Abstract
Objective
To describe the pathophysiology associated with multiple organ dysfunction syndrome (MODS) in children.
Data Sources
Literature review, research data, and expert opinion
Study Selection
Not applicable
Data Extraction
Moderated by an experienced expert from the field, pathophysiological processes associated with MODS in children were described, discussed and debated with a focus on identifying knowledge gaps and research priorities.
Data Synthesis
Summary of presentations and discussion supported and supplemented by relevant literature.
Conclusions
Experiment modeling suggests that persistent macrophage activation may be a pathophysiologic basis for MODS. Children with MODS have 1) reduced cytochrome P450 metabolism inversely proportional to inflammation, 2) increased circulating damage associated molecular pattern molecules (DAMPS) from injured tissues, 3) increased circulating pathogen associated molecular pattern molecules (PAMPS) from infection or endogenous microbiome, and 4) cytokine driven epithelial, endothelial, mitochondrial, and immune cell dysfunction. Cytochrome P450s metabolize endogenous compounds and xenobiotics, many of which ameliorate inflammation, whereas DAMPS and PAMPS alone and together amplify the cytokine production leading to the inflammatory MODS response. Genetic and environmental factors can impede inflammation resolution in children with a spectrum of MODS pathobiology phenotypes. Thrombocytopenia associated MODS patients have extensive endothelial activation and thrombotic microangiopathy with associated oligogenic deficiencies in inhibitory complement and ADAMTS13. Sequential MODS patients have sfasL-fas mediated hepatic failure with associated oligogenic deficiencies in perforin and granzyme signaling. Immune paralysis associated MODS patients have impaired ability to resolve infection, and have associated environmental causes of lymphocyte apoptosis. These inflammation phenotypes can lead to macrophage activation syndrome. Resolution of MODS requires elimination of the source of inflammation. Full recovery of organ functions is noted six to eighteen weeks later when epithelial, endothelial, mitochondrial, and immune cell regeneration and reprogramming is completed.
Keywords: Damage Associated Molecular Pattern molecules (DAMPS), Pathogen Associated Molecular Pattern molecules (PAMPS), Cytochrome P450 metabolism, mitochondria, Thrombocytopenia associated MOF, Sequential MOF, Immunoparalysis, Macrophage Activation Syndrome
STATE OF THE SCIENCE
Overview
Baue et al first described Multiple System Organ Failure (MSOF) in a case series of general surgery patients who died after three days in the intensive care unit (ICU) with sequential respiratory and then hepato-renal organ failures (rather than from shock in the first three days of critical illness) (1–6). At autopsy, these patients had a persistent nidus of inflammation which Baue hypothesized was the catalyst of MSOF. Steinberg subsequently developed an experimental model of MSOF with the pre hoc intention that it be a sterile inflammation model rather than an infection model, and that it induce MSOF with both late survivors as well as late deaths (7). They discovered that only a combined injection of mineral oil plus zymosan (a component of the saccharamycoses A cell wall) induced MSOF, whereas single injections of either zymosan or mineral oil induced little illness. Importantly, this ‘gold standard’ MSOF model exhibits a zymosan dose response effect on degree of organ dysfunction as well as mortality. Mineral oil provides irritation and zymosan provides pathogen associated molecular patterns (PAMPS) which together cause persistent peritoneal macrophage activation that leads to cytokine-mediated epithelial, endothelial, mitochondrial, immune cell and systemic organ dysfunction. The endogenous cytochrome P450 system, which ameliorates inflammation, is protective in this model (8), as is pre-treatment with etoposide (9,10). When studying this model, it is important to note that the term MSOF has evolved to be interchangeable with the term multiple organ failure (MOF) and multiple organ dysfunction syndrome (MODS). Importantly for our purposes, the experimental sterile inflammation intraperitoneal mineral oil and zymosan model has been validated in both ‘adult’ and ‘pediatric’ rodents (11–13).
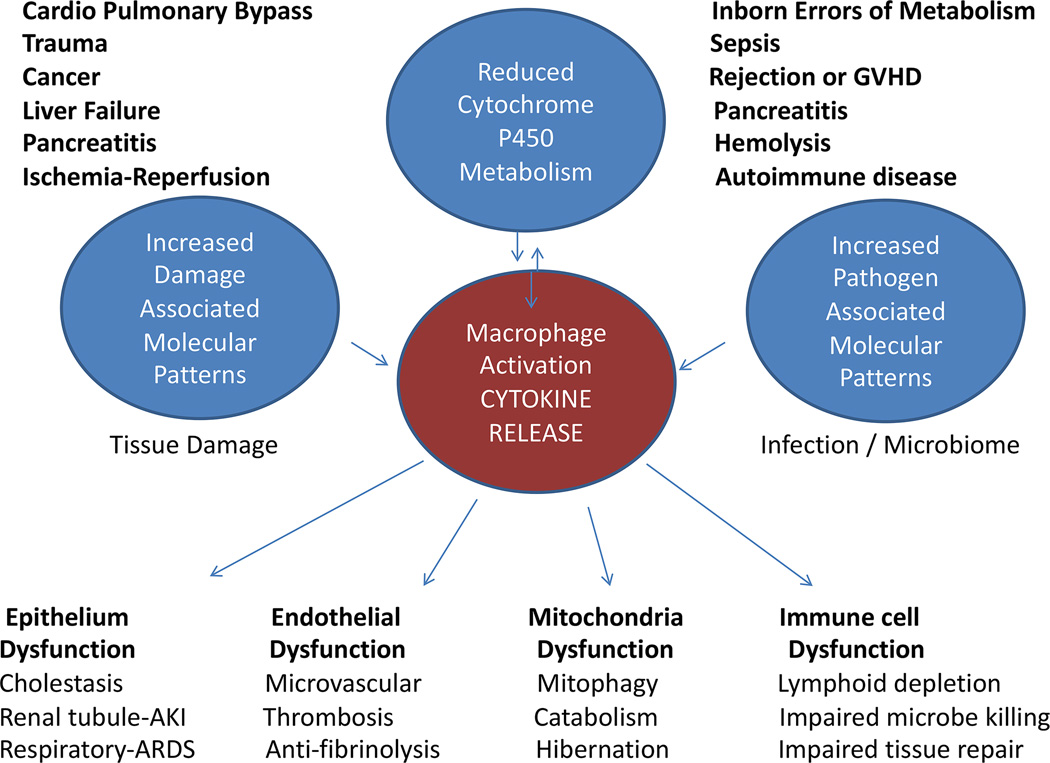
In children, the pathophysiology of MODS has been evaluated in vivo and ex vivo in cohort studies using clinical definitions of persistent (14), progressive, or secondary (15) MOF/MODS described as three or more organ failures at three days, or increasing organs failing or development of multiple organ failure at seven days, respectively. In these clinical studies of children with MODS, the findings are similar to the experimental model. Decreased cytochrome P450 activity has been found to be inversely correlated with degree of cytokinemia and organ dysfunctions, supporting a role of altered metabolism in allowing pathologic inflammation (16). The ‘Danger Hypothesis’ (17), posits that injury to endogenous cells releases damage-associated molecular patterns [DAMPS] that alter antigen presenting cells responses to exogenous antigens or pathogen-associated molecular patterns (PAMPS) in a way that amplifies the cytokine response. This hypothesis is supported by pediatric MODS studies (18–27). Children with MODS have been found to have higher circulating biomarkers of DAMPS, PAMPS, and cytokines that correlate with the degree of organ dysfunctions. The combination of decreased cytochrome P450 metabolism, tissue injury related DAMPS, and circulating PAMPS leading to self-injurious cytokinemia in pediatric MODS can be caused by cardiopulmonary bypass, trauma, cancer, liver failure, burns, pancreatitis, ischemia-reperfusion, inborn errors of metabolism, sepsis, rejection, graft versus host disease, overwhelming hemolysis, or autoimmune disease (Figure 1). Cytokinemia in these children can lead to 1) epithelial cell dysfunction and apoptosis manifested as acute respiratory distress syndrome, hepatobiliary dysfunction and / or acute kidney tubular dysfunction; 2) endothelial cell dysfunction and apoptosis manifested as thrombotic microangiopathy with loss of microvascular homeostasis; 3) mitochondrial autophagy (mitophagy) and dysfunction manifested as catabolism, hibernation, and dysautonomia; and 4) immune cell dysfunction and apoptosis manifested as lymphoid organ depletion with ineffective microbe removal and tissue repair.
Figure 1.
Four conditions are observed in pediatric MODS; 1) reduced cytochrome P450 activity, 2) increased circulating Damage Associated Molecular Pattern molecules (DAMPS), 3) increased circulating Pathogen Associated Molecular Pattern molecules (PAMPS), and 4) macrophage activation driven cytokine release associated with epithelial, endothelial, mitochondrial, and immune cell dysfunction and apoptosis.
Experimental and clinical studies demonstrate that genetic and environmental factors can impede resolution of systemic inflammation in pediatric MODS. A spectrum of three inflammation pathobiology phenotypes has been described (Figures 2 and 3). The first phenotype, thrombocytopenia associated MODS, has low ADAMTS13 activity (formerly known as von Willebrand factor (vWF) cleaving protease), acute kidney injury with extensive endothelial activation, and systemic vWF multimer thrombotic microangiopathy in brain, kidneys, and lungs (28–30). This has been related to oligogenic deficiencies in genes which produce inhibitory complement as well as ADAMTS13, that can lead to complement and thrombosis over-activation (31–41). This form of MODS has been successfully treated with the combination of eculizumab (C5a monoclonal antibody) and plasma exchange (restores ADAMTS13 activity) (42–50). Hemolysis-derived free hemoglobin also drives this phenotype related to both ADAMTS13 inhibition and macrophage activation (51–54). This endothelial activation phenotype can be experimentally produced with monoclonal antibodies to ADAMTS13 or with hemorrhage (DAMP stimulation) and subsequent endotoxin (PAMP stimulation) (55, 56).
Figure 2.
Environmental and genetic factors can impair the ability of the child with MODS to resolve inflammation: 1) Immunoparalysis is a condition in which antigen presenting cells are unable to present and remove microbes and dead tissue, 2) Thrombocytopenia associated multiple organ failure (TAMOF) is a condition in which complement activation is unopposed by inhibitory complement and von Willebrand factor (vWF) microvascular thrombosis is unopposed by ADAMTS13 (vWF cleaving protease), and 3) Sequential MODS is a condition in which CTL and NK cells cannot induce virus, cancer, or activated immune cell death and sFasL-Fas interactions cause liver failure. The common end pathway of uncontrolled inflammation is macrophage activation syndrome which can be associated with one or more of these phenotypes, or an inability to remove the source of inflammation for other reasons, or the presence of other pediatric hyper-inflammatory syndromes including the CAPS (Cryopyrin Associated Autoinflammatory Periodic Syndromes) spectrum.
