Abstract
Background
Stress of different origin is known to alter so called “brain-gut axis” and contributes to a broad array of gastrointestinal disorders including inflammatory bowel disease (IBD), irritable bowel syndrome (IBS) and other functional gastrointestinal diseases. The stressful situations and various stressors including psychosocial events, heat, hypo- and hyperthermia may worsen the course of IBD via unknown mechanism. The aims of this paper were to provide an overview of experimental and clinical evidences that stress activates the brain-gut axis which results in a mucosal mast cells activation and an increase in the production of proinflammatory cytokines and other endocrine and humoral mediators.
Methods
Research and online content related to effects of stress on lower bowel disorders are reviewed and most important mechanisms are delineated.
Results
Brain conveys the neural, endocrine and circulatory messages to the gut via brain-gut axis reflecting changes in corticotrophin releasing hormone, mast cells activity, neurotransmission at the autonomic nerves system and intestinal barrier function all affecting the pathogenesis of animal colitis and human IBD. Stress triggers the hypothalamus-pituitary axis and the activation of the autonomic nervous system, an increase in cortisol levels and proinflammatory cytokines such as tumor necrosis factor-alpha, interleukin-8, interleukin-1beta and interleukin-6.
Conclusion
The acute or chronic stress enhances the intestinal permeability weakening of the tight junctions and increasing bacterial translocation into the intestinal wall. An increased microbial load in the colonic tissue, excessive cytokine release and a partially blunted immune reactivity in response to stress result in its negative impact on IBD.
Keywords: Autonomic nervous system, Brain-gut axis, Cortisol, Enteric nervous system, Histamine, Inflammatory bowel disease, Microbiota, Proinflammatory cytokines, Stress
INTRODUCTION
A complex set of neural, epigenetic and environmental factors is widely recognized to characterize IBD, which is commonly represented by both ulcerative colitis (UC) and Crohn’s disease (CD). These factors lead to severe intestinal disease and long-term discomfort to the patients, sometimes throughout their entire life [1]. An important feature of these diseases is exposure to various degrees of stress and related psychological reactions such as anxiety and depression [2].
Loss of intestinal barrier integrity is a well-recognized medical consequence of a wide range of life-threatening stress conditions, such as heat stroke [3-5], hemorrhagic shock [6], trauma [7], burn injury [8, 9], pancreatitis [10], and septic shock [11, 12]. Interestingly, even milder stress conditions, such as prolonged endurance exercise [13, 14] and chronic psychological stress [15] can induce intestinal barrier dysfunction. Stress can both evoke damage to the gastric mucosa and perpetuate gastric microcirculation [16]. An increased risk of developing stress-related gastric mucosal lesions and gastrointestinal bleeding has been observed in critically ill patients [16]. Consequently, stress ulcerations are defined as acute gastric mucosal hemorrhagic lesions occurring as complications in severely ill patients after burns, sepsis, major surgery, or trauma to the central nervous system [16-20]. Such upper and lower gut consequences of stress insult depend upon the existence of a complex of psychological factors, long-term depression, psychosis, perceived stress, or brain trauma, which may in turn influence individual vulnerability and bi-directionality of brain pathways known to influence autonomic functions [16-18].
Particular stressors were shown to act on both the upper and lower gut via bidirectional communication through the brain-gut axis [17, 18]; however, the relationship between the incidence of such brain episodes, gut function, and severity of the disease in response to stress has not been fully explained. Interestingly, stress can influence not only patients with IBD but also those who suffer from irritable bowel syndrome (IBS), which is of clinical relevance for general practitioners (GPs) and the impetus for frequent consultations with gastroenterologists [2, 21, 22]. It is of particular interest that psychosocial factors such as psychological distress, anxiety, and depression have a higher prevalence not only in IBD but also in functional GI-disorders including IBS and functional dyspepsia [19, 20]. Patients suffering from IBS have been recently recognized as carriers of mucosal inflammation throughout the gut that is most likely either initiated or influenced by life-threatening stress. This stress perpetuates changes in mucosal barrier and intestinal microbiome, which have an impact on the patient’s quality of life and further contribute to progression of inflammation [20, 21]. Recent studies have documented that the occurrence of these IBS type symptoms results in abdominal pain, bloating, and discomfort associated with changes in bowel habit, which in turn are known to exert a negative influence on both mood and quality of life in patients with various forms of IBD [20-22].
The prevalence of IBS symptoms ranges from 10% to 30% in the general population, with higher prevalence among young adult women. Diet-exacerbated IBS could be mediated by a central brain-oriented mechanism. UC in both humans and experimental animal models is predominantly of local origin and usually developed in the lower bowel, i.e. colon and rectum [23-25]. Stress has been shown to complicate both diseases and worsen the outcomes as manifested by greater incidence of flare ups and index severity of changes in the colon [26].
Neural and Endocrine Aspects of Intestinal Disorders. Focus on Stress
The brain-gut axis is an integrative interaction allowing for a bidirectional communication between central centers. This includes central regulation of appetite, the descending autonomic nerves, the enteric nervous system (ENS) with interneurons, sensory neurons, motor neurons, neuro-transmitters, and endocrine pathways [16-18]. The specific centers in the brain including hypothalamus, amygdala, and hippocampus and their interaction with emotional centers localized within limbic system are mainly involved in the control of body reaction in response to stress [27]. The integrative reactions of these centers results in the activation of the hypothalamus-pituitary axis (HPA) and the autonomic nervous system (ANS), a key modulator of the ENS Fig. (1). The efferent communication between the CNS and the gut mucosa include the vagal nerve and pelvic parasympathetic efferents and postganglionic sympathetic neurons [28]. The stress-induced activation of the HPA, widely accepted as a marker of stress response, results in an enhanced secretion of glucocorticosteroids and mineralocorticosteroids from the zona fasciculata and zona glomerulosa of the adrenal cortex, respectively [29, 30]. Another remaining part of ANS, namely, the sympathetic autonomic system becomes also activated. This leads to an increase in the secretion of major adrenal medulla hormones -- mainly catecholamines such as epinephrine and norepinephrine [31]. All these self-perpetuating downstream signals act via neural connections of brain-gut pathway together with reactive oxygen metabolites (ROM), local inflammatory factors, and circulating cytokines such as IL-1β, TNF-α, and IL-6 affecting gut homeostatic functions. Changes in the ecology of gut lumen flora and secretory activities of these microorganisms can also alter the gut function via mechanisms involving change in the local environment and the signaling molecules including catecholamines and neuromediators of ENS Fig. (1). The gut sends off the visceral signals of pain to the brain via ascending pathways involving the spinothalamic, spinoreticular, and spinomesencephalic tracts [27, 28].
Fig. (1).
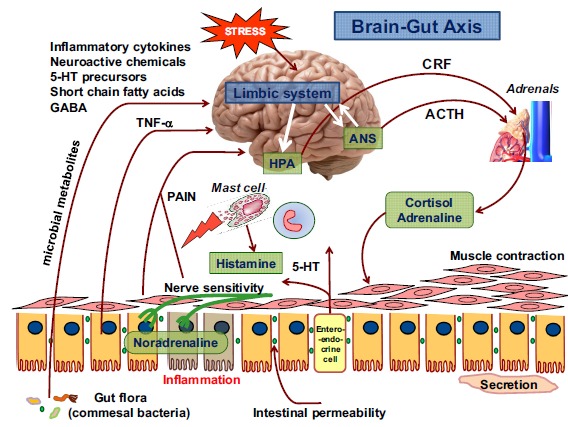
The complexity of stress affecting the function of brain, gut intestinal mucosa and particular functional components of intestinal wall via brain-gut axis. In response to stress, the inflammatory cytokines (TNF-α), various chemicals, short chain fatty acids (SCFA), and microbial products can alter the autonomic nervous system (ANS) leading to secretion of cortisol and adrenaline from adrenals via CRF of and ACTH-dependent pathways. The gut microbiota and neuroactive chemicals including histamine released from mast cells and serotonin (5-hydroxytryptamine) are known to impair the intestinal secretion and intestinal mucosa in response to stress. Various stressors render the intestinal mucosa more permeable, more absorbable to bacterial cytotoxins and neurotransmitters (norepinephrine), and contribute to the development of local inflammation. In conditions associated with stress, bacteria can undergo translocation from intestinal lumen into systemic circulation affecting the central and peripheral organs.
