Abstract
Purpose: To investigate the efficacy of 0.1%, 0.15%, and 0.3% sodium hyaluronate (SH) artificial tears compared with 0.05% cyclosporine (CS) ophthalmic solution for the treatment of dry eye.
Methods: One hundred seventy-six patients were recruited and randomized to receive of 0.1%, 0.15%, and 0.3% SH and 0.05% CS. There was a primary end point which is the changes in the fluorescein corneal staining (FCS) score to determine noninferiority of 0.1%, 0.15%, and 0.3% SH. Secondary objective end points were lissamine green conjunctival staining (LGCS) scores, Schirmer test, and tear film break-up time (TBUT). Secondary subjective end point was ocular surface disease index (OSDI) score. These were evaluated before treatment and 6 and 12 weeks after start of treatment.
Results: In the primary analysis, the mean change from baseline in FCS scores verified noninferiority of 0.1% and 0.15% SH to 0.05% CS and also indicated significant improvement of all groups (P < 0.05). Values for TBUT, LGCS scores, and OSDI scores showed significant improvements in all groups (P < 0.05), although no significant intergroup difference was shown. However, Schirmer test scores in the 0.15% SH group showed a significant tendency for better improvement at week 12 compared with the other groups (P < 0.05). No serious adverse events were observed.
Conclusions: Administration of 0.1%, 0.15%, and 0.3% SH was effective in improving both the objective signs and subjective symptoms of dry eye. Those findings, in addition to the well-tolerated profile of 0.1%, 0.15%, and 0.3% SH, show that it is effective therapeutic method for dry eye.
Keywords: : dry eye syndrome, 0.1% sodium hyaluronate, 0.15% sodium hyaluronate, 0.3% sodium hyaluronate, 0.05% cyclosporine
Introduction
Dry eye is common, affecting ∼10%–20% of the population.1 It is a multifactorial disorder of the tear film and ocular surface, which results in ocular discomfort, visual disturbance, and ocular surface damage. It is caused by disruption of the ocular surface, driven by tear hyperosmolarity and tear film instability.2 The tear film can be destabilized by decreased tear production or altered tear composition, damaging the ocular surface and resulting in inflammation.2 Dryness of the ocular surface epithelium can induce apoptosis of epithelial cells, which eventually leads to aggravation of dry eye.3
The current managements of dry eye include tear supplementation, tear stimulation, anti-inflammatory agents, immunomodulatory agents, and environmental strategies.4 Currently, the main therapy for dry eye is artificial tears, with anti-inflammatory therapy and punctual occlusion therapy as second and third line therapies.5
Sodium hyaluronate (SH) is a glycosaminoglycan with a viscoelastic rheology and a natural component of the tear film. It gained widespread application in lubricants, because it effectively binds water, resists dehydration, and shows excellent biocompatibility.6–8 Previous studies have shown that SH protects corneal epithelial cells against damage, stimulates epithelial migration, and improves the optical quality of the retinal image.6,9
Previous studies reported about the effectiveness of ocular lubricants in protecting cornea from dehydration in a porcine dry eye model,10 the tear film instability in a rabbit model.11 Previous clinical studies also showed that both SH and carboxymethylcellulose (CMC) improve the ocular surface, stabilize precorneal tear film, and ameliorate the intensity of dry eye symptoms.12,13
Topical 0.05% cyclosporine (CS) has an anti-inflammatory and immunomodulatory mode of action and it is an effective treatment for dry eye disorder.14 It showed significant improvement in corneal and conjunctival staining scores.14
However, to the best of our knowledge, there is no report that compares the efficacy of SH and CS in dry eye syndrome. The present study was designed to evaluate the efficacy and safety of 0.1%, 0.15%, and 0.3% SH compared with 0.05% CS in patients with dry eye. We selected 0.1%, 0.15%, and 0.3% concentrations of SH because they are commercially available and commonly used for the treatment of dry eye.
Methods
The study was conducted in accordance with the ethical principles set forth in the Declaration of Helsinki and the Good Clinical Practice Guidelines. The study protocol and informed consent were reviewed and approved by the institutional review board (IRB) before initiation (IRB#SC14MSMV0028). Written informed consent was obtained from each patient before the start of the study, and power analysis was performed to justify the number of patients enrolled in the study. The study was conducted at multiple clinical sites. The name of the trial registry is CRIS (http://cris.nih.go.kr), WHO ICTRP (International Clinical Trials Registry Platform (www.who.int/ictrp), and the registration number of the trial is KCT0001796.
