnyra, A. , Lindberg, A. A. , Scavenger receptor pathway for lipopolysaccharide binding to Kupffer and endothelial liver cells in vitro. Infect. Immun. 1995, 63, 865–873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Niwa, K. , Kado, T. , Sakai, J. , Karino, T. , The effects of a shear flow on the uptake of LDL and acetylated LDL by an EC monoculture and an EC‐SMC coculture. Ann. Biomed. Eng. 2004, 32, 537–543. [DOI] [PubMed] [Google Scholar]
- 30. Shukla, A. A. , Gottschalk, U. , Single‐use disposable technologies for biopharmaceutical manufacturing. Trends Biotechnol. 2013, 31, 147–154. [DOI] [PubMed] [Google Scholar]
- 31. Saam, J. , Erfurth, H. , Hansmann, J. , Schröder, D. , Schwarz, T. , Krziminski, S. , Modulares Bioreaktorsystem, Patent DE102015210609. B3, 2015.


