Abstract
Reactive oxygen species (ROS) are implicated as injurious and as signaling agents in human maladies including inflammation, hyperoxia, ischemia-reperfusion and acute lung injury. ROS produced by the endothelium play an important role in vascular pathology. They quench, for example, nitric oxide, and mediate pro-inflammatory signaling. Antioxidant interventions targeted for the vascular endothelium may help to control these mechanisms. Animal studies have demonstrated superiority of targeting ROS-quenching enzymes catalase and superoxide dismutase to endothelial cells over nontargeted formulations. A diverse arsenal of targeted antioxidant formulations devised in the last decade shows promising results for specific quenching of endothelial ROS. In addition to alleviation of toxic effects of excessive ROS, these targeted interventions suppress pro-inflammatory mechanisms, including endothelial cytokine activation and barrier disruption. These interventions may prove useful in experimental biomedicine and, perhaps, in translational medicine.
Reactive oxygen species & vascular pathology
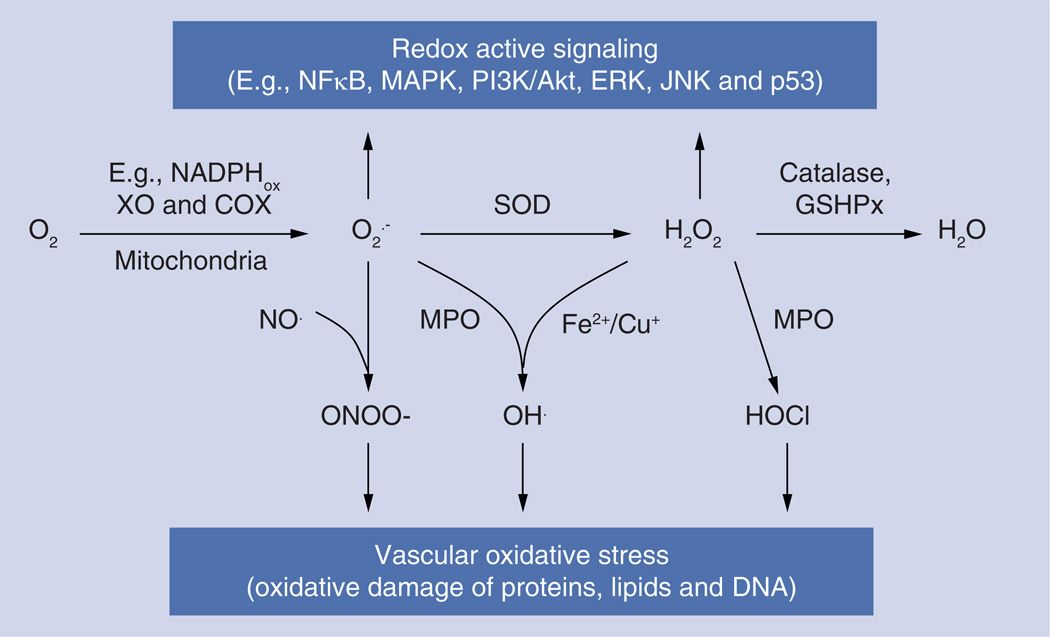
Reactive oxygen species (ROS) superoxide anion (O2•−) and hydrogen peroxide (H2O2) are small molecules implicated as injurious and signaling agents in human maladies including inflammation, hyperoxia, ischemia-reperfusion (I/R) and acute lung injury (ALI) [1]. Activated phagocytes release ROS, causing tissue damage. Endothelial cells (ECs) lining the luminal surface of blood vessels also produce ROS [2] using the mitochondrial respiratory chain [3], membrane-bound NADPH oxidases (NOX) [4], xanthine oxidase [5], uncoupled nitric oxide synthase (NOS) [6] and other enzymatic systems (Figure 1). The mitochondrial respiratory chain is the major producer of injurious ROS that play an important role in apoptosis and cell pathology [7]. ECs play key regulatory functions in the vascular system and, therefore, effects of endothelial ROS, both endogenous and exogenous, are of great biomedical importance [8].
Figure 1. The metabolism and role of reactive oxygen species in signaling and vascular oxidative stress.
