Abstract
Background
The authors, as internists, registered significant difference in the long lasting actions of surgical and chemical (atropine treatment) vagotomy in patients with peptic ulcer during second half of the last century (efficency, gastric acid secretion, gastrointestinal side effects, briefly benefical and harmful actions were examined).
Aims
1. Since the authors participated in the establishing of human clinical pharmacology in this field, they wanted to know more and more facts of the acute and chronic effects of surgical and chemical (atropine treatment) on the gastrointestinal mucosal biochemisms and their actions altered by bioactive compounds and scavengers regarding the development of gastric mucosal damage and protection.
Methods
The observations were carried out in animals under various experimental conditions (in intact, pylorus-ligated rats, in different experimental ulcer models, together with application of various mucosal protecting compounds) without and with surgical vagotomy and chemical vagotomy produced by atropine treatment.
Results
1. No changes were obtained in the cellular energy systems (ATP, ADP, AMP, cAMP, “adenylate pool”, “energy charge“ [(ATP+ 0.5 ADP)/ (ATP+ADP+AMP)] of stomach (glandular part, forestomach) in pylorus ligated rats after surgical vagotomy in contrast to those produced by only chemical vagotomy; 2. The effects of the gastric mucosal protective compounds [atropine, cimetidine, prostaglandins, scavengers (like vitamin A, β-carotene), capsaicin] disappeared after surgical vagotomy; 3. The extents of different chemical agents induced mucosal damaging effects were enhanced by surgical vagotomy and was not altered by chemical vagotomy; 4. The existence of feedback mechanisms of pharmacological (cellular and intracellular) regulatory mechanisms between the membrane-bound ATP-dependent energy systems exists in the gastric mucosa of intact animals, and after chemical vagotomy, but not after surgical vagotomy.
Conclusions
1. Increased vagal nerve activity takes place in the gastric mucosal damage; 2 both surgical and chemical vagotomy result mucosal protective affect on the gastric mucosal in different damaging experimental models; 3. The capsaicin-induced gastric mucosal damage depends on the applied doses, presence of anatomically intact vagal nerve (but independent from the chemical vagotomy), 4. The central and pheripheral neural regulations differ during gastric mucosal damage and protection induced by drugs, bioactive compounds, scavengers.
Keywords: Atropine treatment, Cellular energy systems, Gastric mucosal damage, Mucosal protection, Surgical vagotomy
INTRODUCTION
The number of patients with peptic ulcer is about 10 per cent of population in developed countries world wide. Throughout the last decades medical practice including our therapeutic tools significantly changed in this field (especially from 2005, when Warren and Marshall received Nobel price for the discovery of Helicobacter pylori bacteria as etiologic factor for the development of peptic ulcer disease).
During the first part of the 20th Century, the essential role of vagal nerve has been emphasized in the development of peptic ulcer, producing increased gastric HCl and pepsin secretion. Consequently the reduction of vagal nerve activity was introduced into medical treatment of peptic ulcer patients. At that time most of the peptic ulcer patients were treated medically by internists.
In the 1960-1970s years, the application of anticholinergic agents (as tertiary and quaternary ammonium compounds) was the only tool at the hand of internists to treat patients with classical peptic ulcer in every day medical practice. The drug treatment took about four weeks, while a significant part of these patients healed, however, certain portion of these patients was offered to surgeons for surgical intervention (most patients with unhealed peptic ulcer went under resection of stomach according to the dominant Billroth type II method, in smaller number according to Billroth type I resection or underwent gastroentero-anastomosis). Naturally, there were patients who met surgeons initicially and underwent gastric surgery before any medical therapy by internists.
The surgical vagotomy was introduced by Exner and Schwartzmann (1912) [1] in the treatment of peptic ulcer patients, however, they faced gastric atonia as unwanted complication, tried to applied tube for helping the entry of food into the small intestine). This new method resulted various technical difficulties and significant early and late complications of surgical vagotomy. Dragstedt and Owens [2] opened a new gate for surgical vagotomy, their technic of surgery resulted international revolution of surgical vagotomy and became the most common therapy for peptic ulcer patients. Their influence rapidly arrived to surgeons in Hungary and to world wide [3].
In the 1970-1980s years – in Hungary – the medical (by drug application) and surgical vagotomy offered alternative possibilities in the treatments of peptic ulcer patients.
As internists we wanted to understand the problems of “chemical vagotomy” in peptic ulcer patients, since most of the patients who went over surgical intervention (including the surgical vagotomy) presented different permanent complications (diarrhoea, recidive ulcer, GI bleedings, various forms of malabsorbtion syndromes, stump gastritis and cancer) [4].
Our scientific attention has been focussed to the medical treatment of patients with peptic ulcer diseases in the years from 1960s. We studied in details of atropine treatment (as chemical vagotomy) in patients with peptic ulcer (including its clinical pharmacological problems, actions on gastric acid secretion, ulcer healings, development of “pharmacological denervation phenomenon”, tolerance and cross-tolerance (to similar chemical compounds, most of them never became used in the medical treatment), reversibility of “pharmacological denervation phenomenon” and tolerance (cross-tolerance) after cessation of atropine treatment) [5].
Our results clearly inicated the following main facts: 1. the quaternary ammonium anticholinergic compounds cannot be absorbed from the gastrointestinal tract (consequently we cannot calculate with the real therapeutic actions in the treatment of patients with peptic ulcer); 2. the tertiary ammonium compounds are absorbed from the human GI tract; 3. chronic atropine treatment, the “pharmacological denervation phenomenon” together with development of “tolerance (and cross-tolerance)” appear in the patients during the time of “classical medical treatment” (by other words, the afficacy of medical treatment decreases during a chronic treatment, in association with the increase of patients’ complaints); 4. the “pharmacological denervation phenomenon” and “development of tolerance (cross-tolerance)” disappear in 6 to 10 days after cessation of atropine treatment.
The significant differences exist between the results of “chemical” and “surgical vagotomy” in patients with peptic ulcer, namely the complications of surgical vagotomy are permanent events, meanwhile the unwished phenomena (pharmacological denervation phenonenon and development of drug tolerance) are reversible processes (produced by chronic anticholinergic agents or compounds).
These results drove our attentions to study further the details of differences in actions of “surgical” and “chemical” vagotomy in gastrointestinal tract (especially in stomach). The main points in our attention were to understand the different regulatory mechanisms and biochemical backgrounds in the gastrointestinal tract on dependence of “surgical” and “chemical” vagotomy. Of course, we could study these questions only in animal experimental circumstances, indepedently from that scientific problems (presenting in this short summary) originated from human medical treatments in patients with peptic ulcer disease.
ANIMAL EXPERIMENTS
Many years earlier several clinical pharmacological examinations were carried out in patients with peptic ulcer, sometimes we had to face even harmful effect of drugs (with different mechanisms of actions) producing more gastrointestinal mucosal damage. The reduction of gastric hyperacidity used to be the main goal of human medical (drug or surgical) therapy.
Our attentions were focussed to understand in details of the target organs (stomach and small intestine) – with ulcer, without and with treatments using certain drugs - in the years of 1960-1970s. At that time there was no any knowledge on the biochemical backgrounds of development of gastric acid secretion and ulcer development. The effects of the vagal nerve activity and its surgical (vagotomy) and chemical (atropine) blockage were studied in animal stomach and gastrointestinal mucosa.
Biochemical Methods used in Earlier and Later Time Periods
The biochemical observations (measurements) were carried out in the forestomach (rumen) and glandular (fundic) parts of the rats’ stomach: acid-soluble inorganic and organic phosphates, lipids, ribonucleic acid (RNA) and deoxibonucleic acid (DNA) levels were determined [5].
The measurement of these biochemical examinations represented generally the main components of the cells: lipids (as cell membrane), acid-soluble inorganic and organic phosphates (mitochondrion), RNA (the cytoplasma and nucleus) and DNA (nucleus). By other words, we tried to approach the different compartments of cells. The measurements of amounts of acid-soluble inorganic phosphates in the different tissues are widely used to determine the dephosphorylation [by other words, these compounds are originated from the splitting of adenosine triphosphate (ATP), independently from its pathways]. The components of acid-soluble organic phosphates were not known at that time, meanwhile the presence of adenosine triphosphate (ATP), adenosine diphosphate (ADP), cyclic adenosine monophosphate (cAMP), adenosine monophosphate (AMP), adenine and adenosine were incorporated in this tissue extract. Naturally, these methodologies were updated in the later time by direct measuremenents of these components using thinlayer chromatographic and enzymatical methods.