Study design
This is a prospective, randomized, multicenter active-controlled trial conducted in 3 phases: screening, evaluation, and follow-up. As much as possible, the study was conducted under masked conditions for the investigators; the perfect masked conditions could not be accomplished because the instillation frequency and chemical properties differ between SH and CS ophthalmic solution.
Eligible patients were allocated randomly to receive 0.1% hyaluronic acid (SH) eye drop or 0.15% hyaluronic acid (SH) eye drop or 0.3% hyaluronic acid (SH) eye drop or 0.05% cyclosporine (CS) eye drop. Central randomization was adopted for assigning patients to each group using a dynamic allocation of stratified centers.15
Patients in SH group received 0.1% hyaluronic acid ophthalmic suspension (Hyalein ophthalmic solution 0.1%®; Taejoon, Seoul, Korea) or 0.15% hyaluronic acid ophthalmic suspension (New Hyaluni ophthalmic solution 0.15%; Taejoon, Seoul, Korea) or 0.3% hyaluronic acid ophthalmic suspension (Hyaluni ophthalmic solution 0.3%®; Taejoon, Seoul, Korea), 1 drop in each eye 5–6 times daily. Patients in CS group received 0.05% cyclosporine ophthalmic solution (RESTASIS®; Allergan, Inc., California), 1 drop in each eye twice daily. For both groups, the total treatment period was 12 weeks, and examinations were conducted at week 6 and 12 after the start of treatment.
Study population
Eligible patients were 19 years of age or older who had been diagnosed as dry eye syndrome within 3 months and had dry eye-related symptoms that were present for more than 3 months before the screening examination. Other inclusion criteria were as follows: (1) tear film break-up time (TBUT) value of 5 s or less, (2) fluorescein corneal staining (FCS) score of 4 or more by NEI scale, and (3) Schirmer I test value at 5 min of 10 mm or less. These criteria needed to be met at both the screening and baseline examinations.
Exclusion criteria included (1) anterior ocular disease (such as neurotrophic keratitis or keratoconus), (2) continued use of eye drops, (3) patients who had a punctal plug or had it removed within 1 month before the screening examination, and (4) patients who underwent an operation to the ocular surface or intraocular surgery within 2 months before the screening period.
The following drugs or therapies were prohibited from the screening examination to the end of study treatment: steroids; immunosuppressants; antihistamines; any prescription or over-the-counter ophthalmic drugs; contact lenses; and ocular surgery or any other treatment affecting the dynamics of tear fluid, including its nasolacrimal drainage process.
Randomization
Patients were divided and assigned to 4 groups based on simple randomization. Group 1 was treated with 0.1% SH eye drops for 12 weeks. Group 2 was treated with 0.15% SH eye drops for 12 weeks. Group 3 was treated with 0.3% SH eye drops for 12 weeks. Group 4 was treated with 0.05% CS eye drops for 12 weeks.
At the beginning of the treatment period, the subjects were randomized corresponding to allocation codes generated for SH and CS using the permuted block method. Participants were randomized sequentially at each site.
Assessment of outcome measures
Efficacy assessments
Efficacy was evaluated primarily with an objective measure and secondarily with objective and subjective measures. There was one primary objective end point which is FCS score at week 12 (last observation). Secondary objective end points were TBUT, lissamine green conjunctival staining (LGCS) score, meibomian gland dysfunction (MGD) index, and Schirmer's test value. Secondary subjective end point was ocular surface disease index (OSDI) questionnaire for the grading of the symptom score.16 The sum of the scores was used in the analysis. All of these parameters were assessed at baseline, week 6, week 12, or at treatment discontinuation.
For FCS scores, 5 μL of 2% fluorescein solution was instilled in the conjunctival sac as the patient blinked normally. Corneal staining was examined under standard illumination using a slit-lamp microscope with a cobalt blue filter. According to the National Eye Institute/Industry Workshop report, the cornea was divided into 5 fractions.17 FCS scores were measured on a 0–3 point scale, in the superior, inferior, nasal, temporal, and central corneal zones: 0 (no staining), 1 (mild superficial stippling), 2 (moderate punctate staining, including superficial abrasion of the cornea), and 3 (severe abrasion or corneal erosion, deep corneal abrasion or recurrent erosion). The total score was then calculated.
