Abstract
Nigella sativa (NS) (Ranunculaceae) used as a protective and therapeutic traditional medicine. This study evaluates the effect of NS on inflammation-induced myocardial fibrosis, serum and tissue inflammatory markers, and oxidative stress status in male rats. Fifty male Wistar rats were divided into five groups: (1) control; (2) lipopolysaccharide (LPS), 1 mg/kg/day; (3) LPS + NS (hydroalcoholic extract), 100 mg/kg/day; (4) LPS + NS, 200 mg/kg/day; (5) LPS + NS, 400 mg/kg/day (n = 10 in each group). The duration of LPS administration was two weeks. At the end of the experiment, blood samples were taken and ventricles were homogenized and stained for histological evaluation. Serum nitrite levels were lower in LPS group than the control group (22.98 ± 1.03 vs 28.5 ± 0.93 μmol/L), in which they were significantly increased by NS treatment (P < 0.05). Higher levels of heart interlukine-6 (IL-6) and tumor necrosis factor-α (TNF-α) were observed in LPS group compared to the controls (IL-6: 6805 ± 656 vs 4733 ± 691 pg/mL; TNF-α: 6504 ± 501 vs 5309 ± 452 pg/mL), in which they were reduced by NS 400 mg/kg compared to LPS groups (P < 0.05). A significant increment of malondialdehyde and reduction in heart total thiol, superoxide dismutase and catalase concentrations were observed in LPS group (p < 0.05) which significantly restored with treatment by three doses of NS. Histopathological studies showed higher inflammatory cell infiltrates, cardiac fibrosis, and collagen deposition in LPS group, which were reduced by the administration of NS. Treatment by NS reduced myocardial fibrosis in inflammation-induced fibrosis, possibly through improving oxidative/anti-oxidative balance.
Keywords: Lipopolysacchride, Heart, Collagen, Oxidative stress, Inflammation
INTRODUCTION
Plants are significant sources of new medications, and interest in medicinal plants has expanded because of the effectiveness of new plant-derived drugs. Moreover, as a result of worries about the side effects of conventional medicine, the utilization of natural products as a replacement for synthetic medications has risen considerably in the recent decades. The wide use of herbal medications has encouraged researchers to examine their amazing impacts on health, and an expansive number of therapeutic plants and their active extracted ingredients are broadly considered for their potentials to protect cells from injuries. Among the promising medicinal plants is Nigella sativa (NS) (Ranunculaceae)(1,2).NS is used as a protective and therapeutic traditional medicine in the Middle East and a number of Asian countries(3). It is rich in different phytochemicals whose benefits have been indicated in both in vivo and in vitro studies. This plant can be used as antioxidant, anti-inflammatory, anticancer, glucose lowering, antihistamine, immune booster, antimicrobial, and antiparasitic agent(1,4). NS also has several cardiovascular effects(1,5,6) including hypolipidemic, antiatherogenic, hypotensive, anti-platelet activities.
It also lowers heart rate and improves endothelial function and cardiac contractility(6). In addition, it has cardioprotective effects against chemical cardiotoxicity and hyperhomocysteinemia(1).
Endotoxin (lipopolysaccharide, LPS) is a component of the outer membrane of gram-negative bacteria and is widely used for the induction of inflammation(7). LPS binds to cell membrane receptors (toll-like receptors, or TLRs) of different cells, including endothelial cells and leukocytes, and releases numerous cytokines(8). Cardiac myocytes also have TLRs, especially TLR4(9). It is indicated that LPS brings down the contractile function of the heart, and since TLR4 is the only LPS receptor, it seems that TLR4 plays a role in the heart function(10). The NS oil and thymoquinone (TQ) have been found to exhibit anti-inflammatory effects through lowering the level of pro-inflammatory mediators such as interlukines 1β and 6 (IL-1β and IL-6), tumor necrosis factor-α (TNF-α), interferon-γ (IFN-γ) and increasing anti-inflammatory cytokines such as IL-10(11).