COX: Cycloxigenase; GSHPx: Glutathione peroxidase; MPO: Myeloperoxidase; SOD: Superoxide dismutase; XO: Xanthine oxidase.
Antioxidants, including antioxidant enzymes (AOEs) catalase and superoxide dismutase (SOD), inhibit the effects of ROS in cell culture, animals and, to a limited extent, clinical studies [8,9]. Some forms of chronic mild oxidative stress seem amenable to preventative or prolonged treatment with antioxidants, antioxidant inducers, AOEs (including their polyethylene glycol [PEG] conjugated variants that have enhanced bioavailability) or, in a more distant future, gene therapy [9]. However, effective and specific treatment of acute vascular oxidative stress remains a significant and challenging goal [10]. In acute conditions, such as lung inflammation, I/R and ALI, expedient quenching of ROS in given compartments of target cells is needed. Nontargeted antioxidants do not afford the required spatiotemporal precision of action.
In particular, precise interventions are needed to correct local aberrations of ROS involved in pathological signaling. Inflammatory agents (e.g., cytokines TNF and interleukin-1β) cause abnormal endothelial activation, which manifests, among other signs, by the expression of molecules mediating leukocyte migration (e.g., vascular cell adhesion molecule-1 [VCAM]) [2]. In activated endothelium, NOX releases O2•− in the milieu and cellular organelles including endosomes [11]. O2•− spontaneously transforms into H2O2 and O2 in a fast reaction, which is further accelerated by SOD. Thus, extracellular SOD rapidly quenches O2•− in the milieu [12]. O2•− can cross cell membranes via the chloride channel ClC3 [13]. In turn, H2O2, a more stable molecule, can:
-
▪
Further react directly with cellular components, such as sulfhydryl groups of cell proteins;
-
▪
In the presence of free transition metals, produce extremely reactive hydroxyl radical ·OH;
-
▪
Be degraded by catalase or peroxidases.
Reactions of ROS (in particular O2•−) are compartmentalized within nanometers of the generation site. O2•− released by NOX into endosomes [14] (inaccessible for mitochondrial, cytosolic and extracellular SOD) has been implicated in NFκB-mediated signaling leading to inflammatory changes [14–16].
In order to control these effects of ROS in ECs (and, presumably, other cell types), at least two key intertwined aims must be achieved. First, we need to understand signaling and injurious mechanisms of ROS at a subcellular level. Second, we need means to interfere in these mechanisms at this level in selected cell types and phenotypes; for example, in the signaling endosomes of pathologically activated ECs. This article reviews these two aspects of targeted antioxidant interventions.
ROS pathological signaling in vasculature
General mechanisms of ROS signaling
Many agents including growth factors, cytokines, hormones and neurotransmitters are able to cause transient ROS generation by nonphagocytic cells [17]. In many cases, ROS-mediated signaling in nonphagocytic cells requires endocytosis of a ligand–receptor complex and formation of a signaling endosome that contains activated NOX generating ROS. This mechanism has been demonstrated for pro-inflammatory signaling induced by cytokines, hypoxiareoxygenation, platelet-derived growth factor, epidermal growth factor and angiotensin II [14]. Recent studies of the role of mitochondrial ROS suggest that the regulated production of ROS by respiratory chain complexes I and III may also be involved in numerous signal transduction paths including metabolic signaling, inflammation, apoptosis and autophagy [18,19].
Spatially confined production of ROS allows tuned regulation of redox-mediated signal transduction. ROS, in particular H2O2, oxidize sensitive cysteine residues in target proteins (phosphatases, kinases, small GTPases, transcription factors and ion channels (Figure 2) [2]). Oxidation of specific cysteine in phosphatases can inactivate the enzyme. Blocking homeostatic dephosphorylation can cause activation of corresponding kinases and turn on the specific signaling event. On the other hand, it was demonstrated that some kinases are more active when regulatory cysteine is oxidized by H2O2, which thereby activates signaling.
Figure 2. Molecular mechanisms of reactive oxygen species signaling.
