Abstract
Abstract: Background
The gut-brain axis plays a potential role in numerous physiological and pathological conditions. Several substances link stomach with central nervous system. In particular, hypothalamo-pituitary-adrenocortical axis, thyrotropin-releasing factor-containing nerve fibers and capsaicin-sensitive nerves are principal mediators of the harmful and protective central nervous system-mediated effects on gastric mucosa. Also, existing evidence indicates that nitric oxide, prostaglandins and calcitonin gene-related peptide play a role as final effectors of gastric protection.
Methods
We undertook a structured search of bibliographic databases for peer-reviewed research literature with the aim of focusing on the role of gut-brain axis in gastric damage and protection. In particular, we examined manuscripts dealing with the role of steroids, thyrotropin-releasing hormone, prostaglandins, melatonin, hydrogen sulfide and peptides influencing food intake (i.e. leptin, cholecystokinin, peptide YY, central glucagon–like peptide-1, and ghrelin). Also, the role of GABAergic and glutamatergic pathways in gastric mucosal protection have been examined.
Results
We found and reviewed 61 peer-reviewed papers dealing with the major aspects related to the role of gut brain axis in gastric mucosal damage and protection.
Conclusions
A dense neuronal network links stomach with central nervous system and a number of neurotransmitters and peptides functionally and anatomically related to central nervous system play a major role in contributing to gastric mucosal integrity.
Exploiting the mechanisms underlying the connection between brain and gut may lead to a better understanding of the pathophysiology of gastric mucosal injury and to an improvement in the prevention and, eventually, management of gastric damage.
Keywords: CNS, gastric damage, gastric protection, gut-brain, hydrogen sulfide, melatonin, thyrotropin-releasing hormone
INTRODUCTION
In the last two decades much attention has been focused on the mutual influence of the gastrointestinal tract and Central Nervous System (CNS), the so-called “gut-brain axis”. This communication is made possible by a dense neural network which consists of the afferent nerves sending information from gut to the brain and vice versa. This complex system is formed by a large number of peptides with respective receptors expressed on the afferent terminal in the gut wall. Vagal and spinal nerves are the two main pathways involved in the transmission of stimuli [1]. In this article our attention is directed specifically to the description of the stomach-brain axis.
The stomach is a muscular, hollow, dilated part of the gastrointestinal tract which plays a major role in the initial digestion of food. Sensations such as hunger, satiety and food intake are examples of the connection between the stomach and the brain: the digestive tract senses the luminal contents (volume, wall tensions, acidity, osmolarity) and sends the related information to the CNS. This crosstalk is bidirectional and information coming from the CNS triggered by food may change the secretory activity of the stomach increasing acid production, and may induce the release of gastrointestinal hormones [2].
It is clear that these interactions are active in both physiological and pathological conditions. It is well known that stomach dysfunction could be a risk factor for behavioral disorders in rats [3, 4]. Moreover, psychological distress, as manifested by anxiety and depression, is associated with functional gastrointestinal disorders [5, 6] via limbic system. Furthermore, psychological distress is involved in the regulation of visceral pain. Geeraerts et al. describe an arousal-anxiety-driven failure of pain modulation by means of H2O-positron emission tomography in functional dyspepsia [7].
Gut-brain axis may also be involved in the mechanisms underlying gastric damage and protection. Gastric ulcer can be caused by multiple factors such as Helicobacter pylori (H. pylori) infection, use of nonsteroidal anti-inflammatory drugs (NSAIDs), and smoking. To date, there is no robust evidence indicating that psychological stress may induce gastric lesions. A recent observational 11-years long study, showed a correlation between stress and the onset of ulcer. From 1982 to 1993, 76 patients out of a population of 3379 Danish people developed ulcers. A stress questionnaire was submitted to all participants: the incidence of ulcers was 1.6% in the lowest tertile and 3.5% in the highest tertile (OR 2.2, p = 0.004). Furthermore a multivariate analysis showed that stress, H. pylori infection, smoking and NSAIDs were independent predictors of the onset of ulcer [8]. Similar data had been shown by previous studies which however did not consider altogether most confounding factors, such as NSAIDs, H. pylori infection, and socioeconomic status [9-11]. These results suggest a close link between the brain and the stomach and this is probably made possible through multiple mechanisms.
Gastric Mucosal Protection
Several mechanisms are involved in the ability of the stomach to resist exogenous injury and to recover anatomic and functional integrity after damage. The first line of protection consists of a layer of bicarbonate, mucus gel and surfactant phospholipids. The second line is related to the efficiency of the submucosal blood flow which in turn depends on the production of a number of mediators such as prostaglandins (PGs), sensory neuropeptides and nitric oxide (NO). The ability of the gastric mucosa to resist injury independently of the inhibition of acid secretion is referred to as gastric cytoprotection. For many years, PGs have been regarded to as the only gastric protection mediators. To date it is known that gastric cytoprotection is reduced, or completely abolished, after vagotomy or sympathectomy. This fact demonstrates the presence of additional mediators of protection which are independent of cyclo-oxygenase (COX)-derived products. Generally, we may differentiate the gastric protection activity in PG-dependent (vagus-mediated) and PG-independent (mediated by spinal afferent nerves). In the following paragraphs we analyze in detail the different agents which through the brain-stomach connection may play a role in the maintenance of the integrity of gastric mucosa.
Hypothalamo-Pituitary-Adrenocortical (HPA) System
HPA axis is a major part of the neuroendocrine system that controls reactions to stress and regulates many body processes. For many years its activation was identified as a harmful ulcerogenic factor for strength correlation between glucocorticoids level and gastric ulceration [12, 13]. In the last twenty years, mounting evidence has demonstrated the protective effect of glucocorticoids on the gastric mucosa. The inhibition of stress-induced corticosterone release (i.e., by lesion of hypothalamic paraventricular nucleus, immuno- neutralization of ACTH, cortisol pre-treatment, inhibition of Corticotropin-Releasing Hormone [CRH] synthesis), is associated to the development of gastric erosions in the experimental animal. In particular, Filaretova et al. showed an increase in the number of stress-mediated gastric erosions during glucocorticoid deficiency induced by high dose cortisone through the inhibition of HPA. This effect was reverted by steroid replacement [14, 15]. Stress-induced corticosterone production plays a protective effect on gastric mucosa and it does not cause any damage as it is the case for hormonal therapy at pharmacological doses. The mechanisms underlying the gastroprotective effect of endogenous cortisol are mainly related to the maintenance of submucosal and mucosal blood flow [15], glucose homeostasis during stress, enhancement of mucus production and attenuation of microvascular permeability and gastric motility [16]. Moreover, there are data suggesting a cooperative interaction between glucocorticoids and PGs in gastroprotection. It seems that a deficiency of one protective factor can lead to an apparently compensatory increase of the other [17].
