Abstract
Background: Gestational diabetes mellitus (GDM) is a metabolic disorder characterized by insulin resistance (IR) and altered glucose–lipid metabolism. We propose that ectonucleotide pyrophosphate phosphodiesterase-1 (ENPP1), a protein known to induce adipocyte IR, is a determinant of GDM. Our objective was to study ENPP1 expression in adipose tissue (AT) of obese pregnant women with or without GDM, as well as glucose tolerance in pregnant transgenic (Tg) mice with AT-specific overexpression of human ENPP1.
Methods: AT biopsies and blood were collected from body mass index-matched obese pregnant women non-GDM (n = 6), GDM (n = 7), and nonpregnant controls (n = 6) undergoing cesarian section or elective surgeries, respectively. We measured the following: (1) Expression of key molecules involved in insulin signaling and glucose–lipid metabolism in AT; (2) Plasma glucose and insulin levels and calculation of homeostasis model assessment of IR (HOMA-IR); (3) Intraperitoneal glucose tolerance test in AtENPP1 Tg pregnant mice.
Results: We found that: (1) Obese GDM patients have higher AT ENPP1 expression than obese non-GDM patients, or controls (P = 0.01—ANOVA). (2) ENPP1 expression level correlated negatively with glucose transporter 4 (GLUT4) and positively with insulin receptor substrate-1 (IRS-1) serine phosphorylation, and to other adipocyte functional proteins involved in glucose and lipid metabolism (P < 0.05 each), (3) AT ENPP1 expression levels were positively correlated with HOMA-IR (P = 0.01—ANOVA). (4) Pregnant AT ENPP1 Tg mice showed higher plasma glucose than wild type animals (P = 0.046—t test on area under curve [AUC]glucose).
Conclusions: Our results provide evidence of a causative link between ENPP1 and alterations in insulin signaling, glucose uptake, and lipid metabolism in subcutaneous abdominal AT of GDM, which may mediate IR and hyperglycemia in GDM.
Keywords: : ENPP1, gestational diabetes, insulin signaling, pregnancy
Introduction
Gestational diabetes mellitus (GDM) affects about 14% of pregnant women in the United States. GDM is characterized by maternal peripheral insulin resistance (IR) to glucose disposal,1 dysregulation of adipokines,2–4 altered lipid metabolism,5 low-grade inflammation,6 and elevated circulating free fatty acids.7 All these metabolic alterations contribute to significantly increase the risk for subsequent development of type 2 diabetes mellitus.8,9
Recent research has highlighted the important role of adipose tissue (AT) dysfunction in GDM.10 AT dysfunction is characterized by excessive lipolysis (adipocyte IR) and inflammation, leading to an increase in adipocyte-derived circulating lipids and cytokines that mechanistically play a role in defective total body insulin-mediated glucose utilization mainly in skeletal muscle. In general IR, including adipocyte IR, is acquired with obesity but not all obese individuals develop adipocyte and systemic IR.11 Similarly, although more frequent among obese pregnant women, GDM does not develop in all obese women. A better understanding of the changes in adipose tissue function during pregnancy and the relationship with GDM could help us better understand the potential mechanisms involved in the pathogenesis of this condition.
We recently reported that among obese men, upregulation of ectonucleotide pyrophosphate phosphodiesterase-1 (ENPP1) associates with adipocyte dysfunction and systemic IR.12 ENPP1, also known as plasma cell membrane glycoprotein PC-1, is a class II transmembrane glycoprotein that has been shown to downregulate insulin receptor activation by interaction with β-subunit of insulin receptor and inhibition of downstream signaling. This effect results in cellular insulin resistance as well as in adipocyte maturation arrest and impaired triglyceride (TG) storage.13–15 A mechanistic role of ENPP1 overexpression on IR in vivo is supported by findings obtained in transgenic (Tg) rodent models.16,17 A direct role of AT-specific ENPP1 overexpression on adipocyte IR and tissue functionality is found in the AtENPP1 Tg mice, an animal model displaying systemic IR, ectopic fat deposition in the liver, and glucose intolerance.18
Our study explored the possibility that ENPP1 expression in AT—resulting in adipocyte IR—could differentiate GDM from no GDM in obese women. Our approach combined studies in humans and in the AtENPP1 Tg mice to provide support for a mechanistic role of ENPP1 in the development of IR and hyperglycemia during pregnancy.
Materials and Methods
Human protocol
This was a prospective cross-sectional study approved by the IRB. All study subjects gave written informed consent before enrollment in the study. Obese women (defined as a body mass index [BMI] ≥30 kg/m2) and of reproductive age were enrolled after providing consent. Both pregnant and nonpregnant cohorts were enrolled. Pregnant women desiring a nonurgent cesarean delivery at term were recruited. During routine prenatal care, all women had been tested for GDM using the two-step approach.19 A 1 hr oral glucose screen (50 grams glucola) was first administered. If the result was ≥135 mg/dL, a 3-hr oral glucose tolerance test (GTT; 100 grams glucola) was administered using the Carpenter and Coustan cutoffs (95, 180, 155, and 140 mg/dL).20 When two or more of the values were exceeded, the test was considered positive and GDM was diagnosed. Nonpregnant women of reproductive age who met the criteria for obesity and were normoglycemic and normotensive were recruited at the time of an elective surgical procedure. Exclusion criteria included pre-GDM, urgent cesarean delivery, chorioamnionitis, hemoglobin <8 gm/dL, pre-eclampsia, thrombophilia, autoimmune disorders, chronic infection, metabolic storage disease, thyroid disease, recent glucocorticoid administration, or HIV.
