Abstract
This study investigated the anticonvulsant activity and possible mechanism of action of an aqueous solution of Dorema ammoniacum gum (DAG) which has been used traditionally in the treatment of convulsions. In this study, the anticonvulsant activity of DAG was examined using the pentylentetrazole (PTZ) model in mice. Thirty male albino mice were divided randomly and equally to 5 groups, and pretreated with normal saline, diazepam, or various doses of DAG (500, 700, and 1000 mg/kg, i.p.), prior to the injection of PTZ (60 mg/kg, i.p.). The latency and duration of seizures were recorded 30 min after PTZ injection. Pretreatments with naloxone and flumazenil in different groups were studied to further clarify the mechanisms of the anticonvulsant action. Phytochemical screening and thin layer chromatography (TLC) fingerprinting of ammoniacum gum was also determined. DAG showed significant anticonvulsant activity at all doses used. The gum delayed both the onset and the duration of seizures induced by PTZ. Treatment with flumazenil before DAG (700 mg/kg) inhibited the effect of gum on seizure duration and latency to some extent and administration of naloxone before DAG also significantly inhibited changes in latency and duration of seizure produced by DAG. The percentage inhibition was greater with naloxone than with flumazenil. This study showed that DAG had significant anticonvulsant activity in PTZ-induced seizures, and GABAergic and opioid systems may be involved. More studies are needed to further investigate its detailed mechanism.
Keywords: Dorema ammoniacum, Anticonvulsant, Flumazenil, Naloxane, Pentylentetrazole
INTRODUCTION
Epilepsy is a common neurological disorder which is identified by recurrent spontaneous seizures and affects about 50 million people worldwide(1,2,3,4).
There is an increasing need to explore new natural medicines due to inadequacies of conventional treatments(5).
There are some herbs in Iranian traditional medicine that have been used for the treatment of epilepsy(6). Dorema ammoniacum gum (DAG), known as Oshagh, is recommended for treatment of seizures in Iranian traditional medicine(7,8). It is an oleo gum resin obtained from the stem and leaf of Dorema ammoniacum D. Don (Umbelliferae)(9). Genus Dorema exhibited six species in Iran, among which two are endemic, namely, D. ammoniacum and D. aucheri Boiss(10).
D. ammoniacum grows to a height of 1–2 m in many arid and semi-arid regions of Iran, such as Yazd, Isfahan and Semnan(10,11). This plant produces a medicinal gum resin commonly known as ammoniacum gum which has medicinal and industrial applications(12).
According to our investigations, the antiepileptic effects of DAG have not previously been studied.
Therefore, in the present study, for the first time, the anticonvulsant activity and possible mechanisms of action of DAG were studied using pentylentetrazole (PTZ)-induced seizures in rats.
MATERIALS AND METHODS
Plant material
The DAG was purchased from a local herbal market in Tehran, Iran and its authenticity was approved by Dr. G. Amin, School of Pharmacy, Tehran University of Medical Sciences, Tehran, Iran. A voucher specimen of the plant was deposited in the herbarium of School of Pharmacy (DMP-819). The gum was dissolved in distilled water and the aqueous solution was used for the study.
Animals
One hundred and fourteen healthy male albino mice weighing 25–30 g were housed in the animal unit of Iran University of Medical Sciences under standard laboratory conditions (temperature 23 ± 2 °C) with 12-h dark and 12-h light cycle. The animals had free access to a standard dry pellet diet and tap water ad libitum. All possible steps were taken to avoid animal suffering at each stage of the experiments. All procedures involving animals were conducted according to the guidelines for the care and use of mammals in neuroscience and behavioral research (National Research Council 2003) and guideline of Institutional Animal Ethical Committee (IUMS-AC).