Brain-gut Signals in Stress-induced Functional GI Disorders
Stress-induced release of HPA hormones including adrenocorticotropic hormone (ACTH), corticotropin-releasing factor (CRF), and major catecholamine hormone noradrenalin is inhibited by substance P (SP) [29, 32]. The release of CRF from the hypothalamus in response to activation of the HPA has been shown to stimulate the synthesis of corticotropin in the pituitary gland, which stimulates the adrenal cortex to release cortisol, the major corticosteroid hormone released to the blood stream [31, 32]. Finally, circulating cortisol in the blood stream is made available to every tissue. This facilitates the cooperation between the brain and peripheral effectors known to govern specific GI-functions including motility, secretion, and defense of the gut [34]. The interaction between CRH and its receptor CRF1R is the most important in mediating the behavioral, neuroendocrine, and vasomotor responses to stress. On the other hand, SP is considered to play an important role in the modulation of inflammation during stress: previous studies have convincingly shown that the activity of SP in the central nervous system is increased in response to stress [30, 32]. The comprehensive interplay between nerve endings releasing neurotransmitters and mast cells releasing histamine, white blood elements including eosinophils, or plasma cells has been proposed based on the observation that mast cells identified in the GI-tract as enterochromatoffin-like (ECL) cells are structurally innervated within the intestinal mucosa [28, 29, 33, 34]. Mast cells are known to release histamine which is responsible for blood vessel permeability, secretory functions, and reciprocal release of other peptides observed upon neural stimulation [28, 29]. For instance, calcitonin gene-related peptide (CGRP)-immunoreactivity co-localizes with SP, and both neurotransmitters were found adjacent to mucosal mast cells [32]. This suggests a complex relationship between neural components consisting of the extrinsic afferents (vagal and/or spinal), epithelial component, and immune cells of the gut mucosa [34]. Moreover, the activity of various stressors is directly linked with SP-mediated inflammatory activity in peripheral tissues including the gut [29-32]. The over-expression of SP in the gut potentiates inflammation by several independent mechanisms, including facilitation of leukocyte (mainly white polymorphs) recruitment and subsequent tissue infiltration and stimulation of ROM generation, known to augment tissue damage [33-35]. Interestingly, the activated neutrophils were shown to exert antimicrobial activities and to act as a source of proinflammatory cytokines including IL-17A, IL-23 and IL-6 identified in patients with IBD at the site of inflammation [36]. Under stress conditions, O2•− concentrations rise leading to excessive production of deleterious oxygen metabolite, hydroxyl radical (OH•) through the Haber-Weiss reaction. The excessive formation of OH• is known to inactivate an essential mitochondrial enzyme pyruvate dehydrogenase identified in mitochondria, which depolymerizes enzymatic activity of mucin in the GI-tract. This mechanism can lead to mitochondrial RNA and DNA damage [35]. Apart from influencing mitochondrial metabolism, ROM was shown to influence progression through the cell cycle. ROM in human colon adenocarcinoma cells stimulated expression of p53 which, among other functions, has been shown to play the role of an oxidative response transcription factor, therefore causing S phase arrest [37]. This excessive ROM production undoubtedly contributes to development of mucosal inflammation in IBD patients. These oxygen cytotoxic products generated by phagocytes, neutrophils, and macro-phages have been implicated in the formation of direct epithelial colonic damage in IBD [38]. Several commonly used IBD therapeutics such as immunosuppressants, corticosteroids and anti-TNF-α antibodies could also affect the IBD progression by interfering with cellular oxidative stress and cytokine production [38]. For instance, targeting ROM by mesalazine (5-ASA), a metabolite of sulfasalazine widely used in the treatment of UC in humans, led to the reduction of oxidative stress by inhibiting O2• and H2O2 production as well as preventing mucosal GAPDH inhibition [39].
It is accepted that psychological status and physiological stimuli play an important role in the development of functional GI disorders (FGIDs) such as IBD and IBS [18]. The relationship between adverse symptoms, anxiety, and depression, as well as individual visceral hypersensitivity is critical for the regulation of psychological mood which can influence the generation and perception of regulatory physiological signals [40]. Stress-induced adrenal corticosteroid release is known in turn to impair the cortical function (i.e. hippocampus) leading to depression which can reactivate IBD [41]. This psychological manifestation is associated with a decrease in the synaptic levels of 5-hydroxytryptamine (serotonin) and norepinephrine in various brain region as well as reduced production and release of brain-derived neurotrophic factor (BNDF) in the hippocampus [40, 42].
A recent large prospective population-based study in more than 700 patients with IBD identified perceived stress, negative affect or mood, and major life events to be associated with symptomatic flares [43-45]. Longitudinal follow-up studies have suggested that there is a higher risk of developing anxiety or depression among people without mood disorders who report GI symptoms compatible with IBS at baseline [21, 46]. These studies have also suggested an increased likelihood of developing GI symptoms de novo among people who, at baseline, demonstrate anxiety or depression but not GI symptoms [47]. This bidirectional effect of the brain-gut pathway seen in FGIDs supports the notion that the relationship between the brain and the gut may also be bidirectional in UC, and that coexistent anxiety or depression, if unrecognized or untreated, may have a role in the generation of symptoms compatible with IBS in UC patients. In addition, chronic stress can modify many functions in the gut. These include permeability of intestinal wall, motility, and visceral pain changing also the central circuit of pain signals. The transmission of pain sensations and pain perception are influenced by the gut microbiota supporting the view that stress can interact with intestinal flora via gut-brain axis in complementary or opposing fashion, finally affecting behavior of visceral nociception [48]. Psychosocial stress and depression have been reported as essential factors affecting disease activity index in the pathogenesis of CD and UC [48, 49]. The mechanism of adverse effect of depression could involve the increasing risk of immune-mediated diseases through its effect on the HPA axis and ANS, systemic inflammatory cytokines, or immune cell function [50-52]. The understanding of brain-gut axis in psychosocial stress patients with IBD remains unexplored because studies examining the role of depression in IBD have primarily focused on those with established disease [52]. As mentioned above, patients with IBD are known to experience an increased risk of anxiety and depression [53, 54]. Moreover, patients with IBD can inadequately cope with psychosocial mechanisms: when they are exposed to additional stressful life events and depression, these factors would contribute to risk of disease relapse [49, 55]. Previous studies in humans revealed that depression is associated with elevated levels of C-reactive protein and tumor necrosis factor-alpha (TNF-α), both key mediators of inflammation in patients with IBD [55, 56].
Existing evidence in UC patients who were exposed to acute psychological stress revealed that stress insult enhanced serum and colonic mucosa levels of proinflammatory cytokines [57]. The effect of psychological counseling or treatment with antidepressants has been explored in patients with UC who acted as their own controls before and after receiving institutional consultation or other interventions [58]. In these patients, lower incidence of disease relapse as manifested by greater values of disease activity as well as less frequent utilization of glucocorticosteroids following their introduction has been observed [58, 59]. Additionally, improvement in overall depression scores were found for UC patients in a recent study following treatment with immuno-suppressive therapy with or without probiotics via the brain-gut axis [60]. The use of probiotics in UC patients with IBS-type symptoms may therefore be a viable therapeutic option [61, 62]. However, the efficacy and mechanism of the potential beneficial action of probiotics in IBD patients should be further elucidated. The use of probiotics have been examined in numerous randomized controlled trials concerning human UC; however, there is no satisfactory conclusions due to the large variety of species and strains that had been employed. Therefore, more convincing data are required using additional randomized trials in humans. Interestingly, however, the probiotic bacterial strain of Escherichia coli Nissle 1917 was found beneficial in preventing relapse of disease activity in quiescent UC [61]. The probiotic Escherichia coli Nissle 1917 exerted protective activity in experimental rodent models of cold-stress induced gastric lesions due to an increase in the gastric micro-circulation mediated by protective prostaglandins and nitric oxide-related mechanisms [63]. Unfortunately, none of the studies discussed so far addressed the efficacy of probiotics in the treatment of IBS-type symptoms in patients with UC with or without acute or chronic stress background and history.