A venous blood sample was obtained after an overnight fast (≥8 hrs) before surgery and processed immediately for glucose and lipid panel. Serum samples were aliquoted and stored at −80°C until further analysis. Subcutaneous adipose tissue was obtained from the abdominal region at the time of elective cesarean delivery or nonobstetric elective surgery, snap frozen in liquid nitrogen and stored at −80°C for Western blot analysis.
Assays
Serum total cholesterol, low-density lipoproteins, high-density lipoproteins, triglycerides, and glucose were analyzed using Piccolo Lipid Panel Reagent Discs in Piccolo Express Analyzer (Abaxis, Union City, CA). Serum hormone and cytokine levels were analyzed using the MILLIPLEX Human Adipokine Magnetic Bead Panel 1 and 2 (HADK1MAG-61K, HADK2MAG-61K; Millipore Corporation, Billerica, MA).
Western blot analysis
Proteins were extracted from 100 mg subcutaneous adipose tissue by homogenization in 1× cell lysis buffer (No. 9803; Cell Signaling Technology, Inc., Danvers, MA) containing 1 mM phenylmethylsulfonyl fluoride, 1 × Halt™ protease and phosphatase inhibitor cocktail (ThermoScientific, Rockford, IL) on ice. Lysates were centrifuged at 15,000g at 4°C for 15 min, clear aqueous fraction under fat layer was collected, and protein concentration was measured using the Bio-Rad protein assay kit (Bio-Rad Laboratories, Inc., Hercules, CA). Protein extracts (20 μg) were subjected to 4%–15% sodium dodecyl sulfate-polyacrylamide gel (SDS-PAGE—Criterion™ TGX™; Bio-Rad Laboratories, Inc.), electrophoretically transferred to polyvinylidene difluoride membranes (Immobilon®-P; Millipore), and blocked in 3% bovine serum albumin and Tris-buffered saline (TBS)/0.1% Tween-20 (TBS-T) at room temperature for 1 hr. Primary antibodies used were anti-ENPP1 (No. 2061), insulin receptor substrate (IRS) Antibody Sampler kit (No. 3015), adenosin monophosphate-activated protein kinase (AMPK) and acetyl-CoA carboxylase (ACC) Antibody Sampler kit (No. 9957), phospho-mitogen-activated protein kinase (MAPK) family Antibody Sampler kit (No. 9910), antiadipose triglyceride lipase (ATGL) (No. 2439), anti-Perilipin (No. 9349), antiphosphohormone-sensitive lipase (HSL) (Ser660) (No. 4126), anti-HSL (No. 4107) from Cell Signaling, antiphospho-IRS-1 (Ser307) (07–247), anti-IRS-1 (06–248) from Upstate-Millipore (Millipore), antifatty acid binding protein 4 [ab92501 from Abcam (Cambridge, MA), antiglucose transporter 4 (GLUT4) (2203–1; Epitomics, Burlingame, CA), antiphospho-Perilipin (Ser522) (No. 4856; Valascience, San Diego, CA), and antidiglyceride acyltransferase 1 (DGAT1) (sc-26173), from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA)], anti-Akt substrate 160 kDa (AS160) (MA5-14840; Pierce Biotechnolgy, Rockford, IL), and anti-β-actin (A1978; Sigma-Aldrich, St. Louis, MO). Secondary antibodies were from Southern Biotechnology Associates (Birmingham, AL). Immunoblots were detected using the Electrochemiluminescene Western Blotting Detection Reagents (Amersham Biosciences, Piscataway, NJ). Relative phosphorylation and expression levels were evaluated by quantification of relative density of each band normalized to that of the corresponding nonphosphorylated and β-actin band density, using the NIH ImageJ software version 1.46r (NIH, Bethesda, MD).
Animal protocol
AtENPP1 Tg (n = 6) generated as previously described,18 and C57Bl/6J (wild type, WT–n = 6) female mice mated with C57Bl/6J WT males. Pregnant females were fed regular chow and housed in filter-top cages in a temperature-controlled environment at 22°C, humidity 40%, and a 12:12-hr light–dark cycle. The study was approved by the Animal Care and Use Committee in accordance with guidelines of the National Institutes of Health Guidelines on the use of laboratory animals. At 18 days of gestation, pregnant mice underwent GTT, as previously described.18 Blood glucose levels were measured using an Ascensia Contour glucometer (Bayer). Blood insulin levels were measured using Ultra-Sensitive Mouse Insulin ELISA Kit (cat. no. 90080; Crystal Chem, Downers Grove, IL). Results were compared for the AtENPP1 Tg versus C57Bl/6J pregnant dams.
Statistical analysis
All data were analyzed using SAS version 9.2 (SAS Institute, Cary, NC) and are presented as means ± SEM. Statistical comparisons between study groups were performed using one-way ANOVA Kruskal–Wallis test with Dunn's multiple comparisons. Significance was defined as P < 0.05. Area under curve (AUC) for the glucose and insulin was calculated.
