Abstract
Stress is one of the effective factors in the development of depressive disorders that performs some parts of its effects by affecting hippocampus. Since doxepin has been shown to have neuroprotective effects, in this study, we focused on the effects of doxepin on the expression of involved genes in neuronal survival and plasticity in the rat hippocampus following chronic stress. Male Wistar rats were divided into four groups, the control, the stress, the stress-doxepin 1 mg/kg and the stress-doxepin 5 mg/kg, respectively. To induce stress, the rats were placed within adjustable restraint chambers for 6 h/day, for 21 days. Before daily induction of the stress, rats received an i.p. injection of doxepin. At the end of experiments, expression of Bax, Bad, Bcl-2, tumor necrosis factor alpha (TNF-α), mitogen-activated protein kinase 14 (MAPK14) and serine-threonine protein kinase AKT1 genes were detected by reverse transcription polymerase chain reaction (RT-PCR) in the hippocampus. Results showed significant enhancements in expression of Bax, Bad and Bcl-2 genes in the stressed rats, whereas expression of TNF-α, MAPK14, and AKT1 genes didn’t show significant differences. Doxepin could decrease the expression of Bax and Bad genes in the stress group, but had no significant effects on the expression of other genes. The present findings indicated that doxepin can probably change the pattern of gene expression in the hippocampus to maintain neurons against destructive effects of stress.
Keywords: Doxepin; Stress; Hippocampus; Bcl-2 family, TNF-α, MAP Kinase, AKT
INTRODUCTION
Depression is a physical-mental illness that affects thoughts, moods, feelings, behavior and physical health. Stress is one of the risk factors in depression, but there is little information about its mechanisms(1). Chronic stress changes neural networks and damage brain structure and connectivity(2,3). Studies have shown that stress can directly or indirectly affect genes(4) and consequently cause different effects such as cell death(5). Hippocampus is one of the candidates for the effects of different stresses(6,7). It is an important area for cognitive functions(8,9). It has been demonstrated that stress induces apoptosis and prevents adult neurogenesis in hippocampus(10). Stress damages hippocampal neurons via the adrenal steroids and excitatory neurotransmitters(11). Excitatory neurotransmitters have a role in plasticity in hippocampus; also have an important role in pathological conditions(12). Chronic stress leads to the activation of the hypothalamic-pituitary-adrenal axis (HPA-axis) and increased secretion of corticosteroids(13). Also, stress affects the metabolism of the neurotransmitters that are associated with depression such as serotonin, norepinephrine, and dopamine(14,15). Both of these conditions can lead to depression in susceptible individuals(16,17). Recent studies have shown that depression and antidepressants can influence the processes associated with neuronal plasticity and survival through inhibition or stimulation of intracellular pathways(18,19).
Stress caused morphological and functional changes in the structure of different brain regions(20,21), and antidepressants may prevent the effects of stress through influencing these mechanisms or forming appropriate adaptive responses(22).
Doxepin is a tricyclic antidepressant that is also used to treat sleep disorders, acute and chronic pain, chronic urticaria and atopic dermatitis(13). It seems that doxepin performs parts of its antidepressant and protective effects through mechanisms other than inhibition of monoamine reuptake(23). We have previously observed that doxepin can affect expression of genes involved in neuronal survival and plasticity, and prevent stress-induced learning and memory dysfunction, probably via affecting inflammatory cytokines(13,18,24). Therefore, in this study, we focused on the effects of stress on the expression of the genes that the role of their products in inflammation and neuronal apoptosis has been found, and the probable protective effects of doxepin during induction of stress.
MATERIALS AND METHODS
Subjects
The experiments were carried out on male Wistar rats (200–250 g) that were housed under standard conditions of temperature (22 ± 2 °C) and light (12 h light-dark cycle), with free access to food and water. The Ethic Committee for Animal Experiments at Isfahan University of Medical Sciences approved the study and all experiments were conducted in accordance with the National Institute of Health Guide for the Care and Use of Laboratory Animals (NIH Publications No. 80–23) revised 1996. The animals were randomly divided into four groups of 7 each including the control, the stress, the stress-doxepin 1 mg/kg and the stress-doxepin 5 mg/kg.
