Abstract
Neurodegeneration is an initial process in the development of diabetic retinopathy (DR).
High quantities of glutamate, oxidative stress, induction of the renin-angiotensin system (RAS) and elevated levels of RAGE are crucial elements in the retinal neurodegeneration caused by diabetes mellitus. At least, there is emerging proof to indicate that the equilibrium between the neurotoxic and neuroprotective components will affect the state of the retinal neurons.
Somatostatin (SST), pigment epithelium-derived factor (PEDF), and erythropoietin (Epo) are endogenous neuroprotective peptides that are decreased in the eye of diabetic persons and play an essential role in retinal homeostasis. On the other hand, insulin-like growth factor 1 (IGF-1), and vascular endothelial growth factor (VEGF) are pivotal proteins which participate in the development of new capillaries and finally cause damage to the retinal neurons. During recent years, our knowledge about the function of growth factors in the pathogenesis of retinal neurodegeneration has increased. However, intensive investigations are needed to clarify the basic processes that contribute to retinal neurodegeneration and its association with damage to the capillary blood vessels. The objective of this review article is to show new insights on the role of neurotransmitters and growth factors in the pathogenesis of diabetic retinopathy. The information contained in this manuscript may provide the basis for novel strategies based on the factors of neurodegeneration to diagnose, prevent and treat DR in its earliest phases.
Keywords: Diabetic retinopathy, growth factors, neurotransmitter, retinal neurodegeneration
Introduction
1.1. Retinal Neurodegeneration Pathogenesis
Retinal neurodegeneration pathogenesis diabetic retino- pathy (DR) is a microvascular disorder characterised by microaneurysms, capillary perfusion disorders, intraretinal haemorrhages, intraretinal microvascular abnormalities, and neovascularisation [1-3].
Neurodegeneration is the first phase in the development of DR.
Recent studies have identified neuroretinal abnormalities in diabetic patients, before the evidence of visible micro- vascular changes [4-7]. It has been shown in multicentre studies that over 80% of diabetic patients will develop DR within 20 years [8]. In these patients, reduced reactions in full-field and multifocal electroretinography, decreased blue-yellow colour sensitivity, and contrast sensitivity were usually observed before the occurrence of microvascular lesions [4-8].
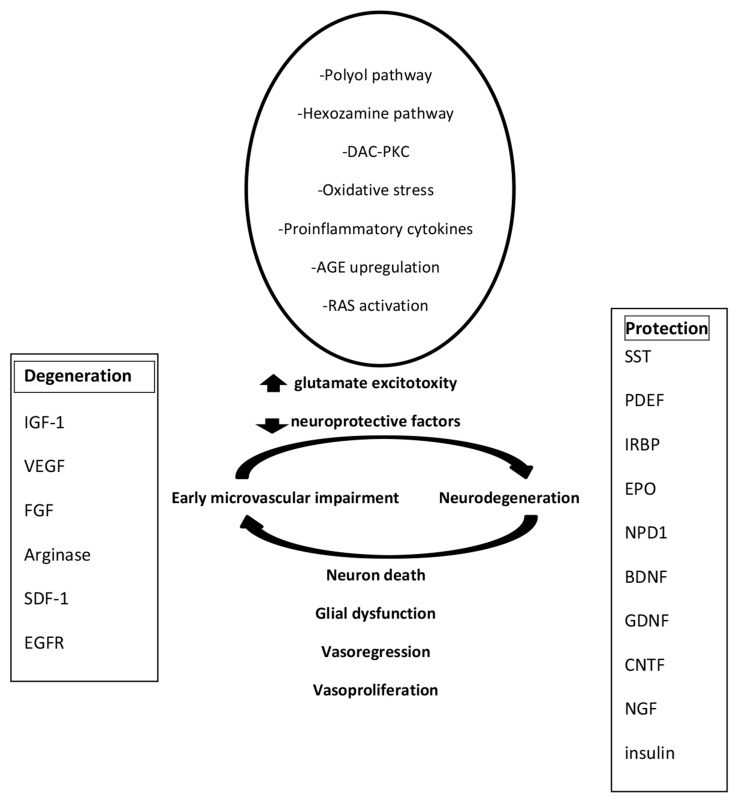
Numerous neuronal cells are detected as damaged in the very early stage of the disease, while microvascular lesions are usually difficult to identify by fundus photography [9]. It is particularly interesting to assess abnormalities in the nerve fibre layer, ganglion cell density, photoreceptor pathological changes, retinal thickness, and evaluation of the extracellular space of the retina [10-12]. Retinal neurodegeneration has also been identified in diabetic rodent models as the very early phase of DR. Rats with streptozotocin-induced type 1 diabetes have exhibited the most accelerated loss of retinal ganglion cells at 8 months after the onset of diabetes [13]. The spontaneous development of diabetes in mice is also associated with changes in neurosensory retina, including apoptosis of retinal ganglion cells as well as marked changes in the pictures of surviving cells, reduction of cholinergic and dopaminergic amacrine cells, and a distinct thinning of the inner plexiform layer and inner nuclear layer [14, 15]. Furthermore, morphological alterations in astrocytes and microglial cells in the inner retina such as impaired glutamate metabolism by Müller cells have been found in rodents [16-18] and mice [19, 20]. Many growth factors participated in pathologic alterations of astrocytes and glial cells and are entailed in the evolution of vascular pathological changes in diabetic retinopathy: vascular endothelial growth factor (VEGF), platelet-derived growth factor (PDGF), insulin-like growth factor (IGF), fibroblast growth factor (FGF), SDF-1, somatostatin, arginase, glutamate, NO, EPO, and others [21-26]. Neural apoptosis is attended by pathological processes in retinal glial cells (astrocytes and Müller cells) described as reactive gliosis.
Currently, it is not clear whether neural apoptosis or reactive gliosis is the initiator of the neurodegenerative changes developed in the retina of diabetic patients. The glial cells play a pivotal role in neuronal survival and normal metabolism. The suggestion that reactive gliosis precedes apoptosis in neural cells is based on the speculation that DR starts from neuronal injury. The biochemical and histo- pathological changes characterised for retinal neuro- degeneration have been noticed in diabetic patients without any pathologic microcirculatory changes in the retinas by ophthalmoscopic examinations [27-29]. The abnormal attitude between survival signalling and pro-apoptotic reactions in the retinas of subjects with diabetes in the initial phases of DR has been described [30]. The neural cells, glial cells, endothelial cells, and pericytes as well as several vasoactive mediators participate in neurovascular inter- actions [31-34]. Nitric oxide (NO) secreted from endothelial cells and neurocytes is involved in process of vasodilatation [32]. The block of NO synthase changes neurovascular complexes [35]. It has been shown that vasodilatation can be reduced by raising NO levels experimentally during suppression of the secretion of vasodilators by glial cells [32]. NO is qualified as a modulator process, rather than a direct mediator [33, 34]. In the literature, other mediators of vasodilatation in the retina have been described e.g. prostanoids, adenosine, ADP, ATP, lactate, glutamate, gamma-aminobutyric acid (GABA), taurine, adrenomedullin (AM), calcitonin gene-related peptide, atrial natriuretic peptide, brain-derived peptide, C-type natriuretic peptide, and retinal relaxing factor [35]. Glial cells play a key function in the haemodynamic processes by the secretion of vasoactive substances [33-39]. Interestingly, at low NO concentrations, glial-induced vasodilating prostanoids are active (i.e., epoxygenase metabolites), whereas vasoconstricting prostanoids (i.e., 20-hydroxyeicosatetraeonic acid) are activated by increased NO levels [35]. The second regulator is the local oxygen concentration; prostanoids can regulate blood flow in association with oxygen concentration, but the mechanism of this process has not been clarified to date [34]. In diabetes mellitus, without microvascular structural changes in the retina, abnormalities in retinal diameter have been reported [40-42]. These observations suggest that the neurovascular unit is damaged at the earliest phase of DR [43]. This problem required studies directed towards the signalling neural or glial cells and the relations between these cells. The mediators of retinal neurodegeneration include
extracellular glutamate accumulation,
oxidative stress,
reduction of neuroprotective substances synthesised by the retina (Fig. 1).
1.2. Diabetic Retinal Neovascularisation
2. Growth factors and neurotrans- mitters involved in the retinal neuro- degeneration
Diabetic retinal neovascularisation is considered a major consequence of retinal ischaemia caused by capillary occlusion similarly to other retinopathies; the mechanism of its development is not clear.
Diabetic retinopathy develops from soft non-proliferative changes, characterised by increased vascular permeability to moderate and severe non-proliferative diabetic retinopathy characterised by vascular obstruction. The next stage is proliferative diabetic retinopathy marked by the growth of new vascular vessels on the retina and posterior surface of the vitreous [10, 11]. Macular oedema defined by retinal thickening from leaky blood vessels can develop at any stage of retinopathy.
The new vascular vessels of DR and contracted concomitant connective tissues deformed the retina and can be cause of partial retinal separation, leading to severe and irreversible vision loss [12]. In DR, many mediators participate in the initial phases of eye complications. Overproduction of advanced glycation end-products through the pathogenesis of diabetes mellitus causes an overload of oxidative stress and activates inflammatory processes. Intensive production of oxygen-free radicals results in a decrease in the thickness of the vessel wall and diminished vascular permeability and elasticity. Inflammation is connected by an increased production of cytokines and is a secondary effect by hyperglycaemia [3-6, 9, 11]. The arterial wall damage in the peripheral and ocular vascular system is caused by chronic inflammation [3-6]. In this pathogenesis, an increase in permeability, leukocyte adhesion, and synthesis of the extracellular matrix is noticed. Inflammation processes lead to the development of retinopathy [3-6]. The prolonged hyperglycaemia causes apoptosis or death of the pericytes and malfunction of the vascular walls. Incompetent blood vessels in the retina characterised swelling. Disturbances of permeability results in the leaking of fluid or blood into the eye, connected with macular oedema or retinal haemorrhages. It is a cause of vision loss. In the late phase of DR, the deficit of oxygen in the retina is observed and stimulates the expression of VEGF and EPO. VEGF and EPO lead to the proliferation of blood vessels, stimulating the growth of new blood vessels on the surface of the retina. The new vessels are fragile, therefore, blood may leak from the ruptured vessels and cause scarring, which can damage eyesight, resulting in blindness [9-11].
2. Growth factors and neurotrans- mitters involved in retinal neurode- generation
2.1. Glutamate
Glutamate is the main stimulating neurotransmitter for the photoreceptor-bipolar-ganglion cell in the retina. Increased glutamate concentration in the retina cause inordinate activation and are entailed in the so-called “excitotoxicity” leading to neurodegeneration [44-46]. The excitotoxicity of glutamate caused the over-activation of ionotropic glutamate receptors, which have been described in rats with streptozotocin-induced diabetes mellitus. 44, 46, 47]. The glutamate, oxidative stress [45-47], advanced glycation end-product receptor regulation [46-48], and renin-angiotensin system activation [44, 48] are pivotal processes in retinal neurodegeneration induced by diabetes mellitus. According to the last investigations, we can suspect that diabetes mellitus–induced down-regulation of neuro- protective factors synthesised by the retina participated in the neurodegenerative process of the DR [49, 50]. On these grounds, we can make the hypothesis that the prevention or arrest of DR development will involve methods based on neuroprotection.
2.2. Somatostatin (SST)
Somatostatin is an endogenous neuroprotective peptide and neurotransmitter that is decreased in the eye structures of diabetic patients [51]. Activation of the glial cells is a major cause of retinal neurodegeneration. Retinal astrocytes in healthy subjects express glial fibrillary acidic protein. In Müller cells, this expression is not noticed. In the course of diabetes mellitus, an abnormal expression of glial fibrillary acidic protein is observed in Müller cells [33]. Müller cells seem to play an important function in the development of diabetic retinal microangiopathy because these cells secrete substances that are capable of regulating blood flow, vascular permeability, and cell survival. Their processes fouled all of the blood vessels in the retina [33, 34]. The human retina secretes SST in significant amounts [52, 53]. The SST-receptors (SSTRs) are also presented in the retina, especially SSTR1 and SSTR2 [54]. SST is an important neuromodulator in the retina: this acts viaintracellular Ca2+-signalling, nitric oxide function, and decreased glutamate secretion from the photoreceptors. SST is a powerful agent against angiogenesis and is involved in the regulation of ion transport and water transport systems [54]. It suspected that SST is crucial in the prevention of both proliferative DR (PDR) and diabetic macular oedema (DME). Eye drops with SST were capable of preventing glial cell activation [51]. Glial activation and apoptosis are the pivotal processes of retinal neurodegeneration [51]. Because SST receptors are expressed on the surface of endothelial cells, SST may directly inhibit angiogenesis [51, 52] and indirectly decrease the production of VEGF or suppress VEGF and other peptide growth factors e.g. IGF-1, epidermal growth factor, and platelet-derived growth factor post-receptor signalling pathways [51]. SST exhibits anti-angiogenic and neuro- protective properties as well, as in the case of PEDF [51, 55]. In PDR and DME the secretion of SST is decreased. In these diseases, low levels of intravitreal SST were noticed [51, 56]. The decrease of SST secretion by the human retina is observed in very early phases of DR and is strictly connected with retinal neurodegeneration [51, 56]. Cortistatin (CST) is a structural and functional neuropeptide that is similar to SST. CST is also decreased in DR [27, 28]. Treatment with SST eye drops has a strong effect in preventing ERG damage, glial cell activation, apoptosis, and abnormal proportions between pro-apoptotic and survival signalling described in rats with STZ-DM. The next observations indicated that SST eye drops decreased glutamate accumulation in the retina inhibited glutamate transporter induction characterised by diabetes mellitus [51]. Topical therapies revolutionised the care of diabetic patients. The phase II-III randomised controlled clinical trial (EUROCONDOR-278040) assessed the efficacy of SST and brimonidine administered topically to prevent or arrest DR [56, 57].