Results
Human study
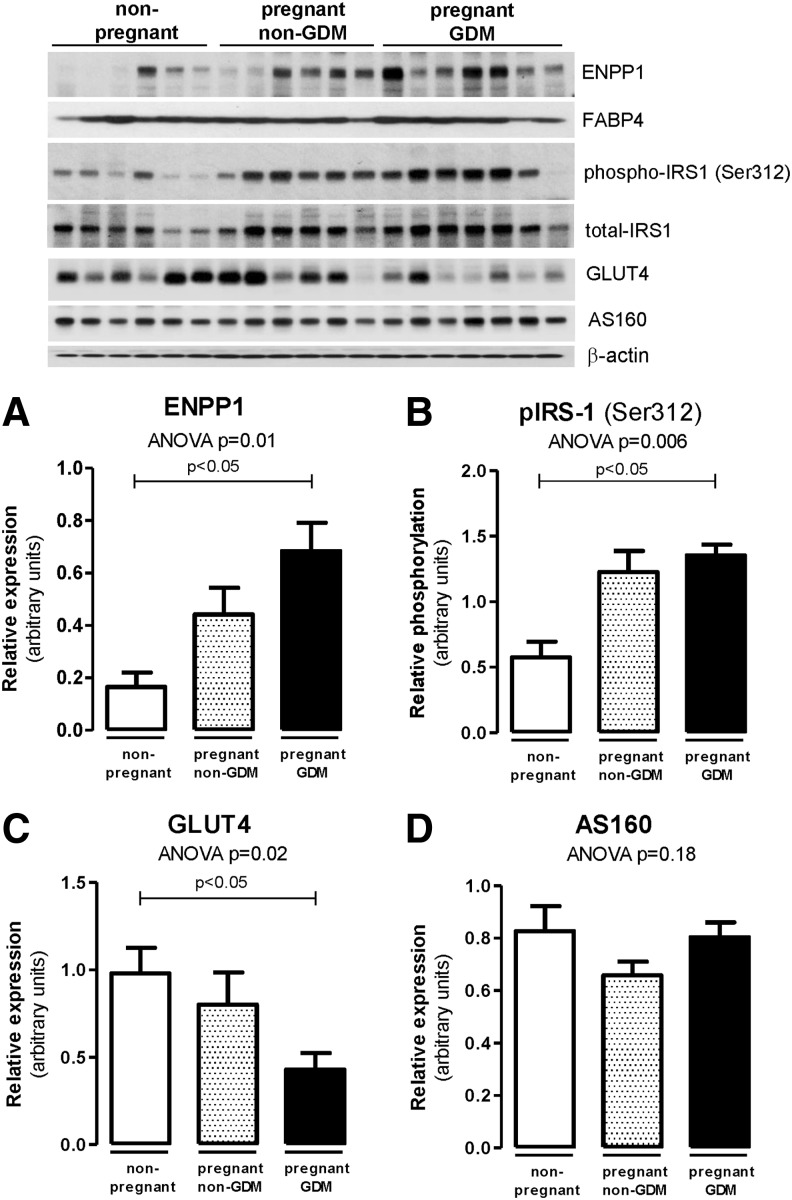
Nineteen obese reproductive age women were enrolled (six pregnant without GDM (BMI 37.7 ± 5.7), seven pregnant with GDM (BMI 41.0 ± 11.4), and six nonpregnant (BMI 42.4 ± 11.4). Serum biochemical analysis results are shown in Table 1. Expression levels of ENPP1 in subcutaneous AT were compared between groups by Western blot. ENPP1 expression was higher in obese pregnant women, particularly among those with GDM (P = 0.01 ANOVA, P < 0.05), than those without GDM and was lowest in the nonpregnant group (Fig. 1A).
Table 1.
Serum Biochemical Analysis at Delivery
| Obese controls | Non-GDM | GDM | P | |
|---|---|---|---|---|
| Total cholesterol (mg/dL) | 188.9 ± 29.7 | 198.6 ± 21.7 | 200.6 ± 27.7 | 0.68 |
| HDL (mg/dL) | 58.6 ± 17.4 | 56.4 ± 8.0 | 61.0 ± 11.9 | 0.81 |
| Triglycerides (mg/dL) | 148.4 ± 53.3 | 231.4 ± 29.0* | 254.1 ± 62.3*,# | 0.002 |
| LDL (mg/dL) | 108.6 ± 20.5 | 95.9 ± 28.3 | 88.7 ± 21.5 | 0.31 |
| VLDL (mg/dL) | 39.7 ± 22.4 | 46.3 ± 5.9 | 50.9 ± 12.5 | 0.95 |
| Adiponectin (μg/mL) | 7.6 ± 3.4 | 9.6 ± 6.8 | 9.4 ± 7.0 | 0.8 |
| Resistin (ng/mL) | 182.7 ± 80.3 | 145.9 ± 32.3 | 144.2 ± 60.3 | 0.46 |
| PAI-1 (ng/mL) | 39.8 ± 32.1 | 31.5 ± 21.0 | 17.7 ± 6.2 | 0.26 |
| Leptin (ng/mL) | 20.7 ± 7.9 | 56.8 ± 22.5* | 46.7 ± 19.1# | 0.004 |
| IL-6 (pg/mL) | 4.6 ± 8.1 | 2.6 ± 1.7 | 3.1 ± 1.1 | 0.75 |
| IL-8 (pg/mL) | 3.0 ± 1.9 | 1.7 ± 0.6 | 2.2 ± 0.7 | 0.21 |
| TNF-α (pg/mL) | 2.2 ± 1.3 | 2.1 ± 0.5 | 1.8 ± 0.6 | 0.73 |
Values are expressed as mean ± SD. Significant differences between groups are marked with * or # (ANOVA with Tukey's multiple comparison test).
GDM, gestational diabetes mellitus; HDL, high-density lipoproteins; LDL, low-density lipoproteins.
FIG. 1.
Adipose tissue ENPP1 and insulin-regulated functional proteins in obese non-GDM, GDM, and nonpregnant controls. Western blots of subcutaneous AT lysates were performed for ENPP1 (A), IRS-1 Ser312 phosphorylation (B), and GLUT4 and AS160 (C, D). Data reported as mean ± SEM in the bar graphs. Statistical analysis was performed using Kruskal–Wallis test with Dunn's multiple comparisons. AS160, Akt substrate of 160 kDa; AT, adipose tissue; ENPP1, ectonucleotide pyrophosphatase/phosphodiesterase 1; GDM, gestational diabetes mellitus; GLUT4, glucose transporter 4; IRS-1, insulin receptor substrate-1.
