Abstract
In spite of the extensive research the complex pathogenesis of diabetic retinopathy (DR) has not been fully elucidated. For many years it has been thought that diabetic retinopathy manifests only with microangiopathic lesions, which are totally responsible for the loss of vision in diabetic patients. In view of the current knowledge on the microangiopathic changes in the fundus of the eye, diabetic retinopathy is perceived as a neurodegenerative disease. Several clinical tools are available to detect neuronal dysfunction at early stages of diabetes. Many functional changes in the retina can be identified before vascular pathology develops, suggesting that they result from a direct effect of diabetes on the neural retina. In the course of diabetes there is a chronic loss of retinal neurons due to increased frequency of apoptosis. The neuronal apoptosis begins very early in the course of diabetes. This observation has led to suggestions that precautions against DR should be implemented immediately after diabetes is diagnosed. Neurodegeneration cannot be reversed; therefore treatments preventing neuronal cell loss in the retina need to be developed to protect diabetic patients. This review is an attempt to summarize what is currently known about the mechanisms of neuronal apoptosis in the context of diabetic retinopathy and vascular degeneration as well as about potential treatments of DR
Keywords: Diabetic retinopathy, Neurodegeneration of neurons, Neuroinflammation, Neuroprotection
1. INTRODUCTION
As reported by the World Health Organization (WHO) currently there are 387 million people worldwide suffering from diabetes, which makes 8.3% of the global human population. By 2030 the number of people with diabetes is estimated to increase by 205 million, with a high percentage of them suffering from type 2 diabetes mellitus (T2DM) [1, 2]. Although clinically it is difficult to overlook type 1 diabetes mellitus (T1DM), many patients with T2DM, which is a slowly progressing disease, are unaware of their condition. It has been shown that in approximately 46% of patients at high risk of diabetes the disease remains undiagnosed. T2DM most commonly affects people aged 40 to 69 years, but over the last decades there has been more and more individuals who suffer from the disease before they are 40 [3-6]. Untreated or poorly controlled diabetes can increase the risk of serious complications, the therapy of which is a great burden to healthcare systems all over the world. The currently available long-term multidisciplinary studies have shown that long-term diabetic complications develop as a result of progressing vascular damage both in small and large blood vessels [7-9].
One of the major complications in patients with diabetes is diabetic retinopathy (DR), a leading cause of blindness worldwide. In spite of the extensive research the complex pathogenesis of diabetic retinopathy has not been fully elucidated [10-15]. For many years it has been thought that DR manifests only with microangiopathic lesions, which are totally responsible for the loss of vision in diabetic patients. In view of the current knowledge on the microangiopathic changes in the fundus of the eye, diabetic retinopathy is perceived as a neurodegenerative disease [13-15]. A large number of cellular and molecular studies in experimental animals and early retinal function tests in DR patients have shown that neurons are vulnerable to damage shortly after onset of diabetes [10, 11, 13, 14]. Several clinical tools such as optical coherence tomography (OCT), multifocal electro-retinogram (mfERG), flash ERG, and short-wavelength automated perimetry have shown functional deficit in the neurological component in the early stages of diabetes. Adaptive optics, a new imaging technique, also showed that loss of photoreceptors is the early change in diabetic retinopathy [16-18]. Results that are generated by mfERG appear similar to those generated by flash ERG. In contrast to flash ERG, which best generates data appropriate for whole-eye disorders. The basic mfERG result is based on the calculated mathematical average of an approximation of the positive deflection component of traditional ERG response, known as the b-wave.
Multifocal ERG programs measure electrical activity from more than a hundred retinal areas per eye, in a few minutes. Through the use of mfERG Semeran et al. has provided evidence suggesting a direct link between neural dysfunction and vascular abnormalities in DR. In this regard, it has been shown that a delayed mfERG implicit time predicts the development of early microvascular abnormalities [17]. A good example is the comparison of mfERGs results to OCT measurements in order to correlate functional mfERG to morphological (CT) change. Early functional changes in the diabetic retina seem to occur before morphological changes in the retinal nerve fiber layer (RNFL) [18].
2. RETINAL NEURODEGENERATION IN THE COURSE OF DIABETES
Retinal neurodegenerative changes underlying the vision impairment in people with diabetes may develop as a result of genetic, chronic inflammation and metabolic disorders.
2.1. Hyperglycaemia and Changes in the Fundus of the Eye
Toxic effects of chronically elevated blood glucose levels include damage to pericytes in the retina of the eye. These cells are the first ones to be affected by changes induced by excess glucose supply [19, 20].
Harmful effects in the pericytes are mediated by activation of several metabolic pathways including nonenzymatic glycation of proteins, which is among the most important biochemical processes playing a key role in the development of angiopathic complications in diabetes. This process results in formation of advanced glycation endproducts (AGEs), which are long-lasting and irreversible products that have the ability to form cross-links between proteins with subsequent modification of blood vessel elasticity [21-24].
In animal studies stimulation of inflammatory response by receptor for the advanced glycation end products (RAGEs) induced mononuclear phagocytes was observed. Moreover in the acute peripheral nerve injury the ligand-RAGE complex can modulate inflammatory response and neurite outgrowth. In subjects suffering from diabetes there is increased synthesis of RAGEs in macrophages and monocytes migrating from the circulation into the tissues. Increased expression of the receptors on the endothelium and smooth muscle cells was observed and shown to be stimulated by increased AGE levels, which resulted in long-term angiopathic complications. This phenomenon was observed in diabetic retinopathy [25-27]. In retinal capillaries of patients with advanced diabetes the endothelial AGE-RAGE complexes stimulated nuclear factor κB (NF-κB) transcription factor. This resulted in increased vascular endothelial growth factor (VEGF) synthesis and leukocyte adhesion to these cells as well as potentiated inflammatory response [27, 28]. Currently available studies revealed AGEs and sRAGE threshold values for the risk of development of retinopathy and nephropathy in children and adolescents with type 1 diabetes Fig. (1a), (1b) [29].
Fig. (1).
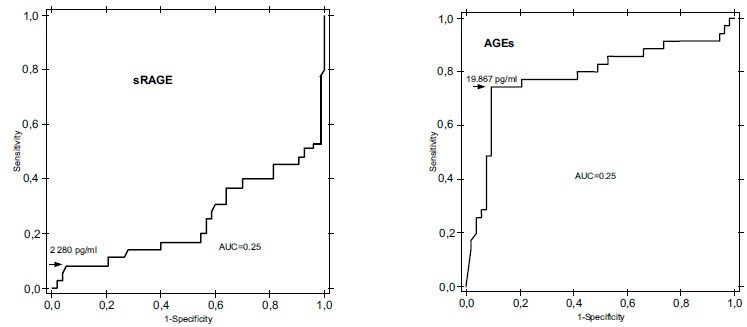
(a, b) shows sRAGE and AGE threshold values in T1DM patients. Based on the ROCAUC analysis the threshold value for sRAGE was calculated to be 2280 pg/ml. In contrast to the serum sRAGE levels, a discriminatory value was found for serum AGEs in studied group of children and adolescents with T1DM. The threshold value for serum AGEs concentrations was established at the level of 19867 pg/ml. ROC, receiver operating characteristic; AUCROC, area under the ROC Curve [29].