Thyrotropin-releasing Hormone (TRH)
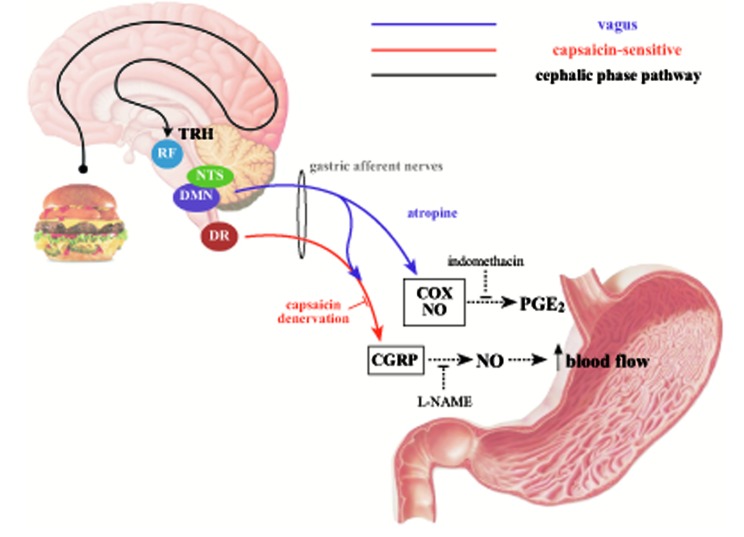
TRH is a trophic hormone, produced by the hypothalamus. TRH stimulates the release of thyroid-stimulating hormone (TSH) and prolactin from the anterior pituitary gland. Its role in gastric protection has been investigated in the past. There are TRH-containing nerve fibers that originate from brainstem nuclei (i.e., raphe pallidus, raphe obscurus and parapyramidal). These fibers innervate the dorsal vagal complex (DVC –formed by dorsal nucleus of the vagus [DMN] and the nucleus tractus solitarius [NTS]) where axons spread to the stomach wall with a direct postsynaptic excitatory action (Fig. 1). In 1994, Tachè et al. showed that injection of high doses of TRH caused vagally mediated gastric injuries similar to those obtained with cold restraint stress. On the other side, an intracisternal injection of a subthreshold dose induced vagally-mediated gastric protection [18]. Also, immune-neutralization of TRH in DVC prevented adaptive gastric protection (i.e., the phenomenon by which pre-treatment with a mild irritant protects the stomach from the subsequent challenge with a strong irritant) thus reinforcing the concept that TRH may play a gastroprotective effect [19]. Recent evidence supports a link between TRH endogenous activation and cephalic phase of digestion. In brief, through diencephalic and telencephalic regions, DVC receives gustatory afferent stimuli from oral cavity. Therefore, these circuits are potential pathways for sensorial cephalic phase information (i.e. of gustatory, olfactory and visual nature) and influence TRH containing fibers in DVC or medullary raphe neurons [20].
To date, it is known that TRH is at the upstream of a peptide cascade that leads, through various routes, to gastric protection. In fact, TRH plays a role in gastric protection both stimulating vagal pathway and recruiting capsaicin-sensitive afferent fibers. This mechanism leads to the release of gastric protection mediators such as PGs, NO and calcitonin gene-related peptide (CGRP) [21].
PGs
PGs are a group of physiologically active lipid compounds having diverse hormone-like effects in animals. They are found in most tissues and organs, are produced by almost all nucleated cells and synthesized in the cell starting from essential fatty acids. It is known that PGs acts on gastric protection trough inhibition of basal and stimulated acid secretion, stimulation of gastric mucus and bicarbonate secretion, and increase in gastric microcirculation. Moreover, PGs stimulate epithelial cell migration and proliferation together with angiogenesis, thus contributing to mucosal healing. PGs are also implicated in adaptative cytoprotection [22]. Although there is evidence of the ability of PGs to modulate catecholaminergic [23], serotoninergic [24], cholinergic [25] and, recently, dopaminergic [26] neurotransmission, the link between central/peripheral PGs and gut-brain axis is not completely clear. In 2005, Dajani et al. concluded that the effects of PGs in the gastrointestinal tract are essentially mediated by direct effects on cells or organs rather than by a direct effect on the CNS [27]. Therefore, local PGs would be the end-effectors of a peptide cascade arising from CNS eventually leading to gastric mucosal protection. On the other hand, Tanaka et al. showed a possible involvement of PGs in the protection against cold restraint stress-induced gastric lesions by prior exposure of the animals to mild stress [28]. The protective effects of preconditioning might also have been contributed to by TRH release.
CGRP
CGRP is the principal effector in capsaicin-sensitive fibers and is widely distributed in the gastrointestinal tract. Cell body of capsaicine-sensitive neurons is contained in dorsal root (DR) ganglions. From there, nervous terminals arise and reach the stomach entering in contact with vagal afferents from DMN. This interaction could explain why pharmacological blockade of peripheral CGRP receptors counteracted the increased gastric mucosal blood flow induced by medullary TRH [29]. It has been reported that nodose ganglion displayed positive immunoreactivity for CGPR supporting the hypothesis that vagal afferents are another source of CGRP [30]. Protective effects on gastric mucosa by CGRP are predominantly mediated by inhibition of acid secretion and increase of gastric mucosal blood. In addition, there are recent evidences of anti-apoptotic, anti-platelet aggregation, anti-oxidant, anti-proliferation and anti-senescence effects of CGRP [31, 32]. An important mediator of CGRP gastroprotective effect is NO. In fact, CGRP increases NO through a decrease in the generation of asymmetric dimethylarginine (ADMA) which is an endogenous NO synthase (NOS) inhibitor [33]. Nitric oxide is released from vascular endothelium, sensory afferent nerves and gastric epithelium, as well. It plays a major role in gastric mucosal protection, mainly by increasing gastric mucosal blood flow.