In addition, the extract of NS seeds and TQ inhibits nitric oxide generation and inducible nitric oxide synthase expression(12). However, the effect of NS on inflammation-induced cardiac fibrosis is not clear. In the present study, we, therefore, evaluated the effect of NS on inflammation-induced myocardial fibrosis, to see whether NS can improve serum and cardiac inflammatory markers, oxidative stress balance, and cardiac fibrosis.
MATERIALS AND METHODS
Animals
Adult male Wistar rats weighing between 250–300 g were obtained from local animal facilities. They were kept under controlled conditions with a 12-h light/dark cycle and free access to drinking water and food throughout the experiment. Rats were handled and divided into five groups that were treated as follows: (1) control (received 1 mL/kg/day saline) intraperitoneally (i.p); (2) LPS (1 mg/kg/day, i.p.); (3) LPS + NS (hydroalcoholic extract) 100 mg/kg (received LPS and NS extract at the dose of 100 mg/kg/day; i.p.); (4) LPS + NS extract 200 mg/kg (received LPS and NS extract at the dose of 200 mg/kg/day, i.p.); (5) LPS + NS extract 400 mg/kg (received LPS and NS extract at the dose of 400 mg/kg/day, i.p.) (n = 10 in each group). The duration of LPS administration was 14 days, and treatment with NS extracts started two days before LPS administration. The study protocol was approved by the bioethics committee of Mashhad University of Medical Sciences (ID, 940840).
At the end of the experiment, all animals were anaesthetized with ketamine (75 mg/kg) and xylazine (7.5 mg/kg), and blood samples were taken from the heart and centrifuged. Then, serum samples were stored at -70 °C for further analysis. The right ventricles were removed, washed with ice-cold saline, and homogenized in the appropriate buffer in a tissue homogenizer. The left ventricles were harvested and maintained in formalin 10% solution for later histological evaluations.
Chemicals
LPS from Escherichia coli (serotype 055:B5) was purchased from Sigma-Aldrich company (USA) The NS seeds were purchased from a local herbal shop in Mashhad, Khorasan province, Iran, and identified by botanists in the herbarium of the Ferdowsi University of Mashhad, Iran and prepared as follows: NS seed powder (100 g) was extracted by Soxhlet extractor with ethanol (70%) and the extract was concentrated under decreased pressure and kept at -20 °C until being utilized(13).
Measurement of inflammatory markers
Serum and heart nitrite levels were measured by Griess reagent method using Elisa kit (Promega Corp. USA; Cat No. G2930)(14,15). IL-6 and TNF-α levels were measured using available Elisa kits (eBioscience, Bender Med systems, GMBH, Austria). Other chemicals which were used for the evaluation of malondialdehyde (MDA), total thiol, superoxide dismutase (SOD) and catalase were purchased from Sigma Co, USA.
Measurement of oxidative stress indicators
MDA level is an index of lipid peroxidation. MDA responds to thiobarbituric acid (TBA) as a thiobarbituric acid receptive substance (TBARS) to deliver a red-hued complex which has top absorbance at 535 nm(16). Two mL of the reagent of TBA/TCA/HCL was added to 1 mL of homogenate, and the arrangement was warmed in a water bath for 40 min. In the wake of cooling, the entire arrangement was centrifuged at 1000 g for 10 min. The absorbance was measured at 535 nm. The MDA concentration was ascertained as follows:
C (m) = Absorbance / (1.65 × 105) (1)
Total thiol groups (SH) were measured using 5,5′-Dithiobis(2-nitrobenzoic acid) (DTNB)) as the reagent. This reagent responds to the thiol groups to create a yellow-hued complex which has maximal absorbance at 412 nm(17). Quickly, 1 mL Tris-EDTA (ethylenediaminetetraacetic acid) buffer (pH = 8.6) was added to 50 μL heart homogenates in cuvettes and the specimen absorbance was perused at 412 nm against Tris-EDTA buffer alone (A1). At that point, 20 μL DTNB reagent (10 mM in methanol) was added to the blend and kept at laboratory temperature. The sample absorbance was measured again after 15 min (A2). The absorbance of DTNB reagent was additionally detrmined as a blank (B). The total thiol concentration (mM) was computed from the following equation:
Total thiol concentration (mM) = (A2 – A1 – B) × 1.07 / (0.05 × 13.6) (2)
SOD was measured utilizing a Ransod kit (Randox Laboratory, UK). Catalase action was measured by a previously described method (18).
