Abstract
Background
The role of preoperative androgen deprivation therapy (ADT) for localized prostate cancer is controversial; prospective assessments have yielded varying results. We sought to define a subset of patients with a higher likelihood of benefit from preoperative ADT.
Methods
An institutional database including consecutive patients receiving definitive surgery for localized prostate cancer was interrogated. Patients recorded as having received preoperative ADT were matched in a 1:2 fashion to patients who had not received prior ADT. Patients were matched on the basis of clinicopathologic characteristics, use of adjuvant treatment strategies, and duration of PSA follow-up. Time to biochemical recurrence (TTBR) was compared using the Kaplan-Meier method and log-rank test for the overall study population and in subsets defined by D’Amico risk.
Results
No significant differences in clinicopathologic characteristics were noted between recipients (n=101) and matched non-recipients (n=196) of preoperative ADT. Although not statistically significant, positive surgical margin rates, seminal vesicle invasion and extracapsular extension were less frequent in patients receiving preoperative ADT. Furthermore, a lesser incidence of perioperative complications was noted in this group (7.4% v 18.4%). No significant differences were noted in TTBR between recipients and non-recipients of preoperative ADT in the overall study population. However, amongst patients with high-risk disease, TTBR was significantly longer in those patients who had received preoperative ADT (P=0.004).
Conclusions
The data presented herein suggest a potential benefit with preoperative ADT in patients with high-risk localized prostate cancer. Consideration should be given to enriching for this subset in preoperative studies of novel endocrine therapies.
Keywords: Preoperative, neoadjuvant, androgen deprivation therapy, prostatectomy
Introduction
Current guidelines for localized prostate facilitate the selection of appropriate patients for either definitive radiation therapy or definitive surgical intervention when treatment is indicated.1,2 Embedded within these guidelines are selected indications for use of androgen deprivation therapy (ADT) in association with these modalities. Based on prospective, randomized trials, patients with intermediate- or high-risk disease who choose to receive definitive radiation therapy should be offered neoadjuvant, adjuvant or concomitant ADT of duration months varying (4–6 for intermediate risk disease versus 2–3 years for high-risk disease).3,4 In contrast, the use of ADT as a preoperative adjunct to definitive surgery is not satisfactorily supported by existing evidence and is therefore absent from the guidelines.
There appears to be renewed interest in exploring the role of ADT in the context of patients receiving radical prostatectomy. In Southwest Oncology Group (SWOG) trial 9921 (S9921), patients with high-risk disease (defined as extraprostatic extension or high Gleason grade) were randomized to receive either ADT alone or ADT with mitoxantrone. Dorff et al reported outcomes for 481 patients receiving ADT in an adjuvant fashion in this study. In this group, biochemical recurrence-free survival (bRFS) was 92.5% at 5-years.5 These values compare favorably to historical standards. Preoperative ADT has also been explored across multiple prospective and retrospective studies.6 However, a cumulative interpretation of this literature is challenged by the (1) varying types of ADT utilized, (2) varying durations of ADT, and (3) disparate risk stratification schema used to classify patients receiving ADT.
Despite the controversy surrounding it, preoperative therapy for prostate cancer remains of substantial interest. Several preoperative trials that have either been reported or are ongoing assess newly approved therapies for metastatic castration-resistant prostate cancer (mCPRC), such as abiraterone or enzalutamide.7,8 As similar studies emerge, it would be ideal to identify the subset of patients most appropriate for preoperative therapy. In the current study, we utilized a large institutional database to achieve these aims.
Methods
Patient Selection
Patients who had received ADT prior to prostatectomy were identified from the City of Hope Prostate Cancer Database. This database was established through an IRB-approved protocol that prospectively captures clinicopathologic data, treatment-related data and a range of outcomes (surgical complications, time to biochemical recurrence with [TTBR], survival, etc.) amongst patients treated robot-assisted radical prostatectomy. The database was established in 2000 and, since 2003, over 5000 robotically assisted cases have been entered. The ADT patient population was selected from among the robotic cases, excluding patients who underwent salvage prostatectomy and those who received neoadjuvant chemotherapy or other pre-operative therapy. Notably, ADT was comprised of LHRH agonist therapy (excluding patients receiving LHRH antagonists) with or without antiandrogen therapy. Durations of therapy referred to herein are specific to the LHRH agonist, not antiandrogen. Notably, no patients in the currently examined cohort received preoperative therapy with novel endocrine agents, such as enzalutamide or abiraterone.