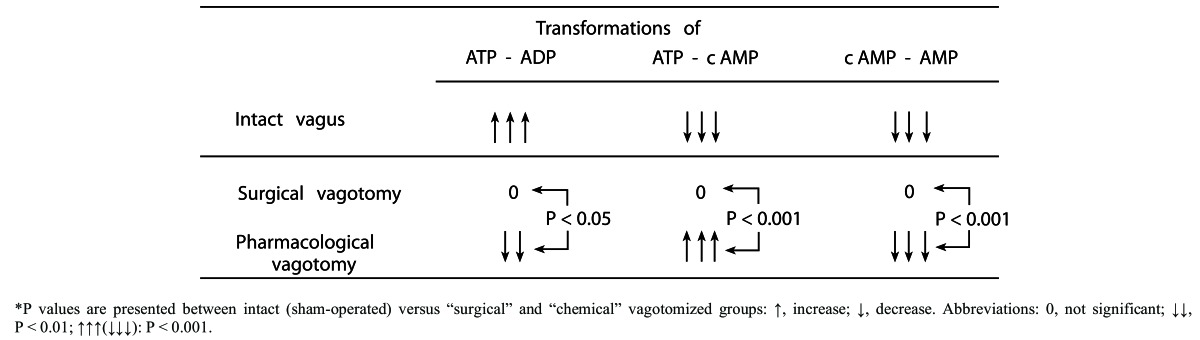
(Table 1). “Surgical” and “chemical” vagotomy-induced changes in the gastric mucosal membrane-bound ATP-dependent energy systems of rats (without application of any necrotizing agents). (Mózsik, Sütő, Vincze: J. Clin. Gastroenterol 14 (Suppl.2): S135-S139, 1992b) (with permission).*
Table 1. "Surgical” and “chemical” vagotomy-induced changes in the gastric mucosal membrane-bound ATP-dependent energy systems of rats (without application of any necrotizing agents). (Mózsik, Sütő, Vincze: J. Clin. Gastroenterol 14 (Suppl.2): S135-S139, 1992b) (with permission).* .
*P values are presented between intact (sham-operated) versus “surgical” and “chemical” vagotomized groups: ↑, increase; ↓, decrease. Abbreviations: 0, not significant; ↓↓, P < 0.01; ↑↑↑(↓↓↓): P < 0.001.
In the year’s form 1969 to 70s, the sodium and potassium-dependent ATPase (Na+-K+-dependent ATPase) and adenylate cyclase were prepared from the rat stomach. The Na+-K+-dependent ATPase enzyme is responsible for the active transport of Na+ (by the splitting of ATP into ADP), meanwile the adenylate cyclase (by the splitting ATP into cAMP) represents the “second messenger system” for the cells. The mitochondrial ATP is a common substrate for both Na+-K+-dependent ATPase and adenylate cyclase in presence of Mg2+ [6-8].
These types of biochemical examinations were carried out in the rat stomach under different experimental conditions.
The Effects of “Surgical” and “Chemical” Vagotomy on the Gastric Mucosal Biochemistry in Intact Animals
The observations were carried out in the gastric mucosal of intact (healthy) rats. The acute effects of bilateral “surgical“and “chemical” vagotomy (1.0 mg/kg atropine s.c.) were determined in 1 hour experiments [9]. The truncal surgical vagotomy and atropine treatment was carried out at 30 min before the start of observations, when these animals received 1 mL saline solution intragastrically. The tissue levels of ATP, ADP, AMP and lactate were measured enzymatically, or by RIA in case of cAMP.
Different metabolic parameters – as ratio of ATP/ADP, adenylate pool (ATP+ADP+AMP), “energy charge” [(ATP + 0.5 ADP) / (ATP+ADP+AMP)] – were also calculated from the direct measurements (expressed to 1 mg protein). The “energy charge” was introduced into the metabolic studies by Atkinson [10]. It represents the phosphorylation rate of adenylate compouds. The value of “energy charge “ is equel to 1, when all adenosine compounds are in phosphorylated form, and this value is 0 when all of the adenosine compounds are in dephosphorylated form.
The results were surprising, since after “surgical vagotomy” there were no changes in the measured biochemical parameters, meanwhile the tissue levels of ATP and AMP, adenylate pool and ratio of ATP/ADP decreased significantly (P<0.001), meanwhile the tissue levels of cAMP and “energy charge” increased significantly (P<0.001) (Table 1)..
In our earlier carried out observations indicated that the acetylcholine, atropine, cAMP and AMP have direct actions of the membrane (only Mg2+-dependent, Mg2+,Na+,K+-depedent and only Na+-K+-dependent) ATPase enzyme prepared from the rat [7, 8] and human [6] gastric mucosa. These results are summarized in (Table 2).
Table 2. Effect of compounds on membrane bound ATP-dependent systems. (Mózsik, Jávor: Dig. Dis. Sci. 33:92-105, 1988; Mózsik, Nagy, Tárnok, Vizi: Acta Med. Acad. Sci. Hung. 36: 1-29, 1979) (with permission).
| Membrane-Bound ATP-Dependent (ATP-Splitting) Systems | |||
|---|---|---|---|
| Mg2+-Na+-K+-dependent ATPase | |||
| Compounds | Mg2+-dependent | Na+-K+-dependent | Adenylate cyclase |
| Acetylcholine | No effect | Stimulation | Inhibition |
| Atropine | No effect | Inhibition | Stimulation |
| cAMP | No effect | Inhibtion | No effect |
| AMP | Stimulation | Inhibition | No effect |
*P values are presented between intact (sham-operated) versus “surgical” and “chemical” vagotomized groups: ↑, increase; ↓, decrease. Abbreviations: 0, not significant; ↓↓, P < 0.01; ↑↑↑(↓↓↓): P < 0.001.
The results of above in vivo and in vitro studies clearly indicated that:
a. The acute surgical and chemical effects differ significantly in the stomach (under intact/healthy circumstances);
b. The biochemical results called our attention to suggest the key role of membrane-bound ATP-dependent energy systems to understand the significance of acute “surgical” and “chemical“ vagotomy in intact/healthy gastric mucosa.
In these studies, the animals did not receive any mucosal damaging agents and other compounds to stimulate the gastric acid secretion.
Observations in Rats after Pylorus Ligation
The pylorus-ligated rats were introduced into the research by Shay et al. [11]. They had primary aims to produce gastric ulceration by stimulating gastric secretion. They studied various pathological factors for the development of gastric ulceration in animal model.
This model reveived many criticisms, dominantly for the fact that the ulcer formation appeared on the non-secretory part of the stomach (forestomach or rumen). It’s true the gastric ulcer clinically detectable in the antrum only in humans, which part also has no acid secretory ability.
This model was very widely used by our team in the different research works. The advantage of this model was that the gastric ulcer appears in presence of gastric hypersecretion, and furthermore, these pathological events appears under the intact vagal nerve function.
The pylorus-ligated rat, as a model, was used as a 24-hour animal model to study the the possible mechanisms involved in the development of gastric hypersecretion and gastric ulceration.
We analyzed the changes of gastric secretion and ulcer development in 24-hour pylorus-ligated rats. It was interesting to note that: a. gastric acid secretion in the stomach reaches its peak at 7 hours after pyloric ligation, meanwhile no ulceration could be detected by this time period Figs. (1 and 2).
Fig. (1).
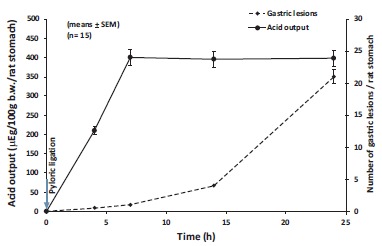
Linkage of gastric acid output and development of gastric mucosal damage in the forestomach of pylorus-ligated rats with intact vagal nerve. Animals were sacrificed at 0, 4, 7, 14 and 24 hours after pyloric ligation. The results were expressed as ±SEM; n=15; µg, microequivalent; b.w., body weight.
Fig. (2).
