Abstract
Purpose
The purpose of this research note is to identify and prioritize diseases important for detection in adult hearing health care delivery systems.
Method
Through literature review and expert consultation, the authors identified 195 diseases likely to occur in adults complaining of hearing loss. Five neurotologists rated the importance of disease on 3 dimensions related to the necessity of detection prior to adult hearing aid fitting.
Results
Ratings of adverse health consequences, diagnostic difficulty, and presence of nonotologic symptoms associated with these diseases resulted in the identification of 104 diseases potentially important for detection prior to adult hearing aid fitting.
Conclusions
Current and evolving health care delivery systems, including direct-to-consumer sales, involve inconsistent means of disease detection vigilance prior to device fitting. The first steps in determining the safety of these different delivery methods are to identify and prioritize which diseases present the greatest risk for poor health outcomes and, thus, should be detected in hearing health care delivery systems. Here the authors have developed a novel multidimensional rating system to rank disease importance. The rankings can be used to evaluate the effectiveness of alternative detection methods and to inform public health policy. The authors are currently using this information to validate a consumer questionnaire designed to accurately identify when pre- fitting medical evaluations should be required for hearing aid patients.
Today, the United States faces a societal challenge of how to best manage the health care of the rapidly growing elderly population. According to the Administration on Aging (2014), the population of adults 65 years of age and older increased by 21% between 2002 and 2012. A growing aging population means a growing population of individuals with hearing loss. The National Institute on Deafness and Other Communication Disorders (NIDCD) estimates 25% of adults age 64–75 and 50% of adults 75 years and older experience disabling hearing loss (NIDCD, 2015). Caring for this growing elderly population with hearing loss presents challenges with regard to accessibility as the growth rate of those needing hearing health care outpaces the entry rate of hearing health care providers into the relevant professions (i.e., physicians, audiologists, hearing instrument specialists) by a significant margin (Freeman, 2009; Health Resources and Services Administration, National Center for Health Workforce Analysis, 2013).
With a growing aging population and no corresponding growth in the ranks of hearing health care providers, the public's access to affordable hearing health care has emerged as a national public health issue. The President's Council of Advisors on Science and Technology (PCAST)'s recent recommendations (PCAST, 2015) encourage a direct-to-consumer approach to amplification device purchase. Hearing aids and hearing aid–related devices are now frequently available for purchase over the Internet or through various large-scale consumer retailers, such as Costco and Walmart. The consumer electronics industry is also expanding rapidly to meet the growing market demand for more affordable devices by selling personal sound amplifier products and hearables. Although these emerging distribution channels and product alternatives offer the promise of greater access to affordable hearing assistive products, they are not well suited for the Food and Drug Administration (FDA)'s recommendation of a prefitting medical examination.
By way of background, the FDA recommends that consumers obtain a medical evaluation, preferably by a physician specializing in diseases of the ear, before purchasing and/or being fit for a hearing aid (FDA, 2015). The purpose of this evaluation is to identify serious ear diseases. Although it is strongly discouraged by the FDA, adult consumers have the option to waive the medical evaluation. When the medical evaluation is waived, detection of otologic disease falls to the consumer or to the person dispensing or selling the hearing device. This FDA policy reflects two competing public health objectives: (a) encouraging the detection and management of potentially serious diseases and (b) making hearing aids more affordable and accessible. There is no empirical evidence evaluating the current policy or other possible disease detection systems.
To begin understanding the risk of using the medical waiver or evaluating the costs and benefits of any disease detection approach used in the process of fitting a hearing aid, the performance of alternative disease detection methods needs to be measured. One way to analyze this problem is through epidemiological data, which suggest that the majority of hearing loss in the U.S. adult population is the result of noise exposure or strongly associated with age-related changes in hearing (Agrawal, Platz, & Niparko, 2008; Cruickshanks et al., 1998; Zapala et al., 2010). For example, using conservative estimates developed by Zapala, Dhar, Nielsen, Griffith, and Kleindienst (2015), the odds are 20:1 against encountering an ear condition that should be treated medically or surgically in individuals seeking hearing aids over the age of 55 years.
Taking epidemiological data into consideration is important, but it does not account for the potential consequences of disease. Despite its low prevalence, some ear diseases are associated with high mortality and morbidity if not detected and treated (e.g., vestibular schwannoma, metastatic cancer affecting a part of the auditory system). Many of these conditions may require evaluation by a specialist (e.g., an otolaryngologist) for an accurate diagnosis and treatment plan.