For LGCS scores, 20 μL of 1% lissamine green solution was instilled in the conjunctival sac, and the conjunctiva was divided into 6 fractions.17 Conjunctival staining was evaluated under low illumination by slit-lamp microscopy and was scored from 0 through 3 for each fraction, then summed to calculate the total score.
For TBUT, 5 μL of 2% fluorescein solution was instilled in the conjunctival sac, and TBUT was then evaluated by slit-lamp microscopy. The elapsed time from a normal blink to the first appearance of a dry spot in the tear film was measured thrice.
Schirmer test was performed without anesthesia to measure tear volume as follows. A Schirmer test strip was placed on the lower eyelid between palpebral conjunctiva and bulbar conjunctiva without touching the cornea. The tear volume then was measured for 5 min. The length in millimeters of tear fluid absorbed on the strip measured from the edge of the strip was recorded as tear volume.
The expressibility of the meibum was scored by the application of the digital pressure to the upper tarsus, and the degree of ease with which the meibum was induced was evaluated semiquantitatively as follows: 0, clear meibum easily expressed; 1, cloudy meibum expressed with mild pressure; 2, cloudy meibum expressed with more than moderate pressure; and 3, meibum not expressed, even with the hard pressure.18
The quality of the meibum was scored by the digital pressure over 8 meibomian glands of the lower lids. The meibum secretion was graded as follows: 0, clear; 1, cloudy; 2, cloudy with granular debris; and 3, thick like toothpaste.
Safety assessments
The safety variable was the occurrence of adverse events (AEs), determined at various visits by means of physical signs and symptoms, external eye examination, slit-lamp microscopy, visual acuity, intraocular pressure, and funduscopy.
Statistical analysis
All patients who were enrolled in the study were included in the efficacy and safety analyses. Considering the multiplicity of the test, closed testing procedures were used to verify noninferiority by FCS score. Noninferiority of SH to CS was determined if the 95% confidence interval (CI) for the intergroup difference was calculated, and if the upper limit did not exceed the 0.34 inferiority margin. In addition, intergroup comparison between SH and CS groups at week 12 was done using the t-test. Furthermore, an analysis of change from baseline of FCS and LGCS score at each visit and secondary end points was performed using the t-test or Wilcoxon signed-rank test.
The incidence rates of AEs and adverse drug reactions observed after starting the treatment period were tabulated for each group, and intergroup homogeneity was analyzed using Fisher's exact test. The significance level for tests related to safety and efficacy was 5% for both sides, with CI of 95% on both sides. SAS software V.9.2 (SAS Institute, Cary, NC) was used for statistical analysis.
Results
Participant characteristics
A total of 176 patients were allocated randomly to receive 1 of the 4 treatments: 43 patients entered the 0.1% SH group, 41 patients entered the 0.15% SH group, 47 patients entered the 0.3% SH group, and 45 patients entered the 0.05% CS group. Demographics and other baseline characteristics are shown in Table 1. Of the total 176 participants, 147 were female (83.52%), and the mean age ± standard deviation (SD) was 45.06 ± 14.83 years.
Table 1.
Initial Characteristics of Each Group with Dry Eye Syndrome Before Treatment
| 0.1% SH | 0.15% SH | 0.3% SH | 0.05% CS | P | |
|---|---|---|---|---|---|
| Gender | |||||
| Male | 6 | 5 | 7 | 11 | 0.4063 |
| Female | 37 | 36 | 40 | 34 | |
| Age | 44.14 ± 13.86 | 46.20 ± 13.98 | 44.77 ± 16.17 | 45.22 ± 15.42 | 0.8217 |
| Main cause of dry eye | |||||
| Tear deficient | |||||
| Primary Sjögren's syndrome | 1 | 0 | 0 | 0 | |
| Non-Sjögren's lacrimal disease (aging) | 12 | 13 | 10 | 16 | 0.4678 |
| Non-Sjögren's lacrimal disease (menopause) | 0 | 1 | 1 | 0 | |
| Evaporative | |||||
| Meibomian gland disease | 11 | 12 | 13 | 16 | 0.5371 |
| Lid surfacing/blinking abnormalities | 2 | 4 | 8 | 6 | |
| Tear deficient + evaporative | 17 | 11 | 15 | 7 | 0.2273 |
Data are presented as number and mean value ± standard error.