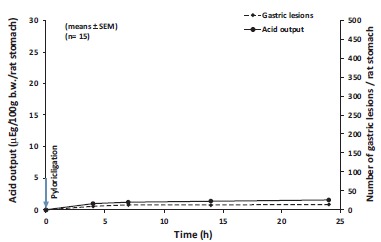
The preventive effect of acute bilateral subdiaphragmatic surgical vagotomy on the gastric acid output and ulceration in plyrus-ligated rats at 0, 4, 7, 14 and 24 hrs after surgical vagotomy and pylorus ligation. The results were expressed as ±SEM; n=15; µg, microequivalent; b.w., body weight.
24. Hours Pylorus-Ligated Rats (H+ Secretion and Ulcer Development) before and after Surgical Vagotomy
It was clear that no gastric hypersecretion and ulcer development can be observed after bilateral surgical vagotomy [12]. It was also interesting to note that tissue level of ATP decreased significantly in gastric fundic mucosa (together with development of gastric hypersecretion) and forestomach (parallel with ulcer development) of 24 hours pylorus-ligated rats, however tissue level of ATP slightly (in the fundic mucosa) or absolutely did not decrease (in forestomach) in 24 hours plyorus-ligated rats after bilateral surgical vagotomy (in association of very small gastric acid secretory responses and no ulceration in the forestomach). By other words, the gastric hypersecretory responses by glandular stomach and gastric ulceration by forestomach appeared in case of significant breakdown of tissue ATP in both parts of rat stomach, meanwhile its level remained practically at the same level in both parts of the rat stomach after bilateral surgical vagotomy. The changes of tissue level of ADP moved parallel with the ATP in both parts of rat stomach under these experimental conditions. These results called our attention to ATP-ADP system, including the other participants ATP-regulatory energy systems. It has been concluded that a significant breakdown of cellular ATP is necessary to produce gastric hypersecretion and ulcer development in pylorus-ligated rats.
New results were obtained in animal experiments as the actual activities of membrane ATPases (Mg2+-dependent, Mg2+-Na+-K+-dependent and only Na+-K+-dependent), gastric acid secretory responses and gastric ulceration were measured in the gastric fundic mucosa and forestomach in rats after different times (0, 4, 7, 18 and 24 hours) after pylorus ligation [13]. All of membrane-bound ATPases reached their peaks at 4 hours in the glandular stomach and the forestomach, the gastric H+ reaches its peak at 7 hours, meanwhile the gastric ulceration appears after 7 hours in the forestomach. These results clearly indicate that: a. The significant increase of all membrane ATPase proceeds the maximal acid secretion and gastric ulceration; b. The gastric acid secretion reaches its peak at 7 hours after pyloric ligation, and thereafter its level remained unchanged to 24 hours; c. The maximal gastric acid secretion by the fundic mucosa proceeds the development of gastric ulceration in the forestomach; d. The gastric acid secretion is a consequence of very active and complex metabolic process in the fundic mucosa; e. The same type of active and complex metabolic process exists in the forestomach before clinically appearance of gastric ulceration in the forestomach.
The strophantin (ouabain) is a specific inhibitor component of Na+-K+-dependent ATPase, its inhibitory action on Na+-K+-ATPase is widely used as one a markers to define classical membrane (Na+-K+-ATPase) ATPase in preparations. (The other criteria are that the enzyme should be located in the plasma membrane and its activity could be enhanced by together application of Na+ and K+ in presence of Mg2+). This enzyme can be found in all types of cells (in the gastric fundic mucosa – including the parietal cells and all other spicialized gastric cells). The sodium pump (Na+-K+-ATPase) contains α (alpha) and β (beta) units, meanwhile the gastric H+-K+-ATPase contents α-subunit and β-subunit [brief summary, see Ref. No. 5]. So there are some similarities between the chemical structures of sodium-pump and H+-K+-ATPase.
Ouabain applied at the time of pyloric ligation inhibited the sodium-pump ATPase, gastric acid secretion and the gastric ulceration. Furthermore, the ouabain-induced inhibitory actions indicated the same time-sequence: sodium pump (4 hours) → gastric acid secretion → gastric ulceration. This time-sequence was also observed in case of increased membrane ATPase activity → gastric acid hypersecretion → gastric ulceration.
It is important to note that the existence of a very complex biochemical mechanism (related to the regulation of tissue level of ATP) is the first reaction in pylorus-ligated rats, followed that the gastric acid secretion and finally the gastric ulceration. This time-sequence of these events are similar for the development of gastric hyperacidity and for gastric ulceration, and also involved in the ouabain inhibition in 24 hours pylorus-ligated rats. There is also important to note that the active biochemical changes can be obtained both in the glandular stomach and in forestomach. Consequently, the gastric ulceration can be clinically detected only after a very active metabolic reaction in the forestomach (by other words, the gastric ulceration in this animal model does not appear only by a passive pathological event). This typical active metabolic adaptation process cannot be found after surgical vagotomy (under acute experimental conditions).
The 4-hour pylorus ligated rat model is an optimal model to study either stimulatory or inhibitory effect of different compounds on the gastric acid secretion.
Actions of “Surgical” and “Chemical” Vagotomy on the Gastric Mucosal Mucosal Biochemistry in 4 Hours Pylorus-Ligated Rats
No gastric acid secretion was observed after bilateral “surgical” vagotomy, meanwhile the atropine (given in doses from 0.2 to 5.0 mg/kg s.c.) dose-dependently decreased the gastric acid secretion [14].
The tissue levels of ATP, ADP, AMP and lactate were enzymatically, meanwhile the cyclic AMP level was measured by RIA in the gastric gastric fundic mucosa. The biochemical results were calculated in accordance to 1.0 mg protein. These observations were done in gastric fundic mucosa of untreated rats: ATP: 11.56±1; ADP: 13.75±1; AMP: 10.73±2 (all of three are expressed as nanomol/mg protein), cAMP: 4.51±0.10 picomol/mg protein), lactate: 277±20 micromol/mg protein) (as means±SEM; n=39), respectively. These biochemical examinations were performed simultaneously from the same tissue samples, and these results were compared with the changes in the gastric fundic mucosa after different experiments in various experimental conditions (sham operated rats, 4 hours pylorus-ligated rats with “surgical” and “chemical” vagotomy with atropine treatment). The preparation of membrane ATPase and adenylate cyclase was not done in these studies, however, the regulation of these membrane-bound enzymes (prepared from rat and human gastric fundic mucosa) had been evaluated in earlier experiments [7, 8].
The in vitro studies with prepared membrane enzymes proved that the active function of these membrane enzymes are working in contraregulatory pathways, since the mitochondial ATP is a common substracte for both membrane ATPase and adenylate cyclase in presence of Mg2+. The membrane ATPase produces an increase in ATP, meanwhile adenylate cyclase results an increase in cAMP. The AMP can be obtained from both ATP breakdown pathways. Various drugs (atropine, epinephrine), compounds (like NaF, which is used to stimulate the ATP-cAMP transformation by adenylate cyclase together with the complex inhibition of all ATPase enzymes), cAMP and AMP were tested in these enzymes. It was interesting to note that actions of these drugs and compounds differ on the membrane ATPase and adenylate cyclase: a. NaF absolutely inhibits the membrane ATPase activity together with maximal stimulation of adenylate cyclase activity; b. cholinergic agents (Ach, cholinesterase inhibitor) do not modify the activity of membrane ATPase, meanwhile the ATP-cAMP transformation is decreased sigificantly; c. atropine and epinephrine inhibit (in smaller molar concentrations) the membrane ATPase activity, and they stimulate the adenylate cyclase (in higher molar concentrations) activity; d. cAMP and AMP (themself as products of ATP breakdown pathways) directly inhibit the membrane ATPase acitivity; e. No increased ATP was obtained by increased oxydative phosphorylation in these experimental conditions [6-8, 15].
We have had possibilities to carried out simultaneous measurements of membrane ATPase, ATP, ADP, AMP in human gastrointestinal tissues [for updated details, see Ref. No 5].
In these above mentioned animal experiments, the tissue levels of ATP, ADP, AMP, cAMP were simultaneously measured from the rat gastric fundic mucosa (no gastric ulceration was found in the forestomach in 4 hours pylorus-ligated rats). Therefore, we received the cross-section on the changes in mitochondrial ATP metabolism.