2.2. Oxidative Stress
The polyol pathway, nonenzymatic glycation of proteins with its endproducts, protein kinase C activation and hexosamine pathway lead to a series of pathological events including increased production of free oxygen radicals, oxidative stress, activation of proangiogenic factors and finally inflammation [30-39]. Hyperglycaemia, due to enhanced production of superoxide anion radicals by electron transport chain in mitochondria triggers a self-perpetuating cycle of production of oxygen free radicals [32-34]. Oxygen free radicals including peroxide anion radical and hydroxyl radical are a part of a whole body of reactive oxygen species (ROS). Peroxide anion radical is generated in mitochondria by electron transport chain in oxidative phosphorylation reaction as a product of non-enzymatic one-electron reaction with oxygen. However the peroxide anion radical is not able to pass cell membranes, remains within the membranes and is deactivated by superoxide dysmutase. The reaction results in hydrogen peroxide which can diffuse from mitochondria and transform into highly reactive hydroxyl radical [35-37]. In all types of cells with possible hyperglycaemia free oxygen radicals cause a decrease in activity of the key glycolytic enzyme glycerylaldehyde 3-phosphate dehydrogenase (GAPDH) [35]. GAPDH is an essential enzyme in the process of glycolysis. Decreased activity of GAPDH results in increased levels of the reaction substrates and intermediate products. Overexpression of glyceraldehyde 3-phosphate dehydrogenase prevents neurovascular degeneration after retinal injury [35]. Moreover oxygen free radicals can increase vascular permeability and reduce the ability of blood vessels to dilate as well as can activate growth factors and platelets resulting in vasoconstriction following endothelial injury [36]. Oxidative stress which is a consequence of prolonged hyperglycaemia is a key link in connecting angiopathic complications in the course of diabetes [30, 31, 40]. It has been shown that oxidative stress is associated with impairment in neuroretinal biochemical homeostasis in patients with PDR, which further augments retinal nitrosative stress and thus worsens the pathogenic process of retinopathy among patients with PDR [40].
2.3. Glutamate Excitotoxicity and Retinal Ganglion Cell Death
In the course of diabetes high blood glucose leads to neuron vulnerability and apoptosis through many mechanisms, with recently explored pathways illustrated in Fig. (2). Glutamate excitotoxicity is indicated as one of the mechanisms that can lead to oxidative stress mediated retinal ganglion cell death in diabetes [41-44]. At physiological conditions the dominant form is the anion form of glutamic acid called glutamate. Glutamate belongs to a class of neuro-transmitters involved in the regulation of neurohormonal activity. Its high levels are found in the central nervous system. Stimulated nerve cells release glutamate molecules from the glutamatergic synaptic vesicles. The neuro-transmitter released at the glutamatergic synapses – glutamate causes depolarization of the postsynaptic neuronal membrane and generates a signal in the neighbouring nerve cell. Glutamate accumulated in the synaptic space is taken up by neighbouring astrocytes, where it is broken down to free glutamine. The process of its resorption is accompanied by sodium ion (Na+) influx into the cell and activation of Na+/K+-ATPase. The active ATPase initiates the process of glycolysis to burn glucose and produce lactate, which is then transported to neurons and used as an energy substrate. Free glutamine obtained in astrocytes is used by neurons as a substrate for glutamate generation. Recently there has been more and more evidence suggesting that glutamate excitotoxicity in the retina leads among others to apoptotic degenerative lesions [45-48].
Fig. (2).
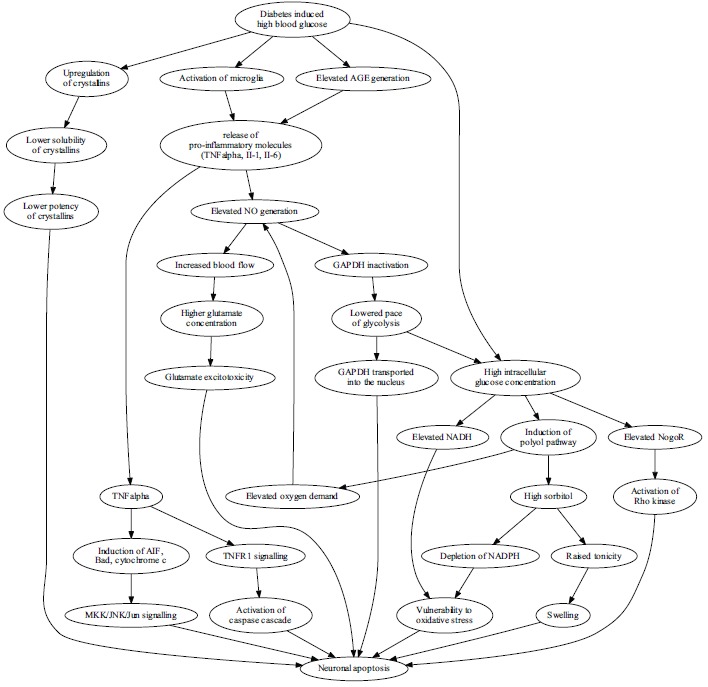
The high blood glucose leads to neuron vulnerability and apoptosis in diabetes through many mechanisms, with recently explored pathways illustrated. Hyperglycemia affects the expression and function of crystallins, a group of chaperones. Crystallins are upregulated, however their solubility is diminished with the net effect of lowered protection. Activated microglia and elevated levels of AGEs induce prolonged inflammation with pro-apoptotic effects on retinal ganglion cells through pathways dependent on TNFα and nitric oxide. Disruption of TNFα balance induces apoptosis via caspase cascade and via Jun kinase, while elevated NO generation leads to vasodilation, increased blood flow, higher glutamate concentrations and results in glutamate excitotoxicity. NO also lowers the pace of glycolysis by inactivating GAPDH, a process that leads to accumulation of GAPDH in the nucleus, which is an apoptosis-associated effect and increases the burden of high intracellular glucose concentration resulting mostly from high blood glucose concentration. Neurons are forced to accommodate the excess glucose by the polyol pathway which uses NADPH to generate sorbitol from glucose. High intracellular glucose concentration additionally leads to NADH (nicotinamide adenine dinucleotide, reduced) accumulation, which, collectively with NADPH (nicotinamide adenine dinucleotide phosphate, reduced) depletion, leads to vulnerability to oxidative stress. Finally, excess intracellular glucose activates the receptor for Nogo, and leads to apoptosis induction via the Rho kinase.
Fig. (1a). AUCROC for serum sRAGE. Fig. (1b). AUCROC for serum AGEs.
Apoptosis is a mechanism to eliminate unnecessary, aging or damaged neurons. The process is regulated by interactions between pro-apoptotic proteins (Bax, Bad, Bmi, Hik, Bak) and anti-apoptotic proteins Bcl-2, Bcl-x, A-1, Mel-1 [48-52]. Pro-apoptotic proteins reduce mitochondrial membrane potential and initiate leakage of apoptosis inducing factor (AIF) and cytochrome c [48]. The appearance of cytochrome c in the cytoplasm and its complexes with apoptotic protease-activating factor-1 (APAF-1) activate procaspase-9. The process triggers a cascade of activation of further caspases, which are proteolytic enzymes, and finally leads to DNA fragmentation and neuron death [47, 53, 54]. Currently available data suggest that the main mechanism underlying the induction of neuron apoptosis under chronic excitotoxic conditions is the mitochondrial apoptosis pathway rather than Fas signalling pathway [48, 53]. Caspases, the enzymes involved in apoptosis are also elevated in retinas of diabetic rats thus making them markers for apoptosis [55-57]. The role of pro-inflammatory cytokine (IL-1) and caspase-1 in diabetes-induced mice has shown that caspase-1/IL-1 signalling pathways play an important role in degeneration of retinal capillaries and its inhibition might represent a new strategy to inhibit capillary degeneration in diabetic retinopathy [55]. The increased expression of anti-apoptotic mediator Bcl-2 in the vascular endothelium inhibits the diabetes-induced degeneration of retinal capillaries and superoxide generation [57].
Tumor necrosis factor-α (TNF-α) has a contradictory impact on cells, it may promote cell survival or death, depending on the presence of other molecules. In the ganglion neurons of a healthy human retina normal levels of TNF-α stimulate the activity and expression only of the pro-survival molecules, for example Cox-2 and Akt, but the diabetic retina expressed also molecules involved in the pro-apoptotic pathways: release of mitochondrial cytochrome c, AIF (apoptosis inducing factor), Bad [52]. In cultured retinal neurons TNF-α works primarily through the TNFR-1 and initiates apoptosis via the caspase system [58]. Aside from the caspases, MKK/JNK/Jun pathway is activated in the apoptotic neurons of retinas of humans and rats, ERK/Fos in human retina and Bax system in rat retina [59, 60].