2.3. PEDF-pigment-epithelial-derived Factor
The retinal production of pigment epithelial-derived factor (PEDF), is decreased in the retina of diabetic patients in comparison with non-diabetic subjects.
PDEF production probably has a neuroprotective effect and acts against neurotoxic factors caused by neurodegeneration. The retinal pigment epithelium (RPE) is the main source of PEDF in eye. This peptide is absolutely crucial in retinal homeostasis due to anti-angiogenic and neuroprotective properties. PEDF decreased oxidative stress and glutamate excitotoxicity [7, 57]. In diabetic patients, PEDF down-regulation is observed in the retina; it seems that the factor causes neurodegeneration and could also mediate early microvascular damage [57].
2.4. IRBP-interphotoreceptor retinoid-binding Protein
IRBP is a glycoprotein produced by photoreceptors and extruded into the interphotoreceptor matrix filling the subretinal space [58]. Besides participation in the visual cycle, IRBP is essential in fatty acid transport and plays a very important role in the care of photoreceptors [57-59]. Significantly decreased levels of IRBP have been noticed in the retinas of diabetic patients at the introduction phase of DR, and retinal neurodegeneration is strictly connected with IRBP down-regulation [56, 57].
2.5. EPO-erythropoietin
EPO and its receptor (Epo-R) are both produced by the human retina (especially in retinal pigment epithelium) [60]. In the vitreous fluid of diabetic eyes, a significant amount of EPO was found and its neuroprotective action was observed [60]. EPO is a strong natural stimulator for mobilisation of endothelial progenitor cells (EPCs) and migration to damaged retinal sites. These cells play an important role in the remodelling of injured retinas [60-64]. The elevated levels of VEGF and EPO are connected with the decreased secretion of neuroprotective substances. On the other hand, in advanced stages of DR, overexpression of VEGF or EPO causes neovascularisation. This process is involved in PDR development [61-63].
EPO raises the influence of VEGF. It is described that the high activity of VEGF and EPO might play a role as protective (in the initial phase DR) and degenerative (in advanced phase DR) substations.
2.6. Other Contributing Factors
Other neuroprotective factors e.g. insulin [64,65], neuroprotectin D1 (NPD1) [66], brain-derived neurotrophic factor (BDNF) [67], glial cell line derived neurotrophic factor (GDNF) [68], ciliary neurotrophic factor (CNTF) [69], nerve growth factor (NGF) [70], and adrenomedullin [71] might contribute to the pathogenesis of neurodegenerative processes in DR.
3. Links of retinal neurodegeneration with microvascular abnormalities
3.1. Inflammation
A newly created aim in DR investigations is a problem with the connection between the activation of subclinical inflammation and neurodegeneration. It has been described that Müller cells indicate inflammation-linked reactions in diabetic patients [72-75]. It has recently been demonstrated that up-regulation of the receptor for AGEs (RAGE - receptor for advanced glycation end-products) is very important in the activation of Müller glia cells and cytokine production induced by hyperglycaemia in DR [73]. The mechanism of action of cytokines is not clearly explained. This cytokine probably participates in neural apoptosis and may contribute to the induction of excitotoxicity, oxidative stress, or mitochondrial dysfunction [75]. Previous investiga- tions indicate that activation of the renin–angiotensin system (RAS) may be crucial in the retinal neurodegeneration developed in diabetic milieu [76-78].
3.2. IGF1-Insulin-like Growth Factor 1
IGF-1 is secreted by numerous cells of the retina: vascular endothelial cells, pericytes, glial cells, retinal ganglion cells, and retinal pigment epithelium [79-81]. IGF-1R (IGF-1 receptor) is expressed in retinal pigment epithelium cells and retinal endothelial cells. Activation of IGF-1R by IGF-1 stimulates the hypoxia-inducible factor 1a protein synthesis and increases the expression of VEGF. The IGF-1 participated in the activation of VEGF in human RPE cells [80, 81]. VEGF is a significant agent contributing to the formation of a novel capillary vessel. IGF-1 is involved in the regulation, growth, maturation and functioning of blood vessels [80, 81]. IGF-1 is a polypeptide hormone that is produced and secreted in the liver and fibroblasts and chondrocytes; it has a similar structure and function to insulin. Insulin increased the secretion of IGF-1 [79]. In healthy subjects, insulin is the main hormone regulating the level of glucose in the blood, and IGF-1 has a subsidiary function.
In diabetic patients, treatment with insulin or oral hypoglycaemic drugs should be a classical treatment for control of blood glucose levels. There were experimental therapies in which IGF-1 was applied in the case of extreme insulin resistance or insensitivity. It was hoped that recombinant IGF-1 treatment might be other method lead to decrease glycaemia and may prevent acute complications of diabetes [80-82]. Unfortunately, treatment with IGF-1 has significant complications: swelling of the optic nerve behind the eye and headaches. The observation of side effects prevent treatment with IGF-1 [81, 82]. The second cause of very careful treatment with IGF-1 and limitation of this is the mitogenic effect. Investigations have shown numerous incidences of proliferative DR as a result of treatment with IGF-1. Nowadays, it has been explained that IGF-1 has an important function in the pathogenesis of DR. IGF-1 during long-term treatment may exacerbate retinal deterioration by promotion of the proliferation of retinal endothelial cells. IGF-1 increased the secretion and action of vascular endothelial growth factor and erythropoietin.
By coupling IGF-1 with IGF-1 receptors, various signalling pathways associated with DR are activated - the phosphatidylinositol 3-kinase/protein kinase B pathway, mitogen-activated protein kinase pathway, and nuclear factor-κB signalling pathway [83]. In animals, the administration of IGF-1 through intravitreal or intracorneal injections accelerates neovascular changes in the retina and cornea. In diabetic subjects with DR, increased levels of IGF-1 have been noticed in the vitreous fluid [84]. These investigations have shown the detrimental activity of IGF-1.
According to our knowledge, in the eye, IGF-1 receptors are found in numerous cells, and treatment with an IGF-1 receptor antagonist [81, 82], with blockade of the action of IGF-1, impedes the development of microvascular changes in the retina [83-89]. Like the effect of IGF-1 on VEGF, IGF-1 induces hypoxia-inducible factor-1, resulting in elevated mRNA levels and the overproduction of EPO [90]. This process probably participates in the development of new blood vessels. The biological function of IGF-1 is to act on IGF-1R and activation of PI3K and several intracellular kinases. PI3K plays an important function in the induction of growth and proliferation of vascular smooth muscle cells. The inhibition of PI3K is caused by wortmannin. This process was observed by a reduction of the early replication of vascular smooth muscle cells (VSMCs) in rats [91]. In normal endothelial cells, IGF-1 might also be efficient in the prevention of apoptosis before new and abnormal capillary vessels are formed.
The mitotic effect of IGF-1 is the main cause of the growth and proliferation of vascular endothelial cells. In this situation, the use of recombinant IGF-1 as a therapeutic agent may cause complications of DM with DR [83]. IGF1 and IGF1R are involved in the pathogenesis or progression of proliferative vitreoretinal disorders [92].
3.3. VEGF-Vascular Endothelial Growth Factor
VEGF is necessary for physiological vascular development and has an important function in maintaining the integrity of endothelial cells, because it is involved in anti-apoptotic signalling. It is a main pathogenic factor for DME and PDR. Moreover, VEGF may have neuroprotective effects. In recent research, following the injection of an antibody that blocks all VEGF isoforms in rats, a dose-dependent decrease in ganglion cells has been reported [93-98]. Other authors of experimental studies have not described any significant neural damage in VEGF knockout mice after blocking the phosphorylation of VEGF receptors in transgenic mice with the sustained expression of VEGF in the photoreceptors [98, 99]. The influence of VEGF on retinal neuroprotection should be clarified in further investigations. Angiogenesis, a process of the development of new capillary networks from pre-existing vessels is a characteristic process of proliferative diabetic retinopathy [94]. Numerous investigations show that vasculogenesis, i.e. de novo formation of blood vessels from circulating bone marrow-derived endothelial progenitor cells (EPCs), can participate in neovascularisation [94, 95]. The circulating bone marrow-derived EPCs that migrate to the ischaemic region differentiate into mature endothelial cells in situ, leading to neovascularisation [93]. In many investigations, it was shown that bone marrow-derived CD133 EPCs are involved in new vessel formation in PDR fibrovascular epiretinal membranes [94, 95]. In angiogenesis and vasculo- genesis, several cytokines/chemokines and their associated tyrosine kinase receptors contribute. A pivotal factor in both of these processes is VEGF [96, 97].
VEGF binds with high affinity and activates two tyrosine kinase receptors, VEGFR-1 (Flt-1) and VEGFR-2 (KDR in humans/Flk-1 in mice) [98]. These receptors participate in the regulation of physiological and pathological angio- genesis. VEGFR-2 is expressed mainly on vascular endothelial cells [98]. VEGFR-2 is also expressed by bone marrow-derived circulating EPCs. VEGFR-2 has intensive tyrosine kinase activity and is the main positive signal transducer for pathological angiogenesis in neoplasm and DR [98]. Activa- tion of VEGFR-2 stimulates endothelial cell proliferation, migration and survival, angiogenesis and microvascular permeability [98]. VEGFR-2 has a short soluble form (sVEGFR-2) that has been reported in mouse and human plasma [99, 100]. Recent evidence shows that the decrease of VEGF, sVEGFR-2, and SCF concentration in the vitreous fluid from patients with PDR are associated with angiogenesis and vasculogenesis in DR [101].
3.4. Fibroblasts Growth Factor (FGF)
The fibroblast growth factor in normal tissue is detected in basement membranes and in the subendothelial extracellular matrix of blood vessels [102]. It is suspected that the action of heparan sulphate-degrading enzymes activates bFGF, and inducing the formation of new blood vessels (angiogenesis) may have an important role in wound healing of normal tissues and during tumour development [102, 103]. FGF is produced by human adipocytes and a correlation between the levels of bFGF and BMI was observed in blood samples. Moreover, bFGF also contributed to the proliferation of preosteoblasts [102, 103]. The fibroblast growth factor family consists of 22 members with a wide range of biological functions in organisms, such as cell growth, development, angiogenesis, and wound healing [104] FGF21 is a member of the endocrine FGF subfamily, which is expressed predominantly in the liver and stimulates glucose uptake through the induction of GLUT1 in adipocytes [105]. The application of FGF21 leads to decreased glucose levels and modulates lipid metabolism in both murine and nonhuman primate models of diabetes and obesity [106]. These investigations have shown that FGF21 has an essential function in the regulation of glucose and lipid metabolism. FGF21 may be used for the effective treatment of diabetes and obesity in the future. Latest investigations have indicated increased serum concentrations of FGF21 in obese subjects and patients with metabolic syndrome and type 2 diabetes mellitus [107, 108]. FGF21 is probably involved in the pathogenesis of retinopathy in diabetic patients but its role is unclear. In patients with type 2 diabetes, increased serum FGF21 levels were noticed, which are higher in patients with diabetic retinopathy than in those without it and connected with the development of diabetes and DR. The prospective studies with greater numbers of patients can explain the relationship between serum FGF21 concentrations and the severity of vascular complications [109, 110].
3.5. Arginase
Vascular epithelial cells contain endothelial nitric oxide synthase (eNOS), an enzyme that hydrolyses L-arginine to form L-citrulline and NO [111]. NO contributes to healthy vascular function as a main signalling molecule [111]. NO induces blood flow by activating guanylyl cyclase within the vascular smooth muscle cells, which leads to the dilation of vessels [112]. The normal production of NO by eNOS is required for the metabolism of healthy vessels, proper blood flow, prevention of leucocyte adhesion and platelet aggregation, and control of smooth muscle cell growth [112]. A decrease in bioavailable NO is a major factor of endothelial cell dysfunction and its implications are shown in diabetic vascular disease [112]. NOS produces NO from its substrate L-arginine. If the level of L-arginine is insufficient, uncoupling of the NOS homodimer can stimulate it to produce superoxide [113]. Superoxide can react with NO to form peroxynitrite, thus reducing levels of NO and increasing oxidative stress [114].
Two isoforms have been described: arginase I is localised in the cytosol and arginase II exists in the mitochondria. Arginase is expressed in epithelial cells. The increase of arginase protein levels and/or activity influenced vascular dysfunction in hypertension, ischaemia reperfusion, ageing, and diabetes [115]. The function of arginase in the DR pathway has not been investigated. Excessive arginase activity has been found to reduce NO production by reducing the L-arginine supply for eNOS [116-118]. Ornithine is a metabolite of arginase activity. It can be further metabolised into polyamines and proline, which are necessary for cell proliferation and collagen synthesis, respectively. Increases in their levels have been connected with vascular remodelling and stiffness [119, 120].
The most recognised feature of retinopathy is retinal neovascularisation, but neuronal dysfunction is also observed. Alterations in polyamine metabolism are involved in neurodegeneration in various diseases. Polyamines participate in the pathogenesis of ischaemic brain damage [121-123]. Arginase activity is increased in various pathologies characterised by vascular dysfunction: diabetes, hypertension and ischaemia-reperfusion injury [122, 123]. High arginase activity and polyamine overproduction have been connected to retinal ganglion cell death due to excessive activation of excitotoxic NMDA receptors and hyperoxia-mediated neuronal death [124-130]. The deletion of arginase strongly reduces retinal degeneration and improves retinal function following hyperoxia treatment in the mouse model of oxygen retinopathy [131]. The mechanism of this neuroprotective effect has been described: deletion of arginase II reduces neuro-glial damage and significantly improves retinal function [131, 132]. Arginase I is a potential therapeutic target for the treatment of DR using arginase inhibitors [133]. Arginase has been linked to DR and is the enzyme with increasing interest in endothelial dysfunction [133].