Protocols of stress induction and treatment
In the stress groups, the rats were individually restrained for 6 h/day (7:30 AM to 1:30 PM), for 21 days in plastic cylinders (20 × 7 cm) which had holes for air exchange(6). In the doxepin groups, doxepin (1 and 5 mg/kg; dissolved in saline; Ray Chemicals Pvt. Ltd, India) was injected intraperitoneally (i.p.) for 21 days(25). In the stress-doxepin groups, doxepin was injected 5 min before the stress process. Animals in the control groups received the same volume of saline.
At the end of experiment, rats were deeply anaesthetized with chloral hydrates (450 mg/kg, i.p.) (26) and decapitated. Brains were rapidly removed and instantly the hippocampi were dissected in ice-cold artificial cerebrospinal fluid containing 145 mM NaCl, 2.7 mM KCl, 1.2 mM CaCl2, 1 mM MgCl2, 2 mM Na2 Hpo4 at pH 7.4(12) and were deep freeze in liquid nitrogen. Then they stored at −80 °C until further studies. Also, the adrenal glands were removed and weighed.
Assessment of gene expression
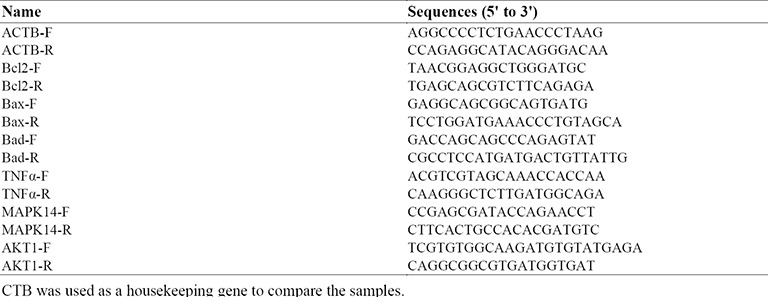
Real-time polymerase chain reaction (PCR) was used to evaluate the expression of Bax, Bad, Bcl-2, TNF-α, MAPK14, and AKT1 genes. Total RNA was isolated from hippocampus tissues using YTA kit (Yekta Tajhiz Azma, Iran), according to the manufacturer's instruction protocol; this method first lyses cells by using a chaotropic salt, then binds RNA to silica-based membranes, washes RNA with ethanol- containing wash buffer and then elutes purified RNA by RNase-free ddH2O. After isolation, the quality of messenger RNA (mRNA) was checked by gel electrophoresis, and RNA quantity was measured using nanodrop (OD 260 nm and 280 nm). At the reverse transcription step, 5 ng of total RNA was used to synthesis the complementary DNA with random hexamers primer using the Reverta-L kit (Amplisens, Moscow, Russia). The real-time PCR was performed using the Step One Plus real-time PCR System (Applied Biosystems, USA). Real Q Plus 2× Master Mix Green with high ROX™ (Ampliqon, Denmark) and specific primers were used (Table 1). Beta-actin (ACTB) was used as an internal control to normalize RNA input. Cycle parameters for real-time PCR included 95 °C for 15 min, 95 °C for 15 s and 60 °C for 60s, the whole process was done 40 cycles and finally melt curve was depicted. All experiments were performed in triplicates for each specimen. The Ct value is defined as the fractional cycle number at which the fluorescence passes the fixed threshold.
Table 1.
Primers used in real-time PCR experiments
The fold change was calculated using the 2−ΔΔCt method presented as the fold expression change in treated experiment group relative to their corresponding control group after normalization to the ACTB endogenous control.
Statistical analysis
Data were analyzed using both the SPSS 21 (IBM Corporation) for Windows and the Rest 2009 (developed by M. Pfaffl (Technical University Munich) and QIAGEN). The results analyzed for gene expression with one sample t-test between the treated and the control groups and with one-way ANOVA between the other groups followed by Tukey's test. The significant level was set at P < 0.05. Results are expressed as means ± SEM.
RESULTS
Weight of adrenal gland
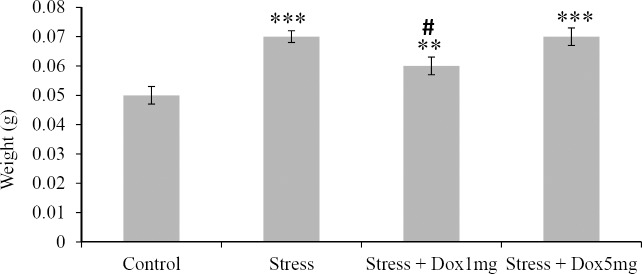
The weight of the adrenal glands was increased significantly (P < 0.001) in the stress group compared to the control group. Doxepin significantly (P < 0.05) decreased weight of adrenal glands only in the stress-doxepin 1 mg/kg comparing to the stress group (Fig. 1).