Group 1 (0.1% SH): 0.1% sodium hyaluronate; group 2 (0.15% SH): 0.15% sodium hyaluronate; group 3 (0.3% SH): 0.3% sodium hyaluronate; group 4 (0.05% CS): 0.05% cyclosporine.
CS, cyclosporine; SH, sodium hyaluronate.
Efficacy evaluation
Primary end point
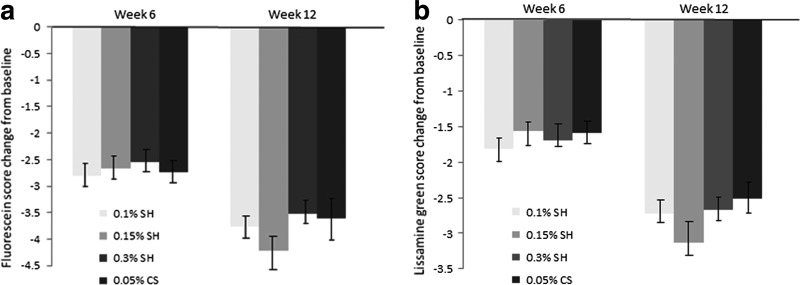
There was a statistically significant improvement in FCS score in all groups at week 6 and 12, although no significant intergroup difference was shown. The mean change from baseline to week 12 in FCS score was −3.77 ± 1.97 for the 0.1% SH group, −4.22 ± 2.73 for the 0.15% SH group, −3.52 ± 2.15 for the 0.3% SH group, and −3.60 ± 3.04 for the 0.05% CS group (Fig. 1a). In the primary analysis, the upper limit was lower than the noninferiority margin of 0.34, and the mean change from baseline in FCS scores verified noninferiority of 0.1% SH and 0.15% SH to 0.05% CS. In addition, the 95% CI did not include 0, indicating the significant improvement of 0.1% and 0.15% SH. At week 12, there were more improvements with 0.15% SH in the FCS scores although no significant intergroup difference was shown.
FIG. 1.
Fluorescein (a) and lissamine green (b) staining score changes from the baseline value. Mean value ± standard error. The decrease in fluorescein and lissamine green staining scores in all groups was statistically significant at week 6 and 12 (P < 0.05).
Secondary end points
LGCS score
LGCS scores showed significant improvement compared with baseline in all groups and at all time points. The mean change from baseline to week 12 in LGCS score was −2.72 ± 3.25 for the 0.1% SH group, −3.14 ± 2.72 for the 0.15% SH group, −2.67 ± 2.95 for the 0.3% SH group, and −2.51 ± 2.95 for the 0.05% CS group (Fig. 1b). At all estimations, there were no significant intergroup differences. However, these scores in the 0.15% SH group showed a tendency for better improvement compared with the other groups at week 12.
TBUT and Schirmer I score
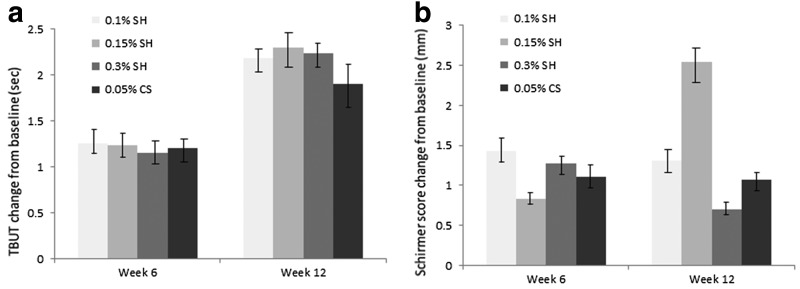
TBUT showed significant improvement compared with baseline in all groups and at all time points. The mean change from baseline to week 12 in TBUT was 2.18 ± 2.63 for the 0.1% SH group, 2.30 ± 2.57 for the 0.15% SH group, 2.24 ± 2.30 for the 0.3% SH group, and 1.90 ± 2.45 for the 0.05% CS group (P < 0.0001; Fig. 2a). There was no statistically significant difference between groups at week 6 and 12 (P > 0.05).