3.6. SDF-1-Stromal Cell-derived Factor-1
Stromal cell-derived factor-1 (SDF-1) is a CXC-chemokine which participates in haematopoiesis. Mice lacking SDF-1 or its receptor CXCR4 die in the foetal period, after developing defects in various organs including the heart, brain, large vessels, and bone marrow. In bone marrow, endothelial cells and stromal cells showed the expression of SDF-1. This protein recruits hematopoietic stem cells to the bone marrow niche, but also supports their survival and proliferation [134, 135]. SDF-1 and the receptor CXCR4 stimulate bone marrow-derived cells to neovascularisation and regeneration sites in the heart, liver and eye [136, 137]. SDF-1 levels are increased in the vitreous in ischaemic ocular diseases, such as proliferative diabetic retinopathy (PDR) and retinopathy of prematurity, retinal vein occlusion [138, 139]. This factor induces the expression of VCAM-1 adhesion molecules in the eye epithelial cells and reduces expression of intramembrane occludin proteins. The level of SDF-1 and its receptor CXCR4 is regulated by VEGF [140]. SDF-1/CXCR4 contributed to the ocular inflammation process by enrolling CD4 T-cells, and is potentially engaged in the formation of proliferative membranes in patients with proliferative vitreoretinopathy [141]. SDF-1 has both beneficial and adverse effects in DR, such as neuro-protection or inflammatory cell accumulation, respectively. Endogenous SDF-1 is a tissue-protective role in RD. Elucidation of the mechanisms underlying the role of SDF-1 in neural protection will support the development of safe and effective treatments [142].
3.7. EGFR-Epidermal Growth Factor Receptor
Inactivation of A disintegrin and metalloproteinase 17 (ADAM17), a membrane-anchored metalloproteinase, which is involved in cleaving ligands of the epidermal growth factor receptor (EGFR) and regulating EGFR signalling, reduces neovascularisation [143-145]. The tissue inhibitor of metalloproteinases-3 (TIMP3) acts as a natural inhibitor of ADAM17 [146-149] and inhibits the release of ligands of EGFR [146-149]. TIMP3 belongs to the family of tissue inhibitors of matrix metalloproteinases (TIMPs) and can be immobiliszed in the extracellular matrix [150]. Mice lacking TIMP3 develop pathologies that can be explained by an increase in the activity of ADAM17, e.g. an enhanced inflammatory response with increased TNFα activity [150, 151]. TIMP3 with ADAM17 regulates angiogenesis in three-dimensional tissue culture assays [151]. The inactivation of ADAM17 in endothelial cells prevents pathological retinal neovascularisation and the growth of heterotopically-injected tumours in mice [152, 153]. The investigations with Timp3−/− mice have demonstrated that TIMP3 regulates choroidal neovascularisation, as well as VEGF-induced corneal neovascularisation and laser-induced choroidal neovas- cularisation. In these mice, the delivery of TIMP3 by adeno-associated viral vectors has been shown to ameliorate ischaemia-induced neovascularisation [152-154]. In addition, TIMP3 may regulate angiogenesis by binding directly to the VEGF receptor 2 (VEGFR2) [152-154]. Moreover, the impact of intravitreal injection of the EGFR inhibitor erlotinib on neovascularisation in the OIR model in wild-type mice has been described [154, 155]. The intravitreally injected TIMP3 probably blocked tuft formation by reducing ADAM17 activity [152-155]. VEGF-A has a very intensive effect in mouse OIR, [156-160] and investigations have shown that VEGF-A/VEGFR2 signalling activates ADAM17 to induce EGFR and stimulate the migration of endothelial cells [154, 155]. Since the injection of TIMP3 might also affect the binding of VEGF-A to VEGFR2, the injection of IMP3 treats pathological neovascularisation by the inhibition of two pivotal components of this pathway: binding of VEGF-A to VEGFR2 and the activation of ADAM17 [161, 162].
4. Therapy strategies
A classical treatment method in diabetes retinopathy is laser therapy. Immunotherapy targeting VEGF was revolutionary in the treatment of DR. Trials of these immunotherapies documented that intraocular injections of anti-VEGF agents are better than laser therapy in preserving and improving vision in DME patients [57]. The anti-VEGF agents ranibizumab, bevacizumab, pegaptanib, and aflibercept have recently been used. Both laser treatment and anti-VEGF antibodies-intraocular injections could be aggregated. In patients receiving combined ranibizumab and laser therapy, the best long-term visual improvement could be achieved with the initiation of injections followed by laser therapy [57]. Currently, nationwide studies by groups such as the Diabetic Retinopathy Clinical Research Network (DRCRnet) explained the role of ocular anti-VEGF therapy for PDR. Intravitreous injections of anti-VEGF agents is an invasive procedure and could even have harmful effects on healthy retina. Besides local side effects, anti-VEGF agents can produce systemic complications due to their capacity to pass into systemic circulation [57].
Neuroprotective factors such as PEDF, SST, NGF, BDNF, and EPO have been applied in experimental DR. Intraocular gene transfer of PEDF significantly increases neuroretinal cell survival after ischaemia-reperfusion injury [163]. In early DR, intravitreal injections of PEDF prevent neuronal derangements and vascular hyperpermeability [164]. SST and SST analogues administered intravitreally protect the retina from AMPA-induced neurotoxicity [165]. Treatment of diabetic rats with NGF prevented apoptosis of ganglion cells and Müller cells [166]. Probably, growth factors can be involved in the regeneration of ganglion cells from stem cells [170].
These results suggest that the increased expression and function of neuroprotective factors synthesised by the retina could be a therapeutic target in DR. Intravitreal or intraperitoneal administration of exogenous EPO and EPO-derived peptides acts against neuroglial and vascular degeneration in diabetic rats [167-169]. EPO or EpoR agonists used in the treatment of DR have neuroprotective properties, cause vessel stability, and increase in tissue repair by the recruitment of EPCs toward the pathological area [168-169]. Nevertheless, in advanced stages, the elevated levels of Epo EPO could enhance the effects of VEGF, thus contributing to neovascularisation and PDR worsening [114, 115]. Probably the next step in this field is the active prevention of neurodegeneration in patients before clinical symptoms. At present, we can diagnose retinal neuro- degeneration in a very early stage in children with diabetes [171].
5. Conclusions
For patients with the early identification of neuro- degeneration, implementing an early treatment based on drugs with a neuroprotective effect will be pivotal. The possibility of using biological treatment against neuro- degenerative factors and special therapy for the activation of neuroprotective growth agents is based on a new and safe strategy for treatment of the early stages of diabetic retinopathy. Therefore, investigations into growth factors in the process of retinal neurodegeneration have important practical aspects. The explanation of the important role of neurodegeneration in the pathogenesis of DR is the basis for new treatment methods. Neuroprotection is an effective method for treating, preventing or arresting DR.
Fig. (1).
Pathogenesis of diabetic retinopathy.
ACKNOWLEDGEMENTs
Source of funding for the study: Grant of Medical University of Lublin Poland: PW 415/2015.
LIST OF ABBREVIATIONS
- ADAM17
metallopeptidase domain 17 also called TACE (tumour necrosis factor-α-converting enzyme)
- AM
adrenomedullin
- AMPA
α-amino-3-hydroxy-5-methyl-4-isoxazole- propionic acid is a compound that is a specific agonist for the AMPA receptor, where it mimics the effects of the neuro- transmitter glutamate
- BDNF
brain-derived neurotrophic factor
- CNTF
ciliary neurotrophic factor
- DAG-PKC
diacylglycerol- protein kinase C
- DR
diabetic retinopathy
- EGFR
epidermal growth factor receptor
- EPO
erythropoietin,
- EPO-R
receptor of erythropoietin
- FGF
fibroblast growth factor
- GABA
gamma-aminobutyric acid
- GDNF
glial cell line derived neurotrophic factor
- IGF 1
insulin-like growth factor 1
- IRBP
interphotoreceptor retinoid-binding protein
- NGF
nerve growth factor
- NO
nitric oxide,
- NPD1
neuroprotectin D1
- PDGF
platelet-derived growth factor
- PEDF
pigment-epithelial-derived factor,
- SDF-1
stromal cell-derived factor-1
- SST
somatostatin,
- VEGF
vascular endothelial growth factor
CONFLICT OF INTEREST
The author confirms that this article content has no conflict of interest.
REFERENCES
- 1.Antonetti D.A., Barber A.J., Hollinger L.A., Wolpert E.B., Gardner T.W. Vascular endothelial growth factor induces rapid phosphorylation of tight junction proteins occludin and zonula occluden 1. A potential mechanism for vascular permeability in diabetic retinopathy and tumors. J. Biol. Chem. 1999;274(33):23463–23467. doi: 10.1074/jbc.274.33.23463. [http://dx.doi.org/10.1074/jbc.274.33.23463]. [PMID: 10438525]. [DOI] [PubMed] [Google Scholar]
- 2.Lopes-Virella M.F., Virella G. The role of immune and inflammatory processes in the development of macrovascular disease in diabetes. Front. Biosci. 2003;8:s750–s768. doi: 10.2741/1141. [http://dx. doi.org/10.2741/1141]. [PMID: 12957881]. [DOI] [PubMed] [Google Scholar]
- 3.Takagi H. Molecular mechanisms of retinal neovascularization in diabetic retinopathy. Intern. Med. 2003;42(3):299–301. doi: 10.2169/internalmedicine.42.299. [http://dx. doi.org/10.2169/internalmedicine.42.299]. [PMID: 12705804]. [DOI] [PubMed] [Google Scholar]
- 4.Antonetti D.A., Barber A.J., Bronson S.K., Freeman W.M., Gardner T.W., Jefferson L.S., Kester M., Kimball S.R., Krady J.K., LaNoue K.F., Norbury C.C., Quinn P.G., Sandirasegarane L., Simpson I.A. Diabetic retinopathy: seeing beyond glucose-induced microvascular disease. Diabetes. 2006;55(9):2401–2411. doi: 10.2337/db05-1635. [http://dx.doi.org/10.2337/db05-1635]. [PMID: 16936187]. [DOI] [PubMed] [Google Scholar]
- 5.Barber A.J., Gardner T.W., Abcouwer S.F. The significance of vascular and neural apoptosis to the pathology of diabetic retinopathy. Invest. Ophthalmol. Vis. Sci. 2011;52(2):1156–1163. doi: 10.1167/iovs.10-6293. [http://dx.doi.org/10.1167/iovs.10-6293]. [PMID: 21357409]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Curtis T.M., Gardiner T.A., Stitt A.W. Microvascular lesions of diabetic retinopathy: clues towards understanding pathogenesis? Eye (Lond.) 2009;23(7):1496–1508. doi: 10.1038/eye.2009.108. [http://dx.doi.org/10.1038/ eye.2009.108]. [PMID: 19444297]. [DOI] [PubMed] [Google Scholar]
- 7.Simó R., Villarroel M., Corraliza L., Hernández C., Garcia-Ramírez M. The retinal pigment epithelium: something more than a constituent of the blood-retinal barrier implications for the pathogenesis of diabetic retinopathy. J. Biomedicine and Biotechnology. 2010. [DOI] [PMC free article] [PubMed]
- 8.Rathmann W., Giani G. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care. 2004;27(10):2568–2569. doi: 10.2337/diacare.27.10.2568. [http://dx.doi.org/10.2337/diacare.27.10.2568]. [PMID: 15451946]. [DOI] [PubMed] [Google Scholar]