Matching Methodology
Patients who had received preoperative ADT were matched in a 1:2 fashion to patients who had received no preoperative ADT, using a computerized matching algorithm GMATCH.9 The following criteria were utilized to optimize matching (in order of priority): (1) clinical T-stage (≤ T1c v > T1c), (2) PSA (0–10 v 10–20 v >20), (3) biopsy Gleason score (≤6 v 7 v >7), (4) use of adjuvant radiotherapy (yes v no), (5) use of adjuvant hormonal therapy (yes v no), and (6) duration of PSA follow-up (< 3 years v 3–5 years v >5 years).
Statistical Analysis
The clinicopathologic characteristics of recipients of neoadjuvant ADT and their matched counterparts were compared using the chi-square or Fisher’s exact test (for categorical variables) or the student’s t-test or the Wilcoxon rank-sum test (for continuous variables), as appropriate. In addition, complications (either perioperative or during a 30-day postoperative period) were compared between the two groups in a similar fashion. Rates of adjuvant therapy use (radiation and androgen deprivation) were also compared. TTBR was characterized as the time from prostatectomy to the first time at which a PSA of > 0.2 ng/ml was recorded. Using the Kaplan-Meier method with the log-rank test, TTBR was compared in recipients and non-recipients of neoadjuvant therapy. The same comparison was then made within subgroups divided by risk (e.g., low, intermediate and high). Risk designations were in accordance with D’Amico criteria. Specifically, low risk features included cT1–T2a disease, Gleason score ≤ 6, and PSA < 10 ng/mL. Intermediate risk features included cT2b disease, Gleason score of 7, or a PSA of 10–20 ng/mL. High risk features included cT2c-T3 disease, Gleason score 8–10, or PSA > 20 ng/mL.
Results
Patient Characteristics
As noted in Table 1, clinicopathologic characteristics of patients treated with preoperative ADT (n=101) and matched patients without preoperative therapy (n=196) were similar. Notably, 3 patients who had received preoperative ADT could not be matched by the criteria noted in Statistical Analysis, but were nonetheless included in the subsequently described results. The median age of the overall cohort was 66, the majority of patients were Caucasian (81%), and based on the Charlson Comorbidity Index (CCI), there was little difference in the extent of comorbidity between the recipients and non-recipients of preoperative therapy (median age-adjusted CCI 5.0 in both groups). No significant differences in Gleason score, clinical T-stage or baseline PSA were noted between groups, and the proportions of patients characterized as low-, intermediate- or high-risk based on D’Amico criteria were similar.
Table 1.
Patient characteristics at baseline.
| ADT (n=101) | No ADT (n=196) | p-value | |
|---|---|---|---|
| Age at Surgery, median (IQR) | 66 (59 – 72) | 65 (58 – 70) | 0.1 |
| Race, n (%) | |||
| Caucasian | 81 (80.2%) | 158 (80.6%) | 0.6 |
| Black | 6 (5.9%) | 8 (4.1%) | |
| Asian | 7 (6.9%) | 9 (4.6%) | |
| Hispanic | 5 (5.0%) | 18 (9.2%) | |
| Other | 2 (2.0%) | 3 (1.5%) | |
| BMI, median (IQR) | 27.1 (25.0 – 31.0) | 27.5 (24.9 – 30.4) | 0.7 |
| PSA, median (IQR)/n (%) | 8.0 (4.9 – 13.0) | 7.2 (5.1 – 12.7) | 1.0 |
| ≤10 | 65 (64.4%) | 128 (65.3%) | 1.0 |
| 10–20 | 26 (25.7%) | 50 (25.5%) | |
| >20 | 10 (9.9%) | 18 (9.2%) | |
