Abstract
The Danish study of Functional Disorders (DanFunD) cohort was initiated to outline the epidemiology of functional somatic syndromes (FSS) and is the first larger coordinated epidemiological study focusing exclusively on FSS. FSS are prevalent in all medical settings and can be defined as syndromes that, after appropriate medical assessment, cannot be explained in terms of a conventional medical or surgical disease. FSS are frequent and the clinical importance varies from vague symptoms to extreme disability. No well-described medical explanations exist for FSS, and how to delimit FSS remains a controversial topic. The specific aims with the cohort were to test delimitations of FSS, estimate prevalence and incidence rates, identify risk factors, delimitate the pathogenic pathways, and explore the consequences of FSS. The study population comprises a random sample of 9,656 men and women aged 18–76 years from the general population examined from 2011 to 2015. The survey comprises screening questionnaires for five types of FSS, ie, fibromyalgia, whiplash-associated disorder, multiple chemical sensitivity, irritable bowel syndrome, and chronic fatigue syndrome, and for the unifying diagnostic category of bodily distress syndrome. Additional data included a telephone-based diagnostic interview assessment for FSS, questionnaires on physical and mental health, personality traits, lifestyle, use of health care services and social factors, and a physical examination with measures of cardiorespiratory and morphological fitness, metabolic fitness, neck mobility, heart rate variability, and pain sensitivity. A biobank including serum, plasma, urine, DNA, and microbiome has been established, and central registry data from both responders and nonresponders are similarly available on morbidity, mortality, reimbursement of medicine, heath care use, and social factors. A complete 5-year follow-up is scheduled to take place from year 2017 to 2020, and further reexaminations will be planned. Several projects using the DanFunD data are ongoing, and findings will be published in the coming years.
Keywords: functional somatic syndromes, medically unexplained symptoms, epidemiology, longitudinal cohort study, pathophysiology, risk factors
Video abstract
Introduction
The Danish study of Functional Disorders (DanFunD) was initiated to outline the epidemiology of what is often referred to as medically unexplained symptoms/syndromes or bodily distress, defined as conditions that cannot be explained in terms of a conventional medical or surgical disease.1–6 They exist in many forms, are clinically important, and are prevalent in all medical settings. The common name for these conditions has been interchanging but will in this article be referred to as functional somatic syndromes (FSS), represented by irritable bowel syndrome,7–10 fibromyalgia,11 chronic fatigue syndrome,12–14 whiplash-associated disorders,15 multiple chemical sensitivity,16 and the unifying diagnostic category of bodily distress syndrome.6,17
A fundamental prerequisite for a rational handling of chronic diseases is a detailed knowledge of the epidemiology of the diseases, which is severely lacking in regard to FSS. A major epidemiological breakthrough came with the Framingham Heart Study (1948–1952), which signifies the foundation of the process that led toward our current understanding of the occurrence, major risk factors, and prognosis for cardiovascular diseases.18–20 Today, >60 years after these first cohort studies, we have an extensive knowledge on the epidemiology of many chronic diseases (cardiovascular diseases, diabetes, lung diseases, and certain cancers), which has led to a solid basis for both rational prevention and treatment.18,20 In spite of this obvious success, the medical fields encompassing the different FSS have not been through such a process yet. Important obstacles are the unclear nosological status of FSS and the ongoing dispute of whether FSS constitute one or several disorders.11 Within the recent years, epidemiological studies of FSS have contributed with valuable new knowledge about FSS.21–25 However, important epidemiological weaknesses of these studies are present, such as recruiting only patients suffering from one single FSS subtype, not using a random sample of the general population or basing the FSS diagnosis on questionnaire data exclusively. Consequently, the estimated prevalence and the incidence rates of FSS have so far been inconsistent,26,27 and our understanding of both the pathogenesis and common risk factors is similarly at a premature stage.28,29 The significance of various physiological, biochemical, and psychological factors has received some scientific attention,30–34 but a systematic approach has been missing. Finally, the impact of FSS both on an individual level and for the society as a whole is difficult to disentangle due to few well-conducted follow-up studies.
The burden on the use of the health care system itself can be calculated indirectly, but there is a need for longitudinally cohort studies linked to registries to obtain reliable data on remission, exacerbation, use of health care services, and development of other diseases. Accordingly, due to the current lack of medical explanations for FSS, patients are often misdiagnosed and offered insufficient health care solutions.5,35 Moreover, they experience being met with mistrust and doubt by health care professionals, the social welfare system, and friends and relatives.16,30 The negative consequences for the patients and society in terms of unmet health care needs, social isolation, and loss of ability to work emphasize the demand for well-designed studies investigating the epidemiology.26
In 2009, motivated by the growing concern about the FSS within the medical community, a “think tank” of scientists, medical experts, and medical social workers were invited by the Danish foundation TrygFonden to discuss possible interdisciplinary and cross-national research studies. The outcome was the formation of the DanFunD Steering Committee with the overall objectives to design and launch a large-scale epidemiological population-based longitudinal study with special focus on FSS, encompassing researchers from basic science, clinical science, biostatistics, and epidemiology.
Objectives
The overall goal of the DanFunD is to unravel the epidemiology of FSS and achieve a similar knowledge to what has been generated for other chronic diseases using epidemiological methods.
More specifically, the objectives of the DanFunD can be divided into the following categories:
To delimitate FSS by means of relevant symptoms in the general population. This will lead to a deeper understanding as to whether we are dealing with one or several disorders and bring forward more transparent delimitations of FSS. A better delimitation of FSS will similarly lead to more precise estimates of the true prevalence and incidence of FSS.
To disentangle the pathophysiology of FSS, thereby supporting the delimitation of the syndrome(s).
To identify risk factors for FSS ranging from the genome and the microbiomes over fitness and lifestyles to psychological factors.
To assess the consequences of FSS, both on an individual level and for the society as a whole, including the course of the syndromes (remission, stability, and exacerbation), development of other diseases, and socioeconomic consequences.
Study population
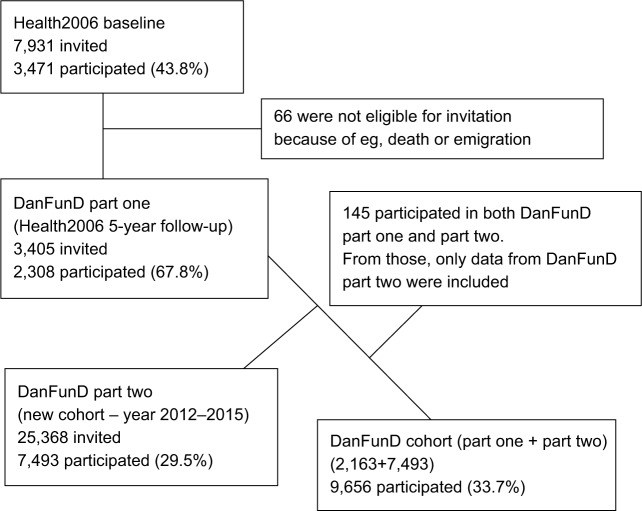
The DanFunD cohort is anchored at the Research Centre for Prevention and Health (RCPH), Glostrup, Denmark and is a random sample of the general adult population. The cohort comprises a total of 9,656 men and women aged 18–76 years, born in Denmark and living in the western part of the greater Copenhagen. The cohort is merged from two cohorts (DanFunD part one and DanFunD part two) and will create the basis for a longitudinal population-based study of FSS. Figure 1 illustrates a flowchart of the DanFunD cohort consisting of
DanFunD part one: a 5-year follow-up examination of the Health2006 cohort (n=2,163);36
DanFunD part two: a new cohort also dedicated to FSS, accomplished from 2012 to 2015 (n=7,493).
Figure 1.
Flowchart of the DanFunD cohort.
Abbreviation: DanFunD, Danish study of Functional Disorders.
DanFunD part one was carried out at RCPH in 2011–2012 as a follow-up examination of the Health2006 study, which is a population-based cohort randomly obtained from the nationwide Danish registries and examined at the RCPH in the period 2006–2008.36 A total of 3,471 individuals had participated in the Health2006 baseline study, of whom 66 individuals were not eligible for invitation for the follow-up examination, and hence 3,405 individuals were invited to participate. A total of 2,308 individuals (67.8%) participated in the follow-up from 2011 to 2012, with an age span from 23 to 76 years.