The proposed mechanisms for the role of high glucose concentrations on the cells of the retina are illustrated in Fig. (2).
3. NEUROINFLAMMATION IN THE COURSE OF DIABETES
In the numerous studies on the pathogenesis of diabetic retinopathy more and more attention is paid to inflammatory and angiogenic factors [7, 8, 61-63]. The development and progression of diabetic retinopathy may result on one hand from stimulation of immune cells in metabolically uncontrolled disease and on the other hand from the presence of proinflammatory cytokines, which reflects the ongoing immune processes initiated already in the phase of prediabetes. Animal studies have shown that as early as after one week of diabetes, before any signs of diabetic retinopathy occur, leukocytes start to adhere to the retinal capillaries with subsequent migration into the retina. Leukocyte-endothelial cell adherence is regulated by integrins expressed on the surface of leukocytes as lymphocyte function-associated antigen-1 (LFA-1), very late activation antigen-4 (VLA-4), CD18 and adhesion molecules expressed on the surface of endothelial cells including intercellular adhesion molecule-1 (ICAM-1), vascular cell adhesion molecule 1 (VCAM-1), P-selectins [64, 65]. The leukocyte-endothelial adhesion initiates the process of releasing proinflammatory cytokines, growth factors and vascular permeability factors. Furthermore endothelial tight junctions are being damaged, which facilitates leukocyte diapedesis to the retina [65, 66]. Moreover currently available studies have shown increased serum levels of proinflammatory cytokines in adolescents with T1DM and diabetic retinopathy [17, 29, 66]. Also increased serum interleukin-6 (IL-6) levels were detected in patients with proliferative diabetic retinopathy (PDR) versus patients with T1DM but without PDR [7, 64]. Markedly increased expression of IL-6 and VEGF was found in the aqueous humour of patients with diabetic macular oedema. Other studies have shown a positive correlation between the aqueous levels of IL-6 and macular thickness, which indicates that IL-6 may play a role in the development of diabetic macular oedema [67]. Higher levels of high-mobility group box-1 protein (HMGB1), VEGF, ICAM-1, VCAM-1, soluble vascular endothelial-cadherin (sVE-cadherin), vascular adhesion protein (VAP-1), erythropoietin (EPO), and chemokines have been recently reported in vitreous fluid from PDR patients compared to nondiabetic patients [68-71]. It is speculated that HMGB1 might be an important link between chronic low-grade inflammation and angiogenesis. HMGB1 functions as a proinflammatory cytokine and exhibits angiogenic effects [68].
Study results from the last years suggest that genetically determined higher production of cytokines may significantly affect the onset and progression of diabetic retinopathy [70-73]. So far, several candidate genes have been implicated in the pathogenesis of diabetic retinopathy via complex interactions of environmental and genetic factors. None of them, however, can be fully and solely responsible for the development of DR. Expression of growth factors, such as VEGF, basic fibroblast growth factor (BFGF), and insulin-like growth factor (IGF), is influenced by single-nucleotide polymorphisms (SNPs) in these genes [74-76].
Furthermore, on the basis of the data published so far we can assume that the development of diabetic retinopathy can be affected by VEGF and increased blood pressure [77, 78]. Persistent elevated HbA1c levels is known to lead to the production and accumulation of AGEs. Formation of AGEs promotes production of pro-inflammatory cytokines, which may further increase VEGF levels and thus indirectly lead to the increased blood pressure. This process results in increased expression of adhesion molecules, cytokines and chemokines that enhance endothelial cell proliferation and extracellular matrix formation with concomitant impairment of its degradation. The consequence of these actions is progressive fibrosis and obliteration of the vascular lumen resulting in increased vascular resistance in these vessels. Increased blood flow resistance leads to further increase in VEGF production and potentiates changes in the structure and proportions of collagen and elastin, thereby leading to increased stiffness of blood vessels and progressing hypertension. Dyslipidemia, smoking and lack of physical activity are among the well-established clinical risk factors for the development and progression of diabetic retinopathy however they are beyond the scope of this article [79-81].
4. POTENTIAL APPROACHES TO DELAY NEURONAL LOSS IN THE COURSE OF DIABETES
Neurodegeneration cannot be reversed, therefore preventing neuronal cell loss in the retina need to be developed to protect diabetic patients [14, 82]. During the last years several interventions with possible neuroprotective effects have been investigated, mostly in rats and mice. The effect of exercise on retinal neurons was investigated on the example of rats running on a treadmill. The physical exercise altered the risk of developing diabetic retinopathy: the number of apoptotic cells in the retina was reduced [83] and the level of VEGF was lowered [84].
In another study by Marchetti et al. umbilical cord blood (UCB) derived CD14+ cells were introduced intravitreally into C57Bl/6J mice with oxygen-induced retinopathy [85]. In this model seven-days-old mice are kept in 75% oxygen atmosphere for five days and returned to normal atmosphere. Elevated oxygen concentrations cause the retinal vasculature to degenerate. Under normal atmosphere tissues with degenerated vasculature become hypoxic and are used as a model of hypoxic injury to the retina. At the interface between perfused and non-perfused retina abnormal lesions develop including pre-laminar, neovascular tufts in C57Bl/6J mice. BALB/cByJ mice revascularize more quickly and develop normal vasculature, which makes them a good control in studies of recovery from hypoxic injury. Human UCB-derived CD14+ cells caused that hypoxic injury in the C57Bl/6J retina healed similarly to the BALB/cByJ retina, while CD14- cells offered only modest improvement. Human CD14+ cells were found around the vasculature and matured into macrophage-like morphology. Their antigens were consistent with those of M2 type macrophages. Moreover human UCB-derived CD14+ cells were matured in vitro into M1 and M2 macrophages and subsequently introduced intravitreally to C57Bl/6J mice with oxygen-induced retinopathy. Both populations were able to protect retinal vasculature against obliteration, however only M2 macrophages prevented formation of neovascular tufts. In transcriptomic analysis human cells showed increased expression of proteins associated with response to oxidative stress (e.g. SOD1, HMOX1, GPX1, ALDOA), anti-inflammatory proteins (Stat3, CCR5, TIMP1), proteins promoting cell survival over apoptosis (e.g. TGF-β, AKT1, NF-κB) and myeloid differentiation, while pro-inflammatory TNF-α was downregulated.
Several diabetes drugs were found to protect retinal neurons against apoptosis. The main effect of rosiglitazone involves sensitization of cells to insulin, therefore its effect on retinal neurons' survival is most likely indirect. Rosiglitazone has been shown to provide anti-apoptotic protection for all layers of retinal neurons [86-90]. The recent years have brought new evidence on rosiglitazone that have changed its position. The medicine has been shown to elevate the risks of myocardial infarction and cardiovascular death [87]. As a result it has been withdrawn from the market in some countries while in the others its sale has considerably declined. Discovering new benefits from using the drug may not be enough to balance the risk of cardiovascular diseases.
Another drug with potential effect on retinal neurons is sitagliptin. Its main mode of action is inhibition of GLP-1 and GIP (gastrointestinal hormones) inactivation with subsequently prolonged insulin secretion after meals. Sitagliptin exerts different effects on diabetes control depending on the type of diabetes in animal models but both in type 1 and type 2 Zucker Diabetic Fatty (ZDF) rats it provides protection to the retinas. Its administration results in increased neuronal viability, tighter blood-retina barrier (BRB) integrity and lower level of inflammation [88-90].