To determine whether altered ENPP1 expression was associated with changes in functional protein expression involved in adipocyte insulin signaling, we examined differences among groups for IRS-1 serine phosphorylation and concomitant inactivation. As shown in Fig. 1B, we detected increased inhibitory IRS-1 phosphorylation (Ser312) in pregnant women with GDM (P = 0.006 ANOVA, P < 0.05 vs. nonpregnant controls). We observed a reduction in insulin-responsive glucose transporter GLUT4 (P = 0.02 ANOVA, P < 0.05) without changes in its regulatory molecule AS160 level, which indicates a downregulation of glucose uptake in subcutaneous AT of obese pregnant women and particularly in those with GDM (Fig. 1C, D).
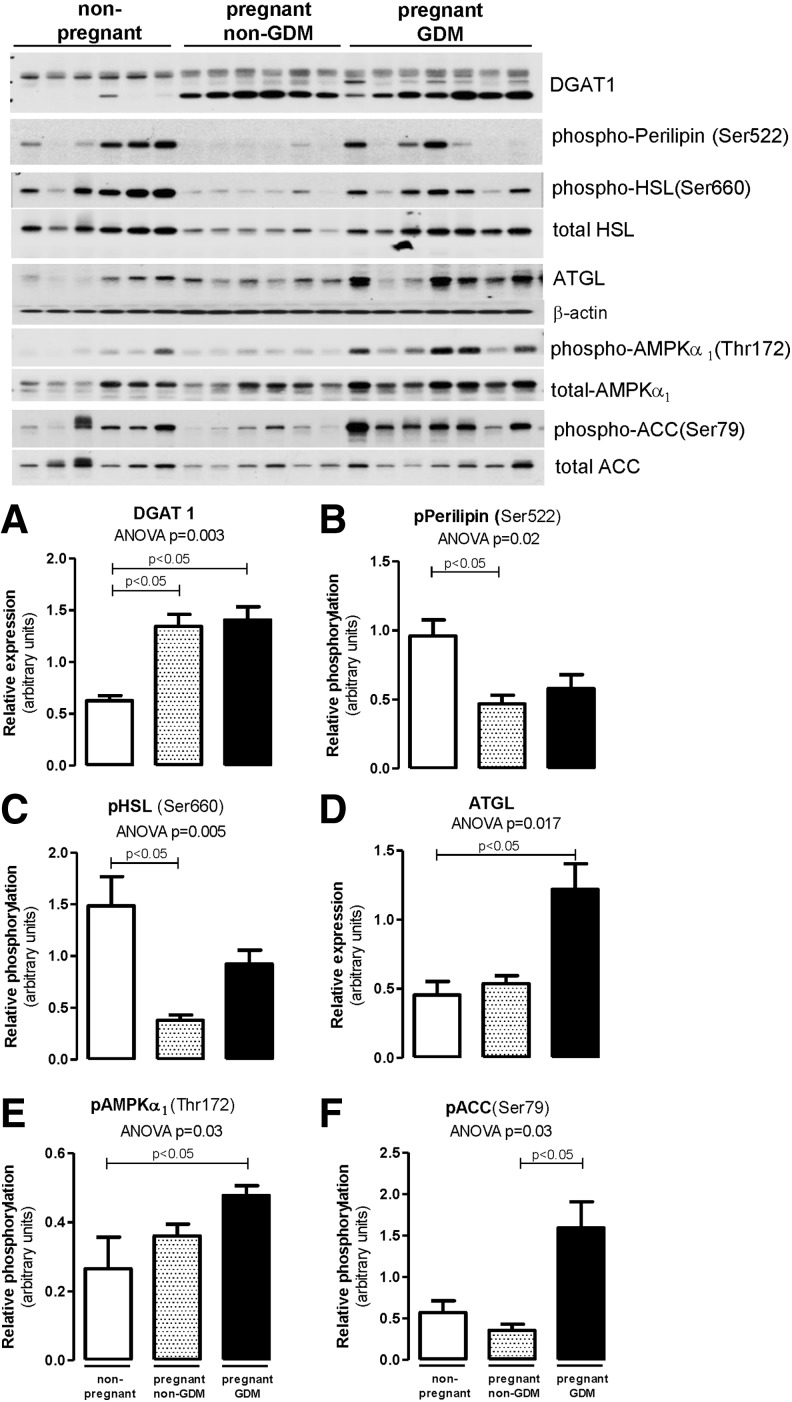
Next, we verified the changes in the expression levels and activation of proteins involved in AT lipid metabolism. We found higher DGAT1 expression (P = 0.003 ANOVA, P < 0.05 vs. nonpregnant controls) in pregnant women with and without GDM than in nonpregnant women (Fig. 2A). The elevated DGAT1 is possibly reflecting an increase in lipogenesis (TG synthesis), which may account for an increase in adipocyte TG storage and weight gain during pregnancy.5 Lipolysis was suppressed in pregnant women without GDM, as evidenced by the decrease in phosphorylation of Perilipin (Ser522) and HSL (Ser660) (Fig. 2B, C). However, sustained activation of the lipolytic pathway in GDM was accompanied with significant increase in ATGL when compared with pregnant women without GDM (Fig. 2D). Furthermore, Fig. 2E and F shows accentuated lipolysis in GDM through activation of AMPK (P = 0.03 ANOVA, P < 0.05) as well as inhibition of lipogenesis through ACC inactivation (P = 0.03 ANOVA, P < 0.05).
FIG. 2.
