Abstract
Ionizing radiation causes DNA damage and chromosome abbreviations on normal cells. The radioprotective effect of celecoxib (CLX) was investigated against genotoxicity induced by ionizing radiation in cultured human blood lymphocytes. Peripheral blood samples were collected from human volunteers and were incubated at different concentrations at 1, 5, 10 and 50 μM of CLX for two hours. At each dose point, the whole blood was exposed in vitro to 150 cGy of X-ray, and then the lymphocytes were cultured with mitogenic stimulation to determine the micronucleus frequency in cytokinesis blocked binucleated lymphocytes. Incubation of the whole blood with CLX exhibited a significant decrease in the incidence of micronuclei in lymphocytes induced by ionizing radiation, as compared with similarly irradiated lymphocytes without CLX treatment. The maximum reduction on the frequency of micronuclei was observed at 50 μM of CLX (65% decrease). This data may have an important possible application for the protection of human lymphocytes from the genetic damage induced by ionizing irradiation in human exposed to radiation.
Keywords: Celecoxib, Genotoxicity, Ionizing radiation, Lymphocyte
INTRODUCTION
Ionizing radiation (IR) produces reactive oxygen species (ROS) and free radicals when is passing through cells. DNA is the most susceptible macromolecule to damage induced by IR. Cell death, apoptosis and autophagy are induced in the cells in response to radiation in cancer cells and in normal cells(1,2). Cyclooxygenase-2 (COX II) is an enzyme induced by a variety of factors such as tumor promoters. It causes radiation-induced G2/M arrest and leads to resistance to IR. COX II is overexpressed by IR in the cells, and it is associated to carcinogenesis and maintenance of progressive tumor growth. COX II and its products may act as radioprotector against cell damage and lead to tumor resistance to IR(3,4). COX II selective inhibitors can radiosensitize cancer cells in a COX II expression-dependent manner. Several studies showed that celecoxib (CLX), as a COX II selective inhibitor, sensitized cancer cells to IR(5,6,7). CLX, as a non-steroid anti-inflammatory drug, is widely used in inflammation-related diseases. It improved tumor response to radiotherapy in some clinical studies(8,9). While the potential of radiosensitizing effect of CLX on tumor cells is established, its effect on normal cells exposed to IR is unclear. DNA double strand breaks (DSBs) occur when the two strands of the DNA are closely broken. DBSs can be induced by genotoxic agents such as IR, chemical and anti-cancer agents. These agents mainly produce ROS on cells result in chromosome breaks and genotoxicity.
High frequency of chromosome damage and genotoxicity has been observed in patients and personnel after exposure to IR(10,11,12). Lymphocytes are sensitive cells to IR. IR exposure to lymphocyte can lead to chromosome abbreviations and DNA breaks. Micronuclei in nucleus arise during cell division and produce from chromosome fragments.
The detection of micronucleus in binucleated lymphocyte cell has been performed as reliable biomarker of in vitro/in vivo exposure to ionizing radiation and other genotoxic agents(13). The aim of this study was to evaluate the effect of CLX against genotoxicity induced by IR in normal human peripheral blood lymphocytes in vitro.
MATERIALS AND METHODS
Human blood treatment
Three healthy nonsmoking human male volunteers, aging 25–30 years, were enrolled in this study. The study protocol was approved by the Research and Ethical Committee of the Mazandaran University of Medical Sciences NO. 91–77. CLX was obtained from Daroupakhsh Pharmaceutical Company (Tehran, Iran). A specified quantity of CLX was dissolved in 800 μL DMSO and made up to 10 mL volume with culture medium to produce a stock solution of CLX at concentration of 1 mM. Working standard concentrations of CLX were prepared by dilution of CLX stock solution with culture medium. The final concentration of DMSO was maintained at 0.4% in all cell culture experiments. Control and irradiated samples were treated with culture medium containing 0.4% DMSO. After overnight fasting, 10 mL of the whole blood were collected from the volunteers in heparinized tubes and divided in tubes each containing 0.9 mL of blood. Blood samples were treated with 100 μL solution of CLX to yield final concentrations of 1, 5, 10 or 50 μM. These samples were incubated for two h at 37° C in a humidified atmosphere of 5% CO2 and 95% air.