HLA - Human leukocyte antigen; TNF – Tumor necrosis factor; LPS – Lipopolysaccharide; TAMOF - Thrombocytopenia associated multiple organ failure; Plt Ct – Platelet count; AKI – Acute kidney injury; DIC – Disseminated intravascular coagulation; SMOF – Sequential multiple organ failure; EBV - Epstein Barr Virus; sFASL - Soluble Fas ligand; IL – Interleukin
Figure 3.
Phenotype specific therapies reported as effective in resolving inflammation and facilitating MODS recovery.
HLA - Human leukocyte antigen; TNF – Tumor necrosis factor; LPS – Lipopolysaccharide; TAMOF - Thrombocytopenia associated multiple organ failure; Plt Ct – Platelet count; AKI – Acute kidney injury; DIC – Disseminated intravascular coagulation; SMOF – Sequential multiple organ failure; IVIG- Intravenous immunoglobulin; PTLD - Post-transplant lymphoproliferative disorder; HLH - Hemophagocytic lymphohistiocytosis; sFASL - Soluble Fas ligand; IRAP - Interleukin-1 (IL-1) receptor antagonist protein
The second phenotype, sequential MODS, develops sFasL-Fas mediated liver failure with associated oligogenic deficiencies in genes related to perforin and granzyme signaling that lead to slow resolution of lymphocyte and macrophage activation and proliferation (57). This can be reproduced experimentally in perforin / granzyme signaling knockout mice which develop MODS when exposed to an otherwise innocuous viral antigen challenge (58–60). Patients with the homozygous mutant form of the disease are treated with chemotherapy including etoposide to target lymphoproliferation, and then eventually bone marrow transplantation to restore cytotoxic T lymphocyte (CTL) / natural killer (NK) cell function (61). Patients with the oligogenic (heterozygous) form are treated with solumedrol, intravenous immunoglobulin (IVIG), and biologics including interleukin-1 receptor antagonist protein (IRAP) (61,62). The third phenotype, immune paralysis associated MODS, has impaired ability to kill infection which can be related in part to environmental factors that induce lymphoid depletion such as chemotherapy, prolonged use of dexamethasone, and overuse of immune suppressants (63–65). Treatments may include immune suppressant tapering and use of granulocyte-macrophage colony-stimulating factor (GM-CSF) (64–67).
Hyper-inflammation among these three phenotypes, whether associated with hyper-complementemia, lack of CTL and NK cell function, or inability to kill infection and mount tissue repair can all result in the macrophage activation syndrome (MAS) manifested clinically as hyper-ferritinemia (> 500 ng/mL), hepatobiliary dysfunction, and disseminated intravascular coagulation. Oligogenic mutations in interleukin-1, interferon γ, NLRP, and CTL/NK signaling (67,68) have been attributed to macrophage activation associated MODS in newborns and children, and IRAP (Interleukin 1 receptor antagonist protein) has been given United States Food and Drug Administration (US FDA) orphan designation for treatment of the CAPS (Cryopyrin Associated Autoinflammatory Periodic Syndromes) spectrum of diseases which include FCAS (Familial Cold Auto-inflammatory Syndrome), Muckle Wells syndrome, and NOMID/CINCA (Neonatal Onset Multisystem Inflammatory Disease/Chronic Inflammatory Neurologic Cutaneous Articular Syndrome). Pediatric MAS-induced MODS has been successfully reversed with methylprednisone, IVIG, and plasma exchange therapy as well as with IRAP (69,70). Cytokine releasing syndrome induced MODS in pediatric cancer patients treated with anti-neoplastic therapies, has been successfully treated with monoclonal antibodies to tumor necrosis factor (TNF), as well as to IL-6 (71,72).
The key to clinical success in preventing and reversing pediatric MODS is removal of the source of inflammation. For patients who have genetic or environmental factors impeding resolution of inflammation, clinicians can consider immune phenotype-specific strategies as well. Once inflammation resolves, the clinician can expect that full organ function recovery will take six to eighteen weeks, which is the time needed for epidermal growth factor, hepatocyte growth factor, vascular endothelial growth factor, stem cell factor, endothelial progenitor cells, hematopoietic stem cells, mesenchymal stem cells, and various resident stem cells to orchestrate epithelial and endothelial cell regeneration, mitochondrial biogenesis, and immune cell reconstitution and reprogramming.
PAMPS and DAMPS
Pathogens express a diverse group of molecular motifs known as pathogen associated molecular patterns (PAMPs) that activate the inflammatory cascade. These motifs are recognized by a limited number of highly conserved pattern recognition receptors (PRRs), which include the Toll-like receptors (TLRs) and nucleotide-binding oligomerization domain (NOD) receptors (73,74). These PRRs also recognize the endogenous danger signals (75) or damage associated molecular patterns (DAMPs). DAMPs are molecules (of many classes: e.g. DNA, RNA, proteins/peptides, lipids, carbohydrates) that are actively secreted or passively released into the extracellular environment from endogenous cells in response to tissue damage, regardless of cause. Since the first description of the cytokine-like properties of high-mobility group box 1 (HMGB1), it has been established as a prototype for DAMPs (76–83). The delayed kinetics of HMGB1 release parallels the onset of lethality in animal models of sepsis. Treatment with neutralizing anti-HMGB1 antibodies can rescue mice from lipopolysaccharide (LPS) or sepsis-induced lethality (80), thereby solidifying its role as a potential therapeutic target. Elevated serum HMGB1 levels have been demonstrated in pediatric patients with multi-organ failure (81). Elevated serum HMGB1 concentrations are also present in adult septic patients with multi-organ failure. However, circulating HMGB1 levels were not different between survivors and non-survivors and failed to predict hospital mortality (82–84). Despite this lack of variation in serum HMGB1 levels between survivors and non-survivors, the currently held opinion is that HMGB1 is a critical late mediator of sepsis and a potential therapeutic target for MODS.
There is growing appreciation that both PAMPs and DAMPs contribute to organ failure and death, although the precise mechanisms are unclear. PAMPs and DAMPs activate immune cells via Toll-like receptors leading to the production of reactive oxygen species (ROS) that promote endothelial dysfunction by the oxidation of crucial cellular signaling proteins (73). Although ROS are important in killing pathogens, excessive or unchecked ROS lead to tissue injury (85). In particular, cytokine and hypoxia-induced production of ROS leads to mitochondrial dysfunction with subsequent development of cellular dysfunction and organ failure (86). Singer reported depressed adenosine triphosphate (ATP) levels in muscle biopsies taken from critically-ill patients who went on to die, in contrast to eventual survivors who demonstrated elevated ATP levels in muscle biopsies (87). Similarly, elevated tissue oxygen tensions have led Fink and others to propose that septic organ failure represents cytopathic hypoxia, i.e. cellular inability to use oxygen rather than a lack of its availability (88,89). Hypercytokinemia activates glycogenolysis and hepatic gluconeogenesis that leads to elevated glucose concentrations; therefore, systemic inflammation can alter ATP production rate and efficiency by altering the substrate availability. ATP depletion accompanied by an inhibited Na+/K+ pump leads to an increase in the cellular Na+ concentration, resulting in cellular gain of electrolytes and water, causing early reversible cellular swelling (90). Inability of the organ to meet the ATP demand with diminished mitochondrial reserve capacity can activate cell death pathways that could lead to organ failure. Thus, key effectors in the pathogenesis of MODS include the inflammatory response that mediates ROS with subsequent reduction in mitochondrial function.
Mitochondria
Mitochondria play a central role in cellular metabolism in all organ systems (except for red blood cells) and are responsible for more than 90% of cellular energy production through oxidative phosphorylation (91). In addition to generating ATP, mitochondria also play an integral role in other cellular pathways, including gene expression, inflammation, immune function, oxidative stress, calcium homeostasis, cell motility, heat production, hormone synthesis, and regulated cell death (91). Mitochondrial function varies in response to both intra- and extra-cellular factors that stress cellular bioenergetic homeostasis.
Perturbations in mitochondrial structure and function have been recognized for decades in animal models and, more recently, in critically ill patients with MODS (92). Under normal conditions, oxygen consumption through the mitochondrial electron transport system is tightly coupled to ATP production and is closely regulated by metabolic demand. In critical illness, acquired deficits in ATP production and other mitochondrial functions as a consequence of hypoxemia, ischemia, and inflammation can impair cellular bioenergetics, accelerate oxidant stress, and disrupt key metabolic pathways (92). Thus, mitochondrial dysfunction has been implicated as a “final common pathway” in the pathogenesis of organ dysfunction in sepsis, trauma, cardiac arrest, and other life-threatening illnesses.
Several lines of evidence support a role for mitochondrial dysfunction in the pathogenesis of MODS. In animal models of sepsis and trauma, mitochondrial abnormalities have been reported across vital organ systems (93,94). In humans, decreased mitochondrial oxygen consumption, low ATP, and mitochondrial gene repression have been linked to illness severity and death (95–98). Metabolomic studies further suggest that energetic substrates related to fatty acid oxidation and the citric acid cycle are less efficiently utilized through mitochondrial aerobic respiration in sepsis non-survivors than in survivors (99). Finally, both spontaneous and pharmacologic restoration of mitochondrial function have been associated with recovery from MODS and improved survival. In particular, enhancement of mitochondrial biogenesis to produce new mitochondria and mitophagy to remove defective mitochondria has been found to restore organ function and promote survival (100).
Mitochondria also play a propagative role that fuels the systemic inflammatory response and contributes to distant organ injury. Mitochondrial DNA (mtDNA) fragmented by oxidative stress can be exported to the cytosol or the extracellular space. In the cytosol, mtDNA promotes the formation of the Nod-like receptor-P3 (NLRP3) inflammasome, a supramolecular platform that up-regulates pro-inflammatory cytokines (101). In the circulation, mtDNA is recognized by the innate immune system as a DAMP and can trigger a systemic inflammatory response (102). Clinical studies have demonstrated an association of circulating levels of mtDNA with adverse outcomes (103,104) and mtDNA has been proposed as a potential biomarker linked to mitochondrial dysfunction (104).
Notably, the term mitochondrial dysfunction, although commonly used, may be somewhat of a misnomer. Experimental evidence suggests that purposive down-regulation of mitochondrial activity likely represents an adaptive response when oxygen and substrate availability are low, as is common in the acute phase of critical illness (92,105). Although this hypometabolic state may manifest clinically as organ dysfunction, it is akin to mammalian hibernation and may help protect cells from a bioenergetic crisis and exposure to high levels of oxidative stress that can precipitate cell death. The observation that organ function rapidly recovers in MODS survivors, even in organs that are poorly regenerative, supports the notion that a coordinated decrease in mitochondrial activity may be both adaptive—at least initially—and reversible (106). The factors coordinating the restoration of mitochondrial respiratory capacity, including mitochondrial biogenesis, fission/fusion, and mitophagy, are an active area of research (100).