To understand the different changes in the gastric mucosal energy metabolism under different experimental conditions, all of the main steps (cellular biochemical components) (ATP, ADP, AMP, cAMP) were measured simultaneously from the same tissue samples. We received an excellent possibility to study the details in the mitochondrial ATP system, including their different extra- and intracellular regulatory feedback mechanisms.
In the first step we studies the changes of mitochondrial ATP system at each (0, 1, 2, 3, 4, 5) hour after pyloric ligation.
It was surprising to note that a very dynamic biochemical mechanism system exists between the changes in the mitochondrial ATP, ADP, AMP, cAMP compounds in pylorus-ligated rats (5 hour time periods) even without application of any drugs or compounds Fig. (3).
Fig. (3).
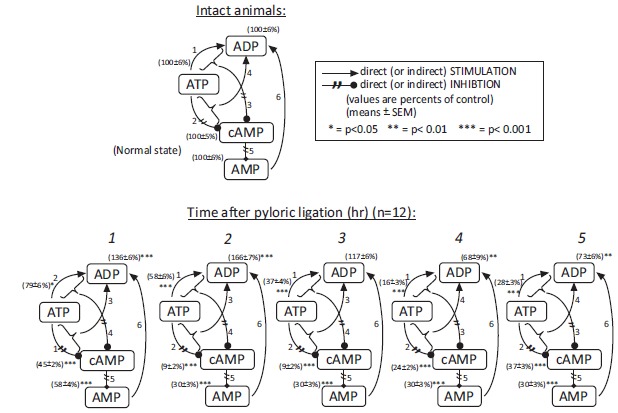
Changes in the cellular ATP, ADP, cAMP and AMP in the time period of first 5 hours on dependence of time after pyloric ligation. The observations were carried out from gastric fundic mucosa. The results obtained in sham-operated (normal state) were taken to be equal to 100 per cent (means±SEM) (Mozsik (2006). Molecular pharmacology and biochemistry of gastroduodenal mucosal damage and protection. In: Mózsik, G.Y. (Ed.) Discoveries in Gastroenterology (1960-2005). Akadémiai Kiadó, Budapest. pp.139-224) (with permission).
The gastric acid secretion is a vagus dependent process proved by pylorus-ligated rat experiments (see above written text). To study the biochemical changes in the membrane-bound ATP-dependent energy systems after “chemical” vagotomy (namely atropine treatment) we used short (5 hour) experiments since atropine’s action lasts about 5 hours after its application.
The 4 hours pyloric ligation of rats was choosen for two reasons for our observations: a. This time period is an optimal to study the actions of different drugs on the gastric acid secretion; b. No gastric ulceration can be found neither in fundic nor in forestomach; c. The application of atropine can cover this time period by its action.
The biochemical paramaters of gastric gastric mucosa were the same in sham-operated rats to those found in control (untreated) rats (see above mentioned results).
The mitochondrial ATP decreased significantly in 4 hours pylorus-ligated rats, together with significant increase of ADP and decrease of cAMP [16].
The gastric acid secretion was found practically zero after surgical vagotomy, and non significant changes were obtaiend in mitochondrial ATP-dependent energy systems of the gastric fundic mucosa.
Atropine treatment decreased significantly the ATP breakdown into ADP, together with increase of cAMP and AMP and decrease of gastric acid secretion in 4 hours pylorus-ligated rats. Interestingly, the components of membrane-bound ATP-dependent energy system changed dose-dependently during this 4 hours periods treated with different (cytoprotective and antisecretory) doses of atropine. Consequently, the intracellular components of ATP-dependent energy systems produced regulatory effect also will change in consequence of the drug actions involved in their intracellular regulations Fig. (4).
Fig. (4).
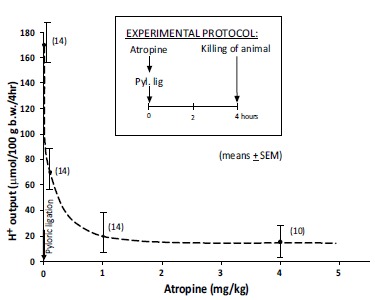
Dose-dependent inhibitory effect of atropine on the gastric acid secretion in 4 hour pylorus-ligated rats (means ±SEM). (Mózsik, Figler, Nagy, Patty, Tárnok (1981): Gastric and small intestinal energy metabolism in mucosal damage. In: Mózsik, G.Y., Hänninen O., Jávor T. (Eds.). Advances in Physiological Sciences.Vol. 29. Gastrointestinal Defence Mechanism. Pergamon Press, Oxford – Akadémiai Kiadó, Budapest.pp. 213-288) (with permission).
Similar changes were found in the forestomach during atropine treatment. It was surprizing, that the gastric mucosal protective effects of atropine produced complete inhibition of sodium pump enzyme (Na+-K+-dependent ATPase) (ATP-ADP transformation by membrane ATPase) together with stimulation of adenylate cyclase (“second messenger system”) enzyme (ATP- cAMP transformation by adenylate cyclase) Fig. (5).
Fig. (5).
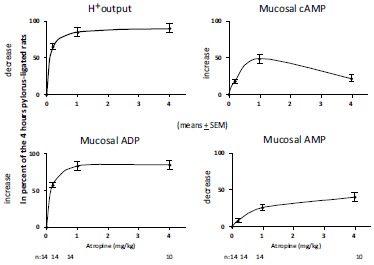
Atropine-induced changes in gastric H+ output, gastric fundic mucosal levels of ATP, ADP and AMP in 4 hours pylorus-ligated rats. The results were expressed as per cent values of obtained immediately after pylorus-ligation (=100 per cent), except of gastric H+ output, which was expressed also in per cent values, however obtained at 4 hours after pylorus-ligation (means ± SEM). (Mózsik, Figler, Nagy, Patty, Tárnok (1981): Gastric and small intestinal energy metabolism in mucosal damage. In: Mózsik, G.Y., Hänninen O., Jávor T. (Eds.). Advances in Physiological Sciences.Vol. 29. Gastrointestinal Defence Mechanism. Pergamon Press, Oxford – Akadémiai Kiadó, Budapest.pp. 213-288) (with permission).
For schematic summary of atropine-induced (given in different doses) gastric fundic mucosal damage in 4 hour pylorus ligated rats see Fig. (6).
Fig. (6).
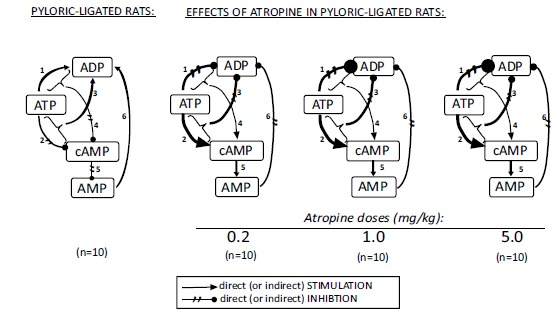
Biochemical changes in the regulatory steps of cellular energy systems in the gastric fundic mucosa produced by different (cytoprotective and antisecretory) doses of atropine in 4 hours pylorus-ligated rats.
These biochemical regulatory mechanisms were found in the rats with intact vagal nerve, however after atropine treatment the regulatory pathway changed toward the generation of cAMP and AMP deplition. It was also interesting note, the biochemical changes in the mitochondrial ATP energy system indicated parallel tendencies (but they differed in their quantities) in its all regulatory steps.
The epinephrine also inhibits the gastric acid secretion together with complete inhibitin of ATP-ADP transformation and with a siginificant increase of cAMP.
There are interesting points in the understanding of correlations in ATP-dependent energy systems observed in gastric fundic mucosa (gastric acid secretion vs. cholinergic and adrenergic drug actions):
a. The gastric acid secretion is primary ATP-ADP dependent process (although the work-team of Sachs’s gave a much more punctual identification of correlation between the classical sodium-pump and H+-K+-ATPase systems due to gastric H+ secretion) [for details, see Ref. No. 5].
b. There is clearcut evidence that the correlation between the sodium-pump (ATP-ADP transformation by Na+-K+-dependent ATPase) and second messenger system (ATP-cAMP transformation by adenylate cyclase) is an antagonist energy mobilization mechanism in the gastric fundic mucosa;
c. The drug actions primary depend on the presence of intact vagal nerve in the gastrointestinal tract (no drug actions can be obtained by drugs in gastric tissues after surgical vagotomy);
d. The extra- and intracellular regulatory mechanism system exists in the gastric tissues under circumstances of the intact vagal nerve.