Fig. 1.
Effects of stress and doxepin on adrenal gland weights. Data are expressed as mean ± SEM (n = 10). **P < 0.01 and ***P < 0.001 with respect to the control group, #P < 0.05 with respect to the stress group.
Levels of gene expression
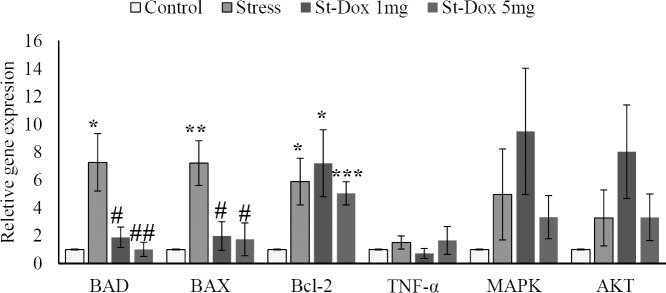
As seen in Fig. 2, the mRNA expression of the genes in the stress group was higher than the control group; Bad 727% (P = 0.024), Bax 722% (P = 0.008), Bcl-2 589% (P = 0.027), TNF-α 151% (P = 0.35), MAPK14 497% (P = 0.27), AKT1 328% (P = 0.3).
Fig. 2.
Effects of doxepin and stress on relative gene expression of Bax, Bad, Bcl-2, TNF-á, MAPK14, and AKT1 in rat hippocampus. The extent of expression was measured by RT-PCR. The mRNA expression data was normalized to the Beta-actin (ACTB) signal. Fold changes relative to the control are presented. Mean ± SEM values of experiments are shown. *P < 0.05, **P < 0.01, and ***P < 0.001 with respect to the control group;#P < 0.05 and##P < 0.01 with respect to the stress group (n = 7).
In the stress-doxepin 1 mg/kg group, doxepin decreased the mRNA expression of Bad 74.14% (P = 0.022), Bax 72.71% (P = 0.017), and TNF-α 52.32% (P = 0.21); and increased the mRNA expression of Bcl-2 22.41% (P = 0.67), MAPK 91.1% (P = 0.43), and AKT 145.12% (P = 0.25) comparing to the stress group.
Doxepin in the stress-doxepin 5 mg/kg group, decreased the mRNA expression of Bad 85.97% (P = 0.006), Bax 75.9% (P = 0.017), Bcl-2 14.26% (P = 0.64), and MAPK 33% (P = 0.83); and increased the mRNA expression of TNF-α 9.93% (P = 0.9), and AKT 1.22% (P = 0.98) comparing to the stress group.
DISCUSSION
Results showed that stress caused significant up-regulation in the expression of Bcl-2, Bad, and Bax in the hippocampus. Although, the up-regulation of gene expression of TNF-α, MAPK 14, and AKT1 also observed following the stress, but these changes were not statistically significant.
In accordance with our results, studies have shown that stress increases the expression of Bcl-2 mRNA and its protein in the hippocampus(27,28) and Bad and Bax genes in neurons(29,30). Bcl-2 is anti-apoptotic and Bax and Bad are pro-apoptotic factors(31). These may indicate that stress through the induction of pro-apoptotic factors could damage the neurons, and the up-regulation of Bcl-2 may be a compensatory response(27,28).
In the present study, changes in TNF-α mRNA expression were not significant. But previously we have seen that chronic restraint stress can increase levels of TNF-α protein in the hippocampus(13). Thus, the expression probably increased in the early stages of stress, and then reduced after the production of its protein. However, more studies are needed to prove this assumption.
The MAPK14 gene encodes P38 mitogen-activated protein kinase. This kinase is activated by various environmental stressors and pro-inflammatory cytokines(32). It has been shown that enhancement of the p38 MAPK activity plays a key role in neuronal apoptosis, and its inhibition can block apoptotic pathway in hippocampus(33). Serine/threonine protein kinase is an enzyme encoded by the AKT1. It is an important mediator of growth factor-induced neuronal survival. This enzyme by phosphorylating a large number of proteins inhibits components of the apoptotic machinery(34). The expression of these both genes wasn’t affected by stress after three weeks from initiation of the stress. However, this does not exclude the possibility of increasing the early and transient expression. Therefore, it is essential to investigate the levels and activity of their proteins.