Melatonin
Melatonin is an indole released by the pineal gland with a circadian rhythm. It is a potent scavenger of reactive oxygen species (ROS) and also attenuates lipid membrane peroxidation-induced mucosal damage. Melatonin is also produced in the gut starting from dietary L-tryptophan through the activity of N-acetytransferase and hydroxylindolo-O-methyltransferase. Melatonin has been reported to exert gastroprotective effects [34]. However, it is not completely clear whether this effect is central or peripheral. In pinealectomized rats there is no change in the melatonin gut concentration and, therefore, central release does not seem to affect the tissue concentration of melatonin in the gut [35]. Otsuka et al. investigated the roles of melatonin and of the pineal gland in the circadian variation of water-immersion restraint stress (WRS)-induced gastric mucosal lesions in rats. Lesion area increased in pinealectomized rats during dark phase, compared to the sham operation; this effect was reversed by intracisternal melatonin pre-administration [36]. Moreover, it is described that intracerebroventricular melatonin injection reduces gastric lesions induced by high dose of TRH [37]. Therefore, melatonin contributes to gastric protection also via a mechanism involving the CNS. Melatonin-dependent protective effect on gastric mucosa is considered to be receptor specific. In fact, this effect is abolished by luzindole, a specific antagonist of melatonin MT2 receptor. In addition to its antioxidant capacity, melatonin action is mediated by activation of PGs and NO with enhancement of gastric microcirculation [38]. Brzozowski et al. showed that indomethacin abolished the protective effect of melatonin, thus supporting the involvement of endogenous PGs in melatonin beneficial effect against gastric injury. The authors also described a reduction of gastric hyperemia induced by melatonin in capsaicin-denervated rats, thus suggesting a CGRP pathway involvement in the attenuation of WRS-induced gastric lesions [39]. Recently, Zhang et al. investigated the effect of melatonin on noise stress-induced gastric ulcer in rats. Rats were exposed to noise stress (120 dB) for 8 hours every day. The authors showed that the area of damaged gastric mucosa was reduced by melatonin treatment respect to untreated control rats. Moreover, melatonin suppressed HPA axis activation and reversed CGRP concentration which was reduced by noise-stress [40]. These data support the hypothesis that melatonin plays a role in gastroprotection and that this effect is mediated by capsaicin sensitive nerves.
Hydrogen Sulfide (H2S)
H2S is an endogenous gas which plays a potential role in systemic inflammation mechanisms. Aboukakr et al. showed, for the first time, that H2S exogenous administration and H2S endogenous release are gastroprotective against cold restraint stress (CRS)-induced gastric injury. NaHS (an H2S donor) attenuated ulcer index by reduction of gastric acid output, mucosal carbonyl content, pepsin activity and ROS generation. H2S also reduced tumoral necrosis factor (TNF)-alfa and myeloperoidase activity [41]. Recently, an attenuation of protective and hyperemic activity of NaHS in rats has been shown following capsaicin denervation. Consequently, it has been suggested that this gaseous mediator activates afferent sensory nerves to release CGRP [42]. Therefore, H2S may be relevant as to the gastroprotection mediated by gut-brain axis.
Peptides Influencing Food Intake
Injection of peptides influencing food intake into the brain (lateral brain ventricle or cisterna magna) may affect gastroprotection. In particular, it seems that cholecystokinin (CCK) and leptin have synergistic interaction in the defense of gastric mucosa. In fact, leptin applied peripherally exerts gastroprotective effects. Also, leptin is released by CCK and mimics CCK action against ethanol injury suggesting that it may mediate the protective and hyperemic action of CCK in the stomach [43].
In addition to adipocytes, placental and gastric production, hypothalamus has been described to express leptin receptor [44] thus being a potential site of leptin action. Brzozowski et al. [45] analyzed effects of this peptide when centrally injected and demonstrated that central CCK and leptin exerted a significant protective effect against ischemia-reperfusion erosions in the stomach. Also, central CCK and leptin injection had different effects on acid gastric secretion, CCK acting as a weak stimulant of gastric secretion (without increase of gastrin levels) while leptin inhibiting gastric-stimulated acid secretion (with enhancement of gastrin). The same authors showed that the protective effect of CCK was accompanied by an elevated plasma leptin level and suggested that enhanced resistance of gastric mucosa against ethanol effect might be mediated by CCK-dependent leptin release [46]. Moreover, capsaicin-induced deactivation of sensory nerves and pretreatment with N-nitro-L-arginine methylester (L-NAME) attenuated leptin and CCK protective activity thus suggesting a role for CGRP and NO in this protective effect.
Peptide YY, another peptide influencing food intake, has been shown to increase gastric resistance to ethanol injury. Kawakubo et al. showed that peptide YY acted trough vagal pathways. Moreover, a synergistic interaction between activation of peptide YY receptor and TRH pathways has been described [47].
Central glucagon–like peptide-1 (GLP-1) injection plays a role in gastric defense decreasing ethanol gastric mucosal damage by 92%. This protective effect is prevented by atropine central injection and peripheral L-NAME administration. Hence, GLP-1 effects on gastric mucosa appear to be dependent on a vagal pathway [48].
Ghrelin also influences gastric mucosa ability to resist injury. Sibilia et al. [49, 50] suggested that NO and PGs represent part of a final common pathway by which central ghrelin injection enables gastric mucosa to withstand the damaging effect of ethanol. Moreover, they showed that ghrelin activates the neuronal circuit involving sensory nerves (CGRP) and NO. In fact, its gastroprotective effects were completely abolished by either capsaicin pretreatment, administration of a CGRP antagonist or by the NOS inhibitor, L-NAME.
Ghrelin plays further roles in the gut-brain axis.During fasting, sympathetic activation (such as that during stress) increases ghrelin secretion by A-like cells in the stomach through norepinephrine or epinephrine stimulation of beta1-adrenergic receptors [51]. Ghrelin is able to cross the blood-brain barrier and acts on brain ghrelin receptors thus affecting HPA axis regulation [52]. Since receptor expression in paraventricular nucleus of the hypothalamus (PVN), apex of the HPA system, is not significant, Kadar et al. suggested that ghrelin regulates the stress response trough different mechanisms and, in particular, by acting indirectly on CRH neurons in PVN and directly in the anterior pituitary gland so to facilitate ACTH release [53]. Through these pathways, ghrelin system may therefore contribute to the development of stress response related to mood disorder. Central ghrelin injection affects the behavior of experimental animals in a time-dependent fashion. Asaka et al. [54] described an anxiogenic effect within 10 minutes of injection. Lutter et al. [55] demonstrated that central ghrelin causes an anxiolytic and antidepressive responses after 45 minutes from the injection.