| Total CCI, median (IQR) | 2 (2 – 3) | 2 (2 – 8) | 0.08 |
| Total CCI - Age Adjusted, median (IQR) | 5 (4 – 6) | 5 (4 – 9) | 0.3 |
| ADT Duration >3 months – all patients, n (%)– high-risk patients, n (%) | 36 (36%) 12 (40%) | ||
| Pre-Operative Gleason Score, n (%) | |||
| ≤6 | 33 (32.7%) | 66 (33.7%) | 0.9 |
| 7 | 44 (43.6%) | 87 (44.4%) | |
| ≥8 | 24 (23.8%) | 43 (21.9%) | |
| Clinical T Stage, n (%) | |||
| T1 | 74 (73.3%) | 146 (74.5%) | 0.4 |
| T2 | 26 (25.7%) | 50 (25.5%) | |
| T3 | 1 (1.0%) | 0 (0.0%) | |
| Damico Risk group, n (%) | |||
| Low | 21 (20.8%) | 42 (21.4%) | 1.0 |
| Intermediate | 50 (49.5%) | 97 (49.5%) | |
| High | 30 (29.7%) | 57 (29.1%) |
Only a minority of patients (36%) received more than 3 months of preoperative ADT in the preoperative therapy group. A comparison of pathologic findings is delineated in Table 2. Patients receiving preoperative therapy had a lower number of lymph nodes retrieved as compared to patients with no preoperative therapy (3 v 4; P=0.001); however, there was no significant difference in the positive lymph node rate between groups (3% for both). Differences in surgical Gleason score were also observed, although it has been advocated that Gleason scores not be provided for patients who receive preoperative therapy (hence a greater extent of missing data in this group). Positive surgical margin rates, seminal vesicle invasion and extracapsular extension were all less frequent in patients receiving preoperative ADT, although these differences were not statistically significant.
Table 2.
Summary of pathologic characteristics of the study population.
| ADT (n=101) |
No ADT (n=196) |
p-value | |
|---|---|---|---|
| Path T Stage, n (%) | |||
| <pT2c | 19 (18.8%) | 23 (11.7%) | 0.4 |
| pT2c | 51 (50.5%) | 112 (57.1%) | |
| pT3a | 15 (14.9%) | 26 (13.3%) | |
| pT3b | 16 (15.8%) | 35 (17.9%) | |
| Total Lymph Nodes Removed, median (IQR) | 3 (0 – 6) | 4 (3 – 6) | 0.001 |
| Total LN Positive, median (IQR) | 2 (1 – 2) | 1.5 (1 – 2) | 1.0 |
| Path Node Status, n (%) | 3 (3.0%) | 6 (3.1%) | 1.0 |
| Surgical Gleason Score, n (%) | |||
| ≤6 | 23 (22.8%) | 40 (20.4%) | <0.0001 |
| 7 | 42 (41.6%) | 123 (62.8%) | |
| ≥8 | 19 (18.8%) | 33 (16.8%) | |
| Surgical Margins, n (%) | 25 (24.8%) | 61 (31.1%) | 0.2 |
| SV Invasion, n (%) | 16 (15.8%) | 35 (17.9%) | 0.3 |
| ExtraCapsular Extension, n (%) | 23 (22.8%) | 53 (27.0%) | 0.3 |
Adjuvant strategies were utilized in only a small proportion of patients, and the extent of use was similar between arms. Approximately 12% of patients received adjuvant radiotherapy, while 15% of patients received adjuvant ADT. Use of adjuvant chemotherapy was minimal.
Operative Morbidity
Operative time was lower in patients receiving preoperative ADT (2.9 hours v 3.0 hours, P=0.03), although the numerical difference was marginal. Intraoperative blood loss was also lower in patients receiving no preoperative therapy (200 mL v 250 mL, P=0.01). As noted in Table 3, perioperative morbidity was limited, although the overall rate of peri-operative complications was lower in patients with preoperative ADT (7.4% v 18.4%). In particular, rates of urinary retention and urine leak appeared to be lower in patients with preoperative ADT as compared to no preoperative ADT (2.1% v 7.7% and 4.3% v 9.7%, respectively). Length of hospital stay was similar between groups.
Table 3.