Irradiation, lymphocyte culture and micronuclei analysis
At each concentration and for each volunteer, tubes containing blood plus CLX were irradiated at 37° C with 6 MV X-ray beam produced by a radiotherapy machine (Linear accelerator, Siemens, Primus, Germany) at a dose of 150 cGy with a dose rate of 200 cGy/min(14,15,16,17,18). In irradiation protocol, the blood samples in microtubes were placed in a box containing water that acted as a phantom for uniformity of radiation exposure to blood samples. Three series of three blood samples, one from each volunteer, were used as the non-irradiated control, irradiated samples with no CLX, and non-irradiated samples containing 50 μM CLX also were included in the experiment. Subsequently, cell culture were performed in duplicate and 0.5 mL of each sample (control and irradiated samples) was added to 4.4 mL of RPMI 1640 culture medium (Gibco, USA), which contained a mixture of 10% fetal calf serum, 100 μL phytohaemagglutinin M (Gibco, USA) and antibiotics (50 U/mL penicillin, 50 μg/mL streptomycin). All cultures were incubated at 37° C in a humidified atmosphere of 5% CO2 and 95% air. Cytochalasin B (Sigma, USA) (final concentration 6 μg/mL) was added 44 h from cell culture establishment. Following 72 h from incubation, the cells were collected by centrifugation, re-suspended in cold potassium chloride. Cells were immediately fixed in a fixative solution of methanol: acetic acid (6:1). The fixed cells were dropped onto clean microscopic slides, air-dried and stained with Giemsa solution.
All slides were evaluated at × 100 magnification in order to determine the frequency of micronuclei in the cytokinesis-blocked binucleated cells with a well-preserved cytoplasm.
The criteria for scoring micronuclei were a diameter between 1/16th and 1/3rd of the main nuclei, non-refractile, not linked to the main nuclei and not overlapping the main nuclei(19). At each concentration and for each volunteer, 1000 binucleated lymphocyte cells were examined from the irradiated and control cultures in duplicate to record the frequency of micronuclei. Twenty one thousands binucleated lymphocytes were examined. In our study, a typical micronucleus in binucleated lymphocyte is shown in Fig. 1.
Fig. 1.
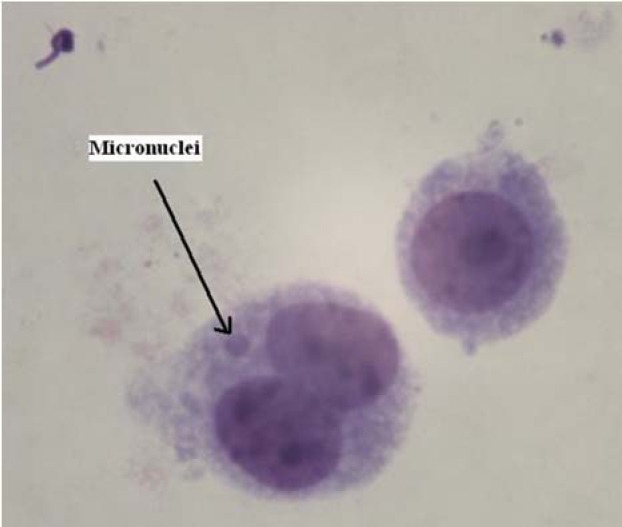
A typical binucleated lymphocyte with micronuclei in our experiments.
Statistical analysis
For each volunteer, at each concentration, the incidence of radiation-induced micronuclei was recorded. The data were analyzed with student t-test. A probability value of 0.05 was accepted to denote significance.
RESULTS
The percentage of micronucleus in binucleated lymphocytes exposed to 1.5 Gy of X-ray was 3.57 ± 0.4, while the percentage in non-treated control lymphocytes was 0.33 ± 0.07. There was a significant increase of 10-fold in frequency of micronuclei in samples treated with X-ray (P < 0.001) in comparison with the control non-irradiated samples (Fig. 2).
Fig. 2.