DanFunD part two was carried out at RCPH from 2012 to 2015. For this new cohort, a total of 25,368 men and women aged 18–72 years with the same background population characteristics as those of DanFunD part one were randomly obtained from the nationwide Danish registries and invited to participate. A total of 7,493 (29.5%) individuals agreed to participate. A total of 145 individuals participated in both DanFunD part one and DanFunD part two, and for those individuals, only data obtained from DanFunD part two were included in the overall study.
All participants were asked to meet fasting at the day of examination and to abstain from smoking at least 1 hour prior to examination. Exclusion criteria were as follows: not born in Denmark, not being a Danish citizen, and pregnancy. All participants had a general health examination and completed a premailed questionnaire as well as an additional questionnaire at RCPH. A written informed consent form was obtained from all participants, and the study was approved by the Ethical Committee of Copenhagen County (Ethics Committee: KA-2006-0011, H-3-2011-081, and H-3-2012-0015) and the Danish Data Protection Agency.
Plans for follow-up
A follow-up is scheduled to take place in 2017–2020. Further reexaminations will be planned, and there are options of nested case–control or case–cohort studies in the future. The entire (both responders and nonresponders) cohort will continuously be followed up in the nationwide Danish registries on the development of diseases (both somatic and psychiatric), reimbursement of medicine, contact to general practitioners, and social factors.
Measurements
The data collection procedure was similar for both DanFunD part one and DanFunD part two, and most measures are available for the entire merge cohort. Some examinations have been included only for DanFunD part two, and the numbers of participants for those measures are provided where applicable. A summary of all measurements can be found in Table 1.
Table 1.
Summary of data collected for the DanFunD
| General questionnaire-based information | Social factors, lifestyle factors, physical activity and sedentarism, general health, use of health care system, and chronic diseases |
| Diet | Dietary Quality Score based on a self-administered food frequency questionnaire |
| Mental fitness component | Mental vulnerability, somatization, anxiety, and depression, illness perception, health anxiety, life events (DanFunD part two), fatigue, personality traits, coping resources, and perceived stress |
| FSS | Fibromyalgia, chronic fatigue syndrome, irritable bowel syndrome and dyspepsia, multiple chemical sensitivity, whiplash-associated disorders, and bodily distress syndrome |
| Diagnostic assessment of FSS | Simplified version of the Schedules for Clinical Assessment in Neuropsychiatry interview for FSS and common mental disorders (n=1,609 from DanFunD part two) |
| Morphological fitness and muscle fitness | Weight, height, waist and hip circumference, bioelectrical impedance, and hand grip |
| Cardiorespiratory fitness | Blood pressure, resting heart rate, step test, lung function by spirometry, and respiratory rate |
| Mobility of the neck test | Mobility of the neck measured by cervical-range-of-motion (DanFunD part two only) |
| Metabolic and nutritional biomarkers | Glucose, glycated hemoglobin (HbA1c), lipids (total cholesterol, LDL, HDL, and VLDL cholesterol, and triglycerides), sodium, alanine aminotransferase, albumin, calcium, carbamide, creatinine, potassium, and eGFR. Urine levels of sodium, calcium, albumin, and the albumin/creatinine ratio |
| Heart rate viability | Heart rate viability was measured at rest and during deep breathing (DanFunD part two only) and before and during the cold pressor test (n=2,199) |
| Pain perception | Pressure pain thresholds and conditioning pain modulation measures using quantitative sensory testing (n= first 2,199 participants of DanFunD part two) |
| Biobank | Serum, plasma, urine, and DNA from the entire cohort and feces (n=2,464, volunteers from DanFunD part two) |
| Genetics | About 800,000 single nucleotide polymorphisms |
| Gut microbiome characterization | Gut microbiome characterization using 16S ribosomal RNA gene marker sequencing (n=2,464) |
| Linkage to nationwide Danish registries | Hospital admissions – both general and psychiatric, reimbursement of medicine and primary health care, the diabetes registry, the cancer registry, and the social registries covering education, income, employment, and cohabitation |
Abbreviations: DanFunD, Danish study of Functional Disorders; eGFR, estimated glomerular filtration rate; FSS, functional somatic syndromes; LDL, low-density lipoprotein; HDL, high-density lipoprotein; VLDL, very-low-density lipoprotein.
Questionnaires
Validated questions on a number of parameters routinely assessed in the RCPH cohorts were measured in a premailed questionnaire. Participants were asked questions regarding social factors, mental vulnerability,37 general health, use of the health care system, occurrence of chronic diseases, and lifestyle factors such as smoking, alcohol consumption, physical activity, sedentarism,36 and dietary intake (estimated using a self-administered 26-item food frequency questionnaire [FFQ]).38 Furthermore, validated questionnaires were filled in at RCPH on the five-factor personality traits (NEO Five-Factor Inventory),39 coping resources (general self-efficacy scale),40 self-perceived stress (Cohen’s Perceived Stress Scale),41 life events (cumulative lifetime adversity measure, DanFunD part two only),42 fatigue (the Chalder Fatigue scale),43,44 mental health (Symptom Checklist 90 [somatization, anxiety, and depression]),45 health anxiety (the Whiteley-7 scale),46 illness perception (Brief Illness Perception Questionnaire),47 and Kinesiophobia (Tampa scale).48 Questionnaires regarding a large variety of relevant symptoms were also administered regarding fibromyalgia,49,50 chronic fatigue syndrome,43 irritable bowel syndrome and dyspepsia,7–9 multiple chemical sensitivity,51,52 whiplash-associated disorders, and bodily distress syndrome.6,17,53
Interview
To establish FSS diagnosis and validate the symptom questionnaires, a stratified sample of ~10% of all DanFunD part two participants (n=706) and all DanFunD part two high scores on the bodily distress syndrome symptoms questionnaire17 and Whitely-7 scale46 (n=903) completed a diagnostic assessment by FSSminiSCAN, which is a brief version of the Schedules for Clinical Assessment in Neuropsychiatry.54 The interviews were conducted by experienced primary care physicians.
Examinations
Basic physiological examinations
A basic examination routinely conducted in all cohorts at RCPH was included55 regarding cardiorespiratory fitness (blood pressure, resting heart rate, step test,56 lung function by spirometry, and respiratory rate), morphological fitness (weight, height, waist and hip circumference, and bioelectrical impedance), and muscle fitness (hand grip).
Cervical range of motion
For the DanFunD part two, a “mobility of the neck” test was included, measured by cervical range of motion.57,58 Participants were placed in a low, fixed chair without armrest with the cervical-range-of-motion device installed on the head. Active head movement in six different directions were measured, ie, bending, backward bending, rotation to the right and left sides, and side bending to the right and left sides. The six measured values were used to compute an overall measure of total active cervical range of motion.
Pain perception
A pain modulation test was completed in a subgroup represented by the first 2,199 individuals examined as part of the DanFunD part two. Two quantitative sensory testing methods were applied:59,60 manually applied pressure stimulation to examine pressure pain thresholds and descending pain control as assessed by a conditioning pain modulation paradigm polymorphisms.61
Heart rate variability
Heart rate variability is used to monitor dynamics of the autonomic, cardiac innervation and is used to reflect the general tone in the autonomic nervous system (ANS). Heart rate variability examined as beat-to-beat variation serves as an indirect measure of the ANS.62,63 In DanFunD part two, heart rate variability was measured at rest and during deep breathing. In the subgroup selected for the pain modulation test, heart rate variability was also monitored before and during the cold pressor test to quantify the autonomic response to pain.64
Biochemical material and measures
Biobank
A biobank has been established at RCPH including serum, plasma, urine, and DNA for the entire cohort and feces for a subgroup of DanFunD part two (n=2,464). This biobank allows for future hypothesis testing, and various biomarkers will be of interest, such as markers for autoimmune diseases, infectious disease markers, persistent organic pollutants, long-chain fatty acids, and low-grade inflammatory markers.
Metabolic markers
Fasting blood samples and urine were analyzed for metabolic markers as listed in Table 1.
Genetics
Genotyping applying Human OmniExpress Bead array has been conducted on human leukocyte DNA from the entire cohort, covering ~800,000 single nucleotides subsequently. The genotyping data have been imputed from national and international genotype panels.
Gut microbiome characterization
The fecal samples were collected under standardized conditions, and microbial DNA has been extracted for 16S rRNA gene marker studies of the gut microbiome (n=2,464). Aliquots of feces have been stored in a research biobank for subsequent analysis of fecal biomarkers and metabolites.
