Abstract
Diabetic retinopathy is generally considered as a microvascular disease which develops as a result of chronic hyperglycaemia. However, the neuronal apoptosis and reactive gliosis are recently postulated as early changes in diabetic retinopathy. This phenomenon is described as a neurodegeneration and suggests that diabetic retinopathy should be recognized as a neurovascular complication. In this review, we discuss the mechanisms leading to the neurodegeneration of the retina in diabetic patients including: low-grade inflammatory process, oxidative stress, activation of polymorphonuclear neutrophils, glutamate excitotoxicity and imbalance in the neuroprotective factors. Secondly, we point out the clinical significance of measuring the retinal neurodegeneration.
Keywords: Diabetes mellitus, diabetic retinopathy, inflammatory process, neurodegeneration, oxidative stress, retina
INTRODUCTION
Diabetic retinopathy is still the leading cause of blindness in adult population of the Western countries. It is generally considered as a microvascular disease which develops as a result of chronic hyperglycaemia. However, the neuronal apoptosis and reactive gliosis are recently postulated as early changes in diabetic retinopathy. This phenomenon is described as a neurodegeneration and suggests that diabetic retinopathy should be recognized as a neurovascular complication [1-3]. In this review, we discuss the mechanisms leading to the neurodegeneration of the retina in diabetic patients. Secondly, we present the summary of data from clinical studies indicating the importance of measuring the retinal neurodegeneration.
Main Features of the Neurodegeneration in Diabetic Retina
The retina is a structure with several layers of neurons and a monolayer of retinal pigment epithelium situated between the neuroretina and the choroids. The only neurons that are directly sensitive to light are the photoreceptor cells (rods and cones). Neural signals from the rods and cones undergo processing by other neurons of the retina. The output takes the form of action potentials in retinal ganglion cells (RGC) whose axons form the optic nerve. In the course of diabetes the neural apoptosis is firstly detected in RGC. It results in a reduction of the retinal nerve fiber layer. Probably elimination of neurons is preceded by functional abnormalities within the retina [4].
The neurodegeneration is accompanied by the changes in glial cells which consist of macroglia (Müller cells and astrocytes) and microglia. Müller cells play an important role in the glutamate metabolism, extracellular ionic balance and neuronal function. The characteristic changes in glial cells in diabetic patients are called reactive gliosis. This contains overexpression of glial acidic fibrilar protein (GFAP) by Müller cells, impaired astrocyte function, activation of microglial cells which start to release cytokines that contribute to neuronal cell death.
The retinal neural tissue and the glial cells are supplied with oxygen by dual blood circulation. The endothelial cells of the microvessels constitute the blood retinal barrier which protects the neuroretina from the circulatory inflammatory cells and released cytokines. The interaction between all types of cells in the retina are essential for normal vision. Therefore, the metabolic processes taking place in the vessels and neurons in diabetes can not be separated. However, emerging evidence suggests that neurodegeneration precedes overt vascular complications in the retina [2, 3].
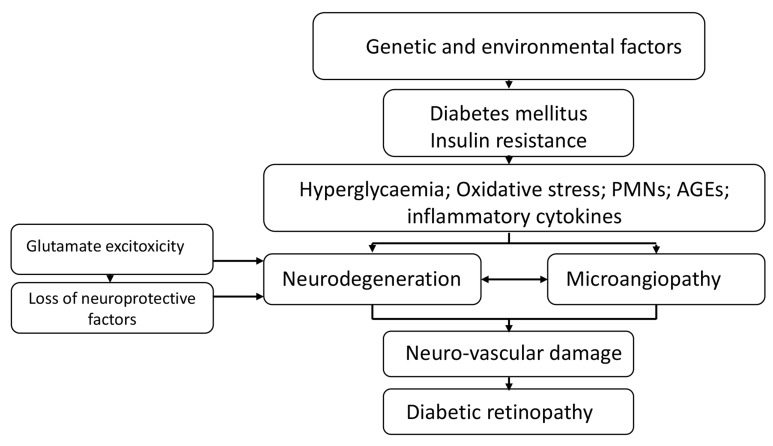
Neurodegeneration as an Early Event in the Pathogenesis of Diabetic Retinopathy-Potential Mechanisms (Fig. 1)
Fig. (1).
The key mechanisms leading to diabetic retinopathy.