FIG. 2.
TBUT (a) and Schirmer I score (b) change from the baseline. Mean value ± standard error. The increase in TBUT (s) in all groups was statistically significant at week 6 and 12 (P < 0.05). There was no significant difference in the increase in the TBUT between groups at week 6 and 12. The increase in the Schirmer I score in 0.15% SH was statistically significant at week 12 (P < 0.05). SH, sodium hyaluronate; TBUT, tear film break-up time.
The mean change from baseline to week 12 in Schirmer test results was 1.31 ± 6.04 for the 0.1% SH group, 2.54 ± 6.46 for the 0.15% SH group, 0.70 ± 6.39 for the 0.3% SH group, and 1.07 ± 6.57 for the 0.05% CS group (Fig. 2b). However, scores in the 0.15% SH group showed a significant tendency for better improvement at week 12 compared with the 0.1% SH, 0.3% SH, and 0.05% CS group (P < 0.05). There was no statistically significant difference in the improvement in Schirmer I score between groups at week 6 and 12 (P > 0.05).
OSDI score
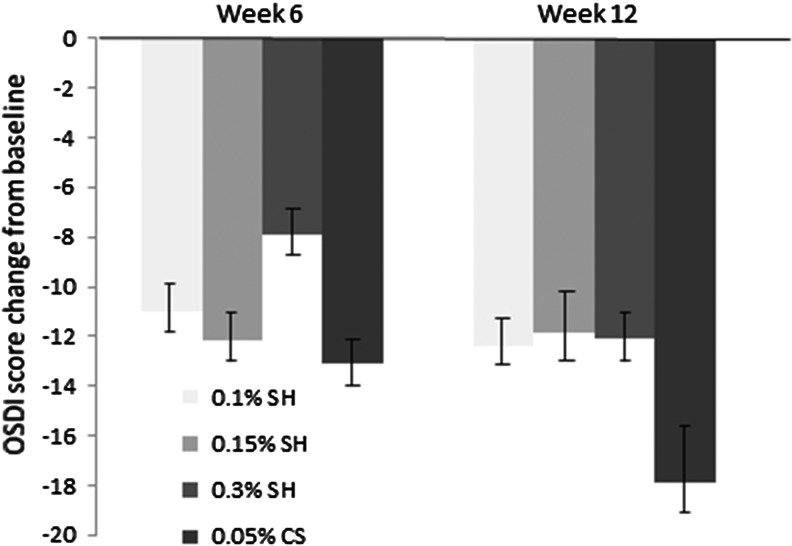
There was a statistically significant improvement in the mean change from baseline to week 6 and 12 in the OSDI score. The mean change from baseline to week 12 was −12.39 ± 19.21 for the 0.1% SH group, −11.86 ± 14.48 for the 0.15% SH group, −12.07 ± 18.49 for the 0.3% SH group, and −17.93 ± 20.58 for the 0.05% CS group (P < 0.0001; Fig. 3). There was no statistically significant difference in the improvement in OSDI between groups at week 6 and 12 (P > 0.05).
FIG. 3.
OSDI score change from the baseline. Mean value ± standard error. The decrease in the OSDI score in all groups was statistically significant at week 6 and 12 (P < 0.05). OSDI, ocular surface disease index.
There was no significant difference in all groups for change from baseline to week 12 in parameters of MGD.
Safety evaluation
Treatment-related AEs were observed in 6 patients (13.33%) in the 0.1% SH group, 9 patients (19.57%) in the 0.15% SH group, 6 patients (12.77%) in the 0.3% SH group, and 15 patients (31.25%) in the 0.05% CS group. No significant intergroup difference was shown. The most frequently observed AE was ocular pain, which was observed in 2 patients (4.44%) in the 0.1% SH group, 2 patients (4.35%) in the 0.15% SH group, 1 patient (2.13%) in the 0.3% SH group, and in 2 patients (4.17%) in the 0.05% CS group. All eye disorders were mild in severity and resolved either with appropriate treatment or with no treatment. No deaths and no serious AEs were observed in this study.