Registries
The entire DanFunD cohort including responders and non-responders is linked to the nationwide Danish registries using the unique person number system (The Danish Civil Registration System) making individual linkage possible. These registries cover hospital admissions and outpatient contacts – both general and psychiatric – reimbursement of medicine, use of primary health care, and a variety of social parameters (eg, education, income, employment, cohabitation, and ethnicity). The cohort will similarly be linked to the Danish diabetes registry and cancer registry.
Cohort characteristics and ongoing projects
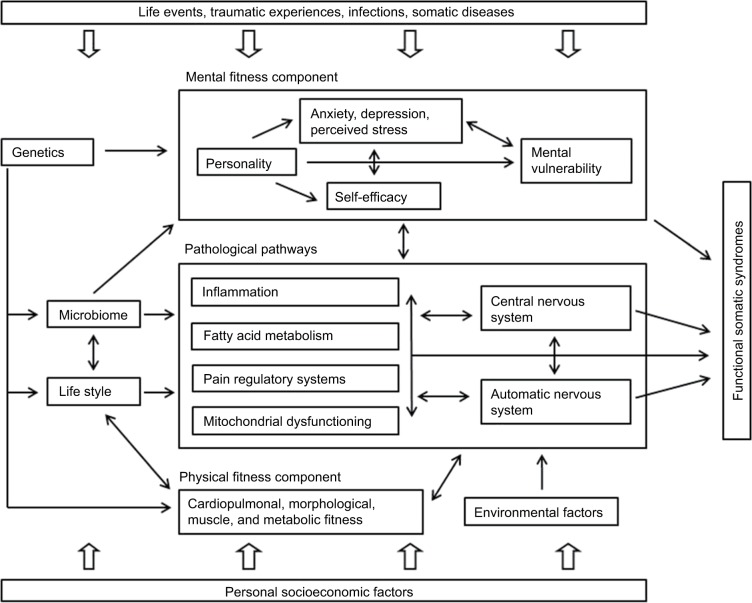
Selected characteristics of the DanFunD cohort are depicted in Table 2 (lifestyle factors), Table 3 (self-reported symptoms commonly associated with FSS), and Table 4 (self-reported FSS), providing an overview of parameters of relevance for FSS research. As described in the following sections, a number of projects using DanFunD data have already been initiated to fulfill the primary objectives, and findings will be published in the coming years. A theoretical framework for the development of FSS has been computed as depicted in Figure 2, and as more knowledge about various aspects of FSS becomes available, new hypotheses will be tested.
Table 2.
Lifestyle factors
| Variables | Men, age (years), n (%)
|
Women, age (years), n (%)
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| All | 19–39 | 40–49 | 50–59 | 60–76 | All | 19–39 | 40–49 | 50–59 | 60–76 | |
| Sex | 4,453 (46.1) | 719 (16.2) | 902 (20.3) | 1,130 (25.4) | 1,702 (38.2) | 5,203 (53.9) | 905 (17.4) | 1,139 (21.9) | 1,401 (26.9) | 1,758 (33.8) |
| BMI | ||||||||||
| Underweight (<18.5 kg/m2) | 26 (0.6) | 13 (1.8) | 2 (0.2) | 4 (0.4) | 7 (0.4) | 116 (2.2) | 44 (4.9) | 13 (1.1) | 25 (1.8) | 34 (1.9) |
| Normal (≥18.5–25 kg/m2) | 1,583 (35.6) | 413 (57.4) | 352 (39.1) | 353 (31.3) | 465 (27.4) | 2,698 (51.9) | 567 (63.7) | 621 (54.5) | 699 (49.9) | 802 (45.6) |
| Overweight (≥25–30 kg/m2) | 2,028 (45.6) | 234 (32.5) | 432 (47.9) | 535 (47.4) | 827 (48.7) | 1,552 (29.8) | 190 (21.0) | 319 (28.0) | 452 (32.3) | 591 (33.6) |
| Obese (≥30 kg/m2) | 810 (18.2) | 59 (8.2) | 115 (12.8) | 236 (20.9) | 400 (23.5) | 834 (16.0) | 94 (10.4) | 186 (16.3) | 224 (16.0) | 330 (18.8) |
| Physical activity, leisure time (self-rated) | ||||||||||
| Sedentary | 595 (13.5) | 118 (16.5) | 119 (13.3) | 150 (13.4) | 208 (12.4) | 696 (13.5) | 164 (18.2) | 143 (12.6) | 201 (14.4) | 187 (10.8) |
| Low activity | 2,284 (51.8) | 270 (37.8) | 382 (42.8) | 591 (52.6) | 1,041 (62.0) | 3,003 (58.1) | 456 (50.6) | 593 (52.2) | 821 (59.0) | 1,133 (65.2) |
| Medium activity | 1,425 (32.3) | 289 (40.5) | 353 (39.5) | 365 (32.5) | 418 (24.9) | 1,430 (27.7) | 262 (29.0) | 389 (34.3) | 363 (26.1) | 416 (23.9) |
| High activity | 105 (2.4) | 37 (5.2) | 39 (4.4) | 17 (1.5) | 12 (0.7) | 39 (0.8) | 20 (2.2) | 10 (0.9) | 7 (0.5) | 2 (0.1) |
| Diet (self-rated)a | ||||||||||
| Healthy | 774 (17.6) | 111 (15.8) | 168 (18.8) | 218 (19.5) | 277 (16.5) | 1,364 (26.4) | 160 (17.8) | 281 (24.7) | 420 (30.2) | 503 (28.8) |
| Average | 2,935 (66.8) | 459 (65.5) | 588 (65.9) | 728 (64.9) | 1,160 (69.0) | 3,390 (65.6) | 649 (72.2) | 756 (66.5) | 879 (63.2) | 1,106 (63.3) |
| Unhealthy | 686 (15.6) | 131 (18.7) | 136 (15.2) | 175 (15.6) | 244 (14.5) | 418 (8.1) | 90 (10.0) | 99 (8.7) | 92 (6.6) | 137 (7.8) |
| Smoking | ||||||||||
| Daily smokers | 593 (13.4) | 101 (14.2) | 101 (11.3) | 173 (15.4) | 218 (12.9) | 670 (12.9) | 115 (12.7) | 145 (12.8) | 203 (14.6) | 207 (11.8) |
| Occasional smokers | 165 (3.7) | 71 (10.0) | 32 (3.6) | 30 (2.7) | 32 (1.9) | 154 (3.0) | 70 (7.8) | 33 (2.9) | 33 (2.4) | 18 (1.0) |
| Ex-smokers | 1,647 (37.2) | 129 (18.1) | 247 (27.6) | 416 (36.9) | 855 (50.5) | 1,999 (38.6) | 232 (25.7) | 428 (37.7) | 605 (43.4) | 734 (41.9) |
| Never been a smoker | 2,024 (45.7) | 411 (57.7) | 516 (57.6) | 508 (45.1) | 589 (34.8) | 2,360 (45.5) | 485 (53.8) | 530 (46.7) | 552 (39.6) | 793 (45.3) |
| Alcohol (self-reported) | ||||||||||
| >14 (men)/7 (women) units/week | 1,111 (24.8) | 67 (9.9) | 130 (15.1) | 311 (28.7) | 603 (36.8) | 1,202 (24.7) | 84 (10.2) | 142 (13.2) | 365 (27.6) | 611 (37.3) |
Note:
Based on Dietary Quality Score, developed as a crude index of the overall quality of the dietary habits.
Abbreviation: BMI, body mass index.
Table 3.