Relationship between Stress, Intestinal Inflammation and Gut Microbiota in Experimental Colitis and the Course of IBD in Humans
Evidence to support the role of brain-gut axis in IBD -- including UC in humans that would depend upon a bidirectional relationship between the brain and gut -- comes mainly from studies in animal models with experimental colitis. Chronic GI inflammation involves behavioral changes that mimic alterations in mood disorders in humans [64]. It has been demonstrated that the induction of depression can reactivate inflammation of the colonic mucosa in murine models of quiescent colitis [41, 65]. Since this effect can be attenuated by the administration of antidepressant drugs, interference with the inhibition of proinflammatory macrophage activity by the vagus nerve has been proposed to explain the therapeutic efficacy of these drugs in experimental colitis [41, 62, 65].
Although animal models such as dextran sulfate sodium (DSS)-induced colitis do not represent the complexity of the human disease, recent attempts prompting the determination of intestinal inflammation in animal models of IBD have further uncovered signaling pathways during colon inflammation, thus suggesting the potential for novel advancements in the therapy of these intestinal disorders. In a DDS murine model of colitis, induction of depression by olfactory bulbectomy or intracerebroventricular injection of reserpine was associated with reactivation of inflammation in mice with previously established quiescent chronic inflammation [41]. Moreover, this effect was mediated in part by an increase in proinflammatory cytokine secretion by macrophages [41]. Interestingly, administration of tricyclic antidepressants prevented reactivation of colitis among depressed mice but not non-depressed mice [38, 41]. The mechanism of deleterious influence of stress on intestinal disorders may involve the activation of interleukin (IL)-6, IL-10, IL-1β, and tumor necrosis factor-α (TNF-α) production in response to stimulation by bacterial lipopolysaccharide [41, 44]. In addition, acute experimental stress in animals and stressful life events in humans can also alter the number and function of CD4 and CD8 lymphocytes and natural killer as well as modulate platelet function and their activation [41, 44, 66]. The higher number of important stress events was recorded in a period preceding exacerbation of ulcerative colitis in patients with clinically and endoscopically inactive ulcerative colitis evaluated prospectively for one year [67]. Duffy et al. [68] have also shown that there is a positive temporal relationship between incidence of stress and exacerbation of IBD. Disease activity stratified by an increased risk of clinical exacerbation was higher among stress-exposed patients compared with unexposed patients [68].
Stress and inflammation are interacting together as clearly demonstrated in animal studies; however, it is less clear whether stress is directly associated with inflammation in IBD patients. Recently, Targownik et al. [69] demonstrated that perceived stress was strongly associated with symptomatic based disease activity both in CD patients and those with UC. However, there was no correlation between symptom scores and the degree of inflammation for participants with CD, and only a weak correlation for those with UC. Furthermore, perceived stress was not inversely correlated with intestinal inflammation as measured in their study by fecal calprotectin [69]. They concluded that the association between perceived stress and IBD symptoms may occur independently of inflammatory activity associated with IBD [69].
Even the accumulated evidence on relationship between stress and IBD in human is controversial and not fully explained. Several studies have implicated stress as an important factor in both predisposition and reactivation of colitis in hapten-induced animal models [70, 71]. Indeed, both acute and chronic stress applied to laboratory animals have been shown to increase ion and water secretion and intestinal permeability in the jejunum and the colon of laboratory animals. These changes were paralleled by a marked increase in epithelial macromolecular permeability, and were mimicked by the intraperitoneal injection of CRF through mast cells and neural pathways [70, 71]. For instance, mild restraint stress has been found to exaggerate the inflammatory response and disease activity when TNBS was administered [67]. Interestingly, when animals were exposed to stress which had been applied after administration of TNBS, this also resulted in an aggravation of colitis in these species [71]. Therefore, stress may predispose the organism to organ injury such as intestinal damage by either an increase in mucosal susceptibility to an inflammatory stimulus through altering intestinal physiological processes (for example, epithelial permeability) or by affecting the balance between mucosal oxidant and antioxidant mechanisms [72]. In another study, the mild restraint stress reactivated the inflammatory process in rats which have recovered from TNBS-induced colitis: this effect was reflected in increases in both MPO activity and inflammatory infiltrates in animals exposed to stress without accompanying structural damage [73]. The increased mucosal infiltration of neutrophils and mononuclear cells in the colonic mucosa and increased MPO activity observed in animals exposed to stress seem to suggest that white blood cells may be an initiating factor in intestinal inflammation and impairment of the mucosal defense mechanisms [73]. The relation between inflammation and cancer involves key inflammatory mediators, such as NF-κB-targeted gene products including TNF-α and COX-2 [52, 74]. The interaction of microbe-gene-inflammation involves an excessive production of TNF-α which has been implicated in both animals with experimental anxiety and depressive human subjects [75]. Interestingly, gut bacteria can also influence the change in behavior of animals [75]. This is supported by the theory of dysbiosis originating from observations in germ-free mice or pathogen-free mice [76]. Gut bacteria are hypothesized to act directly on lower bowel motility, sensitivity, and inflammation causing overgrowth and changes in the relative population of detrimental phyla such as Firmicutes and Bacteroides [76]. The most severe consequences of damage to the intestinal barrier involve translocation of bacteria or bacterial wall products into the circulation. These bacterial products can activate the innate immune system, leukocyte activation and CD4+ lymphocytes that were reported to induce pro- and anti-inflammatory cascades [77]. In agreement with the leucocyte and microbial concept in IBD, Qui et al. [77] reported that mechanism of the stress-induced reactivation of colitis could involve a transfer of information on the increased intestinal susceptibility by a CD-4-enriched population of lymphocytes from the spleen and the lymph nodes. When inflammation is sufficiently severe, shock -- and ultimately, multiple organ dysfunction syndrome (MODS) -- can occur [78]. In line with these evidences, a high incidence of bacteremia and/or endotoxemia was observed in patients who suffer from hemorrhagic shock or burn injury [79, 80].
This microbe-gut-brain communication helps to understand the pathogenesis of FGIDs and concept of the feedback loop linking the gut-brain to the brain-gut as shown in Fig. (2). In a study by Zwolinska-Wcislo et al., [74], TNBS-induced colitis in rats was associated with inflammation and upregulation of gene expression for proinflammatory COX-2. Treatment with antibiotics such as amoxicillin and probiotics such as Lacidofil accelerated healing of these colonic lesions during the time course of experimental colitis, and subsequently down-regulated the expression of COX-2 mRNA in colonic mucosa [74]. Moreover, administration of aspirin (ASA), the most potent NSAID, significantly increased the area of colonic damage, produced an increase in colonic tissue weight, and elevated the stress-related plasma cytokine IL-1β and TNF-α levels as well as the mRNA expression of these proinflammatory cytokines in the colonic mucosa in comparison with rats not treated with ASA [74]. It was, therefore, proposed that the inhibition of COX-2 enzyme by therapeutic agents such as celecoxib to prevent colonic damage by ROM and/or proinflammatory stimuli such as TNF-α and IL-1β in rodent model of colitis [74] could be at least in part translated to IBD patients and considered as an alternative strategy for treatment of human UC as well as intestinal cancer chemoprevention in humans.
Fig. (2).
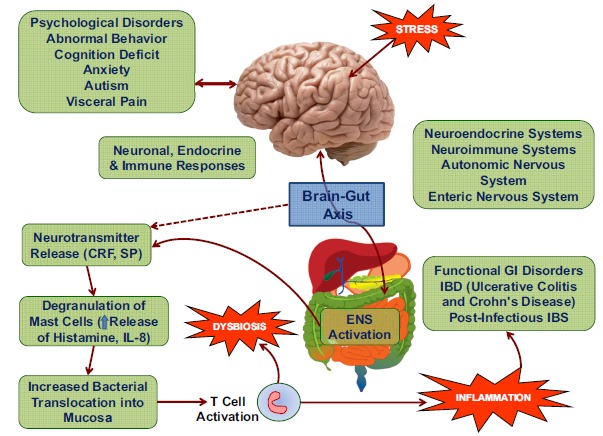
Schematic presentation of psychoneurological, endocrine, and immune responses to stress leading to intestinal inflammation, dysbiosis, and functional gastrointestinal disorders (FGIDs) including inflammatory bowel disease (IBD). Stress-induced activation of brain-gut axis and both autonomic and enteric systems (ANS and ENS) causes intestinal inflammation mediated by degranulation of mast cells releasing histamine and proinflammatory cytokines, T lymphocyte cell activation and bacterial translocation from mucosa to blood stream and peripheral organs.