Preliminary phytochemical screening
Phytochemical investigation of DAG was carried out using standard methods and tests as published previously (13–15). The test for tannins was carried out by subjecting 1 g of extract in 2 mL of distilled water; the mixture was filtered and ferric chloride reagent was added to the filtrate. The gum was subjected to the “frothing” test for the identification of saponins and to fehling's test for glycosides. Alkaloids were detected in the alkaloid fraction obtained by a classical acid:base extraction procedure for alkaloids and analyzed by TLC using chloroform: methanol:ammonia solution 25% (8:2:0.5) as solvent system, spots were detected by spraying with Dragendorff's reagent. The presence of flavonoids was determined by adding 1% aluminum chloride solution to the extract and examining for yellow coloration. In another test for flavonoids, diluted ammonia (5 mL) was added to the extract followed by concentrated sulfuric acid (1 mL). Steroids were detected by adding 1 mL of acetic anhydride to 0.25 g of a methanolic extract of sample followed by 1 mL H2 SO4. A color change from violet to blue or green indicated the presence of steroids. The test for anthraquinones was performed by combining 0.5 g of extract boiled with 10 mL sulfuric acid and filtered; the filtrate was then shaken with 5 mL chloroform and the chloroform layer was transferred to another tube and 1 mL of ammonia was added and the color change was observed. Detection of terpenoids was carried out by adding 2 mL of chloroform to 0.5 g of extract and then carefully adding concentrated sulfuric acid (3 mL) to form a layer with a reddish to brown color at the interface.
High performance thin layer chromatography analysis
A finger print profile of the DAG extract was established using high performance thin layer chromatography analysis (HPTLC) (CAMAG, Switzerland). The method was described by Rajani, et al. The gum extract was dissolved in methanol (100 μL sample + 900 μL methanol) as stock solution. 10 μL of solution was applied on a precoated plate (Silica gel 60F 254) using a TLC spotter (CAMAG Automatic TLC Sampler 4 (ATS4)) and developed in a solvent system of n-hexane:ethylacetate:methanol (8:2:1 v/v). The plate was dried and analyzed with a TLC scanner (CAMAG Scanner 3) at 254 and 366 nm, and chromatograms and absorption spectra were recorded(9).
Chemicals
PTZ (Sigma, USA), diazepam (Darupakhsh, Iran) and flumazenil (Darupakhsh, Iran) were purchased from commercial sources.
Experimental studies
Anticonvulsant activity
Seizures were induced in mice with the standard convulsing agent, PTZ (60 mg/kg, i.p.) according to the method described by Corda, et al.(16). The animals were randomly divided into five groups of 6 each. Group I served as the control and received normal saline (10 mL/kg, i.p.), Group II was treated with diazepam (1 mg/kg, i.p.), as a positive control group and groups III to V received DAG at doses of 500, 700, 1000 mg/kg i.p. After 30 min, all groups received PTZ. Animals which did not exhibit seizure after 30 min were considered as protected. The latency and duration of seizures were recorded 30 min after PTZ injection in the unprotected animals. Also, animals were observed for mortality for 24 h after PTZ administration.
The percentage protection was determined comparing the gum solution to diazepam, as established anti-seizure agent. The protection of diazepam considered to be 100 % and other agents were compared to diazepam.
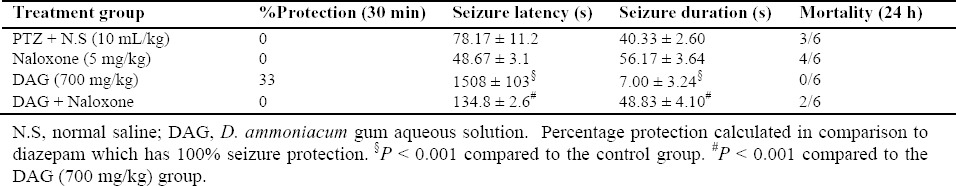
Effect of flumazenil on the anticonvulsant activity of D. ammoniacum gum
The effect of flumazenil, a benzodiazepine receptor antagonist, on the anticonvulsant activity of DAG was studied in order to investigate the possible involvement of benzodiazepine receptors(17). Six groups of mice were selected (ten mice in each group); the first group, was given flumazenil (2 mg/kg) 5 min before the administration of DAG (700 mg/kg, i.p.) and 35 min before the injection of PTZ. In the second group, the animals received flumazenil (2 mg/kg) 5 min before the administration of diazepam (1 mg/kg). Also, three groups were injected with diazepam (1 mg/kg, i.p.), flumazenil (2 mg/kg) or normal saline 30 min before the administration of PTZ (60 mg/kg, i.p.) respectively. The latency and duration of convulsions at 30 min after injection of PTZ, protection percentage (the percentage which is determined comparing to diazepam as established anti-seizure agent) and also mortality rate during the first 24 h were recorded. The anticonvulsant activity of DAG and diazepam in mice pretreated with flumazenil was assessed and compared with normal saline (10 mL/kg), flumazenil (2 mg/kg), diazepam (0.5 mg/kg) and DAG (700 mg/kg, i.p.) treated animals.