Surgical Vagotomy, Actions of Necrotizing Agents and Gastric Cytoprotection in the Stomach
The research work of André Robert (Kalamazoo, Michigan, USA) introduced the “terminology of gastric cytoprotection” [17, 18]. There is a very brief story on the background of this very interesting scientific discovery. He was invited to give a review lecture by the American Society of Surgery on the mucosal protective effects of prostaglandins in the late 1970s. He was an excellent researcher, and he wanted to show some experimental data on the possible defensive mechanisms of prostaglandins in the stomach. He accepted the key role of gastric acid in the development of gastric mucosal damage, so he applied intragastrically by gastric tube 0.1 M concentration of HCl in the rats. He believed that after administration of 0.1 M HCl (in 1 mL volume), he will produce gastric mucosal damage in rats. But he found no mucosal damage in the stomach of experimental animals. He increased the concentration of intragastrically applied H+ to 0.6 M concentration, when he obtained dominantly diffuse mucosal damage in the glandular part (however, he never received meleana in the rats). He gave very small quanties (1-5 µg /kg) of PGE2/kg at 30 min before the application of 0.6 M HCl (very important to note, that the PGE2 did not decrease the gastric acid secretion), and surprisingly these small doses of PGE2 completely prevented the HCl-induced gastric mucosal damage (without presence of any gastric acid inhibitory effect). Since the concentration of intragastrically applied (0.6 M) HCl was totally un-physiological, therefore he applied other compounds [0.2 M NaOH, 25% NaCl solution (as hyperomolaric agent), ethanol (96%)] intagastrically by gastric tube, and he received the same morphological types of gastric fundic mucosal damage as that was produced by 0.6 M HCl in rats. However, all these agent-induced gastric mucosal damage could be completely inhibited (prevented) by small doses of PGE2 application. This type of mucosal protection caused by very different agent given in doses did not alter gastric acid secretion. The registration of the existence of gastric mucosal damage vs. gastric acid secretion and the prevention of gastric mucosal damage without any inbition of gastric acid scetion opened an absolutely new field in experimental and clinical research of peptic ulcer disease. This phenomenon was named as “gastric cytoprotection”.
We have to mention that the existence of the “cytoprotection” was proved by Robert et al. in 1979 in rats, meanwhile we observed earlier that the duodenal ulcer in duodenal ulcer patients healed completely without any changes of gastric acid secretory responses by chronic atropine treatment [19]. Our observations, unfortunately, did not become known worldwide.
Thereafter, we went deeper in the research of “gastric cytoprotection”, which involved more experimental work. We wanted to know more and more new date on the denfesive factors impaired during in human peptic ulcer development and those could be strengthened as treatment or prevention.
Our attention was focussed to discover the primary similarities and differences between the mechanims between the “gastrointestinal “surgical” and “chemical” vagotomy (independently from the progress of drug research of the field involving H2RA, gastrin antagonist or propton pump inhibitors). We widely used different experimental models to approach the details of similarities and differences of “surgical” and “chemical” vagotomy. As first step we tested the coadministrations of various drugs (as epinephrine, atropine, dinitrophenol, actinomycin D, histamine, mannomustine, pentagastrin, tetracycline, ouabain) with prostacyclin (PGI2).
“Surgical” Vagotomy Failured the PGI2 -Induced Gastric Mucosal Protective effect in Rats
The surgical vagotomy alone cannot produce ulcer development in the rat stomach, however, Sikiric and co-workers found ulcer appearance in the rat stomach [20-22].
After bilateral surgical vagotomy the extent of IND-induced gastric mucosal lesion was found to be enhanced, meanwhile the chemical vagotomy significantly decreased the gastric mucosal damage induced by chemicals.
The interpretation of these results offered to conclude the following points:
a. the intact vagal nerve is basically necessary for the action of certain protective mechanisms against different chemicals-induced gastric mucosal damage;
b. the gastric mucosal protective effects significantly differ from each other against chemical- induced gastric mucosal damage.
These results were presented in a Satellite Symposium of the Congress if International Union of Physiological Sciences (IUPS) (Budapest, Hungary; 1980) [20] Fig. (7).
Fig. (7).
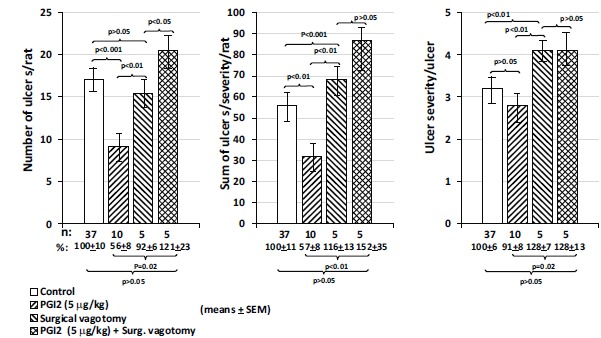
The presentation (firstly in the World) of PGI2 gastric protective effect dipappears after bilateral surgical vagotomy in rats treated with ETOH (96 v/v) (means ±SEM) [Jávor et al., (1981) Gastric mucosal resistance to physical and chemical stress. In: Mózsik, G.Y., Hänninen O., Jávor T. (Eds.) Advances in the Physiological Sciences. Vol.29. Gastrointestinal Mucosal Defence. Pergamon Press, Oxford- Akadémiai Kiadó, Budapest. pp. 141-159] (with permission).
These observations were published in journal of Prostaglandins, Leucotrienes Medicine [23], in which we also emphasized the essential role of intact vagal nerve for development of gastric mucosal preventive effect of prostacyclin againts the chemical-induced mucosal damage, e.g. the protective effect of prostacycline was not present in these animal models after vagotomy. We concluded from the results of these observations that the intact vagal nerve is basically necessary for the gastric mucosal protection.
Similar observations were published by Miller [24], and Henagen, Seidel, Miller cited our results in his paper [25, 26] (however, our priority on the key-role of intact vagal nerve in the gastric mucosal defense mechanisms has lasted by time [22, 27-35].
However, it is important to emphysize that the “chemical vagotomy” by atropine treatment does not inhibit clinically the appearance of prostaglandin and prostacyclin-induced gastric mucosal protection against the chemical-induced damage in animals.
We need to emphasize that the surgical vagotomy had been widely used in the clinical practice for the treatment of patients with peptic ulcer in the 1970-80s years. We newer accepted the application the surgical vagotomy in the medical practice, and we emphasized the application of “chemical vagotomy” instead of surgical vagotomy in the medical treatment of patients with peptic ulcer [36].
Our first gastric cytoprotection study was carried out in 1981 with PGI2 and different drugs as well as surgical vagotomy [23, 37], then the correlations between the changes in the membrane-bound ATP-dependent energy systems vs gastric mucosal damage produced by NaOH, hypertonic NaCl, HCl, and alcohol vs. prostacyclin-induced gastric cytoprotection in rats [38].
It was interesting to see that all members in the membrane-boud ATP-dependent system were the same during the development of gastric mucosal damage induced by different chemical agents and during the development of prostacyclin-induced gastric mucosal protection.
Surgical Vagotomy vs. Different Chemicals Induced Gastric Mucosal Damage
The extent (number and severity) of gastric mucosal damage produced by 0.6 M HCl, 0.2 M NaOH, 25% NaCl and 96% ethanol was enhanced by surgical vagotomy and acute adrenalectomy in rats [32, 39]. The mucosal protective effects of these compounds could be reversed by glucocorticoid suplementation in rats after acute adrenalectomy (Fig. 8 and 9).
Fig. (8).
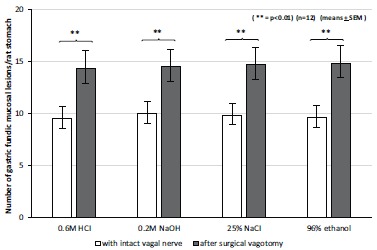
The effect of acute bilateral subdiaphragmatic surgical vagotomy on the development of gastric fundic mucosal lesions produced by intragastriccally administered 0.6 M HCl, 0.2 M NaOH, 25% NaCl, 96% ethanol, The results were expressed as ±SEM; n=12;** P<0.01.
Fig. (9).