Hyperglycaemia, Inflammation and Oxidative Stress
Inflammation is defined as a cascade of phenomena induced in response to different pathological stimuli. It plays an important role in the development of diabetes and its late complications including diabetic retinopathy. Acute and chronic hyperglycaemia and high variability of glycaemia are associated with low grade inflammation and oxidative stress. In hyperglycaemic conditions glucose metabolism is enhanced both by the main and alternative pathways [5]. It results in enhanced glycolysis and mitochondrial overproduction of superoxide anions (O2-) that are a very important substrate for other reactive oxygen species (ROS). Mitochondria are source of endogenous ROS in all retinal cell types, and due to their high reactivity and local production the mitochondrial components such as mtDNA are also the first to be exposed and damaged by ROS. This cause decreasing mitochondrial energy production leading to the onset and progression of retinal degeneration. Moreover, hyperglycaemia results in increased polyol pathway flux leading to accumulation of sorbitol, decreases the NADPH/NADP and increases the NADH/NAD+ ratio. The decline in cellular NADPH may decrease the generation of nitric oxide in cells and alter the cellular redox balance [6]. The hyperglycaemia-induced increase in NADH/NAD ratio is generally characteristic of hypoxia and called “metabolic pseudohypoxia”. All cells of the retina are sensitive to oxygen deficiency.
To counterbalance ROS damage antioxidant defense system exists. However, in retina of diabetic patients with bad glycaemic control, endogenous antioxidants including superoxide dismutase, catalase, glutathione, glutathione reductase and glutathione peroxidase, are not sufficiently effective [7].
Hyperglycaemia enhances non-enzymatic glycosylation (glycation) of proteins and production of poorly reversible early glycation and completely irreversible Advanced Glycation End products (AGEs). AGEs modify intra- and extracellular proteins. Moreover, through specific receptors (RAGE) they activate inflammatory cells such as monocytes, macrophages, endothelial cells [6]. Moreover, they may interact with Müller cells that become additional source of inflammatory cytokines such as VEGF [8]. Biochemical disturbances such as hyperglycaemic pseudohypoxia, glucose auto-oxidation and increased production of AGEs create specific constellation of different molecules with the central position of protein kinase C (PKC) and nuclear factor kappa B (NF-kB) activation [9]. This leads to increased production of proinflammatory cytokines, chemokines and growth factors, causing further damage of retina.
Polymorphonuclear Neutrophils
The peripheral polymorphonuclear neutrophils are one of the main inflammatory cells and seem to significantly influence the damage of endothelium. Mechanisms of the injurious effect of activated neutrophils on endothelium are related to the release of large amounts of reactive oxygen species, proteolytic enzymes and cytokines. Additionally, increased neutrophil aggregation and adherence to endothelium were shown to result in leucoembolisation and capillary plugging with subsequent impairment of blood flow and tissue ischaemia. Physiologically PMNs die by programmed cell death named apoptosis (unstimulated cells) or by necrosis (activated cells) [10,11]. In diabetic patients, an increasing activity of peripheral circulating PMNs was noticed and simultaneously their worse response to stimuli. A number of circulating PMNs with expression of specific receptors CD11b/CD18, reflecting activation of cells, was significantly higher in diabetic patients compared with healthy subjects [12-14]. O2– and H2O2 production by PMNs is rigidly connected with NADPH oxidase activation. It was clearly shown that the activity of this enzyme was increased markedly both in hyperglycaemic and hypoglycaemic conditions and reflects low grade inflammation that plays crucial role in neurodegeneration.
Glutamate Excitotoxicity
Glutamate is a key neurotransmitter in the retina. Müller cells are mainly responsible for glutamate level both intra- and extracellular in retina. There are two metabolic fates of glutamate: amidation to glutamine and conversion by transamination to alfa-ketoglutarate. Numerous studies indicate that excessive levels of glutamate are associated with neuronal cell loss in the central nervous system and retina. The balance of glutamate and glutamine between glial cells and neurons is altered in diabetes and seems to play important role in retinal neurodegeneration. There are several potential mechanisms of this phenomenon in diabetes. Firstly, in hyperglycaemic conditions the activity of glutamine synthetase in Müller cells is reduced. Secondly, glutamate oxidation to alfa-ketoglutarate is imapaired and finally, glutamate uptake by Müller cells is diminished, which leads to an extracellular accumulation of glutamate in the neuroretina [1].