The predominant negative consequence of acute injury to the intestinal mucosa induced by bacteria, stressors such as endotoxins, heat stroke, hemorrhagic shock, or NSAID ingestion is the translocation of bacteria or bacterial wall fragments into the bloodstream [81-83]. However, recent studies have demonstrated that other inflammatory mediators besides endotoxin (e.g. proteases) can translocate by crossing the intestinal barrier. Such translocations from the intestine may be equally -- if not more -- important in initiating proinflammatory cascades responsible for cytokine activation and release as well as behavioral alterations and MODS [81, 84-86].
Animal studies revealed that the chronic exposure to stress contributes to changes in the expression pattern of intestinal hexose and lipid transporters because the coordinated and site-specific increase in expression of glucose transporters SLC5A1 and SLC2A2 may correlate with pathological changes induced by stress [87]. These clearly indicates that chronic stress not only promotes intestinal inflammation, but also may promote metabolic alterations including sugar uptake and glycaemia. This is corroborative with the observation that hyperglycemia and the development of metabolic syndrome have been implicated in some human phenotypes of IBD [87]. Moderate exercise can also influence the course of experimental colitis in rodents both with standard diet and in those fed with diet-induced obesity [88, 89]. Lipid-rich diet augmented the severity of experimental colitis, while the beneficial effects of physical exercise accelerated healing of colitis by modifying muscle-adipose tissue crosstalk -- including the release by skeletal muscle of a newly discovered protein named irisin [88]. Exercise can also affect the development of IBD since Elsenbruch et al. [90] recently reported significantly higher mental and physical health scores and significantly greater improvements on the IBD Questionnaire (IBDQ) for subjects in an exercise group than controls post-intervention. Further studies are needed to confirm these interesting findings in terms of the benefits of moderate vs strenuous exercise (perceived as stressor) in large scale patient population with functional GI disorders.
In summary, the interaction between the predisposition of the host due to genetic and environmental factors, and an inappropriate immune response to bacterial flora in the intestinal lumen are the major factors in pathogenesis of IBD including Crohn’s disease and ulcerative colitis. The role of stress-induced gut mucosal pathophysiology has not been fully clarified but recent evidence indicates that chronic stress may be implicated in the development of disease. The scientific evidence for an epidemiologic connection between IBD and stress is conflicting. In this review we provided existing evidence that persistent stress and life events increase the risk for exacerbations of both ulcerative colitis and, to a lesser extent, CD; however, the epidemiological as well as mechanical evidence is less convincing. The brain conveys neural, endocrine and circulatory messages to the gut via brain-gut axis. These messages reflect changes in CRH, mast cells activity, neurotransmission at the ANS nerve endings, and intestinal barrier function determined so far in both stress animal models and human IBD. Such signals are transduced from the CNS to the gut mucosa and in opposite direction from gut to the brain via vagal afferent pathways, thus constituting a loop between central and peripheral GI organs. The mechanism through which stress from different origin can affect multiple aspects of functional GI disorders -- including on the emerging role of microbiome and probiotic bacteria -- should be further explored under both experimental conditions and human clinical trials.
ACKNOWLEDGEMENTS
This study was supported by statutory grant K/DSC/002035 for young investigators from Jagiellonian University Medical College and grant from National Research Centre in Poland (UMO-2013/09/B/NZ4/01566). Authors are thankful to Mr. Cyrus Sani for critical comments and helpful suggestions to improve this paper.
CONFLICT OF INTEREST
The authors confirm that this article content has no conflict of interest.
REFERENCES
- 1.Barbara G., Cremon C., Stanghellini V. Inflammatory bowel disease and irritable bowel syndrome: similarities and differences. Curr. Opin. Gastroenterol. 2014;30(4):352–358. doi: 10.1097/MOG.0000000000000070. [DOI] [PubMed] [Google Scholar]
- 2.Gracie D.J., Ford A.C. IBS-like symptoms in patients with ulcerative colitis. Clin. Exp. Gastroenterol. 2015;8:101–109. doi: 10.2147/CEG.S58153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shapiro Y., Alkan M., Epstein Y., Newman F., Magazanik A. Increase in rat intestinal permeability to endotoxin during hyper- thermia. Eur. J. Appl. Physiol. Occup. Physiol. 1986;55(4):410–412. doi: 10.1007/BF00422742. [DOI] [PubMed] [Google Scholar]
- 4.Yu J., Yin P., Liu F., Cheng G., Guo K., Lu A., Zhu X., Luan W., Xu J. Effect of heat stress on the porcine small intestine: a morphological and gene expression study. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2010;156(1):119–128. doi: 10.1016/j.cbpa.2010.01.008. [DOI] [PubMed] [Google Scholar]
- 5.Oliver S.R., Phillips N.A., Novosad V.L., Bakos M.P., Talbert E.E., Clanton T.L. Hyperthermia induces injury to the intestinal mucosa in the mouse: evidence for an oxidative stress mechanism. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2012;302(7):R845–R853. doi: 10.1152/ajpregu.00595.2011. [http://dx.doi.org/10.1152/ajpregu.00595.2011]. [PMID: 22237593]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thuijls G., de Haan J.J., Derikx J.P., Daissormont I., Hadfoune M., Heineman E., Buurman W.A. Intestinal cytoskeleton degradation precedes tight junction loss following hemorrhagic shock. Shock. 2009;31(2):164–169. doi: 10.1097/SHK.0b013e31817fc310. [DOI] [PubMed] [Google Scholar]
- 7.Ding L.A., Li J.S., Li Y.S., Zhu N.T., Liu F.N., Tan L. Intestinal barrier damage caused by trauma and lipopolysaccharide. World J. Gastroenterol. 2004;10(16):2373–2378. doi: 10.3748/wjg.v10.i16.2373. [http://dx.doi.org/10.3748/wjg.v10.i16.2373]. [PMID: 15285022]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jones W.G., II, Minei J.P., Barber A.E., Fahey T.J., III, Shires G.T., III, Shires G.T. Splanchnic vasoconstriction and bacterial translocation after thermal injury. Am. J. Physiol. 1991;261(4 Pt 2):H1190–H1196. doi: 10.1152/ajpheart.1991.261.4.H1190. [PMID: 1928402]. [DOI] [PubMed] [Google Scholar]