Discussion
Patients with dry eye complain of blurriness and glare although they have normal visual acuity.19 This deterioration in vision may be the consequence of the tear film breakup, causing an irregular tear surface.20 In addition, tear hyperosmolarity, ocular surface inflammation, and damage to the ocular surface were observed in patients with dry eye.21
In this phase 4 trial, SH eye drop demonstrated statistically significant efficacy and improvements as 0.05% CS for the treatment of dry eye. SH eye drop was effective at improving both the objective signs and the subjective symptoms of dry eye. Study data were obtained from a population representative of that seen in normal clinical practice, because dry eye commonly affects women who are middle-aged and older.22,23
Our study revealed that the effects of 0.1% SH, 0.15% SH, and 0.3% SH versus 0.05% CS were thoroughly comparable during the whole study period. In addition, in within-group analysis, dry eye patients who received 0.15% SH exhibited better improvement versus baseline in FCS score at week 12 although it was not statistically significant. FCS is a measurement of corneal damage reflecting corneal epithelium integrity and LGCS reflecting conjunctival epithelium integrity. Therefore, these improvements at week 6 and 12 showed that 0.1% SH, 0.15% SH, 0.3% SH, and 0.05% CS objectively improved the health of the ocular surface in the primary analysis. Our results are in good agreement with previous clinical data, which reported that SH-containing eye drops stimulate healing of the corneal epithelium.24
TBUT is considered a surrogate measure of tear-film stability. The improvements in TBUT with use of SH suggest improved integrity of the tear film, which can prevent evaporation, hyperosmolarity, and progression of dry eye disease due to its rheological, mucomimetic, and water-retention properties.25
In addition to its benefits on objective measures, 0.1% SH, 0.15% SH, and 0.3% SH were as effective as 0.05% CS on subjective outcomes, showing improvement in symptoms. The subjective improvements in dry eye symptoms observed in this study were supported by improvements in clinical outcome measures. Evaluating the treatment success in dry eye syndrome is a major challenge because of the weak correlation between signs and symptoms in dry eye syndrome, and the high variability of objective signs such as Schirmer test.26 Therefore, the assessment of efficacy using subjective symptoms, as well as objective signs, is particularly important in patients with dry eye.
However, OSDI scores in the 0.05% CS group showed a tendency for better improvement compared with the other groups although the intergroup difference was not significant. This finding could be partly explained by the higher degrees of dry eye symptoms in the CS group at the beginning of the study although it was not statistically significant. Schirmer test values in the 0.15% SH group showed a significant tendency for better improvement at week 12 compared with those of the 0.1% SH, 0.3% SH, and 0.05% CS group (P < 0.05). This is most likely to be attributed to unique anti-inflammatory properties of SH indirectly leading to tear secretion.
Previous study found that SH and CMC had significantly higher ocular surface retention times than physiological saline solution, and of these, 0.3% SH had the highest ability to protect the corneal epithelial cells from desiccation, which was concentration dependent.27 However, in this study, the efficacy of SH eye drops did not reveal concentration dependent results. The difference between our results and previous study may come from dissimilarities in type and dry eye severity. The participants in this study had a combined aqueous tear-deficient and evaporative dry eye with the severity of moderate to severe grading according to DEWS classification.2 Therefore, a single dose of SH did not seem to be significantly effective in moderate to severe dry eye patients. If our study enrolled patients with mild dry eye, concentration dependent results may have been shown. In addition, only 0.05% CS without any kinds of artificial tears is insufficient to allow epithelial healing and reduce dry eye symptoms in moderate to severe dry eye disease and is, therefore, unlikely to cause noticeable alterations after treatment. Artificial tears are typically recommended to use together with anti-inflammatory or immunomodulatory ophthalmic solutions as primary treatment options for moderate dry eye and in combination with other therapies for severe dry eye in clinical practice.28
0.1% SH, 0.15% SH, 0.3% SH, and 0.05% CS were well tolerated. All AEs were resolved by the end of the study period. Some of the AEs were not distinguishable from exacerbations or fluctuations of dry eye symptoms clearly. No serious events occurred and no safety issues were raised.