Self-reported symptoms commonly associated with functional somatic syndromes
| Variables | Men, age (years), n (%)
|
Women, age (years), n (%)
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| All | 18–39 | 40–49 | 50–59 | 60–72 | All | 18–39 | 40–49 | 50–59 | 60–72 | |
| Have you within the last 12 months experienced pain from shoulders, arms, or hands? | ||||||||||
| Yes | 1,287 (29.5) | 165 (23.3) | 247 (27.8) | 365 (32.8) | 510 (30.5) | 2,131 (41.4) | 248 (27.7) | 467 (41.3) | 660 (47.5) | 756 (43.8) |
| No | 3,088 (70.6) | 542 (76.6) | 640 (72.2) | 745 (67.2) | 1,161 (69.5) | 3,011 (58.6) | 648 (72.3) | 663 (58.7) | 729 (52.5) | 971 (56.2) |
| How frequently are you bothered by pain from shoulders, arms, or hands? | ||||||||||
| Never | 3,117 (71.2) | 553 (78.2) | 643 (72.5) | 749 (67.5) | 1,172 (70.1) | 2,994 (58.2) | 655 (73.1) | 665 (58.9) | 722 (52.0) | 951 (55.1) |
| Occasionally | 638 (14.6) | 102 (14.4) | 141 (15.9) | 169 (15.1) | 226 (13.5) | 834 (16.2) | 119 (13.2) | 189 (16.7) | 238 (17.1) | 288 (16.7) |
| Frequently | 460 (10.5) | 39 (5.5) | 85 (9.6) | 141 (12.7) | 195 (11.7) | 894 (17.4) | 94 (10.5) | 182 (16.1) | 275 (19.8) | 343 (19.9) |
| Almost constantly | 160 (3.7) | 13 (1.8) | 18 (1.9) | 51 (4.6) | 78 (4.7) | 421 (8.2) | 28 (3.1) | 94 (8.3) | 154 (11.1) | 145 (8.3) |
| Have you within the last 12 months experienced pain from legs or feet? | ||||||||||
| Yes | 1,177 (26.9) | 144 (20.4) | 216 (24.4) | 312 (28.1) | 505 (30.2) | 1,734 (33.7) | 166 (18.5) | 336 (29.7) | 565 (40.7) | 667 (38.6) |
| No | 3,198 (73.1) | 563 (79.6) | 671 (75.6) | 798 (71.9) | 1,166 (69.8) | 3,408 (66.3) | 730 (81.5) | 794 (70.3) | 824 (59.3) | 1,060 (61.4) |
| How frequently are you bothered by pain from legs or feet? | ||||||||||
| Never | 3,200 (73.1) | 571 (80.8) | 676 (76.2) | 798 (71.9) | 1,155 (69.1) | 3,384 (65.8) | 735 (82.0) | 793 (70.2) | 820 (59.0) | 1,036 (60.0) |
| Occasionally | 600 (13.7) | 82 (11.6) | 115 (13.0) | 172 (15.5) | 231 (13.8) | 691 (13.4) | 82 (9.2) | 149 (13.2) | 220 (15.8) | 240 (13.9) |
| Frequently | 391 (8.9) | 43 (6.1) | 70 (7.9) | 92 (8.3) | 186 (11.1) | 717 (13.9) | 53 (5.9) | 124 (11.0) | 233 (16.8) | 307 (17.8) |
| Almost constantly | 184 (4.2) | 11 (1.6) | 26 (2.9) | 48 (4.3) | 99 (5.9) | 350 (6.8) | 26 (2.9) | 64 (5.7) | 116 (8.4) | 144 (8.3) |
| Have you within the last 12 months experienced pain from the neck, chest or back? | ||||||||||
| Never | 2,778 (63.5) | 486 (68.7) | 543 (61.2) | 642 (57.9) | 1,107 (66.2) | 2,711 (52.7) | 572 (63.8) | 569 (50.4) | 663 (47.7) | 907 (52.5) |
| Occasionally | 953 (21.8) | 140 (19.8) | 212 (23.9) | 276 (24.8) | 325 (19.4) | 1,225 (23.8) | 167 (18.6) | 283 (25.0) | 357 (25.8) | 418 (24.2) |
| Frequently | 459 (10.5) | 56 (7.9) | 93 (10.5) | 137 (12.4) | 173 (10.4) | 825 (16.0) | 110 (12.3) | 195 (17.3) | 253 (18.2) | 267 (15.5) |
| Almost constantly | 185 (4.2) | 25 (3.5) | 39 (4.4) | 55 (5.0) | 66 (3.9) | 381 (7.4) | 47 (5.2) | 83 (7.3) | 116 (8.4) | 135 (7.8) |
| Have you within the last 12 months experienced stomach pain or stomach cramps? | ||||||||||
| Never | 2,964 (68.0) | 479 (67.8) | 600 (67.9) | 745 (67.4) | 1,140 (68.5) | 2,580 (50.5) | 329 (37.0) | 558 (49.5) | 702 (51.0) | 991 (57.7) |
| Occasionally | 1,195 (27.4) | 197 (27.9) | 240 (27.1) | 303 (27.3) | 455 (27.3) | 2,029 (39.7) | 433 (48.7) | 445 (39.5) | 556 (40.4) | 595 (34.6) |
| Frequently | 180 (4.1) | 27 (3.8) | 41 (4.6) | 55 (5.0) | 57 (3.4) | 445 (8.7) | 110 (12.4) | 117 (10.4) | 102 (7.4) | 116 (6.8) |
| Almost constantly | 22 (0.5) | 3 (0.4) | 3 (0.3) | 3 (0.3) | 13 (0.8) | 56 (1.1) | 17 (1.9) | 7 (0.6) | 16 (1.2) | 16 (0.9) |
| In the last 12 months, how much have you been bothered by precordial discomfort or chest pain? | ||||||||||
| Not at all | 3,390 (76.9) | 525 (73.5) | 681 (76.3) | 847 (75.6) | 1,337 (79.5) | 3,962 (77.1) | 659 (73.5) | 893 (79.3) | 1,066 (76.7) | 1,344 (77.8) |
| A bit | 827 (18.8) | 147 (20.6) | 176 (19.7) | 221 (19.6) | 283 (16.8) | 897 (17.5) | 179 (20.0) | 175 (15.5) | 232 (16.7) | 311 (18.0) |
| Somewhat | 147 (3.3) | 34 (4.8) | 28 (3.1) | 42 (3.8) | 43 (2.5) | 199 (3.9) | 36 (4.0) | 42 (3.7) | 67 (4.8) | 54 (3.1) |
| Quite a bit | 37 (0.8) | 8 (1.1) | 7 (0.8) | 7 (0.6) | 15 (0.9) | 75 (1.5) | 22 (2.5) | 14 (1.2) | 23 (1.7) | 16 (0.9) |
| A lot | 9 (0.2) | 0 (0.0) | 1 (0.1) | 4 (0.4) | 4 (0.2) | 6 (0.1) | 1 (0.1) | 2 (0.2) | 1 (0.1) | 2 (0.1) |
| In the last 12 months, how much have you been bothered by headaches? | ||||||||||
| Not at all | 2,944 (66.7) | 428 (59.98) | 530 (59.3) | 740 (66.2) | 1,246 (73.8) | 2,262 (43.8) | 251 (28.0) | 342 (30.2) | 587 (42.0) | 1,082 (62.0) |
| A bit | 1,138 (25.8) | 212 (29.7) | 276 (30.9) | 293 (26.1) | 357 (21.1) | 1,901 (36.8) | 368 (41.1) | 489 (43.2) | 542 (38.8) | 502 (28.8) |
| Somewhat | 221 (5.0) | 56 (7.8) | 58 (6.5) | 53 (4.7) | 54 (3.2) | 608 (11.8) | 160 (17.9) | 175 (15.5) | 169 (12.1) | 104 (6.0) |
| Quite a bit | 87 (2.0) | 14 (2.0) | 24 (2.7) | 23 (2.1) | 26 (1.5) | 307 (5.9) | 94 (10.5) | 95 (8.4) | 76 (5.4) | 42 (2.4) |
| A lot | 24 (0.5) | 4 (0.6) | 6 (0.7) | 9 (0.8) | 5 (0.3) | 92 (1.8) | 23 (2.6) | 31 (2.7) | 23 (1.6) | 15 (0.9) |
| In the last 12 months, how much have you been bothered by excessive fatigue? | ||||||||||
| Not at all | 1,620 (36.7) | 160 (22.4) | 284 (31.8) | 410 (36.6) | 766 (45.4) | 1,390 (26.8) | 106 (11.8) | 235 (20.7) | 334 (23.9) | 715 (40.6) |
| A bit | 1,854 (42.0) | 317 (44.4) | 414 (46.3) | 481 (42.9) | 642 (38.0) | 2,157 (41.7) | 355 (39.5) | 502 (44.3) | 620 (44.4) | 680 (38.9) |
| Somewhat | 630 (14.3) | 166 (23.2) | 135 (15.1) | 147 (13.1) | 182 (10.8) | 922 (17.8) | 246 (27.7) | 215 (19.0) | 246 (17.7) | 215 (12.3) |
| Quite a bit | 254 (5.8) | 51 (7.1) | 54 (6.0) | 64 (5.7) | 85 (5.0) | 525 (10.1) | 136 (15.1) | 132 (11.7) | 151 (10.8) | 106 (6.1) |
| A lot | 58 (1.3) | 20 (2.8) | 7 (0.8) | 18 (1.6) | 13 (0.8) | 183 (3.5) | 55 (6.1) | 49 (4.3) | 46 (3.3) | 33 (1.9) |
| In the last 12 months, how much have you been bothered by concentration difficulties? | ||||||||||