Adipose tissue protein activation related to lipid metabolism in obese non-GDM, GDM, and nonpregnant controls. Adipose tissue was obtained from subcutaneous abdominal areas and Western blots were performed for DGAT1 (A), phospho-Perilipin Ser522 (B), phospho-HSL Ser660 (C), ATGL (D), phospho-AMPKα Thr172 (E), and phospho-ACC Ser79 (F). Data reported as mean ± SEM in the bar graphs. Statistical analysis was performed using Kruskal–Wallis test with Dunn's multiple comparisons. ACC, acetyl-CoA carboxylase; AMPK, AMP-activated protein kinase; ATGL, adipose triglyceride lipase; DGAT1, diacylglycerol acyltransferase 1; HSL, hormone-sensitive lipase.
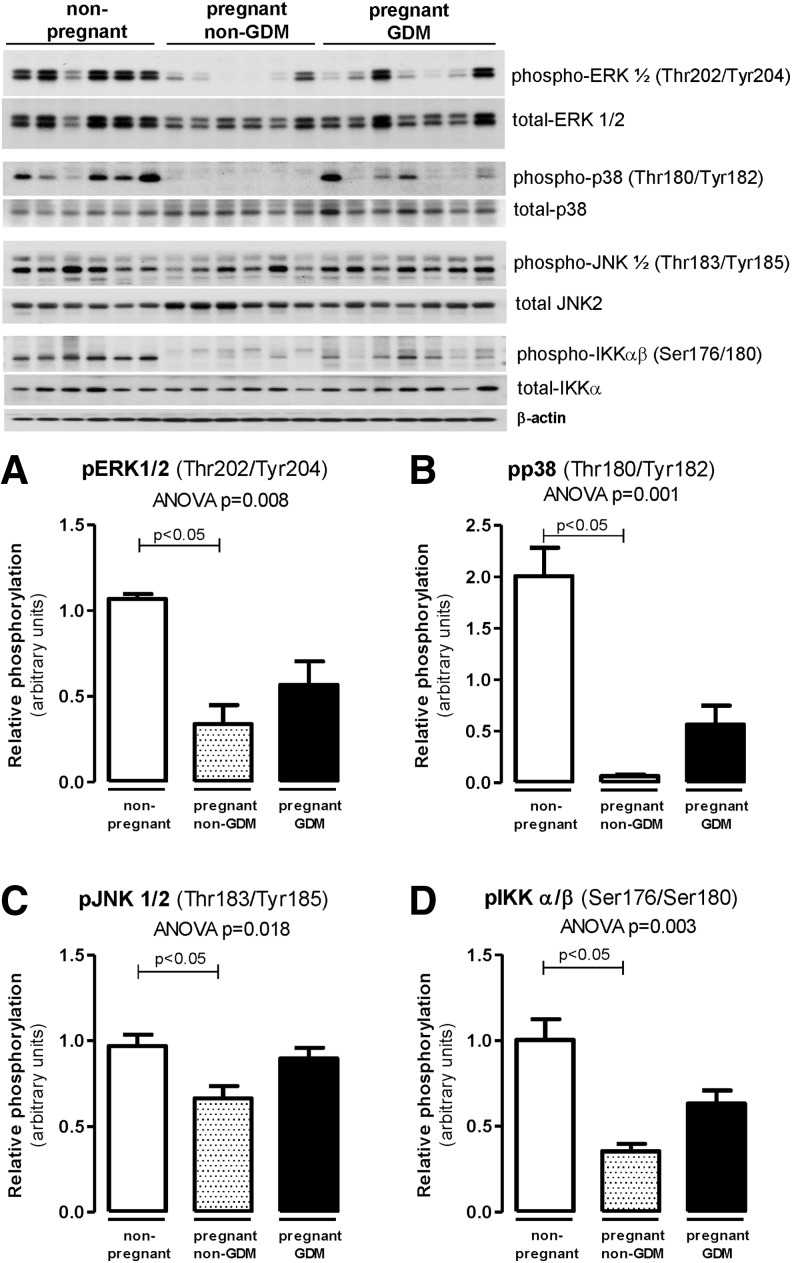
Figure 3 illustrates the group differences in activation of inflammatory pathways that may affect IRS-1 serine phosphorylation and downstream insulin signaling related to glucose and lipid metabolism. The MAPK activation [phospho-ERK (Thr202/Tyr204—Fig. 3A), phospho-p38 (Thr180/Tyr182—Fig. 3B), phospho-JNK (Thr183/Tyr185—Fig. 3C), and phospho-IKKα/β (Ser176/Tyr185—Fig. 3D)] was lower in pregnant women without GDM but similar in pregnant with GDM and in nonpregnant women (Fig. 3).
FIG. 3.
Adipose tissue protein activation related to inflammation in obese non-GDM, GDM, and nonpregnant controls. Western blots of subcutaneous AT lysates were performed for phospho-ERK1/2 (Thr202/Tyr204) (A), phospho-p38 (Thr180/Tyr182) (B), phospho-JNK (Thr183/Tyr185) (C), and phospho-IKKαβ (Ser176/Ser180) (D). Data reported as mean ± SEM in the bar graphs. Statistical analysis was performed using Kruskal–Wallis test with Dunn's multiple comparisons.