Other drugs used in diabetes for reasons other than glycaemic control can also improve retinal health. Cilostazol is commonly used in management of diabetic complications and protects rat retinas against ischemia [91]. Acetylsalicylic acid alleviated microangiopathy in a process different from reducing inflammation and antithrombotic effect [92]. A nonpsychotropic cannabinoid, cannabidiol, reduced BRB breakdown, neuronal apoptosis and inflammation [93]. Propranolol can either reduce or intensify vascular changes depending on the model, but in animal in vivo experiments it caused mostly improvement mediated by reduction of VEGF overexpression. This effect in turn may raise neuronal apoptosis [94].
Herbal preparations that have been investigated in this setting include KIOM-79, Purendan superfine powder and Litsea japonica extract. KIOM-79 and Litsea japonica extract reduced AGE accumulation and in consequence improved neuronal viability; Purendan superfine powder altered cell survival signalling which resulted in lower number of apoptotic retinal ganglion cells [95-97].
CONCLUSION
In conclusion, neurodegeneration, as above mentioned, cannot be reversed and therefore new neuroprotective agents are expected to provide a new strategy of treatment for early stages of diabetic retinopathy. There is a growing need for clinical studies to evaluate the efficacy of noninvasive treatment approaches with neuroprotective drugs. The research on diabetic animal models that developed clinical manifestations of retinopathy is needed as they may provide a correlation of the systemic metabolic profiles and retinal pathology with human studies. In view of the complexity of diabetic retinopathy, it seems likely that combinatory therapy will be required with pathways targeting different cell types at different stages of the disease process. Hopefully in the nearest future neuroprotection will become an effective therapeutic option in the “armoury˝ against diabetic retinopathy.
ACKNOWLEDGEMENTS
This work was supported by The State Committee for Scientific Research ST-108 (Medical University of Gdańsk).
CONFLICT OF INTEREST
The authors confirm that this article content has no conflict of interest.
REFERENCES
- 1.Wild S., Roglic G., Green A., Sicree R., King H. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care. 2004;27(5):1047–1053. doi: 10.2337/diacare.27.5.1047. [DOI] [PubMed] [Google Scholar]
- 2.Hartwig S., Greiser K.H., Medenwald D., Tiller D., Herzog B., Schipf S., Ittermann T., Völzke H., Müller G., Haerting J., Kluttig A. Association of Change of Anthropometric Measurements With Incident Type 2 Diabetes Mellitus: A Pooled Analysis of the Prospective Population-Based CARLA and SHIP Cohort Studies. Medicine (Baltimore) 2015;94(34):e1394. doi: 10.1097/MD.0000000000001394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Imperatore G., Boyle J.P., Thompson T.J., Case D., Dabelea D., Hamman R.F., Lawrence J.M., Liese A.D., Liu L.L., Mayer-Davis E.J., Rodriguez B.L., Standiford D. Projections of type 1 and type 2 diabetes burden in the U.S. population aged <20 years through 2050: dynamic modeling of incidence, mortality, and population growth. Diabetes Care. 2012;35(12):2515–2520. doi: 10.2337/dc12-0669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Triolo T.M., Chase H.P., Barker J.M. Diabetic subjects diagnosed through the Diabetes Prevention Trial-Type 1 (DPT-1) are often asymptomatic with normal A1C at diabetes onset. Diabetes Care. 2009;32(5):769–773. doi: 10.2337/dc08-1872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wierusz-Wysocka B., Zozulińska D., Knast B., Pisarczyk-Wiza D. Pol. Arch. Med. Wewn. 2001;106(3):815–821. [Appearance of undiagnosed diabetes mellitus in the population of professionally active people in the urban areas]. [PubMed] [Google Scholar]
- 6.Pantalone K.M., Hobbs T.M., Wells B.J., Kong S.X., Kattan M.W., Bouchard J., Yu C., Sakurada B., Milinovich A., Weng W., Bauman J.M., Zimmerman R.S. Clinical characteristics, complications, comorbidities and treatment patterns among patients with type 2 diabetes mellitus in a large integrated health system. BMJ Open Diabetes Res. Care. 2015;3(1):e000093. doi: 10.1136/bmjdrc-2015-000093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tomić M., Ljubić S., Kaštelan S., Gverović Antunica A., Jazbec A., Poljičanin T. Inflammation, haemostatic disturbance, and obesity: possible link to pathogenesis of diabetic retinopathy in type 2 diabetes. Mediators Inflamm. 2013 doi: 10.1155/2013/818671. Article ID 818671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wegner M., Araszkiewicz A., Piorunska-Stolzmann M., Wierusz-Wysocka B., Zozulinska-Ziolkiewicz D. Association between IL-6 concentration and diabetes-related variables in DM1 patients with and without microvascular complications. Inflammation. 2013;36(3):723–728. doi: 10.1007/s10753-013-9598-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zorena K., Myśliwska J., Myśliwiec M., Balcerska A., Lipowski P., Raczyńska K. Klin. Oczna. 2007;109(4-6):155–159. [Interleukin-12, vascular endothelial growth factor and tumor necrosis factor-alpha in the process of neoangiogenesis of diabetic retinopathy in children]. [PMID: 17725275]. [PubMed] [Google Scholar]
- 10.Lieth E., Barber A.J., Xu B., Dice C., Ratz M.J., Tanase D., Strother J.M. Glial reactivity and impaired glutamate metabolism in short-term experimental diabetic retinopathy. Diabetes. 1998;47(5):815–820. doi: 10.2337/diabetes.47.5.815. [DOI] [PubMed] [Google Scholar]
- 11.Howell S.J., Mekhail M.N., Azem R., Ward N.L., Kern T.S. Degeneration of retinal ganglion cells in diabetic dogs and mice: relationship to glycemic control and retinal capillary degeneration. Mol. Vis. 2013;19:1413–1421. PMID: 23825921. [PMC free article] [PubMed] [Google Scholar]
- 12.Myśliwska J., Zorena K., Myśliwiec M., Malinowska E., Raczyńska K., Balcerska A. The -174GG interleukin-6 genotype is protective from retinopathy and nephropathy in juvenile onset type 1 diabetes mellitus. Pediatr. Res. 2009;66(3):341–345. doi: 10.1203/PDR.0b013e3181b1bd05. [DOI] [PubMed] [Google Scholar]
- 13.Barber A.J. Diabetic retinopathy: recent advances towards understanding neurodegeneration and vision loss. Sci. China Life Sci. 2015;58(6):541–549. doi: 10.1007/s11427-015-4856-x. [DOI] [PubMed] [Google Scholar]
- 14.Jindal V. Neurodegeneration as a primary change and role of neuro- protection in diabetic retinopathy. Mol. Neurobiol. 2015;51(3):878–884. doi: 10.1007/s12035-014-8732-7. [DOI] [PubMed] [Google Scholar]