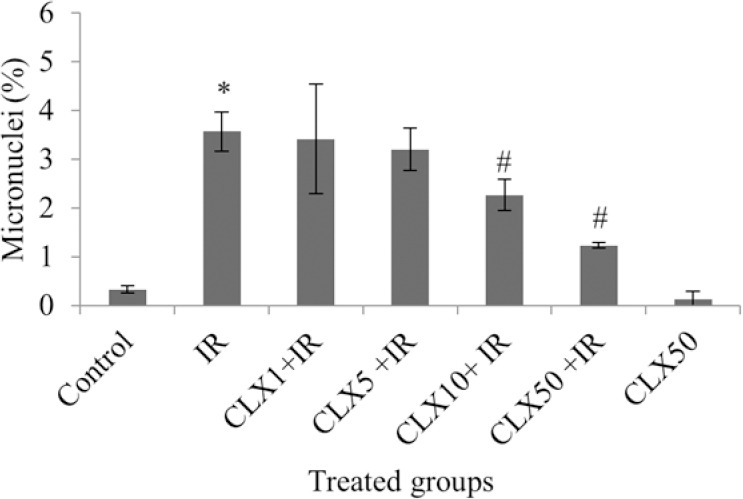
In vitro protection by celecoxib (CLX) at different concentrations (1, 5, 10 and 50 μM) against radiation-induced genetic damage induced by X-ray (IR; 1.5 Gy) in cultured blood lymphocyte. The data represent average ± standard deviation of three human volunteers. *P < 0.001; Irradiated lymphocytes from the blood sample treated with IR compared to non-irradiated control samples. #P < 0.05; CLX10 + IR and CLX50 + IR samples compared to IR sample.
The frequency of micronuclei after pre-treatment with CLX at doses of 1, 5, 10 and 50 μM was 3.42 ± 1.13, 3.20 ± 0.44, 2.27 ± 0.32 and 1.23 ± 0.06, respectively (Fig. 2).
The data demonstrated that human blood incubated with CLX for two h, and then exposed in vitro to 1.5 Gy of X-ray radiation, exhibited a significant reduction in micronuclei frequency compared to blood samples incubated with X-ray alone. CLX significantly mitigated the frequency of micronuclei at doses 10 and 50 μM in lymphocytes exposed to IR (P < 0.05). Total micronuclei values were reduced by 4%, 10%, 36% and 65% in samples treated with CLX and irradiation at concentrations of 1, 5, 10 and 50 μM, respectively, compared to irradiated sample control (Fig. 2). CLX by itself without radiation exposure did not induce any genotoxicity effect in cultured lymphocytes treated with 50 μM concentration.
DISCUSSION
In this study we showed that CLX has a radioprotective effect against genotoxicity induced by IR in human lymphocytes. CLX reduced the frequency of micronuclei in binucleated lymphocytes which was induced by IR. CLX exhibited a protective effect at concentrations of 10 and 50 μM by factors 1.6 and 2.9, respectively. It was not observed any protective effect of CLX at low concentrations (1 and 5 μM). In this study, CLX concentrations were selected based on plasma concentration of CLX after oral administration of this drug. CLX showed a plasma concentration of 1–8.5 μM at a single dosage administration of 200 mg or 400 mg (20,2122), then we selected the concentrations of 1, 10 μM and also two different concentrations as 5 and 50 μM for comparing with CLX plasma concentrations. In this study, CLX showed radioprotective effect at high concentrations then it is recommended that patients receive at least 400 mg of CLX for radioprotection. COX II expression levels are increased in several cancer cells such as breast, colorectal and prostate cancers. Previous studies exhibited that CLX had beneficial effects through killing of cancer cells. CLX effectively inhibited the cell survival and colony formation and increased IR-induced apoptosis in cancer cells. CLX significantly enhanced tumor radiosensitivity which is related to COX II inhibition by CLX(23,24). Cancer cells increase expression of COX II in response to IR, and it contributes in pro-inflammatory process and resistance to killing effect of IR by tumor cells. CLX has a synergistic effect with IR on killing of cancer cells (3, 25). IR can cause side effects on normal tissues. IR can activate inflammatory process that causes dysfunction in normal cells(26). The cytokinesis-block micronucleus assay in peripheral blood lymphocytes is one of the best standard and validated techniques for assessment of the absorbed radiation doses in human(27,28). Human T lymphocytes express the COX II enzyme and involves in the inflammatory