Effect of naloxone on the anticonvulsant activity of D. ammoniacum gum
Four groups of mice were selected (six mice in each) for further investigation of the possible involvement of opioid receptors in the anticonvulsant activity of DAG(18,19). Naloxone, an opioid receptor antagonist, was given at a dose of 5 mg/kg, 5 min before the administration of DAG (700 mg/kg i.p.) and 35 min before the injection of PTZ in groups of six mice each. The anticonvulsant activity of DAG in the groups pretreated with naloxone was assessed and compared with animals pretreated with DAG alone (700 mg/kg i.p.), with the naloxone (5 mg/kg) and normal saline (10 mL/kg) groups.
Statistical analysis
The results are reported as mean ± S.E.M and tested with one way ANOVA followed by the multiple comparison test of Tukey-Kramer. Results with P < 0.05 were considered significant.
RESULTS
Preliminary phytochemical screening
The values obtained from the preliminary phytochemical study of DAG is described in Table 1.
Table 1.
The results of a qualitative phytochemical screening of D. ammoniacum gum.
The TLC fingerprint of D. ammoniacum gum
Ammoniacum gum extract was identified by TLC fingerprinting and the details are given in Table 2 and Fig. 1. The TLC fingerprint was comparable to that found in a previous study on DAG(9).
Table 2.
Thin layer chromatography fingerprint of D. ammoniacum gum.
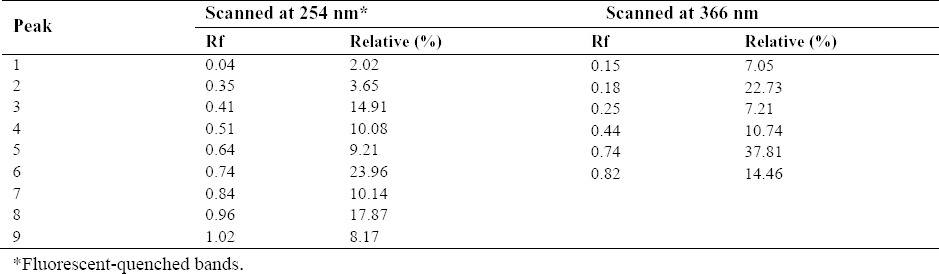
Fig. 1.
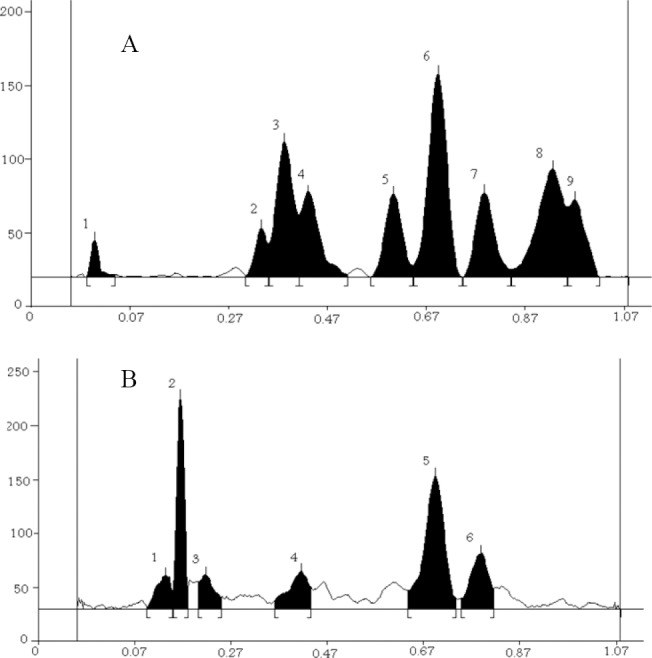
High performance thin layer chromatography analysis of D. ammoniacum gum extract. Thin layer chromatography analysis scanned at (A) UV 254 nm and (B) UV 366 nm.