The disease detection processes recommended by the FDA are paradoxical (i.e., giving options of specialist assessment or waiver of medical assessment) and not well suited to meeting the needs of the aging population pursuing hearing devices. Thus, we perceive a need for new, efficient screening procedures for serious ear diseases. These new procedures should present to the consumer a data-driven, easy-to-follow recommendation on the basis of his or her probability of having a hearing-related disease. We envision the possibility of inexpensive tools, such as questionnaires, that can effectively estimate risk of ear disease, thereby serving as a triage tool prior to the fitting of hearing aids. The construction of such tools, however, is critically dependent on the accurate cataloging of diseases to be detected, ideally ranked according to the severity of the prognosis and availability of treatment. In this research note, we describe such a catalog of ear diseases on the basis of literature review and expert input. Future investigators and policymakers with interests in building tools to maintain patient safety while increasing accessibility and affordability of hearing devices can benefit from our identification and prioritization of diseases. In addition, we describe how our three-dimensional rating of diseases by consequences of missed identification, chance of isolated hearing loss, and diagnostic difficulty might be used to balance risk tolerance with financial constraints in today's rapidly evolving health care system.
Method
We established content domain development for our disease rating system in four steps. First, through a review of classic textbooks, we identified 195 diseases and conditions that might be encountered in consumers complaining of hearing loss (Adunka & Buchman, 2011; Becker, Naumann, & Rudolf, 1989; Jackler & Brackman, 2004; Merchant & Nadol, 2010; Zarandy & Rutka, 2010). These diseases were categorized into conditions with primarily otologic symptoms and conditions with systemic symptoms that included hearing loss. We added foil conditions (n = 15), such as broken bone, heart attack, or anxiety, which would not be thought to have hearing loss as a presenting symptom. These foil conditions were added to assist in calibrating the rating of diseases.
Second, we created a system to rate conditions using a scale from 0 to 4 for each of three dimensions: (a) consequences of missed identification; (b) diagnostic difficulty; and (c) likelihood that hearing loss would be the primary, only, or initial condition/symptom, abbreviated as the likelihood of isolated hearing loss in the remainder of the research note (see Table 1 for details).
Table 1.
Description of each dimension and scoring criteria presented to the panel of neurologists when ranking each of the 210 conditions and foils.
| Dimension 1: Diagnostic difficulty | |
| Prompt: “We want to know how difficult it is to diagnose each of the presented diseases or conditions. We may want to exclude diseases or conditions that are impossible or improbable to diagnose. For example, there may be an obscure genetic mutation that predisposes an individual to develop age-related hearing loss. We might not be so concerned about holding a consumer-centered questionnaire responsible for identifying risk for the genetic mutation if the diagnostic tests are not readily available.” | |
| Score | Description |
| 0 | History and physical exam alone |
| 1 | … + a comprehensive review of prior audiologic test data |
| 2 | ..… + commonly available adjunctive studies (computed tomography, MRI, vestibular testing) |
| 3 | ….… + consultation with other medical specialists (e.g., neurology, oncology, infectious disease) |
| 4 | It is currently not possible to routinely make this diagnosis with existing technologies prior to death (e.g., requires temporal bone studies). |
| Dimension 2: Consequences of missed diagnosis | |
| Prompt: “It is conceivable that, at some point, hearing aids may be purchased over the Internet without the requirement for a medical or audiological evaluation. If that happens, hearing aids may be purchased by individuals who have no access to medical care. This dimension attempts to quantify the risk of missing nonotogenic diseases or conditions that may cause hearing loss even though those conditions might be easily identified by a primary care provider or other health care practitioner.” | |
| Score | Description |
| 0 | No consequence |
| 1 | Mild short-term discomfort at most, no long-term morbidity |
| 2 | Moderate possibility of long-term morbidity, no threat to life |
| 3 | Severe possibility of permanent morbidity or potential threat to life |
| 4 | Critical, definite permanent morbidity and potential threat to life |
| Dimension 3: Likelihood of hearing loss as the primary, only, or initial condition/symptom | |
| Prompt: “If, in the future, hearing aids can be purchased without audiologic or otolaryngologic evaluation but the consumer has access to primary care services, systemic causes of hearing loss or hearing loss with signs and symptoms of disease might be readily detected by primary care providers. However, some conditions, such as otosclerosis or early vestibular schwannoma, might not produce worrisome signs and symptoms on primary care evaluation. This dimension attempts to identify those conditions for which otologic and neurotologic disease presents as hearing loss alone at some point in the course of disease. The rationale is that a premium might be placed on a consumer's or hearing aid dispenser's ability to identify these conditions prior to hearing aid fitting.” | |
| Score | Description |
| 0 | Never, essentially 0% of the time |
| 1 | Rarely |
| 2 | Sometimes, essentially 50% of the time |
| 3 | Often |
| 4 | Always, essentially 100% of the time |
Note. Descriptions for each score for individual dimensions were presented to the rating experts. Note that the descriptions are additive for Dimension 1. Each increment in the score is cumulatively included in the previous descriptions. MRI = magnetic resonance imaging.