Immunoparalysis
In the current era of critical care, many children survive the acute stages of critical illness from a myriad of triggers (e.g. sepsis, trauma, cardiopulmonary bypass), only to experience progressive organ dysfunction and delayed death. Most initial critical insults are characterized by the pro-inflammatory host response. It is increasingly evident, however, that the subacute course of critical illness is associated with an attenuated response of the host immune system, so-called “immunoparalysis” (Figure 4). Investigators have identified infections from opportunistic pathogens, unresolved sources of infection at autopsy, and reactivation of latent viruses all consistent with a functional alteration of host immunity following the acute insult (107).
Figure 4.
The dynamic immune response in MODS. Children who experience an uncomplicated recovery (black bars) frequently demonstrate prompt resolution of systemic inflammation with mild and transient reduction in immune function. Children with complicated courses (gray bars) often have persistently high levels of systemic inflammation concomitant with markedly reduced immune function. *Elevations in levels of suppressor cells have been demonstrated in critically ill adults, but have not yet been found in children.
HLA - Human leukocyte antigen; IL – Interleukin; LPS – Lipopolysaccharide; Treg - Regulatory T cell; MDSC - Myeloid-derived suppressor cell, TNF - Tumor necrosis factor
It is now recognized that the host immune response in critical illness is highly dynamic, with systemic inflammation often concomitant with suppression of leukocyte numbers and function. The latter phenomenon represents the compensatory anti-inflammatory response syndrome (CARS) which, if transient, serves to prevent runaway inflammation (108). If persistent or severe, however, the CARS response represents an important form of acquired immune deficiency which can greatly complicate recovery from MODS. The development of impaired innate (e.g. monocyte, macrophage, dendritic cell) and adaptive (e.g. lymphocyte) immunity has been described in the aftermath of sepsis, critical viral infections, trauma, and cardiopulmonary bypass in children (109–113). Severe or persistent immune impairment has been associated with increased risk for secondary infection, MODS, and death in these settings.
The simultaneous elaboration of pro- and anti-inflammatory mediators in the storm of critical illness has been termed immunologic dissonance (108). This is a result of complex interactions of signal transduction pathways triggered by host exposure to PAMPS and endogenous DAMPS. These molecules bind to leukocytes as well as other tissues and utilize a variety of pathways to transmit their signals to the nucleus. The propagation of signals in these pathways relies on interconnected networks of multifunctional, signaling molecules which ultimately elicit a gene expression response that impacts cellular functions. For each of these signaling pathways, there exist negative regulatory mechanisms, including decoy molecules and inhibitory proteins, which can re-polarize the cell to an anti-inflammatory phenotype. The literature suggests that in settings such as sepsis and critical trauma, downregulation of leukocyte gene expression does occur, with the degree of suppression associated with mortality risk (116,117). There are host-specific factors which can predispose patients to immunoparalysis. Family studies have demonstrated heritable tendencies toward increased anti-inflammatory cytokine production (118), although specific polymorphisms have not been identified. Epigenetics also likely plays a role, with an anti-inflammatory “gene on” histone methylation signature demonstrated in immunoparalysis following pediatric cardiopulmonary bypass (113). Disease or pathogen-specific factors are also important determinants of the risk for immunoparalysis. In addition to diseases that overtly affect immune function (e.g. primary immunodeficiency, leukemia), some forms of pediatric critical illness appear to be particularly immunosuppressive. These include severe traumatic brain injury (111) and infection with Staphylococcus aureus (110).
Lastly, treatment-related factors can contribute to the development of immunoparalysis. The use of immunosuppressive medications such as glucocorticoids, anti-rejection drugs, and chemotherapy impair immune function. Many of the medications and therapies that are routinely used in the pediatric ICU, including sedatives and red blood cell transfusions, can negatively modulate the immune response as well (119). In this complicated setting, it is therefore crucial to have immune function tests that can identify the patient’s place on the spectrum of immune suppression or competence. This is particularly important because evidence suggests that immunoparalysis can be reversible through the use of medications such as GM-CSF or interferon gamma with beneficial effects on outcomes in properly selected patients (66,107,120).
Innate immune function in critical illness has been measured through the quantitation of monocyte antigen presenting capacity and/or cytokine production capacity. Expression of human leukocyte antigen (HLA)-DR, an important antigen presenting molecule, on the surface of monocytes can be quantified by flow cytometry. Data from critically ill adults and children suggest that risks for adverse outcomes increase if less than 30% of monocytes strongly express HLA-DR (121). Studies using a newer quantitative flow cytometry methodology suggest a similar threshold at less than 8,000 HLA-DR molecules per monocyte (122). Whole blood from patients with immunoparalysis will not respond robustly to ex vivo LPS stimulation, with reduced TNF-α production capacity being similarly associated with secondary infection, and mortality risk in pediatric MODS (66). While TNF-α production in the laboratory will vary depending on the volume of blood used, the type of LPS, and the incubation duration, standardized protocols have been developed that permit single- and multi-center immune monitoring studies (110,123). New microfluidic technology promises to reduce the blood volumes and times required for cytokine production capacity to be determined. At present, similar to HLA-DR measurement, no assay for TNF-α quantitation is currently FDA-approved for clinical use in the United States.
Adaptive immune function has also been found to be reduced in critical illness, both in terms of lymphocyte function and numbers. Prolonged lymphopenia, with absolute lymphocyte counts < 1,000 cells/mm3, has been reported to independently predict secondary infection and mortality risks in pediatric MODS (65). Autopsy studies have demonstrated marked lymphocyte apoptosis in lymphoid organs from nonsurvivors of sepsis-induced MODS (124,125). Reduced capacity of lymphocytes to produce pro-inflammatory cytokines such as interferon-γ and IL-2 has been associated with increased risk of infectious complications in septic children (126). While cell counts should be a part of the routine clinical assessment of immune competence, it is unclear which markers of lymphocyte function are best for use in the ICU. It is possible that measurement of the negative co-stimulatory cell surface molecule, programmed death (PD)-1 or its ligands, PD-L1 and PD-L2, on lymphocytes and antigen presenting cells respectively, may have a role in ICU immune monitoring. High levels of PD-1, PD-L1, and PD-L2 expression have been associated with immunoparalysis, and murine data suggest that they may be good therapeutic targets in future clinical trials (125,127), potentially in combination with IL-7 therapy (128).
Hyperinflammatory Immune Mechanisms in MODS
Over the last decade, it has been convincingly demonstrated that immune responsiveness is downregulated during MODS induced by sepsis, trauma (including traumatic brain injury), and other entities; however, there are notable patients who have “hyperinflammatory” conditions. Persistent inflammation can occur related to failure to achieve activated immune cell death (AICD). Two signal transduction systems which mediate AICD are particularly important, the Fas-Fas-ligand signaling pathway, and the CTL/NK cell signaling pathway. The Fas-Fas ligand (Fas and FasL) molecules are among the key regulators of apoptosis of activated immune cells (129). Fas is a type 1 transmembrane protein of the TNF-receptor family. It is widely expressed constitutively and can be induced during the inflammatory response. Ligation of Fas by the FasL triggers a signaling pathway that leads to AICD (130). Fas can be cleaved from the cell surface into a soluble form (sFas) much like the TNF receptor and may serve as a decoy binding FasL and preventing its interaction with Fas (130). The expression of FasL is mostly restricted to T and NK cells (130). Its production is induced during inflammation and it has its own pro-inflammatory properties including induction of IL-8, IL-1β, MCP1, TNF-α, and others, and it has chemotactic properties bringing neutrophils and macrophages into inflamed areas (131). Impairment of AICD by defective Fas-FasL function can lead to autoimmunity, and in the autoimmune lymphoproliferative syndrome (ALPS), Fas and FasL mutations are thought to be responsible (132). In children with sepsis-induced MODS (57), sFas levels were found to be highest in children with persistent (> 3 days) or sequential MODS (respiratory failure followed by hepato-renal failure) whereas soluble FasL (sFasL) levels were only elevated in sequential MODS. Soluble FasL was associated with viral infection related lymphoproliferative disease and the development of hepatic failure (57). Autopsy findings revealed hepatic lymphocytic infiltration, Epstein Barr Virus (EBV) infection, and lymphoproliferative disease in children with sFasL levels greater than 200 ng/mL, and hepatic necrosis in children with sFasL levels greater than 500 ng/mL. In hepatocyte cell culture experiments, incubation with exogenous sFasL > 500 ng/mL results in hepatocyte necrosis. These data support a role for lymphoproliferation generated sFasL inducing hepatic injury in sequential multiple organ failure patients (57). Other investigators have also reported the presence of hepatocytes expressing Fas with FasL positively stained lymphocytic infiltration at the site of tissue injury in acute hepatitis / liver failure patients (133). Upregulation of the sFas-FasL system has been observed in MODS related to acute respiratory distress syndrome (134,135), inflammatory bowel disease (136), graft versus host disease (137), trauma (138), thrombotic thrombocytopenic purpura and disseminated intravascular coagulation (139), burns (140), MAS (141), and hemophagocytic lymphohistiocytosis (HLH) (142). All of these entities have a hyperinflammatory response. It is unclear whether Fas-FasL has an important role in these syndromes related to failed AICD, or to sFasL being directly injurious to tissues.
Another important mechanism to achieve AICD is CTL and NK cell cytolysis of target cells (143). HLH and MAS (also known as secondary or reactive HLH) share many features and are characterized by persistent hyperinflammation with hypercytokinemia. In familial HLH, there are mutations of genes involved with NK cell degranulation of perforin and granzyme (cytolytic mechanisms) (144). MAS can occur with oligogenic mutations most often observed in children with autoinflammatory conditions such as systemic juvenile idiopathic arthritis (SJIA) and its adult equivalent Adult Onset Still Disease, numerous autoimmune diseases, malignancy, viral infections, and Kawasaki disease (145–147). Both HLH and MAS are characterized by low NK cell activity per cell, high levels of the CTL activation marker soluble CD25 (IL-2 receptor), and accumulation of CD8+ CTLs and macrophages (146). Despite defective cytolytic activity, proliferation and cytokine production of these cells is robust leading to a prolonged and exaggerated inflammatory response (143,144). Experimental and clinical studies have demonstrated that interferon-γ is a key mediator in this process (147,148). The precise mechanisms leading to defective NK and CD8 cytolytic functions are unknown; however, one model of MAS in a genetically normal rodent demonstrates that it can be induced by repeated TLR-9 stimulation using CPG (cytosine-phosphate guanine, a microbial DNA or PAMP mimicker) (149). Autoimmune disease, malignancy, and some persistent viral infections result in TLR-9 stimulation and can provoke these syndromes. Most viral infections induce robust interferon-γ production which sensitizes macrophages to TLR ligand stimulation. It is plausible that viral infections trigger HLH /MAS because of interferon-γ induction in the setting of genetic susceptibility or other unknown predispositions. Recently, whole exome sequencing of patients with SJIA and MAS revealed several (oligogenic) heterozygous protein–altering rare variants within some of the homozygous genetic mutations in the cytolytic pathway present in familial HLH. These findings were more common in SJIA positive MAS compared with SJIA without MAS (36% vs 14%, respectively) (150).