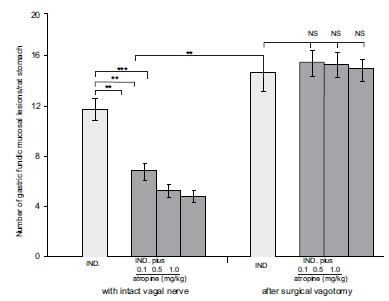
The protective effect of atropine on the subcutaneously administered indomethacin-induced gastric mucoal lesions in intact and in vagotomised rats. The results were expressed as ±SEM; n=12; NS, not significant; ** P<0.01, *** P<0.001.
It was also important to note, that gastric mucosal protection or cytoprotection induced by atropine, cimetidine (as receptor antagonists), β-carotene (as scavenger) and prostacyclin (as endogenously regulatory bioactive compound) disappeared after surgical vagotomy [31, 35]. Similar observations were obtained with indomethacin in surgically vagotomized rats [40].
The indomethacin is a widely used as nonstreroidal anti-inflammatory drug in the every day medical practice. That was the reason why we studied it in more detailed forms and its damaging mechanims (vascular permeability, clinical manifestations of the gastrointestinal mucosal damage) in animal experiments under different experimental conditions of acute surgical and chemical vagotomy [22].
Indomethacin (20 mg/kg s.c.given) produced gastric, small intestinal and colonic mucosal damage in rats. Most of the researchers used to emphasize the clinical evidence only to gastric mucosa, meanwhile the highest number and severity of mucosal damage can be obtained in the small intestine. However mucosal damage can be also observed in the large bowel (in smaller extent). These primary observations were performed in fasted (24 and 48 hours starvation) intact animal with intact vagal nerve. The mucosal damage found in these three part of GI tract was associated with increased vascular permeabilty (measured by the Evan’s blue concentration and by intragastric quantities of exudate into the stomach) (Fig. 10).
Fig. (10).
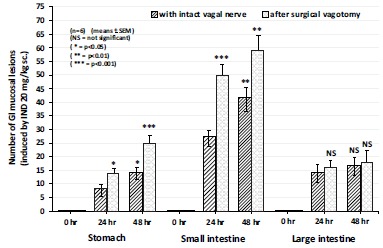
The effect of acute bilateral vagotomy on the number of indomethacin-induced (20 mg/kg s.c.) gastric, small intestine and large bowel lesions in the 0, 24 and 48 h experiments in rats. Each value was calculated by semiquantitative estimation using a scoring system. Data expressed as means as ±SEM; n=6. NS, not significant; * P<0.05, ** P<0.01, *** P<0.001 between the 24 and 48 h experiments without and with surgical vagotomy.
The acute and chronic (14 days) surgical vagotomy enhanced the mucosal damage both in number and severity, increased vascular permeability and excreted Evan’s blue in the stomach, small intestine, meanwhile “chemical vagotomy” by atropine (given it at 0, 5, 10, 15 and 20 hours after the indometacin administration – in doses of 0.1, 0.5 and 1.0 mg/kg i.p.) siginificantly and dose-dependently inhibited the above mentioned parameters in indomethacin-induced mucosal damage in the stomach, small intestine, meanwhile the extent of mucosal damage in large bowel was not enhanced by surgical vagotomy and prevented by chemical vagotomy.
The gastric mucosal preventive affect of cytoprotective dose of atropine (0.1 mg/kg i.p.given) completely disappeared after acute surgical vagotomy in the stomach and small intestine, but no such action was obtained in the large bowel.
Thereafter, the large bowel was devided proximal, middle and distal parts for same experimental procedures. Surpisingly, the proximal part of large bowel incicated the same results with the stomach and small intestine, meawhile no any changes were found in the ulceration (number and severity), mucosal permeability in the middle and distal parts of large bowel after acute and chronic surgical vagotomy or chemical vagotomy. The discrepancies obtained in the changes of mucosal damage and vascular permeability – between the proximal vs. middle and distal parts of the large bowel – can be explained by that no vagal innervation reaches in the middle and distal parts of the large bowel (Figs. 11 and 12).
Fig. (11).
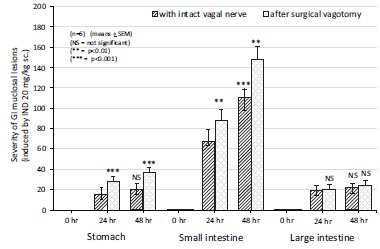
The effect of acute bilateral vagotomy on the severity of indomethacin-induced (20 mg/kg s.c.) gastric, small intestine and large bowel lesions in the 0, 24 and 48 h experiments in rats. Each value was calculated by semiquantitative estimation using a scoring system. Data expressed as means as ±SEM; n=6. NS, not significant; * P<0.05, ** P<0.01, *** P<0.001 between the 24 and 48 h experiments without and with surgical vagotomy.
Fig. (12).
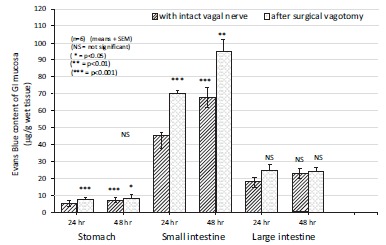
The changes in gastric, small intestine and large bowel mucosal Evans Blue content (μg/g wet tissue) during the development of indomethacin-induced (20 mg/kg s.c.) mucosal damage after bilateral vagotomy in 24 and 48 hr experiments in rats. Data expressed as means as ±SEM; n=6. NS, not significant; * P<0.05, ** P<0.01, *** P<0.001 between the 24 and 48 h experiments without and with surgical vagotomy.
Gastroprotection by Capsaicin vs. Intact Vagal Nerve
The capsaicin acts dose-dependently on the capsaicin-sensitive afferent nerves – on dependence of its applied doses [40]. The chemical vagotomy cannot abolish the capsaicin’s actions in the gastrointestinal tract, gastro- protection (by cimetidine, atropine) and cytoprotection (by sucralfat, β-carotene and postacyclin) remains intact besides the gastroprotective affects of capsaicin [42] (Fig. 13).
Fig. (13).
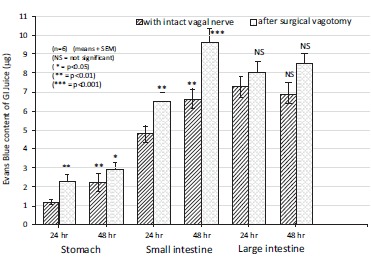
The changes in gastric, small intestine and large bowel juice Evans Blue content (μg) during the development of indomethacin-induced (20 mg/kg s.c.) mucosal damage after bilateral vagotomy in 24 and 48 hr experiments in rats. Data expressed as means as ±SEM; n=6. NS, not significant; * P<0.05, ** P<0.01, *** P<0.001 between the 24 and 48 h experiments without and with surgical vagotomy.
DISCUSSION
The presented results emphasized the essential role of vagal nerve in the development of gastrointestinal mucosal damage. This scientific statement has been emphysized in the development of peptic ulcer in patients, and the key role of vagotomy (both “chemical” and “surgical” vagotomy) was widely used in the medical treatment of peptic ulcer patients in the years of 1970s.
Following this time period the medical treatment of peptic ulcer patients significantly changed by the rapid development of drug reseach (H2RA, gastrin antagonist, proton pump inhibitors) before the 2005, when Marschall and Warren reveived Nobel prize for the discovery of the role of Helicobacter pylori in the development of peptic ulcer and gastritis. Although some arguments of H. pylori infection can criticise its main pathogenic role (for these vulnerable point see Ref. No. 43).
Our scientific problems originated from the surgical and chemical vagotomy of patients with peptic ulcer (especially by results of human clinical pharmacology with anticholinergic agents). Our primary aims to clear up some part of stomach biochemistry after acute and chronic surgical and chemical vagotomy. We performed animal experiments to determine the changes of the membrane-bound ATP-dependent energy systems, chemical agent-induced gastrointestinal mucosal damage, the role of various drugs, scavengers and endogenous mucosal protective compounds done applied alone or after surgical and chemical vagotomy (under different experimental conditions).