Third, we had five experienced neurotologists rate the 210 diseases and conditions (with foils) using our three-dimensional rating system. The neurotologists comprised the entire neurotology staff of the Mayo Clinic Arizona, Florida, and Minnesota at the time of the study. They all completed fellowships in neurotology; maintain subspecialty certification in neurotology; are board certified in otolaryngology; and are fellows of the American Academy of Otolaryngology, American Neurotology Society, and American Otological Society.
Last, median ratings across the experts were calculated for each of the 210 diseases and foils for the three dimensions. Then the original list of 195 diseases was reduced to identify the potential diseases critical for detection in the adult population. This was done by eliminating genetic disorders and systemic diseases with little or no association with hearing loss. Genetic conditions considered disorders of syndromic and nonsyndromic hearing loss, chromosomal, mitochondrial, and x-linked disorders and prenatal causes of hearing loss (n = 65) were removed as most manifest before the onset of age-related hearing loss. Systemic diseases with tenuous evidence for otologic symptoms were also removed (n = 26) when a comprehensive literature review confirmed these diseases demonstrated no convincing evidence of a causal relationship between the condition and hearing loss (e.g., ampicillin intoxication). The remaining 104 diseases and conditions presented with primarily otologic and nonotologic signs and symptoms in adults and were designated targeted diseases for identification prior to the fitting of a hearing aid. For these 104 diseases, inter-rater agreement was evaluated using intraclass correlation coefficients (Kim, 2013; McGraw & Wong, 1996; Shrout & Fleiss, 1979) for each of the dimensions.
Results
The 104 diseases and conditions comprising our final targeted diseases are listed in Table 2 with their median ratings for the three dimensions. The diseases are listed in order of severity for consequences of missed diagnosis. Sufficient reliability was found between the raters for each of the diseases. The average intraclass correlation coefficient measure was .86 with a 95% confidence interval from .82 to .90, F(103, 412) = 7.65, p < .001, for the dimension of diagnostic difficulty; .73 with a 95% confidence interval from .61 to .81, F(103, 412) = 7.65, p < .001, for the dimension of consequences of missed diagnosis; and .78 with a 95% confidence interval from .71 to .84, F(103, 412) = 7.65, p < .001, for the likelihood for isolated hearing loss.
Table 2.
List of 104 primary otologic and systemic conditions identified as targeted diseases for detection prior to hearing aid fitting.
| Primary otologic |
Primary systemic |
||||||
|---|---|---|---|---|---|---|---|
| Condition | Consequences without services a | Diagnostic difficulty | Isolated otologic symptoms | Condition | Consequences without services a | Diagnostic difficulty | Isolated otologic symptoms |
| Epidermoid carcinoma | 4 | 2 | 1 | Cerebellar infarction | 4 | 3 | 0 |
| Malignant external otitis | 4 | 3 | 0 | Inferior pontine syndromes | 4 | 3 | 0 |
| Otitic meningitis | 4 | 3 | 1 | Intracranial hemorrhage | 4 | 3 | 0 |
| Acute mastoiditis | 3 | 2 | 1 | Leukemia | 4 | 3 | 1 |
| Acute petrositis (Gradenigo) | 3 | 2 | 1 | Lymphoma | 4 | 3 | 0 |
| Benign neoplasms | 3 | 2 | 1 | Major vessels | 4 | 3 | 0 |
| Complications of chronic active OM–cholesteatoma | 3 | 1 | 2 | Occlusion of AICA | 4 | 3 | 0 |
| Complications of chronic active OM–labyrinth fistula | 3 | 2 | 0 | Salicylates | 4 | 3 | 1 |