ROS produced by a family of NADPH oxidase enzymes or by mitochondria (complexes I and III of the respiratory chain) are able to oxidize cysteine residues of several components of signaling pathways. This can cause an increase in ion-channel activity or activation of kinase activity. The most studied ROS signaling mechanism is the inhibition of phosphatase activity by H2O2, which in turn leads to kinase activation. ROS can also increase DNA binding to the transcription factor.
NOX: NADPH oxidase; ROS: Reactive oxygen species.
ROS in hypertension
Superoxide generated in activated endothelium by NOX or the mitochondrial respiratory chain quenches a key vasodilating mediator NO and produces damaging reactive nitrogen species peroxynitrite, ONOO− (Figure 3). Moreover, peroxynitrite oxidizes tetrahydrobiopterin, a key cofactor of endothelial NOS, which in the absence of this cofactor generates superoxide instead of NO [6]. Thus, ROS cause vasoconstriction and, therefore, oxidative stress is a pathological factor in hypertension [20]; for example, in response to activation of the rennin– angiotensin system [21]. The key agent of this system, angiotensin II, boosts superoxide production in the vasculature, which leads to vasospasm [22]. Excessive ROS also cause endothelial dysfunction, inflammation, vascular hypertrophy, apoptosis, vascular remodeling and fibrosis [23]. In vivo studies detected high ROS production in hypertensive conditions [24–26]. In contrast, reduction of ROS or inhibition of ROS-generated enzymes in experimental conditions has vasodilatory effect. Nevertheless, clinical trials of antioxidants in hypertensive patients did not produce significant improvements, perhaps due to failure to interfere effectively in ROS signaling [20].
Figure 3. Mechanism of AngII-induced hypertension.
AngII binds to its receptor AT1, which activates NOX2 and results in superoxide generation. Other systems that produce superoxide in an AngII responsive manner include the mitochondrial respiration chain and nitric oxide synthase uncoupling and NOX1, induced in pathological conditions. Superoxide scavenges NO, a critical vasodilator. Consequent depletion of NO causes development of hypertension.
NOX: NADPH oxidase; ROS: Reactive oxygen species.
Vascular oxidative stress & thrombosis
Thrombosis is the leading cause of morbidity and mortality in cardiovascular pathology and stroke. Thrombus formation is initiated by activation of a coagulation cascade leading to thrombin formation, and by activation of platelets, leading to their adhesion and aggregation [27]. Several in vivo studies showing the inhibitory effect of antioxidants on platelet activation and recruitment to growing thrombus provide evidence for the involvement of ROS in thrombosis (Figure 4) [28–30]. Exposure of platelets to ROS can decrease the threshold for agonist-induced platelet activation and, therefore, promote thrombosis [31,32]. Several lines of evidence suggest that ROS modulate activity of platelet-signaling molecules, including Ca2+-ATPase and inositol 1,3,5-trisphosphate receptors [33,34]. The αIIbβ3 integrin receptor, a key mediator of platelet aggregation and further coagulation, is also regulated by ROS [35]. In addition, vascular ROS can regulate platelet function indirectly; for example, via quenching NO, a known inhibitor of platelet aggregation [36]. ROS can also regulate platelet function via oxidation of low-density lipoproteins (LDL), lipids and their derivatives. Oxidized LDL (oxLDL) induce platelet aggregation and secretion [37]. The oxLDL play a key role in the development and progression of atherosclerosis [38]. Within atherosclerotic lesions, oxLDL inhibit anticoagulant β2-glycoprotein 1 [39–41]. The auto-antibodies against oxLDL/β2-glycoprotein 1 complexes correlate with arterial thrombosis in autoimmune patients [42]. Furthermore, hypercholesterolemia aggravates platelet adhesiveness via ROS formed by the NOX [43].