Imbalance in the Neuroprotective Factors Synthesis
Neuronal survival, growth and function depend on the local availability of neurotrophins and growth factors. Dysregulation of neurotrophic factors is considered as the major future of retinal neurodegeneration in the course of diabetes. Retinal factors that exhibit neuroprotective properties include pigment epithelial derived factor (PEDF), somatostatin, interstitial retinol-binding protein (IRBP), brain derived neurotrophic factor (BDNF) and nerve growth factor (NGF). In diabetes, the efficacy and concentration of listed above neuroprotective factors are reduced [15]. Interestingly, neurotrophins such as vascular endothelial growth factor (VEGF), insulin growth factor-1 (IGF-1) and erythropoetin also decrease shortly after diabetes onset, but all increase with the development of proliferative retinopathy. The nature of this biphasic response remains uncertain.
The main source of PEDF and somatostatin is retinal pigment epithelium. Both have antiangiogenic and neuroprotective properties. PEDF protects against oxidative stress and glutamate excitotoxicity [16,17].
IRBP is produced by the photoreceptors and participate in the visual cycle. This factor is essential for the maintenance of the photoreceptors [18]. BDNF and NGF are synthesized and secreted mainly by glial and microglial cells. BDNF provides trophic support for retinal neurons and amacrine cells. Restoring NGF level prevent early apoptosis of neuronal death.
VEGF is a well described factor that play an important role in the development of diabetic macular edema and neovascularization in proliferative diabetic retinopathy. The overexpression of VEGF is accompanied by downregulation of neuroprotective factors, especially PEDF.
All neurotrophins and many growth factors are responsible for functional and structural picture of retina. They have potent effects on neuronal differentiation, survival, neurite outgrowth, synaptic formation, and plasticity [19]. There is emerging evidence that imbalance in the neuroprotective factors synthesis is an early and crucial phenomenon in the diabetic retinal neurodegeneration. These findings are interesting, having clinical implications, but require further research to better understanding.
Retinal Neurodegeneration-Clinical Methods of Detection
A number of different instruments are available for the diagnosis of diseases and disorders affecting the retina. Obviously, various studies have been conducted using animal models of diabetes as well as human donor tissues to explore the molecular mechanisms of retinopathy [20]. In the clinical evaluation ophthalmoscopy and fundus photography are used to examine the retina, however these techniques allow the assessment of microvessels. The electroretinogram is used to measure non-invasively the retina's electrical activity. Whereas, optical coherence tomography (OCT) allows to obtain a 3D volumetric or high resolution cross-sectional tomograms of the retina.
Optical Coherence Tomography (OCT)
Optical coherence tomography is a non-invasive imaging test that uses light waves to take cross-section pictures of the retina. With OCT, each of the retina’s distinctive layers can be seen, allowing to map and measure their thickness. Optical coherence tomography is based on low-coherence interferometry, typically employing near-infrared light. The use of relatively long wavelength light allows it to penetrate into the scattering medium. Image resolutions of 1 to 15 µm can be achieved one to two orders of magnitude higher than conventional ultrasound. Imaging can be performed in situ and in real time.
Multifocal Electroretinogram (mfERG)
The electroretinogram is a measure of electro- physiological activity in the retina that measures changes in field potentials elicited by retinal neurons. The multifocal electroretinogram (mfERG) technique allows local ERG responses to be recorded simultaneously from many regions of the retina.
Neurodegeneration of the Retina In Diabetic Patients-Clinical Significance
Due to introduction of new methods of assessment the changes in the retina, the evaluation of neurodegeneration gained clinical significance. It has been shown in clinical studies in diabetic patients that the measurement of retinal nerve fiber layer (RNFL) and ganglion cell layer (GCL) thickness in OCT could serve as simple early sign of neurodegeneration in diabetic retina. There are studies conducted in type 2 diabetic (DM2) subject that revealed retinal nerve fiber layer defect in patients with early diabetic retinopathy [21]. However, the studies using OCT in type 2 diabetes concentrated mostly on the assessment of macular oedema [22-24]. Cabrera et al. showed reduced RNFL and GCL thickness in diabetic patients with mild retinopathy as compared to subjects without retinopathy [25]. However, the study group of Cabrera et al. was not homogeneous with very wide patients’ age range. The nasal part of RNFL thickness was also significantly thinner in subjects with gestational diabetes mellitus (GDM) than in the healthy pregnant group. Acmaz et al. suggest that it might be the first retinal change in women with GDM [26]. It is suggested that neurodegeneration of the retina is reflects the general pathology of the nerves in diabetes. Therefore, Shahidi et al. aimed to correlate RNFL thickness with the presence of peripheral neuropathy in DM2 patients. The authors found a significant reduction of 1.5 μm for every unit increase in neuropathy disability score for the inferior RNFL quadrant [27]. Probably this quadrant of the retina presents worse adaptation to metabolic stress in diabetes due to its thickness.