| Congenital cholesteatoma | 3 | 2 | 3 | Stroke | 4 | 2 | 0 |
| Facial neuroma | 3 | 2 | 1 | Alzheimer's | 3 | 3 | 0 |
| Glandular neoplasms | 3 | 2 | 1 | Dementia (non-Alzheimer's) | 3 | 3 | 0 |
| Metastatic neoplasms (breast, kidney, lung, stomach, larynx, prostate, thyroid) | 3 | 3 | 2 | Diabetes | 3 | 3 | 0 |
| Neurofibromatosis 1 | 3 | 3 | 1 | Giant cell arteritis | 3 | 3 | 1 |
| Neurofibromatosis 2 | 3 | 3 | 2 | Langerhans cell histiocytosis | 3 | 3 | 1 |
| Other lesions CPA | 3 | 2 | 1 | Lymphomatoid granulomatosis | 3 | 3 | 1 |
| Otitic hydrocephalus | 3 | 3 | 1 | Multiple myeloma | 3 | 3 | 1 |
| Otosyphilis | 3 | 3 | 1 | Occlusion of PICA (Wallenberg) | 3 | 3 | 0 |
| Penetrating injury | 3 | 1 | 0 | Polyarteritis nodosa | 3 | 3 | 0 |
| Perilymph fistula | 3 | 3 | 0 | Quinine & chlorquinine | 3 | 3 | 1 |
| Sarcoma | 3 | 2 | 1 | Susac's syndrome | 3 | 3 | 1 |
| Serous/toxic labyrinthitis | 3 | 3 | 0 | Systemic lupus erythmatosus | 3 | 3 | 1 |
| Sinus thrombophlebitis | 3 | 2 | 1 | Waldenstrom's macroglobulinemia | 3 | 3 | 1 |
| Spontaneous CSF leak | 3 | 3 | 0 | Wegener's granulomatosis | 3 | 3 | 1 |
| Sudden HL of vascular etiology | 3 | 2 | 1 | Aminoglycoside | 2 | 1 | 3 |
| Suppurative labyrinthitis | 3 | 3 | 1 | Cholesterol granuloma | 2 | 2 | 1 |
| Tuberculous OM | 3 | 3 | 1 | Cisplatin | 2 | 3 | 3 |
| Vestibular schwannoma | 3 | 2 | 3 | Cogan's syndrome | 2 | 3 | 2 |
| Automobile air bag | 2 | 1 | 2 | Erythromycin | 2 | 1 | 2 |
| Chronic active OM | 2 | 0 | 1 | Fibrous dysplasia | 2 | 3 | 1 |
| Chronic petrositis | 2 | 2 | 1 | Loop diuretics | 2 | 1 | 3 |
| Complications of chronic active OM–ossicular resorption | 2 | 2 | 1 | Multiple sclerosis | 2 | 3 | 1 |
| Complications of chronic active OM–facial paresis | 2 | 2 | 2 | Naproxen | 2 | 2 | 2 |
| Congenital aural atresia | 2 | 0 | 2 | Opiod + acetaminophen combination | 2 | 1 | 3 |
| Endolymphatic hydrops | 2 | 2 | 2 | Paget's disease | 2 | 3 | 1 |
| Fluctuant SNHL | 2 | 2 | 4 | Polycythemia vera | 2 | 2 | 3 |
| Fracture of temporal bone | 2 | 2 | 1 | Relapsing polychondritis | 2 | 1 | 2 |
| Fungal infections of temporal bone | 2 | 2 | 1 | Rheumatoid arthritis | 2 | 1 | 2 |
| Glomus body | 2 | 2 | 1 | Subarachnoid hemorrhage | 2 | 3 | 3 |
| Hemolabyrinth | 2 | 2 | 1 | Vogt–Koyanagi–Harada syndrome | 2 | 3 | 0 |
| Idiopathic sudden sensorineural hearing loss | 2 | 2 | 4 | Degenerative arthritis of ossicular joints | 1 | 2 | 1 |
| Irradiation | 2 | 1 | 1 | Peripheral vascular disease | 0 | 0 | 0 |
| Labyrinthine concussion | 2 | 2 | 1 | ||||
| Large vestibular aqueduct | 2 | 2 | 3 | ||||
| Osteogenesis imperfecta | 2 | 3 | 1 | ||||
| Otosclerosis | 2 | 2 | 4 | ||||
| Perforation | 2 | 0 | 2 | ||||
| Perilylmph gusher | 2 | 2 | 1 | ||||
| Petrification of auricle | 2 | 3 | 1 | ||||
| Primary autoimmune hearing loss | 2 | 1 | 2 | ||||
| Retraction | 2 | 3 | 0 | ||||
| Acute external otitis | 1 | 0 | 1 | ||||
| Acute myringitis | 1 | 0 | 1 | ||||
| Acute OM | 1 | 0 | 1 | ||||
| Auricular deformities | 1 | 0 | 1 | ||||
| Barotitis | 1 | 1 | 2 | ||||
| Chronic external otitis | 1 | 0 | 1 | ||||
| Chronic myringitis | 1 | 0 | 1 | ||||
| Exostoses of external auditory canal | 1 | 0 | 1 | ||||
| False fundus | 1 | 0 | 1 | ||||
| Hemotympanum | 1 | 1 | 2 | ||||
| Otitis media with effusion | 1 | 1 | 3 | ||||
| Dimeric membrane | 0 | 0 | 0 | ||||
| Tympanosclerosis | 0 | 0 | 1 | ||||
Note. OM = otitis media; AICA = anterior inferior cerebellar artery; CPA = cerebellopontine angle; PICA = posterior inferior cerebellar artery; CSF = cerebrospinal fluid; HL = hearing loss; SNHL = sensorineural hearing loss.
Diseases are listed by highest consequence of missed diagnosis.