Secondarily, we evaluated the potential effects of doxepin on the expression of these genes in the hippocampus of the stressed rats. Interestingly, doxepin could decrease the increased levels of expression of the Bad and Bax genes in stress group, but had no significant effects on the Bcl-2 gene. In our previous study, we demonstrated that doxepin could decrease gene expression of Bad in the hippocampus of intact rats(24). This suggests that doxepin mainly decreases the RNA expression of pro-apoptotic genes, however, according to the initial effects of stress on the rise of expression of Bcl-2 gene that inhibits apoptosis, it can be concluded that doxepin could prevent neuronal apoptosis effectively in stress.
Although in our previous study, we observed that low dose of doxepin (1 mg/kg) decreased the increased levels of TNF-α protein in the hippocampus of the stressed rats(13), but present results show that both stress and doxepin have no significant effects on the gene expression of TNF-α, and also MAPK and AKT after a 21-day period of stress and treatments. Therefore, over a long period, the expression of these genes cannot be a candidate for the effects of stress and doxepin, although this suggestion needs to be elucidated.
Another interesting result was the effect of doxepin at 1 mg/kg on suppression of HPA axis and reduction of adrenal glands weight. As both doses of doxepin exhibited similar effects on the gene expression, it can be postulated that its effects on gene expression is independent of HPA axis and glucocorticoids.
CONCLUSION
To determine the potential neurobiological effects of doxepin, we applied a chronic stress protocol and then evaluated the hippocampus for molecular indices that may be involved in this psychopathology. We observed that stress mainly affects the expression of Bcl-2 family genes in the hippocampus and that doxepin changes the pattern of that expression to maintain neuronal survival. Thus, doxepin can be considered as a neuroprotective agent against stress.
ACKNOWLEDGEMENTS
The content of this paper is extracted from the MSc. thesis of student Nastaran Eidelkhani (No. 393045) and a research project (No. 293251) which was financially supported by Applied Physiology Research Center, Isfahan University of Medical Sciences, Isfahan, Iran.
REFERENCES
- 1.Lee TH, Kim K, Shin MS, Kim CJ, Lim BV. Treadmill exercise alleviates chronic mild stress-induced depression in rats. J Exerc Rehabil. 2015;11(6):303–310. doi: 10.12965/jer.150265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lupien SJ, McEwen BS, Gunnar MR, Heim C. Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nat Rev Neurosci. 2009;10(6):434–445. doi: 10.1038/nrn2639. [DOI] [PubMed] [Google Scholar]
- 3.Roozendaal B, McEwen BS, Chattarji S. Stress, memory and the amygdala. Nat Rev Neurosci. 2009;10(6):423–433. doi: 10.1038/nrn2651. [DOI] [PubMed] [Google Scholar]
- 4.Tsolakidou A, Czibere L, Putz B, Trumbach D, Panhuysen M, Deussing JM, et al. Gene expression profiling in the stress control brain region hypothalamic paraventricular nucleus reveals a novel gene network including amyloid beta precursor protein. BMC Genomics. 2010;11:546. doi: 10.1186/1471-2164-11-546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Reimertz C, Kogel D, Rami A, Chittenden T, Prehn JH. Gene expression during ER stress-induced apoptosis in neurons: induction of the BH3-only protein Bbc3/PUMA and activation of the mitochondrial apoptosis pathway. J Cell Biol. 2003;162(4):587–597. doi: 10.1083/jcb.200305149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sadeghi M, Radahmadi M, Reisi P. Effects of repeated treatment with cholecystokinin sulfated octapeptide on passive avoidance memory under chronic restraint stress in male rats. Adv Biomed Res. 2015;4:150. doi: 10.4103/2277-9175.161577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moghaddasi M, Javanmard SH, Reisi P, Tajadini M, Taati M. The effect of regular exercise on antioxidant enzyme activities and lipid peroxidation levels in both hippocampi after occluding one carotid in rat. J Physiol Sci. 2014;64(5):325–332. doi: 10.1007/s12576-014-0322-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hamidi G, Arabpour Z, Shabrang M, Rashidi B, Alaei H, Sharifi MR, et al. Erythropoietin improves spatial learning and memory in streptozotocin model of dementia. Pathophysiology. 