| Not at all | 2,733 (61.8) | 312 (43.6) | 541 (60.5) | 678 (60.5) | 1,202 (71.2) | 2,670 (51.6) | 356 (39.7) | 549 (48.5) | 685 (49.1) | 1,080 (61.8) |
| A bit | 1,270 (28.7) | 276 (38.6) | 273 (30.5) | 340 (30.3) | 380 (22.5) | 1,782 (34.5) | 359 (39.9) | 413 (36.5) | 493 (35.4) | 517 (29.6) |
| Somewhat | 297 (6.7) | 88 (12.3) | 59 (6.7) | 72 (6.4) | 78 (4.6) | 479 (9.3) | 126 (14.0) | 107 (9.5) | 137 (9.8) | 109 (6.2) |
| Quite a bit | 95 (2.1) | 27 (3.8) | 19 (2.1) | 24 (2.1) | 25 (1.5) | 181 (3.5) | 39 (4.3) | 48 (4.2) | 59 (4.2) | 35 (2.0) |
| A lot | 24 (0.5) | 12 (1.7) | 2 (0.2) | 7 (0.6) | 3 (0.2) | 59 (1.1) | 17 (1.9) | 15 (1.3) | 20 (1.4) | 7 (0.4) |
| In the last 12 months, how much have you been bothered by impairment of memory? | ||||||||||
| Not at all | 2,695 (61.0) | 448 (62.7) | 571 (63.9) | 690 (61.6) | 986 (58.5) | 2,743 (53.0) | 486 (54.1) | 595 (52.5) | 697 (50.0) | 965 (55.2) |
| A bit | 1,314 (29.8) | 185 (25.9) | 240 (26,8) | 331 (29.4) | 558 (33.1) | 1,703 (32.9) | 260 (28.9) | 361 (31.9) | 490 (35.2) | 592 (33.9) |
| Somewhat | 283 (6.4) | 54 (7.6) | 61 (6.8) | 66 (5.9) | 102 (6.0) | 476 (9.2) | 105 (11.7) | 103 (9.1) | 130 (9.3) | 138 (7.9) |
| Quite a bit | 100 (2.3) | 18 (2.5) | 19 (2.1) | 27 (2.4) | 36 (2.1) | 183 (3.5) | 37 (4.1) | 51 (4.5) | 53 (3.8) | 42 (2.4) |
| A lot | 24 (0.5) | 9 (1.3) | 3 (0.3) | 8 (0.7) | 4 (0.2) | 68 (1.3) | 11 (1.2) | 23 (2.0) | 24 (1.7) | 10 (0.6) |
| In the last 12 months, how much have you been bothered by dizziness? | ||||||||||
| Not at all | 3,541 (80.2) | 573 (80.3) | 733 (82.1) | 910 (81.3) | 1,325 (78.4) | 3,564 (69.0) | 554 (61.8) | 761 (67.4) | 985 (70.7) | 1,264 (72.3) |
| A bit | 683 (15.5) | 110 (15.4) | 130 (14.6) | 161 (14.3) | 282 (16.7) | 1,203 (23.3) | 235 (26.2) | 281 (24.9) | 312 (22.4) | 375 (21.5) |
| Somewhat | 136 (3.1) | 23 (3.2) | 19 (2.1) | 39 (3.5) | 55 (3.3) | 269 (5.2) | 75 (8.4) | 53 (4.7) | 70 (5.0) | 71 (4.1) |
| Quite a bit | 51 (1.2) | 8 (1.1) | 11 (1.2) | 8 (0.7) | 24 (1.4) | 105 (2.0) | 29 (3.2) | 26 (2.3) | 21 (1.5) | 29 (1.7) |
| A lot | 5 (0.1) | 0 (0.0) | 0 (0.0) | 2 (0.2) | 3 (0.2) | 26 (0.5) | 4 (0.4) | 8 0.7) | 5 (0.4) | 9 (0.5) |
| Have you ever experienced unpleasant reactions upon inhalation of odors or chemicalsa | ||||||||||
| Yes | 2,045 (47.2) | 275 (39.1) | 380 (43.0) | 515 (46.6) | 875 (53.3) | 3,010 (59.0) | 509 (57.3) | 653 (58.3) | 828 (60.0) | 1,020 (59.5) |
Notes:
From one or more of the four most commonly reported symptom triggers among persons with multiple chemical sensitivity: car exhausts, other persons wearing of perfume, aftershave or deodorant, cleaning agents, or freshly printed papers or magazines. Please visit Berg et al for further reading about the nature of the four most commonly reported symptom triggers.52
Table 4.
| Variables | Men, age (years), n (%)
|
Women, age (years), n (%)
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Allb (3,456) |
18–39 (602) |
40–49 (677) |
50–59 (894) |
60–72 (1,283) |
Allb (4,037) |
18–39 (734) |
40–49 (872) |
50–59 (1,097) |
60–72 (1,334) |
|
| Fibromyalgia | 7 (0.2) | 1 (0.2) | 1 (0.1) | 4 (0.5) | 1 (0.1) | 52 (1.3) | 5 (0.7) | 16 (1.8) | 14 (1.3) | 17 (1.3) |
| Irritable bowel syndrome | 261 (7.7) | 18 (3.0) | 42 (6.3) | 70 (7.9) | 131 (10.5) | 602 (15.1) | 82 (11.3) | 95 (10.9) | 170 (15.8) | 255 (19.6) |
| Chronic fatigue syndrome | 39 (1.1) | 4 (0.7) | 1 (0.1) | 14 (1.6) | 20 (1.6) | 50 (1.3) | 4 (0.6) | 13 (1.5) | 13 (1.2) | 20 (1.5) |
| Multiple chemical sensitivity | 36 (1.1) | 2 (0.3) | 0 (0.0) | 6 (0.7) | 28 (2.2) | 124 (3.1) | 13 (1.8) | 15 (1.7) | 34 (3.2) | 62 (4.8) |
| Whiplash- associated disorders | 71 (2.1) | 17 (2.8) | 16 (2.4) | 18 (2.1) | 20 (1.6) | 146 (3.7) | 21 (2.9) | 39 (4.5) | 51 (4.7) | 35 (2.7) |
| Report one of the above | 344 (10.0) | 40 (6.6) | 51 (7.5) | 84 (9.4) | 169 (13.2) | 678 (16.8) | 102 (13.9) | 117 (13.4) | 199 (18.1) | 260 (19.5) |
| Report two or more of the above | 31 (0.9) | 1 (0.2) | 4 (0.6) | 12 (1.3) | 14 (1.1) | 134 (3.3) | 11 (1.5) | 28 (3.2) | 36 (3.3) | 59 (4.4) |
Notes:
Have you ever been told by a physician that you suffer from any of the following conditions?
Question about self-reported functional somatic syndromes were only included in DanFunD part two.
Abbreviation: DanFunD, Danish study of Functional Disorders.
Figure 2.
A theoretical framework for development of FSS.
Abbreviation: FSS, functional somatic syndromes.
Delimitation of FSS
This part follows the following two integrated trails: validation of the various classical definitions of FSS based on an FSSminiSCAN interview for FSS in a nested case-based design54 and the use of advanced statistical methods and an exploratory approach to identify and describe the associations among somatic symptoms, showing the frequency of symptoms and how they are associated in a complex structure. Findings from the two parallel trails will be used to assess whether other delimitations of FSS may be more relevant than currently available.65
Pathogenic pathways
ANS is suspected to be a central player in FSS pathologies, with altered ANS reactivity being associated with chronic pain and sympathetic predominance found in conditions such as fibromyalgia, chronic fatigue syndrome, and irritable bowel syndrome.62,63,66,67 The functioning of the ANS is studied by means of heart rate variability as an indirect measure of ANS functioning. Persistent pain is similarly common in most FSS, and changes in pain sensitivity are frequently reported.10,13,30,68,69 The status of specific pain mechanisms can be assessed experimentally by standardized activation of different pathways in the nociceptive system and quantitative assessment of the evoked responses, also known as quantitative sensory testing. By means of heart rate viability and quantitative sensory testing measures, the role of and interplay between altered pain perception, regulation of ANS, and a number of relevant biomarkers will be examined in relation to the pathophysiology of FSS.