Anticonvulsant activity on pentylentetrazole induced seizure
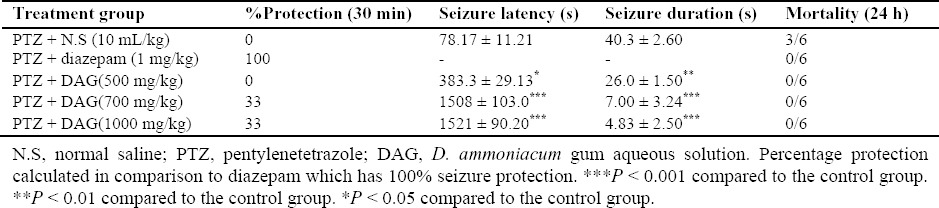
Injection of the DAG (500 mg/kg) significantly prolonged the onset of seizure activity (P < 0.05) and decreased seizure duration (P < 0.01) compared to the group treated with saline.
Furthermore, DAG at doses of 700 mg/kg and 1000 mg/kg also prolonged latency and decreased duration significantly (P < 0.001). In addition, DAG at doses of 700 and 1000 mg/kg, exhibited protection against seizures in 33% of animals and reduced mortality when compared to the control group.
Diazepam (1 mg/kg) protected 100% of the mice from PTZ-induced seizures and mortality (Table 3).
Table 3.
Effect of D. ammoniacum gum solution on pentylentetrazole-induced seizures in mice (data expressed as mean ± S.E.M., n = 6).
The effect of flumazenil on the anticonvulsant activity of D. ammoniacum gum
Administration of flumazenil (2 mg/kg) 5 min before DAG (700 mg/kg) and 20 min before the injection of PTZ resulted in inhibition of the prolongation of latency and reduction in the duration of seizures exhibited by the gum solution (P < 0.01). Thus, flumazenil attenuated the action of DAG to some extent. Flumazenil also antagonized the anticonvulsant activity of diazepam (Table 4).
Table 4.
Effect of flumazenil on the anticonvulsant activity of D. ammoniacum gum solution in pentylentetrazole-induced convulsion in mice (data expressed as mean ± S.E.M., n = 6).
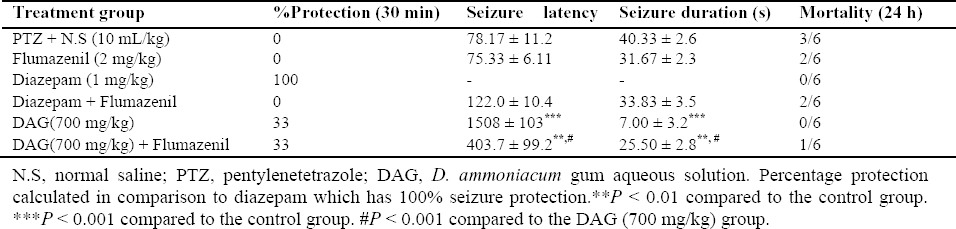
The effect of naloxone on the anticonvulsant activity of D. ammoniacum gum
In the experimental group receiving naloxone (5 mg/kg) before DAG (700 mg/kg) and PTZ, there were no significant changes in latency or duration of seizures compared to the control group. Naloxone reduced the protective effects of DAG in prolonging seizure latency and reducing the duration of seizures and this inhibitory effect was more pronounced than that of flumazenil (Table 5).
Table 5.