Summary of intraoperative and perioperative morbidity.
| ADT Patients (n=101) |
No ADT Patients (n=196) |
P value | |
|---|---|---|---|
| Operative Time, median (IQR) | 2.9 (2.5 – 3.3) | 3.0 (2.6 – 3.5) | 0.03 |
| Intraoperative Blood Loss, median (IQR) | 200 (150 – 300) | 250 (190 – 300) | 0.01 |
| IntraOp Complications, n (%) | 1 (1.0%) | 1 (0.5%) | |
| IntraOp Rectotomy, n (%) | 0 (0.0%) | 1 (0.5%) | |
| IntraOp Other, n (%) | 1 (1.0%) | 0 (0.0%) | |
| IntraOp Transfusion, n (%) | 1 (1.0%) | 3 (1.5%) | |
| PeriOp Complications, n (%) | 7 (7.4%) | 37 (18.9%) | |
| PeriOp DVT, n (%) | 1 (1.1%) | 3 (1.5%) | |
| PeriOp Hernia, n (%) | 0 (0.0%) | 4 (2.1%) | |
| PeriOp Wound Infection, n (%) | 0 (0.0%) | 6 (3.1%) | |
| PeriOp Ileus, n (%) | 3 (3.4%) | 5 (2.6%) | |
| PeriOp UVA Stricture, n (%) | 0 (0.0%) | 1 (0.6%) | |
| PeriOp Lymphocele, n (%) | 1 (1.1%) | 1 (0.5%) | |
| PeriOp Urinary Retention, n (%) | 2 (2.1%) | 15 (7.7%) | |
| PeriOp Transfusion, n (%) | 1 (1.0%) | 1 (0.5%) | |
| Urine Leak, n (%) | 4 (4.3%) | 19 (9.7%) | |
| Hospital Stay, n (%) | |||
| 1–2 | 87 (86.1%) | 169 (86.2%) | 1.0 |
| 3+ | 14 (13.9%) | 27 (13.8%) |
Clinical Outcome
The median length of PSA follow-up was slightly longer in patients with preoperative ADT as compared to patients with no preoperative ADT, although this difference was not statistically significant (48.2 months v 37.8 months, P=0.4). Median TTBR was not reached for either cohort, and no significant difference was identified in comparing TTBR between these groups (Figure 1). However, several interesting trends were noted when analyses were separately conducted within subgroups based on D’Amico risk. First, amongst patients with low-risk disease, TTBR was improved in those patients who did not receive preoperative ADT (P=0.02) (Figure 2a). This particular analysis was limited by a low number of recorded events and early censoring of many patients within this subgroup. Amongst patients with intermediate-risk disease, no significant difference in TTBR was noted (Figure 2b). However, amongst patients with high-risk disease, there was a significant improvement in TTBR with the use of preoperative ADT (P=0.004) (Figure 2c).
Figure 1.
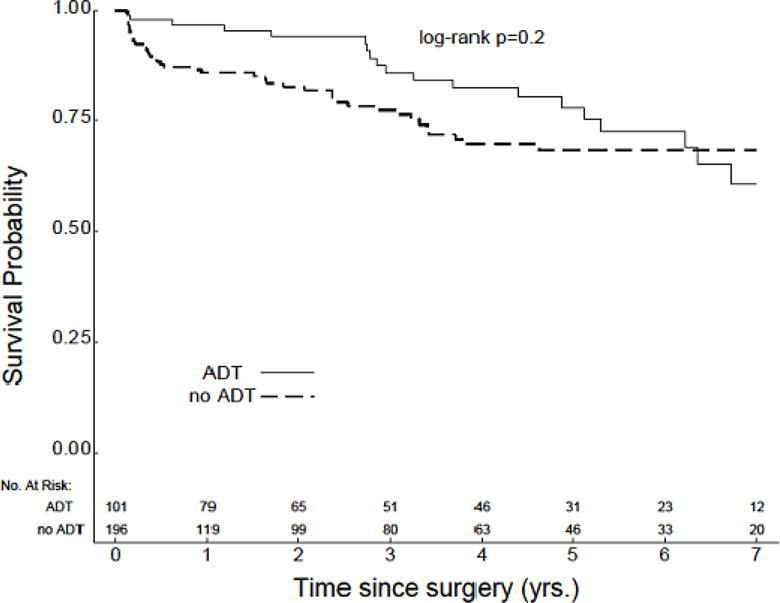
TTBR for the overall study population (n=297).
Figure 2.