Currently, treatment of HLH includes high dose steroids, cyclosporine, and etoposide, all of which have substantial toxicities (151). Other biological therapies being explored for MAS are anti-cytokine in nature including anti-IL-1, anti-TNF, and anti-IL-6 with some case reports of paradoxical MAS (with anti-IL-1 or anti-IL-6) during treatment for SJIA (152). In EBV-induced lymphoproliferation, anti-CD20 (rituximab) has been found to be successful (153). Because of the compelling experimental and clinical data implicating interferon-γ, clinical trials using interferon-γ blocking strategies are currently being conducted (152).
Summary
MODS pathophysiology occurs when damaged tissue molecules (DAMPS), infection or bacterial toxin molecules (PAMPS), and reduced protective CYP 450/ mitochondrial metabolism lead to uncontrolled inflammation that perturbs endothelial, epithelial, immune, and mitochondrial cell homeostasis resulting in multiple organ system failures/dysfunctions. Altered coagulation with bleeding and thrombosis, and immune dysregulation with immune depression and macrophage activation, are associated with several MODS phenotypes related to environmental exposures and host genetics. In addition to organ support, pathophysiology based MODS therapies may include 1) removal of damaged and necrotic tissues (e.g. surgery) 2) removal of infection and toxin sources (e.g. timely administration of appropriate antimicrobials and anti-toxins) and 3) MODS phenotype specific therapies (e.g. immune modulation for immunoparalysis; eculizumab and/or plasma exchange for thrombocytopenia associated MOF; IVIG and/or rituximab for lymphoproliferative sequential MOF; and IVIG, methylprednisone, and/or anti-inflammatory biologics for macrophage activation syndrome). It is hoped that with further study, important knowledge gaps may be bridged that will enhance the understanding of the pathophysiology of this life-threatening condition and result in improved outcomes (Table 1 and Table 2).
Table 1.
Identified Knowledge Gaps and Potential Opportunities for Study.
|
Table 2.
Reported therapies for MODS subtypes
| Subtype | Treatment | Study Population | Design | Study | Outcome |
|---|---|---|---|---|---|
| Immune paralysis |
Immune suppressant Withdrawal |
Case reports |
Infection and MODS resolution |
||
| GM-CSF 66,118,120 | Children with ≥ 3 organ failure and ex vivo TNF response < 168 pg/mL; N = 14; GM-CSF N =7, Standard N = 7 |
Randomized Controlled Trial |
Prospective Single Center66 |
GM-CSF reversed immune paralysis, and reduced the onset of nosocomial infection from 8 infections in 7 patients with placebo patients with GM-CSF (p < 0.05). |
|
| Adults with septic shock/severe sepsis/MODS and Immune paralysis defined by low monocyte HLA-DR expression N = 38; GM-CSF N = 19, placebo N = 19 |
Randomized Placebo Controlled Trial |
Prospective Multiple Center Study118 |
GM-CSF reversed immune paralysis, increased ventilator free days and improved Physiologic Severity/MODS Score (p < 0.05). |
||
|
Interferon gamma154 |
Intubated adults with severe multiple trauma and immune paralysis N=21; Inhaled Interferon gamma N = 11, Inhaled placebo N = 10 |
Randomized Placebo Controlled Trial |
Prospective Single Center Study154 |
Inhaled Interferon gamma reduced ventilator associated pneumonia (p< 0.5) and restored alveolar macrophage HLA-DR expression |
|
|
Thrombocytopenia Associated MOF |
Plasma Exchange 30,31,48,49,155 |
Pediatric TAMOF N =42 15 plasma exchange; 27 standard care |
Cohort Study Plasma Exchange vs Standard Therapy |
Prospective Multiple Center Analysis49 |
28 day Mortality decreased from 70.4% to 26.7%; Multivariate analysis found improved survival controlling for PRISM, OFI, PELOD, neurologic failure (p = 0.048). |
| Pediatric TAMOF N=10; plasma exchange N = 5; standard therapy N = 5 |
Randomized Controlled Trial Plasma Exchange vs Standard Therapy |
Prospective Single Center30 |
Plasma exchange restored organ function, improved ADAMTS13 levels, and reduced 28 day mortality from 80% to 0% (p < 0.05) |
||
| Adult TAMOF N = 37; Plasma infusion N = 22, Plasma exchange N = 15 |
Randomized Trial Plasma Infusion Vs Plasma Exchange |
Prospective Single Center155 |
Plasma exchange reduced hospital mortality from 32% to 0% (p < 0.001) |
||
| Adult TAMOF N=102; Plasma infusion N = 51, Plasma Exchange N = 51 |
Randomized Trial Plasma Infusion vs Plasma Exchange |
Prospective Multiple Center31 |
Plasma exchange Reduced hospital mortality from 16% to 4% (p = 0.035); and 6 month mortality from 37% to 22% (p = 0.035) |
||
|
C5a antibody (Ecullizumab)42–47 |
Two small Phase II trials; age > 12 years with atypical Hemolytic Uremic Syndrome |
Open Label Single Arm; Year Long Treatment |
Prospective Multiple Center42 |
Improved renal function over time and loss of plasma exchange dependence led to FDA approval as orphan drug |
|
| Case series N=3 of children with HUS- STEC-related MODS treated with plasma exchange/ Eculizumab rescue |
Open Label Single Arm Two Week Treatment |
Retro- spective Single Center Case Series47 |
Improved MODS resolution and renal function thought to be temporally related to Eculizumab |
||
| Sequential MOF | Rituximab156,157 | Phase II trial of N = 43 adults with PTLD unresponsive to holding immune suppression subsequently treated with Rituximab |
Open Label Single Arm |
Prospective Multiple Center Study156 |
86% survival at 80 days; 62% survival at 1 year |
| Phase II trial of adding Rituximab to low dose chemotherapy N = 55 in children with PTLD already receiving low dose cytoxan and prednisone |
Open Label Single Arm |
Prospective Multiple Center Study157 |
83% survival at 4.8 years |
||
|
Antivirals/IVIG/ Methylprednisone |
Case reports |
Infection and MODS resolution |
|||
| HLH protocol158 | Case series treated with HLH-94 protocol |
Registry, Open label Single Arm |
Retro- spective, Multiple Center158 |
5 year probability of survival is 54% |
|
|
Macrophage Activation Syndrome |
Methylprednisone/ IVIG / Plasma Exchange69 |
Pediatric secondary hemophagocytic lymphohistiocytosis /sepsis/multiple organ dysfunction syndrome/macro- phage activation syndrome N = 23; HLH chemotherapy protocol N =6, IVIG / Methylprednisone N = 17 |
Cluster Randomized Trial Comparing HLH Protocol With Plasma Exchange To IVIG/ Methyl- Prednisone With Plasma Exchange |
Prospective Multiple Center Analysis69 |
Plasma exchange and treatment with IVIG/Methyl- prednisone reduced hospital mortality from 50% to 0% (p = 0.002). |
| IRAP70,159 | Adult MODS with disseminated intravascular coagulation and hepatobiliary dysfunction |
Randomized Double Blinded Placebo Controlled Trial |
Post Hoc Multiple Center Analysis159 |
28 day mortality decreased from 64.7% to 34.6% hazard ratio 0.28 [95% Confidence Interval I 0.11–0.0071] p = 0.007 |
|
| Pediatric secondary hemophagocytic lymphohistiocytosis /sepsis/multiple organ dysfunction syndrome/macro- phage activation syndrome treated with IRAP N=8 |
Case series | Post-Hoc Single Center70 |
Considered to be temporally related to improvement of MODS. Hospital survival 100%. |
||
| Tocilizumab71,160 | Pediatric patients with cytokine releasing syndrome after CART treated with tocilizumab N = 13 |
Case series | Post-Hoc Single Center160 |
Considered to be temporally related to improvement of MODS |
|
MODS- Multiple Organ Dysfunction Syndrome, GM-CSF- Granulocyte Macrophage Colony Stimulating Factor, N – number of patients, HLA-DR – Human leukocyte antigen DR, TAMOF – Thrombocytopenia associated Multiple Organ Failure, PRISM – Pediatric Risk of Mortality, OFI – Organ Failure Index, C5a – Complement component 5a, HUS-STEC – Hemolytic Uremic Syndrome-Shiga Toxin producing Escherichia Coli, FDA – Food and Drug Administration, PTLD – Post Transplant Lympho Proliferative Disease, IVIG – Intra Venous Immune Globulin , HLH – Hemophagocytic Lympho Histiocytosis, IRAP – Interleukin 1 receptor antagonist protein, CART – Chimeric Antigen Receptor T cell therapy
Acknowledgments
We thank the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) and their Office of Science Policy, Analysis and Communications for their support of this Workshop.