Effect of naloxone on the anticonvulsant activity of D. ammoniacumgumsolution in pentylentetrazole induced convulsion in mice (the data were expressed as mean ± S.E.M., n = 6).
DISCUSSION
Given that the treatment of epilepsy with currently available antiepileptic medications is frequently sub-optimal, interest in discovering new alternative drugs from natural sources has increased (5,20). There are some herbs and herbal compounds which showed anticonvulsant activity (16). Iranian traditional medicine has utilized some herbs or herbal preparations such as D. ammoniacum (Called Oshagh) for the treatment of epilepsy(7,8).
Ammoniacum gum has been traditionally used for different purposes in Iran. It is also used for some CNS disorders such as epilepsy and for reducing joint pain(7,12,21). Previous studies showed antimicrobial, cytotoxic and acetylcholinesterase inhibitory activity of Dorema ammoniacum(22,23,24)
The TLC fingerprinting of ammoniacum gum was confirmed using HPTLC and it was comparable to that from a previous study on ammoniacum gum(9). In this study, DAG produced a statistically significant reduction in seizures’ duration and an increase in the latency period of seizures induced by PTZ in the mouse model. This effect was appeared in all doses used. No death was recorded even at highest dose of DAG used (1000 mg/kg). This suggested that the gum aqueous solution is relatively safe and non-toxic in mice even at the highest dose used in this study.
The main mechanism of PTZ-induced seizures involves the reduction in the level of γ-aminobutyric acid (GABA) in the cortex. GABA is considered to be a major inhibitory neurotransmitter in the CNS of mammals and decreased GABA activity has been implicated in convulsions, as GABA mediates the inhibition of neuronal responsiveness and activity by increasing chloride-ion conductance through the opening of the chloride-ion channel,(16,25). The findings of the present study suggest that DAG might have delayed the occurrence of PTZ-induced seizure activity and reduced its duration by acting on the GABAergic system. Since opiates have been involved in many functions in CNS, naloxone was used to evaluate the possible role of opioid system in effects of DAG. Naloxone decreased the prolongation of seizure latency induced by DAG and also antagonized the effect of DAG on decreasing the duration of seizures in the PTZ model. This attenuation of the anticonvulsant effect of DAG by an opiate receptor antagonist suggests the involvement of opiate receptor activation in the effects of DAG(26,27,28). Administration of flumazenil before DAG led to an increase in the latency and a reduction in the duration of seizures compared to the control group. This shows that flumazenil antagonizes the effect of DAG on latency time and duration of seizures in the PTZ model. These results, suggest that the anticonvulsant effect of DAG probably involves activation of benzodiazepine receptors. Since benzodiazepine receptors are present on the GABA receptor complex, it can be deduced that the effect of DAG might involve binding sites modulating the activity of GABA receptor complex. It has been found that many flavonoids can act as benzodiazepine-like molecules in the central nervous system (CNS) and modulate GABA-generated chloride currents in animal models of anxiety, sedation and convulsion(29). It is possible that the anticonvulsant activities of DAG are related to its flavonoid content (Table 1). Finally the results obtained from this work suggested that an aqueous solution of DAG had anticonvulsant activity, hence supports the traditional use of the plant in the treatment of convulsive disorders. This observed activity of the plant extract on PTZ-induced seizures could be due to the involvement of the GABAergic pathway. Further investigation is required to determine which components in the DAG extract may be responsible for the observed activity and determine their specific mechanism of action(25).
CONCLUSION
This study showed that DAG had significant anticonvulsant activity in PTZ-induced seizures, in a dose dependent manner via involvement of GABAergic and opioid systems. More studies are needed to further elucidate its detailed underlying mechanism.
ACKNOWLEDGMENTS
This work was supported by Iran University of Medical Sciences (Grant Number 27535). We also thank Mr. Hosseinzadeh for his technical assistance in HPTLC analysis.