Risk factors
The analysis of risk factors will comprise a broad range of physical, emotional, cognitive, social, and health care variables (Figure 2). Studies have found that cognitive and behavioral factors, such as illness worrying, symptom catastrophizing, and pain avoidance behavior, are important risk factors and determinants of FSS.32,33,70–72 However, the mechanisms behind these associations between psychological factors and FSS, as well as the impact of personality, self-efficacy, and perceived stress in particular, are still unclear. Analyses will be conducted to assess whether personality traits and general self-efficacy act as independent risk factors for FSS, and self-perceived stress, illness worrying, pain avoidance behavior, and physical activity will be investigated as potential mediating factors.
Consequences
Utilizing the improved delimitations of FSS phenotypes proposed based on DanFunD, the consequences of FSS will be reassessed on an individual level and for the society as a whole, focusing on the course of the syndromes, development of other diseases, and the socioeconomic consequences. Linking to central registries, it will be tested to which extent individuals with FSS compared to individuals without FSS use the health care system, their use of antidepressive medication or minor tranquilizers, their incidences of somatic and psychiatric diseases, and whether they suffer negative social changes (eg, disability pension).73
Strengths and weaknesses
DanFunD is the first attempt in Denmark to bring experts within epidemiology, clinicians working with FSS, and basic researchers and biostatisticians together in a joint effort to unravel the epidemiology of FSS by establishing a new large population-based cohort dedicated to research in FSS. Succeeding the data collection procedure, a comprehensive and coordinated research plan will be anchored in the epidemiological institution, RCPH, creating the fundament for research into the epidemiology of FSS for the years to come.
The DanFunD has some methodological strengths and limitations, which should be considered when analyzing and interpreting future data and implications of results. An important strength of this study is the large random sample recruited from the general adult population using the nationwide Danish registries and comprising both men and women over a 50-year age span. Furthermore, all data collected are based on validated questionnaires and examinations and are being linked to national registers, by which participants can be followed and their data updated lifelong. Finally, the biobank makes it possible to test the upcoming hypothesis.
A concern is the relatively low rate of participation (Figure 1), which is a general trend in these years. Furthermore, some differences between responders and non-responders are also expected. A nonresponder analyses of the Health2006 (baseline) study stated that nonresponders differed from responders with respect to sociodemographic characteristics, education, and use of health services. Nonresponders had lower socioeconomic status, lower educational attainment, and lower personal income,36 and the same characteristics are expected to be applicable to the DanFunD cohort as well. However, we will be able to perform weighted analyses using the information obtained from nationwide Danish registries, which includes data from both the responders and the nonresponders.
Steering committee
DanFunD is anchored at RCPH. The center has >50 years of experience in conducting large-scale population-based surveys and epidemiological research as well as a permanent staff capable of conducting large-scale data collections in the general population. The principal investigator of the DanFunD, Torben Jørgensen, from RCPH leads the coordination of projects and activities and is responsible for the operation of DanFunD, including scientific, administrative, financial, ethics, and communication tasks. The steering committee consists of Professor Torben Jørgensen, Professor Per Fink, senior scientist Sine Skovbjerg, chief physician Jesper Mehlsen, and chief physician Lene Falgaard Eplov. The steering committee reviews the scientific progress and oversees the direction and progress of the scientific objectives.
Access to data
The DanFunD includes a growing number of national and international collaborating research partners representing various areas of expertise. It is the ambition with DanFunD to use a multidisciplinary approach in order to fulfill its objectives, and all researchers with an interest in the field of FSS and a high quality research proposal are encouraged to make contact. Access to data and biological material can be granted by the DanFunD steering committee. Submitted applications must be accompanied by a research proposal that comply with Danish regulations on ethical approval and data protection. For more information, please contact DanFunD administrative and scientific officer Thomas Dantoft or visit our webpage at http://www.danfund.org.
Acknowledgments
This study was supported by TrygFonden (7-11-0213), the Lundbeck Foundation (R155-2013-14070), Novo Nordisk Foundation (NNF15OC0015896), and RCPH.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Wessely S, Nimnuan C, Sharpe M. Functional somatic syndromes: one or many? Lancet. 1999;354(9182):936–939. doi: 10.1016/S0140-6736(98)08320-2. [DOI] [PubMed] [Google Scholar]
- 2.Rosmalen JG, Tak LM, de Jonge P. Empirical foundations for the diagnosis of somatization: implications for DSM-5. Psychol Med. 2011;41(6):1133–1142. doi: 10.1017/S0033291710001625. [DOI] [PubMed] [Google Scholar]
- 3.Henningsen P, Zipfel S, Herzog W. Management of functional somatic syndromes. Lancet. 2007;369(9565):946–955. doi: 10.1016/S0140-6736(07)60159-7. [DOI] [PubMed] [Google Scholar]
- 4.Creed F, Guthrie E, Fink P, et al. Is there a better term than “medically unexplained symptoms”? J Psychosom Res. 2010;68(1):5–8. doi: 10.1016/j.jpsychores.2009.09.004. [DOI] [PubMed] [Google Scholar]
- 5.Creed F, Henningsen P, Fink P. Medically Unexplained Symptoms, Somatisation, and Bodily Distress: Developing Better Clinical Services. Cambridge: Cambridge University Press; 2011. [Google Scholar]
- 6.Fink P, Toft T, Hansen MS, Ornbol E, Olesen F. Symptoms and syndromes of bodily distress: an exploratory study of 978 internal medical, neurological, and primary care patients. Psychosom Med. 2007;69(1):30–39. doi: 10.1097/PSY.0b013e31802e46eb. [DOI] [PubMed] [Google Scholar]
- 7.Kay L, Jorgensen T. Redefining abdominal syndromes. Results of a population-based study. Scand J Gastroenterol. 1996;31(5):469–475. doi: 10.3109/00365529609006767. [DOI] [PubMed] [Google Scholar]
- 8.Kay L, Jorgensen T, Lanng C. Irritable bowel syndrome: which definitions are consistent? J Intern Med. 1998;244(6):489–494. [PubMed] [Google Scholar]
- 9.Videlock EJ, Chang L. Irritable bowel syndrome: current approach to symptoms, evaluation, and treatment. Gastroenterol Clin North Am. 2007;36(3):665–685. x. doi: 10.1016/j.gtc.2007.07.002. [DOI] [PubMed] [Google Scholar]
- 10.Chey WD, Kurlander J, Eswaran S. Irritable bowel syndrome: a clinical review. JAMA. 2015;313(9):949–958. doi: 10.1001/jama.2015.0954. [DOI] [PubMed] [Google Scholar]
- 11.Schroder A, Fink P. The proposed diagnosis of somatic symptom disorders in DSM-V: two steps forward and one step backward? J Psychosom Res. 2010;68(1):95–96. doi: 10.1016/j.jpsychores.2009.06.013. author reply 99–100. [DOI] [PubMed] [Google Scholar]