- 15.Ola M.S., Alhomida A.S. Neurodegeneration in diabetic retina and its potential drug targets. Curr. Neuropharmacol. 2014;12(4):380–386. doi: 10.2174/1570159X12666140619205024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sutter E.E. Noninvasive Testing Methods: Multifocal Electro- physiology. In: Dartt D.A., editor. Encyclopedia of the Eye. Vol. 3. Oxford: Academic Press; 2010. pp. 142–160. [http://dx.doi.org/10.1016/B978-0-12-374203-2.00226-8] [Google Scholar]
- 17.Semeran K., Pawłowski P., Lisowski Ł., Szczepaniak I., Wójtowicz J., Ławicki S., Bakunowicz-Łazarczyk A., Bossowski A. Plasma levels of IL-17, VEGF, and adrenomedullin and S-cone dysfunction of the retina in children and adolescents without signs of retinopathy and with varied duration of diabetes. Mediators Inflamm. 2013 doi: 10.1155/2013/274726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lung J.C., Swann P.G., Wong D.S., Chan H.H. Global flash multifocal electroretinogram: early detection of local functional changes and its correlations with optical coherence tomography and visual field tests in diabetic eyes. Doc. Ophthalmol. 2012;125(2):123–135. doi: 10.1007/s10633-012-9343-0. [DOI] [PubMed] [Google Scholar]
- 19.Aghdam S.Y., Gurel Z., Ghaffarieh A., Sorenson C.M., Sheibani N. High glucose and diabetes modulate cellular proteasome function: Implications in the pathogenesis of diabetes complications. Biochem. Biophys. Res. Commun. 2013;432(2):339–344. doi: 10.1016/j.bbrc.2013.01.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hammes H.P. Pericytes and the pathogenesis of diabetic retinopathy. Horm. Metab. Res. 2005;37(Suppl. 1):39–43. doi: 10.1055/s-2005-861361]. [DOI] [PubMed] [Google Scholar]
- 21.Simó R., Hernández C. Neurodegeneration is an early event in diabetic retinopathy: therapeutic implications. Br. J. Ophthalmol. 2012;96(10):1285–1290. doi: 10.1136/bjophthalmol-2012-302005. [DOI] [PubMed] [Google Scholar]
- 22.Zorena K., Raczyńska D., Raczyńska K. Biomarkers in diabetic retinopathy and the therapeutic implications. Mediators Inflamm. 2013 doi: 10.1155/2013/193604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mancini J.E., Ortiz G., Croxatto J.O., Gallo J.E. Retinal upregulation of inflammatory and proangiogenic markers in a model of neonatal diabetic rats fed on a high-fat-diet. BMC Ophthalmol. 2013;13:14. doi: 10.1186/1471-2415-13-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yamagishi S., Matsui T. Soluble form of a receptor for advanced glycation end products (sRAGE) as a biomarker. Front. Biosci. (Elite Ed.) 2010;2:1184–1195. doi: 10.2741/e178. [DOI] [PubMed] [Google Scholar]
- 25.Beisswenger P.J. Glycation and biomarkers of vascular complications of diabetes. Amino Acids. 2012;42(4):1171–1183. doi: 10.1007/s00726-010-0784-z. [DOI] [PubMed] [Google Scholar]
- 26.Yan S.F., Yan S.D., Ramasamy R., Schmidt A.M. Tempering the wrath of RAGE: an emerging therapeutic strategy against diabetic complications, neurodegeneration, and inflammation. Ann. Med. 2009;41(6):408–422. doi: 10.1080/07853890902806576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bharadwaj A.S., Appukuttan B., Wilmarth P.A., Pan Y., Stempel A.J., Chipps T.J., Benedetti E.E., Zamora D.O., Choi D., David L.L., Smith J.R. Role of the retinal vascular endothelial cell in ocular disease. Prog. Retin. Eye Res. 2013;32:102–180. doi: 10.1016/j.preteyeres.2012.08.004. [http://dx.doi.org/10.1016/j.preteyeres.2012.08.004]. [PMID: 22982179]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fu S., Dong S., Zhu M., Sherry D.M., Wang C., You Z., Haigh J.J., Le Y.Z. Müller glia are a major cellular source of survival signals for retinal neurons in diabetes. Diabetes. 2015;64(10):3554–3563. doi: 10.2337/db15-0180. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zorena K., Kula M., Malinowska E., Raczyńska D., Myśliwiec M., Raczyńska K. Threshold serum concentrations of tumour necrosis factor alpha (TNFα) as a potential marker of the presence of microangiopathy in children and adolescents with type 1 diabetes mellitus (T1DM). Hum. Immunol. 2013;74(1):75–81. doi: 10.1016/j.humimm.2012.10.002. [http://dx.doi.org/10.1016/j.humimm.2012.10.002]. [PMID: 23073299]. [DOI] [PubMed] [Google Scholar]
- 30.Giacco F., Brownlee M. Oxidative stress and diabetic complications. Circ. Res. 2010;107(9):1058–1070. doi: 10.1161/CIRCRESAHA.110.223545. [http://dx.doi.org/10.1161/CIRCRESAHA.110.223545]. [PMID: 21030723]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kowluru R.A., Chan P.S. Oxidative stress and diabetic retinopathy. 2007. [DOI] [PMC free article] [PubMed]
- 32.Zhang L., Yu C., Vasquez F.E., Galeva N., Onyango I., Swerdlow R.H., Dobrowsky R.T. Hyperglycemia alters the schwann cell mitochondrial proteome and decreases coupled respiration in the absence of superoxide production. J. Proteome Res. 2010;9(1):458–471. doi: 10.1021/pr900818g. [http://dx.doi.org/10.1021/pr900818g]. [PMID: 19905032]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Herlein J.A., Fink B.D., Sivitz W.I. Superoxide production by mitochondria of insulin-sensitive tissues: mechanistic differences and effect of early diabetes. Metabolism. 2010;59(2):247–257. doi: 10.1016/j.metabol.2009.07.021. [http://dx.doi.org/10.1016/j.metabol.2009.07.021]. [PMID: 19765776]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Busik J.V., Mohr S., Grant M.B. Hyperglycemia-induced reactive oxygen species toxicity to endothelial cells is dependent on paracrine mediators. Diabetes. 2008;57(7):1952–1965. doi: 10.2337/db07-1520. [http://dx.doi.org/10.2337/db07-1520]. [PMID: 18420487]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cai R., Xue W., Liu S., Petersen R.B., Huang K., Zheng L. Overexpression of glyceraldehyde 3-phosphate dehydrogenase prevents neurovascular degeneration after retinal injury. FASEB J. 2015;29(7):2749–2758. doi: 10.1096/fj.14-265801. [http://dx.doi.org/10.1096/fj.14-265801]. [PMID: 25805836]. [DOI] [PubMed] [Google Scholar]
- 36.Bandyopadhyay M., Rohrer B. Matrix metalloproteinase activity creates pro-angiogenic environment in primary human retinal pigment epithelial cells exposed to complement. Invest. Ophthalmol. Vis. Sci. 2012;53(4):1953–1961. doi: 10.1167/iovs.11-8638. [http://dx.doi.org/10.1167/iovs. 11-8638]. [PMID: 22408008]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kanwar M., Chan P.S., Kern T.S., Kowluru R.A. Oxidative damage in the retinal mitochondria of diabetic mice: possible protection by superoxide dismutase. Invest. Ophthalmol. Vis. Sci. 2007;48(8):3805–3811. doi: 10.1167/iovs.06-1280. [http://dx.doi.org/10.1167/iovs.06-1280]. [PMID: 17652755]. [DOI] [PubMed] [Google Scholar]