The surgical vagotomy produced irreversible changes in the regulation of membrane-bound ATP-dependent energy systems (namely no metabolic adaptation was obtained in the ATP breakdown in the gastric mucosa, in association with the significant decrease of gastric acid secretion and gastric ulceration in rats, it enhances the development of gastric mucosal damage produced by different chemicals and decrease of gastric mucosal damage facilitated by different drugs, scavengers, and bioactive compounds). All of these metioned phenomena are absent after chemical vagotomy, consequently the regulations of the membrane-bound ATP-dependent energy systems, gastric mucosal protective effects of certain drugs, scavengers and bioactive compounds (prostacyclin, capsaicin) remained to be intact in the gastrointestinal mucosal tissues.
By other words, the intact vagal nerve participates both in the development of gastrointestinal damage and development of gastrointestinal mucosal mucosal protection (produced by drugs, scavengers, bioactive compounds).
The surgical vagotomy produces ablation of afferent and efferent nerves (including capsaicin-sensitive afferent nerves), meanwhile the atropine acts only at the level of efferent nerve of target organs.
If no afferention and efferentation exists in the nerves (including the vagal nerve), consequently there will be no protective effect of cytoprotective drugs. These results offer us a remarkable information on the function of intact or chemically modified vagal nerve.
ACKNOWLEDGEMENTS
The present scientific contribution is dedicated to the 650th anniversary of the foundation of the University of Pécs in Hungary.
CONFLICT OF INTEREST
The authors confirm that this article content has no conflict of interest.
REFERENCES
- 1.Ihász M. Budapest: Vagotomy. Akadémiai Kiadó; 1980. 1912. p. 12. [Google Scholar]
- 2.Dragstedt L.R., Owens F.M. Supradiaphragmatic section of the vagus nerves in the treatment of duodenal ulcer. Proc Soc Exp Biol (N Y) 1943;53:152–161. [http://dx.doi.org/10.3181/00379727-53-14227]. [Google Scholar]
- 3.Ihász M. Vagotomy. Budapest: Akadémiai Kiadó; 1980. [Google Scholar]
- 4.Mózsik G.Y., Jávor T., Beró T., Kutas J., Palotai Z., Polyák L. Late complications of gastric surgery. In: Louhija A., Valtonen C., editors. Internal Medicine: Topics 1976. Basel: Karger; 1978. pp. 200–208. [Google Scholar]
- 5.Mózsik G.Y., Szabó I.L. Membrane-Bound ATP-Dependent Energy Systems in the Gastrointestinal Mucosal Damage and Prevention. Rijeka: INTECH open science, open mind Publishers.; 2016. [Google Scholar]
- 6.Mózsik G.Y., Øye I. The preparation of Na+-K+-dependent ATPase from human gastric mucosa. Biophys.Biochim. Acta (Amsterdam) 1969;183:640–641. doi: 10.1016/0005-2736(69)90178-3. [http://dx.doi.org/10.1016/0005-2736(69) 90178-3]. [DOI] [PubMed] [Google Scholar]
- 7.Mózsik G. Some feed-back mechanisms by drugs in the interrelationship between the active transport system and adenyl cyclase system localized in the cell membrane. Eur. J. Pharmacol. 1969;7(3):319–327. doi: 10.1016/0014-2999(69)90099-5. [http://dx.doi.org/10.1016/0014-2999(69) 90099-5]. [PMID: 4242776]. [DOI] [PubMed] [Google Scholar]
- 8.Mózsik G. Direct inhibitory effect of adenosine monophosphates on Na+-K+-dependent ATPase from human gastric mucosa. Eur. J. Pharmacol. 1970;9:207–210. doi: 10.1016/0014-2999(70)90301-8. [http://dx.doi.org/10.1016/0014-2999(70)90301-8]. [PMID: 4315595]. [DOI] [PubMed] [Google Scholar]
- 9.Mózsik G., Sütö G., Vincze A. Correlations between the acute chemical and surgical vagotomy-induced gastric mucosal biochemistry in rats. J. Clin. Gastroenterol. 1992;14(Suppl. 1):S135–S139. doi: 10.1097/00004836-199206001-00024. [http:// dx.doi.org/10.1097/00004836-199206001-00024]. [PMID: 1629569]. [DOI] [PubMed] [Google Scholar]
- 10.Atkinson D.E. The energy charge of the adenylate pool as a regulatory parameter. Interaction with feedback modifiers. Biochemistry. 1968;7(11):4030–4034. doi: 10.1021/bi00851a033. [http://dx.doi.org/10.1021/ bi00851a033]. [PMID: 4972613]. [DOI] [PubMed] [Google Scholar]
- 11.Kolm R., Komarov S.A., Shay H. Experimental studies on the excretion of neutral red by the stomach. Gastroenterology. 1945;5:303–319. [PMID: 21007058]. [PubMed] [Google Scholar]
- 12.Mózsik G., Vizi F. Surgical vagotomy and stomach wall ATP and ADP in pylorus--ligated rats. Am. J. Dig. Dis. 1977;22(12):1072–1075. doi: 10.1007/BF01072860. [http://dx.doi.org/10.1007/BF01072860]. [PMID: 930905]. [DOI] [PubMed] [Google Scholar]
- 13.Mózsik G., Vizi F. Examination of stomach wall Mg2+-Na+-K+-dependent ATPase, ATP, and ADP in pylorus-ligated rats. Am. J. Dig. Dis. 1976;21(8):649–654. doi: 10.1007/BF01071959. [http://dx.doi.org/10.1007/ BF01071959]. [PMID: 133608]. [DOI] [PubMed] [Google Scholar]
- 14.Mózsik G.Y., Nagy L., Patty I., Tárnon F. Advances in Physiological Sciences. Vol. 29. Gastrointestinal Defense Mechanism. Kiadó Budapest: Pergamon Press, Oxford-Akadémiai; 1981. Gastric and small intestinal energy metabolism in mucosal damage. pp. 213–288. [Google Scholar]
- 15.Mózsik G.Y. PhD Thesis. Pécs: 1969. Data to the action of mechanism of anticholinegicagents in patients and animals; pp. 213–288. [Google Scholar]
- 16.Mózsik G., Nagy L., Tárnok F. Interrelationships between the gastric mucosal cAMP and gastric H+ secretion in pylorus-ligated rats. Gastroenterology. 1978;75(1):164. [PMID: 45580]. [PubMed] [Google Scholar]
- 17.Robert A. Cytoprotection by prostaglandins. Gastroenterology. 1979;77(4 Pt 1):761–767. [PMID: 38173]. [PubMed] [Google Scholar]
- 18.Robert A., Nemasis E.J., Lanchaster C., Hauchar J. Cyto- protection of gastric necrosis produced by alcohol, HCl, NaOH, hypertonic NaCl and thermal injury. Gastroenterology. 1979;77:433–443. [PMID: 456839]. [PubMed] [Google Scholar]
- 19.Mózsik G.Y., Jávor T., Dobi S. Clinical pharmacological analysis of long term parasympatholytic treatment. In: Magyar I., editor. Acta Terii Conventus Medicinae Internae. Budapest: Gastroenterologia. Akadémiai Kiadó; 1965. pp. 709–715. [Google Scholar]
- 20.Mózsik G.Y., Hänninen O., Jávor T., editors. Gastrointestinal Defence Mechanism. Pergamon Press. Vol. 29. Budapest: Oxford: Pergamon Press; Akadémiai Kiadó; 1981. Advances in Physiological Sciences. [Google Scholar]
- 21.Mózsik G., Garamszegi M., Jávor T., Nagy L., Németh M., Sütö G., Vincze A. Chemical (alcohol, NaOH, NaCl and HCl)-induced changes in the gastric mucosal membrane-bound ATP-dependent energy systems. Acta Physiol. Hung. 1990;75(Suppl.):217–218. [PMID: 2164753]. [PubMed] [Google Scholar]
- 22.Karádi O., Mózsik G.Y. Surgical and Chemical Vagotomy on the Gastrointestinal Mucosal Defense. Budapest: Akadémiai Kiadó; 2000. [Google Scholar]