Histopathological evaluation of heart
The left ventricles were put in formalin 10%, embedded in paraffin, sliced at 5 mm, deparaffinized, and stained with hematoxylin and eosin. An average number of 10 randomly selected fields in each slide in six rats per group were analyzed by two investigators who were unaware of the animal groups. The sections were also stained with Masson's Trichrome. A blue color indicated positive staining for collagen. Cardiac fibrosis was determined according to the findings from 10 randomly selected high-power fields (400×) for each tissue slide. The percentage of collagen content was evaluated by an image analyzer (Image J, NIH).
Statistical analysis
Data are presented as mean ± SEM. One-Way ANOVA test was used to compare data between groups using LSD post hoc test. SPSS software V. 21 was used for data analysis. P < 0.05 was considered statistically significant.
RESULTS
Serum and cardiac inflammatory marker concentrations
The results of heart nitrite levels showed that there was no significant difference between LPS and control groups. The heart nitrite levels in the animals of NS-treated groups were significantly higher than those of the LPS group (Fig. 1A). LPS group had a lower serum nitrite concentration than the control group (P < 0.05, Fig. 1B). Treatment by all doses of NS significantly increased serum nitrite concentration in LPS-receiving groups (P < 0.05, Fig. 1B).
Fig. 1.
(A) serum and (B) heart nitrite concentrations in experimental groups. (n = 10 in each group). *P < 0.05 compared to LPS; **P < 0.01 compared to LPS.
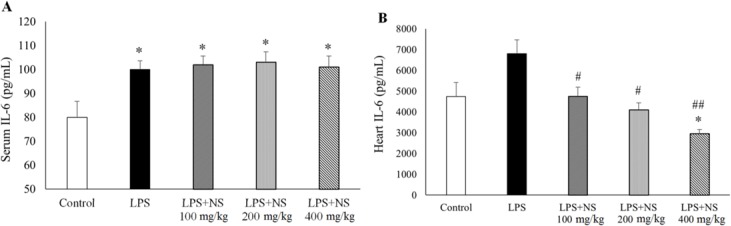
Evaluation of serum and heart IL-6 indicated higher levels of this inflammatory marker in LPS group compared to the controls (P < 0.05). Treatment by NS could not alter serum IL-6 levels; however, it significantly reduced heart IL-6 content in LPS groups, dose-dependently (P < 0.05, Figs. 2A and 2B). NS at the dose of 400 mg/kg decreased heart IL-6 content to a level that was significantly lower than that of the controls (P < 0.05), while there were no significant differences in heart IL-6 between the LPS + NS (100 mg/kg/day) and LPS + NS (200 mg/kg/day) groups compared to the control group (P > 0.05). Serum TNF-α level in LPS group was higher than the controls, although it was not statistically significant (P > 0.05).
Fig. 2.
(A) serum and (B) heart IL-6 level in control, LPS, LPS-NS 100, 200, 400 mg/kg treated groups. (n = 10 in each group). *P < 0.05 compared to control. #P < 0.05 compared to LPS; ##P < 0.01 compared to LPS.
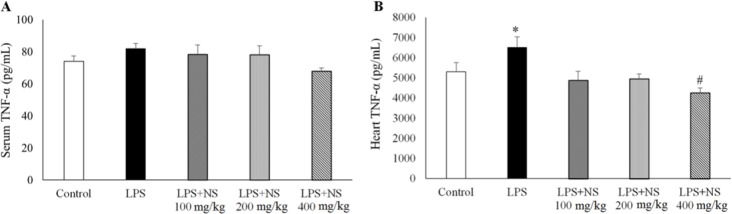
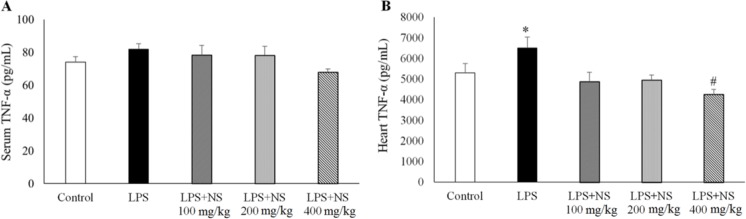
Treatment by all three doses of NS could not alter it significantly (Fig. 3A). However, heart TNF-α content in LPS group was higher than the controls (P < 0.05). NS, especially at the dose of 400 mg/kg, significantly lowered heart TNF-α concentrations in LPS-receiving groups (P < 0.05).