2013;20(2):153–158. doi: 10.1016/j.pathophys.2013.01.001. [DOI] [PubMed] [Google Scholar]
- 9.Hosseini N, Alaei H, Reisi P, Radahmadi M. The effect of treadmill running on memory before and after the NBM-lesion in rats. J Bodyw Mov Ther. 2013;17(4):423–429. doi: 10.1016/j.jbmt.2012.12.005. [DOI] [PubMed] [Google Scholar]
- 10.Pham K, Nacher J, Hof PR, McEwen BS. Repeated restraint stress suppresses neurogenesis and induces biphasic PSA-NCAM expression in the adult rat dentate gyrus. Eur J Neurosci. 2003;17(4):879–886. doi: 10.1046/j.1460-9568.2003.02513.x. [DOI] [PubMed] [Google Scholar]
- 11.Popoli M, Yan Z, McEwen BS, Sanacora G. The stressed synapse: the impact of stress and glucocorticoids on glutamate transmission. Nat Rev Neurosci. 2012;13(1):22–37. doi: 10.1038/nrn3138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Reisi P, Alaei H, Babri S, Sharifi MR, Mohaddes G, Soleimannejad E. Determination of the extracellular basal levels of glutamate and GABA at dentate gyrus of streptozotocin-induced diabetic rats. Pathophysiology. 2009;16(1):63–66. doi: 10.1016/j.pathophys.2009.03.003. [DOI] [PubMed] [Google Scholar]
- 13.Azadbakht AA, Radahmadi M, Javanmard SH, Reisi P. The effects of doxepin on stress-induced learning, memory impairments, and TNF-alpha level in the rat hippocampus. Res Pharm Sci. 2015;10(5):460–465. [PMC free article] [PubMed] [Google Scholar]
- 14.Bliss EL, Ailion J, Zwanziger J. Metabolism of norepinephrine, serotonin and dopamine in rat brain with stress. J Pharmacol Exp Ther. 1968;164(1):122–134. [PubMed] [Google Scholar]
- 15.Roth KA, Mefford IM, Barchas JD. Epinephrine, norepinephrine, dopamine and serotonin: differential effects of acute and chronic stress on regional brain amines. Brain Res. 1982;239(2):417–424. doi: 10.1016/0006-8993(82)90519-4. [DOI] [PubMed] [Google Scholar]
- 16.Uchihara Y, Tanaka K, Asano T, Tamura F, Mizushima T. Superoxide dismutase overexpression protects against glucocorticoid-induced depressive-like behavioral phenotypes in mice. Biochem Biophys Res Commun. 2016;469(4):873–877. doi: 10.1016/j.bbrc.2015.12.085. [DOI] [PubMed] [Google Scholar]
- 17.Weiss JM, Goodman PA, Losito BG, Corrigan S, Charry JM, Bailey WH. Behavioral depression produced by an uncontrollable stressor: relationship to norepinephrine, dopamine, and serotonin levels in various regions of rat brain. Brain Res Rev. 1981;3(2):167–205. [Google Scholar]
- 18.Eidelkhani N, Radahmadi M, Kazemi M, Rafiee L, Alaei H, Reisi P. Effects of doxepin on brain-derived neurotrophic factor, tumor necrosis factor alpha, mitogen-activated protein kinase 14, and AKT1 genes expression in rat hippocampus. Adv Biomed Res. 2015;4:203. doi: 10.4103/2277-9175.166139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hisaoka-Nakashima K, Kajitani N, Kaneko M, Shigetou T, Kasai M, Matsumoto C, et al. Amitriptyline induces brain-derived neurotrophic factor (BDNF) mRNA expression through ERK-dependent modulation of multiple BDNF mRNA variants in primary cultured rat cortical astrocytes and microglia. Brain Res. 2016;1634:57–67. doi: 10.1016/j.brainres.2015.12.057. [DOI] [PubMed] [Google Scholar]
- 20.Jevtic G, Nikolic T, Mircic A, Stojkovic T, Velimirovic M, Trajkovic V, et al. Mitochondrial impairment, apoptosis and autophagy in a rat brain as immediate and long-term effects of perinatal phencyclidine treatment – influence of restraint stress. Prog Neuropsychopharmacol Biol Psychiatry. 2016;66:87–96. doi: 10.1016/j.pnpbp.2015.11.014. [DOI] [PubMed] [Google Scholar]