responses. Pro-inflammatory processes have cross-talk with COX II. Inflammatory processes induced by IR have different effects in cancer cells and normal cells. In cancer cells, enhanced inflammatory process activates some cellular signaling pathways that lead to tumor growth. Then CLX as a COX II inhibitor can reduce tumor proliferation or increase cell death. While in normal cells, inflammatory processes activate cellular signaling pathways involved in cell death, so CLX as COX II inhibitor can lead to protect normal cells against toxicity induced by IR. Recently it was reported that mefenamic acid as an anti-inflammatory drug exhibited radioprotective effects on genotoxicity induced by IR(14). CLX probably attenuates DNA damage induced by IR through suppression of pro-inflammatory process in lymphocytes. CLX was reported to reduce oxidative stress(29) and also enhanced the activities of antioxidant enzymes, superoxide dismutase, glutathione peroxidase and the total antioxidant status in hypercholesterolemic rabbits(30). CLX exhibited a protective effect against DNA fragmentation induced by hydrogen peroxide as an oxidative stress agent on normal mucosa cell(31). These results show CLX probably has anti-oxidative stress properties on reduction of DNA damage.
CONCLUSION
In this study, we showed that CLX significantly reduced DNA damage induced by IR on human normal lymphocytes. Although CLX can sensitize cancer cells to IR, it likely protects normal cell against genotoxicity induced by IR. This result provides a new indication of CLX on normal cells exposed to IR and is promising in treatment of cancer patients during radiation therapy.
ACKNOWLEDGEMENTS
This study was supported by a grant NO. 91–77 from Mazandaran University of Medical Sciences.
REFERENCES
- 1.Panganiban RA, Snow AL, Day RM. Mechanisms of radiation toxicity in transformed and non-transformed cells. Int J Mol Sci. 2013;14:15931–15958. doi: 10.3390/ijms140815931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hosseinimehr SJ. Flavonoids and genomic instability induced by ionizing radiation. Drug Discovery Today. 2010;15:907–918. doi: 10.1016/j.drudis.2010.09.005. [DOI] [PubMed] [Google Scholar]
- 3.Kim YM, Shin YK, Jun HJ, Rha SY, Pyo H. Systematic analyses of genes associated with radiosensitizing effect by celecoxib, a specific cyclooxygenase-2 inhibitor. J Radiat Res. 2011;52:752–765. doi: 10.1269/jrr.10146. [DOI] [PubMed] [Google Scholar]
- 4.Milas L. Cyclooxygenase-2 (COX-2) enzyme inhibitors as potential enhancers of tumor radioresponse. Semin Radiat Oncol. 2001;11:290–299. doi: 10.1053/srao.2001.26018. [DOI] [PubMed] [Google Scholar]
- 5.Kim YM, Jeong IH, Pyo H. Celecoxib enhances the radiosensitizing effect of 7-hydroxystaurosporine (UCN-01) in human lung cancer cell lines. Int J Radiat Oncol Biol Phys. 2012;83:e399–407. doi: 10.1016/j.ijrobp.2012.01.001. [DOI] [PubMed] [Google Scholar]
- 6.Kim YM, Pyo H. Different cell cycle modulation by celecoxib at different concentrations. Cancer Biother Radiopharm. 2013;28(2):138–145. doi: 10.1089/cbr.2012.1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Suzuki K, Gerelchuluun A, Hong Z, Sun L, Zenkoh J, Moritake T, et al. Celecoxib enhances radiosensitivity of hypoxic glioblastoma cells through endoplasmic reticulum stress. Neuro Oncol. 2013;15(9):1186–1199. doi: 10.1093/neuonc/not062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xue WP, Bai SM, Luo M, Bi ZF, Liu YM, Wu SK. Phase I clinical trial of nasopharyngeal radiotherapy and concurrent celecoxib for patients with locoregionally advanced nasopharyngeal carcinoma. Oral Oncol. 2011;47:753–757. doi: 10.1016/j.oraloncology.2011.06.002. [DOI] [PubMed] [Google Scholar]