- 9.Gardner T.W., Abcouwer S.F., Barber A.J., Jackson G.R. An integrated approach to diabetic retinopathy research. Arch. Ophthalmol. 2011;129(2):230–235. doi: 10.1001/archophthalmol.2010.362. [http://dx.doi.org/10.1001/ archophthalmol.2010.362]. [PMID: 21320973]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Varma R., Choudhury F., Klein R., Chung J., Torres M., Azen S.P. Four-year incidence and progression of diabetic retinopathy and macular edema: the Los Angeles Latino Eye Study. 2010. [DOI] [PMC free article] [PubMed]
- 11.Harris Nwanyanwu K., Talwar N., Gardner T.W., Wrobel J.S., Herman W.H., Stein J.D. Predicting development of proliferative diabetic retinopathy. Diabetes Care. 2013;36(6):1562–1568. doi: 10.2337/dc12-0790. [http://dx.doi.org/10.2337/dc12-0790]. [PMID: 23275374]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Davidović S.P., Nikolić S.V., Curić N.J., Latinović S.L., Drašković D.O., Cabarkapa V.S., Stošić Z.Z. Changes of serum VEGF concentration after intravitreal injection of Avastin in treatment of diabetic retinopathy. Eur. J. Ophthalmol. 2012;22(5):792–798. doi: 10.5301/ejo.5000118. [PMID: 22344470]. [DOI] [PubMed] [Google Scholar]
- 13.Gastinger M.J., Kunselman A.R., Conboy E.E., Bronson S.K., Barber A.J. Dendrite remodeling and other abnormalities in the retinal ganglion cells of Ins2 Akita diabetic mice. Invest. Ophthalmol. Vis. Sci. 2008;49(6):2635–2642. doi: 10.1167/iovs.07-0683. [http://dx.doi.org/ 10.1167/iovs.07-0683]. [PMID: 18515593]. [DOI] [PubMed] [Google Scholar]
- 14.Barber A.J., Antonetti D.A., Kern T.S., Reiter C.E., Soans R.S., Krady J.K., Levison S.W., Gardner T.W., Bronson S.K. The Ins2Akita mouse as a model of early retinal complications in diabetes. Invest. Ophthalmol. Vis. Sci. 2005;46(6):2210–2218. doi: 10.1167/iovs.04-1340. [http://dx.doi.org/10.1167/iovs.04-1340]. [PMID: 15914643]. [DOI] [PubMed] [Google Scholar]
- 15.Gastinger M.J., Singh R.S., Barber A.J. Loss of cholinergic and dopaminergic amacrine cells in streptozotocin-diabetic rat and Ins2Akita-diabetic mouse retinas. Invest. Ophthalmol. Vis. Sci. 2006;47(7):3143–3150. doi: 10.1167/iovs.05-1376. [http://dx.doi.org/10.1167/iovs.05-1376]. [PMID: 16799061]. [DOI] [PubMed] [Google Scholar]
- 16.Krady J.K., Basu A., Allen C.M., Xu Y., LaNoue K.F., Gardner T.W., Levison S.W. Minocycline reduces pro- inflammatory cytokine expression, microglial activation, and caspase-3 activation in a rodent model of diabetic retinopathy. Diabetes. 2005;54(5):1559–1565. doi: 10.2337/diabetes.54.5.1559. [http://dx.doi.org/10.2337/ diabetes.54.5.1559]. [PMID: 15855346]. [DOI] [PubMed] [Google Scholar]
- 17.Lieth E., Barber A.J., Xu B., Dice C., Ratz M.J., Tanase D., Strother J.M. Glial reactivity and impaired glutamate metabolism in short-term experimental diabetic retinopathy. Diabetes. 1998;47(5):815–820. doi: 10.2337/diabetes.47.5.815. [http://dx.doi.org/10.2337/diabetes.47.5.815]. [PMID: 9588455]. [DOI] [PubMed] [Google Scholar]
- 18.Rungger-Brändle E., Dosso A.A., Leuenberger P.M. Glial reactivity, an early feature of diabetic retinopathy. Invest. Ophthalmol. Vis. Sci. 2000;41(7):1971–1980. [PMID: 10845624]. [PubMed] [Google Scholar]
- 19.Kodama H., Fujita M., Yamaguchi I. Development of hyperglycaemia and insulin resistance in conscious genetically diabetic (C57BL/KsJ-db/db) mice. Diabetologia. 1994;37(8):739–744. doi: 10.1007/BF00404329. [http://dx.doi.org/10.1007/BF00404329]. [PMID: 7988774]. [DOI] [PubMed] [Google Scholar]
- 20.Barile G.R., Pachydaki S.I., Tari S.R., Lee S.E., Donmoyer C.M., Ma W., Rong L.L., Buciarelli L.G., Wendt T., Hörig H., Hudson B.I., Qu W., Weinberg A.D., Yan S.F., Schmidt A.M. The RAGE axis in early diabetic retinopathy. Invest. Ophthalmol. Vis. Sci. 2005;46(8):2916–2924. doi: 10.1167/iovs.04-1409. [http://dx.doi.org/10.1167/iovs. 04-1409]. [PMID: 16043866]. [DOI] [PubMed] [Google Scholar]
- 21.Gündüz K., Bakri S.J. Management of proliferative diabetic retinopathy. Compr. Ophthalmol. Update. 2007;8(5):245–256. [PMID: 18201511]. [PubMed] [Google Scholar]
- 22.Abbate M., Cravedi P., Iliev I., Remuzzi G., Ruggenenti P. Prevention and treatment of diabetic retinopathy: evidence from clinical trials and perspectives. Curr. Diabetes Rev. 2011;7(3):190–200. doi: 10.2174/157339911795843168. [http://dx.doi.org/10.2174/157339911795843168]. [PMID: 21438851]. [DOI] [PubMed] [Google Scholar]
- 23.Abhary S., Burdon K.P., Gupta A., Lake S., Selva D., Petrovsky N., Craig J.E. Common sequence variation in the VEGFA gene predicts risk of diabetic retinopathy. Invest. Ophthalmol. Vis. Sci. 2009;50(12):5552–5558. doi: 10.1167/iovs.09-3694. [http://dx.doi.org/ 10.1167/iovs.09-3694]. [PMID: 19553626]. [DOI] [PubMed] [Google Scholar]
- 24.Ruberte J., Ayuso E., Navarro M., Carretero A., Nacher V., Haurigot V., George M., Llombart C., Casellas A., Costa C., Bosch A., Bosch F. Increased ocular levels of IGF-1 in transgenic mice lead to diabetes-like eye disease. J. Clin. Invest. 2004;113(8):1149–1157. doi: 10.1172/JCI19478. [http://dx.doi.org/10.1172/JCI19478]. [PMID: 15085194]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Praidou A., Papakonstantinou E., Androudi S., Georgiadis N., Karakiulakis G., Dimitrakos S. Vitreous and serum levels of vascular endothelial growth factor and platelet-derived growth factor and their correlation in patients with non-proliferative diabetic retinopathy and clinically significant macula oedema. Acta Ophthalmol. 2011;89(3):248–254. doi: 10.1111/j.1755-3768.2009.01661.x. [http://dx.doi.org/10.1111/ j.1755-3768.2009.01661.x]. [PMID: 19799585]. [DOI] [PubMed] [Google Scholar]
- 26.Zakareia F.A., Alderees A.A., Al Regaiy K.A., Alrouq F.A. Correlation of electroretinography b-wave absolute latency, plasma levels of human basic fibroblast growth factor, vascular endothelial growth factor, soluble fatty acid synthase, and adrenomedullin in diabetic retinopathy. J. Diabetes Complications. 2010;24(3):179–185. doi: 10.1016/j.jdiacomp.2008.12.007. [http://dx.doi.org/10.1016/j.jdiacomp.2008.12.007]. [PMID: 19216096]. [DOI] [PubMed] [Google Scholar]
- 27.Carrasco E., Hernández C., Miralles A., Huguet P., Farrés J., Simó R. Lower somatostatin expression is an early event in diabetic retinopathy and is associated with retinal neurodegeneration. Diabetes Care. 2007;30(11):2902–2908. doi: 10.2337/dc07-0332. [http://dx.doi.org/10. 2337/dc07-0332]. [PMID: 17704349]. [DOI] [PubMed] [Google Scholar]
- 28.Carrasco E., Hernández C., de Torres I., Farrés J., Simó R. Lowered cortistatin expression is an early event in the human diabetic retina and is associated with apoptosis and glial activation. Mol. Vis. 2008;14:1496–1502. [PMID: 18709137]. [PMC free article] [PubMed] [Google Scholar]
- 29.Garcia-Ramírez M., Hernández C., Villarroel M., Canals F., Alonso M.A., Fortuny R., Masmiquel L., Navarro A., García-Arumí J., Simó R. Interphotoreceptor retinoid-binding protein (IRBP) is downregulated at early stages of diabetic retinopathy. Diabetologia. 2009;52(12):2633–2641. doi: 10.1007/s00125-009-1548-8. [http://dx.doi.org/10. 1007/s00125-009-1548-8]. [PMID: 19823802]. [DOI] [PubMed] [Google Scholar]
- 30.Valverde A.M., Miranda S., García-Ramírez M., González-Rodriguez Á., Hernández C., Simó R. Proapoptotic and survival signaling in the neuroretina at early stages of diabetic retinopathy. Mol. Vis. 2013;19:47–53. [PMID: 23335850]. [PMC free article] [PubMed] [Google Scholar]
- 31.Han Y., Schneck M.E., Bearse M.A., Jr, Barez S., Jacobsen C.H., Jewell N.P., Adams A.J. Formulation and evaluation of a predictive model to identify the sites of future diabetic retinopathy. Invest. Ophthalmol. Vis. Sci. 2004;45(11):4106–4112. doi: 10.1167/iovs.04-0405. [http://dx. doi.org/10.1167/iovs.04-0405]. [PMID: 15505062]. [DOI] [PubMed] [Google Scholar]
- 32.Harrison W.W., Bearse M.A., Jr, Ng J.S., Jewell N.P., Barez S., Burger D., Schneck M.E., Adams A.J. Multifocal electro- retinograms predict onset of diabetic retinopathy in adult patients with diabetes. Invest. Ophthalmol. Vis. Sci. 2011;52(2):772–777. doi: 10.1167/iovs.10-5931. [http://dx.doi.org/10.1167/iovs.10-5931]. [PMID: 20926810]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Metea M.R., Newman E.A. Signalling within the neurovascular unit in the mammalian retina. Exp. Physiol. 2007;92(4):635–640. doi: 10.1113/expphysiol.2006.036376. [http://dx.doi.org/10.1113/expphysiol.2006.036376]. [PMID: 17434916]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kur J., Newman E.A., Chan-Ling T. Cellular and physiological mechanisms underlying blood flow regulation in the retina and choroid in health and disease. Prog. Retin. Eye Res. 2012;31(5):377–406. doi: 10.1016/j.preteyeres.2012.04.004. [http://dx.doi.org/10.1016/j.preteyeres.2012.04.004]. [PMID: 22580107]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Buerk D.G., Riva C.E., Cranstoun S.D. Nitric oxide has a vasodilatory role in cat optic nerve head during flicker stimuli. Microvasc. Res. 1996;52(1):13–26. doi: 10.1006/mvre.1996.0040. [http://dx.doi.org/10.1006/ mvre.1996.0040]. [PMID: 8812749]. [DOI] [PubMed] [Google Scholar]
- 36.Maenhaut N., Boussery K., Delaey C., Van de Voorde J. Control of retinal arterial tone by a paracrine retinal relaxing factor. Microcirculation. 2007;14(1):39–48. doi: 10.1080/10739680601072131. [http://dx.doi.org/10.1080/ 10739680601072131]. [PMID: 17365660]. [DOI] [PubMed] [Google Scholar]
- 37.Nakahara T., Mori A., Kurauchi Y., Sakamoto K., Ishii K. Neurovascular interactions in the retina: physiological and pathological roles. J. Pharmacol. Sci. 2013;123(2):79–84. doi: 10.1254/jphs.13r03cp. [http://dx.doi.org/10.1254/jphs.13R03CP]. [PMID: 24067498]. [DOI] [PubMed] [Google Scholar]
- 38.Metea M.R., Newman E.A. Glial cells dilate and constrict blood vessels: a mechanism of neurovascular coupling. J. Neurosci. 2006;26(11):2862–2870. doi: 10.1523/JNEUROSCI.4048-05.2006. [http://dx.doi.org/10.1523/JNEUROSCI. 4048-05.2006]. [PMID: 16540563]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wurm A., Pannicke T., Iandiev I., Francke M., Hollborn M., Wiedemann P., Reichenbach A., Osborne N.N., Bringmann A. Purinergic signaling involved in Müller cell function in the mammalian retina. Prog. Retin. Eye Res. 2011;30(5):324–342. doi: 10.1016/j.preteyeres.2011.06.001. [http:// dx.doi.org/10.1016/j.preteyeres.2011.06.001]. [PMID: 21689780]. [DOI] [PubMed] [Google Scholar]
- 40.Lecleire-Collet A., Audo I., Aout M., Girmens J.F., Sofroni R., Erginay A., Le Gargasson J.F., Mohand-Saïd S., Meas T., Guillausseau P.J., Vicaut E., Paques M., Massin P. Evaluation of retinal function and flicker light-induced retinal vascular response in normotensive patients with diabetes without retinopathy. Invest. Ophthalmol. Vis. Sci. 2011;52(6):2861–2867. doi: 10.1167/iovs.10-5960. [http://dx.doi.org/ 10.1167/iovs.10-5960]. [PMID: 21282578]. [DOI] [PubMed] [Google Scholar]