GABAergic and Glutamatergic Neurotransmission
Gamma aminobutyric acid (GABA) is the most abundant inhibitory neurotransmitter in mammalian brain. Its effects are mediated through the interaction with two receptors, namely GABA(A)R and GABA(B)R. L-glutamate plays a major role in the synaptic excitatory neurotransmission in the CNS and its effects are mediated by the interaction with ionotropic (i.e., α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid [AMPA], and N-methyl-D-aspartate [NMDA] receptors) and metabotropic receptors (i.e. metabotropic glutamate receptors [mGluRs]). Namiki et al. studied the effects of GABA and L-glutamate on gastric secretion and gastric defensive mechanisms in rats by microinfusion of the GABA(A)R agonist muscimol and L-glutamate in lateral hypothalamic area. They found that stimulation of GABAergic pathway increased gastric acid secretion, gastric mucosal blood flow and transepithelial potential difference, an index of gastric mucosal barrier, whereas activation of L-glutamate-dependent pathway only increased gastric mucosal blood flow [56]. Intragastric administration of clonazepam, an agonist at the benzodiazepine sites of GABA(A)R has been shown to attenuate ethanol-induced gastric damage in isolated and perfused rat stomach thus suggesting a local protective effect of GABA(A)R agonists at local GABA(A) receptors located in the rat stomach [57]. Similarly oral administration of the GABA(A)R agonist abamectin has been demonstrated to be protective against ethanol-induced gastric injury in therat [58]. However, the injection of the GABA(B)R agonist baclofen or of the GABA(A)R agonist muscimol into cerebellar fastigial nucleus has recently been shown to exacerbate stress-induced gastric mucosal damage in the rat [59,60]. Finally microinjection of L-glutamate into cerebellar interpositus nucleus has recently been shown to prevent gastric ischemia-reperfusion-induced injury in rats and this effect was associated with a significant increase in gastric mucosal blood flow, together with a decrease in gastric cell apoptosis and increase in gastric cell proliferation [61].
CONCLUSION
The gut-brain axis plays a potential role in many physiological and pathological conditions. In particular, a dense neuronal network links stomach with CNS. Main communication mechanisms involve spinal and vagal system. HPA axis, TRH-containing nerve fibers and capsaicin-sensitive nerves are principal mediators of the ability of CNS to contribute to the maintenance of gastric mucosal integrity. Therefore, NO, PGs and CGPR play a major role as the final effectors of the cascade of events that follow neuronal activation eventually leading to gastric mucosal protection.
While physiological events arising in CNS may affect GI function and, in particular, the gastric mucosal barrier, to date, only limited evidence exists linking stress to gastric mucosa damage. However, many peptides, functionally or anatomically linked to the CNS, exert gastric mucosal protective effects. Exploiting the mechanisms underlying the connection between brain and gut may lead to a better understanding of the pathophysiology of gastric mucosal injury and, as a consequence, to an improvement in the prevention and, eventually, management of gastric damage.
Fig. (1).
Gastric mucosal protection and brain neuropeptides. Gustatory, olfactory and visual informations, by diencephalic and telencephalic projections, reach raphe pallidus and activate thyrotropin-releasing factor (TRH)-containing fibers. TRH stimulates dorsal vagal complex [formed by dorsal nucleus of the vagus (DMN) and nucleus tractus solitarius (NTS)], where vagal-mediated activation of endogenous gastric prostaglandin E2 (PGE2) and nitric oxide (NO) arise. Moreover, vagal afferent pathway recruits calcitonin gene-related peptides (CGRP)-containing capsaicine-sensitive splanchnic gastric afferents. The spinal sensory neurons have their cell bodies in the dorsal root (DR) ganglia and reach the stomach via the splanchnic and mesenteric nerves. In contrast to vagally-mediated gastroprotective effects, protection following stimulation of afferent sensory neurons by capsaicin is independent of the gastric PG system. NO appears to be the final effector of CGRP pathway; in fact, protective effect is blocked by a NO-inhibitor, N-nitro-L-arginine methylester (L-NAME).
ACKNOWLEDGEMENTs
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
LIST OF ABBREVIATIONS
- ADMA
Asymmetric Dimethylarginine
- AMPA
α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid
- CCK
Cholecystokinin
- CGRP
Calcitonin Gene-Related Peptides
- CNS
Central Nervous System
- COX
Cyclo-oxygenase
- CRH
Corticotropin-Releasing Hormone
- CRS
Cold Restraint Stress
- DMN
Dorsal Nucleus of the vagus
- DR
Dorsal Root
- DVC
Dorsal Vagal Complex
- GABA
Gamma aminobutyric acid
- GLP-1
Central Glucagon–Like Peptide-1
- H pylori
Helicobacter Pylori
- H2S
Hydrogen Sulfide
- HPA
Hypothalamo-Pituitary-Adrenocortical
- L-NAME
N-Nitro-L-Arginine Methylester
- mGluRs
Metabotropic Glutamate Receptors
- NMDA
N-methyl-D-aspartate
- NO
Nitric Oxide
- NOS
Nitric Oxide Synthase
- NSAIDs
NonSteroidal Anti-Inflammatory Drugs
- NTS
Nucleus TractusSolitarius
- PGs
Prostaglandins
- PVN
Nucleus Of The Hypothalamus
- ROS
Reactive Oxygen Species
- TNF
Tumoral Necrosis Factor
- TRH
Thyrotropin-Releasing Hormone
- TSH
Thyroid-Stimulating Hormone
- WRS
Water-Immersion Restraint Stress
CONFLICT OF INTEREST
The authors confirm that this article content has no conflict of interest.