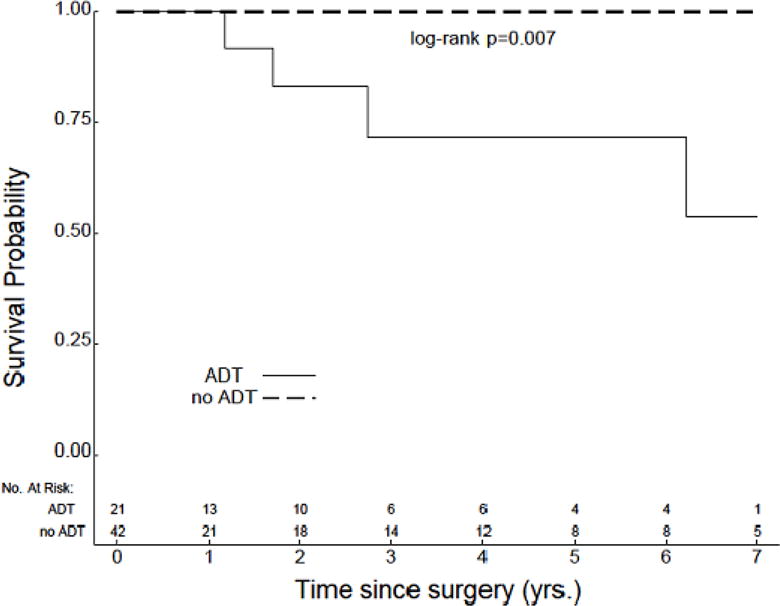
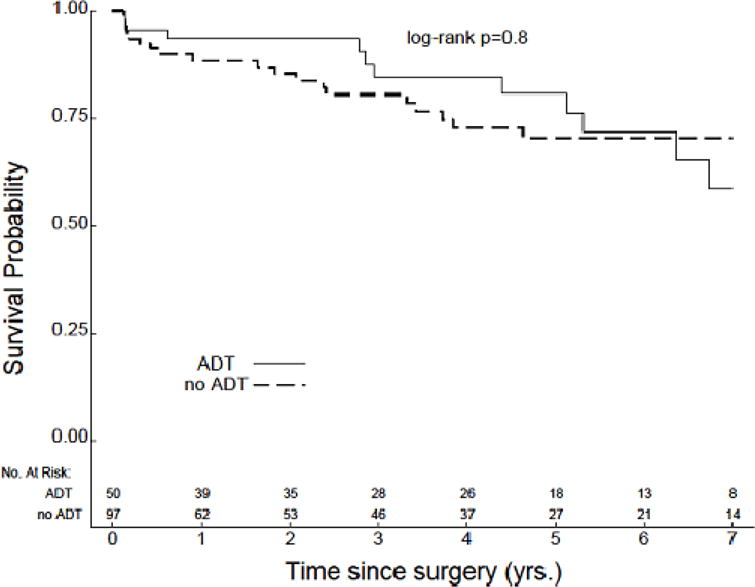
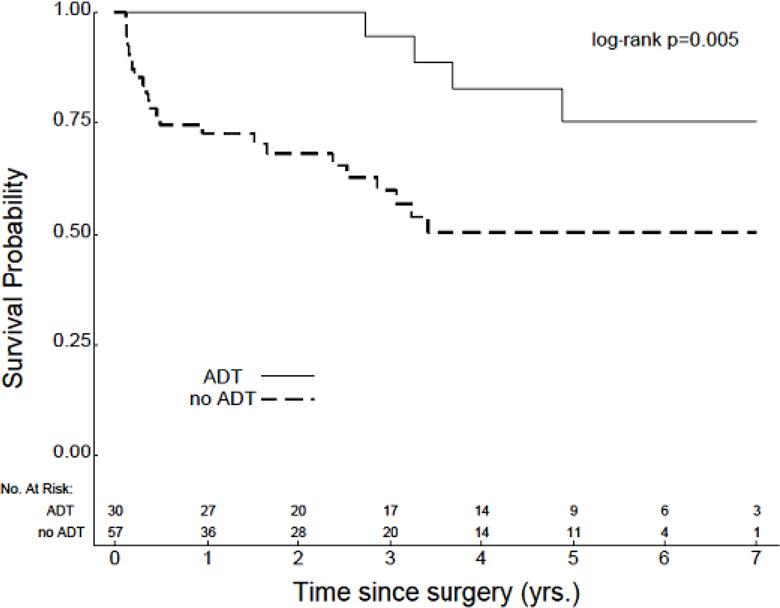
TTBR for patients with (a) low-risk (n=63), (b) intermediate-risk (n=147), and (c) high-risk disease (n=87).