- 41.Tyrberg M., Lindblad U., Melander A., Lövestam-Adrian M., Ponjavic V., Andréasson S. Electrophysiological studies in newly onset type 2 diabetes without visible vascular retinopathy. Doc. Ophthalmol. 2011;123(3):193–198. doi: 10.1007/s10633-011-9298-6. [http://dx.doi.org/10.1007/ s10633-011-9298-6]. [PMID: 22057379]. [DOI] [PubMed] [Google Scholar]
- 42.Nguyen T.T., Kawasaki R., Wang J.J., Kreis A.J., Shaw J., Vilser W., Wong T.Y. Flicker light-induced retinal vasodilation in diabetes and diabetic retinopathy. Diabetes Care. 2009;32(11):2075–2080. doi: 10.2337/dc09-0075. [http://dx.doi.org/10.2337/dc09-0075]. [PMID: 19641162]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Antonetti D.A., Klein R., Gardner T.W. Diabetic retinopathy. N. Engl. J. Med. 2012;366(13):1227–1239. doi: 10.1056/NEJMra1005073. [http://dx.doi.org/10. 1056/NEJMra1005073]. [PMID: 22455417]. [DOI] [PubMed] [Google Scholar]
- 44.Ola M.S., Nawaz M.I., Siddiquei M.M., Al-Amro S., Abu El-Asrar A.M. Recent advances in understanding the biochemical and molecular mechanism of diabetic retinopathy. J. Diabetes Complications. 2012;26(1):56–64. doi: 10.1016/j.jdiacomp.2011.11.004. [http://dx.doi.org/10.1016/ j.jdiacomp.2011.11.004]. [PMID: 22226482]. [DOI] [PubMed] [Google Scholar]
- 45.Abu El-Asrar A.M., Al-Mezaine H.S., Ola M.S. Pathophysiology and management of diabetic retinopathy. Expert Rev. Ophthalmol. 2009;4:627–647. [http://dx.doi.org/10.1586/eop.09.52]. [Google Scholar]
- 46.Ola M.S., Berkich D.A., Xu Y., King M.T., Gardner T.W., Simpson I., LaNoue K.F. Analysis of glucose metabolism in diabetic rat retinas. Am. J. Physiol. Endocrinol. Metab. 2006;290(6):E1057–E1067. doi: 10.1152/ajpendo.00323.2005. [http://dx.doi.org/10.1152/ajpendo.00323. 2005]. [PMID: 16380392]. [DOI] [PubMed] [Google Scholar]
- 47.Diederen R.M., La Heij E.C., Deutz N.E., Kijlstra A., Kessels A.G., van Eijk H.M., Liem A.T., Dieudonné S., Hendrikse F. Increased glutamate levels in the vitreous of patients with retinal detachment. Exp. Eye Res. 2006;83(1):45–50. doi: 10.1016/j.exer.2005.10.031. [http://dx.doi. org/10.1016/j.exer.2005.10.031]. [PMID: 16530753]. [DOI] [PubMed] [Google Scholar]
- 48.Yu X.H., Zhang H., Wang Y.H., Liu L.J., Teng Y., Liu P. Time-dependent reduction of glutamine synthetase in retina of diabetic rats. Exp. Eye Res. 2009;89(6):967–971. doi: 10.1016/j.exer.2009.08.006. [http://dx. doi.org/10.1016/j.exer.2009.08.006]. [PMID: 19699197]. [DOI] [PubMed] [Google Scholar]
- 49.Ola M.S., Hosoya K., LaNoue K.F. Regulation of glutamate metabolism by hydrocortisone and branched chain keto acids in cultured rat retinal Müller cells (TR-MUL). Neurochem. Int. 2011;59(5):656–663. doi: 10.1016/j.neuint.2011.06.010. [http://dx.doi.org/10.1016/j.neuint.2011.06.010]. [PMID: 21756956]. [DOI] [PubMed] [Google Scholar]
- 50.Zhang Y., Bhavnani B.R. Glutamate-induced apoptosis in neuronal cells is mediated via caspase-dependent and independent mechanisms involving calpain and caspase-3 proteases as well as apoptosis inducing factor (AIF) and this process is inhibited by equine estrogens. BMC Neurosci. 2006;7:49–57. doi: 10.1186/1471-2202-7-49. [http://dx.doi. org/10.1186/1471-2202-7-49]. [PMID: 16776830]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hernández C., García-Ramírez M., Corraliza L., Fernández-Carneado J., Farrera-Sinfreu J., Ponsati B., González-Rodríguez A., Valverde A.M., Simó R. Topical administration of somatostatin prevents retinal neurodegeneration in experimental diabetes. Diabetes. 2013;62(7):2569–2578. doi: 10.2337/db12-0926. [http://dx.doi.org/ 10.2337/db12-0926]. [PMID: 23474487]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Simó R., Lecube A., Sararols L., García-Arumí J., Segura R.M., Casamitjana R., Hernández C. Deficit of somatostatin-like immunoreactivity in the vitreous fluid of diabetic patients: possible role in the development of proliferative diabetic retinopathy. Diabetes Care. 2002;25(12):2282–2286. doi: 10.2337/diacare.25.12.2282. [http://dx.doi.org/10. 2337/diacare.25.12.2282]. [PMID: 12453974]. [DOI] [PubMed] [Google Scholar]
- 53.Hernández C., Carrasco E., Casamitjana R., Deulofeu R., García-Arumí J., Simó R. Somatostatin molecular variants in the vitreous fluid: a comparative study between diabetic patients with proliferative diabetic retinopathy and nondiabetic control subjects. Diabetes Care. 2005;28(8):1941–1947. doi: 10.2337/diacare.28.8.1941. [http://dx.doi.org/10.2337/ diacare.28.8.1941]. [PMID: 16043736]. [DOI] [PubMed] [Google Scholar]
- 54.Cervia D., Casini G., Bagnoli P. Physiology and pathology of somatostatin in the mammalian retina: a current view. Mol. Cell. Endocrinol. 2008;286(1-2):112–122. doi: 10.1016/j.mce.2007.12.009. [http://dx.doi.org/10.1016/ j.mce.2007.12.009]. [PMID: 18242820]. [DOI] [PubMed] [Google Scholar]
- 55.Zong H., Ward M., Madden A., Yong P.H., Limb G.A., Curtis T.M., Stitt A.W. Hyperglycaemia-induced pro-inflammatory responses by retinal Müller glia are regulated by the receptor for advanced glycation end-products (RAGE). Diabetologia. 2010;53(12):2656–2666. doi: 10.1007/s00125-010-1900-z. [http://dx.doi.org/10.1007/s00125-010-1900-z]. [PMID: 20835858]. [DOI] [PubMed] [Google Scholar]
- 56.Simó R., Hernández C. Neurodegeneration is an early event in diabetic retinopathy: therapeutic implications. Br. J. Ophthalmol. 2012;96(10):1285–1290. doi: 10.1136/bjophthalmol-2012-302005. [http://dx.doi.org/10.1136/bjophthalmol-2012-302005]. [PMID: 22887976]. [DOI] [PubMed] [Google Scholar]
- 57.Simó R., Hernández C. Neurodegeneration in the diabetic eye: new insights and therapeutic perspectives. Trends Endocrinol. Metab. 2014;25(1):23–33. doi: 10.1016/j.tem.2013.09.005. [http://dx.doi.org/10.1016/j.tem.2013. 09.005]. [PMID: 24183659]. [DOI] [PubMed] [Google Scholar]
- 58.Gonzalez-Fernandez F. Interphotoreceptor retinoid-binding protein--an old gene for new eyes. Vision Res. 2003;43(28):3021–3036. doi: 10.1016/j.visres.2003.09.019. [http://dx.doi.org/10.1016/j.visres.2003.09.019]. [PMID: 14611938]. [DOI] [PubMed] [Google Scholar]
- 59.Gonzalez-Fernandez F., Ghosh D. Focus on Molecules: interphotoreceptor retinoid-binding protein (IRBP). Exp. Eye Res. 2008;86(2):169–170. doi: 10.1016/j.exer.2006.09.003. [http://dx.doi.org/10.1016/j.exer.2006.09. 003]. [PMID: 17222825]. [DOI] [PubMed] [Google Scholar]
- 60.Becerra S.P., Amaral J. Erythropoietin--an endogenous retinal survival factor. N. Engl. J. Med. 2002;347(24):1968–1970. doi: 10.1056/NEJMcibr022629. [http://dx.doi.org/10.1056/NEJMcibr022629]. [PMID: 12477950]. [DOI] [PubMed] [Google Scholar]
- 61.Rex T.S., Wong Y., Kodali K., Merry S. Neuroprotection of photoreceptors by direct delivery of erythropoietin to the retina of the retinal degeneration slow mouse. Exp. Eye Res. 2009;89(5):735–740. doi: 10.1016/j.exer.2009.06.017. [http://dx.doi.org/10.1016/j.exer.2009.06.017]. [PMID: 19591826]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Shen J., Wu Y., Xu J.Y., Zhang J., Sinclair S.H., Yanoff M., Xu G., Li W., Xu G.T. ERK- and Akt-dependent neuroprotection by erythropoietin (EPO) against glyoxal-AGEs via modulation of Bcl-xL, Bax, and BAD. Invest. Ophthalmol. Vis. Sci. 2010;51(1):35–46. doi: 10.1167/iovs.09-3544. [http://dx.doi.org/10.1167/iovs.09-3544]. [PMID: 19628748]. [DOI] [PubMed] [Google Scholar]
- 63.Chen J., Connor K.M., Aderman C.M., Smith L.E. Erythropoietin deficiency decreases vascular stability in mice. J. Clin. Invest. 2008;118(2):526–533. doi: 10.1172/JCI33813. [PMID: 18219389]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Grant M.B., Boulton M.E., Ljubimov A.V. Erythropoietin: when liability becomes asset in neurovascular repair. J. Clin. Invest. 2008;118(2):467–470. doi: 10.1172/JCI34643. [PMID: 18219388]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Reiter C.E., Wu X., Sandirasegarane L., Nakamura M., Gilbert K.A., Singh R.S., Fort P.E., Antonetti D.A., Gardner T.W. Diabetes reduces basal retinal insulin receptor signaling: reversal with systemic and local insulin. Diabetes. 2006;55(4):1148–1156. doi: 10.2337/diabetes.55.04.06.db05-0744. [http://dx.doi.org/10.2337/diabetes.55.04.06.db05-0744]. [PMID: 16567541]. [DOI] [PubMed] [Google Scholar]
- 66.Gordon W.C., Bazan N.G. Mediator lipidomics in ophthalmology: targets for modulation in inflammation, neuroprotection and nerve regeneration. Curr. Eye Res. 2013;38(10):995–1005. doi: 10.3109/02713683.2013.827211. [http://dx. doi.org/10.3109/02713683.2013.827211]. [PMID: 23981028]. [DOI] [PubMed] [Google Scholar]
- 67.Seki M., Tanaka T., Nawa H., Usui T., Fukuchi T., Ikeda K., Abe H., Takei N. Involvement of brain-derived neurotrophic factor in early retinal neuropathy of streptozotocin-induced diabetes in rats: therapeutic potential of brain-derived neurotrophic factor for dopaminergic amacrine cells. Diabetes. 2004;53(9):2412–2419. doi: 10.2337/diabetes.53.9.2412. [http://dx.doi.org/10.2337/diabetes.53.9.2412]. [PMID: 15331553]. [DOI] [PubMed] [Google Scholar]
- 68.Wang N., Wang L., Deng Q.Q., Wu X.H., Yu J., Yang X.L., Zhong Y.M. Up-regulation of glutamate-aspartate transporter by glial cell line-derived neurotrophic factor ameliorates cell apoptosis in neural retina in streptozotocin-induced diabetic rats. 2015. [DOI] [PMC free article] [PubMed]
- 69.Aizu Y., Katayama H., Takahama S., Hu J., Nakagawa H., Oyanagi K. Topical instillation of ciliary neurotrophic factor inhibits retinal degeneration in streptozotocin-induced diabetic rats. Neuroreport. 2003;14(16):2067–2071. doi: 10.1097/00001756-200311140-00012. [http://dx.doi.org/10.1097/ 00001756-200311140-00012]. [PMID: 14600499]. [DOI] [PubMed] [Google Scholar]
- 70.Ali T.K., Al-Gayyar M.M., Matragoon S., Pillai B.A., Abdelsaid M.A., Nussbaum J.J., El-Remessy A.B. Diabetes-induced peroxynitrite impairs the balance of pro-nerve growth factor and nerve growth factor, and causes neurovascular injury. Diabetologia. 2011;54(3):657–668. doi: 10.1007/s00125-010-1935-1. [http://dx.doi.org/10.1007/ s00125-010-1935-1]. [PMID: 20957344]. [DOI] [PubMed] [Google Scholar]
- 71.Blom J.J., Giove T.J., Favazza T.L., Akula J.D., Eldred W.D. Inhibition of the adrenomedullin/nitric oxide signaling pathway in early diabetic retinopathy. J. Ocul. Biol. Dis. Infor. 2011;4(1-2):70–82. doi: 10.1007/s12177-011-9072-8. [http://dx.doi.org/10.1007/s12177-011-9072-8]. [PMID: 23316263]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Joussen A.M., Poulaki V., Le M.L., Koizumi K., Esser C., Janicki H., Schraermeyer U., Kociok N., Fauser S., Kirchhof B., Kern T.S., Adamis A.P. A central role for inflammation in the pathogenesis of diabetic retinopathy. FASEB J. 2004;18(12):1450–1452. doi: 10.1096/fj.03-1476fje. [PMID: 15231732]. [DOI] [PubMed] [Google Scholar]
- 73.Kern T.S. Contributions of inflammatory processes to the development of early stages of diabetic retinopathy. 2007. [DOI] [PMC free article] [PubMed]
- 74.Gerhardinger C., Costa M.B., Coulombe M.C., Toth I., Hoehn T., Grosu P. Expression of acute-phase response proteins in retinal Müller cells in diabetes. Invest. Ophthalmol. Vis. Sci. 2005;46(1):349–357. doi: 10.1167/iovs.04-0860. [http://dx.doi.org/10.1167/iovs.04-0860]. [PMID: 15623795]. [DOI] [PubMed] [Google Scholar]