The knowledge concerning clinical usefulness of retinal thickness measurement in type 1 diabetes (DM1) is more controversial. Ciresi et al. found no difference between DM1 patients with and without diabetic retinopathy and the control group for all OCT parameters investigated. The authors suggest that retinopathy without macular oedema in type 1 diabetic patients cannot be detected with OCT [28]. Opposite to Ciresi, Biallosterski et al. showed significantly decreased pericentral retinal thickness in patients with retinopathy compared to healthy controls [29]. Similarly, Van Dijk et al. compared DM1 subjects with retinopathy to the control group and showed thinning of the total retina [30]. We have found previously the thinning of the retina, as well as the particular neuroglial layers in type 1 diabetic subjects with retinopathy as compared to DM1 patients without retinopathy [31]. Moreover, the amplitude of oscillatory potentials measured in electroretinogram was reduced in young patients with DM1 and short disease duration before development of vascular changes [32]. Similar results were described in the adolescents by Juen et al. [33].
Interestingly, we have found the negative correlations between thickness of retinal layers in OCT and duration of the disease [31]. The results are consistent with the works of Asefzadeh et al., however conducted in type 2 diabetes and with Biallosterski et al. in DM1. Similarly, in the work of Chihara et al. the risk factors for nerve fiber layer thinning included high systolic blood pressure and patient's age, but not glycated haemoglobin level [21]. Degeneration of the neurons and ganglion cells seems to be a gradual process, progressing with time. It could be optimistic for type 1 diabetic patients that this neurodegeneration is not dominantly dependent on glycaemic control. However, it might be that HbA1c level reflects only mean values of glycaemia from the last three months without showing the fluctuations of glycaemia and is just not a perfect determinant of good metabolic control. There are strong data in the literature that the combination of high metabolic demand and minimal vascular supply may limit the neural inner retina layers’ ability to adapt to the metabolic stress of diabetes [34]. These aspects may partially explain the pathogenesis of neuro-degeneration as an additive to microvascular disease element of diabetic retinopathy as well as the lack of correlation between HbA1c and OCT parameters presented in the literature.
CONCLUSION
In the development of diabetic retinopathy the neurodegenerative processes are involved. All types of cells in the retina are affected, including primarily retinal ganglion cells and glial cells. Since recently, noninvasive precise assessment of the retinal thickness was widely introduced into clinical practice. This could allow for early assessment of the retinal changes in patients with diabetes and will have potential therapeutic implications.
ACKNOWLEDGEMENTS
Declared none.
CONFLICT OF INTEREST
The authors confirm that this article content has no conflict of interest.