REFERENCES
- 1.Moshi MJ, Kagashe GA, Mbwambo ZH. Plants used to treat epilepsy by Tanzanian traditional healers. J Ethnopharmacol. 2005;97(2):327–336. doi: 10.1016/j.jep.2004.11.015. [DOI] [PubMed] [Google Scholar]
- 2.Arab MR, Suratgar AA, Martínez-Hernández VM, Rezaei Ashtian A. Electroencephalogram signals processing for the diagnosis of petit mal and grand mal epilepsies using an artificial neural network. JART. 2010;8(1):120–129. [Google Scholar]
- 3.Hadizadeh F, Rahimi B, Taghiabadi E, Razavi M, Karimi G. Evaluation of anticonvulsant effect of two novels 4-[1-(4-fluorobenzyl)-5-imidazolyl] dihydropyridine derivatives in mice. Res Pharm Sci. 2013;8(2):91–95. [PMC free article] [PubMed] [Google Scholar]
- 4.Palizvan MR, Ghaznavi-Rad E. Naloxane enhanced inhibitory effect of verapamil on seizure induced by pentylenetetrazol in male rats. Res Pharm Sci. 2014;9(4):295–299. [PMC free article] [PubMed] [Google Scholar]
- 5.Engel J., Jr Surgery for seizures. N Engl J Med. 1996;334(10):647–653. doi: 10.1056/NEJM199603073341008. [DOI] [PubMed] [Google Scholar]
- 6.Abdollahi Fard M, Shojaii A. Efficacy of Iranian traditional medicine in the treatment of epilepsy. BioMed Res Int. 2013;2013:692751. doi: 10.1155/2013/692751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Khorasani MA. Research institute for Islamic and Complementary Medicine. Tehran, Iran: Iran University of Medical Sciences; 2001. Makhzan al Advieh. Bavardaran Press; pp. 135–136. [Google Scholar]
- 8.Tonkaboni MM. In: Tohfeh Al- Momenin. Rahimi R., Shams M. R., Ardekani A, editors. Tehran, Iran.: Shahid Beheshti University of Medical Sciences; 2007. p. 53. [Google Scholar]
- 9.Rajani M, Ravishankara M, Shrivastava N, Padh H. HPTLC-aided phytochemical fingerprinting analysis as a tool for evaluation of herbal drugs. A case study of Ushaq (Ammoniacum gum) JPC J Pl Chrom, Modern TLC. 2001;14(1):34–41. [Google Scholar]
- 10.Mozaffarian V. A dictionary of Iranian plant names: Latin, English. Persian: Farhang Mo’aser; 1996. pp. 190–191. [Google Scholar]
- 11.Rechinger KH, Browicz K, Persson K, Wendelbo P. Flora iranica: Akademische Druck-u. Verlagsanstalt. 1969:51. [Google Scholar]
- 12.Amin GR. Popular medicinal plants of Iran: Iranian Research Institute of Medicinal Plants Tehran. 1991:262. [Google Scholar]
- 13.Tona L, Kambu K, Ngimbi N, Cimanga K, Vlietinck A. Antiamoebic and phytochemical screening of some Congolese medicinal plants. J Ethnopharmacol. 1998;61(1):57–65. doi: 10.1016/s0378-8741(98)00015-4. [DOI] [PubMed] [Google Scholar]
- 14.Ayoola G, Coker H, Adesegun S, Adepoju-Bello A, Obaweya K, Ezennia E, et al. Phytochemical screening and antioxidant activities of some selected medicinal plants used for malaria therapy in Southwestern Nigeria. Trop J Pharm Res. 2008;7(3):1019–1024. [Google Scholar]
- 15.Jia Q, Su W, Peng W, Li P, Wang Y. Anti-diarrhoea and analgesic activities of the methanol extract and its fractions of Jasminum amplexicaule Buch.-Ham.(Oleaceae) J Ethnopharmacol. 2008;119(2):299–304. doi: 10.1016/j.jep.2008.07.014. [DOI] [PubMed] [Google Scholar]
- 16.Corda MG GO, Longoni B, Orlandi M, Biggio G. Decrease in the function of the gamma-aminobutyric acid-coupled chloride channel produced by the repeated administration of pentylenetetrazol to rats. J Neurochem. 1990;55(4):1216–1221. doi: 10.1111/j.1471-4159.1990.tb03127.x. [DOI] [PubMed] [Google Scholar]