- 9.Jiang J.X., Tian K.L., Chen H.S., Zhu P.F., Wang Z.G. Plasma cytokines and endotoxin levels in patients with severe injury and their relationship with organ damage. Injury. 1997;28(8):509–513. doi: 10.1016/s0020-1383(97)00057-0. [http://dx.doi.org/10.1016/S0020-1383(97)00057-0]. [PMID: 9616386]. [DOI] [PubMed] [Google Scholar]
- 10.Wang X.D., Wang Q., Andersson R., Ihse I. Alterations in intestinal function in acute pancreatitis in an experimental model. Br. J. Surg. 1996;83(11):1537–1543. doi: 10.1002/bjs.1800831113. [http://dx.doi.org/10.1002/ bjs.1800831113]. [PMID: 9026331]. [DOI] [PubMed] [Google Scholar]
- 11.Xu D., Qi L., Guillory D., Cruz N., Berg R., Deitch E.A. Mechanisms of endotoxin-induced intestinal injury in a hyperdynamic model of sepsis. J. Trauma. 1993;34(5):676–682. doi: 10.1097/00005373-199305000-00010. [DOI] [PubMed] [Google Scholar]
- 12.Wang J.F., Gao Y.Q., Lippton H., Hyman A., Spitzer J.J. The roles of nitric oxide and hydrogen peroxide production in lipo- polysaccharide-induced intestinal damage. Shock. 1994;2(3):185–191. doi: 10.1097/00024382-199409000-00005. [DOI] [PubMed] [Google Scholar]
- 13.Pals K.L., Chang R.T., Ryan A.J., Gisolfi C.V. Effect of running intensity on intestinal permeability. (1985) J. Appl. Physiol. 1997;82(2):571–576. doi: 10.1152/jappl.1997.82.2.571. [DOI] [PubMed] [Google Scholar]; van Wijck K., Lenaerts K., van Loon L.J., Peters W.H., Buurman W.A., Dejang C.H. Exercise-induced splanchnic hypoperfusion results in gut dysfunction in healthy man. PLoS ONE. 2011;6(7):e22366. doi: 10.1371/journal.pone.0022366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Söderholm J.D., Yang P.C., Ceponis P., Vohra A., Riddell R., Sherman P.M., Perdue M.H. Chronic stress induces mast cell-dependent bacterial adherence and initiates mucosal inflammation in rat intestine. Gastroenterology. 2002;123(4):1099–1108. doi: 10.1053/gast.2002.36019. [http://dx.doi.org/10.1053/gast.2002.36019]. [PMID: 12360472]. [DOI] [PubMed] [Google Scholar]
- 16.Konturek P.C., Brzozowski T., Konturek S.J. Stress and the gut: pathophysiology, clinical consequences, diagnostic approach and treatment options. J. Physiol. Pharmacol. 2011;62(6):591–599. [PMID: 22314561]. [PubMed] [Google Scholar]
- 17.Koloski N.A., Jones M., Kalantar J., Weltman M., Zaguirre J., Talley N.J. The brain--gut pathway in functional gastrointestinal disorders is bidirectional: a 12-year prospective population-based study. Gut. 2012;61(9):1284–1290. doi: 10.1136/gutjnl-2011-300474. [http://dx.doi.org/10.1136/ gutjnl-2011-300474]. [PMID: 22234979]. [DOI] [PubMed] [Google Scholar]
- 18.Jones M.P., Dilley J.B., Drossman D., Crowell M.D. Brain-gut connections in functional GI disorders: anatomic and physiologic relationships. Neurogastroenterol. Motil. 2006;18(2):91–103. doi: 10.1111/j.1365-2982.2005.00730.x. [http://dx.doi.org/10.1111/j.1365-2982.2005.00730.x]. [PMID: 16420287]. [DOI] [PubMed] [Google Scholar]
- 19.Whitehead W.E., Palsson O., Jones K.R. Systematic review of the comorbidity of irritable bowel syndrome with other disorders: what are the causes and implications? Gastroenterology. 2002;122(4):1140–1156. doi: 10.1053/gast.2002.32392. [http://dx.doi.org/10.1053/gast.2002.32392]. [PMID: 11910364]. [DOI] [PubMed] [Google Scholar]
- 20.Henningsen P., Zimmermann T., Sattel H. Medically unexplained physical symptoms, anxiety, and depression: a meta-analytic review. Psychosom. Med. 2003;65(4):528–533. doi: 10.1097/01.psy.0000075977.90337.e7. [http://dx.doi.org/10.1097/01.PSY.0000075977.90337.E7]. [PMID: 12883101]. [DOI] [PubMed] [Google Scholar]
- 21.Graff L.A., Walker J.R., Bernstein C.N. Depression and anxiety in inflammatory bowel disease: a review of comorbidity and management. Inflamm. Bowel Dis. 2009;15(7):1105–1118. doi: 10.1002/ibd.20873. [http://dx.doi.org/10.1002/ibd.20873]. [PMID: 19161177]. [DOI] [PubMed] [Google Scholar]
- 22.Lackner J.M., Ma C.X., Keefer L., Brenner D.M., Gudleski G.D., Satchidanand N., Firth R., Sitrin M.D., Katz L., Krasner S.S., Ballou S.K., Naliboff B.D., Mayer E.A. Type, rather than number, of mental and physical comorbidities increases the severity of symptoms in patients with irritable bowel syndrome. Clin. Gastroenterol. Hepatol. 2013;11(9):1147–1157. doi: 10.1016/j.cgh.2013.03.011. [http://dx. doi.org/10.1016/j.cgh.2013.03.011]. [PMID: 23524278]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mittermaier C., Dejaco C., Waldhoer T., Oefferlbauer-Ernst A., Miehsler W., Beier M., Tillinger W., Gangl A., Moser G. Impact of depressive mood on relapse in patients with inflammatory bowel disease: a prospective 18-month follow-up study. Psychosom. Med. 2004;66(1):79–84. doi: 10.1097/01.psy.0000106907.24881.f2. [http://dx.doi.org/10.1097/01.PSY. 0000106907.24881.F2]. [PMID: 14747641]. [DOI] [PubMed] [Google Scholar]
- 24.Panara A.J., Yarur A.J., Rieders B., Proksell S., Deshpande A.R., Abreu M.T., Sussman D.A. The incidence and risk factors for developing depression after being diagnosed with inflammatory bowel disease: a cohort study. Aliment. Pharmacol. Ther. 2014;39(8):802–810. doi: 10.1111/apt.12669. [http://dx.doi.org/10.1111/apt.12669]. [PMID: 24588323]. [DOI] [PubMed] [Google Scholar]
- 25.Shah E., Rezaie A., Riddle M., Pimentel M. Psychological disorders in gastrointestinal disease: epiphenomenon, cause or consequence? Ann. Gastroenterol. 2014;27(3):224–230. [PMID: 24974805]. [PMC free article] [PubMed] [Google Scholar]
- 26.McEwen B.S. Physiology and neurobiology of stress and adaptation: central role of the brain. Physiol. Rev. 2007;87(3):873–904. doi: 10.1152/physrev.00041.2006. [http://dx.doi.org/10.1152/physrev.00041.2006]. [PMID: 17615391]. [DOI] [PubMed] [Google Scholar]
- 27.Drossman D.A. Functional abdominal pain syndrome. Clin. Gastroenterol. Hepatol. 2004;2(5):353–365. doi: 10.1016/s1542-3565(04)00118-1. [http://dx.doi.org/10. 1016/S1542-3565(04)00118-1]. [PMID: 15118972]. [DOI] [PubMed] [Google Scholar]
- 28.Keightley P.C., Koloski N.A., Talley N.J. Pathways in gut-brain communication: evidence for distinct gut-to-brain and brain-to-gut syndromes. Aust. N. Z. J. Psychiatry. 2015;49(3):207–214. doi: 10.1177/0004867415569801. [http://dx.doi.org/10.1177/0004867415569801]. [PMID: 25710826]. [DOI] [PubMed] [Google Scholar]
- 29.Israeli E., Hershcovici T., Berenshtein E., Zannineli G., Wengrower D., Weiss O., Chevion M., Goldin E. The effect of restraint stress on the normal colon and on intestinal inflammation in a model of experimental colitis. Dig. Dis. Sci. 2008;53(1):88–94. doi: 10.1007/s10620-007-9827-z. [http://dx.doi.org/10.1007/s10620-007-9827-z]. [PMID: 17565472]. [DOI] [PubMed] [Google Scholar]