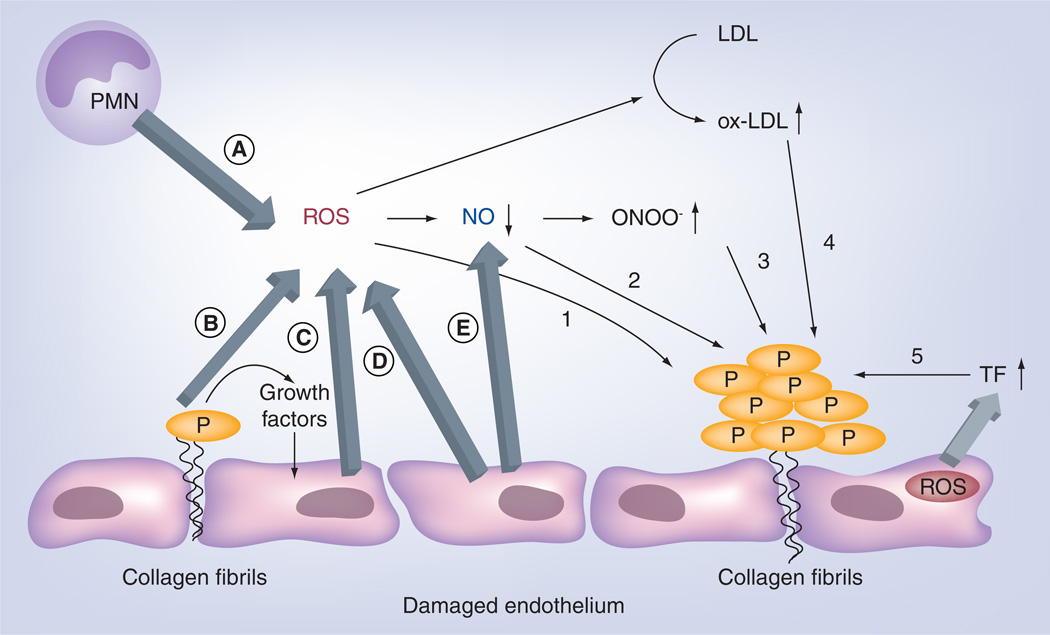
Figure 4. The involvement of vascular reactive oxygen species in thrombosis.
ROS in vascular lumen are derived from various vascular cells, including (A) activated leukocytes, (B) adherent platelets and (C,D) endothelial cells. ROS themselves can directly stimulate platelet activation and aggregation (1). ROS can also indirectly regulate formation of thrombosis by decreasing bioavailability of NO (2), which is generated by endothelial cells (E), increasing the levels of ONOO− (3), oxidized LDL (4) and TF that initiates explosive coagulation cascade (5).
LDL: Low-density lipoprotein; NOX: NADPH oxidase; Ox-LDL: Oxidized LDL; P: Platelet; PMN: Polymorphonuclear leukocyte; ROS: Reactive oxygen species; TF: Tissue factor.
In turn, activated platelets secrete platelet-derived growth factor, transforming growth factor-β1, EGF and VEGF, all known to stimulate ROS generation by vascular NOX [44,45]. Therefore, platelet activation and vascular ROS flux form a vicious cycle. In addition, oxidants inactivate anti-thrombotic endothelial protein thrombomodulin and cause ECs to release inhibitors of fibrinolysis [46]. Vascular ROS also contribute to thrombosis through regulation of the extrinsic coagulation cascade initiated by the tissue factor [47], via upregulation of tissue factor expression in vascular cells [48].
ROS signaling in inflammation
Pathological inflammation is an important factor in the mechanisms of many diseases including cancer, diabetes and atherosclerosis [49]. ROS produced by activated phagocytes have long been implicated in inflammation. However, ROS produced by nonphagocytic cells, first of all ECs, play an important role as well [50].
Inhibitory effects of antioxidants on cell stimulation by cytokines were observed decades ago [51]. Subsequent studies revealed that ROS are important elements of signaling pathways and involved in cell proliferation, differentiation and migration [52]. NF-κB transcription factor plays a central role in inflammation and innate immunity. Canonical pathways for NF-κB activation are mediated by Toll-like receptors, a family of TNF receptors and other cytokine receptors. NF-κB, a dimer of p50 and p65 subunits, is normally restricted from entering the nucleus by inhibitory proteins. NF-κB-induced transcription leads to synthesis of pro-inflammatory proteins including adhesion molecules mediating migration of leukocytes releasing ROS. In turn, ROS modulate NF-κB signaling in several ways including endosomal signaling (Figure 5). Thus, ROS and NF-κB positively affect each other, forming a vicious cycle of pro-inflammatory activation [53]. However, excessive ROS may also inhibit NF-κB signaling [54].