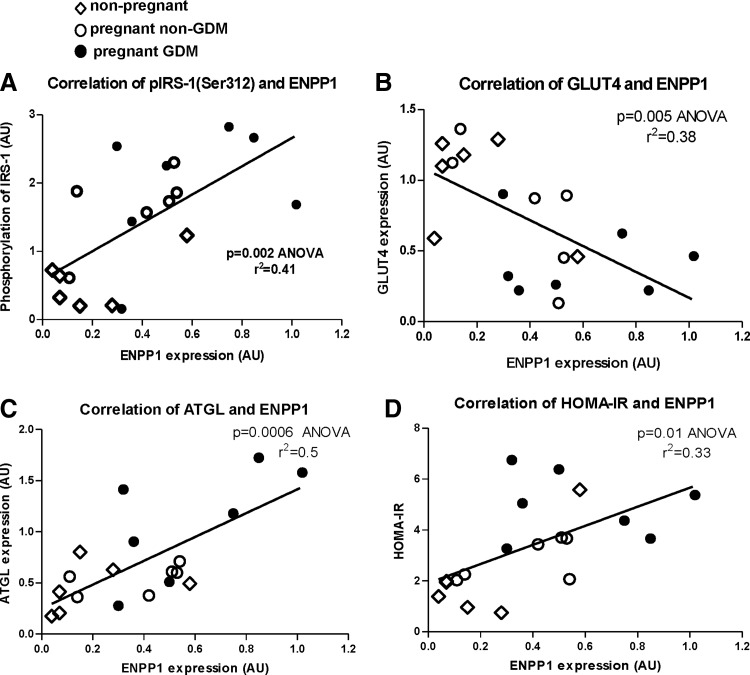
Figure 4 depicts the positive correlation between AT ENPP1 and proteins involved in adipocyte and systemic IR (Fig. 4A, C, D). It also shows a negative correlation between AT ENPP1 and GLUT4 (Fig. 4B). These results may indicate a possible (causative) direct effect of elevated AT ENPP1 of pregnant women with GDM in development of more accentuated IR. To lend support to this mechanistic view, we conducted studies on glucose metabolism during pregnancy in AtENPP1 Tg mice.
FIG. 4.
Correlation between AT ENPP1 and parameters of AT insulin signaling (A) IRS-1 phosphorylation, (B) GLUT4, (C) ATGL, and (D) HOMA-IR. HOMA-IR, homeostasis model assessment of insulin resistance.
Animal study
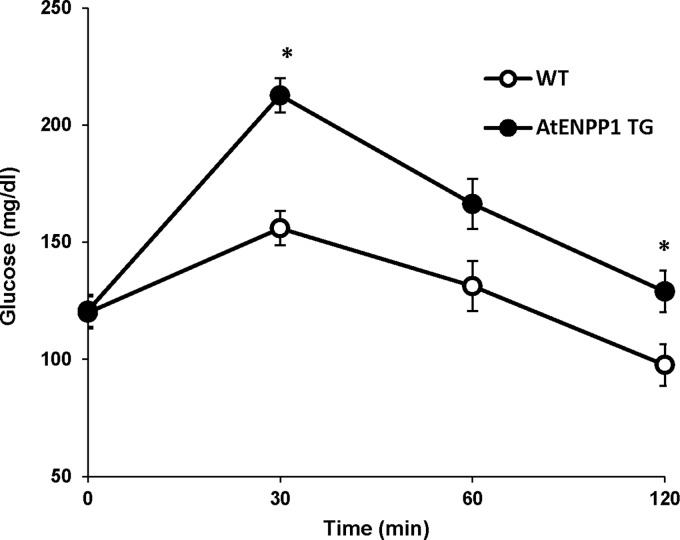
We have previously shown that male AtENPP1 Tg mice have selective adipocyte IR, which determines systemic glucose intolerance. In this study, we found that female pregnant AtENPP1 Tg mice, even when on regular diet, have higher blood glucose during intraperitoneal GTT (AUCglucose = 19,554 ± 1313 vs. 15,320 ± 926; P = 0.046) than WT littermates, indicating decreased glucose tolerance in presence of elevated AT ENPP1 expression during pregnancy (Fig. 5).
FIG. 5.
Hyperglycemia in pregnant AtENPP1 Tg mice. On IPGTT, Tg mice showed a significant increase in glucose levels at 30 and 120 min compared with WT littermates (*P ≤ 0.03 each). This was associated with significant decrease in insulin levels (data not shown). IPGTT, intraperitoneal glucose tolerance test; Tg, transgenic; WT, wild type.
Discussion
Taken together, the results of this study support the view that adipocyte IR is excessive in obese women who develop GDM compared with those without GDM, and that increased AT-ENPP1 may mechanistically contribute to this metabolic abnormality. Increased ENPP1-mediated adipocyte IR could significantly contribute to systemic IR through excessive lipolysis and inflammatory activation of AT.
Pregnancy is a status of physiological IR characterized by compensatory regulation in glucose and lipid metabolism that optimizes substrates utilization for fetal growth without compromising maternal health. Placental hormones are known to contribute to the pathogenesis of such physiological IR during pregnancy. However, obesity during pregnancy is more likely to alter this balance and significantly increases the risk for developing GDM, a condition of pathological IR in which systemic glucose tolerance becomes compromised. However, similarly to the metabolically healthy and metabolically unhealthy obese,21 only a fraction of obese pregnant women develop GDM.22 Although previous work has shown that IR usually pre-exists pregnancy in those who develop GDM, metabolic determinants of pathological IR that distinguish obese GDM from obese non-GDM remain largely unknown.
Recent literature has been accumulating supporting the idea that AT function in obesity may differentiate GDM women from non-GDM women.10 For example, AT expandability during pregnancy has been proposed to play a role in the etiology of GDM as supported by gene expression and histological properties of GDM subcutaneous AT.23,24 According to this view, similarly to what is found in men with metabolic syndrome,12 decreased ability to store energy in adipocytes plays a role in accentuating IR during pregnancy. Along these lines, our study proposes that ENPP1 upregulation could functionally mediate impairment in AT expandability just as previously shown in the AtENPP1 Tg mice.18 The ENPP1-induced adipocyte IR reduces TG storage in adipocytes, thus diminishing the AT function as a buffer for excessive caloric intake in obesity. Increased ENPP1 expression in AT resulted in glucose intolerance during pregnancy in AtENPP1 Tg mice (Fig. 5) and supports the view that increased ENpp1 in AT of obese pregnant with GDM is not just an associative finding but could be a true determinant of this condition.