This manuscript is funded in part by R01GM008619 (JAC), R01GM094203 (M.W.H.), K12HD047349 and K23GM110496 (SLW), R01GM112806 (TCN)
References
- 1.Baue AE. Multiple, progressive, or sequential systems failure. A syndrome of the 1970s. Arch Surg. 1975;110:779–781. doi: 10.1001/archsurg.1975.01360130011001. [DOI] [PubMed] [Google Scholar]
- 2.Baue AE. Recovery from multiple organ failure. Am J Surg. 1985;149:420–421. doi: 10.1016/s0002-9610(85)80124-0. [DOI] [PubMed] [Google Scholar]
- 3.Baue AE. Nutrition and metabolism in sepsis and multisystem organ failure. Surg Clin North Am. 1991;71:549–565. doi: 10.1016/s0039-6109(16)45433-2. [DOI] [PubMed] [Google Scholar]
- 4.Baue AE. The horror autotoxicus and multiple-organ failure. Arch Surg. 1992;127:1451–1462. doi: 10.1001/archsurg.1992.01420120085016. [DOI] [PubMed] [Google Scholar]
- 5.Chandel B, Shapiro MJ, Kurtz M, et al. MEGX (monoethylglycinexylidide): a novel in vivo test to measure early hepatic dysfunction after hypovolemic shock. Shock. 1995;3:51–53. doi: 10.1097/00024382-199501000-00008. discussion 54–55. [DOI] [PubMed] [Google Scholar]
- 6.Baue AE. MOF/MODS, SIRS: an update. Shock. 1996;(6 Suppl 1):S1–S5. [PubMed] [Google Scholar]
- 7.Steinberg S, Flynn W, Kelley K, et al. Development of a bacteria-independent model of the multiple organ failure syndrome. Arch Surg. 1989;124:1390–1395. doi: 10.1001/archsurg.1989.01410120036008. [DOI] [PubMed] [Google Scholar]
- 8.Carcillo JA, Korzekwa KR, Jones GS, et al. The cytochrome P450 suicide inhibitor, 1-aminobenzotriazole, sensitizes rats to zymosan-induced toxicity. Res Commun Mol Pathol Pharmacol. 1998;102:57–68. [PubMed] [Google Scholar]
- 9.Remichkova M, Yordanov M, Dimitrova P. Etoposide attenuates zymosan-induced shock in mice. Inflammation. 2008;31:57–64. doi: 10.1007/s10753-007-9049-8. [DOI] [PubMed] [Google Scholar]
- 10.Bender JW. The effect of VP 16-213 on NBT reduction in the normal polymorphonuclear neutrophil. Cancer Biochem Biophys. 1980;4:233–236. [PubMed] [Google Scholar]
- 11.Jackson RJ, Johnson DD, Maxson RT, Thomas R, Smith SD. A comparison of neonatal and adult multiorgan failure in a rat model. J Pediatr Surg. 2000;35:428–431. doi: 10.1016/s0022-3468(00)90207-0. [DOI] [PubMed] [Google Scholar]
- 12.Thomas NJ, Umstead TM, Phelps DS. Altered chemokine response in an animal model of multiple organ dysfunction syndrome induced by zymosan. J Pediatr Surg. 2005;40:464–469. doi: 10.1016/j.jpedsurg.2004.11.042. [DOI] [PubMed] [Google Scholar]
- 13.Whitmore LC, Goss KL, Newell EA, Hilkin BM, Hook JS, Moreland JG. NOX2 protects against progressive lung injury and multiple organ dysfunction syndrome. Am J Physiol Lung Cell Mol Physiol. 2014;307:L71–L82. doi: 10.1152/ajplung.00054.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Doughty L, Carcillo JA, Kaplan S, Janosky J. Plasma nitrite and nitrate concentrations and multiple organ failure in pediatric sepsis. Crit Care Med. 1998;26:157–162. doi: 10.1097/00003246-199801000-00032. [DOI] [PubMed] [Google Scholar]
- 15.Proulx F, Fayon M, Farrell CA, Lacroix J, Gauthier M. Epidemiology of sepsis and multiple organ dysfunction syndrome in children. Chest. 1996;109:1033–1037. doi: 10.1378/chest.109.4.1033. [DOI] [PubMed] [Google Scholar]
- 16.Carcillo JA, Doughty L, Kofos D, et al. Cytochrome P450 mediated-drug metabolism is reduced in children with sepsis-induced multiple organ failure. Intensive Care Med. 2003;29:980–984. doi: 10.1007/s00134-003-1758-3. [DOI] [PubMed] [Google Scholar]
- 17.Matzinger P. An innate sense of danger. Semin Immunol. 1998;10:399–415. doi: 10.1006/smim.1998.0143. [DOI] [PubMed] [Google Scholar]
- 18.Doughty LA, Kaplan SS, Carcillo JA. Inflammatory cytokine and nitric oxide responses in pediatric sepsis and organ failure. Crit Care Med. 1996;24:1137–1143. doi: 10.1097/00003246-199607000-00012. [DOI] [PubMed] [Google Scholar]
- 19.Doughty L, Carcillo JA, Kaplan S, Janosky J. The compensatory anti-inflammatory cytokine interleukin 10 response in pediatric sepsis-induced multiple organ failure. Chest. 1998;113:1625–1631. doi: 10.1378/chest.113.6.1625. [DOI] [PubMed] [Google Scholar]
- 20.Whalen MJ, Doughty LA, Carlos TM, Wisniewski SR, Kochanek PM, Carcillo JA. Intercellular adhesion molecule-1 and vascular cell adhesion molecule-1 are increased in the plasma of children with sepsis-induced multiple organ failure. Crit Care Med. 2000;28:2600–2607. doi: 10.1097/00003246-200007000-00070. [DOI] [PubMed] [Google Scholar]
- 21.Thomas NJ, Carcillo JA, Herzer WA, Mi Z, Jackson EK. Chronic type IV phosphodiesterase inhibition protects glomerular filtration rate and renal and mesenteric blood flow in a zymosan-induced model of multiple organ dysfunction syndrome treated with norepinephrine. J Pharmacol Exp Ther. 2001;296:168–174. [PubMed] [Google Scholar]
- 22.Despond O, Proulx F, Carcillo JA, Lacroix J. Pediatric sepsis and multiple organ dysfunction syndrome. Curr Opin Pediatr. 2001;13:247–253. doi: 10.1097/00008480-200106000-00006. [DOI] [PubMed] [Google Scholar]
- 23.Green J, Doughty L, Kaplan SS, Sasser H, Carcillo JA. The tissue factor and plasminogen activator inhibitor type-1 response in pediatric sepsis-induced multiple organ failure. Thromb Haemost. 2002;87:218–223. [PubMed] [Google Scholar]
- 24.Wheeler DS, Fisher LE, Jr, Catravas JD, Jacobs BR, Carcillo JA, Wong HR. Extracellular hsp70 levels in children with septic shock. Pediatr Crit Care Med. 2005;6:308–311. doi: 10.1097/01.PCC.0000161075.97355.2E. [DOI] [PubMed] [Google Scholar]
- 25.Wheeler DS, Lahni P, Odoms K, et al. Extracellular heat shock protein 60 (Hsp60) levels in children with septic shock. Inflamm Res. 2007;56:216–219. doi: 10.1007/s00011-007-6108-4. [DOI] [PubMed] [Google Scholar]
- 26.Giuliano JS, Jr, Lahni PM, Harmon K, et al. Admission angiopoietin levels in children with septic shock. Shock. 2007;28:650–654. [PMC free article] [PubMed] [Google Scholar]
- 27.Carcillo JA. Pediatric septic shock and multiple organ failure. Crit Care Clin. 2003;19:413–440. doi: 10.1016/s0749-0704(03)00013-7. [DOI] [PubMed] [Google Scholar]
- 28.Nguyen TC, Carcillo JA. Bench-to-bedside review: thrombocytopenia-associated multiple organ failure--a newly appreciated syndrome in the critically ill. Crit Care. 2006;10:235. doi: 10.1186/cc5064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nguyen TC, Carcillo JA. Understanding the role of von Willebrand factor and its cleaving protease ADAMTS13 in the pathophysiology of critical illness. Pediatr Crit Care Med. 2007;8:187–189. doi: 10.1097/01.CCM.0000257468.75474.D4. [DOI] [PubMed] [Google Scholar]
- 30.Nguyen TC, Han YY, Kiss JE, et al. Intensive plasma exchange increases a disintegrin and metalloprotease with thrombospondin motifs-13 activity and reverses organ dysfunction in children with thrombocytopenia-associated multiple organ failure. Crit Care Med. 2008;36:2878–2887. doi: 10.1097/ccm.0b013e318186aa49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rock GA, Shumak KH, Buskard NA, et al. Comparison of plasma exchange with plasma infusion in the treatment of thrombotic thrombocytopenic purpura. Canadian Apheresis Study Group. N Engl J Med. 1991;325:393–397. doi: 10.1056/NEJM199108083250604. [DOI] [PubMed] [Google Scholar]
- 32.Madach K, Aladzsity I, Szilagyi A, et al. 4G/5G polymorphism of PAI-1 gene is associated with multiple organ dysfunction and septic shock in pneumonia induced severe sepsis: prospective, observational, genetic study. Crit Care. 2010;14:R79. doi: 10.1186/cc8992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Levy GG, Nichols WC, Lian EC, et al. Mutations in a member of the ADAMTS gene family cause thrombotic thrombocytopenic purpura. Nature. 2001;413(6855):488–494. doi: 10.1038/35097008. [DOI] [PubMed] [Google Scholar]
- 34.Kokame K, Matsumoto M, Soejima K, et al. Mutations and common polymorphisms in ADAMTS13 gene responsible for von Willebrand factor-cleaving protease activity. Proc Natl Acad Sci USA. 2002;99:11902–11907. doi: 10.1073/pnas.172277399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Matsumoto M, Kokame K, Soejima K, et al. Molecular characterization of ADAMTS13 gene mutations in Japanese patients with Upshaw-Schulman syndrome. Blood. 2004;103:1305–1310. doi: 10.1182/blood-2003-06-1796. [DOI] [PubMed] [Google Scholar]
- 36.Pimanda JE, Maekawa A, Wind T, Paxton J, Chesterman CN, Hogg PJ. Congenital thrombotic thrombocytopenic purpura in association with a mutation in the second CUB domain of ADAMTS13. Blood. 2004;103:627–629. doi: 10.1182/blood-2003-04-1346. [DOI] [PubMed] [Google Scholar]
- 37.Schneppenheim R, Budde U, Oyen F, et al. von Willebrand factor cleaving protease and ADAMTS13 mutations in childhood TTP. Blood. 2003;101:1845–1850. doi: 10.1182/blood-2002-08-2399. [DOI] [PubMed] [Google Scholar]
- 38.Uchida T, Wada H, Mizutani M, et al. Identification of novel mutations in ADAMTS13 in an adult patient with congenital thrombotic thrombocytopenic purpura. Blood. 2004;104:2081–2083. doi: 10.1182/blood-2004-02-0715. [DOI] [PubMed] [Google Scholar]
- 39.Noris M, Mescia F, Remuzzi G. STEC-HUS, atypical HUS and TTP are all diseases of complement activation. Nat Rev Nephrol. 2012;8:622–633. doi: 10.1038/nrneph.2012.195. [DOI] [PubMed] [Google Scholar]
- 40.Dragon-Durey MA, Sethi SK, Bagga A, et al. Clinical features of anti-factor H autoantibody-associated hemolytic uremic syndrome. J Am Soc Nephrol. 2010;21:2180–2187. doi: 10.1681/ASN.2010030315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ricklin D, Hajishengallis G, Yang K, Lambris JD. Complement: a key system for immune surveillance and homeostasis. Nat Immunol. 2010;11:785–797. doi: 10.1038/ni.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Legendre CM, Licht C, Muus P, et al. Terminal complement inhibitor eculizumab in atypical hemolytic-uremic syndrome. N Engl J Med. 2013;368:2169–2181. doi: 10.1056/NEJMoa1208981. [DOI] [PubMed] [Google Scholar]