REFERENCES
- 1.Kirkup A.J., Brunsden A.M., Grundy D. Receptors and transmission in the brain-gut axis: potential for novel therapies. I. Receptors on visceral afferents. Am. J. Physiol. Gastrointest. Liver Physiol. 2001;280(5):G787–G794. doi: 10.1152/ajpgi.2001.280.5.G787. [PMID: 11292585]. [DOI] [PubMed] [Google Scholar]
- 2.Katschinski M., Dahmen G., Reinshagen M., Beglinger C., Koop H., Nustede R., Adler G. Cephalic stimulation of gastrointestinal secretory and motor responses in humans. Gastroenterology. 1992;103(2):383–391. doi: 10.1016/0016-5085(92)90825-j. [http://dx.doi.org/10. 1016/0016-5085(92)90825-J]. [PMID: 1634057]. [DOI] [PubMed] [Google Scholar]
- 3.Luo J., Wang T., Liang S., Hu X., Li W., Jin F. Experimental gastritis leads to anxiety- and depression-like behaviors in female but not male rats. Behav. Brain Funct. 2013;17:9–46. doi: 10.1186/1744-9081-9-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu L., Li Q., Sapolsky R., Liao M., Mehta K., Bhargava A., Pasricha P.J. Transient gastric irritation in the neonatal rats leads to changes in hypothalamic CRF expression, depression- and anxiety-like behavior as adults. PLoS One. 2011;12:6–e19498. doi: 10.1371/journal.pone.0019498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jones M.P., Coppens E., Vos R., Holvoet L., Luyten P., Tack J., Van Oudenhove L. A multidimensional model of psycho- biological interactions in functional dyspepsia: a structural equation modelling approach. Gut. 2013;62(11):1573–1580. doi: 10.1136/gutjnl-2012-302634. [http://dx.doi. org/10.1136/gutjnl-2012-302634]. [PMID: 22917658]. [DOI] [PubMed] [Google Scholar]
- 6.Clauwaert N., Jones M.P., Holvoet L., Vandenberghe J., Vos R., Tack J., Van Oudenhove L. Associations between gastric sensorimotor function, depression, somatization, and symptom-based subgroups in functional gastroduodenal disorders: are all symptoms equal? Neurogastroenterol. Motil. 2012;24(12):1088–e565. doi: 10.1111/j.1365-2982.2012.01985.x. [http://dx.doi.org/10.1111/j.1365-2982.2012.01985.x]. [PMID: 22816492]. [DOI] [PubMed] [Google Scholar]
- 7.Geeraerts B., Van Oudenhove L., Dupont P., Vanderghinste D., Bormans G., Van Laere K., Tack J. Different regional brain activity during physiological gastric distension compared to balloon distension: a H2 15O-PET study. Neurogastroenterol. Motil. 2011;23(6):533–e203. doi: 10.1111/j.1365-2982.2010.01642.x. [http://dx.doi.org/10.1111/j.1365-2982.2010.01642.x]. [PMID: 21155950]. [DOI] [PubMed] [Google Scholar]
- 8.Levenstein S., Rosenstock S., Jacobsen R.K., Jorgensen T. Psychological stress increases risk for peptic ulcer, regardless of Helicobacter pylori infection or use of nonsteroidal anti-inflammatory drugs. Clin. Gastroenterol. Hepatol. 2015;13(3):498–506.e1. doi: 10.1016/j.cgh.2014.07.052. [http://dx.doi.org/10.1016/j.cgh.2014.07.052]. [PMID: 25111233]. [DOI] [PubMed] [Google Scholar]
- 9.Levenstein S., Kaplan G.A., Smith M. Sociodemographic characteristics, life stressors, and peptic ulcer. A prospective study. J. Clin. Gastroenterol. 1995;21(3):185–192. doi: 10.1097/00004836-199510000-00004. [http://dx.doi.org/ 10.1097/00004836-199510000-00004]. [PMID: 8648050]. [DOI] [PubMed] [Google Scholar]
- 10.Levenstein S., Kaplan G.A., Smith M.W. Psychological predictors of peptic ulcer incidence in the Alameda County Study. J. Clin. Gastroenterol. 1997;24(3):140–146. doi: 10.1097/00004836-199704000-00004. [http://dx.doi.org/ 10.1097/00004836-199704000-00004]. [PMID: 9179731]. [DOI] [PubMed] [Google Scholar]
- 11.Räihä I., Kemppainen H., Kaprio J., Koskenvuo M., Sourander L. Lifestyle, stress, and genes in peptic ulcer disease: a nationwide twin cohort study. Arch. Intern. Med. 1998;158(7):698–704. doi: 10.1001/archinte.158.7.698. [http://dx.doi.org/10.1001/archinte.158.7.698]. [PMID: 9554675]. [DOI] [PubMed] [Google Scholar]
- 12.Weiss J.M. Effects of coping behavior in different warning signal conditions on stress pathology in rats. J. Comp. Physiol. Psychol. 1971;77(1):1–13. doi: 10.1037/h0031583. [http://dx.doi.org/10.1037/h0031583]. [PMID: 5166076]. [DOI] [PubMed] [Google Scholar]
- 13.Murphy H.M., Wideman C.H., Brown T.S. Plasma corticosterone levels and ulcer formation in rats with hippocampal lesions. Neuroendocrinology. 1979;28(2):123–130. doi: 10.1159/000122852. [http://dx.doi.org/ 10.1159/000122852]. [PMID: 431775]. [DOI] [PubMed] [Google Scholar]
- 14.Filaretova L.P., Filaretov A.A. The effect of large corticosteroid doses on stomach ulceration: a new hypothesis. SechenovPhysiol J. 1992;78:77–83. [PubMed] [Google Scholar]
- 15.Filaretova L., Maltcev N., Bogdanov A., Levkovich Y. Role of gastric microcirculation in the gastroprotection by glucocorticoids released during water-restraint stress in rats. Chin. J. Physiol. 1999;42(3):145–152. [PMID: 10707888]. [PubMed] [Google Scholar]
- 16.Filaretova L., Tanaka A., Miyazawa T., Kato S., Takeuchi K. Mechanisms by which endogenous glucocorticoid protects against indomethacin-induced gastric injury in rats. Am. J. Physiol. Gastrointest. Liver Physiol. 2002;283(5):G1082–G1089. doi: 10.1152/ajpgi.00189.2002. [http:// dx.doi.org/10.1152/ajpgi.00189.2002]. [PMID: 12381521]. [DOI] [PubMed] [Google Scholar]