- 38.Li X., Zhang M., Zhou H. The morphological features and mitochondrial oxidative stress mechanism of the retinal neurons apoptosis in early diabetic rats. J. Diabetes Res. 2014 doi: 10.1155/2014/678123. 2014, 678123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jivabhai Patel S., Bany-Mohammed F., McNally L., Valencia G.B., Lazzaro D.R., Aranda J.V., Beharry K.D. Exogenous Superoxide Dismutase Mimetic Without Scavenging H2O2 Causes Photoreceptor Damage in a Rat Model for Oxygen-Induced Retinopathy. Invest. Ophthalmol. Vis. Sci. 2015;56(3):1665–1677. doi: 10.1167/iovs.14-15321. [http://dx.doi.org/10.1167/iovs.14-15321]. [PMID: 25670494]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mandal L.K., Choudhuri S., Dutta D., Mitra B., Kundu S., Chowdhury I.H., Sen A., Chatterjee M., Bhattacharya B. Oxidative stress-associated neuroretinal dysfunction and nitrosative stress in diabetic retinopathy. Can. J. Diabetes. 2013;37(6):401–407. doi: 10.1016/j.jcjd.2013.05.004. [http://dx.doi.org/10.1016/j.jcjd.2013.05.004]. [PMID: 24321721]. [DOI] [PubMed] [Google Scholar]
- 41.Lee J.J., Hsiao C.C., Yang I.H., Chou M.H., Wu C.L., Wei Y.C., Chen C.H., Chuang J.H. High-mobility group box 1 protein is implicated in advanced glycation end products-induced vascular endothelial growth factor A production in the rat retinal ganglion cell line RGC-5. Mol. Vis. 2012;18:838–850. [PMID: 22511847]. [PMC free article] [PubMed] [Google Scholar]
- 42.Magistretti P.J. Role of glutamate in neuron-glia metabolic coupling. Am. J. Clin. Nutr. 2009;90(3):875S–880S. doi: 10.3945/ajcn.2009.27462CC. [DOI] [PubMed] [Google Scholar]
- 43.Orellana J.A., Sáez P.J., Cortés-Campos C., Elizondo R.J., Shoji K.F., Contreras-Duarte S., Figueroa V., Velarde V., Jiang J.X., Nualart F., Sáez J.C., García M.A. Glucose increases intracellular free Ca(2+) in tanycytes via ATP released through connexin 43 hemichannels. Glia. 2012;60(1):53–68. doi: 10.1002/glia.21246. [http://dx.doi.org/10.1002/ glia.21246]. [PMID: 21987367]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang L., Deng Q.Q., Wu X.H., Yu J., Yang X.L., Zhong Y.M. Upregulation of glutamate-aspartate transporter by glial cell line-derived neurotrophic factor ameliorates cell apoptosis in neural retina in streptozotocin-induced diabetic rats. CNS Neurosci. Ther. 2013;19(12):945–953. doi: 10.1111/cns.12150. [http://dx.doi.org/10.1111/cns.12150]. [PMID: 23870489]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Al-Shabrawey M., Smith S. Prediction of diabetic retinopathy: role of oxidative stress and relevance of apoptotic biomarkers. EPMA J. 2010;1(1):56–72. doi: 10.1007/s13167-010-0002-9. [http://dx.doi.org/10.1007/s13167-010-0002-9]. [PMID: 23199041]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pothecary C.A., Thompson H., Salt T.E. Changes in glutamate receptor function in synaptic input to the superficial superior colliculus (SSC) with aging and in retinal degeneration in the Royal College of Surgeons (RCS) rat. Neurobiol. Aging. 2005;26(6):965–972. doi: 10.1016/j.neurobiolaging.2004.07.004. [http://dx.doi.org/10.1016/j.neurobiolaging.2004.07.004]. [PMID: 15718056]. [DOI] [PubMed] [Google Scholar]
- 47.Kannan K., Jain S.K. Oxidative stress and apoptosis. Pathophysiology. 2000;7(3):153–163. doi: 10.1016/s0928-4680(00)00053-5. [http://dx.doi.org/10.1016/ S0928-4680(00)00053-5]. [PMID: 10996508]. [DOI] [PubMed] [Google Scholar]
- 48.Nogueira T.C., Paula F.M., Villate O., Colli M.L., Moura R.F., Cunha D.A., Marselli L., Marchetti P., Cnop M., Julier C., Eizirik D.L. GLIS3, a susceptibility gene for type 1 and type 2 diabetes, modulates pancreatic beta cell apoptosis via regulation of a splice variant of the BH3-only protein Bim. PLoS Genet. 2013;9(5):e1003532. doi: 10.1371/journal.pgen.1003532. [http://dx.doi.org/10.1371/journal.pgen.1003532]. [PMID: 23737756]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Khalfaoui T., Basora N., Ouertani-Meddeb A. Apoptotic factors (Bcl-2 and Bax) and diabetic retinopathy in type 2 diabetes. J. Mol. Histol. 2010;41(2-3):143–152. doi: 10.1007/s10735-010-9271-9. [http://dx.doi.org/10.1007/s10735-010-9271-9]. [PMID: 20532811]. [DOI] [PubMed] [Google Scholar]
- 50.Romeo G., Liu W.H., Asnaghi V., Kern T.S., Lorenzi M. Activation of nuclear factor-kappaB induced by diabetes and high glucose regulates a proapoptotic program in retinal pericytes. Diabetes. 2002;51(7):2241–2248. doi: 10.2337/diabetes.51.7.2241. [http://dx.doi.org/10.2337/ diabetes.51.7.2241]. [PMID: 12086956]. [DOI] [PubMed] [Google Scholar]
- 51.Valverde A.M., Miranda S., García-Ramírez M., González-Rodriguez Á., Hernández C., Simó R. Proapoptotic and survival signaling in the neuroretina at early stages of diabetic retinopathy. Mol. Vis. 2013;19:47–53. [PMID: 23335850]. [PMC free article] [PubMed] [Google Scholar]
- 52.Abu El-Asrar A.M., Dralands L., Missotten L., Geboes K. Expression of antiapoptotic and proapoptotic molecules in diabetic retinas. Eye (Lond.) 2007;21(2):238–245. doi: 10.1038/sj.eye.6702225. [http://dx.doi.org/10.1038/sj.eye.6702225]. [PMID: 16424911]. [DOI] [PubMed] [Google Scholar]
- 53.Yang P., Peairs J.J., Tano R., Zhang N., Tyrell J., Jaffe G.J. Caspase-8-mediated apoptosis in human RPE cells. Invest. Ophthalmol. Vis. Sci. 2007;48(7):3341–3349. doi: 10.1167/iovs.06-1340. [DOI] [PubMed] [Google Scholar]
- 54.Bhattacharya S., Manna P., Gachhui R., Sil P.C. D-saccharic acid-1,4-lactone ameliorates alloxan-induced diabetes mellitus and oxidative stress in rats through inhibiting pancreatic β-cells from apoptosis via mitochondrial dependent pathway. Toxicol. Appl. Pharmacol. 2011;257(2):272–283. doi: 10.1016/j.taap.2011.09.013. [http://dx.doi.org/10.1016/ j.taap.2011.09.013]. [PMID: 21982801]. [DOI] [PubMed] [Google Scholar]
- 55.Mohr S. Caspase-1/interleukin-1beta signaling in diabetic retinopathy. Acta Ophthalmologica. 2008;86:S243. [http://dx.doi.org/10.1111/j.1755-3768.2008.6114.x]. [Google Scholar]
- 56.Ortis F., Cardozo A.K., Crispim D., Störling J., Mandrup-Poulsen T., Eizirik D.L. Cytokine-induced proapoptotic gene expression in insulin-producing cells is related to rapid, sustained, and nonoscillatory nuclear factor-kappaB activation. Mol. Endocrinol. 2006;20(8):1867–1879. doi: 10.1210/me.2005-0268. [http://dx.doi.org/10.1210/me.2005-0268]. [PMID: 16556731]. [DOI] [PubMed] [Google Scholar]
- 57.Kern T.S., Du Y., Miller C.M., Hatala D.A., Levin L.A. Overexpression of Bcl-2 in vascular endothelium inhibits the microvascular lesions of diabetic retinopathy. Am. J. Pathol. 2010;176(5):2550–2558. doi: 10.2353/ajpath.2010.091062. [http://dx.doi.org/10.2353/ajpath.2010.091062]. [PMID: 20363911]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Costa G.N., Vindeirinho J., Cavadas C., Ambrósio A.F., Santos P.F. Contribution of TNF receptor 1 to retinal neural cell death induced by elevated glucose. Mol. Cell. Neurosci. 2012;50(1):113–123. doi: 10.1016/j.mcn.2012.04.003. [http://dx.doi.org/10.1016/j.mcn.2012.04.003]. [PMID: 22522145]. [DOI] [PubMed] [Google Scholar]