- 75.Zhong Y., Li j., Chen Y., Wang J.J., Ratan R., Zhang S.X. Activation of endoplasmic reticulum stress by hyperglycaemia is essential for Müller cell-derived inflammatory cytokine production in diabetes. Diabetes. 2012;61:492–504. doi: 10.2337/db11-0315. [http://dx.doi.org/ 10.2337/db11-0315]. [PMID: 22228718]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wilkinson-Berka J.L. Angiotensin and diabetic retinopathy. Int. J. Biochem. Cell Biol. 2006;38(5-6):752–765. doi: 10.1016/j.biocel.2005.08.002. [http://dx.doi.org/ 10.1016/j.biocel.2005.08.002]. [PMID: 16165393]. [DOI] [PubMed] [Google Scholar]
- 77.Downie L.E., Pianta M.J., Vingrys A.J., Wilkinson-Berka J.L., Fletcher E.L. AT1 receptor inhibition prevents astrocyte degeneration and restores vascular growth in oxygen-induced retinopathy. Glia. 2008;56(10):1076–1090. doi: 10.1002/glia.20680. [http://dx.doi.org/10. 1002/glia.20680]. [PMID: 18442090]. [DOI] [PubMed] [Google Scholar]
- 78.Kurihara T., Ozawa y., Nagai N., Shinoda K., Noda K., Imamura Y., Tsubota K., Okano H., Oike Y., Ishida S. Angiotensin II type 1 receptor signalling contributes to synaptophysin degradation and neuronal dysfunction in the diabetic retina. Diabetes. 2008;57:2191–2198. doi: 10.2337/db07-1281. [http://dx.doi.org/10.2337/ db07-1281]. [PMID: 18487452]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Pierce A.L., Breves J.P., Moriyama S., Hirano T., Grau E.G. Differential regulation of Igf1 and Igf2 mRNA levels in tilapia hepatocytes: effects of insulin and cortisol on GH sensitivity. J. Endocrinol. 2011;211(2):201–210. doi: 10.1530/JOE-10-0456. [http://dx.doi.org/10.1530/ JOE-10-0456]. [PMID: 21803836]. [DOI] [PubMed] [Google Scholar]
- 80.Chu Q., Moreland R., Yew N.S., Foley J., Ziegler R., Scheule R.K. Systemic Insulin-like growth factor-1 reverses hypoalgesia and improves mobility in a mouse model of diabetic peripheral neuropathy. Mol. Ther. 2008;16(8):1400–1408. doi: 10.1038/mt.2008.115. [http://dx.doi.org/ 10.1038/mt.2008.115]. [PMID: 18545223]. [DOI] [PubMed] [Google Scholar]
- 81.Genovese S., Riccardi G. The role of modulation of GH/IGF-I axis in the development of diabetic proliferative retinopathy. J. Endocrinol. Invest. 2003;26(8) Suppl.:114–116. [PMID: 15233225]. [PubMed] [Google Scholar]
- 82.Frank R.N. Treating diabetic retinopathy by inhibiting growth factor pathways. Curr. Opin. Investig. Drugs. 2009;10(4):327–335. [PMID: 19337953]. [PubMed] [Google Scholar]
- 83.Wang H., Xu J., Chen J., Little P.J., Zheng W. 2015 [Google Scholar]
- 84.Inokuchi N., Ikeda T., Imamura Y., Sotozono C., Kinoshita S., Uchihori Y., Nakamura K. Vitreous levels of insulin-like growth factor-I in patients with proliferative diabetic retinopathy. Curr. Eye Res. 2001;23(5):368–371. doi: 10.1076/ceyr.23.5.368.5441. [http://dx.doi.org/10.1076/ceyr. 23.5.368.5441]. [PMID: 11910526]. [DOI] [PubMed] [Google Scholar]
- 85.Smith L.E., Shen W., Perruzzi C., Soker S., Kinose F., Xu X., Robinson G., Driver S., Bischoff J., Zhang B., Schaeffer J.M., Senger D.R. Regulation of vascular endothelial growth factor-dependent retinal neovascularization by insulin-like growth factor-1 receptor. Nat. Med. 1999;5(12):1390–1395. doi: 10.1038/70963. [http://dx.doi.org/ 10.1038/70963]. [PMID: 10581081]. [DOI] [PubMed] [Google Scholar]
- 86.Lee H.C., Lee K.W., Chung C.H., Chung Y.S., Lee E.J., Lim S.K., Kim K.R., Huh K.B., Lee S.C., Kwon O.W. IGF-I of serum and vitreous fluid in patients with diabetic proliferative retinopathy. Diabetes Res. Clin. Pract. 1994;24(2):85–88. doi: 10.1016/0168-8227(94)90024-8. [http:// dx.doi.org/10.1016/0168-8227(94)90024-8]. [PMID: 7956713]. [DOI] [PubMed] [Google Scholar]
- 87.Sall J.W., Klisovic D.D., O’Dorisio M.S., Katz S.E. Somatostatin inhibits IGF-1 mediated induction of VEGF in human retinal pigment epithelial cells. Exp. Eye Res. 2004;79(4):465–476. doi: 10.1016/j.exer.2004.06.007. [http://dx.doi.org/10.1016/j.exer.2004.06.007]. [PMID: 15381031]. [DOI] [PubMed] [Google Scholar]
- 88.Fukuda R., Hirota K., Fan F., Jung Y.D., Ellis L.M., Semenza G.L. Insulin-like growth factor 1 induces hypoxia-inducible factor 1-mediated vascular endothelial growth factor expression, which is dependent on MAP kinase and phosphatidylinositol 3-kinase signaling in colon cancer cells. J. Biol. Chem. 2002;277(41):38205–38211. doi: 10.1074/jbc.M203781200. [http://dx.doi.org/10.1074/jbc.M203781200]. [PMID: 12149254]. [DOI] [PubMed] [Google Scholar]
- 89.Thurston G. Complementary actions of VEGF and angiopoietin-1 on blood vessel growth and leakage. J. Anat. 2002;200(6):575–580. doi: 10.1046/j.1469-7580.2002.00061.x. [http://dx.doi.org/10.1046/j.1469-7580.2002.00061.x]. [PMID: 12162725]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kim I., Kim C.H., Yim Y.S., Ahn Y.S. Autocrine function of erythropoietin in IGF-1-induced erythropoietin biosynthesis. Neuroreport. 2008;19(17):1699–1703. doi: 10.1097/WNR.0b013e32831743fb. [http://dx.doi.org/10.1097/ WNR.0b013e32831743fb]. [PMID: 18841090]. [DOI] [PubMed] [Google Scholar]
- 91.Mehrhof F.B., Schmidt-Ullrich R., Dietz R., Scheidereit C. Regulation of vascular smooth muscle cell proliferation: role of NF-kappaB revisited. Circ. Res. 2005;96(9):958–964. doi: 10.1161/01.RES.0000166924.31219.49. [http://dx. doi.org/10.1161/01.RES.0000166924.31219.49]. [PMID: 15831813]. [DOI] [PubMed] [Google Scholar]
- 92.Romaniuk D., Kimsa M.C., Kabiesz A., Strzalka-Mrozik B., Romaniuk W., Mazurek U. Gene Expression of IGF1, IGF1R, and IGFBP3 in Epiretinal Membranes of Patients with Proliferative Diabetic Retinopathy: Preliminary Study. Mediators of Inflammation. 2015. [DOI] [PMC free article] [PubMed]
- 93.Dome B., Dobos J., Tovari J., Paku S., Kovacs G., Ostoros G., Timar J. Circulating bone marrow-derived endothelial progenitor cells: characterization, mobilization, and therapeutic considerations in malignant disease. Cytometry A. 2008;73(3):186–193. doi: 10.1002/cyto.a.20480. [http://dx.doi.org/10.1002/cyto.a.20480]. [PMID: 18000872]. [DOI] [PubMed] [Google Scholar]
- 94.Abu El-Asrar A.M., Struyf S., Verbeke H., Van Damme J., Geboes K. Circulating bone-marrow-derived endothelial precursor cells contribute to neovascularization in diabetic epiretinal membranes. Acta Ophthalmol. 2011;89(3):222–228. doi: 10.1111/j.1755-3768.2009.01700.x. [http://dx. doi.org/10.1111/j.1755-3768.2009.01700.x]. [PMID: 19764917]. [DOI] [PubMed] [Google Scholar]
- 95.Abu El-Asrar A.M., Struyf S., Opdenakker G., Van Damme J., Geboes K. Expression of stem cell factor/c-kit signaling pathway components in diabetic fibrovascular epiretinal membranes. Mol. Vis. 2010;16:1098–1107. [PMID: 20596251]. [PMC free article] [PubMed] [Google Scholar]
- 96.Kalka C., Masuda H., Takahashi T., Gordon R., Tepper O., Gravereaux E., Pieczek A., Iwaguro H., Hayashi S.I., Isner J.M., Asahara T. Vascular endothelial growth factor(165) gene transfer augments circulating endothelial progenitor cells in human subjects. Circ. Res. 2000;86(12):1198–1202. doi: 10.1161/01.res.86.12.1198. [http://dx.doi.org/ 10.1161/01.RES.86.12.1198]. [PMID: 10864908]. [DOI] [PubMed] [Google Scholar]
- 97.Li B., Sharpe E.E., Maupin A.B., Teleron A.A., Pyle A.L., Carmeliet P., Young P.P. VEGF and PlGF promote adult vasculogenesis by enhancing EPC recruitment and vessel formation at the site of tumor neovascularization. FASEB J. 2006;20(9):1495–1497. doi: 10.1096/fj.05-5137fje. [http://dx.doi.org/10.1096/fj.05-5137fje]. [PMID: 16754748]. [DOI] [PubMed] [Google Scholar]
- 98.Shibuya M. Differential roles of vascular endothelial growth factor receptor-1 and receptor-2 in angiogenesis. J. Biochem. Mol. Biol. 2006;39(5):469–478. doi: 10.5483/bmbrep.2006.39.5.469. [http://dx.doi.org/10.5483/BMBRep.2006. 39.5.469]. [PMID: 17002866]. [DOI] [PubMed] [Google Scholar]
- 99.Ebos J.M., Bocci G., Man S., Thorpe P.E., Hicklin D.J., Zhou D., Jia X., Kerbel R.S. A naturally occurring soluble form of vascular endothelial growth factor receptor 2 detected in mouse and human plasma. Mol. Cancer Res. 2004;2(6):315–326. [PMID: 15235107]. [PubMed] [Google Scholar]
- 100.Ebos J.M., Lee C.R., Bogdanovic E., Alami J., Van Slyke P., Francia G., Xu P., Mutsaers A.J., Dumont D.J., Kerbel R.S. Vascular endothelial growth factor-mediated decrease in plasma soluble vascular endothelial growth factor receptor-2 levels as a surrogate biomarker for tumor growth. Cancer Res. 2008;68(2):521–529. doi: 10.1158/0008-5472.CAN-07-3217. [http://dx.doi.org/10.1158/0008-5472.CAN-07-3217]. [PMID: 18199548]. [DOI] [PubMed] [Google Scholar]
- 101.Abu El-Asrar A.M., Siddiquei M.M., Nawaz D., Kangave K.G. Angiogenic and Vasculogenic Factors in the Vitreous from Patients with Proliferative Diabetic Retinopathy. Journal of Diabetes Research. 2013:1–9. doi: 10.1155/2013/539658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kühn M.C., Willenberg H.S., Schott M., Papewalis C., Stumpf U., Flohé S., Scherbaum W.A., Schinner S. Adipocyte-secreted factors increase osteoblast proliferation and the OPG/RANKL ratio to influence osteoclast formation. Mol. Cell. Endocrinol. 2012;349(2):180–188. doi: 10.1016/j.mce.2011.10.018. [http://dx.doi.org/10.1016/j.mce.2011.10.018]. [PMID: 22040599]. [DOI] [PubMed] [Google Scholar]
- 103.Fang L., Barber A.J., Shenberger J.S. Regulation of fibroblast growth factor 2 expression in oxygen-induced retinopathy. Invest. Ophthalmol. Vis. Sci. 2015;56(1):207–215. doi: 10.1167/iovs.14-15616. [http://dx.doi.org/ 10.1167/iovs.14-15616]. [PMID: 25525167]. [DOI] [PubMed] [Google Scholar]
- 104.Belovand A.A., Mohammadi M. Molecular mechanisms of fibroblast growth factor signalling in physiology and pathology. Cold Spring Harbor Perspectives in Biology. 2013;5(6) doi: 10.1101/cshperspect.a015958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Cantó C., Auwerx J. Cell biology. FGF21 takes a fat bite. Science. 2012;336(6082):675–676. doi: 10.1126/science.1222646. [http://dx.doi.org/10.1126/science. 1222646]. [PMID: 22582248]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Smith R., Duguay A., Weiszmann J., Stanislaus S., Belouski E., Cai L., Yie J., Xu J., Gupte J., Wu X., Li Y. A novel approach to improve the function of FGF21. BioDrugs. 2013;27(2):159–166. doi: 10.1007/s40259-013-0013-x. [http://dx.doi.org/10.1007/s40259-013-0013-x]. [PMID: 23456652]. [DOI] [PubMed] [Google Scholar]
- 107.Xiao Y., Xu A., Law L.S., Chen C., Li H., Li X., Yang L., Liu S., Zhou Z., Lam K.S. Distinct changes in serum fibroblast growth factor 21 levels in different subtypes of diabetes. J. Clin. Endocrinol. Metab. 2012;97(1):E54–E58. doi: 10.1210/jc.2011-1930. [http://dx.doi.org/10. 1210/jc.2011-1930]. [PMID: 22013098]. [DOI] [PubMed] [Google Scholar]
- 108.Kharitonenkov A., Shiyanova T.L., Koester A., Ford A.M., Micanovic R., Galbreath E.J., Sandusky G.E., Hammond L.J., Moyers J.S., Owens R.A., Gromada J., Brozinick J.T., Hawkins E.D., Wroblewski V.J., Li D.S., Mehrbod F., Jaskunas S.R., Shanafelt A.B. FGF-21 as a novel metabolic regulator. J. Clin. Invest. 2005;115(6):1627–1635. doi: 10.1172/JCI23606. [http://dx.doi.org/10.1172/ JCI23606]. [PMID: 15902306]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Yuan L., Xiu-juan L., Zhu-Mei X., Qing-yan X., Lei Q., Yu-bin W., Xiu-juan L., Ma-li Z., Rui-sheng Z., Yi Z., Xiang-dong X., Bo-le W., Zhu-mei X., Xiang-hong L . Serum Fibroblast Growth Factor 21 Levels Are Correlated with the Severity of Diabetic Retinopathy. J. Diab. Res. 2014. [DOI] [PMC free article] [PubMed]