Fig. 3.
Changes of (A) serum and (B) heart TNF-α levels. (n= 10 in each group). *P < 0.05 compared to other groups. #P < 0.05 compared to control and LPS groups.
A significant reduction in heart TNF-α was observed after treatment with NS 400 mg/kg which reduced heart TNF-α to a level that was lower than that of the control group (P < 0.05) (Fig. 3B).
Oxidative stress markers
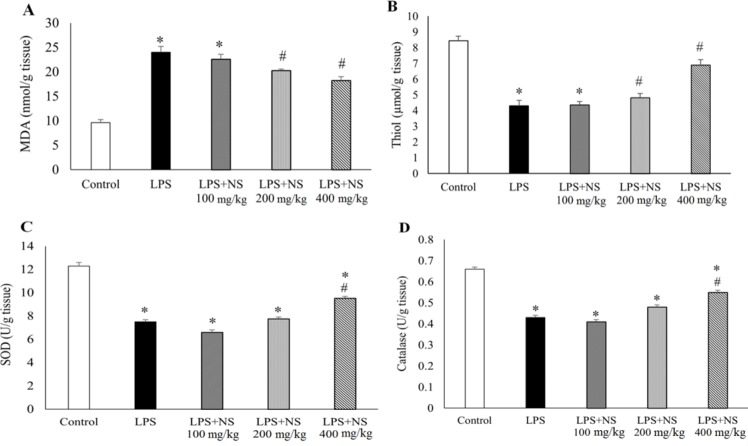
The results showed that there was a significant increase in MDA concentration in the heart tissue of LPS group (P < 0.05, Fig. 4A). Treatment by NS lowered heart MDA level, especially by doses of 200 and 400 mg/kg (P < 0.05). Compared to the controls, there was a significant reduction in heart total thiol concentrations in LPS group (P < 0.05, Fig. 4B). Likewise, the concentrations of heart SOD and catalase in LPS group were lower than those of the controls (P < 0.05, Figs. 4C and 4D).
Fig. 4.
(A) Comparison of MDA, (B) total thiol, (C) SOD, and (D) catalase levels in all groups. (n = 10 in each group). * P < 0.05 compared to control, # P < 0.05 compared to LPS, ## P < 0.01 compared to LPS.
The animals treated with three doses of NS exhibited a trend of reduction in MDA and increase in total thiol, SOD and catalase concentrations. In LPS group treated with NS 400 mg/kg, heart MDA and total thiol levels were significantly reduced (P < 0.05). Likewise, a significant increase in heart SOD and catalase concentrations were observed after treatment with NS 400 mg/kg (P < 0.05).
Histopathological findings
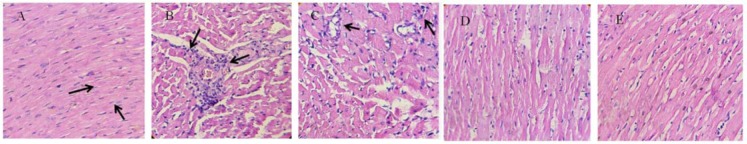
The Hemotoxylin and Eosin (H&E) stained histopathological examination showed a normal ventricular muscle structure in the control group. In contrast, after LPS stimulation, inflammatory cell infiltrates were increased, and disarrangement was observed in cardiac muscles. Administration of NS showed a trend of improvement in histological changes in the cardiac tissue of LPS groups (Fig. 5).
Fig. 5.
Effect of NS on LPS-induced inflammation in heart, stained with H&E (400× magnification). Arrows indicate inflammatory cells. (A) control, (B) LPS, (C) LPS + NS 100 mg/kg, (D) LPS + NS 200 mg/kg, and (E) LPS + NS 400 mg/kg.