- 17.Hosseinzadeh H, Parvardeh S. Anticonvulsant effects of thymoquinone, the major constituent of Nigella sativa seeds, in mice. Phytomed. 2004;11(1):56–64. doi: 10.1078/0944-7113-00376. [DOI] [PubMed] [Google Scholar]
- 18.Lauretti GR, Ahmad I, Pleuvry B. The activity of opioid analgesics in seizure models utilizing N-methyl-DL-aspartic acid, kainic acid, bicuculline and pentylenetetrazole. Neuropharmacol. 1994;33(2):155–160. doi: 10.1016/0028-3908(94)90002-7. [DOI] [PubMed] [Google Scholar]
- 19.Kaminski RM, Witkin JM, Shippenberg TS. Pharmacological and genetic manipulation of kappa opioid receptors: Effects on cocaine-and pentylenetetrazol-induced convulsions and seizure kindling. Neuropharmacol. 2007;52(3):895–903. doi: 10.1016/j.neuropharm.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 20.Arab M, Suratgar A, Ashtiani A. Electroencephalogram signals processing for topographic brain mapping andepilepsies classification. Comput Biol Med. 2010;40:733–739. doi: 10.1016/j.compbiomed.2010.06.001. [DOI] [PubMed] [Google Scholar]
- 21.Zargari A. Medicinal Plants: Tehrari University Publications. ISBN. 1995:602–605. [Google Scholar]
- 22.Rajani M, Saxena N, Ravishankara M, Desai N, Padh H. Evaluation of the antimicrobial activity of ammoniacum gum from Dorema ammoniacum. Pharm Biol. 2002;40(7):534–541. [Google Scholar]
- 23.Yousefzadi M, Heidari M, Akbarpour M, Mirjalili MH, Zeinali A, Parsa M. In vitro cytotoxic activity of the essential oil of Dorema ammoniacum D. Don. Middle-East J Sci Res. 2011;7(4):511–514. [Google Scholar]
- 24.Adhami H-R, Lutz J, Kählig H, Zehl M, Krenn L. Compounds from Gum ammoniacum with acetylcholinesterase inhibitory activity. Sci Pharm. 2013;81(3):793–805. doi: 10.3797/scipharm.1306-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Riazi K, Honar H, Homayoun H, Rashidi N, Dehghani M, Sadeghipour H, et al. Sex and estrus cycle differences in the modulatory effects of morphine on seizure susceptibility in mice. Epilepsia. 2004;45(9):1035–1042. doi: 10.1111/j.0013-9580.2004.69903.x. [DOI] [PubMed] [Google Scholar]
- 26.AliMohammadi B, Moslem A, Azhdari Zarmehri H, Kamranian H. The roles of opioid receptors on anticonvulsant properties of hydro alcoholic extract of scrophularia striata Boiss in mice. J Sabzevar Uni Med Sci. 2016;23(2):204–213. [Google Scholar]
- 27.Nassiri-Asl M, Shariati-Rad s, Zamansoltani F. Anticonvulsant effects of aerial parts of Passiflora incarnata extract in mice: involvement of benzodiazepine and opioid receptors. BMC Complem Altern M. 2007;7(26):1–6. doi: 10.1186/1472-6882-7-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jamilu Yu, Abdullahi Hamza Y, Aliyu MM, Ahmed A, Sani MY, Ben Ahmadu C. Mechanisms of anticonvulsant action of residual aqueous fraction (RAF) of the ethanol root bark extract of Carissa edulis. J Nat Sci Res. 2014;4(22):43–48. [Google Scholar]
- 29.Fernández SP, Wasowski C, Loscalzo LM, Granger RE, Johnston GA, Paladini AC, et al. Central nervous system depressant action of flavonoid glycosides. Eur J Pharm. 2006;539(3):168–176. doi: 10.1016/j.ejphar.2006.04.004. [DOI] [PubMed] [Google Scholar]