We found that AT ENPP1 expression is higher in GDM women than in non-GDM women or nonpregnant controls. Although the mechanisms involved in such elevation are currently unknown, pregnancy-related increase of AT ENPP1 was associated with significant decrease in expression levels of insulin responsive functional protein GLUT4. It had been previously reported that GLUT4 and glucose uptake is lower in AT of GDM patients.25 Here we also find a negative correlation between ENPP1 and GLUT4 without changes in its regulatory molecule (AS160) levels. This may indicate a specific role of ENPP1 in downregulation of glucose uptake in GDM. In addition, AT-specific ENPP1 overexpression determined suppression in GLUT4 expression in AtENPP1 Tg mice (Supplementary Fig. S1; Supplementary Data are available online at www.liebertpub.com/met). Interestingly, gene–gene interaction between maternal ENPP1 and fetal glucose transporter encoding SLC2A2 was recently reported26 and may provide a mechanism for ENPP1-regulated GLUT4 expression in AT.
AT of GDM showed insulin signaling inhibition at the level of IRS-1 Ser312 phosphorylation. We observed a relative increase of inflammatory pathway activation in the AT of GDM versus non-GDM but an overall reduction related to the pregnancy status. Although no differences in plasma adipokines levels were found in the three study groups, likely because of the relatively small number of study subjects for this outcome variable (data not shown), increased inflammation in AT of GDM versus non-GDM could contribute to systemic impairment in glucose metabolism through paracrine effects on skeletal muscle cells insulin signaling activation.27
Altered lipid metabolism resulting from adipocyte IR may also play a role in skeletal muscle and systemic IR. Our data suggest that pregnancy in obese women is associated with an increase of DGAT1 expression regardless of metabolic status. This increase in DGAT1 is possibly reflecting an attempt to increase lipogenesis (TG synthesis) during pregnancy, which is, however, counteracted by activation of lipolytic enzymes in GDM. The AT-specific lipase, ATGL, was expressed at the same levels in non-GDM women as compared with nonpregnant obese women, but was significantly increased in GDM women. Furthermore, we observed accentuated lipolysis in GDM through activation of AMPK, and inhibition of lipogenesis through ACC phosphorylation as previously shown by others.28–30 Taken together, our findings support the view that in late pregnancy, failure to fully inhibit lipolysis results in net increased release of free fatty acids, which in obese individuals with GDM may explain the observed elevation of circulating non-esterified fatty acid,1 mediator of skeletal muscle IR to glucose disposal. Our study directly links these metabolic changes to AT ENPP1 and can provide a mechanism for AT-mediated systemic IR. Accordingly, we observed a positive correlation between AT ENPP1 expression level and homeostasis model assessment of IR, which clearly displays linkage of AT ENPP1 expression level to pregnancy-induced IR and GDM.
The small sample size of this study was driven by the limitations related to protein quantification methodology. This reduced our ability to identify circulating markers of AT dysfunction as well as studies of AT histology. However, the approach of combining well-defined study groups with animal studies strengthens our conclusions on the role of ENPP1 as a mechanistic target.
Although we have previously demonstrated that ENPP1 is linked to IR in men, and in the AtENNP1 Tg mice,12,18 this is the first study to show a correlation between ENPP1 and IR in women. Future investigations could clarify the mechanisms regulating ENPP1 in AT and perhaps assess the potential role of this protein as a novel target of therapeutic intervention to prevent GDM and its associated complications for both mother and newborn health.
Supplementary Material
Acknowledgments
This research was supported, in part, by intramural funding provided by the University of Texas Medical Branch to N.A., and by the National Institutes of Health, CTSA, and the Institute for Translational Sciences at the University of Texas Medical Branch at Galveston: 1UL1RR029876 UTMB CTSA (ARB).
Author Disclosure Statement
The authors have nothing to disclose. A.P. is currently working for the Department of Navy. The views expressed in this presentation are those of the authors and do not necessarily reflect the official policy or position of the Department of Navy, Department of Defense, or the U.S. Government.