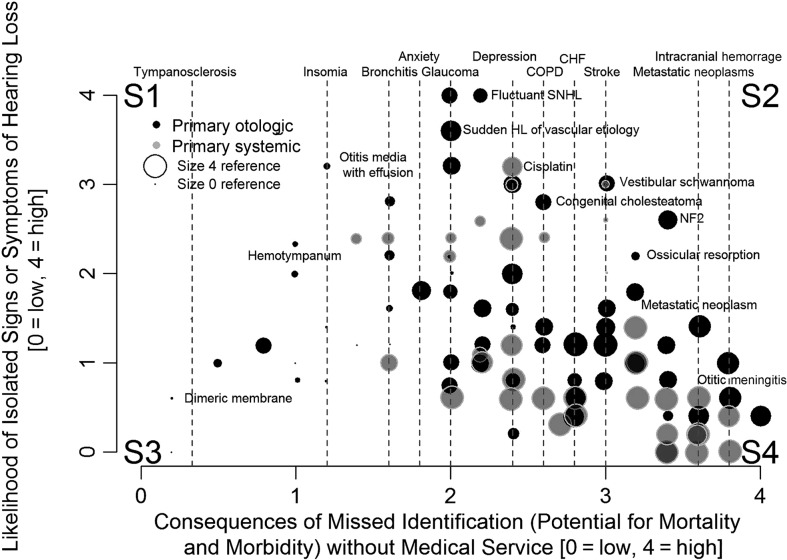
The ratings of the 104 targeted diseases for consequences of missed diagnosis as a function of the ratings of the likelihood of isolated hearing loss are presented in three dimensions in Figure 1. The size of each symbol represents the relative rating of diagnostic difficulty, with increasing size signifying increasing difficulty. None of the diseases/conditions received the highest rating of four for diagnostic difficulty. Corners of the figure were marked with S1 through S4, partitioning the graph into four sections for discussion. Section S1 represents diseases with lower consequence of missed diagnosis and higher likelihood of isolated hearing loss, S2 represents diseases with a higher consequence of missed diagnosis and higher likelihood of isolated hearing loss, S3 represents diseases with lower consequence of missed diagnosis and lower likelihood of isolated hearing loss, and S4 represents higher consequence of missed diagnosis and lower likelihood of isolated hearing loss. The labeled diseases and conditions at the top horizontal axis correspond with the dashed vertical lines and were provided as a meaningful reference of the rating system. There are several noteworthy aspects of Figure 1. First, most of the data are in sections S3 and S4, and data density is the highest in S4. Section S2 is relatively sparsely populated. In S4, there is a high concentration of larger dots, indicating a moderate level of diagnostic difficulty. Diseases in S4 carry serious consequences. However, very few conditions in S4 present with isolated signs or symptoms of hearing loss; instead, there is a high concentration of systemic conditions in this section.
Figure 1.
Scatterplot of diseases and conditions associated with hearing loss were selected as adult targeted diseases. The diseases are ranked by consequences of missed identification (x-axis) and chance of isolated hearing loss (y-axis). Black and gray symbols are used to indicate primarily otologic (black) versus systemic conditions (gray), respectively. The size of the symbol is determined by the rating of diagnostic difficulty for the particular condition. The smaller the size, the easier to diagnose, and the larger the symbol size, the more diagnostically difficult. Reference conditions are marked along the horizontal axis with vertical dashed lines and labeled along the top horizontal axis. Four sections are labeled S1, S2, S3, and S4 for convenience of general discussion. Their boundaries are not defined because how and where the boundaries are set depends on risk tolerance and the importance given to the presence of nonotologic symptoms and ease of diagnosis in the hearing health care delivery system in which the plot is used. SNHL = sensorineural hearing loss; HL = hearing loss; NF 2 = neurofibromatosis II; COPD = chronic obstructive pulmonary disease; CHF = congestive heart failure.
Discussion
Evidence is needed to determine the importance and validity of disease detection alternatives in current and future hearing health care delivery systems. Prospective U.S. consumers of hearing aids are recommended to either obtain a medical evaluation or sign a waiver prior to the fitting. This disease detection policy is inconsistent, giving only options for a detailed specialist assessment or waiver of assessment with no middle ground. An alternative detection method might ensure that serious diseases are detected early while not incurring unnecessary medical costs or creating obstacles to the acquisition of hearing aids. But in order to contemplate alternatives, one needs to understand what diseases should be detected prior to hearing aid purchase (i.e., the public health benefit) and at what cost. We have provided a starting point to determine what diseases might be critical to identify prior to hearing aid purchase using three dimensions: (a) consequences of missed identification, (b) diagnostic difficulty, and (c) likelihood of isolated hearing loss. The assumption is that any prepurchase disease detection method for hearing aid patients should identify conditions that are diagnosable by otolaryngologists and consequential if missed.