- 12.Carruthers BB, Jain AK, De Meirleir KL, et al. Myaligic encephalomyelitis/chronic fatigue syndrome: clinical working case definition, diagnostic and treatment protocols. J Chronic Fatigue Syndr. 2003;11(1):7. [Google Scholar]
- 13.Prins JB, van der Meer JW, Bleijenberg G. Chronic fatigue syndrome. Lancet. 2006;367(9507):346–355. doi: 10.1016/S0140-6736(06)68073-2. [DOI] [PubMed] [Google Scholar]
- 14.Fukuda K, Straus SE, Hickie I, Sharpe MC, Dobbins JG, Komaroff A. The chronic fatigue syndrome: a comprehensive approach to its definition and study. International Chronic Fatigue Syndrome Study Group. Ann Intern Med. 1994;121(12):953–959. doi: 10.7326/0003-4819-121-12-199412150-00009. [DOI] [PubMed] [Google Scholar]
- 15.Spitzer WO, Skovron ML, Salmi LR, et al. Scientific monograph of the Quebec Task Force on Whiplash-Associated Disorders: redefining “whiplash” and its management. Spine (Phila Pa 1976) 1995;20(Suppl 8):1S–73S. [PubMed] [Google Scholar]
- 16.Dantoft TM, Andersson L, Nordin S, Skovbjerg S. Chemical intolerance. Curr Rheumatol Rev. 2015;11(2):167–184. doi: 10.2174/157339711102150702111101. [DOI] [PubMed] [Google Scholar]
- 17.Budtz-Lilly A, Schroder A, Rask MT, Fink P, Vestergaard M, Rosendal M. Bodily distress syndrome: a new diagnosis for functional disorders in primary care? BMC Fam Pract. 2015;16:180. doi: 10.1186/s12875-015-0393-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mahmood SS, Levy D, Vasan RS, Wang TJ. The Framingham Heart Study and the epidemiology of cardiovascular disease: a historical perspective. Lancet. 2014;383(9921):999–1008. doi: 10.1016/S0140-6736(13)61752-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dawber TR, Meadors GF, Moore FE., Jr Epidemiological approaches to heart disease: the Framingham Study. Am J Public Health Nations Health. 1951;41(3):279–281. doi: 10.2105/ajph.41.3.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Niiranen TJ, Vasan RS. Epidemiology of cardiovascular disease: recent novel outlooks on risk factors and clinical approaches. Expert Rev Cardiovasc Ther. 2016;14(7):855–869. doi: 10.1080/14779072.2016.1176528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kato K, Sullivan PF, Evengard B, Pedersen NL. A population-based twin study of functional somatic syndromes. Psychol Med. 2009;39(3):497–505. doi: 10.1017/S0033291708003784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Walitt B, Fitzcharles MA, Hassett AL, Katz RS, Hauser W, Wolfe F. The longitudinal outcome of fibromyalgia: a study of 1555 patients. J Rheumatol. 2011;38(10):2238–2246. doi: 10.3899/jrheum.110026. [DOI] [PubMed] [Google Scholar]
- 23.Joustra ML, Janssens KA, Bultmann U, Rosmalen JG. Functional limitations in functional somatic syndromes and well-defined medical diseases. Results from the general population cohort LifeLines. J Psychosom Res. 2015;79(2):94–99. doi: 10.1016/j.jpsychores.2015.05.004. [DOI] [PubMed] [Google Scholar]
- 24.Stolk RP, Rosmalen JG, Postma DS, et al. Universal risk factors for multifactorial diseases: lifelines: a three-generation population-based study. Eur J Epidemiol. 2008;23(1):67–74. doi: 10.1007/s10654-007-9204-4. [DOI] [PubMed] [Google Scholar]
- 25.Creed FH, Davies I, Jackson J, et al. The epidemiology of multiple somatic symptoms. J Psychosom Res. 2012;72(4):311–317. doi: 10.1016/j.jpsychores.2012.01.009. [DOI] [PubMed] [Google Scholar]
- 26.Rosmalen JG. The way forward: a case for longitudinal population-based studies in the field of functional somatic syndromes. J Psychosom Res. 2010;68(5):399–401. doi: 10.1016/j.jpsychores.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 27.Jones GT, Atzeni F, Beasley M, Fluss E, Sarzi-Puttini P, Macfarlane GJ. The prevalence of fibromyalgia in the general population: a comparison of the American College of Rheumatology 1990, 2010, and modified 2010 classification criteria. Arthritis Rheumatol. 2015;67(2):568–575. doi: 10.1002/art.38905. [DOI] [PubMed] [Google Scholar]
- 28.Jones GT. Psychosocial vulnerability and early life adversity as risk factors for central sensitivity syndromes. Curr Rheumatol Rev. 2016;12(2):140–153. doi: 10.2174/1573397112666151231113438. [DOI] [PubMed] [Google Scholar]
- 29.Creed F. Exploding myths about medically unexplained symptoms. J Psychosom Res. 2016;85:91–93. doi: 10.1016/j.jpsychores.2016.02.007. [DOI] [PubMed] [Google Scholar]
- 30.Yunus MB. Editorial review: an update on central sensitivity syndromes and the issues of nosology and psychobiology. Curr Rheumatol Rev. 2015;11(2):70–85. doi: 10.2174/157339711102150702112236. [DOI] [PubMed] [Google Scholar]
- 31.Mayer EA, Labus JS, Tillisch K, Cole SW, Baldi P. Towards a systems view of IBS. Nat Rev Gastroenterol Hepatol. 2015;12(10):592–605. doi: 10.1038/nrgastro.2015.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Christensen SS, Frostholm L, Ornbol E, Schroder A. Changes in illness perceptions mediated the effect of cognitive behavioural therapy in severe functional somatic syndromes. J Psychosom Res. 2015;78(4):363–370. doi: 10.1016/j.jpsychores.2014.12.005. [DOI] [PubMed] [Google Scholar]
- 33.Rief W, Broadbent E. Explaining medically unexplained symptoms-models and mechanisms. Clin Psychol Rev. 2007;27(7):821–841. doi: 10.1016/j.cpr.2007.07.005. [DOI] [PubMed] [Google Scholar]
- 34.Korkina L, Scordo MG, Deeva I, Cesareo E, De Luca C. The chemical defensive system in the pathobiology of idiopathic environment-associated diseases. Curr Drug Metab. 2009;10(8):914–931. doi: 10.2174/138920009790274568. [DOI] [PubMed] [Google Scholar]
- 35.Fink P, Rosendal M. Recent developments in the understanding and management of functional somatic symptoms in primary care. Curr Opin Psychiatry. 2008;21(2):182–188. doi: 10.1097/YCO.0b013e3282f51254. [DOI] [PubMed] [Google Scholar]
- 36.Thuesen BH, Cerqueira C, Aadahl M, et al. Cohort profile: the Health2006 cohort, research centre for prevention and health. Int J Epidemiol. 2014;43(2):568–575. doi: 10.1093/ije/dyt009. [DOI] [PubMed] [Google Scholar]
- 37.Eplov LF, Petersen J, Jorgensen T, et al. The Mental Vulnerability Questionnaire: a psychometric evaluation. Scand J Psychol. 2010;51(6):548–554. doi: 10.1111/j.1467-9450.2010.00834.x. [DOI] [PubMed] [Google Scholar]
- 38.Toft U, Kristoffersen LH, Lau C, Borch-Johnsen K, Jorgensen T. The Dietary Quality Score: validation and association with cardiovascular risk factors: the Inter99 study. Eur J Clin Nutr. 2007;61(2):270–278. doi: 10.1038/sj.ejcn.1602503. [DOI] [PubMed] [Google Scholar]
- 39.Costa PT, Jr, McCrae RR. Domains and facets: hierarchical personality assessment using the revised NEO personality inventory. J Pers Assess. 1995;64(1):21–50. doi: 10.1207/s15327752jpa6401_2. [DOI] [PubMed] [Google Scholar]
- 40.Luszczynska A, Scholz U, Schwarzer R. The general self-efficacy scale: multicultural validation studies. J Psychol. 2005;139(5):439–457. doi: 10.3200/JRLP.139.5.439-457. [DOI] [PubMed] [Google Scholar]
- 41.Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav. 1983;24(4):385–396. [PubMed] [Google Scholar]
- 42.Seery MD, Leo RJ, Holman EA, Silver RC. Lifetime exposure to adversity predicts functional impairment and healthcare utilization among individuals with chronic back pain. Pain. 2010;150(3):507–515. doi: 10.1016/j.pain.2010.06.007. [DOI] [PubMed] [Google Scholar]
- 43.Chalder T, Berelowitz G, Pawlikowska T, et al. Development of a fatigue scale. J Psychosom Res. 1993;37(2):147–153. doi: 10.1016/0022-3999(93)90081-p. [DOI] [PubMed] [Google Scholar]