This study has some limitations. First, the duration was short. It may be insufficient to compare the impact of the SH and cyclosporine treatment on long-term dry eye disease since CS eye drop is believed to effect subconjunctival inflammation by preventing the recruitment of T cells, which may take 3–6 months. However previous study has shown that although CS eye drop improves objective and subjective measures of the dry eye, it may take up to 4–8 weeks to show its maximum effect.29 Therefore even though this study is limited in duration, it still justifies the results. A larger and longer study is warranted to more thoroughly address important clinical issues, including the effects of SH and CS eye drops on controlling ocular surface inflammation, reducing tear hyperosmolarity, and repairing corneal epithelium. Second, the difference of instillation frequency between SH and CS may influence the outcomes. Third, the lack of a placebo for the SH and CS ophthalmic solution could be the weakness in this study. Further studies will be required to investigate whether the improvements reported with SH are maintained in the longer term.
Conclusions
In conclusion, this is the first study to evaluate the efficacy and safety of the 0.1% SH, 0.15% SH, and 0.3% SH compared with 0.05% CS in patients with dry eye. Our study showed that a 12-week, 4–6 times daily treatment with 0.1% SH, 0.15% SH, and 0.3% SH was as effective as 0.05% CS in improving the objective signs and subjective symptoms of dry eye. These results suggest that it may lead to improved treatment of corneal and conjunctival epithelial damage and improvement in symptoms in patients with dry eye. Such efficacy, in addition to the well-tolerated profile of 0.1% SH, 0.15% SH, and 0.3% SH, makes them potentially useful treatment options for dry eye in clinical practice.
Acknowledgment
This study was supported by an unrestricted educational grant from Taejoon Pharm (Seoul, Korea). The sponsor or funding organization had no role in the design or conduct of this research.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Johnson M.E., and Murphy P.J. Changes in the tear film and ocular surface from dry eye syndrome. Prog. Retin. Eye Res. 23:449–474, 2004 [DOI] [PubMed] [Google Scholar]
- 2.International Dry Eye WorkShop Study Group. The definition and classification of dry eye disease: report of the Definition and Classification Subcommittee of the International Dry Eye WorkShop. Ocul. Surf. 5:75–92, 2007 [DOI] [PubMed] [Google Scholar]
- 3.Yeh S., Song X.J., Farley W., et al. Apoptosis of ocular surface cells in experimentally induced dry eye. Invest. Ophthalmol. Vis. Sci. 44:124–129, 2003 [DOI] [PubMed] [Google Scholar]
- 4.Management and therapy of dry eye disease: report of the management and therapy Subcommittee of the International Dry Eye Work Shop. Ocul. Surf. 5:163–178, 2007 [DOI] [PubMed] [Google Scholar]
- 5.Babić G.S., Zlatanović G., Jocić J.D., et al. Therapeutical approach to dry eye syndrome. Med. Pregl. 63:793–800, 2010 [DOI] [PubMed] [Google Scholar]
- 6.Johnson M.E., Murphy P.J., and Boulton M. Effectiveness of sodium hyaluronate eye drops in the treatment of dry eye. Graefes Arch. Clin. Exp. Ophthalmol. 244:109–112, 2006 [DOI] [PubMed] [Google Scholar]
- 7.Nakamura M., Hikida M., Nakano T., et al. Characterization of water retentive properties of hyaluronan. Cornea. 12:433–436, 1993 [DOI] [PubMed] [Google Scholar]
- 8.Nakamura M., Mishima H., Nishida T., et al. Binding of hyaluronan to plasma fibronectin increases the attachment of corneal epithelial cells to a fibronectin matrix. J. Cell Physiol. 159:415–422, 1994 [DOI] [PubMed] [Google Scholar]
- 9.Montés-Micó R, Cerviño A, Ferrer-Blasco T., García-Lázaro S., and Orti-Navarro S. Optical quality after instillation of eye drops in dry eye syndrome. J. Cataract Refract. Surg. 36:935–940, 2010 [DOI] [PubMed] [Google Scholar]
- 10.Choy E.P., Cho P., Benzie I.F., and Choy C.K. Investigation of corneal effect of different types of artificial tears in a simulated dry eye condition using a novel porcine dry eye model (pDEM). Cornea. 25:1200–1204, 2006 [DOI] [PubMed] [Google Scholar]