- 30.Chowdrey H.S., Jessop D.S., Lightman S.L. Substance P stimulates arginine vasopressin and inhibits adrenocorticotropin release in vivo in the rat. Neuroendocrinology. 1990;52(1):90–93. doi: 10.1159/000125544. [http://dx.doi.org/10.1159/000125544]. [PMID: 1697661]. [DOI] [PubMed] [Google Scholar]
- 31.Soderholm J.D. Effect of stress on intestinal mucosal functions. In: Johnson L.R., editor. Physiology of the Gastrointestinal Tract, Two volume set. 2012. pp. 1979–2000. [http://dx.doi.org/10.1016/B978-0-12-382026-6.00074-9] [Google Scholar]
- 32.Faria M., Navarra P., Tsagarakis S., Besser G.M., Grossman A.B. Inhibition of CRH-41 release by substance P, but not substance K, from the rat hypothalamus in vitro. Brain Res. 1991;538(1):76–78. doi: 10.1016/0006-8993(91)90378-9. [http://dx.doi.org/10.1016/0006-8993(91)90378-9]. [PMID: 1708306]. [DOI] [PubMed] [Google Scholar]
- 33.Mazelin L., Theodorou V., More J., Emonds-Alt X., Fioramonti J., Bueno L. Comparative effects of nonpeptide tachykinin receptor antagonists on experimental gut inflammation in rats and guinea-pigs. Life Sci. 1998;63(4):293–304. doi: 10.1016/s0024-3205(98)00271-9. [http://dx.doi.org/10.1016/S0024-3205(98)00271-9]. [PMID: 9698038]. [DOI] [PubMed] [Google Scholar]
- 34.Martínez C., González-Castro A., Vicario M., Santos J. Cellular and molecular basis of intestinal barrier dysfunction in the irritable bowel syndrome. Gut Liver. 2012;6(3):305–315. doi: 10.5009/gnl.2012.6.3.305. [http://dx.doi.org/10.5009/gnl.2012.6.3.305]. [PMID: 22844557]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Halliwell B. Oxygen and nitrogen are pro-carcinogens. Damage to DNA by reactive oxygen, chlorine and nitrogen species: measurement, mechanism and the effects of nutrition. Mutat. Res. 1999;443(1-2):37–52. doi: 10.1016/s1383-5742(99)00009-5. [http://dx.doi.org/10.1016/S1383-5742(99) 00009-5]. [PMID: 10415430]. [DOI] [PubMed] [Google Scholar]
- 36.McKenzie S.J., Baker M.S., Buffinton G.D., Doe W.F. Evidence of oxidant-induced injury to epithelial cells during inflammatory bowel disease. J. Clin. Invest. 1996;98(1):136–141. doi: 10.1172/JCI118757. [http://dx.doi.org/10.1172/JCI118757]. [PMID: 8690784]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sun L., Wang X., Yao H., Li W., Son Y.O., Luo J., Liu J., Zhang Z. Reactive oxygen species mediate Cr(VI)-induced S phase arrest through p53 in human colon cancer cells. J. Environ. Pathol. Toxicol. Oncol. 2012;31(2):95–107. doi: 10.1615/jenvironpatholtoxicoloncol.v31.i2.20. [http://dx.doi.org/10. 1615/JEnvironPatholToxicolOncol.v31.i2.20]. [PMID: 23216635]. [DOI] [PubMed] [Google Scholar]
- 38.Piechota-Polanczyk A., Fichna J. Review article: the role of oxidative stress in pathogenesis and treatment of inflammatory bowel diseases. Naunyn Schmiedebergs Arch. Pharmacol. 2014;387(7):605–620. doi: 10.1007/s00210-014-0985-1. [http://dx.doi.org/10.1007/s00210-014-0985-1]. [PMID: 24798211]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Campregher C., Luciani M.G., Biesenbach P., Evstatiev R., Lyakhovich A., Gasche C. The position of the amino group on the benzene ring is critical for mesalamine’s improvement of replication fidelity. Inflamm. Bowel Dis. 2010;16(4):576–582. doi: 10.1002/ibd.21112. [http://dx.doi.org/10.1002/ibd.21112]. [PMID: 19821510]. [DOI] [PubMed] [Google Scholar]
- 40.Ananthakrishnan A.N., Khalili H., Pan A., Higuchi L.M., de Silva P., Richter J.M., Fuchs C.S., Chan A.T., Chan A.T. Association between depressive symptoms and incidence of Crohn’s disease and ulcerative colitis: results from the Nurses’ Health Study. Clin. Gastroenterol. Hepatol. 2013;11(1):57–62. doi: 10.1016/j.cgh.2012.08.032. [http://dx.doi.org/10.1016/j.cgh.2012.08.032]. [PMID: 22944733]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ghia J.E., Blennerhassett P., Deng Y., Verdu E.F., Khan W.I., Collins S.M. Reactivation of inflammatory bowel disease in a mouse model of depression. Gastroenterology. 2009;136(7):2280–2288. doi: 10.1053/j.gastro.2009.02.069. [http://dx.doi.org/10.1053/j.gastro.2009.02.069]. [DOI] [PubMed] [Google Scholar]
- 42.Duman R.S., Monteggia L.M. A neurotrophic model for stress-related mood disorders. Biol. Psychiatry. 2006;59(12):1116–1127. doi: 10.1016/j.biopsych.2006.02.013. [http://dx.doi.org/10.1016/j.biopsych.2006.02.013]. [PMID: 16631126]. [DOI] [PubMed] [Google Scholar]
- 43.Bernstein C.N., Singh S., Graff L.A., Walker J.R., Miller N., Cheang M. A prospective population-based study of triggers of symptomatic flares in IBD. Am. J. Gastroenterol. 2010;105(9):1994–2002. doi: 10.1038/ajg.2010.140. [http://dx.doi.org/10.1038/ajg.2010.140]. [PMID: 20372115]. [DOI] [PubMed] [Google Scholar]
- 44.Söderholm J.D., Perdue M.H. Stress and gastrointestinal tract. II. Stress and intestinal barrier function. Am. J. Physiol. Gastrointest. Liver Physiol. 2001;280(1):G7–G13. doi: 10.1152/ajpgi.2001.280.1.G7. [PMID: 11123192]. [DOI] [PubMed] [Google Scholar]
- 45.Wallon C., Persborn M., Jönsson M., Wang A., Phan V., Lampinen M., Vicario M., Santos J., Sherman P.M., Carlson M., Ericson A.C., McKay D.M., Söderholm J.D. Eosinophils express muscarinic receptors and corticotropin-releasing factor to disrupt the mucosal barrier in ulcerative colitis. Gastroenterology. 2011;140(5):1597–1607. doi: 10.1053/j.gastro.2011.01.042. [http://dx.doi.org/10.1053/j.gastro.2011. 01.042]. [PMID: 21277851]. [DOI] [PubMed] [Google Scholar]
- 46.North C.S., Alpers D.H., Helzer J.E., Spitznagel E.L., Clouse R.E. Do life events or depression exacerbate inflammatory bowel disease? A prospective study. Ann. Intern. Med. 1991;114(5):381–386. doi: 10.7326/0003-4819-114-5-381. [http://dx.doi.org/10.7326/0003-4819-114-5-381]. [PMID: 1992880]. [DOI] [PubMed] [Google Scholar]
- 47.Nahon S., Lahmek P., Durance C., Olympie A., Lesgourgues B., Colombel J.F., Gendre J.P. Risk factors of anxiety and depression in inflammatory bowel disease. Inflamm. Bowel Dis. 2012;18(11):2086–2091. doi: 10.1002/ibd.22888. [http://dx.doi.org/10.1002/ibd.22888]. [PMID: 22294486]. [DOI] [PubMed] [Google Scholar]
- 48.Mawdsley J.E., Rampton D.S. The role of psychological stress in inflammatory bowel disease. Neuroimmunomodulation. 2006;13(5-6):327–336. doi: 10.1159/000104861. [http://dx.doi.org/10.1159/000104861]. [PMID: 17709955]. [DOI] [PubMed] [Google Scholar]
- 49.Rampton D. Does stress influence inflammatory bowel disease? The clinical data. Dig. Dis. 2009;27(Suppl. 1):76–79. doi: 10.1159/000268124. [http://dx.doi.org/10.1159/000268124]. [PMID: 20203500]. [DOI] [PubMed] [Google Scholar]