- 17.Filaretova L.P., Podvigina T.T., Bagaeva T.R., Tanaka A., Takeuchi K. Gastroprotective action of glucocorticoid hormones during NSAID treatment. Inflammopharmacology. 2005;13(1-3):27–43. doi: 10.1163/156856005774423746. [http://dx.doi.org/10.1163/156856005774423746]. [PMID: 16259726]. [DOI] [PubMed] [Google Scholar]
- 18.Taché Y., Yoneda M., Kato K., Király A., Sütö G., Kaneko H. Intracisternal thyrotropin-releasing hormone-induced vagally mediated gastric protection against ethanol lesions: central and peripheral mechanisms. J. Gastroenterol. Hepatol. 1994;9(Suppl. 1):S29–S35. doi: 10.1111/j.1440-1746.1994.tb01298.x. [http://dx.doi.org/10.1111/j.1440-1746.1994.tb01298.x]. [PMID: 7881015]. [DOI] [PubMed] [Google Scholar]
- 19.Kato K., Arakawa Y., Kaneko K., Taché Y. Medullary thyrotropin-releasing hormone TRH is involved in gastric adaptive cytoprotection induced by ethanol in urethane anesthetized rats. Gastroenterology. 1995;108:A129. [http://dx.doi.org/10.1016/ 0016-5085(95)23179-X]. [Google Scholar]
- 20.Taché Y., Adelson D., Yang H. TRH/TRH-R1 receptor signaling in the brain medulla as a pathway of vagally mediated gut responses during the cephalic phase. Curr. Pharm. Des. 2014;20(16):2725–2730. doi: 10.2174/13816128113199990578. [http://dx.doi.org/10.2174/13816128113199990578]. [PMID: 23886382]. [DOI] [PubMed] [Google Scholar]
- 21.Taché Y. Brainstem neuropeptides and vagal protection of the gastric mucosal against injury: role of prostaglandins, nitric oxide and calcitonin-gene related peptide in capsaicin afferents. Curr. Med. Chem. 2012;19(1):35–42. doi: 10.2174/092986712803414097. [http://dx.doi.org/10.2174/ 092986712803414097]. [PMID: 22300074]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Konturek S.J., Brzozowski T., Piastucki I., Radecki T., Dembiński A., Dembińska-Kieć A. Role of locally generated prostaglandins in adaptive gastric cytoprotection. Dig. Dis. Sci. 1982;27(11):967–971. doi: 10.1007/BF01391740. [http://dx.doi.org/10.1007/BF01391740]. [PMID: 6754295]. [DOI] [PubMed] [Google Scholar]
- 23.Narumiya S., Ogorochi T., Nakao K., Hayaishi O. Prostaglandin D2 in rat brain, spinal cord and pituitary: basal level and regional distribution. Life Sci. 1982;31(19):2093–2103. doi: 10.1016/0024-3205(82)90101-1. [http://dx.doi.org/ 10.1016/0024-3205(82)90101-1]. [PMID: 6960222]. [DOI] [PubMed] [Google Scholar]
- 24.Brus R., Herman Z.S., Szkilnik R., Zabawska J. Mediation of central prostaglandin effects by serotoninergic neurons. Psychopharmacology (Berl.) 1979;64(1):113–120. doi: 10.1007/BF00427355. [http://dx.doi.org/ 10.1007/BF00427355]. [PMID: 113822]. [DOI] [PubMed] [Google Scholar]
- 25.Saito R., Kamiya H.O., Ono N. Role of the central muscarinic receptor of prostaglandin I2 in cardiovascular function in rat. Brain Res. 1985;330(1):167–169. doi: 10.1016/0006-8993(85)90021-6. [http://dx.doi.org/10.1016/0006-8993 (85)90021-6]. [PMID: 3886074]. [DOI] [PubMed] [Google Scholar]
- 26.Naidu P.S., Kulkarni S.K. Differential effects of cyclooxygenase inhibitors on haloperidol-induced catalepsy. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2002;26(5):819–822. doi: 10.1016/s0278-5846(01)00289-5. [http://dx.doi. org/10.1016/S0278-5846(01)00289-5]. [PMID: 12369252]. [DOI] [PubMed] [Google Scholar]
- 27.Dajani E.Z., Shahwan T.G., Dajani N.E. Prostaglandins and brain-gut axis. J. Physiol. Pharmacol. 2003;54(Suppl. 4):155–164. [PMID: 15075457]. [PubMed] [Google Scholar]
- 28.Tanaka A., Hatazawa R., Takahira Y., Izumi N., Filaretova L., Takeuchi K. Preconditioning stress prevents cold restraint stress-induced gastric lesions in rats: roles of COX-1, COX-2, and PLA2. Dig. Dis. Sci. 2007;52(2):478–487. doi: 10.1007/s10620-006-9394-8. [http://dx.doi.org/10.1007/ s10620-006-9394-8]. [PMID: 17226073]. [DOI] [PubMed] [Google Scholar]
- 29.Kato K., Yang H., Taché Y. Role of peripheral capsaicin-sensitive neurons and CGRP in central vagally mediated gastro- protective effect of TRH. Am. J. Physiol. 1994;266(5 Pt 2):R1610–R1614. doi: 10.1152/ajpregu.1994.266.5.R1610. [PMID: 8203640]. [DOI] [PubMed] [Google Scholar]
- 30.Luo X.J., Liu B., Dai Z., Yang Z.C., Peng J. Stimulation of calcitonin gene-related peptide release through targeting capsaicin receptor: a potential strategy for gastric mucosal protection. Dig. Dis. Sci. 2013;58(2):320–325. doi: 10.1007/s10620-012-2362-6. [PMID: 22918689]. [DOI] [PubMed] [Google Scholar]
- 31.Zhao F.P., Guo Z., Wang P.F. Calcitonin gene related peptide (CGRP) inhibits norepinephrine induced apoptosis in cultured rat cardiomyocytes not via PKA or PKC pathways. Neurosci. Lett. 2010;482(2):163–166. doi: 10.1016/j.neulet.2010.07.025. [http://dx.doi.org/10.1016/j.neulet.2010.07.025]. [PMID: 20650306]. [DOI] [PubMed] [Google Scholar]
- 32.Zhou Z., Hu C.P., Wang C.J., Li T.T., Peng J., Li Y.J. Calcitonin gene-related peptide inhibits angiotensin II-induced endothelial progenitor cells senescence through up-regulation of klotho expression. Atherosclerosis. 2010;213(1):92–101. doi: 10.1016/j.atherosclerosis.2010.08.050. [http://dx. doi.org/10.1016/j.atherosclerosis.2010.08.050]. [PMID: 20832068]. [DOI] [PubMed] [Google Scholar]
- 33.Liu Y.Z., Zhou Y., Li D., Wang L., Hu G.Y., Peng J., Li Y.J. Reduction of asymmetric dimethylarginine in the protective effects of rutaecarpine on gastric mucosal injury. Can. J. Physiol. Pharmacol. 2008;86(10):675–681. doi: 10.1139/y08-073. [http://dx.doi.org/10.1139/ Y08-073]. [PMID: 18841172]. [DOI] [PubMed] [Google Scholar]