Functional outcomes were comparable between the two study populations. Among patients who were considered continent prior to undergoing prostatectomy and who were postsurgically assessed for continence (n=266), 84.2% of ADT patients and 89.7% of patients not receiving pre-operative ADT regained continence following prostatectomy (p=0.2).
Discussion
The results presented herein suggest that although preoperative ADT may not uniformly render benefit for localized prostate cancer, there may be a particular benefit amongst those patients with high-risk disease. These results support subset analyses focused on high-risk patients enrolled in separate prospective assessments of preoperative ADT by SWOG and CUOG.10,11 Ultimately, these data may support current investigations of novel therapies within this space. For instance, a recently reported study of preoperative abiraterone (a novel CYP17 lyase inhibitor) included patients with ≥ cT3a disease, baseline PSA ≥ 20, Gleason grade ≥ 7 (4+3) or a PSA velocity > 2 ng/mL/year. Although these criteria differ from the D’Amico criteria used herein, there are some consistencies. The current study also provides data pertaining to the utility of neoadjuvant therapy in patients receiving robotic prostatectomy – all patients in the current series were treated with this approach. Others have summarized neoadjuvant data specifically in the setting of laparoscopic surgery; these studies have suggested similar efficacy and surgical morbidity.12
Outside of the findings related to TTBR in risk-based subsets, several other differences between recipients and non-recipients of preoperative therapy deserve mention. First, on pathologic analysis, there was a non-significant trend towards lower positive surgical margin rates, lower frequency of seminal vesicle invasion and lower frequency of extracapsular extension. Of note, randomized trials assessing neoadjuvant therapy have previously reported lower positive margin rates.13,14 While the use of adjuvant strategies in the study population may confound analysis of distant endpoints, such as TTBR, they would have little impact on pathologic findings. Interestingly, the rate of perioperative complications and blood loss was lower in patients with prior ADT. Although entirely speculative, it is possible that a reduction in tumor burden with preoperative ADT may facilitate surgical intervention. Notably, there was little difference in functional outcomes (i.e., continence) amongst the matched cohorts.
Several limitations of this study should be noted. First, due to the retrospective nature of this study, all findings should be considered hypothesis-generating. Several prospective studies have addressed preoperative ADT approaches. However, as alluded to in the introduction, these studies have included highly heterogeneous patients, and the ADT approach has varied widely. For example, in one of the largest randomized experiences to date, Schulman et al randomized 402 patients with cT2–T3 disease and a baseline PSA of < 100 ng/mL to either goserelin/flutamide × 3 months followed by radical prostatectomy, or radical prostatectomy alone.13 In contrast, Soloway et al randomized 282 patients with cT2b disease with a baseline PSA of < 50 ng/mL to either leuprolide/flutamide × 3 months followed by radical prostatectomy or prostatectomy alone.14 With the markedly differing (and broad) criteria used in these studies, it is challenging to ascertain the cumulative effect of preoperative ADT – these studies report varying clinical outcomes.10,13–20
The retrospective nature of the current study also challenges the ability to precisely characterize the optimal duration and timing of androgen deprivation therapy. Patients were characterized as having greater than or less than 3 months of preoperative ADT. In an exploratory analysis, the duration (segregated by these categories) had no impact on TTBR. Recent reports have suggested the critical importance of the extent of preoperative ADT – even the short courses used in this setting appear to have an impact on functional outcomes following prostatectomy.21 If preoperative therapy will at some point be adopted as a standard of care, it will be critical to refine and standardize the duration of therapy.