- 50.Mawdsley J.E., Rampton D.S. Psychological stress in IBD: new insights into pathogenic and therapeutic implications. Gut. 2005;54(10):1481–1491. doi: 10.1136/gut.2005.064261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rampton D.S. The influence of stress on the development and severity of immune-mediated diseases. J. Rheumatol. Suppl. 2011;88:43–47. doi: 10.3899/jrheum.110904. [http://dx.doi.org/10.3899/jrheum.110904]. [PMID: 22045978]. [DOI] [PubMed] [Google Scholar]
- 52.Singh S., Graff L.A., Bernstein C.N. Do NSAIDs, antibiotics, infections, or stress trigger flares in IBD? Am. J. Gastroenterol. 2009;104(5):1298–1313. doi: 10.1038/ajg.2009.15. [http://dx.doi.org/10.1038/ajg.2009.15]. [PMID: 19337242]. [DOI] [PubMed] [Google Scholar]
- 53.Maunder R.G. Evidence that stress contributes to inflammatory bowel disease: evaluation, synthesis, and future directions. Inflamm. Bowel Dis. 2005;11(6):600–608. doi: 10.1097/01.mib.0000161919.42878.a0. [http://dx.doi.org/10.1097/01. MIB.0000161919.42878.a0]. [PMID: 15905709]. [DOI] [PubMed] [Google Scholar]
- 54.Goodhand J.R., Wahed M., Mawdsley J.E., Farmer A.D., Aziz Q., Rampton D.S. Mood disorders in inflammatory bowel disease: relation to diagnosis, disease activity, perceived stress, and other factors. Inflamm. Bowel Dis. 2012;18(12):2301–2309. doi: 10.1002/ibd.22916. [http://dx. doi.org/10.1002/ibd.22916]. [PMID: 22359369]. [DOI] [PubMed] [Google Scholar]
- 55.Ford D.E., Erlinger T.P. Depression and C-reactive protein in US adults: data from the Third National Health and Nutrition Examination Survey. Arch. Intern. Med. 2004;164:1010–1014. doi: 10.1001/archinte.164.9.1010. [http://dx.doi.org/10.1001/archinte.164.9.1010]. [DOI] [PubMed] [Google Scholar]
- 56.Tuglu C., Kara S.H., Caliyurt O., Vardar E., Abay E. Increased serum tumor necrosis factor-alpha levels and treatment response in major depressive disorder. Psychopharmacology (Berl.) 2003;170(4):429–433. doi: 10.1007/s00213-003-1566-z. [DOI] [PubMed] [Google Scholar]
- 57.Mawdsley J.E., Macey M.G., Feakins R.M., Langmead L., Rampton D.S. The effect of acute psychologic stress on systemic and rectal mucosal measures of inflammation in ulcerative colitis. Gastroenterology. 2006;131(2):410–419. doi: 10.1053/j.gastro.2006.05.017. [DOI] [PubMed] [Google Scholar]
- 58.Goodhand J.R., Greig F.I., Koodun Y., McDermott A., Wahed M., Langmead L., Rampton D.S. Do antidepressants influence the disease course in inflammatory bowel disease? A retrospective case-matched observational study. Inflamm. Bowel Dis. 2012;18(7):1232–1239. doi: 10.1002/ibd.21846. [http://dx.doi.org/10.1002/ibd.21846]. [PMID: 22234954]. [DOI] [PubMed] [Google Scholar]
- 59.Wahed M., Corser M., Goodhand J.R., Rampton D.S. Does psychological counseling alter the natural history of inflammatory bowel disease? Inflamm. Bowel Dis. 2010;16(4):664–669. doi: 10.1002/ibd.21098. [http://dx.doi.org/10.1002/ibd.21098]. [PMID: 19774642]. [DOI] [PubMed] [Google Scholar]
- 60.Horst S., Chao A., Rosen M., Nohl A., Duley C., Wagnon J.H., Beaulieu D.B., Taylor W., Gaines L., Schwartz D.A. Treatment with immunosuppressive therapy may improve depressive symptoms in patients with inflammatory bowel disease. Dig. Dis. Sci. 2015;60(2):465–470. doi: 10.1007/s10620-014-3375-0. [http://dx.doi.org/10.1007/s10620-014-3375-0]. [PMID: 25274158]. [DOI] [PubMed] [Google Scholar]
- 61.Matthes H., Krummenerl T., Giensch M., Wolff C., Schulze J. Clinical trial: probiotic treatment of acute distal ulcerative colitis with rectally administered Escherichia coli Nissle 1917 (EcN). BMC Complement. Altern. Med. 2010;10:13. doi: 10.1186/1472-6882-10-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Henker J., Müller S., Laass M.W., Schreiner A., Schulze J. Probiotic Escherichia coli Nissle 1917 (EcN) for successful remission maintenance of ulcerative colitis in children and adolescents: an open-label pilot study. Z. Gastroenterol. 2008;46(9):874–875. doi: 10.1055/s-2008-1027463. [http://dx.doi.org/10.1055/s-2008-1027463]. [PMID: 18810672]. [DOI] [PubMed] [Google Scholar]
- 63.Konturek P.C., Sliwowski Z., Koziel J., Ptak-Belowska A., Burnat G., Brzozowski T., Konturek S.J. Probiotic bacteria Escherichia coli strain Nissle 1917 attenuates acute gastric lesions induced by stress. J. Physiol. Pharmacol. 2009;60(Suppl. 6):41–48. [PMID: 20224150]. [PubMed] [Google Scholar]
- 64.Bercik P., Verdu E.F., Foster J.A., Macri J., Potter M., Huang X., Malinowski P., Jackson W., Blennerhassett P., Neufeld K.A., Lu J., Khan W.I., Corthesy-Theulaz I., Cherbut C., Bergonzelli G.E., Collins S.M. Chronic gastrointestinal inflammation induces anxiety-like behavior and alters central nervous system bio- chemistry in mice. Gastroenterology. 2010;139(6):2102–2112.e1. doi: 10.1053/j.gastro.2010.06.063. [http://dx.doi.org/10.1053/j.gastro.2010.06.063]. [PMID: 20600016]. [DOI] [PubMed] [Google Scholar]
- 65.Ghia J.E., Blennerhassett P., Collins S.M. Impaired parasympathetic function increases susceptibility to inflammatory bowel disease in a mouse model of depression. J. Clin. Invest. 2008;118(6):2209–2218. doi: 10.1172/JCI32849. [http://dx.doi.org/10.1172/JCI32849]. [PMID: 18451995]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Funderburg N.T., Stubblefield Park S.R., Sung H.C., Hardy G., Clagett B., Ignatz-Hoover J., Harding C.V., Fu P., Katz J.A., Lederman M.M., Levine A.D. Circulating CD4(+) and CD8(+) T cells are activated in inflammatory bowel disease and are associated with plasma markers of inflammation. Immunology. 2013;140(1):87–97. doi: 10.1111/imm.12114. [http://dx.doi.org/10.1111/imm.12114]. [PMID: 23600521]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bitton A., Sewitch M.J., Peppercorn M.A. deB Edwardes, M.D.; Shah, S.; Ransil, B.; Locke, S.E. Psychosocial determinants of relapse in ulcerative colitis: a longitudinal study. Am. J. Gastroenterol. 2003;98(10):2203–2208. doi: 10.1111/j.1572-0241.2003.07717.x. [http://dx.doi.org/10. 1111/j.1572-0241.2003.07717.x]. [PMID: 14572569]. [DOI] [PubMed] [Google Scholar]
- 68.Duffy L.C., Zielezny M.A., Marshall J.R., Byers T.E., Weiser M.M., Phillips J.F., Calkins B.M., Ogra P.L., Graham S. Relevance of major stress events as an indicator of disease activity prevalence in inflammatory bowel disease. Behav. Med. 1991;17(3):101–110. doi: 10.1080/08964289.1991.9937553. [http://dx.doi.org/10.1080/08964289.1991.9937553]. [PMID: 1932843]. [DOI] [PubMed] [Google Scholar]
- 69.Targownik L.E., Sexton K.A., Bernstein M.T., Beatie B., Sargent M., Walker J.R., Graff L.A., Graff L.A. The relationship among perceived stress, symptoms, and inflammation in persons with inflammatory bowel disease. Am. J. Gastroenterol. 2015;110(7):1001–1012. doi: 10.1038/ajg.2015.147. [http://dx.doi.org/10.1038/ajg.2015.147]. [PMID: 26077178]. [DOI] [PubMed] [Google Scholar]