- 11.Nakamura S., Okada S., Umeda Y., and Saito F. Development of a rabbit model of tear film instability and evaluation of viscosity of artificial tear preparations. Cornea. 23:390–397, 2004 [DOI] [PubMed] [Google Scholar]
- 12.Aragona P., Papa V., Micali A., et al. Long term treatment with sodium hyaluronate-containing artificial tears reduces ocular surface damage in patients with dry eye. Br. J. Ophthalmol. 86:181–184, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grene R.B., Lankston P., Mordaunt J., et al. Unpreserved carboxymethylcellulose artificial tears evaluated in patients with keratoconjunctivitis sicca. Cornea. 11:294–301, 1992 [DOI] [PubMed] [Google Scholar]
- 14.Sall K., Stevenson O.D., Mundorf T.K., and Reis B.L. Two multicenter, randomized studies of the efficacy and safety of cyclosporine ophthalmic emulsion in moderate to severe dry eye disease. CsA Phase 3 Study Group. Ophthalmology. 107:631–639, 2000 [DOI] [PubMed] [Google Scholar]
- 15.Pocock S.J., and Simon R. Sequential treatment assignment with balancing for prognostic factors in the controlled clinical trial. Biometrics. 31:103–115, 1975 [PubMed] [Google Scholar]
- 16.Schiffman R.M., Christianson M.D., Jacobsen G., et al. Reliability and validity of the Ocular Surface Disease Index. Arch. Ophthalmol. 118:615–621, 2000 [DOI] [PubMed] [Google Scholar]
- 17.Lemp M.A. Report of the National Eye Institute/Industry workshop on clinical trials in dry eyes. CLAO J. 21:221–232, 1995 [PubMed] [Google Scholar]
- 18.Shimazaki J., Sakata M., and Tsubota K. Ocular surface changes and discomfort in patients with meibomian gland dysfunction. Arch. Ophthalmol. 113:1266–1270, 1995 [DOI] [PubMed] [Google Scholar]
- 19.Ridder WH, III, Tomlinson A., Huang J.F., and Li J. Impaired visual performance in patients with dry eye. Ocul. Surf. 9:42–55, 2011 [DOI] [PubMed] [Google Scholar]
- 20.Liu H., Thibos L., Begley C.G., and Bradley A. Measurement of the time course of optical quality and visual deterioration during tear break-up. Invest. Ophthalmol. Vis. Sci. 51:3318–3326, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ingram P.R., Pitt A.R., Wilson C.G., Olejnik O., and Spickett C.M. A comparison of the effects of ocular preservatives on mammalian and microbial ATP and glutathione levels. Free Radic. Res. 38:739–750, 2004 [DOI] [PubMed] [Google Scholar]
- 22.Schaumberg D.A., Dana R., Buring J.E., and Sullivan D.A. Prevalence of dry eye disease among US men: estimates from the Physicians' Health Studies. Arch. Ophthalmol. 27:763–768, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.The epidemiology of dry eye disease: report of the Epidemiology Subcommittee of the International Dry Eye WorkShop. Ocul. Surf. 5:93–107, 2007 [DOI] [PubMed] [Google Scholar]
- 24.Shimmura S., Ono M., Shinozaki K., et al. Sodium hyaluronate eyedrops in the treatment of dry eyes. Br. J. Ophthalmol. 79:1007–1011, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Prabhasawat P., Tesavibul N., and Kasetsuwan N. Performance profile of sodium hyaluronate in patients with lipid tear deficiency: randomised, double-blind, controlled, exploratory study. Br. J. Ophthalmol. 91:47–50, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sullivan B.D., Crews L.A., Messmer E.M., et al. Correlations between commonly used objective signs and symptoms for the diagnosis of dry eye disease: clinical implications. Acta Ophthalmol. 92:161–166, 2014 [DOI] [PubMed] [Google Scholar]
- 27.Zheng X., Goto T., and Ohashi Y. Comparison of in vivo efficacy of different ocular lubricants in dry eye animal models. Invest. Ophthalmol. Vis. Sci. 55:3454–3460, 2014 [DOI] [PubMed] [Google Scholar]
- 28.Behrens A., Doyle J.J., Stern L., et al. Dysfunctional Tear Syndrome Study Group. Dysfunctional tear syndrome: a Delphi approach to treatment recommendations. Cornea. 25:900–907, 2006 [DOI] [PubMed] [Google Scholar]
- 29.Fakhraie G., Lopes J., Spaeth G., et al. Effects of postoperative cyclosporine ophthalmic emulsion 0.05% (Restasis) following glaucoma surgery. Clin. Exp. Ophthalmol. 37:842–848, 2009 [DOI] [PubMed] [Google Scholar]