- 44.Jackson C. The Chalder Fatigue Scale (CFQ 11) Occup Med (Lond) 2015;65(1):86. doi: 10.1093/occmed/kqu168. [DOI] [PubMed] [Google Scholar]
- 45.Olsen LR, Mortensen EL, Bech P. The SCL-90 and SCL-90R versions validated by item response models in a Danish community sample. Acta Psychiatr Scand. 2004;110(3):161–162. doi: 10.1111/j.1600-0447.2004.00399.x. [DOI] [PubMed] [Google Scholar]
- 46.Fink P, Ewald H, Jensen J, et al. Screening for somatization and hypochondriasis in primary care and neurological in-patients: a seven-item scale for hypochondriasis and somatization. J Psychosom Res. 1999;46(3):261–273. doi: 10.1016/s0022-3999(98)00092-0. [DOI] [PubMed] [Google Scholar]
- 47.Broadbent E, Petrie KJ, Main J, Weinman J. The brief illness perception questionnaire. J Psychosom Res. 2006;60(6):631–637. doi: 10.1016/j.jpsychores.2005.10.020. [DOI] [PubMed] [Google Scholar]
- 48.Kori SH, Miller RP, Todd DD. Kinesiophobia: a new view of chronic pain behaviour. Pain Management. 1990;3:35–43. [Google Scholar]
- 49.Branco JC, Bannwarth B, Failde I, et al. Prevalence of fibromyalgia: a survey in five European countries. Semin Arthritis Rheum. 2010;39(6):448–453. doi: 10.1016/j.semarthrit.2008.12.003. [DOI] [PubMed] [Google Scholar]
- 50.White KP, Harth M, Speechley M, Ostbye T. Testing an instrument to screen for fibromyalgia syndrome in general population studies: the London Fibromyalgia Epidemiology Study Screening Questionnaire. J Rheumatol. 1999;26(4):880–884. [PubMed] [Google Scholar]
- 51.Berg ND, Linneberg A, Dirksen A, Elberling J. Prevalence of self-reported symptoms and consequences related to inhalation of airborne chemicals in a Danish general population. Int Arch Occup Environ Health. 2008;81(7):881–887. doi: 10.1007/s00420-007-0282-0. [DOI] [PubMed] [Google Scholar]
- 52.Berg ND, Linneberg A, Dirksen A, Elberling J. Phenotypes of individuals affected by airborne chemicals in the general population. Int Arch Occup Environ Health. 2009;82(4):509–517. doi: 10.1007/s00420-008-0352-y. [DOI] [PubMed] [Google Scholar]
- 53.Fink P, Schroder A. One single diagnosis, bodily distress syndrome, succeeded to capture 10 diagnostic categories of functional somatic syndromes and somatoform disorders. J Psychosom Res. 2010;68(5):415–426. doi: 10.1016/j.jpsychores.2010.02.004. [DOI] [PubMed] [Google Scholar]
- 54.Wing JK, Babor T, Brugha T, et al. SCAN Schedules for clinical assessment in neuropsychiatry. Arch Gen Psychiatry. 1990;47(6):589–593. doi: 10.1001/archpsyc.1990.01810180089012. [DOI] [PubMed] [Google Scholar]
- 55.Osler M, Linneberg A, Glumer C, Jorgensen T. The cohorts at the Research Centre for Prevention and Health, formerly ‘The Glostrup Population Studies’. Int J Epidemiol. 2011;40(3):602–610. doi: 10.1093/ije/dyq041. [DOI] [PubMed] [Google Scholar]
- 56.Aadahl M, Zacho M, Linneberg A, Thuesen BH, Jorgensen T. Comparison of the Danish step test and the watt-max test for estimation of maximal oxygen uptake: the Health2008 study. Eur J Prev Cardiol. 2013;20(6):1088–1094. doi: 10.1177/2047487312462825. [DOI] [PubMed] [Google Scholar]
- 57.Kasch H, Bach FW, Jensen TS. Handicap after acute whiplash injury: a 1-year prospective study of risk factors. Neurology. 2001;56(12):1637–1643. doi: 10.1212/wnl.56.12.1637. [DOI] [PubMed] [Google Scholar]
- 58.Youdas JW, Carey JR, Garrett TR. Reliability of measurements of cervical spine range of motion – comparison of three methods. Phys Ther. 1991;71(2):98–104. doi: 10.1093/ptj/71.2.98. [DOI] [PubMed] [Google Scholar]
- 59.Yarnitsky D. Conditioned pain modulation (the diffuse noxious inhibitory control-like effect): its relevance for acute and chronic pain states. Curr Opin Anaesthesiol. 2010;23(5):611–615. doi: 10.1097/ACO.0b013e32833c348b. [DOI] [PubMed] [Google Scholar]
- 60.Graven-Nielsen T, Arendt-Nielsen L. Assessment of mechanisms in localized and widespread musculoskeletal pain. Nat Rev Rheumatol. 2010;6(10):599–606. doi: 10.1038/nrrheum.2010.107. [DOI] [PubMed] [Google Scholar]
- 61.Yarnitsky D, Bouhassira D, Drewes AM, et al. Recommendations on practice of conditioned pain modulation (CPM) testing. Eur J Pain. 2015;19(6):805–806. doi: 10.1002/ejp.605. [DOI] [PubMed] [Google Scholar]
- 62.Heart rate variability: standards of measurement, physiological interpretation and clinical use. Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Task Force of the European Society of Cardiology the North American Society of Pacing Electrophysiology. Circulation. 1996;93(5):1043–1065. [PubMed] [Google Scholar]
- 63.Van Cauwenbergh D, Nijs J, Kos D, Van Weijnen L, Struyf F, Meeus M. Malfunctioning of the autonomic nervous system in patients with chronic fatigue syndrome: a systematic literature review. Eur J Clin Invest. 2014;44(5):516–526. doi: 10.1111/eci.12256. [DOI] [PubMed] [Google Scholar]
- 64.Peng RC, Yan WR, Zhou XL, Zhang NL, Lin WH, Zhang YT. Time-frequency analysis of heart rate variability during the cold pressor test using a time-varying autoregressive model. Physiol Meas. 2015;36(3):441–452. doi: 10.1088/0967-3334/36/3/441. [DOI] [PubMed] [Google Scholar]
- 65.Eliasen M, Kreiner S, Ebstrup JF, et al. Somatic symptoms: prevalence, co-occurrence and associations with self-perceived health and limitations due to physical health – a Danish population-based study. PLoS One. 2016;11(3):e0150664. doi: 10.1371/journal.pone.0150664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Martinez-Martinez LA, Mora T, Vargas A, Fuentes-Iniestra M, Martinez-Lavin M. Sympathetic nervous system dysfunction in fibro-myalgia, chronic fatigue syndrome, irritable bowel syndrome, and interstitial cystitis: a review of case–control studies. J Clin Rheumatol. 2014;20(3):146–150. doi: 10.1097/RHU.0000000000000089. [DOI] [PubMed] [Google Scholar]
- 67.Tak LM, Riese H, de Bock GH, Manoharan A, Kok IC, Rosmalen JG. As good as it gets? A meta-analysis and systematic review of methodological quality of heart rate variability studies in functional somatic disorders. Biol Psychol. 2009;82(2):101–110. doi: 10.1016/j.biopsycho.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 68.Clauw DJ. Fibromyalgia: a clinical review. JAMA. 2014;311(15):1547–1555. doi: 10.1001/jama.2014.3266. [DOI] [PubMed] [Google Scholar]
- 69.Lewis GN, Rice DA, McNair PJ. Conditioned pain modulation in populations with chronic pain: a systematic review and meta-analysis. J Pain. 2012;13(10):936–944. doi: 10.1016/j.jpain.2012.07.005. [DOI] [PubMed] [Google Scholar]
- 70.Chalder T, Goldsmith KA, White PD, Sharpe M, Pickles AR. Rehabilitative therapies for chronic fatigue syndrome: a secondary mediation analysis of the PACE trial. Lancet Psychiatry. 2015;2(2):141–152. doi: 10.1016/S2215-0366(14)00069-8. [DOI] [PubMed] [Google Scholar]
- 71.Bushnell MC, Ceko M, Low LA. Cognitive and emotional control of pain and its disruption in chronic pain. Nat Rev Neurosci. 2013;14(7):502–511. doi: 10.1038/nrn3516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Carroll LJ, Holm LW, Ferrari R, Ozegovic D, Cassidy JD. Recovery in whiplash-associated disorders: do you get what you expect? J Rheumatol. 2009;36(5):1063–1070. doi: 10.3899/jrheum.080680. [DOI] [PubMed] [Google Scholar]
- 73.Heinsvig Poulsen C, Falgaard Eplov L, Hjorthoj C, et al. Gastrointestinal symptoms related to the irritable bowel syndrome – a longitudinal population-based register study. Scand J Gastroenterol. 2016;51(4):420–426. doi: 10.3109/00365521.2015.1117652. [DOI] [PubMed] [Google Scholar]