- 9.Smith MR, Manola J, Kaufman DS, Oh WK, Bubley GJ, Kantoff PW. Celecoxib versus placebo for men with prostate cancer and a rising serum prostate-specific antigen after radical prostatectomy and/or radiation therapy. J Clin Oncol. 2006;24:2723–2728. doi: 10.1200/JCO.2005.03.7804. [DOI] [PubMed] [Google Scholar]
- 10.Rothkamm K, Barnard S, Moquet J, Ellender M, Rana Z, Burdak-Rothkamm S. DNA damage foci: Meaning and significance. Environ Mol Mutagen. 2015;56:491–504. doi: 10.1002/em.21944. [DOI] [PubMed] [Google Scholar]
- 11.Borrego-Soto G, Ortiz-Lopez R, Rojas-Martinez A. Ionizing radiation-induced DNA injury and damage detection in patients with breast cancer. Genet Mol Biol. 2015;38(4):420–432. doi: 10.1590/S1415-475738420150019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Geisel D, Zimmermann E, Rief M, Greupner J, Laule M, Knebel F, et al. DNA double-strand breaks as potential indicators for the biological effects of ionising radiation exposure from cardiac CT and conventional coronary angiography: a randomised, controlled study. Eur Radiol. 2012;22(8):1641–1650. doi: 10.1007/s00330-012-2426-1. [DOI] [PubMed] [Google Scholar]
- 13.Rastkhah E, Zakeri F, Ghoranneviss M, Rajabpour MR, Farshidpour MR, Mianji F, et al. The cytokinesis-blocked micronucleus assay: dose-response calibration curve, background frequency in the population and dose estimation. Radiat Environ Biophys. 2016;55:41–51. doi: 10.1007/s00411-015-0624-3. [DOI] [PubMed] [Google Scholar]
- 14.Hosseinimehr SJ, Nobakht R, Ghasemi A, Pourfallah TA. Radioprotective effect of mefenamic acid against radiation-induced genotoxicity in human lymphocytes. Radiat Oncol J. 2015;33:256–260. doi: 10.3857/roj.2015.33.3.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Beetstra S, Thomas P, Salisbury C, Turner J, Fenech M. Folic acid deficiency increases chromosomal instability, chromosome 21 aneuploidy and sensitivity to radiation-induced micronuclei. Mutat Res. 2005;578(1-2):317–326. doi: 10.1016/j.mrfmmm.2005.05.012. [DOI] [PubMed] [Google Scholar]
- 16.Cinkilic N, Tuzun E, Cetintas SK, Vatan O, Yilmaz D, Cavas T, et al. Radio-protective effect of cinnamic acid, a phenolic phytochemical, on genomic instability induced by X-rays in human blood lymphocytes in vitro. Mutat Res Genet Toxicol Environ Mutagen. 2014;770:72–79. doi: 10.1016/j.mrgentox.2014.04.025. [DOI] [PubMed] [Google Scholar]
- 17.Rostami A, Moosavi SA, Dianat Moghadam H, Bolookat ER. Micronuclei Assessment of the radioprotective effects of melatonin and vitamin c in human lymphocytes. Cell J. 2016;18:46–51. doi: 10.22074/cellj.2016.3986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wu J, Lyons GH, Graham RD, Fenech MF. The effect of selenium, as selenomethionine, on genome stability and cytotoxicity in human lymphocytes measured using the cytokinesis-block micronucleus cytome assay. Mutagenesis. 2009;24:225–232. doi: 10.1093/mutage/gen074. [DOI] [PubMed] [Google Scholar]
- 19.Fenech M. The in vitro micronucleus technique. Mutat Res. 2000;455:81–95. doi: 10.1016/s0027-5107(00)00065-8. [DOI] [PubMed] [Google Scholar]