- 110.De Caterina R., Libby P., Peng H.B., Thannickal V.J., Rajavashisth T.B., Gimbrone M.A., Jr, Shin W.S., Liao J.K. Nitric oxide decreases cytokine-induced endothelial activation. Nitric oxide selectively reduces endothelial expression of adhesion molecules and proinflammatory cytokines. J. Clin. Invest. 1995;96(1):60–68. doi: 10.1172/JCI118074. [http://dx.doi.org/10.1172/JCI118074]. [PMID: 7542286]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Cohen R.A., Tong X. Vascular oxidative stress: the common link in hypertensive and diabetic vascular disease. J. Cardiovasc. Pharmacol. 2010;55(4):308–316. doi: 10.1097/fjc.0b013e3181d89670. [http://dx.doi.org/10.1097/ FJC.0b013e3181d89670]. [PMID: 20422735]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Xia Y., Zweier J.L. Superoxide and peroxynitrite generation from inducible nitric oxide synthase in macrophages. Proc Natl Acad Sci. 1997;94:6954–6958. doi: 10.1073/pnas.94.13.6954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Pacher P., Obrosova I.G., Mabley J.G., Szabó C. Role of nitrosative stress and peroxynitrite in the pathogenesis of diabetic complications. Emerging new therapeutical strategies. Curr. Med. Chem. 2005;12(3):267–275. doi: 10.2174/0929867053363207. [http://dx.doi.org/10.2174/ 0929867053363207]. [PMID: 15723618]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Hein T.W., Zhang C., Wang W., Chang C.I., Thengchaisri N., Kuo L. Ischemia-reperfusion selectively impairs nitric oxide-mediated dilation in coronary arterioles: counteracting role of arginase. FASEB J. 2003;17(15):2328–2330. doi: 10.1096/fj.03-0115fje. [PMID: 14563685]. [DOI] [PubMed] [Google Scholar]
- 115.Topal G., Brunet A., Walch L., Boucher J.L., David-Dufilho M. Mitochondrial arginase II modulates nitric-oxide synthesis through nonfreely exchangeable L-arginine pools in human endothelial cells. J. Pharmacol. Exp. Ther. 2006;318(3):1368–1374. doi: 10.1124/jpet.106.103747. [http:// dx.doi.org/10.1124/jpet.106.103747]. [PMID: 16801455]. [DOI] [PubMed] [Google Scholar]
- 116.Wu G., Morris S.M. Jr Arginine metabolism: nitric oxide and beyond. Biochem. J. 1998;336(Pt 1):1–17. doi: 10.1042/bj3360001. [http://dx.doi.org/ 10.1042/bj3360001]. [PMID: 9806879]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Zhang W., Baban B., Rojas M., Tofigh S., Virmani S.K., Patel C., Behzadian M.A., Romero M.J., Caldwell R.W., Caldwell R.B. Arginase activity mediates retinal inflammation in endotoxin-induced uveitis. Am. J. Pathol. 2009;175(2):891–902. doi: 10.2353/ajpath.2009.081115. [http://dx.doi.org/10.2353/ajpath.2009.081115]. [PMID: 19590038]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Berkowitz D.E., White R., Li D., Minhas K.M., Cernetich A., Kim S., Burke S., Shoukas A.A., Nyhan D., Champion H.C., Hare J.M. Arginase reciprocally regulates nitric oxide synthase activity and contributes to endothelial dysfunction in aging blood vessels. Circulation. 2003;108(16):2000–2006. doi: 10.1161/01.CIR.0000092948.04444.C7. [http://dx.doi.org/ 10.1161/01.CIR.0000092948.04444.C7]. [PMID: 14517171]. [DOI] [PubMed] [Google Scholar]
- 119.Ryoo S., Berkowitz D.E., Lim H.K. Endothelial arginase II and atherosclerosis. Korean J. Anesthesiol. 2011;61(1):3–11. doi: 10.4097/kjae.2011.61.1.3. [http://dx.doi.org/10.4097/kjae.2011.61.1.3]. [PMID: 21860744]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Zahedi K., Huttinger F., Morrison R., Murray-Stewart T., Casero R.A., Strauss K.I. Polyamine catabolism is enhanced after traumatic brain injury. J. Neurotrauma. 2010;27(3):515–525. doi: 10.1089/neu.2009.1097. [http://dx.doi.org/10.1089/neu.2009.1097]. [PMID: 19968558]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Wood P.L., Khan M.A., Kulow S.R., Mahmood S.A., Moskal J.R. Neurotoxicity of reactive aldehydes: the concept of “aldehyde load” as demonstrated by neuroprotection with hydroxylamines. Brain Res. 2006;1095(1):190–199. doi: 10.1016/j.brainres.2006.04.038. [http://dx.doi.org/10.1016/ j.brainres.2006.04.038]. [PMID: 16730673]. [DOI] [PubMed] [Google Scholar]
- 122.Takano K., Ogura M., Nakamura Y., Yoneda Y. Neuronal and glial responses to polyamines in the ischemic brain. Curr. Neurovasc. Res. 2005;2(3):213–223. doi: 10.2174/1567202054368335. [http://dx.doi.org/10.2174/ 1567202054368335]. [PMID: 16181115]. [DOI] [PubMed] [Google Scholar]
- 123.Chen B., Calvert A.E., Cui H., Nelin L.D. Hypoxia promotes human pulmonary artery smooth muscle cell proliferation through induction of arginase. Am. J. Physiol. Lung Cell. Mol. Physiol. 2009;297(6):L1151–L1159. doi: 10.1152/ajplung.00183.2009. [http://dx.doi.org/10.1152/ajplung. 00183.2009]. [PMID: 19801451]. [DOI] [PubMed] [Google Scholar]
- 124.Jin Y., Calvert T.J., Chen B., Chicoine L.G., Joshi M., Bauer J.A., Liu Y., Nelin L.D., Bauer J.A., Liu Y., Nelin L.D. Mice deficient in Mkp-1 develop more severe pulmonary hypertension and greater lung protein levels of arginase in response to chronic hypoxia. Am. J. Physiol. Heart Circ. Physiol. 2010;298(5):H1518–H1528. doi: 10.1152/ajpheart.00813.2009. [http://dx.doi.org/10.1152/ajpheart.00813.2009]. [PMID: 20173047]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Pernet V., Bourgeois P., Di Polo A. A role for polyamines in retinal ganglion cell excitotoxic death. J. Neurochem. 2007;103(4):1481–1490. doi: 10.1111/j.1471-4159.2007.04843.x. [http://dx.doi.org/10.1111/j.1471-4159.2007. 04843.x]. [PMID: 17714450]. [DOI] [PubMed] [Google Scholar]
- 126.Fulton A.B., Hansen R.M. Photoreceptor function in infants and children with a history of mild retinopathy of prematurity. J. Opt. Soc. Am. A Opt. Image Sci. Vis. 1996;13(3):566–571. doi: 10.1364/josaa.13.000566. [http://dx. doi.org/10.1364/JOSAA.13.000566]. [PMID: 8627413]. [DOI] [PubMed] [Google Scholar]
- 127.Fulton A.B., Hansen R.M., Moskowitz A., Barnaby A.M. Multifocal ERG in subjects with a history of retinopathy of prematurity. Doc. Ophthalmol. 2005;111(1):7–13. doi: 10.1007/s10633-005-2621-3. [http://dx.doi. org/10.1007/s10633-005-2621-3]. [PMID: 16502302]. [DOI] [PubMed] [Google Scholar]
- 128.Fulton A.B., Hansen R.M., Petersen R.A., Vanderveen D.K. The rod photoreceptors in retinopathy of prematurity: an electro- retinographic study. Arch. Ophthalmol. 2001;119(4):499–505. doi: 10.1001/archopht.119.4.499. [http://dx.doi.org/10.1001/archopht.119.4.499]. [PMID: 11296015]. [DOI] [PubMed] [Google Scholar]
- 129.Wu W.C., Lin R.I., Shih C.P., Wang N.K., Chen Y.P., Chao A.N., Chen K.J., Chen T.L., Hwang Y.S., Lai C.C., Huang C.Y., Tsai S. Visual acuity, optical components, and macular abnormalities in patients with a history of retinopathy of prematurity. Ophthalmology. 2012;119(9):1907–1916. doi: 10.1016/j.ophtha.2012.02.040. [http://dx. doi.org/10.1016/j.ophtha.2012.02.040]. [PMID: 22578258]. [DOI] [PubMed] [Google Scholar]
- 130.Dembinska O., Rojas L.M., Varma D.R., Chemtob S., Lachapelle P. Graded contribution of retinal maturation to the development of oxygen-induced retinopathy in rats. Invest. Ophthalmol. Vis. Sci. 2001;42(5):1111–1118. [PMID: 11274093]. [PubMed] [Google Scholar]
- 131.Morris S.M., Jr Recent advances in arginine metabolism: roles and regulation of the arginases. Br. J. Pharmacol. 2009;157(6):922–930. doi: 10.1111/j.1476-5381.2009.00278.x. [http://dx.doi.org/10.1111/j.1476-5381.2009.00278.x]. [PMID: 19508396]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Narayanan S.P., Xu Z., Putluri N., Sreekumar A., Lemtalsi T., Caldwell R.W., Caldwell R.B. Arginase 2 deficiency reduces hyperoxia-mediated retinal neurodegeneration through the regulation of polyamine metabolism. 2014. [DOI] [PMC free article] [PubMed]
- 133.Elms S.C., Toque H.A., Rojas M., Xu Z., Caldwell R.W., Caldwell R.B. The role of arginase I in diabetes-induced retinal vascular dysfunction in mouse and rat models of diabetes. Diabetologia. 2013;56(3):654–662. doi: 10.1007/s00125-012-2789-5. [http://dx.doi.org/10.1007/ s00125-012-2789-5]. [PMID: 23232640]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Burger J.A., Kipps T.J. CXCR4: a key receptor in the crosstalk between tumor cells and their microenvironment. Blood. 2006;107(5):1761–1767. doi: 10.1182/blood-2005-08-3182. [http://dx.doi.org/10.1182/blood-2005-08-3182]. [PMID: 16269611]. [DOI] [PubMed] [Google Scholar]
- 135.Lataillade J.J., Clay D., Dupuy C., Rigal S., Jasmin C., Bourin P., Le Bousse-Kerdilès M.C. Chemokine SDF-1 enhances circulating CD34(+) cell proliferation in synergy with cytokines: possible role in progenitor survival. Blood. 2000;95(3):756–768. [PMID: 10648383]. [PubMed] [Google Scholar]
- 136.Ruiz de Almodovar C., Luttun A., Carmeliet P. An SDF-1 trap for myeloid cells stimulates angiogenesis. Cell. 2006;124(1):18–21. doi: 10.1016/j.cell.2005.12.023. [http://dx.doi.org/10.1016/j.cell.2005.12.023]. [PMID: 16413476]. [DOI] [PubMed] [Google Scholar]
- 137.Askari A.T., Unzek S., Popovic Z.B., Goldman C.K., Forudi F., Kiedrowski M., Rovner A., Ellis S.G., Thomas J.D., DiCorleto P.E., Topol E.J., Penn M.S. Effect of stromal-cell-derived factor 1 on stem-cell homing and tissue regeneration in ischaemic cardio- myopathy. Lancet. 2003;362(9385):697–703. doi: 10.1016/S0140-6736(03)14232-8. [http://dx.doi.org/ 10.1016/S0140-6736(03)14232-8]. [PMID: 12957092]. [DOI] [PubMed] [Google Scholar]
- 138.Butler J.M., Guthrie S.M., Koc M., Afzal A., Caballero S., Brooks H.L., Mames R.N., Segal M.S., Grant M.B., Scott E.W. SDF-1 is both necessary and sufficient to promote proliferative retinopathy. J. Clin. Invest. 2005;115(1):86–93. doi: 10.1172/JCI22869. [http://dx.doi.org/10.1172/JCI22869]. [PMID: 15630447]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Sonmez K., Drenser K.A., Capone A., Jr, Trese M.T. Vitreous levels of stromal cell-derived factor 1 and vascular endothelial growth factor in patients with retinopathy of prematurity. Ophthalmology. 2008;115(6):1065–1070.e1. doi: 10.1016/j.ophtha.2007.08.050. [http://dx.doi. org/10.1016/j.ophtha.2007.08.050]. [PMID: 18031819]. [DOI] [PubMed] [Google Scholar]
- 140.Ki-I Y., Arimura N., Noda Y., Yamakiri K., Doi N., Hashiguchi T., Maruyama I., Shimura M., Sakamoto T. Stromal-derived factor-1 and inflammatory cytokines in retinal vein occlusion. Curr. Eye Res. 2007;32(12):1065–1072. doi: 10.1080/02713680701758727. [http://dx.doi. org/10.1080/02713680701758727]. [PMID: 18085471]. [DOI] [PubMed] [Google Scholar]
- 141.Curnow S.J., Wloka K., Faint J.M., Amft N., Cheung C.M., Savant V., Lord J., Akbar A.N., Buckley C.D., Murray P.I., Salmon M. Topical glucocorticoid therapy directly induces up-regulation of functional CXCR4 on primed T lymphocytes in the aqueous humor of patients with uveitis. J. Immunol. 2004;172(11):7154–7161. doi: 10.4049/jimmunol.172.11.7154. [http://dx.doi.org/10.4049/jimmunol.172.11. 7154]. [PMID: 15153539]. [DOI] [PubMed] [Google Scholar]