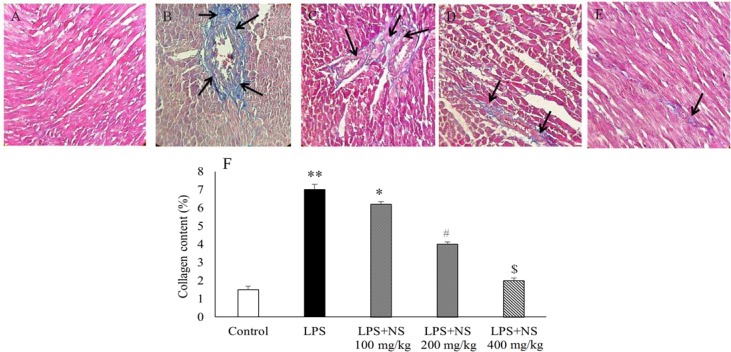
To investigate whether LPS induces cardiac fibrosis, the amount of total collagen was detected using Masson Trichrome staining. As illustrated in Figs. 6A and B, cardiac fibrosis and collagen content were more apparent in LPS group as compared with the control group. Therefore, administration of NS resulted in a reduction of cardiac fibrosis and collagen deposition (P < 0.05) (Figs. 6C–F).
Fig. 6.
Left ventricle wall fibrosis. (A-E) representative images of myocardium at ×400 magnification, stained with Masson's Trichrome. Blue color indicates collagen fibers (black arrow). (A) control, (B) LPS, (C) LPS + NS 100 mg/kg, (D) LPS + NS 200 mg/kg, (E) LPS + NS 400 mg/kg, (F) NS treatment caused a significant decrease in collagen deposition in left ventricle wall. * P < 0.05 compared to control and LPS + NS 100 mg/kg, ** P < 0.01 compared to control, LPS + NS 200 and 400 mg/kg, # P < 0.05 compared to other groups, $ P < 0.05 compared to other groups except control.
DISCUSSION
The cardiovascular health benefits of NS have been established in several studies(1,5,6). These include hypolipidemic, antidiabetic, hypotensive, antiplatelet, and bradycardiac effects, and improving endothelial function. In the present study, we found that in inflammation-induced fibrosis, NS reduced cardiac fibrosis, serum and tissue inflammatory markers, possibly through its antioxidant activity.
LPS is a component of the outer membrane of gram-negative bacteria and is widely used for induction of inflammation(7,19). Our results showed that the animals which received LPS had higher serum and heart inflammatory markers and cardiac fibrosis. Treatment with NS, especially with the highest dose, reduced cardiac fibrosis and returned inflammatory markers to the control level. The anti-inflammatory effect of NS has been demonstrated in several inflammation-based models such as asthma and rheumatoid arthritis(20). In a clinical study, asthmatic patients were treated with NS seed extract with a three-month follow-up(21). It was found that asthma symptoms and pulmonary function test values were significantly improved compared to the controls. Similar results were observed in rheumatoid arthritis in both animal and human studies.
To the best of our knowledge, no study has investigated the effect of NS on cardiac fibrosis, at least in an inflammation-induced fibrosis model. However, previous studies have demonstrated that thymoquinone, the biologically active compound of NS seeds(22), reduces liver and pulmonary fibrosis and inflammation, and suggested it as a potential candidate for fibrosis therapy. Nevertheless, the exact effects of thymoquinone on fibrosis in organs are not yet clear(23).
To examine the possible mechanism for cardiac fibrosis, we evaluated the oxidative stress status in the heart tissue. We found that NS had a dose-dependent antioxidative property. Kanter, et al. showed that in alcohol-induced mucosal injury, NS oil and thymoquinone protect gastric mucosa, which is partly attributed to their free-radical scavenging activity(24). Moreover, the ethanolic extract of NS protects radiation-induced oxidative damage in mice(23). Several mechanisms for antioxidative properties of NS have been suggested (1). It is demonstrated that it preserves the activity of catalase, glutathione peroxidase, and glutathione-S-transferase. It also inhibits microsomal lipid peroxidation in the experimental model of nephropathy(25). In a study on gentamicin-induced nephrotoxicity, thymoquinone resulted in increased levels of glutathione peroxidase, catalase, and ATP(26). That study also showed complete reversal of the gentamicin-induced increased blood urea nitrogen, creatinine, thiobarbituric acid-reactive substances, and total nitrate/nitrite to normal range.