- 23.Mózsik G., Morón F., Jávor T. Cellular mechanisms of the development of gastric mucosal damage and of gastrocyto- protection induced by prostacyclin in rats. A pharmacological study. Prostaglandins Leukot. Med. 1982;9(1):71–84. doi: 10.1016/0262-1746(82)90074-9. [http://dx. doi.org/10.1016/0262-1746(82)90074-9]. [PMID: 6752955]. [DOI] [PubMed] [Google Scholar]
- 24.Miller. Protective effects of prostaglandins against gastric mucosal damage: current knowledge and proposed mechanisms. Am. J. Physiol. 1983;245:601–623. doi: 10.1152/ajpgi.1983.245.5.G601. [DOI] [PubMed] [Google Scholar]
- 25.Henagan J.M., Smith G.S., Seidel E.R., Miller T.A. Influence of vagotomy on mucosal protection against alcohol-induced gastric damage in the rat. Gastroenterology. 1984;87(4):903–908. [PMID: 6468878]. [PubMed] [Google Scholar]
- 26.Schmidt K.L., Henagan J.M., Smith G.S., Hilburn P.J., Miller T.A. Prostaglandin cytoprotection against ethanol-induced gastric injury in the rat. A histologic and cytologic study. Gastroenterology. 1985;88(3):649–659. doi: 10.1016/0016-5085(85)90132-5. [http://dx.doi.org/10.1016/0016-5085(85) 90132-5]. [PMID: 3967802]. [DOI] [PubMed] [Google Scholar]
- 27.Karádi O., Nagy Z., Bódis B., Mózsik G. Atropine-induced gastrointestinal cytoprotection dependences to the intact of vagal nerve against indomethacin-induced gastrointestinal mucosal and microvascular damage in rats. J. Physiol. Paris. 2001;95(1-6):29–33. doi: 10.1016/s0928-4257(01)00006-7. [http://dx.doi.org/10.1016/S0928-4257(01)00006-7]. [PMID: 11595415]. [DOI] [PubMed] [Google Scholar]
- 28.Király Á., Sütő G., Vincze Á., Jávor T., Mózsik G.Y. Effect of acute (ASV) and chronic (CSV) surgical vagotomy on the vascular permeability of the gastric mucosa after 96% ethanol (ETOH) treatment in rats. Acta Physiol. Hung. 1992;80:221–226. [Google Scholar]
- 29.Mózsik G.Y. Molecular pharmacology and biochemistry of gastroduodenal mucosal damage and protection. In: Mózsik G.Y., editor. Discoveries in Gastroenterology (1960-2005). Budapest: Akadémiai Kiadó; 2006. pp. 139–224. [Google Scholar]
- 30.Mózsik G.Y., Garamszegi M., Jávor T., Nagy L., Patty I., Sütö G., Vincze A. A biochemical and pharmacological approach to the genesis of ulcer disease II. A model study of stress-induced injury to gastric mucosa in rats. Ann. N. Y. Acad. Sci. 1990;597:264–281. doi: 10.1111/j.1749-6632.1990.tb16175.x. [http://dx.doi.org/10.1111/j.1749-6632.1990.tb16175.x]. [PMID: 2167035]. [DOI] [PubMed] [Google Scholar]
- 31.Mózsik G., Király Á., Garamszegi M., Jávor T., Nagy L., Sütő G. Tóth Gy, Vincze Á. Failure of prostacyclin, carotene, atropine and cimetidine to produce gastric cyto- and general mucosal protection in surgically vagotomized rats. Life Sci. 1991;49:1383–1389. doi: 10.1016/0024-3205(91)90389-s. [http://dx.doi.org/10.1016/0024-3205(91)90389-S]. [PMID: 1943444]. [DOI] [PubMed] [Google Scholar]
- 32.Mózsik G., Karádi O., Király A., Debreceni A., Figler M., Nagy L., Pár A., Pár G., Süto G., Vincze A. The key-role of vagal nerve and adrenals in the cytoprotection and general gastric mucosal integrity. J. Physiol. Paris. 2001;95(1-6):229–237. doi: 10.1016/s0928-4257(01)00030-4. [http:// dx.doi.org/10.1016/S0928-4257(01)00030-4]. [PMID: 11595442]. [DOI] [PubMed] [Google Scholar]
- 33.Vincze Á., Király Á., Sütő G., Mózsik G.Y. Acute surgical vagotomy (ASV) is an aggressor of gastric mucosa with and without indomethacin treatment? Acta Physiol. Hung. 1992;80:197–205. [PubMed] [Google Scholar]
- 34.Vincze A., Király A., Sütö G., Mózsik G. Changes of gastric mucosal biochemistry in ethanol-treated rats with and without acute surgical vagotomy. J. Physiol. Paris. 1993;87(5):339–341. doi: 10.1016/0928-4257(93)90041-q. [http:// dx.doi.org/10.1016/0928-4257(93)90041-Q]. [PMID: 8298612]. [DOI] [PubMed] [Google Scholar]
- 35.Vincze Á., Király Á., Sütő G., Karádi O., Mózsik G.Y. Role of neurohumoral and local mucosal factors in β-carotene-induced gastroprotection in the rat. In: Mózsik G.Y., Nagy L., Király Á., editors. Twenty Five Years of Peptic Ulcer research in Hungary: from Basic Sciences to Clinical Practice. Budapest: Akadémiai Kiadó; 1997. pp. 265–273. [Google Scholar]
- 36.Mózsik G.Y., Nagy L., Tárnok F. Ten years of the intermittent treatment with atropine in patients with duodenal ulcer. Drugs Exp. Clin. Res. 1979;5:185–195. [Google Scholar]
- 37.Jávor T., Bata M., Kutor G., Lovász L., Mózes G.Y., Mózsik G.Y. Gastric mucosal resistance to physical and chemical stress. In: Mózsik G.Y., Hanninen O., Jávor T., editors. Gastrointestinal Defense Mechanism. Pergamon Press. Vol. 29. Oxford: Akadémiai Kiadó Budapest; 1981. pp. 141–159. (Advances in Physiological Sciences). [Google Scholar]
- 38.Mózsik G., Morón F., Fiegler M., Jávor T., Nagy L., Patty I., Tárnok F. Interrelationships between membrane-bound ATP-dependent energy systems, gastric mucosal damage produced by NaOH, hypertonic NaCl, HCl, and alcohol, and prostacyclin-induced gastric cytoprotection in rats. Prostaglandins Leukot. Med. 1983;12(4):423–436. doi: 10.1016/0262-1746(83)90032-x. [http://dx.doi.org/10.1016/0262-1746(83) 90032-X]. [PMID: 6322205]. [DOI] [PubMed] [Google Scholar]
- 39.Mózsik G.Y., Bódis B., Garamszegi M., Karádi O., Király Á., Nagy L., Sütő G., Tóth G., Vincze Á. Role of the vagal nerve in the development of gastric mucosal injury and its prevention by atropine, cimetidine, β-carotene and prostacyclin in rats. In: Szabó S., Taché I., editors. Neuroendocrinology of Gastrointestinal Ulceration. New York: Plenum Press; 1995. pp. 175–190. [http://dx.doi.org/10.1007/978-1-4615-1867-9_18] [Google Scholar]
- 40.Karádi O., Bódis B., Király Á., Abdel-Salam O.M., Sütő G., Vincze Á., Mózsik G.Y. Surgical vagotomy enhances the indomethacin-induced gastrointestinal mucosal damage. Inflammopharmacology. 1994;2:389–399. [http://dx.doi.org/10.1007/BF02678605]. [Google Scholar]
- 41.Szabo I.L., Czimmer J., Mózsik G.Y. From mucosal energy metabolism to capsaicin – Strategies for understanding mucosal protection. In: Filaretova L.P., Takeuchi K., editors. Cell/Tissue Injury and Cytoprotection/Organo- protection in the Gastrointestinal Tract. Mechanisms, Prevention and Treatment. Front Gastrointest. Res. Vol. 30. Basel: Karger; 2012. pp. 230–241. [http://dx.doi. org/10.1159/000338463] [Google Scholar]
- 42.Mózsik G.Y., Abdel-Salam O.M., Szolcsányi J. Capsaicin-sensitive Afferent Nerves in Gastric Mucosal Damage and Protection. Budapest: Akadémiai Kiadó; 1997. [Google Scholar]
- 43.Mózsik G.Y., Szabo I.L., Czimmer J. Vulnerable points of the Helicobater pylori story-based on Animal and human observations (1975-2012) In: Buzas GyM., editor. Helicobater Pylori: A Worldwide Perspectives 2013; Oak Park, IL, USA: Benthan Science Publishers:; 2014. pp. 429–480. [Google Scholar]