- 70.Gué M., Bonbonne C., Fioramonti J., Moré J., Del Rio-Lachèze C., Coméra C., Buéno L. Stress-induced enhancement of colitis in rats: CRF and arginine vasopressin are not involved. Am. J. Physiol. 1997;272(1 Pt 1):G84–G91. doi: 10.1152/ajpgi.1997.272.1.G84. [PMID: 9038880]. [DOI] [PubMed] [Google Scholar]
- 71.Million M., Taché Y., Anton P. Susceptibility of Lewis and Fischer rats to stress-induced worsening of TNB-colitis: protective role of brain CRF. Am. J. Physiol. 1999;276(4 Pt 1):G1027–G1036. doi: 10.1152/ajpgi.1999.276.4.G1027. [PMID: 10198347]. [DOI] [PubMed] [Google Scholar]
- 72.Tüzün A., Erdil A., Inal V., Aydin A., Bağci S., Yeşilova Z., Sayal A., Karaeren N., Dağalp K. Oxidative stress and antioxidant capacity in patients with inflammatory bowel disease. Clin. Biochem. 2002;35(7):569–572. doi: 10.1016/S0009-9120(02)00361-2. [DOI] [PubMed] [Google Scholar]
- 73.Collins S.M., McHugh K., Jacobson K., Khan I., Riddell R., Murase K., Weingarten H.P. Previous inflammation alters the response of the rat colon to stress. Gastroenterology. 1996;111(6):1509–1515. doi: 10.1016/s0016-5085(96)70012-4. [http://dx.doi.org/10.1016/S0016-5085(96)70012-4]. [PMID: 8942729]. [DOI] [PubMed] [Google Scholar]
- 74.Zwolinska-Wcislo M., Krzysiek-Maczka G., Ptak-Belowska A., Karczewska E., Pajdo R., Sliwowski Z., Urbanczyk K., Drozdowicz D., Konturek S.J., Pawlik W.W., Brzozowski T. Antibiotic treatment with ampicillin accelerates the healing of colonic damage impaired by aspirin and coxib in the experimental colitis. Importance of intestinal bacteria, colonic microcirculation and proinflammatory cytokines. J. Physiol. Pharmacol. 2011;62(3):357–368. [PMID: 21893697]. [PubMed] [Google Scholar]
- 75.Neufeld K.M., Kang N., Bienenstock J., Foster J.A. Reduced anxiety-like behavior and central neurochemical change in germ-free mice. Neurogastroenterol. Motil., 2011, 23(3), 255-264, e119. 2011;23(3):255–264. doi: 10.1111/j.1365-2982.2010.01620.x. [DOI] [PubMed] [Google Scholar]
- 76.Cremon C., Carini G., De Giorgio R., Stanghellini V., Corinaldesi R., Barbara G. Intestinal dysbiosis in irritable bowel syndrome: etiological factor or epiphenomenon? Expert Rev. Mol. Diagn. 2010;10(4):389–393. doi: 10.1586/erm.10.33. [DOI] [PubMed] [Google Scholar]
- 77.Qiu B.S., Vallance B.A., Blennerhassett P.A., Collins S.M. The role of CD4+ lymphocytes in the susceptibility of mice to stress-induced reactivation of experimental colitis. Nat. Med. 1999;5(10):1178–1182. doi: 10.1038/13503. [http://dx.doi.org/10.1038/13503]. [PMID: 10502822]. [DOI] [PubMed] [Google Scholar]
- 78.Novosad V.L., Richards J.L., Phillips N.A., King M.A., Clanton T.L. Regional susceptibility to stress-induced intestinal injury in the mouse. Am. J. Physiol. Gastrointest. Liver Physiol. 2013;305(6):G418–G426. doi: 10.1152/ajpgi.00166.2013. [http://dx.doi.org/10.1152/ajpgi.00166.2013]. [PMID: 23868412]. [DOI] [PubMed] [Google Scholar]
- 79.Rush B.F., Jr, Sori A.J., Murphy T.F., Smith S., Flanagan J.J., Jr, Machiedo G.W. Endotoxemia and bacteremia during hemorrhagic shock. The link between trauma and sepsis? Ann. Surg. 1988;207(5):549–554. doi: 10.1097/00000658-198805000-00009. [http://dx.doi.org/10.1097/00000658-198805000-00009]. [PMID: 3377565]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Winchurch R.A., Thupari J.N., Munster A.M. Endotoxemia in burn patients: levels of circulating endotoxins are related to burn size. Surgery. 1987;102(5):808–812. [PMID: 3672321]. [PubMed] [Google Scholar]
- 81.Swidsinski A., Loening-Baucke V., Herber A. Mucosal flora in Crohn’s disease and ulcerative colitis - an overview. J. Physiol. Pharmacol. 2009;60(Suppl. 6):61–71. [PMID: 20224153]. [PubMed] [Google Scholar]
- 82.Strus M., Gosiewski T., Fyderek K., Wedrychowicz A., Kowalska-Duplaga K., Kochan P., Adamski P., Heczko P.B. A role of hydrogen peroxide producing commensal bacteria present in colon of adolescents with inflammatory bowel disease in perpetuation of the inflammatory process. J. Physiol. Pharmacol. 2009;60(Suppl. 6):49–54. [PMID: 20224151]. [PubMed] [Google Scholar]
- 83.Szkudlapski D., Labuzek K., Pokora Z., Smyla N., Gonciarz M., Mularczyk A., Maluch P., Okopien B. The emering role of helminths in treatment of the inflammatory bowel disorders. J. Physiol. Pharmacol. 2014;65(6):741–751. [PMID: 25554978]. [PubMed] [Google Scholar]
- 84.Deitch E.A. Intestinal permeability is increased in burn patients shortly after injury. Surgery. 1990;107(4):411–416. [PMID: 2108508]. [PubMed] [Google Scholar]
- 85.Schmid-Schönbein G.W. 2008 Landis Award lecture. Inflammation and the autodigestion hypothesis. Microcirculation. 2009;16(4):289–306. doi: 10.1080/10739680902801949. [http://dx.doi.org/10.1080/10739680902801949]. [PMID: 19384726]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.DeLano F.A., Hoyt D.B., Schmid-Schönbein G.W. Pancreatic digestive enzyme blockade in the intestine increases survival after experimental shock. Sci. Transl. Med. 2013;5(169):169ra11. doi: 10.1126/scitranslmed.3005046. [http://dx.doi.org/10.1126/scitranslmed.3005046]. [PMID: 23345609]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lee C.Y. Chronic restraint stress induces intestinal inflammation and alters the expression of hexose and lipid transporters. Clin. Exp. Pharmacol. Physiol. 2013;40(6):385–391. doi: 10.1111/1440-1681.12096. [http://dx.doi.org/10.1111/1440-1681.12096]. [PMID: 23586523]. [DOI] [PubMed] [Google Scholar]
- 88.Cook M.D., Martin S.A., Williams C., Whitlock K., Wallig M.A., Pence B.D., Woods J.A. Forced treadmill exercise training exacerbates inflammation and causes mortality while voluntary wheel training is protective in a mouse model of colitis. Brain Behav. Immun. 2013;33:46–56. doi: 10.1016/j.bbi.2013.05.005. [http://dx.doi.org/10.1016/j.bbi. 2013.05.005]. [PMID: 23707215]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Bilski J., Mazur-Bialy A.I., Wierdak M., Brzozowski T. The impact of physical activity and nutrition on inflammatory bowel disease: the potential role of cross talk between adipose tissue and skeletal muscle. J. Physiol. Pharmacol. 2013;64(2):143–155. [PMID: 23756389]. [PubMed] [Google Scholar]
- 90.Elsenbruch S., Langhorst J., Popkirowa K., Müller T., Luedtke R., Franken U., Paul A., Spahn G., Michalsen A., Janssen O.E., Schedlowski M., Dobos G.J. Effects of mind-body therapy on quality of life and neuroendocrine and cellular immune functions in patients with ulcerative colitis. Psychother. Psychosom. 2005;74(5):277–287. doi: 10.1159/000086318. [http://dx.doi.org/10.1159/000086318]. [PMID: 16088265]. [DOI] [PubMed] [Google Scholar]