REFERENCES
- 1.Stem M.S., Gardner T.W. Neurodegeneration in the pathogenesis of diabetic retinopathy: molecular mechanisms and therapeutic implications. Curr. Med. Chem. 2013;20(26):3241–3250. doi: 10.2174/09298673113209990027. [http://dx.doi.org/10.2174/09298673113209990027]. [PMID: 23745549]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Simó R., Hernández C. Neurodegeneration in the diabetic eye: new insights and therapeutic perspectives. Trends Endocrinol. Metab. 2014;25(1):23–33. doi: 10.1016/j.tem.2013.09.005. [http://dx.doi.org/10.1016/j.tem.2013. 09.005]. [PMID: 24183659]. [DOI] [PubMed] [Google Scholar]
- 3.Gardner T.W., Antonetti D.A., Barber A.J., LaNoue K.F., Levison S.W. Diabetic retinopathy: more than meets the eye. Surv. Ophthalmol. 2002;47(Suppl. 2):S253–S262. doi: 10.1016/s0039-6257(02)00387-9. [http://dx.doi.org/10. 1016/S0039-6257(02)00387-9]. [PMID: 12507627]. [DOI] [PubMed] [Google Scholar]
- 4.Barber A.J. Diabetic retinopathy: recent advances towards understanding neurodegeneration and vision loss. Sci. China Life Sci. 2015;58(6):541–549. doi: 10.1007/s11427-015-4856-x. [http://dx.doi.org/10.1007/s11427-015-4856-x]. [PMID: 25951929]. [DOI] [PubMed] [Google Scholar]
- 5.Sheetz M.J., King G.L. Molecular understanding of hyperglycemia’s adverse effects for diabetic complications. JAMA. 2002;288(20):2579–2588. doi: 10.1001/jama.288.20.2579. [http://dx.doi.org/10.1001/jama.288.20. 2579]. [PMID: 12444865]. [DOI] [PubMed] [Google Scholar]
- 6.Brownlee M. The pathobiology of diabetic complications: a unifying mechanism. Diabetes. 2005;54(6):1615–1625. doi: 10.2337/diabetes.54.6.1615. [http://dx. doi.org/10.2337/diabetes.54.6.1615]. [PMID: 15919781]. [DOI] [PubMed] [Google Scholar]
- 7.Eshaq R.S., Wright W.S., Harris N.R. Oxygen delivery, consumption, and conversion to reactive oxygen species in experimental models of diabetic retinopathy. Redox Biol. 2014;2:661–666. doi: 10.1016/j.redox.2014.04.006. [http://dx. doi.org/10.1016/j.redox.2014.04.006]. [PMID: 24936440]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zong H., Ward M., Madden A., Yong P.H., Limb G.A. [DOI] [PubMed]; Curtis T.M., Stitt A.W. Hyperglycaemia-induced pro-inflammatory responses by retinal Müller glia are regulated by the receptor for advanced glycation end-products (RAGE). Diabetologia. 2010;53(12):2656–2666. doi: 10.1007/s00125-010-1900-z. [http://dx.doi.org/10.1007/s00125-010-1900-z]. [PMID: 20835858]. [DOI] [PubMed] [Google Scholar]
- 9.Zozulinska D., Wierusz-Wysocka B. Hyperglycaemia and Inflammation are culprits of late diabetic complications. Arch. Med. Sci. 2005;1:115–118. [Google Scholar]
- 10.Hirata F., Yoshida M., Ogura Y. High glucose exacerbates neutrophil adhesion to human retinal endothelial cells. Exp. Eye Res. 2006;82(1):179–182. doi: 10.1016/j.exer.2005.08.022. [http://dx.doi.org/10.1016/j.exer.2005. 08.022]. [PMID: 16202408]. [DOI] [PubMed] [Google Scholar]
- 11.Schmid-Schönbein G.W. The damaging potential of leukocyte activation in the microcirculation. Angiology. 1993;44(1):45–56. doi: 10.1177/000331979304400108. [http://dx.doi.org/10.1177/000331979304400108]. [PMID: 8380959]. [DOI] [PubMed] [Google Scholar]
- 12.Wierusz-Wysocka B., Wysocki H., Siekierka H., Wykretowicz A., Szczepanik A., Klimas R. Evidence of polymorphonuclear neutrophils (PMN) activation in patients with diabetes mellitus. J. Leukoc. Biol. 1987;42:519–524. doi: 10.1002/jlb.42.5.519. [PMID: 2824647]. [DOI] [PubMed] [Google Scholar]