- 21.McLaughlin KJ, Gomez JL, Baran SE, Conrad CD. The effects of chronic stress on hippocampal morphology and function: an evaluation of chronic restraint paradigms. Brain Res. 2007;1161:56–64. doi: 10.1016/j.brainres.2007.05.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jafary L, Reisi P, Naghsh N. Effects of fluoxetine on memory under forced treadmill exercise conditions in male rats. Adv Biomed Res. 2015;4:235. doi: 10.4103/2277-9175.167962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ji BS, Ji H, Liu GQ. Doxepin protects cultured neurons against oxidative stress-induced injury. Acta Pharmacol Sin. 2004;25(3):297–300. [PubMed] [Google Scholar]
- 24.Eidelkhani N, Radahmadi M, Rafiee L, Gharzi M, Alaei H, Reisi P. Effects of doxepin on spatial memory, TNF-α and Bcl-2 family genes expression in rat hippocampus. Physiol Pharmacol. 2015;19(3):185–192. [Google Scholar]
- 25.Gharzi M, Dolatabadi HR, Reisi P, Javanmard SH. Effects of different doses of doxepin on passive avoidance learning in rats. Adv Biomed Res. 2013;2:66. doi: 10.4103/2277-9175.115823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hosseini N, Alaei H, Reisi P, Radahmadi M. The effect of treadmill running on passive avoidance learning in animal model of Alzheimer disease. Int J Prev Med. 2013;4(2):187–192. [PMC free article] [PubMed] [Google Scholar]
- 27.Moretti M, Budni J, Dos Santos DB, Antunes A, Daufenbach JF, Manosso LM, et al. Protective effects of ascorbic acid on behavior and oxidative status of restraint-stressed mice. J Mol Neurosci. 2013;49(1):68–79. doi: 10.1007/s12031-012-9892-4. [DOI] [PubMed] [Google Scholar]
- 28.Murthy SR, Thouennon E, Li WS, Cheng Y, Bhupatkar J, Cawley NX, et al. Carboxypeptidase E protects hippocampal neurons during stress in male mice by up-regulating prosurvival BCL2 protein expression. Endocrinology. 2013;154(9):3284–3293. doi: 10.1210/en.2013-1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gerecke KM, Kolobova A, Allen S, Fawer JL. Exercise protects against chronic restraint stress-induced oxidative stress in the cortex and hippocampus. Brain Res. 2013;1509:66–78. doi: 10.1016/j.brainres.2013.02.027. [DOI] [PubMed] [Google Scholar]
- 30.Huang P, Li C, Fu T, Zhao D, Yi Z, Lu Q, et al. Flupirtine attenuates chronic restraint stress-induced cognitive deficits and hippocampal apoptosis in male mice. Behav Brain Res. 2015;288:1–10. doi: 10.1016/j.bbr.2015.04.004. [DOI] [PubMed] [Google Scholar]
- 31.Alabsi AM, Lim KL, Paterson IC, Ali-Saeed R, Muharram BA. Cell cycle arrest and apoptosis induction via modulation of mitochondrial integrity by Bcl-2 family members and caspase dependence in dracaena cinnabari-treated H400 human oral squamous cell carcinoma. Biomed Res Int. 2016;2016:4904016. doi: 10.1155/2016/4904016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Han D, Scott EL, Dong Y, Raz L, Wang R, Zhang Q. Attenuation of mitochondrial and nuclear p38alpha signaling: a novel mechanism of estrogen neuroprotection in cerebral ischemia. Mol Cell Endocrinol. 2015;400:21–31. doi: 10.1016/j.mce.2014.11.010. [DOI] [PubMed] [Google Scholar]
- 33.Yang S, Zhou G, Liu H, Zhang B, Li J, Cui R, et al. Protective effects of p38 MAPK inhibitor SB202190 against hippocampal apoptosis and spatial learning and memory deficits in a rat model of vascular dementia. Biomed Res Int. 2013;2013:215798. doi: 10.1155/2013/215798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pan J, Pei DS, Yin XH, Hui L, Zhang GY. Involvement of oxidative stress in the rapid Akt1 regulating a JNK scaffold during ischemia in rat hippocampus. Neurosci Lett. 2006;392(1-2):47–51. doi: 10.1016/j.neulet.2005.08.057. [DOI] [PubMed] [Google Scholar]