CONCLUSION
Administration of NS improved myocardial fibrosis in an inflammation-induced fibrosis model. It seems that it acts through alteration of oxidative/antioxidative balance and raising antioxidative enzymes. More studies are guaranteed to clarify the beneficial effects and the underlying mechanism(s) of NS seed extract on myocardial fibrosis after common clinical cardiovascular diseases especially myocardial infarction.
ACKNOWLEDGEMENTS
The author thanks Vice Chancellor of Mashhad University of Medical Sciences for their financial support (grant number: 940840).
REFERENCES
- 1.Shabana A, El-Menyar A, Asim M, Al-Azzeh H, Al Thani H. Cardiovascular benefits of black cumin (Nigella sativa) Cardiovasc Toxicol. 2013;13(1):9–21. doi: 10.1007/s12012-012-9181-z. [DOI] [PubMed] [Google Scholar]
- 2.Yarnell E, Abascal K. Nigella sativa: holy herb of the Middle East. Altern Compl Therap. 2011;17:99–105. [Google Scholar]
- 3.Salem ML. Immunomodulatory and therapeutic properties of the Nigella sativa L. seed. Int Immunopharmacol. 2005;5(13-14):1749–1770. doi: 10.1016/j.intimp.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 4.Majdalawieh AF, Fayyad MW. Immunomodulatory and anti-inflammatory action of Nigella sativa and thymoquinone: A comprehensive review. Int Immunopharmacol. 2015;28(1):295–304. doi: 10.1016/j.intimp.2015.06.023. [DOI] [PubMed] [Google Scholar]
- 5.Kanter M, Demir H, Karakaya C, Ozbek H. Gastroprotective activity of Nigella sativa L oil and its constituent, thymoquinone against acute alcohol-induced gastric mucosal injury in rats. World J Gastroenterol. 2005;11(42):6662–6666. doi: 10.3748/wjg.v11.i42.6662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shafiq H, Ahmad A, Masud T, Kaleem M. Cardio-protective and anti-cancer therapeutic potential of Nigella sativa. Iran J Basic Med Sci. 2014;17(12):967–979. [PMC free article] [PubMed] [Google Scholar]
- 7.Doi K, Leelahavanichkul A, Yuen PS, Star RA. Animal models of sepsis and sepsis-induced kidney injury. J Clin Invest. 2009;119(10):2868–2878. doi: 10.1172/JCI39421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Abbas AK, Lichtman AHH. Basic immunology updated edition: functions and disorders of the immune system. 3rd edition. Saunders Elsevier; 2006. pp. 11–13. [Google Scholar]
- 9.Frantz S, Kobzik L, Kim YD, Fukazawa R, Medzhitov R, Lee RT, et al. Toll4 (TLR4) expression in cardiac myocytes in normal and failing myocardium. J Clin Invest. 1999;104(3):271–280. doi: 10.1172/JCI6709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thompson M, Kliewer A, Maass D, Becker L, White DJ, Bryant D, et al. Increased cardiomyocyte intracellular calcium during endotoxin-induced cardiac dysfunction in guinea pigs. Pediatr Res. 2000;47(5):669–676. doi: 10.1203/00006450-200005000-00019. [DOI] [PubMed] [Google Scholar]
- 11.Umar S, Zargan J, Umar K, Ahmad S, Katiyar CK, Khan HA. Modulation of the oxidative stress and inflammatory cytokine response by thymoquinone in the collagen induced arthritis in Wistar rats. ChemBiol Interact. 2012;197(1):40–46. doi: 10.1016/j.cbi.2012.03.003. [DOI] [PubMed] [Google Scholar]
- 12.Mahmood MS, Gilani AH, Khwaja A, Rashid A, Ashfaq MK. The in vitro effect of aqueous extract of Nigella sativa seeds on nitric oxide production. Phytother Res. 2003;17(8):921–924. doi: 10.1002/ptr.1251. [DOI] [PubMed] [Google Scholar]