- 43.Zuber J, Fakhouri F, Roumenina LT, Loirat C, Fremeaux-Bacchi V French Study Group for a HCG. Use of eculizumab for atypical haemolytic uraemic syndrome and C3 glomerulopathies. Nat Rev Nephrol. 2012;8:643–657. doi: 10.1038/nrneph.2012.214. [DOI] [PubMed] [Google Scholar]
- 44.Colic E, Dieperink H, Titlestad K, Tepel M. Management of an acute outbreak of diarrhoea-associated haemolytic uraemic syndrome with early plasma exchange in adults from southern Denmark: an observational study. Lancet. 2011;378:1089–1093. doi: 10.1016/S0140-6736(11)61145-8. [DOI] [PubMed] [Google Scholar]
- 45.Kim JJ, Goodship TH, Tizard J, Inward C. Plasma therapy for atypical haemolytic uraemic syndrome associated with heterozygous factor H mutations. Pediatr Nephrol. 2011;26:2073–2076. doi: 10.1007/s00467-011-1944-4. [DOI] [PubMed] [Google Scholar]
- 46.Kielstein JT, Beutel G, Fleig S, et al. Best supportive care and therapeutic plasma exchange with or without eculizumab in Shiga-toxin-producing E. coli O104:H4 induced haemolytic-uraemic syndrome: an analysis of the German STEC-HUS registry. Nephrol Dial Transplant. 2012;27:3807–3815. doi: 10.1093/ndt/gfs394. [DOI] [PubMed] [Google Scholar]
- 47.Lapeyraque AL, Malina M, Fremeaux-Bacchi V, et al. Eculizumab in severe Shiga-toxin-associated HUS. N Engl J Med. 2011;364:2561–2563. doi: 10.1056/NEJMc1100859. [DOI] [PubMed] [Google Scholar]
- 48.Qu L, Kiss JE, Dargo G, Carcillo JA. Outcomes of previously healthy pediatric patients with fulminant sepsis-induced multisystem organ failure receiving therapeutic plasma exchange. J Clin Apher. 2011;26:208–213. doi: 10.1002/jca.20296. [DOI] [PubMed] [Google Scholar]
- 49.Sevketoglu E, Yildizdas D, Horoz OO, et al. Use of therapeutic plasma exchange in children with thrombocytopenia-associated multiple organ failure in the Turkish thrombocytopenia-associated multiple organ failure network. Pediatr Crit Care Med. 2014;15:e354–359. doi: 10.1097/PCC.0000000000000227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nguyen TC, Carcillo JA. Therapeutic plasma exchange as a strategy to reverse multiple organ dysfunction syndrome in patients receiving extracorporeal life support. Pediatr Crit Care Med. 2015;16:383–385. doi: 10.1097/PCC.0000000000000372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhou Z, Yee DL, Guchhait P. Molecular link between intravascular hemolysis and vascular occlusion in sickle cell disease. Curr Vasc Pharmacol. 2012;10:756–761. doi: 10.2174/157016112803520738. [DOI] [PubMed] [Google Scholar]
- 52.Zhou Z, Han H, Cruz MA, López JA, Dong JF, Guchhait P. Haemoglobin blocks von Willebrand factor proteolysis by ADAMTS-13: a mechanism associated with sickle cell disease. Thromb Haemost. 2009;101:1070–1077. [PubMed] [Google Scholar]
- 53.Larkin D, de Laat B, Jenkins PV, et al. Severe Plasmodium falciparum malaria is associated with circulating ultra-large von Willebrand multimers and ADAMTS13 inhibition. PLoS Pathog. 2009;5:e1000349. doi: 10.1371/journal.ppat.1000349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Vinchi F, Costa da Silva M, Ingoglia G, et al. Hemopexin therapy reverts heme-induced proinflammatory phenotypic switching of macrophages in a mouse model of sickle cell disease. Blood. 2016;127:473–486. doi: 10.1182/blood-2015-08-663245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Feys HB, Roodt J, Vandeputte N, et al. Thrombotic thrombocytopenic purpura directly linked with ADAMTS13 inhibition in the baboon (Papio ursinus) Blood. 2010;116:2005–2010. doi: 10.1182/blood-2010-04-280479. [DOI] [PubMed] [Google Scholar]
- 56.Bockmeyer CL, Reuken PA, Simon TP, et al. ADAMTS13 activity is decreased in a septic porcine model. Significance for glomerular thrombus deposition. Thromb Haemost. 2011;105:145–153. doi: 10.1160/TH10-03-0153. [DOI] [PubMed] [Google Scholar]
- 57.Doughty L, Clark RS, Kaplan SS, Sasser H, Carcillo J. sFas and sFas ligand and pediatric sepsis-induced multiple organ failure syndrome. Pediatr Res. 2002;52:922–927. doi: 10.1203/00006450-200212000-00018. [DOI] [PubMed] [Google Scholar]
- 58.Jordan MB, Hildeman D, Kappler J, Marrack P. An animal model of hemophagocytic lymphohistiocytosis (HLH): CD8+ T cells and interferon gamma are essential for the disorder. Blood. 2004;104:735–743. doi: 10.1182/blood-2003-10-3413. [DOI] [PubMed] [Google Scholar]
- 59.Johnson TS, Terrell CE, Millen SH, Katz JD, Hildeman DA, Jordan MB. Etoposide selectively ablates activated T cells to control the immunoregulatory disorder hemophagocytic lymphohistiocytosis. J Immunol. 2014;192:84–91. doi: 10.4049/jimmunol.1302282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kögl T, Müller J, Jessen B, et al. Hemophagocytic lymphohistiocytosis in syntaxin-11-deficient mice: T-cell exhaustion limits fatal disease. Blood. 2013;121:604–613. doi: 10.1182/blood-2012-07-441139. [DOI] [PubMed] [Google Scholar]
- 61.Castillo L, Carcillo J. Secondary hemophagocytic lymphohistiocytosis and severe sepsis/ systemic inflammatory response syndrome/multiorgan dysfunction syndrome / macrophage activation syndrome share common intermediate phenotypes on a spectrum of inflammation. Pediatr Crit Care Med. 2009;10:387–392. doi: 10.1097/PCC.0b013e3181a1ae08. [DOI] [PubMed] [Google Scholar]
- 62.Simon DW, Aneja R, Carcillo JA, Halstead ES. Plasma exchange, methylprednisolone, IV immune globulin, and now anakinra support continued PICU equipoise in management of hyperferritinemia-associated sepsis/multiple organ dysfunction syndrome/macrophage activation syndrome/secondary hemophagocytic lymphohistiocytosis syndrome. Pediatr Crit Care Med. 2014;15:486–488. doi: 10.1097/PCC.0000000000000098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Muszynski JA, Thakkar R, Hall MW. Inflammation and innate immune function in critical illness. Curr Opin Pediatr. 2016;28:267–273. doi: 10.1097/MOP.0000000000000352. [DOI] [PubMed] [Google Scholar]
- 64.Doughty L. Adaptive immune function in critical illness. Adaptive immune function in critical illness. Curr Opin Pediatr. 2016;28:274–280. doi: 10.1097/MOP.0000000000000357. [DOI] [PubMed] [Google Scholar]
- 65.Felmet KA, Hall MW, Clark RS, Jaffe R, Carcillo JA. Prolonged lymphopenia, lymphoid depletion, and hypoprolactinemia in children with nosocomial sepsis and multiple organ failure. J Immunol. 2005;174:3765–3772. doi: 10.4049/jimmunol.174.6.3765. [DOI] [PubMed] [Google Scholar]
- 66.Hall MW, Knatz NL, Vetterly C, et al. Immunoparalysis and nosocomial infection in children with multiple organ dysfunction syndrome. Intensive Care Med. 2011;37:525–532. doi: 10.1007/s00134-010-2088-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Halstead ES, Carcillo JA, Schilling B, Greiner RJ, Whiteside TL. Reduced frequency of CD56 dim CD16 pos natural killer cells in pediatric systemic inflammatory response syndrome/sepsis patients. Pediatr Res. 2013;74:427–432. doi: 10.1038/pr.2013.121. [DOI] [PubMed] [Google Scholar]
- 68.Zhang M, Behrens EM, Atkinson TP, Shakoory B, Grom AA, Cron RQ. Genetic defects in cytolysis in macrophage activation syndrome. Curr rheumatol Rep. 2014;16:439. doi: 10.1007/s11926-014-0439-2. [DOI] [PubMed] [Google Scholar]
- 69.Demirkol D, Yildizdas D, Bayrakci B, et al. Turkish Secondary HLH/MAS Critical Care Study Group Hyperferritinemia in the critically ill child with secondary hemophagocytic lymphohistiocytosis/sepsis/multiple organ dysfunction syndrome/macrophage activation syndrome: what is the treatment? Crit Care. 2012;16:R52. doi: 10.1186/cc11256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Rajasekaran S, Kruse K, Kovey K, et al. Therapeutic role of anakinra, an interleukin-1 receptor antagonist, in the management of secondary hemophagocytic lymphohistiocytosis /sepsis/multiple organ dysfunction/macrophage activating syndrome in critically ill children. Pediatr Crit Care Med. 2014;15:401–408. doi: 10.1097/PCC.0000000000000078. [DOI] [PubMed] [Google Scholar]
- 71.Teachey DT, Rheingold SR, Maude SL, et al. Cytokine release syndrome after blinatumomab treatment related to abnormal macrophage activation and ameliorated with cytokine-directed therapy. Blood. 2013;121:5154–5157. doi: 10.1182/blood-2013-02-485623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lee DW, Gardner R, Porter DL, et al. Current concepts in the diagnosis and management of cytokine release syndrome. Blood. 2014;124:188–195. doi: 10.1182/blood-2014-05-552729. Erratum in: Blood 2015;126:1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Akira S, Takeda K. Toll-like receptor signaling. Nat Rev Immunol. 2004;4:499–511. doi: 10.1038/nri1391. [DOI] [PubMed] [Google Scholar]
- 74.Franchi L, McDonald C, Kanneganti T-D, Amer A, Nunez G. Nucleotide-binding oligomerization domain-like receptors: Intracellular pattern recognition molecules for pathogen detection and host defense. J Immunol. 2006;177:3507–3513. doi: 10.4049/jimmunol.177.6.3507. [DOI] [PubMed] [Google Scholar]
- 75.Zhang X, Mosser DM. Macrophage activation by endogenous danger signals. J Pathol. 2008;214:161–178. doi: 10.1002/path.2284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wang H, Yang H, Czura CJ, Sama AE, Tracey KJ. HMGB1 as a late mediator of lethal systemic inflammation. Am J Respir Crit Care Med. 2001;164(10 Pt 1):1768–1773. doi: 10.1164/ajrccm.164.10.2106117. [DOI] [PubMed] [Google Scholar]
- 77.Bianchi ME. DAMPs, PAMPs and alarmins: all we need to know about danger. J Leukoc Biol. 2007;81:1–5. doi: 10.1189/jlb.0306164. [DOI] [PubMed] [Google Scholar]
- 78.Castiglioni A, Canti V, Rovere-Querini P, Manfredi AA. High-mobility group box 1 (HMGB1) as a master regulator of innate immunity. Cell Tissue Res. 2011;343:189–199. doi: 10.1007/s00441-010-1033-1. [DOI] [PubMed] [Google Scholar]
- 79.Gallucci S, Matzinger P. Danger signals: SOS to the immune system. Curr Opin Immunol. 2001;13:114–119. doi: 10.1016/s0952-7915(00)00191-6. [DOI] [PubMed] [Google Scholar]
- 80.Wang H, Bloom O, Zhang M, et al. HMG-1 as a late mediator of endotoxin lethality in mice. Science. 1999;285:248–251. doi: 10.1126/science.285.5425.248. [DOI] [PubMed] [Google Scholar]