The likelihood that the disease would present with hearing loss as the sole or initial symptom helps determine if the burden of detecting the disease rests solely on the hearing aid distribution channel or if it could be identified through routine primary care or the patient's medical home, a health care model in which the patient has a long-term, collaborative relationship with a primary care provider or team of providers (Hesse, Nilsen, & Hunter, 2012). A few diseases are serious in the sense that they carry a high risk of mortality or morbidity and initially present with no symptoms beyond hearing loss. An example would be vestibular schwannoma, with which the initial hearing evaluation may be the best opportunity for early detection. Other diseases may carry a high risk for mortality or morbidity but may only cause hearing loss later in the disease. In these conditions, multiple nonotologic signs and symptoms are typically present well before hearing may be involved. An example would be hearing loss from metastatic cancer or the suspected relationship between diabetes and hearing loss. In these cases, the patients' medical home might have a primary role in detecting the disease or condition, and the hearing aid distributer would play a secondary role. It is certain that the pre–hearing aid disease detection method should place a priority on detecting those diseases and conditions that would carry a high risk for mortality or morbidity and present with hearing loss as an isolated symptom (conditions in Section S2 of Figure 1).
Our disease rating system can also help to identify potential conditions that should not be the target of disease screening efforts. Some diseases may present with little long-term risk of a poor health outcome but require a specialist medical evaluation to detect. They may be academically noteworthy but in isolation would warrant no medical intervention. An example would be tympanosclerosis without corresponding hearing loss.
In a similar manner, the disease rating system helps assess the relative risk of disease. When identified and treated early, a disease can pose little long-term risk (e.g., otitis media with effusion). Yet, if ignored, it may evolve into a more serious and life-threatening condition (e.g., mastoiditis). In the U.S. health care system, progression of many otologic diseases is rare. For instance, approximately 45 deaths were attributed to chronic otitis media in 2010 (1.6/100,000). However, in Sub-Saharan Africa, the death rate is almost 100 times higher (1/1,000; Monasta et al., 2012). If we experienced the same death rate as in Sub-Saharan Africa, we would see 4,000 deaths each year. Therefore, the risk of mortality or morbidity related to any condition varies significantly with access to health care. To help assess the relative risk of ear disease in the U.S. health care system, we included ratings on conditions that were not associated with hearing loss and would not be targeted in a prepurchase disease detection method for hearing aid patients. For instance, chronic otitis media has the same relative risk of mortality or morbidity as diabetes. Acute otitis media has the same risk as insomnia and vestibular schwannoma the same as stroke. These foil conditions can be informative by suggesting the amount of risk society is willing to bear in the allocation of resources to detect the condition.
The cost of health care delivery is influenced by many factors, including the prevalence of disease, the cost of early treatment, the cost of delayed treatment, and the ease with which the condition can be detected. In general, the cost of health care delivery increases with the level of training and specialization of the providers. Costs may also increase when diseases are not detected early as might occur when the appropriate providers are not involved in care delivery. The evaluation by a physician specialized in the detection of otologic diseases versus signing a medical waiver prior to a hearing aid fitting are at opposite extremes of cost, risk, and accessibility. The current FDA recommendation of a medical exam, particularly one with an otolaryngologist, would be capable of detecting most of the targeted diseases in Figure 1 but would be costly and may create an obstacle to obtaining hearing aids, and the waiver system, when not accompanied by an audiological exam, would likely not detect any of the targeted diseases in individuals pursuing hearing aids. Between these extremes, there are multiple opportunities for accomplishing efficient and effective disease detection. For instance, the FDA and the American Academy of Otolaryngology–Head and Neck Surgery (AAO-HNS) advocate for the use of published lists of red flag conditions that should trigger a medical referral prior to the fitting of a hearing aid when hearing health care services are not provided by a physician (FDA, 1977; AAO-HNS, 2002). In theory, using red flag conditions to instigate a medical referral might help reduce health care costs and increase accessibility to hearing health care. The validity of these lists, however, is unknown. Worse yet, it is unknown how often they are used by hearing aid dispensers and other settings outside the clinic. Evidence to determine the importance and validity of disease detection is needed to inform public health policies related to hearing health care delivery.
The distribution in Figure 1 shows the targeted diseases most essential for detection would be those conditions that fall into sections S2 and S4 on the right side of the figure, the conditions with higher consequence for missed diagnosis. The effectiveness of any disease detection method that misses S2 and S4 conditions must be questioned. This would include methods that rely on consumer recognition of disease symptoms or the use of red flag criteria by any hearing health care providers, such as audiologists. In a similar manner, the efficiency of a disease detection method that uses higher cost providers to detect S1 and S3 conditions should also be challenged; this might be the case when all hearing aid seekers obtain specialty medical evaluations.