- 13.Zozulińska D.A., Wierusz-Wysocka B., Wysocki H., Majchrzak A.E., Wykretowicz A. The influence of insulin-dependent diabetes mellitus (IDDM) duration on superoxide anion and hydrogen peroxide production by polymorphonuclear neutrophils. Diabetes Res. Clin. Pract. 1996;33(3):139–144. doi: 10.1016/0168-8227(96)01289-2. [http://dx.doi.org/10.1016/ 0168-8227(96)01289-2]. [PMID: 8922534]. [DOI] [PubMed] [Google Scholar]
- 14.Grykiel K., Zozulińska D., Kostrzewa A., Wiktorowicz K., Wierusz-Wysocka B. Pol. Arch. Med. Wewn. 2001;105(5):377–381. [Evaluation of expression of polymorpho- nuclear neutrophil surface receptors in patients with type 1 diabetes]. [PMID: 11865589]. [PubMed] [Google Scholar]
- 15.Carmeliet P., Tessier-Lavigne M. Common mechanisms of nerve and blood vessel wiring. Nature. 2005;436(7048):193–200. doi: 10.1038/nature03875. [http://dx.doi.org/10.1038/nature03875]. [PMID: 16015319]. [DOI] [PubMed] [Google Scholar]
- 16.Elahy M., Baindur-Hudson S., Cruzat V.F., Newsholme P., Dass C.R. Mechanisms of PEDF-mediated protection against reactive oxygen species damage in diabetic retinopathy and neuropathy. J. Endocrinol. 2014;222(3):R129–R139. doi: 10.1530/JOE-14-0065. [http://dx. doi.org/10.1530/JOE-14-0065]. [PMID: 24928938]. [DOI] [PubMed] [Google Scholar]
- 17.Kiagiadaki F., Savvaki M., Thermos K. Activation of somatostatin receptor (sst 5) protects the rat retina from AMPA-induced neurotoxicity. Neuropharmacology. 2010;58(1):297–303. doi: 10.1016/j.neuropharm.2009.06.028. [http://dx.doi.org/10.1016/j.neuropharm.2009.06.028]. [PMID: 19576912]. [DOI] [PubMed] [Google Scholar]
- 18.Wu Q., Blakeley L.R., Cornwall M.C., Crouch R.K., Wiggert B.N., Koutalos Y. Interphotoreceptor retinoid-binding protein is the physiologically relevant carrier that removes retinol from rod photoreceptor outer segments. Biochemistry. 2007;46(29):8669–8679. doi: 10.1021/bi7004619. [http://dx.doi.org/10.1021/bi7004619]. [PMID: 17602665]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Whitmire W., Al-Gayyar M.M., Abdelsaid M., Yousufzai B.K., El-Remessy A.B. Alteration of growth factors and neuronal death in diabetic retinopathy: what we have learned so far. Mol. Vis. 2011;17:300–308. [PMID: 21293735]. [PMC free article] [PubMed] [Google Scholar]
- 20.Eisma J.H., Dulle J.E., Fort P.E. Current knowledge on diabetic retinopathy from human donor tissues. World J. Diabetes. 2015;6(2):312–320. doi: 10.4239/wjd.v6.i2.312. [http://dx.doi.org/10.4239/wjd.v6.i2.312]. [PMID: 25789112]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chihara E., Matsuoka T., Ogura Y., Matsumura M. Retinal nerve fiber layer defect as an early manifestation of diabetic retinopathy. Ophthalmology. 1993;100(8):1147–1151. doi: 10.1016/s0161-6420(93)31513-7. [http://dx. doi.org/10.1016/S0161-6420(93)31513-7]. [PMID: 8341494]. [DOI] [PubMed] [Google Scholar]
- 22.Lattanzio R., Brancato R., Pierro L., Bandello F., Iaccher B., Fiore T., Maestranzi G. Macular thickness measured by optical coherence tomography (OCT) in diabetic patients. Eur. J. Ophthalmol. 2002;12(6):482–487. doi: 10.1177/112067210201200606. [PMID: 12510717]. [DOI] [PubMed] [Google Scholar]
- 23.Goebel W., Kretzchmar-Gross T. Retinal thickness in diabetic retinopathy: a study using optical coherence tomography (OCT). Retina. 2002;22(6):759–767. doi: 10.1097/00006982-200212000-00012. [http://dx.doi.org/10.1097/00006982-200212000-00012]. [PMID: 12476103]. [DOI] [PubMed] [Google Scholar]
- 24.Yang C.S., Cheng C.Y., Lee F.L., Hsu W.M., Liu J.H. Quantitative assessment of retinal thickness in diabetic patients with and without clinically significant macular edema using optical coherence tomography. Acta Ophthalmol. Scand. 2001;79(3):266–270. doi: 10.1034/j.1600-0420.2001.790311.x. [http://dx.doi.org/10.1034/j.1600-0420.2001.790311.x]. [PMID: 11401636]. [DOI] [PubMed] [Google Scholar]