- 34.De La Lastra C.A., Cabeza J., Motilva V., Martin M.J. Melatonin protects against gastric ischemia-reperfusion injury in rats. J. Pineal Res. 1997;23(2):47–52. doi: 10.1111/j.1600-079x.1997.tb00334.x. [http://dx.doi.org/10.1111/ j.1600-079X.1997.tb00334.x]. [PMID: 9392441]. [DOI] [PubMed] [Google Scholar]
- 35.Bubenik G.A., Brown G.M. Pinealectomy reduces melatonin levels in the serum but not in the gastrointestinal tract of rats. Biol. Signals. 1997;6(1):40–44. doi: 10.1159/000109107. [http://dx.doi.org/10.1159/000109107]. [PMID: 9098522]. [DOI] [PubMed] [Google Scholar]
- 36.Otsuka M., Kato K., Murai I., Asai S., Iwasaki A., Arakawa Y. Roles of nocturnal melatonin and the pineal gland in modulation of water-immersion restraint stress-induced gastric mucosal lesions in rats. J. Pineal Res. 2001;30(2):82–86. doi: 10.1034/j.1600-079x.2001.300203.x. [http://dx.doi.org/10.1034/ j.1600-079X.2001.300203.x]. [PMID: 11270483]. [DOI] [PubMed] [Google Scholar]
- 37.Kato K., Murai I., Asai S., Komuro S., Matsuno Y., Matsukawa Y., Kurosaka H., Iwasaki A., Ishikawa K., Arakawa Y. Central effect of melatonin against stress-induced gastric ulcers in rats. Neuroreport. 1997;8(9-10):2305–2309. doi: 10.1097/00001756-199707070-00041. [http://dx.doi.org/ 10.1097/00001756-199707070-00041]. [PMID: 9243630]. [DOI] [PubMed] [Google Scholar]
- 38.Brzozowska I., Konturek P.C., Brzozowski T., Konturek S.J., Kwiecien S., Pajdo R., Drozdowicz D., Pawlik M., Ptak A., Hahn E.G. Role of prostaglandins, nitric oxide, sensory nerves and gastrin in acceleration of ulcer healing by melatonin and its precursor, L-tryptophan. J. Pineal Res. 2002;32(3):149–162. doi: 10.1034/j.1600-079x.2002.1o811.x. [http://dx.doi. org/10.1034/j.1600-079x.2002.1o811.x]. [PMID: 12074098]. [DOI] [PubMed] [Google Scholar]
- 39.Brzozowski T., Konturek P.C., Zwirska-Korczala K., Konturek S.J., Brzozowska I., Drozdowicz D., Sliwowski Z., Pawlik M., Pawlik W.W., Hahn E.G. Importance of the pineal gland, endogenous prostaglandins and sensory nerves in the gastroprotective actions of central and peripheral melatonin against stress-induced damage. J. Pineal Res. 2005;39(4):375–385. doi: 10.1111/j.1600-079X.2005.00264.x. [http://dx.doi.org/10.1111/ j.1600-079X.2005.00264.x]. [PMID: 16207293]. [DOI] [PubMed] [Google Scholar]
- 40.Zhang L., Gong J.T., Zhang H.Q., Song Q.H., Xu G.H., Cai L., Tang X.D., Zhang H.F., Liu F.E., Jia Z.S., Zhang H.W. Melatonin Attenuates Noise Stress-induced Gastrointestinal Motility Disorder and Gastric Stress Ulcer: Role of Gastrointestinal Hormones and Oxidative Stress in Rats. J. Neurogastroenterol. Motil. 2015;21(2):189–199. doi: 10.5056/jnm14119. [http://dx.doi.org/10.5056/jnm14119]. [PMID: 25537679]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Aboubakr E.M., Taye A., El-Moselhy M.A., Hassan M.K. Protective effect of hydrogen sulfide against cold restraint stress-induced gastric mucosal injury in rats. Arch. Pharm. Res. 2013;36(12):1507–1515. doi: 10.1007/s12272-013-0194-3. [http://dx.doi.org/10.1007/s12272-013-0194-3]. [PMID: 23812778]. [DOI] [PubMed] [Google Scholar]
- 42.Magierowski M., Jasnos K., Kwiecien S., Drozdowicz D., Surmiak M., Strzalka M., Ptak-Belowska A., Wallace J.L., Brzozowski T. Endogenous prostaglandins and afferent sensory nerves in gastroprotective effect of hydrogen sulfide against stress-induced gastric lesions. PLoS One. 2015;10(3):e0118972. doi: 10.1371/journal.pone.0118972. [http:// dx.doi.org/10.1371/journal.pone.0118972]. [PMID: 25774496]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Brzozowski T., Konturek P.C., Konturek S.J., Pajdo R., Duda A., Pierzchalski P., Bielański W., Hahn E.G. Leptin in gastro- protection induced by cholecystokinin or by a meal. Role of vagal and sensory nerves and nitric oxide. Eur. J. Pharmacol. 1999;374(2):263–276. doi: 10.1016/s0014-2999(99)00314-3. [http://dx.doi.org/10.1016/S0014-2999(99)00314-3]. [PMID: 10422768]. [DOI] [PubMed] [Google Scholar]
- 44.Hâkansson M.L., Brown H., Ghilardi N., Skoda R.C., Meister B. Leptin receptor immunoreactivity in chemically defined target neurons of the hypothalamus. J. Neurosci. 1998;18(1):559–572. doi: 10.1523/JNEUROSCI.18-01-00559.1998. [PMID: 9412531]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Brzozowski T., Konturek P.C., Pajdo R., Kwiecień S., Ptak A., Sliwowski Z., Drozdowicz D., Pawlik M., Konturek S.J., Hahn E.G. Brain-gut axis in gastroprotection by leptin and cholecystokinin against ischemia-reperfusion induced gastric lesions. J. Physiol. Pharmacol. 2001;52(4 Pt 1):583–602. [PMID: 11787760]. [PubMed] [Google Scholar]
- 46.Brzozowski T., Konturek P.C., Konturek S.J., Pierzchalski P., Bielanski W., Pajdo R., Drozdowicz D., Kwiecień S., Hahn E.G. Central leptin and cholecystokinin in gastroprotection against ethanol-induced damage. Digestion. 2000;62(2-3):126–142. doi: 10.1159/000007805. [http://dx.doi.org/10.1159/000007805]. [PMID: 11025360]. [DOI] [PubMed] [Google Scholar]
- 47.Kawakubo K., Yang H., Taché Y. Intracisternal PYY inhibits gastric lesions induced by ethanol in rats: role of PYY-preferring receptors? Brain Res. 2000;854(1-2):30–34. doi: 10.1016/s0006-8993(99)02293-3. [http://dx.doi.org/ 10.1016/S0006-8993(99)02293-3]. [PMID: 10784103]. [DOI] [PubMed] [Google Scholar]