- 142.Abu El-Asrar A.M., Struyf S., Kangave D., Geboes K., Van Damme J. Chemokines in proliferative diabetic retinopathy and proliferative vitreoretinopathy. Eur. Cytokine Netw. 2006;17(3):155–165. [PMID: 17194635]. [PubMed] [Google Scholar]
- 143.Peschon J.J., Slack J.L., Reddy P., Stocking K.L., Sunnarborg S.W., Lee D.C., Russell W.E., Castner B.J., Johnson R.S., Fitzner J.N., Boyce R.W., Nelson N., Kozlosky C.J., Wolfson M.F., Rauch C.T., Cerretti D.P., Paxton R.J., March C.J., Black R.A. An essential role for ectodomain shedding in mammalian development. Science. 1998;282(5392):1281–1284. doi: 10.1126/science.282.5392.1281. [http://dx.doi. org/10.1126/science.282.5392.1281]. [PMID: 9812885]. [DOI] [PubMed] [Google Scholar]
- 144.Sahin U., Weskamp G., Kelly K., Zhou H.M., Higashiyama S., Peschon J., Hartmann D., Saftig P., Blobel C.P. Distinct roles for ADAM10 and ADAM17 in ectodomain shedding of six EGFR ligands. J. Cell Biol. 2004;164(5):769–779. doi: 10.1083/jcb.200307137. [http://dx.doi.org/10. 1083/jcb.200307137]. [PMID: 14993236]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Blobel C.P. ADAMs: key components in EGFR signalling and development. Nat. Rev. Mol. Cell Biol. 2005;6(1):32–43. doi: 10.1038/nrm1548. [http:// dx.doi.org/10.1038/nrm1548]. [PMID: 15688065]. [DOI] [PubMed] [Google Scholar]
- 146.Mahmoodi M., Sahebjam S., Smookler D., Khokha R., Mort J.S. Lack of tissue inhibitor of metalloproteinases-3 results in an enhanced inflammatory response in antigen-induced arthritis. Am. J. Pathol. 2005;166(6):1733–1740. doi: 10.1016/S0002-9440(10)62483-2. [http://dx.doi.org/10.1016/ S0002-9440(10)62483-2]. [PMID: 15920158]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Mohammed F.F., Smookler D.S., Taylor S.E., Fingleton B., Kassiri Z., Sanchez O.H., English J.L., Matrisian L.M., Au B., Yeh W.C., Khokha R. Abnormal TNF activity in Timp3-/- mice leads to chronic hepatic inflammation and failure of liver regeneration. Nat. Genet. 2004;36(9):969–977. doi: 10.1038/ng1413. [http://dx.doi.org/10.1038/ng1413]. [PMID: 15322543]. [DOI] [PubMed] [Google Scholar]
- 148.Murthy A., Defamie V., Smookler D.S., Di Grappa M.A., Horiuchi K., Federici M., Sibilia M., Blobel C.P., Khokha R. Ectodomain shedding of EGFR ligands and TNFR1 dictates hepatocyte apoptosis during fulminant hepatitis in mice. J. Clin. Invest. 2010;120(8):2731–2744. doi: 10.1172/JCI42686. [http://dx.doi.org/10.1172/ JCI42686]. [PMID: 20628198]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Smookler D.S., Mohammed F.F., Kassiri Z., Duncan G.S., Mak T.W., Khokha R. Tissue inhibitor of metalloproteinase 3 regulates TNF-dependent systemic inflammation. J. Immunol. 2006;176(2):721–725. doi: 10.4049/jimmunol.176.2.721. [http://dx.doi.org/10.4049/jimmunol.176.2.721]. [PMID: 16393953]. [DOI] [PubMed] [Google Scholar]
- 150.Horiuchi K., Le Gall S., Schulte M. Substrate selectivity of EGF-receptor ligand sheddases and their regulation by phorbol esters and calcium influx. Mol. Biol. Cell. 2007;18:176–188. doi: 10.1091/mbc.E06-01-0014. [http://dx.doi.org/10.1091/mbc.E06-01-0014]. [PMID: 17079736]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Le Gall S., Bobe P., Reiss K. ADAMs 10 and 17 represent differentially regulated components of a general shedding machinery for membrane proteins such as TGFα, L-Selectin and TNFα. Mol. Biol. Cell. 2009;20:1785–1794. doi: 10.1091/mbc.E08-11-1135. [http://dx.doi.org/ 10.1091/mbc.E08-11-1135]. [PMID: 19158376]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Blobel C.P. ADAMs: key components in EGFR signalling and development. Nat. Rev. Mol. Cell Biol. 2005;6(1):32–43. doi: 10.1038/nrm1548. [http:// dx.doi.org/10.1038/nrm1548]. [PMID: 15688065]. [DOI] [PubMed] [Google Scholar]
- 153.Franzke C.W., Cobzaru C., Triantafyllopoulou A., Löffek S., Horiuchi K., Threadgill D.W., Kurz T., van Rooijen N., Bruckner-Tuderman L., Blobel C.P. Epidermal ADAM17 maintains the skin barrier by regulating EGFR ligand-dependent terminal keratinocyte differentiation. J. Exp. Med. 2012;209(6):1105–1119. doi: 10.1084/jem.20112258. [http://dx.doi.org/10.1084/jem.20112258]. [PMID: 22565824]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Jackson L.F., Qiu T.H., Sunnarborg S.W., Chang A., Zhang C., Patterson C., Lee D.C. Defective valvulogenesis in HB-EGF and TACE-null mice is associated with aberrant BMP signaling. EMBO J. 2003;22(11):2704–2716. doi: 10.1093/emboj/cdg264. [http://dx.doi.org/10.1093/emboj/ cdg264]. [PMID: 12773386]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Sternlicht M.D., Sunnarborg S.W., Kouros-Mehr H., Yu Y., Lee D.C., Werb Z. Mammary ductal morphogenesis requires paracrine activation of stromal EGFR via ADAM17-dependent shedding of epithelial amphiregulin. Development. 2005;132(17):3923–3933. doi: 10.1242/dev.01966. [http://dx.doi.org/10.1242/dev.01966]. [PMID: 16079154]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Chalaris A., Adam N., Sina C., Rosenstiel P., Lehmann-Koch J., Schirmacher P., Hartmann D., Cichy J., Gavrilova O., Schreiber S., Jostock T., Matthews V., Häsler R., Becker C., Neurath M.F., Reiss K., Saftig P., Scheller J., Rose-John S. Critical role of the disintegrin metalloprotease ADAM17 for intestinal inflammation and regeneration in mice. J. Exp. Med. 2010;207(8):1617–1624. doi: 10.1084/jem.20092366. [http://dx.doi.org/10.1084/jem.20092366]. [PMID: 20603312]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Xiang N., Zhao M.J., Li X.Y., Zheng H.H., Li G.G., Li B. Redundant mechanisms for vascular growth factors in retinopathy of prematurity in vitro. Ophthalmic Res. 2011;45(2):92–101. doi: 10.1159/000316134. [http://dx.doi.org/10.1159/000316134]. [PMID: 20720439]. [DOI] [PubMed] [Google Scholar]
- 158.Zhao M., Shi X., Liang J., Miao Y., Xie W., Zhang Y., Li X. Expression of pro- and anti-angiogenic isoforms of VEGF in the mouse model of oxygen-induced retinopathy. Exp. Eye Res. 2011;93(6):921–926. doi: 10.1016/j.exer.2011.10.013. [http://dx.doi.org/10.1016/j.exer.2011.10.013]. [PMID: 22067127]. [DOI] [PubMed] [Google Scholar]
- 159.McColm J.R., Geisen P., Hartnett M.E. VEGF isoforms and their expression after a single episode of hypoxia or repeated fluctuations between hyperoxia and hypoxia: relevance to clinical ROP. Mol. Vis. 2004;10:512–520. [PMID: 15303088]. [PMC free article] [PubMed] [Google Scholar]
- 160.Maretzky T., Evers A., Zhou W., Swendeman S.L., Wong P.M., Rafii S., Reiss K., Blobel C.P. Migration of growth factor-stimulated epithelial and endothelial cells depends on EGFR transactivation by ADAM17. Nat. Commun. 2011;2:229. doi: 10.1038/ncomms1232. [http://dx.doi.org/10.1038/ncomms1232]. [PMID: 21407195]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Swendeman S., Mendelson K., Weskamp G., Horiuchi K., Deutsch U., Scherle P., Hooper A., Rafii S., Blobel C.P. VEGF-A stimulates ADAM17-dependent shedding of VEGFR2 and crosstalk between VEGFR2 and ERK signaling. Circ. Res. 2008;103(9):916–918. doi: 10.1161/CIRCRESAHA.108.184416. [http://dx.doi.org/10.1161/CIRCRESAHA.108. 184416]. [PMID: 18818406]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Maretzky T., Evers A., Zhou W., Swendeman S.L., Wong P.M., Rafii S., Reiss K., Blobel C.P. Migration of growth factor-stimulated epithelial and endothelial cells depends on EGFR transactivation by ADAM17. Nat. Commun. 2011;2:229. doi: 10.1038/ncomms1232. [CrossRef]. [PubMed]. [http://dx.doi.org/10.1038/ncomms1232]. [PMID: 21407195]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Takita H., Yoneya S., Gehlbach P.L., Duh E.J., Wei L.L., Mori K. Retinal neuroprotection against ischemic injury mediated by intraocular gene transfer of pigment epithelium-derived factor. Invest. Ophthalmol. Vis. Sci. 2003;44(10):4497–4504. doi: 10.1167/iovs.03-0052. [http://dx.doi.org/10.1167/iovs.03-0052]. [PMID: 14507898]. [DOI] [PubMed] [Google Scholar]
- 164.Yoshida Y., Yamagishi S., Matsui T., Jinnouchi Y., Fukami K., Imaizumi T., Yamakawa R. Protective role of pigment epithelium-derived factor (PEDF) in early phase of experimental diabetic retinopathy. Diabetes Metab. Res. Rev. 2009;25(7):678–686. doi: 10.1002/dmrr.1007. [http://dx.doi.org/10.1002/dmrr.1007]. [PMID: 19685553]. [DOI] [PubMed] [Google Scholar]
- 165.Kiagiadaki F., Savvaki M., Thermos K. Activation of somatostatin receptor (sst 5) protects the rat retina from AMPA-induced neurotoxicity. Neuropharmacology. 2010;58(1):297–303. doi: 10.1016/j.neuropharm.2009.06.028. [http://dx.doi.org/10.1016/j.neuropharm.2009.06.028]. [PMID: 19576912]. [DOI] [PubMed] [Google Scholar]
- 166.Gong Y., Chang Z.P., Ren R.T., Wei S., Zhou H.F., Chen X., Hou B., Jin X., Zhang M. Protective effect of adeno-associated virus mediated brain-derived neurotrophic factor expression on retinal ganglion cells in diabetic rats. Cell. Mol. Neurobiol. 2012;32:467–475. doi: 10.1007/s10571-011-9779-x. [http://dx.doi.org/10.1007/s10571-011-9779-x]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Zhang J., Wu Y., Jin Y., Ji F., Sinclair S.H., Luo Y., Xu G., Lu L., Dai W., Yanoff M., Li W., Xu G.T. Intravitreal injection of erythropoietin protects both retinal vascular and neuronal cells in early diabetes. Invest. Ophthalmol. Vis. Sci. 2008;49(2):732–742. doi: 10.1167/iovs.07-0721. [http://dx.doi.org/10.1167/iovs.07-0721]. [PMID: 18235022]. [DOI] [PubMed] [Google Scholar]
- 168.Wang Q., Gorbey S., Pfister F., Höger S., Dorn-Beineke A., Krügel K., Berrone E., Wu L., Korff T., Lin J., Busch S., Reichenbach A., Feng Y. Hammes H.P. Long-term treatment with sub-erythropoietic Epo is vaso- and neuroprotective in experimental diabetic retinopathy. Cell. Physiol. Biochem. 2011;27:769–782. doi: 10.1159/000330085. [http://dx.doi.org/10.1159/000330085]. [PMID: 21691094]. [DOI] [PubMed] [Google Scholar]
- 169.McVicar C.M., Hamilton R., Colhoun L.M., Gardiner T.A., Brines M., Cerami A., Stitt A.W. Intervention with an erythropoietin-derived peptide protects against neuroglial and vascular degeneration during diabetic retinopathy. Diabetes. 2011;60(11):2995–3005. doi: 10.2337/db11-0026. [http://dx.doi.org/10.2337/db11-0026]. [PMID: 21911748]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Singhal S., Bhatia B., Jayaram H., Becker S., Jones M.F., Cottrill P.B., Khaw P.T., Salt T.E., Limb G.A. Human Müller glia with stem cell characteristics differentiate into retinal ganglion cell (RGC) precursors in vitro and partially restore RGC function in vivo following transplantation. Stem Cells Transl. Med. 2012;1(3):188–199. doi: 10.5966/sctm.2011-0005. [http://dx.doi.org/10.5966/sctm.2011-0005]. [PMID: 23197778]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Semeran K., Pawłowski P., Lisowski Ł., Szczepaniak I., Wójtowicz J., Ławicki S., Bakunowicz-Łazarczyk A., Bossowski A. Plasma levels of IL-17, VEGF, and adrenomedullin and S-cone dysfunction of the retina in children and adolescents without signs of retinopathy and with varied duration of diabetes. Mediators Inflamm. 2015:274726. doi: 10.1155/2013/274726. accessed 31.08.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]