References
- 1.Catalano PM, Nizielski SE, Shao J, et al. . Downregulated IRS-1 and PPARgamma in obese women with gestational diabetes: Relationship to FFA during pregnancy. Am J Physiol Endocrinol Metab 2002;282:E522–E533 [DOI] [PubMed] [Google Scholar]
- 2.Miehle K, Stepan H, Fasshauer M. Leptin, adiponectin and other adipokines in gestational diabetes mellitus and pre-eclampsia. Clin Endocrinol (Oxf) 2012;76:2–11 [DOI] [PubMed] [Google Scholar]
- 3.Ramirez VI, Miller E, Meireles CL, et al. . Adiponectin and IGFBP-1 in the development of gestational diabetes in obese mothers. BMJ Open Diabetes Res Care 2014;2:e000010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wurst U, Ebert T, Kralisch S, et al. . Serum levels of the adipokine Pref-1 in gestational diabetes mellitus. Cytokine 2015;71:161–164 [DOI] [PubMed] [Google Scholar]
- 5.Herrera E, Ortega-Senovilla H. Disturbances in lipid metabolism in diabetic pregnancy—Are these the cause of the problem? Best Pract Res Clin Endocrinol Metab 2010;24:515–525 [DOI] [PubMed] [Google Scholar]
- 6.Lappas M, Jinks D, Ugoni A, et al. . Postpartum plasma C-peptide and ghrelin concentrations are predictive of type 2 diabetes in women with previous gestational diabetes mellitus. J Diabetes 2015;7:506–511 [DOI] [PubMed] [Google Scholar]
- 7.Sivan E, Boden G. Free fatty acids, insulin resistance, and pregnancy. Curr Diab Rep 2003;3:319–322 [DOI] [PubMed] [Google Scholar]
- 8.Kim C, Newton KM, Knopp RH. Gestational diabetes and the incidence of type 2 diabetes: A systematic review. Diabetes Care 2002;25:1862–1868 [DOI] [PubMed] [Google Scholar]
- 9.Shah BR, Retnakaran R, Booth GL. Increased risk of cardiovascular disease in young women following gestational diabetes mellitus. Diabetes Care 2008;31:1668–1669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Simas TAM, Corvera S. The roles of adipose tissue and inflammation in gestational diabetes mellitus. Int Med 2014. (S6:010) [Google Scholar]
- 11.Catalano PM, Tyzbir ED, Roman NM, et al. . Longitudinal changes in insulin release and insulin resistance in nonobese pregnant women. Am J Obstet Gynecol 1991;165:1667–1672 [DOI] [PubMed] [Google Scholar]
- 12.Chandalia M, Davila H, Pan W, et al. . Adipose tissue dysfunction in humans: A potential role for the transmembrane protein ENPP1. J Clin Endocrinol Metab 2012;97:4663–4672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maddux BA, Goldfine ID. Membrane glycoprotein PC-1 inhibition of insulin receptor function occurs via direct interaction with the receptor alpha-subunit. Diabetes 2000;49:13–19 [DOI] [PubMed] [Google Scholar]
- 14.Maddux BA, Sbraccia P, Kumakura S, et al. . Membrane glycoprotein PC-1 and insulin resistance in non-insulin-dependent diabetes mellitus. Nature 1995;373:448–451 [DOI] [PubMed] [Google Scholar]
- 15.Liang J, Fu M, Ciociola E, et al. . Role of ENPP1 on adipocyte maturation. PLoS One 2007;2:e882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dong H, Maddux BA, Altomonte J, et al. . Increased hepatic levels of the insulin receptor inhibitor, PC-1/NPP1, induce insulin resistance and glucose intolerance. Diabetes 2005;54:367–372 [DOI] [PubMed] [Google Scholar]
- 17.Maddux BA, Chang YN, Accili D, et al. . Overexpression of the insulin receptor inhibitor PC-1/ENPP1 induces insulin resistance and hyperglycemia. Am J Physiol Endocrinol Metab 2006;290:E746–E749 [DOI] [PubMed] [Google Scholar]
- 18.Pan W, Ciociola E, Saraf M, et al. . Metabolic consequences of ENPP1 overexpression in adipose tissue. Am J Physiol Endocrinol Metab 2011;301:E901–E911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Committee on Practice B-O. Practice Bulletin No. 137: Gestational diabetes mellitus. Obstet Gynecol 2013;122:406–416 [DOI] [PubMed] [Google Scholar]
- 20.Bulletins—Obstetrics CoP. Practice Bulletin No. 137: Gestational diabetes mellitus. Obstet Gynecol 2013;122:406–416 [DOI] [PubMed] [Google Scholar]
- 21.Blüher M. The distinction of metabolically ‘healthy’ from ‘unhealthy’ obese individuals. Curr Opin Lipidol 2010;21:38–43 [DOI] [PubMed] [Google Scholar]
- 22.Catalano PM, Huston L, Amini SB, et al. . Longitudinal changes in glucose metabolism during pregnancy in obese women with normal glucose tolerance and gestational diabetes mellitus. Am J Obstet Gynecol 1999;180:903–916 [DOI] [PubMed] [Google Scholar]
- 23.Rojas-Rodriguez R, Lifshitz LM, Bellve KD, et al. . Human adipose tissue expansion in pregnancy is impaired in gestational diabetes mellitus. Diabetologia 2015;58:2106–2114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lappas M. Effect of pre-existing maternal obesity, gestational diabetes and adipokines on the expression of genes involved in lipid metabolism in adipose tissue. Metabolism 2014;63:250–262 [DOI] [PubMed] [Google Scholar]
- 25.Colomiere M, Permezel M, Lappas M. Diabetes and obesity during pregnancy alter insulin signalling and glucose transporter expression in maternal skeletal muscle and subcutaneous adipose tissue. J Mol Endocrinol 2010;44:213–223 [DOI] [PubMed] [Google Scholar]
- 26.Lupo PJ, Mitchell LE, Canfield MA, et al. . Maternal-fetal metabolic gene-gene interactions and risk of neural tube defects. Mol Genet Metab 2014;111:46–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Friedman JE, Ishizuka T, Shao J, et al. . Impaired glucose transport and insulin receptor tyrosine phosphorylation in skeletal muscle from obese women with gestational diabetes. Diabetes 1999;48:1807–1814 [DOI] [PubMed] [Google Scholar]
- 28.Long YC, Zierath JR. AMP-activated protein kinase signaling in metabolic regulation. J Clin Invest 2006;116:1776–1783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sullivan JE, Brocklehurst KJ, Marley AE, et al. . Inhibition of lipolysis and lipogenesis in isolated rat adipocytes with AICAR, a cell-permeable activator of AMP-activated protein kinase. FEBS Lett 1994;353:33–36 [DOI] [PubMed] [Google Scholar]
- 30.Yin W, Mu J, Birnbaum MJ. Role of AMP-activated protein kinase in cyclic AMP-dependent lipolysis In 3T3-L1 adipocytes. J Biol Chem 2003;278:43074–43080 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.