- 48.Isbil-Buyukcoskun N., Gulec G. Investigation of the mechanisms involved in the central effects of glucagon-like peptide-1 on ethanol-induced gastric mucosal lesions. Regul. Pept. 2005;128(1):57–62. doi: 10.1016/j.regpep.2004.12.019. [http://dx.doi.org/10.1016/j.regpep.2004.12.019]. [PMID: 15721488]. [DOI] [PubMed] [Google Scholar]
- 49.Sibilia V., Rindi G., Pagani F., Rapetti D., Locatelli V., Torsello A., Campanini N., Deghenghi R., Netti C. Ghrelin protects against ethanol-induced gastric ulcers in rats: studies on the mechanisms of action. Endocrinology. 2003;144(1):353–359. doi: 10.1210/en.2002-220756. [http://dx.doi.org/10.1210/en.2002-220756]. [PMID: 12488364]. [DOI] [PubMed] [Google Scholar]
- 50.Sibilia V., Pagani F., Rindi G., Lattuada N., Rapetti D., De Luca V. Campanini. N.; Bulgarelli, I.; Locatelli, V.; Guidobono, F.; Netti, C. Central ghrelingastroprotectioninvolvesnitricoxide/ prostaglandin cross-talk. Br. J. Pharmacol. 2008;154:688–697. doi: 10.1038/bjp.2008.120. [http://dx.doi.org/10.1038/bjp.2008.120]. [PMID: 18414388]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhao T.J., Sakata I., Li R.L., Liang G., Richardson J.A., Brown M.S., Goldstein J.L., Zigman J.M. Ghrelin secretion stimulated by beta1-adrenergic receptors in cultured ghrelinoma cells and in fasted mice. Proc. Natl. Acad. Sci. USA. 2010;107(36):15868–15873. doi: 10.1073/pnas.1011116107. [http://dx.doi.org/10.1073/pnas.1011116107]. [PMID: 20713709]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Raspopow K., Abizaid A., Matheson K., Anisman H. Psychosocial stressor effects on cortisol and ghrelin in emotional and non-emotional eaters: influence of anger and shame. Horm. Behav. 2010;58(4):677–684. doi: 10.1016/j.yhbeh.2010.06.003. [http://dx.doi.org/10.1016/j.yhbeh. 2010.06.003]. [PMID: 20540943]. [DOI] [PubMed] [Google Scholar]
- 53.Kádár A., Sánchez E., Wittmann G., Singru P.S., Füzesi T., Marsili A., Larsen P.R., Liposits Z., Lechan R.M., Fekete C. Distribution of hypophysiotropic thyrotropin-releasing hormone (TRH)-synthesizing neurons in the hypothalamic paraventricular nucleus of the mouse. J. Comp. Neurol. 2010;518(19):3948–3961. doi: 10.1002/cne.22432. [http://dx.doi.org/10.1002/cne.22432]. [PMID: 20737594]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Asakawa A., Inui A., Kaga T., Yuzuriha H., Nagata T., Fujimiya M., Katsuura G., Makino S., Fujino M.A., Kasuga M. A role of ghrelin in neuroendocrine and behavioral responses to stress in mice. Neuroendocrinology. 2001;74(3):143–147. doi: 10.1159/000054680. [http://dx.doi.org/10.1159/000054680]. [PMID: 11528215]. [DOI] [PubMed] [Google Scholar]
- 55.Lutter M., Sakata I., Osborne-Lawrence S., Rovinsky S.A., Anderson J.G., Jung S., Birnbaum S., Yanagisawa M., Elmquist J.K., Nestler E.J., Zigman J.M. The orexigenic hormone ghrelin defends against depressive symptoms of chronic stress. Nat. Neurosci. 2008;11(7):752–753. doi: 10.1038/nn.2139. [http://dx.doi.org/10.1038/nn. 2139]. [PMID: 18552842]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Namiki T., Egawa M., Tominaga S., Inoue S., Takamura Y. Effects of GABA and L-glutamate on the gastric acid secretion and gastric defensive mechanisms in rat lateral hypothalamus. J. Auton. Nerv. Syst. 1993;44(2-3):217–223. doi: 10.1016/0165-1838(93)90034-r. [http://dx.doi.org/10.1016/ 0165-1838(93)90034-R]. [PMID: 7901262]. [DOI] [PubMed] [Google Scholar]
- 57.Najim R.A., Saaed H.K. Drugs acting at the GABAA receptor attenuate ethanol-induced gastric mucosal damage in vitro. Clin. Exp. Pharmacol. Physiol. 2003;30(7):495–500. doi: 10.1046/j.1440-1681.2003.03866.x. [http://dx.doi.org/ 10.1046/j.1440-1681.2003.03866.x]. [PMID: 12823265]. [DOI] [PubMed] [Google Scholar]
- 58.Liu M.Y., Chiang J.P., Hsu D.Z., Deng J.F. Abamectin attenuates gastric mucosal damage induced by ethanol through activation of vagus nerve in rats. Alcohol. 2003;30(1):61–65. doi: 10.1016/s0741-8329(03)00094-6. [http:// dx.doi.org/10.1016/S0741-8329(03)00094-6]. [PMID: 12878275]. [DOI] [PubMed] [Google Scholar]
- 59.Zhu J.Z., Fei S.J., Zhang J.F., Zhu S.P., Liu Z.B., Li T.T., Qiao X. Lateral hypothalamic area mediated the aggravated effect of microinjection of Baclofen into cerebellar fastigial nucleus on stress gastric mucosal damage in rats. Neurosci. Lett. 2012;509(2):125–129. doi: 10.1016/j.neulet.2011.12.057. [http://dx.doi.org/10.1016/j.neulet.2011.12.057]. [PMID: 22240102]. [DOI] [PubMed] [Google Scholar]
- 60.Zhu J.Z., Fei S.J., Zhang J.F., Zhu S.P., Liu Z.B., Li T.T., Qiao X. Muscimol microinjection into cerebellar fastigial nucleus exacerbates stress-induced gastric mucosal damage in rats. Acta Pharmacol. Sin. 2013;34(2):205–213. doi: 10.1038/aps.2012.152. [http://dx.doi.org/10.1038/ aps.2012.152]. [PMID: 23247592]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhu S.P., Fei S.J., Zhang J.F., Zhu J.Z., Li Y., Liu Z.B., Qiao X., Li T.T. Lateral hypothalamic area mediated the protective effects of microinjection of glutamate into interpositus nucleus on gastric ischemia-reperfusion injury in rats. Neurosci. Lett. 2012;525(1):39–43. doi: 10.1016/j.neulet.2012.07.035. [http://dx.doi.org/10.1016/j.neulet.2012.07.035]. [PMID: 22842393]. [DOI] [PubMed] [Google Scholar]