Another limitation of our study was the duration of PSA follow-up. The duration of followup (48.1 months in patients with preoperative therapy v 36.8 months in patients with no preoperative therapy, P=0.4) was comparable to other similar experiences, and may, in fact, be sufficient for the assessment of patients with high-risk disease. However, given the extended TTBR anticipated with low-risk disease following prostatectomy, this may not yield sufficient follow-up to capture events. Furthermore, as depicted in Figure 2a, there were multiple patients with low-risk disease with early censoring. The poor follow-up additionally makes this particular subset analysis somewhat unreliable. However, long-term outcomes were more consistently Captured amongst patients with intermediate- and high-risk disease, increasing our confidence in the findings related to these subsets.
Conclusions
There has been renewed interest in assessing the role of neoadjuvant therapy, primarily in the context of emerging agents (i.e., enzalutamide and abiraterone) that demonstrate substantial efficacy and a high therapeutic potential in the metastatic, castration-resistant setting. Already, preliminary data related to neoadjuvant abiraterone therapy has been presented by Taplin et al, with encouraging rates of pathologic response.7 Similar studies of enzalutamide are also underway. As these studies move forward, it will be critical to focus development of neoadjuvant therapies in relevant subsets of patients with localized prostate cancer. Our study provides preliminary evidence that such studies may be ideal in patients with high-risk disease as defined by D’Amico criteria.
Clinical Practice Points.
Multiple studies (both prospective and retrospective) have assessed the role of androgen deprivation therapy (ADT)
A cumulative interpretation of this literature is challenge by (1) varying types of ADT utilized, (2) varying durations of ADT, and (3) disparate risk stratification schema used to classify patients receiving ADT
In one of the largest retrospective series to date, we compare the clinical outcome of patients who have received preoperative ADT to risk-matched patients who have not received preoperative ADT
Although preoperative ADT did not impact clinical outcome for the population at large, there was an improvement in time to biochemical recurrence in patients with high-risk disease
Future studies of neoadjuvant preoperative therapy may thus enrich for patients with high-risk disease
Acknowledgments
Dr. Pal’s efforts are supported by the NIH Loan Repayment Plan (LRP) and NIH K12 2K12CA001727-16A1.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines): Prostate Cancer Version 2.2013. Available at http://www.nccn.org; last accessed April 2, 2013.
- 2.Mohler JL, Armstrong AJ, Bahnson RR, et al. Prostate Cancer, Version 3.2012 Featured Updates to the NCCN Guidelines. Journal of the National Comprehensive Cancer Network. 2012;10:1081–7. doi: 10.6004/jnccn.2012.0114. [DOI] [PubMed] [Google Scholar]
- 3.Bolla M, de Reijke TM, Van Tienhoven G, et al. Duration of androgen suppression in the treatment of prostate cancer. N Engl J Med. 2009;360:2516–27. doi: 10.1056/NEJMoa0810095. [DOI] [PubMed] [Google Scholar]
- 4.Bolla M, Gonzalez D, Warde P, et al. Improved survival in patients with locally advanced prostate cancer treated with radiotherapy and goserelin. N Engl J Med. 1997;337:295–300. doi: 10.1056/NEJM199707313370502. [DOI] [PubMed] [Google Scholar]
- 5.Dorff TB, Flaig TW, Tangen CM, et al. Adjuvant androgen deprivation for high-risk prostate cancer after radical prostatectomy: SWOG S9921 study. J Clin Oncol. 2011;29:2040–5. doi: 10.1200/JCO.2010.32.2776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hu J, Hsu J, Bergerot PG, Yuh BE, Stein CA, Pal SK. Preoperative therapy for localized prostate cancer: a comprehensive overview. Maturitas. 2013;74:3–9. doi: 10.1016/j.maturitas.2012.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Taplin M-E, Montgomery RB, Logothetis C, et al. Effect of neoadjuvant abiraterone acetate (AA) plus leuprolide acetate (LHRHa) on PSA, pathological complete response (pCR), and near pCR in localized high-risk prostate cancer (LHRPC): Results of a randomized phase II study. ASCO Meeting Abstracts. 2012;30:4521. [Google Scholar]