- 81.Aneja R, Killeen M, Bayir H, et al. High Mobility Group Box 1(HMGB1) clearance with plasma exchange in pediatric patients with sepsis and thrombocytopenia-associated with multiple organ failure. Crit Care Med. 2007;35:A264. [Google Scholar]
- 82.Sunden-Cullberg J, Norrby-Teglund A, Rouhiainen A, et al. Persistent elevation of high mobility group box-1 protein (HMGB1) in patients wih severe sepsis and septic shock. Crit Care Med. 2005;33:564–573. doi: 10.1097/01.ccm.0000155991.88802.4d. [DOI] [PubMed] [Google Scholar]
- 83.van Zoelen MA, Laterre PF, van Veen SQ, et al. Systemic and local high mobility group box 1 concentrations during severe infection. Crit Care Med. 2007;35:2799–2804. doi: 10.1097/01.CCM.0000287588.69000.97. [DOI] [PubMed] [Google Scholar]
- 84.Karlsson S, Pettila V, Tenhunen J, Laru-Sompa R, Hynninen M, Ruokonen E. HMGB1 as a predictor of organ dysfunction and outcome in patients with severe sepsis. Intensive Care Med. 2008;34:1046–1053. doi: 10.1007/s00134-008-1032-9. [DOI] [PubMed] [Google Scholar]
- 85.Doctor A, Zimmerman JJ, Agus M, et al. Pediatric Multiple Organ Dysfunction Syndrome: Promising Therapies. Pediatr Crit Care Med. 2016;17(Suppl) doi: 10.1097/PCC.0000000000001053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kuznetsov AV, Kehrer I, Kozlov AV, et al. Mitochondrial ROS production under cellular stress: comparison of different detection methods. Anal Bioanal Chem. 2011;400:2383–2390. doi: 10.1007/s00216-011-4764-2. [DOI] [PubMed] [Google Scholar]
- 87.Brealey D, Brand M, Hargreaves I, et al. Association between mitochondrial dysfunction and severity and outcome of septic shock. Lancet. 2002;360:219–223. doi: 10.1016/S0140-6736(02)09459-X. [DOI] [PubMed] [Google Scholar]
- 88.Fink MP. Cytopathic hypoxia. Is oxygen use impaired in sepsis as a result of an acquired intrinsic derangement in cellular respiration? Crit Care Clin. 2002;18:165–175. doi: 10.1016/s0749-0704(03)00071-x. [DOI] [PubMed] [Google Scholar]
- 89.Fink MP. Bench-to-bedside review: Cytopathic hypoxia. Crit Care. 2002;6:491–499. doi: 10.1186/cc1824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kozlov AV, Bahrami S, Calzia E, et al. Mitochondrial dysfunction and biogenesis: do ICU patients die from mitochondrial failure? Ann Intensive Care. 2011;1:41. doi: 10.1186/2110-5820-1-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Picard M, Taivassalo T, Gouspillou G, et al. Mitochondria: isolation, structure and function. J Physiol. 2011;589:4413–4421. doi: 10.1113/jphysiol.2011.212712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Singer M. Mitochondrial function in sepsis: acute phase versus multiple organ failure. Crit Care Med. 2007;35(9 Suppl):S441–S448. doi: 10.1097/01.CCM.0000278049.48333.78. [DOI] [PubMed] [Google Scholar]
- 93.Crouser ED. Mitochondrial dysfunction in septic shock and multiple organ dysfunction syndrome. Mitochondrion. 2004;4:729–741. doi: 10.1016/j.mito.2004.07.023. [DOI] [PubMed] [Google Scholar]
- 94.Villarroel JP, Guan Y, Werlin E, et al. Hemorrhagic shock and resuscitation are associated with peripheral blood mononuclear cell mitochondrial dysfunction and immunosuppression. J Trauma Acute Care Sur. 2013;75:24–31. doi: 10.1097/TA.0b013e3182988b1f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Belikova I, Lukaszewicz AC, Faivre V, et al. Oxygen consumption of human peripheral blood mononuclear cells in severe human sepsis. Crit Care Med. 2007;35:2702–2708. doi: 10.1097/01.ccm.0000295593.25106.c4. [DOI] [PubMed] [Google Scholar]
- 96.Brealey D, Brand M, Hargreaves I, et al. Association between mitochondrial dysfunction and severity and outcome of septic shock. Lancet. 2002;360:219–223. doi: 10.1016/S0140-6736(02)09459-X. [DOI] [PubMed] [Google Scholar]
- 97.Weiss SL, Selak MA, Tuluc F, et al. Mitochondrial dysfunction in peripheral blood mononuclear cells in pediatric septic shock. Pediatr Crit Care Med. 2015;16:e4–e12. doi: 10.1097/PCC.0000000000000277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Weiss SL, Cvijanovich NZ, Allen GL, et al. Differential expression of the nuclear-encoded mitochondrial transcriptome in pediatric septic shock. Crit Care. 2014;18:623. doi: 10.1186/s13054-014-0623-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Langley RJ, Tsalik EL, van Velkinburgh JC, et al. An integrated clinico-metabolomic model improves prediction of death in sepsis. Sci Transl Med. 2013;5 doi: 10.1126/scitranslmed.3005893. 195ra195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Cherry AD, Piantadosi CA. Regulation of mitochondrial biogenesis and its intersection with inflammatory responses. Antioxid Redox Signal. 2015;22:965–976. doi: 10.1089/ars.2014.6200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kepp O, Galluzzi L, Kroemer G. Mitochondrial control of the NLRP3 inflammasome. Nat Immunol. 2011;12:199–200. doi: 10.1038/ni0311-199. [DOI] [PubMed] [Google Scholar]
- 102.Zhang Q, Raoof M, Chen Y, et al. Circulating mitochondrial DAMPs cause inflammatory responses to injury. Nature. 2010;464:104–107. doi: 10.1038/nature08780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Nakahira K, Kyung SY, Rogers AJ, et al. Circulating mitochondrial DNA in patients in the ICU as a marker of mortality: derivation and validation. PLoS Med. 2013;10:e1001577. doi: 10.1371/journal.pmed.1001577. discussion e1001577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Malik AN, Czajka A. Is mitochondrial DNA content a potential biomarker of mitochondrial dysfunction? Mitochondrion. 2013;13:481–492. doi: 10.1016/j.mito.2012.10.011. [DOI] [PubMed] [Google Scholar]
- 105.Levy RJ. Mitochondrial dysfunction, bioenergetic impairment, and metabolic down-regulation in sepsis. Shock. 2007;28:24–28. doi: 10.1097/01.shk.0000235089.30550.2d. [DOI] [PubMed] [Google Scholar]
- 106.Schumacker PT, Gillespie MN, Nakahira K, et al. Mitochondria in lung biology and pathology: more than just a powerhouse. Am J Physiol Lung Cell Mol Physiol. 2014;306:L962–L974. doi: 10.1152/ajplung.00073.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Hall MW, Muszynski JA. Immune modulation in sepsis. J Pediatr Infect Dis. 2009;4:127–136. [Google Scholar]
- 108.Bone RC. Immunologic dissonance: a continuing evolution in our understanding of the systemic inflammatory response syndrome (SIRS) and the multiple organ dysfunction syndrome (MODS) Ann of Internal Med. 1996;125:680–687. doi: 10.7326/0003-4819-125-8-199610150-00009. [DOI] [PubMed] [Google Scholar]
- 109.Mella C, Suarez-Arrabal MC, Lopez S, et al. Innate immune dysfunction is associated with enhanced disease severity in infants with severe respiratory syncytial virus bronchiolitis. J Infect Dis. 2013;207:564–573. doi: 10.1093/infdis/jis721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Hall MW, Geyer SM, Guo CY, et al. Innate immune function and mortality in critically ill children with influenza: a multicenter study. Crit Care Med. 2013;41:224–236. doi: 10.1097/CCM.0b013e318267633c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Muszynski JA, Nofziger R, Greathouse K, et al. Innate immune function predicts the development of nosocomial infection in critically injured children. Shock. 2014;42:313–321. doi: 10.1097/SHK.0000000000000217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Allen ML, Hoschtitzky JA, Peters MJ, et al. Interleukin-10 and its role in clinical immunoparalysis following pediatric cardiac surgery. Crit Care Med. 2006;34:2658–2665. doi: 10.1097/01.CCM.0000240243.28129.36. [DOI] [PubMed] [Google Scholar]
- 113.Cornell TT, Sun L, Hall MW, et al. Clinical implications and molecular mechanisms of immunoparalysis after cardiopulmonary bypass. J Thorac Cardiovasc Surg. 2012;143:1160–1166. doi: 10.1016/j.jtcvs.2011.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Wong HR, Cvijanovich NZ, Anas N, et al. Developing a clinically feasible personalized medicine approach to pediatric septic shock. Am J Respir Critical Care Med. 2015;191:309–315. doi: 10.1164/rccm.201410-1864OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Xiao W, Mindrinos MN, Seok J, et al. A genomic storm in critically injured humans. J Exp Med. 2011;208:2581–2590. doi: 10.1084/jem.20111354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Westendorp RG, Langermans JA, Huizinga TW, Verweij CL, Sturk A. Genetic influence on cytokine production in meningococcal disease. Lancet. 1997;349:1912–1913. doi: 10.1016/s0140-6736(05)63910-4. [DOI] [PubMed] [Google Scholar]
- 117.Muszynski JA, Frazier E, Nofziger R, et al. Pediatric Critical Care Blood Research Network (Blood Net) subgroup of the Pediatric Acute Lung Injury and Sepsis Investigators (PALISI). Red blood cell transfusion and immune function in critically ill children: a prospective observational study. Transfusion. 2015;55:766–774. doi: 10.1111/trf.12896. [DOI] [PubMed] [Google Scholar]
- 118.Meisel C, Schefold JC, Pschowski R, et al. Granulocyte-macrophage colony-stimulating factor to reverse sepsis-associated immunosuppression: a double-blind, randomized, placebo-controlled multicenter trial. Am J Respir Critical Care Med. 2009;180:640–648. doi: 10.1164/rccm.200903-0363OC. [DOI] [PubMed] [Google Scholar]
- 119.Volk HD, Reinke P, Krausch D, et al. Monocyte deactivation--rationale for a new therapeutic strategy in sepsis. Intensive Care Med. 1996;22(Suppl 4):S474–S481. doi: 10.1007/BF01743727. [DOI] [PubMed] [Google Scholar]
- 120.Docke WD, Hoflich C, Davis KA, et al. Monitoring temporary immunodepression by flow cytometric measurement of monocytic HLA-DR expression: a multicenter standardized study. Clin Chem. 2005;51:2341–2347. doi: 10.1373/clinchem.2005.052639. [DOI] [PubMed] [Google Scholar]
- 121.Albert M, Williamson D, Muscedere J, et al. Candida in the respiratory tract secretions of critically ill patients and the impact of antifungal treatment: a randomized placebo controlled pilot trial (CANTREAT study) Intensive Care Med. 2014;40:1313–1322. doi: 10.1007/s00134-014-3352-2. [DOI] [PubMed] [Google Scholar]