- 59.Oshitari T., Brown D., Roy S. SiRNA strategy against overexpression of extracellular matrix in diabetic retinopathy. Exp. Eye Res. 2005;81(1):32–37. doi: 10.1016/j.exer.2005.01.006. [DOI] [PubMed] [Google Scholar]
- 60.Oshitari T., Yamamoto S., Roy S. Increased expression of c-Fos, c-Jun and c-Jun N-terminal kinase associated with neuronal cell death in retinas of diabetic patients. Curr. Eye Res. 2014;39(5):527–531. doi: 10.3109/02713683.2013.833248. [http://dx.doi.org/10.3109/02713683.2013.833248]. [PMID: 24073601]. [DOI] [PubMed] [Google Scholar]
- 61.Rogowicz-Frontczak A., Pilacinski S., Araszkiewicz A., Zozulinska-Ziolkiewicz D., Wykretowicz A., Wierusz-Wysocka B. C-Reactive protein and soluble intracellular adhesion molecule-1 are related to pulse wave reflection in type 1 diabetes 1C-1. J. Diabetes. 2014;6(6):577–585. doi: 10.1111/1753-0407.12129. [http://dx.doi.org/10.1111/1753-0407.12129]. [PMID: 24456036]. [DOI] [PubMed] [Google Scholar]
- 62.Yu Y., Chen H., Su S.B. Neuroinflammatory responses in diabetic retinopathy. J. Neuroinflammation. 2015;12:141. doi: 10.1186/s12974-015-0368-7. [http://dx.doi.org/10.1186/s12974-015-0368-7]. [PMID: 26245868]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Abcouwer S.F., Gardner T.W. Diabetic retinopathy: loss of neuroretinal adaptation to the diabetic metabolic environment. Ann. N. Y. Acad. Sci. 2014;1311:174–190. doi: 10.1111/nyas.12412. [http://dx.doi.org/10.1111/ nyas.12412]. [PMID: 24673341]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Crane I.J., Liversidge J. Mechanisms of leukocyte migration across the blood-retina barrier. Semin. Immunopathol. 2008;30(2):165–177. doi: 10.1007/s00281-008-0106-7. [http://dx.doi.org/10.1007/s00281-008-0106-7]. [PMID: 18305941]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cahoon J.M., Olson P.R., Nielson S., Miya T.R., Bankhead P., McGeown J.G., Curtis T.M., Ambati B.K. Acridine orange leukocyte fluorography in mice. Exp. Eye Res. 2014;120:15–19. doi: 10.1016/j.exer.2013.12.002. [http://dx.doi.org/10.1016/j.exer.2013.12.002]. [PMID: 24333760]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zorena K., Malinowska E., Raczyńska D., Myśliwiec M., Raczyńska K. Serum concentrations of transforming growth factor-Beta 1 in predicting the occurrence of diabetic retinopathy in juvenile patients with type 1 diabetes mellitus. J. Diabetes Res. 2013 doi: 10.1155/2013/614908. 2013, 614908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Oh I.K., Kim S.W., Oh J., Lee T.S., Huh K. Inflammatory and angiogenic factors in the aqueous humor and the relationship to diabetic retinopathy. Curr. Eye Res. 2010;35(12):1116–1127. doi: 10.3109/02713683.2010.510257. [http://dx.doi.org/10.3109/02713683.2010.510257]. [PMID: 21121809]. [DOI] [PubMed] [Google Scholar]
- 68.Abu El-Asrar AM., Nawaz MI., Kangave D., Abouammoh M, Mohammad G. High-mobility group box-1 and endothelial cell angiogenic markers in the vitreous from patients with proliferative diabetic retinopathy. 2012. [DOI] [PMC free article] [PubMed]
- 69.Petrovič M.G., Korošec P., Košnik M., Hawlina M. Vitreous levels of interleukin-8 in patients with proliferative diabetic retino- pathy. Am. J. Ophthalmol. 2007;143(1):175–176. doi: 10.1016/j.ajo.2006.07.032. [DOI] [PubMed] [Google Scholar]
- 70.Hernández C., Fonollosa A., García-Ramírez M., Higuera M., Catalán R., Miralles A., García-Arumí J., Simó R. Erythropoietin is expressed in the human retina and it is highly elevated in the vitreous fluid of patients with diabetic macular edema. Diabetes Care. 2006;29(9):2028–2033. doi: 10.2337/dc06-0556. [http://dx.doi.org/10.2337/dc06-0556]. [PMID: 16936148]. [DOI] [PubMed] [Google Scholar]
- 71.Ersoy L., Ristau T., Hahn M., Karlstetter M., Langmann T., Dröge K., Caramoy A., den Hollander A.I., Fauser S. Genetic and environmental risk factors for age-related macular degeneration in persons 90 years and older. Invest. Ophthalmol. Vis. Sci. 2014;55(3):1842–1847. doi: 10.1167/iovs.13-13420. [http://dx.doi.org/10.1167/iovs.13-13420]. [PMID: 24576882]. [DOI] [PubMed] [Google Scholar]
- 72.Doria A. Genetics of diabetes complications. Curr. Diab. Rep. 2010;10(6):467–475. doi: 10.1007/s11892-010-0147-x. [http://dx.doi.org/10.1007/s11892-010-0147-x]. [PMID: 20835900]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Cilenšek I., Hercegovac A., Vukojević K., Babić M.S., Milutinović Živin A. Polymorphisms of interleukin-4, -10 and 12B genes and diabetic retinopathy. Cent. Eur. J. Biol. 2011;6:558–564. [Google Scholar]
- 74.Petrovič D. Candidate genes for proliferative diabetic retinopathy. BioMed Res. Int. 2013 doi: 10.1155/2013/540416. 2013, 540416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Awata T., Inoue K., Kurihara S., Ohkubo T., Watanabe M., Inukai K., Inoue I., Katayama S. A common polymorphism in the 5′-untranslated region of the VEGF gene is associated with diabetic retinopathy in type 2 diabetes. Diabetes. 2002;51(5):1635–1639. doi: 10.2337/diabetes.51.5.1635. [DOI] [PubMed] [Google Scholar]
- 76.Choudhuri S., Chowdhury I.H., Das S., Dutta D., Saha A., Sarkar R., Mandal L.K., Mukherjee S., Bhattacharya B. Role of NF-κB activation and VEGF gene polymorphisms in VEGF up regulation in non-proliferative and proliferative diabetic retinopathy. Mol. Cell. Biochem. 2015;405(1-2):265–279. doi: 10.1007/s11010-015-2417-z. [http://dx.doi.org/10.1007/s11010-015-2417-z]. [PMID: 25956512]. [DOI] [PubMed] [Google Scholar]
- 77.Barengo N.C., Tuomilehto J.O. Blood pressure treatment target in patients with diabetes mellitus--current evidence. Ann. Med. 2012;44(Suppl. 1):S36–S42. doi: 10.3109/07853890.2012.679961. [http://dx.doi.org/10.3109/07853890.2012.679961]. [PMID: 22713147]. [DOI] [PubMed] [Google Scholar]