- 13.Mousavi SH, Tayarani-Najaran Z, Asghari M, Sadeghnia HR. Protective effect of Nigella sativa extract and thymoquinone on serum/glucose deprivation-induced PC12 cells death. Cell Mol Neurobiol. 2010;30(4):591–598. doi: 10.1007/s10571-009-9484-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Elmi S, Sallam NA, Rahman MM, Teng X, Hunter AL, Moien-Afshari F, et al. Sulfaphenazole treatment restores endothelium-dependent vasodilation in diabetic mice. Vascul Pharmacol. 2008;48(1):1–8. doi: 10.1016/j.vph.2007.09.001. [DOI] [PubMed] [Google Scholar]
- 15.Nematollahi S, Nematbakhsh M, Haghjooyjavanmard S, Khazaei M, Salehi M. Inducible nitric oxide synthase modulates angiogenesis in ischemic hindlimb of rat. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2009;153(2):125–129. doi: 10.5507/bp.2009.021. [DOI] [PubMed] [Google Scholar]
- 16.Buege JA, Aust SD. Microsomal lipid peroxidation. Methods Enzymol. 1978;52:302–310. doi: 10.1016/s0076-6879(78)52032-6. [DOI] [PubMed] [Google Scholar]
- 17.Hosseinzadeh H, Sadeghnia HR. Safranal, a constituent of Crocus sativus (saffron), attenuated cerebral ischemia induced oxidative damage in rat hippocampus. J Pharm Pharm Sci. 2005;8(3):394–399. [PubMed] [Google Scholar]
- 18.Aebi H. Catalase in vitro. Methods Enzymol. 1984;105:121–126. doi: 10.1016/s0076-6879(84)05016-3. [DOI] [PubMed] [Google Scholar]
- 19.Tahergorabi Z, Khazaei M. The relationship between inflammatory markers, angiogenesis, and obesity. ARYA atheroscler. 2013;9(4):247–253. [PMC free article] [PubMed] [Google Scholar]
- 20.Sturzu A, Sheikh S, Echner H, Nagele T, Deeg M, Amin B, et al. Rhodamine-marked bombesin: a novel means for prostate cancer fluorescence imaging. Invest New Drugs. 2014;32(1):37–46. doi: 10.1007/s10637-013-9975-2. [DOI] [PubMed] [Google Scholar]
- 21.Kokku SB, Mahapatra B, Tucker S, Saggurti N, Prabhakar P. Effect of public-private partnership in treatment of sexually transmitted infections among female sex workers in Andhra Pradesh, India. Indian J Med Res. 2014;139(2):285–293. [PMC free article] [PubMed] [Google Scholar]
- 22.Wilson H, Carvalho B, Granot M, Landau R. Temporal stability of conditioned pain modulation in healthy women over four menstrual cycles at the follicular and luteal phases. Pain. 2013;154(12):2633–2638. doi: 10.1016/j.pain.2013.06.038. [DOI] [PubMed] [Google Scholar]
- 23.Carvalho BM, Abdalla Saad MJ. Influence of gut microbiota on subclinical inflammation and insulin resistance. Mediators Inflamm. 2013;2013 doi: 10.1155/2013/986734. ID 986734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kanter M, Coskun O, Budancamanak M. Hepatoprotective effects of Nigella sativa L and Urtica dioica L on lipid peroxidation, antioxidant enzyme systems and liver enzymes in carbon tetrachloride-treated rats. World J Gastroenterol. 2005;11(42):6684–6688. doi: 10.3748/wjg.v11.i42.6684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Khozoie C, Borland MG, Zhu B, Baek S, John S, Hager GL, et al. Analysis of the peroxisome proliferator-activated receptor-beta/delta (PPARbeta/delta) cistrome reveals novel co-regulatory role of ATF4. BMC Genomics. 2012;13:665. doi: 10.1186/1471-2164-13-665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gonzalez F. Adrenal dysfunction in polycystic ovary syndrome: has it been lost to follow-up? Fertil Steril. 2013;99(2):352–353. doi: 10.1016/j.fertnstert.2012.10.045. [DOI] [PubMed] [Google Scholar]