When hearing loss evolves as part of a systemic disease (conditions toward the bottom of Figure 1), one could reasonably anticipate that the presentation of multiple nonotologic signs and symptoms could be readily diagnosed by the primary care provider or the afflicted person himself or herself. By focusing on diseases toward the right of Figure 1, tools or practice guidelines might be developed that are tailored to the specific capabilities of a health care system. In the absence of a primary or specialist medical exam, hearing health care providers and consumers themselves need to have tools to identify the targeted diseases in sections S2 and S4 so that they can be examined by a physician and treated. When primary care services are available, hearing health care providers and consumers still must have tools that identify S2 targeted diseases as these conditions may present with isolated hearing loss and may only be detected through an audiological evaluation.
The disease ranking system presented herein is a potential first step in the systematic evaluation of emerging disease detection policies as contemplated by PCAST and the FDA. This system can be used to evaluate the performance of possible changes in public health policy, such as if the prepurchase medical evaluation requirement for hearing aid patients was removed or if the waiver was used in a medically underserved region. The rating system allows us to evaluate the risks of missed disease in a method not previously used before. The system can also serve as a validation tool for the development of alternative disease detection systems that may be considered for use in the hearing health care delivery system.
We approached our goal of generating a ranked categorization of ear diseases using a comprehensive literature review and quantitative ratings by content area experts. Using a list of diseases from published expert sources, we asked our network of the highest qualified experts for their observations of what they experience clinically regarding risk and outcomes of these diseases. This process was designed to mimic the FDA-approved current practice standard of obtaining a physician's opinion prior to hearing aid purchase. The limitations of the FDA system are not the physician's accuracy in identifying and realizing the importance of diseases but its cost and barriers to accessibility. Our method also follows the time-tested use of expert committees to generate health care consensus guidelines serving as the gold standard for clinical applications. Furthermore, our methodology is often the recourse when other designs are not easily applicable to a specific problem. For example, McArthur, Klugarova, Yan, and Florescu (2015) stated, “Systematic reviews of text and opinion may be considered as legitimate sources of evidence, especially when there is an absence of other research designs” (p. 195). Although a small group of raters were used in the study, every one of the raters was a neurotologist, a provider with the highest degree of training. In addition, the strength of the agreement between raters gives us confidence in our results. Last, our choice of providers at a tertiary care facility, such as the Mayo Clinic, provided the greatest opportunity for the raters to have had sufficient encounters with diseases that are of extreme low prevalence (e.g., vestibular schwannoma or other retrocochlear masses at 0.002% and cholesteatoma at 0.01%. See Zapala et al., 2010, for details and other examples).
Summary
We have presented a catalog of ear diseases that would be important to identify in a hearing health care system to assure patient safety. Our results establish a set of 104 critical targeted diseases rated by five experienced neurotologists on three dimensions: (a) adverse health consequences of missed diagnosis, (b) diagnostic difficulty, and (c) the presence of isolated hearing loss. These ratings help to classify diseases that should be identified even at higher cost and which are less critical to be identified when cost is high or accessibility is poor. Our ranking system can support the construction of future tools that are customized for different health care settings with providers of varied backgrounds and skill levels. Given the changing climate of hearing health care delivery and the overwhelming need to provide services to a greater proportion of the population, our results will provide the basis for the development of appropriate tools that improve the accessibility and affordability of hearing health care while maintaining public safety.
Acknowledgments
This work was supported by grants from the National Institute on Deafness and Other Communication Disorders (Grant R21 DC013115-02, awarded to Sumitrajit Dhar and David A. Zapala), the Knowles Hearing Center at Northwestern University (awarded to Sumitrajit Dhar, Donald W. Nielsen, and David A. Zapala), and by the James Russell and Martha Crawford Endowed Clinical Research Fellowship in Otolaryngology (awarded to Samantha J. Kleindienst). We thank Rachael Baiduc and Chun Chan for assistance with data collection and analysis and Greta Stamper and Dania Rishiq for their assistance in manuscript preparation. Conflicts of interest and source of funding: All authors declare there is no conflict of interest. Portions of this study were presented at the annual meeting of the American Auditory Society (2015).
Funding Statement
This work was supported by grants from the National Institute on Deafness and Other Communication Disorders (Grant R21 DC013115-02, awarded to Sumitrajit Dhar and David A. Zapala), the Knowles Hearing Center at Northwestern University (awarded to Sumitrajit Dhar, Donald W. Nielsen, and David A. Zapala), and by the James Russell and Martha Crawford Endowed Clinical Research Fellowship in Otolaryngology (awarded to Samantha J. Kleindienst).