- 122.Hotchkiss RS, Tinsley KW, Swanson PE, et al. Sepsis-induced apoptosis causes progressive profound depletion of B and CD4+ T lymphocytes in humans. J Immunol. 2001;166:6952–6963. doi: 10.4049/jimmunol.166.11.6952. [DOI] [PubMed] [Google Scholar]
- 123.Boomer JS, To K, Chang KC, et al. Immunosuppression in patients who die of sepsis and multiple organ failure. JAMA. 2011;306:2594–2605. doi: 10.1001/jama.2011.1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Muszynski JA, Nofziger R, Greathouse K, et al. Early adaptive immune suppression in children with septic shock: a prospective observational study. Crit Care. 2014;18:R145. doi: 10.1186/cc13980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Chang KC, Burnham CA, Compton SM, et al. Blockade of the negative co-stimulatory molecules PD-1 and CTLA-4 improves survival in primary and secondary fungal sepsis. Crit Care. 2013;17:R85. doi: 10.1186/cc12711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Shindo Y, Unsinger J, Burnham CA, Green JM, Hotchkiss RS. Interleukin-7 and anti-programmed cell death 1 antibody have differing effects to reverse sepsis-induced immunosuppression. Shock. 2015;43:334–343. doi: 10.1097/SHK.0000000000000317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Arakaki R, Yamada A, Kudo Y, Hayashi Y, Ishimaru N. Mechanism of activation-induced cell death of T cells and regulation of FasL expression. Crit Rev Immunol. 2014;34:301–314. doi: 10.1615/critrevimmunol.2014009988. [DOI] [PubMed] [Google Scholar]
- 128.Lettau M, Paulsen M, Kabelitz D, Janssen O. Storage, expression and function of Fas ligand, the key death factor of immune cells. Curr Med Chem. 2008;15:1684–1696. doi: 10.2174/092986708784872384. [DOI] [PubMed] [Google Scholar]
- 129.Wajant H, Pfizenmaier K, Scheurich P. Non-apoptotic Fas signaling. Cytokine Growth Factor Rev. 2003;14:53–66. doi: 10.1016/s1359-6101(02)00072-2. [DOI] [PubMed] [Google Scholar]
- 130.Li P, Huang P, Yang Y, Hao M, Peng H, Li F. Updated understanding of autoimmune lymphoproliferative syndrome (ALPS) Clin Rev Allergy Immunol. 2016;50:55–63. doi: 10.1007/s12016-015-8466-y. [DOI] [PubMed] [Google Scholar]
- 131.Kondo T, Suda T, Fukuyama H, Adachi M, Nagata S. Essential roles of the Fas ligand in the development of hepatitis. Nat Med. 1997;3:409–413. doi: 10.1038/nm0497-409. [DOI] [PubMed] [Google Scholar]
- 132.Hashimoto S, Kobayashi A, Kooguchi K, Kitamura Y, Onodera H, Nakajima H. Upregulation of two death pathways of perforin/granzyme and FasL/Fas in septic acute respiratory distress syndrome. Am J Respir Critical Care Med. 2000;161:237–243. doi: 10.1164/ajrccm.161.1.9810007. [DOI] [PubMed] [Google Scholar]
- 133.Matute-Bello G, Liles WC, Steinberg KP, et al. Soluble Fas ligand induces epithelial cell apoptosis in humans with acute lung injury (ARDS) J Immunol. 1999;163:2217–2225. [PubMed] [Google Scholar]
- 134.Pinkoski MJ, Brunner T, Green DR, Lin T. Fas and Fas ligand in gut and liver. Am J Physiol Gastrointest Liver Physiol. 2000;278:G354–G366. doi: 10.1152/ajpgi.2000.278.3.G354. [DOI] [PubMed] [Google Scholar]
- 135.Wu Q, Chen H, Fang J, Xie W, Hong M, Xia L. Elevated Fas/FasL system and endothelial cell microparticles are involved in endothelial damage in acute graft-versus-host disease: a clinical analysis. Leuk Res. 2012;36:275–280. doi: 10.1016/j.leukres.2011.08.005. [DOI] [PubMed] [Google Scholar]
- 136.Paunel-Gorgulu A, Flohe S, Scholz M, Windolf J, Logters T. Increased serum soluble Fas after major trauma is associated with delayed neutrophil apoptosis and development of sepsis. Crit Care. 2011;15:R20. doi: 10.1186/cc9965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Hori Y, Wada H, Mori Y, et al. Plasma sFas and sFas ligand levels in patients with thrombotic thrombocytopenic purpura and in those with disseminated intravascular coagulation. Am J Hematol. 1999;61:21–25. doi: 10.1002/(sici)1096-8652(199905)61:1<21::aid-ajh5>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 138.Yamada Y, Endo S, Nakae H, et al. Examination of soluble Fas (sFas) and soluble Fas ligand (sFasL) in patients with burns. Burns. 2003;29:799–802. doi: 10.1016/s0305-4179(03)00201-8. [DOI] [PubMed] [Google Scholar]
- 139.Emmenegger U, Zehnder R, Frey U, Reimers A, Spaeth PJ, Neftel KA. Elevation of soluble Fas and soluble Fas ligand in reactive macrophage activation syndromes. Am J Hematol. 2000;64:116–119. doi: 10.1002/(sici)1096-8652(200006)64:2<116::aid-ajh8>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 140.Takada H, Nomura A, Ohga S, Hara T. Interleukin-18 in hemophagocytic lymphohistiocytosis. Leuk Lymphoma. 2001;42:21–28. doi: 10.3109/10428190109097673. [DOI] [PubMed] [Google Scholar]
- 141.Meeths M, Chiang SC, Lofstedt A, et al. Pathophysiology and spectrum of diseases caused by defects in lymphocyte cytotoxicity. Exp Cell Res. 2014;325:10–17. doi: 10.1016/j.yexcr.2014.03.014. [DOI] [PubMed] [Google Scholar]
- 142.Brisse E, Wouters CH, Matthys P. Hemophagocytic lymphohistiocytosis (HLH): A heterogeneous spectrum of cytokine-driven immune disorders. Cytokine Growth Factor Rev. 2015;26:263–280. doi: 10.1016/j.cytogfr.2014.10.001. [DOI] [PubMed] [Google Scholar]
- 143.Janka GE, Lehmberg K. Hemophagocytic syndromes--an update. Blood Rev. 2014;28:135–142. doi: 10.1016/j.blre.2014.03.002. [DOI] [PubMed] [Google Scholar]
- 144.Usmani GN, Woda BA, Newburger PE. Advances in understanding the pathogenesis of HLH. Br J Haematol. 2013;161:609–622. doi: 10.1111/bjh.12293. [DOI] [PubMed] [Google Scholar]
- 145.Atteritano M, David A, Bagnato G, et al. Haemophagocytic syndrome in rheumatic patients. A systematic review. Eur Rev Med Pharmacol Sci. 2012;16:1414–1424. [PubMed] [Google Scholar]
- 146.Terrell CE, Jordan MB. Perforin deficiency impairs a critical immunoregulatory loop involving murine CD8(+) T cells and dendritic cells. Blood. 2013;121:5184–5191. doi: 10.1182/blood-2013-04-495309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Takada H, Takahata Y, Nomura A, Ohga S, Mizuno Y, Hara T. Increased serum levels of interferon-gamma-inducible protein 10 and monokine induced by gamma interferon in patients with haemophagocytic lymphohistiocytosis. Clin Exp Immunol. 2003;133:448–453. doi: 10.1046/j.1365-2249.2003.02237.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Jordan MB, Hildeman D, Kappler J, Marrack P. An animal model of hemophagocytic lymphohistiocytosis (HLH): CD8+ T cells and interferon gamma are essential for the disorder. Blood. 2004;104:735–743. doi: 10.1182/blood-2003-10-3413. [DOI] [PubMed] [Google Scholar]
- 149.Behrens EM, Canna SW, Slade K, et al. Repeated TLR9 stimulation results in macrophage activation syndrome-like disease in mice. J Clin Invest. 2011;121:2264–2277. doi: 10.1172/JCI43157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Kaufman KM, Linghu B, Szustakowski JD, et al. Whole-exome sequencing reveals overlap between macrophage activation syndrome in systemic juvenile idiopathic arthritis and familial hemophagocytic lymphohistiocytosis. Arthritis Rheumatol. 2014;66:3486–3495. doi: 10.1002/art.38793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Henter JI, Horne A, Arico M, et al. HLH-2004: Diagnostic and therapeutic guidelines for hemophagocytic lymphohistiocytosis. Pediatr Blood Cancer. 2007;48:124–131. doi: 10.1002/pbc.21039. [DOI] [PubMed] [Google Scholar]
- 152.Schulert GS, Grom AA. Pathogenesis of macrophage activation syndrome and potential for cytokine- directed therapies. Annu Rev Med. 2015;66:145–159. doi: 10.1146/annurev-med-061813-012806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Giraldi E, Provenzi M, Conter V, et al. Risk-adapted treatment for severe B-lineage posttransplant lymphoproliferative disease after solid organ transplantation in children. Transplantation. 2016;100:437–445. doi: 10.1097/TP.0000000000000845. [DOI] [PubMed] [Google Scholar]
- 154.Darmon M, Azoulay E, Thiery G, et al. Time course of organ dysfunction in thrombotic microangiopathy patients receiving either plasma perfusion or plasma exchange. Crit Care Med. 2006;34:2127–2133. doi: 10.1097/01.CCM.0000227659.14644.3E. [DOI] [PubMed] [Google Scholar]
- 155.Nakos G, Malamou-Mitsi VD, Lachana A, et al. Immunoparalysis in patients with severe trauma and the effect of inhaled interferon-gamma. Crit Care Med. 2002;30:1488–1494. doi: 10.1097/00003246-200207000-00015. [DOI] [PubMed] [Google Scholar]
- 156.Choquet S, Leblond V, Herbrecht R, et al. Efficacy and safety of rituximab in B-cell post-transplant lymphoproliferative disorders: results of a prospective multicentre phase II study. Blood. 2006;107:3053–3057. doi: 10.1182/blood-2005-01-0377. [DOI] [PubMed] [Google Scholar]
- 157.Gross TG, Orjuela MA, Perkins SL, et al. Low-dose chemotherapy and rituximab for post transplant lymphoproliferative disease (PTLD): a Children’s Oncology Group Report. Am J Transplant. 2012;12:3069–3075. doi: 10.1111/j.1600-6143.2012.04206.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Trottestam H, Horne A, Aricò M, et al. Chemoimmunotherapy for hemophagocytic lymphohistiocytosis: long-term results of the HLH-94 treatment protocol. Blood. 2011;118:4577–4584. doi: 10.1182/blood-2011-06-356261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Shakoory B, Carcillo JA, Chatham WW, et al. Interleukin-1 Receptor Blockade Is Associated With Reduced Mortality in Sepsis Patients With Features of Macrophage Activation Syndrome: Reanalysis of a Prior Phase III Trial. Crit Care Med. 2016;44:275–281. doi: 10.1097/CCM.0000000000001402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Fitzgerald JC, Weiss SL, Maude SL, et al. Cytokine Release Syndrome After Chimeric Antigen Receptor T Cell Therapy for Acute Lymphoblastic Leukemia. Crit Care Med. 2016 Sep 14; doi: 10.1097/CCM.0000000000002053. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]