- 20.Dongari N, Sauter ER, Tande BM, Kubatova A. Determination of Celecoxib in human plasma using liquid chromatography with high resolution time of flight-mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci. 2014;955-956:86–92. doi: 10.1016/j.jchromb.2014.02.012. [DOI] [PubMed] [Google Scholar]
- 21.Park MS, Shim WS, Yim SV, Lee KT. Development of simple and rapid LC-MS/MS method for determination of celecoxib in human plasma and its application to bioequivalence study. J Chromatogr B Analyt Technol Biomed Life Sci. 2012;902:137–141. doi: 10.1016/j.jchromb.2012.06.016. [DOI] [PubMed] [Google Scholar]
- 22.Zarghi A, Shafaati A, Foroutan SM, Khoddam A. Simple and rapid high-performance liquid chromatographic method for determination of celecoxib in plasma using UV detection: application in pharmacokinetic studies. J Chromatogr B Analyt Technol Biomed Life Sci. 2006;835:100–104. doi: 10.1016/j.jchromb.2006.03.021. [DOI] [PubMed] [Google Scholar]
- 23.Chen KH, Hsu CC, Song WS, Huang CS, Tsai CC, Kuo CD, et al. Celecoxib enhances radiosensitivity in medulloblastoma-derived CD133-positive cells. Childs Nerv Syst. 2010;26:1605–1612. doi: 10.1007/s00381-010-1190-2. [DOI] [PubMed] [Google Scholar]
- 24.Kang KB, Wang TT, Woon CT, Cheah ES, Moore XL, Zhu C, et al. Enhancement of glioblastoma radioresponse by a selective COX-2 inhibitor celecoxib: inhibition of tumor angiogenesis with extensive tumor necrosis. Int J Radiat Oncol Biol Phys. 2007;67:888–896. doi: 10.1016/j.ijrobp.2006.09.055. [DOI] [PubMed] [Google Scholar]
- 25.Pruthi RS, Derksen JE, Moore D, Carson CC, Grigson G, Watkins C, et al. Phase II trial of celecoxib in prostate-specific antigen recurrent prostate cancer after definitive radiation therapy or radical prostatectomy. Clin Cancer Res. 2006;12:2172–2177. doi: 10.1158/1078-0432.CCR-05-2067. [DOI] [PubMed] [Google Scholar]
- 26.Mukherjee D, Coates PJ, Lorimore SA, Wright EG. Responses to ionizing radiation mediated by inflammatory mechanisms. J Pathol. 2014;232(2):289–299. doi: 10.1002/path.4299. [DOI] [PubMed] [Google Scholar]
- 27.De Sanctis S, De Amicis A, Di Cristofaro S, Franchini V, Regalbuto E, Mammana G, et al. Cytokinesis-block micronucleus assay by manual and automated scoring: calibration curves and dose prediction. Health Phys. 2014;106:745–749. doi: 10.1097/HP.0000000000000052. [DOI] [PubMed] [Google Scholar]
- 28.Heylmann D, Rodel F, Kindler T, Kaina B. Radiation sensitivity of human and murine peripheral blood lymphocytes, stem and progenitor cells. Biochim Biophys Acta. 2014;1846:121–129. doi: 10.1016/j.bbcan.2014.04.009. [DOI] [PubMed] [Google Scholar]
- 29.Senbel AM, AbdelMoneim L, Omar AG. Celecoxib modulates nitric oxide and reactive oxygen species in kidney ischemia/reperfusion injury and rat aorta model of hypoxia/reoxygenation. Vascul Pharmacol. 2014;62:24–31. doi: 10.1016/j.vph.2014.04.004. [DOI] [PubMed] [Google Scholar]
- 30.Ahmed S, Gul S, Gul H, Zia-Ul-Haq M, Ercisli S. Cyclooxygenase-2 inhibition improves antioxidative defense during experimental hypercholesterolemia. Bosn J Basic Med Sci. 2014;14:63–69. doi: 10.17305/bjbms.2014.2264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Matthias C, Schuster MT, Zieger S, Harreus U. COX-2 inhibitors celecoxib and rofecoxib prevent oxidative DNA fragmentation. Anticancer Res. 2006;26:2003–2007. [PubMed] [Google Scholar]