- 78.Izzo R., de Simone G., Trimarco V., Gerdts E., Giudice R., Vaccaro O., De Luca N., Trimarco B. Hypertensive target organ damage predicts incident diabetes mellitus. Eur. Heart J. 2013;34(44):3419–3426. doi: 10.1093/eurheartj/eht281. [http://dx.doi.org/10.1093/eurheartj/eht281]. [PMID: 23882068]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zorena K., Myśliwska J., Myśliwiec M., Rybarczyk-Kapturska K., Malinowska E., Wiśniewski P., Raczyńska K. Association between vascular endothelial growth factor and hypertension in children and adolescents type I diabetes mellitus. J. Hum. Hypertens. 2010;24(11):755–762. doi: 10.1038/jhh.2010.7. [http://dx.doi.org/10.1038/ jhh.2010.7]. [PMID: 20164848]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Pons M., Marin-Castaño M.E. Cigarette smoke-related hydro- quinone dysregulates MCP-1, VEGF and PEDF expression in retinal pigment epithelium in vitro and in vivo. PLoS One. 2011;6(2):e16722. doi: 10.1371/journal.pone.0016722. [http://dx.doi.org/10.1371/journal.pone.0016722]. [PMID: 21386905]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Csiszar A., Gautam T., Sosnowska D., Tarantini S., Banki E., Tucsek Z., Toth P., Losonczy G., Koller A., Reglodi D., Giles C.B., Wren J.D., Sonntag W.E., Ungvari Z. Caloric restriction confers persistent anti-oxidative, pro-angiogenic, and anti-inflammatory effects and promotes anti-aging miRNA expression profile in cerebromicrovascular endothelial cells of aged rats. Am. J. Physiol. Heart Circ. Physiol. 2014;307(3):H292–H306. doi: 10.1152/ajpheart.00307.2014. [http://dx.doi.org/10.1152/ajpheart.00307.2014]. [PMID: 24906921]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Uruska A., Araszkiewicz A., Uruski P., Zozulinska-Ziolkiewicz D. Higher risk of microvascular complications in smokers with type 1 diabetes despite intensive insulin therapy. Microvasc. Res. 2014;92:79–84. doi: 10.1016/j.mvr.2014.01.002. [http://dx.doi.org/10.1016/j.mvr.2014.01.002]. [PMID: 24423616]. [DOI] [PubMed] [Google Scholar]
- 83.Lieth E., Gardner T.W., Barber A.J., Antonetti D.A. Retinal neurodegeneration: early pathology in diabetes. Clin. Experiment. Ophthalmol. 2000;28(1):3–8. doi: 10.1046/j.1442-9071.2000.00222.x. [http://dx.doi.org/10.1046/j.1442-9071.2000.00222.x]. [PMID: 11345341]. [DOI] [PubMed] [Google Scholar]
- 84.Kim D.Y., Jung S.Y., Kim C.J., Sung Y.H., Kim J.D. Treadmill exercise ameliorates apoptotic cell death in the retinas of diabetic rats. Mol. Med. Rep. 2013;7(6):1745–1750. doi: 10.3892/mmr.2013.1439. [PMID: 23620139]. [DOI] [PubMed] [Google Scholar]
- 85.Ji E.S., Ko I.G., Cho J.W., Davis R.W., Hwang G.Y., Jee Y.S., Lim B.V. Treadmill exercise inhibits apoptotic neuronal cell death with suppressed vascular endothelial growth factor expression in the retinas of the diabetic rats. J. Exerc. Rehabil. 2013;9(3):348–353. doi: 10.12965/jer.130043. [http://dx.doi.org/10.12965/jer.130043]. [PMID: 24278883]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Marchetti V., Yanes O., Aguilar E., Wang M., Friedlander D., Moreno S., Storm K., Zhan M., Naccache S., Nemerow G., Siuzdak G., Friedlander M. Differential macrophage polarization promotes tissue remodeling and repair in a model of ischemic retinopathy. Sci. Rep. 2011;1:76. doi: 10.1038/srep00076. [http://dx.doi.org/10.1038/ srep00076]. [PMID: 22355595]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Li P., Xu X., Zheng Z., Zhu B., Shi Y., Liu K. Protective effects of rosiglitazone on retinal neuronal damage in diabetic rats. Curr. Eye Res. 2011;36(7):673–679. doi: 10.3109/02713683.2011.572220. [http://dx.doi.org/10.3109/ 02713683.2011.572220]. [PMID: 21599458]. [DOI] [PubMed] [Google Scholar]
- 88.Nissen S.E., Wolski K. Effect of rosiglitazone on the risk of myocardial infarction and death from cardiovascular causes. N. Engl. J. Med. 2007;356(24):2457–2471. doi: 10.1056/NEJMoa072761. [http://dx.doi.org/ 10.1056/NEJMoa072761]. [PMID: 17517853]. [DOI] [PubMed] [Google Scholar]
- 89.Gonçalves A., Leal E., Paiva A., Teixeira Lemos E., Teixeira F., Ribeiro C.F., Reis F., Ambrósio A.F., Fernandes R. Protective effects of the dipeptidyl peptidase IV inhibitor sitagliptin in the blood-retinal barrier in a type 2 diabetes animal model. Diabetes Obes. Metab. 2012;14(5):454–463. doi: 10.1111/j.1463-1326.2011.01548.x. [http://dx.doi.org/10.1111/j.1463-1326.2011.01548.x]. [PMID: 22151893]. [DOI] [PubMed] [Google Scholar]
- 90.Gonçalves A., Marques C., Leal E., Ribeiro C.F., Reis F., Ambrósio A.F., Fernandes R. Dipeptidyl peptidase-IV inhibition prevents blood-retinal barrier breakdown, inflammation and neuronal cell death in the retina of type 1 diabetic rats. Biochim. Biophys. Acta. 2014;1842(9):1454–1463. doi: 10.1016/j.bbadis.2014.04.013. [http://dx.doi.org/10.1016/j.bbadis.2014.04.013]. [PMID: 24769045]. [DOI] [PubMed] [Google Scholar]
- 91.Jung K.I., Kim J.H., Park H.Y., Park C.K. Neuroprotective effects of cilostazol on retinal ganglion cell damage in diabetic rats. J. Pharmacol. Exp. Ther. 2013;345(3):457–463. doi: 10.1124/jpet.113.203067. [http://dx.doi.org/10.1124/jpet.113.203067]. [PMID: 23536314]. [DOI] [PubMed] [Google Scholar]
- 92.Sun W., Gerhardinger C., Dagher Z., Hoehn T., Lorenzi M. Aspirin at low-intermediate concentrations protects retinal vessels in experimental diabetic retinopathy through non-platelet-mediated effects. Diabetes. 2005;54(12):3418–3426. doi: 10.2337/diabetes.54.12.3418. [http://dx.doi.org/10.2337/diabetes.54.12.3418]. [PMID: 16306357]. [DOI] [PubMed] [Google Scholar]
- 93.El-Remessy A.B., Al-Shabrawey M., Khalifa Y., Tsai N.T., Caldwell R.B., Liou G.I. Neuroprotective and blood-retinal barrier-preserving effects of cannabidiol in experimental diabetes. Am. J. Pathol. 2006;168(1):235–244. doi: 10.2353/ajpath.2006.050500. [http://dx.doi.org/10.2353/ ajpath.2006.050500]. [PMID: 16400026]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Casini G., Dal Monte M., Fornaciari I., Filippi L., Bagnoli P. The β-adrenergic system as a possible new target for phar- macologic treatment of neovascular retinal diseases. Prog. Retin. Eye Res. 2014;42:103–129. doi: 10.1016/j.preteyeres.2014.06.001. [http://dx.doi.org/10.1016/j.preteyeres. 2014.06.001]. [PMID: 24933041]. [DOI] [PubMed] [Google Scholar]
- 95.Sohn E.J., Kim Y.S., Kim C.S., Lee Y.M., Kim J.S. KIOM-79 prevents apoptotic cell death and AGEs accumulation in retinas of diabetic db/db mice. J. Ethnopharmacol. 2009;121(1):171–174. doi: 10.1016/j.jep.2008.09.036. [http://dx.doi.org/10.1016/j.jep.2008.09.036]. [PMID: 19013511]. [DOI] [PubMed] [Google Scholar]
- 96.Dong Z., Tao X., Fu X., Wang H., Wang D., Zhang T. Protective effects of Purendan superfine powder on retinal neuron apoptosis in a rat model of type 2 diabetes mellitus. Neural Regen. Res. 2012;7(3):202–206. doi: 10.3969/j.issn.1673-5374.2012.03.008. [PMID: 25767500]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kim J., Kim C.S., Lee Y.M., Sohn E., Jo K., Kim J.S. Litsea japonica extract inhibits neuronal apoptosis and the accumulation of advanced glycation end products in the diabetic mouse retina. Mol. Med. Rep. 2015;12(1):1075–1081. doi: 10.3892/mmr.2015.3543. [PMID: 25815519]. [DOI] [PMC free article] [PubMed] [Google Scholar]