- 25.Cabrera DeBuc D., Somfai G.M. Early detection of retinal thickness changes in diabetes using Optical Coherence Tomography. Med. Sci. Monit. 2010;16(3):MT15–MT21. [PMID: 20190693]. [PubMed] [Google Scholar]
- 26.Acmaz G., Atas M., Gulhan A., Acmaz B., Atas F., Aksoy H., Zararsiz G., Gokce G. Assessment of Macular Peripapillary Nerve Fiber Layer and Choroidal Thickness Changes in Pregnant Women with Gestational Diabetes Mellitus, Healthy Pregnant Women, and Healthy Non-Pregnant Women. Med. Sci. Monit. 2015;21:1759–1764. doi: 10.12659/MSM.893221. [http://dx.doi.org/10.12659/MSM.893221]. [PMID: 26084958]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shahidi A.M., Sampson G.P., Pritchard N., Edwards K., Vagenas D., Russell A.W., Malik R.A., Efron N. Retinal nerve fibre layer thinning associated with diabetic peripheral neuropathy. Diabet. Med. 2012;29(7):e106–e111. doi: 10.1111/j.1464-5491.2012.03588.x. [http://dx.doi.org/10.1111/ j.1464-5491.2012.03588.x]. [PMID: 22269030]. [DOI] [PubMed] [Google Scholar]
- 28.Ciresi A., Amato M.C., Morreale D., Morreale R., Di Giovanna F., Carità S., Lodato G., Galluzzo A., Giordano C. OCT is not useful for detection of minimal diabetic retinopathy in type 1 diabetes. Acta Diabetol. 2010;47(3):259–263. doi: 10.1007/s00592-010-0193-5. [http://dx.doi.org/ 10.1007/s00592-010-0193-5]. [PMID: 20454812]. [DOI] [PubMed] [Google Scholar]
- 29.Biallosterski C., van Velthoven M.E., Michels R.P., Schlingemann R.O., DeVries J.H., Verbraak F.D. Decreased optical coherence tomography-measured pericentral retinal thickness in patients with diabetes mellitus type 1 with minimal diabetic retinopathy. Br. J. Ophthalmol. 2007;91(9):1135–1138. doi: 10.1136/bjo.2006.111534. [http://dx.doi.org/10.1136/bjo.2006.111534]. [PMID: 17383994]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.van Dijk H.W., Kok P.H., Garvin M., Sonka M., Devries J.H., Michels R.P., van Velthoven M.E., Schlingemann R.O., Verbraak F.D., Abràmoff M.D. Selective loss of inner retinal layer thickness in type 1 diabetic patients with minimal diabetic retinopathy. Invest. Ophthalmol. Vis. Sci. 2009;50(7):3404–3409. doi: 10.1167/iovs.08-3143. [http://dx.doi.org/10.1167/iovs.08-3143]. [PMID: 19151397]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Araszkiewicz A., Zozulińska-Ziółkiewicz D., Meller M., Bernardczyk-Meller J., Piłaciński S., Rogowicz-Frontczak A., Naskręt D., Wierusz-Wysocka B. Neurodegeneration of the retina in type 1 diabetic patients. Pol. Arch. Med. Wewn. 2012;122(10):464–470. [PMID: 22910230]. [PubMed] [Google Scholar]
- 32.Barber A.J. A new view of diabetic retinopathy: a neuro- degenerative disease of the eye. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2003;27(2):283–290. doi: 10.1016/S0278-5846(03)00023-X. [http://dx.doi.org/10.1016/ S0278-5846(03)00023-X]. [PMID: 12657367]. [DOI] [PubMed] [Google Scholar]
- 33.Juen S., Kieselbach G.F. Electrophysiological changes in juvenile diabetics without retinopathy. Arch. Ophthalmol. 1990;108(3):372–375. doi: 10.1001/archopht.1990.01070050070033. [http://dx.doi.org/10.1001/archopht.1990.01070050070033]. [PMID: 2310337]. [DOI] [PubMed] [Google Scholar]
- 34.Antonetti D.A., Barber A.J., Bronson S.K., Freeman W.M., Gardner T.W., Jefferson L.S., Kester M., Kimball S.R., Krady J.K., LaNoue K.F., Norbury C.C., Quinn P.G., Sandirasegarane L., Simpson I.A. Diabetic retinopathy: seeing beyond glucose-induced microvascular disease. Diabetes. 2006;55(9):2401–2411. doi: 10.2337/db05-1635. [http://dx.doi.org/10.2337/db05-1635]. [PMID: 16936187]. [DOI] [PubMed] [Google Scholar]