References
- Administration on Aging. (2014). A profile of older Americans: 2013. Washington, DC: U.S. Department of Health and Human Services. [Google Scholar]
- Adunka O. F., & Buchman C. A. (2011). Otology, neurotology, and lateral skull base surgery. New York, NY: Thieme. [Google Scholar]
- Agrawal Y., Platz E. A., & Niparko J. K. (2008). Prevalence of hearing loss and differences by demographic characteristics among US adults. Archives of Internal Medicine, 168, 1522–1530. [DOI] [PubMed] [Google Scholar]
- American Academy of Otolaryngology-Head and Neck Surgery. (2002). Position statement: Red flags-warning of ear disease. Retrieved from http://www.entnet.org/content/position-statement-red-flags-warning-ear-disease
- Becker W., Naumann H. H., & Rudolf C. R. (1989). Ear, nose, and throat diseases. New York, NY: Thieme. [Google Scholar]
- Cruickshanks K. J., Wiley T. L., Tweed T. S., Klein B. E. K., Klein R., Mares-Perlman J., & Nondahl D. M. (1998). Prevalence of hearing loss in older adults in Beaver Dam, Wisconsin: The Epidemiology of Hearing Loss Study. American Journal of Epidemiology, 148, 879–886. [DOI] [PubMed] [Google Scholar]
- Food and Drug Administration. (1977, February 15). U.S. Food and Drug Administration rules and regulations regarding hearing aid devices: Professional and patient labeling and conditions for sale, part IV. Federal Register, 42, 9286–9296. [Google Scholar]
- Food and Drug Administration. (2015). Code of Federal Regulations, Title 21, Volume 8, 21CFR801.421, Retrieved from https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?fr=801.421
- Freeman B. (2009). The coming crisis in audiology. American Journal of Audiology, 21, 46–52. [Google Scholar]
- Health Resources and Services Administration, National Center for Health Workforce Analysis. (2013). Projecting the supply and demand for primary care practitioners through 2020. Rockville, MD: U.S. Department of Health and Human Services. [Google Scholar]
- Hesse B. W., Nilsen W. J., & Hunter C. M. (2012). News from NIH: The patient-centered medical home. Translational Behavioral Medicine, 2, 255–256. doi:10.1007/s13142-012-0144-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackler R. K., & Brackman D. (2004). Neurology (2nd ed.). St. Louis, MO: Mosby. [Google Scholar]
- Kim H. (2013). Statistical notes for clinical researchers: Evaluation of measurement error 1: Using intraclass correlation coefficients. Restorative Dentistry & Endodonics, 38, 98–102. doi:10.5395/rde.2013.38.2.98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McArthur A., Klugarova J., Yan H., & Florescu S. (2015). Innovations in the systematic review of text and opinion. International Journal of Evidence-Based Healthcare, 13, 188–195. doi:10.1097/XEB.0000000000000060 [DOI] [PubMed] [Google Scholar]
- McGraw K. O., & Wong S. P. (1996). Forming inferences about some intraclass correlation coefficients. Psychological Methods, 1, 30–46. doi:10.1037/1082-989X.1.1.30 [Google Scholar]
- Merchant S. N., & Nadol J. B. (2010). Schuknecht's pathology of the ear (3rd ed.). Shelton, CT: People's Medical Publishing House. [Google Scholar]
- Monasta L., Ronfani L., Marchetti F., Montico M., Vecchi Brumatti L., Bavcar A., … Tamburlini G. (2012). Burden of disease caused by otitis media: Systematic review and global estimates. PLoS ONE, 7, e36226 doi:10.1371/journal.pone.0036226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Institute on Deafness and Other Communication Disorders. (2015). Quick statistics about hearing. Retrieved from http://www.nidcd.nih.gov/health/statistics/pages/quick.aspx
- President's Council of Advisors on Science and Technology. (2015). Aging America & hearing loss: Imperatives of improved hearing technologies. Retrieved from https://www.whitehouse.gov/sites/default/files/microsites/ostp/PCAST/pcast_hearing_tech_letterreport_final3.pdf
- Shrout P. E., & Fleiss J. L. (1979). Intraclass correlations: Use in assessing rater reliability. Psychological Bulletin, 86, 420–428. [DOI] [PubMed] [Google Scholar]
- Zapala D. A., Dhar S., Nielsen D., Griffith J. W., & Kleindienst S. J. (2015). Managing disease risk in hearing healthcare delivery [PowerPoint]. Retrieved from http://iom.nationalacademies.org/~/media/Files/Activity%20Files/HealthServices/HearingHealthCare/Meeting%203/David%20Zapala.pdf
- Zapala D. A., Stamper G. C., Shelfer J. S., Walker D. A., Karatayli-Ozgursoy S., Ozgursoy O. B., & Hawkins D. B. (2010). Safety of audiology access for Medicare patients complaining of impaired hearing. Journal of the American Academy of Audiology, 21, 365–379. doi:10.3766/jaaa.21.6.2 [DOI] [PubMed] [Google Scholar]
- Zarandy M. M., & Rutka J. (2010). Diseases of the inner ear: A clinical, radiologic, and pathologic atlas. New York, NY: Springer. [Google Scholar]