- 8.Demetri GD, Huang X, Garrett CR, et al. Novel statistical analysis of long-term survival to account for crossover in a phase III trial of sunitinib (SU) vs. placebo (PL) in advanced GIST after imatinib (IM) failure. J Clin Oncol (Meeting Abstracts) 2008;26:10524. [Google Scholar]
- 9.Bergstralh E, Kosanke J. GMATCH SAS macro. Mayo Clinic; Rochester, MN: 2003. [Google Scholar]
- 10.Klotz LH, Goldenberg SL, Jewett MA, et al. Long-term followup of a randomized trial of 0 versus 3 months of neoadjuvant androgen ablation before radical prostatectomy. J Urol. 2003;170:791–4. doi: 10.1097/01.ju.0000081404.98273.fd. [DOI] [PubMed] [Google Scholar]
- 11.Berglund RK, Tangen CM, Powell IJ, et al. Ten-year follow-up of neoadjuvant therapy with goserelin acetate and flutamide before radical prostatectomy for clinical T3 and T4 prostate cancer: update on Southwest Oncology Group Study 9109. Urology. 2012;79:633–7. doi: 10.1016/j.urology.2011.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Naiki T, Kawai N, Okamura T, et al. Neoadjuvant hormonal therapy is a feasible option in laparoscopic radical prostatectomy. BMC urology. 2012;12:36. doi: 10.1186/1471-2490-12-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schulman CC, Debruyne FM, Forster G, Selvaggi FP, Zlotta AR, Witjes WP. 4-Year follow-up results of a European prospective randomized study on neoadjuvant hormonal therapy prior to radical prostatectomy in T2–3N0M0 prostate cancer. European Study Group on Neoadjuvant Treatment of Prostate Cancer. Eur Urol. 2000;38:706–13. doi: 10.1159/000020366. [DOI] [PubMed] [Google Scholar]
- 14.Soloway MS, Pareek K, Sharifi R, et al. Neoadjuvant androgen ablation before radical prostatectomy in cT2bNxMo prostate cancer: 5-year results. J Urol. 2002;167:112–6. [PubMed] [Google Scholar]
- 15.Cookson MS, Sogani PC, Russo P, et al. Pathological staging and biochemical recurrence after neoadjuvant androgen deprivation therapy in combination with radical prostatectomy in clinically localized prostate cancer: results of a phase II study. Br J Urol. 1997;79:432–8. doi: 10.1046/j.1464-410x.1997.00022.x. [DOI] [PubMed] [Google Scholar]
- 16.Fair WR, Rabbani F, Bastar A, Betancourt J. Neoadjuvant Hormone Therapy Before Radical Prostatectomy: Update on the Memorial Sloan-Kettering Cancer Center Trials. Mol Urol. 1999;3:253–60. [PubMed] [Google Scholar]
- 17.Gleave M, Kelly WK. High-risk localized prostate cancer: a case for early chemotherapy. J Clin Oncol. 2005;23:8186–91. doi: 10.1200/JCO.2005.03.3068. [DOI] [PubMed] [Google Scholar]
- 18.Lee F, Siders DB, McHug TA, Solomon MH, Klamerus ML. Long-term follow-up of stages T2–T3 prostate cancer pretreated with androgen ablation therapy prior to radical prostatectomy. Anticancer Res. 1997;17:1507–10. [PubMed] [Google Scholar]
- 19.Prezioso D, Lotti T, Polito M, Montironi R. Neoadjuvant hormone treatment with leuprolide acetate depot 3.75 mg and cyproterone acetate, before radical prostatectomy: a randomized study. Urol Int. 2004;72:189–95. doi: 10.1159/000077113. [DOI] [PubMed] [Google Scholar]
- 20.Witjes WP, Schulman CC, Debruyne FM. Preliminary results of a prospective randomized study comparing radical prostatectomy versus radical prostatectomy associated with neoadjuvant hormonal combination therapy in T2–3 N0 M0 prostatic carcinoma. The European Study Group on Neoadjuvant Treatment of Prostate Cancer. Urology. 1997;49:65–9. doi: 10.1016/s0090-4295(97)00171-4. [DOI] [PubMed] [Google Scholar]
- 21.Mazzola CR, Deveci S, Heck M, Mulhall JP. Androgen deprivation therapy before radical prostatectomy is associated with poorer postoperative erectile function outcomes. BJU Int. 2012;110:112–6. doi: 10.1111/j.1464-410X.2011.10728.x. [DOI] [PubMed] [Google Scholar]