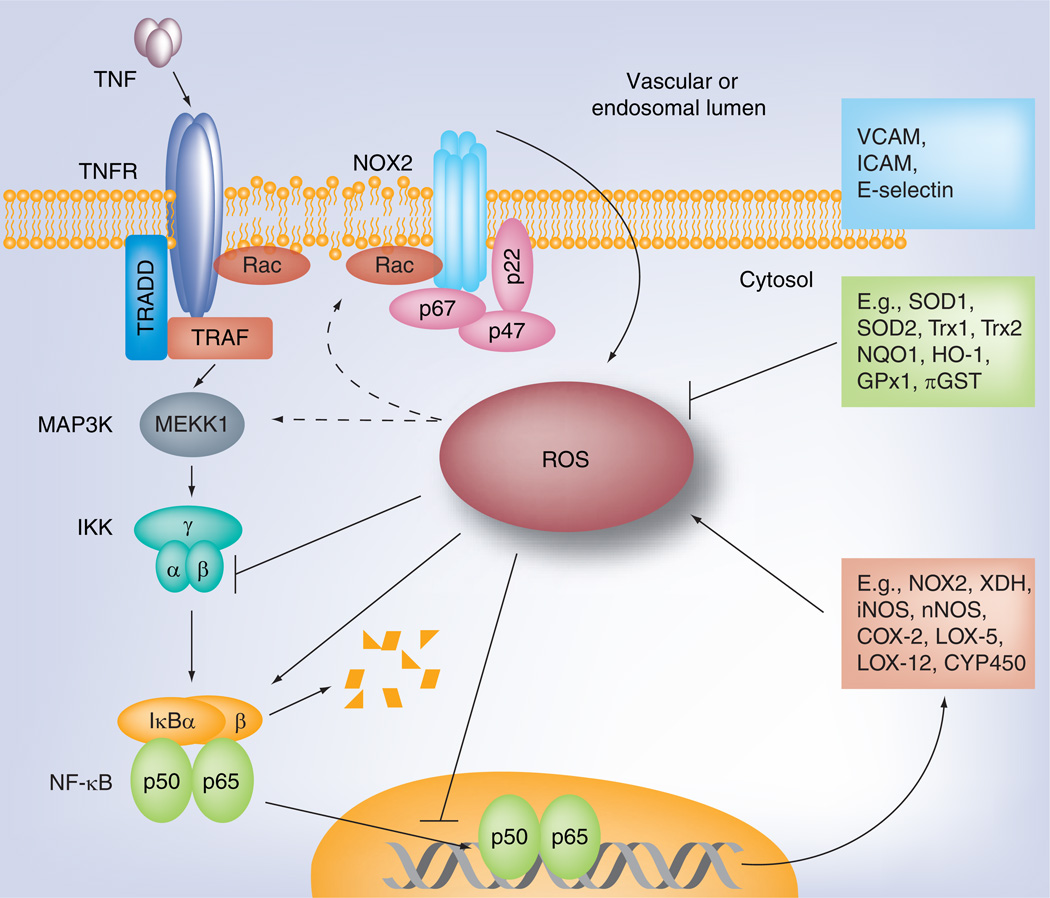
Figure 5. Relationship between reactive oxygen species and inflammatory NF-κB signaling.
The activation of NF-κB produces cell-specific set of target proteins, including cell adhesion molecules (specific for endothelial cells, top box), antioxidant proteins (middle box) and pro-oxidant proteins (bottom box). ROS may modulate NF-κB function by either its activation or, in some cases, by inhibition. Outcome of NF-κB regulation by ROS will depend on cell type and its redox status, localization, production time and type of ROS.
COX: Cycloxigenase; ICAM: Intercellular adhesion molecule; NOX: NADPH oxidase; ROS: Reactive oxygen species; TRADD: Tumor necrosis factor receptor type 1-associated death domain protein; TRAF: Tumor necrosis factor receptor-associated factor; VCAM: Vascular cell adhesion molecule-1.
Oxidative impairment of endothelial barrier function
A surplus of vascular ROS has been implicated in endothelial barrier dysfunction [55]. An increased EC permeability is the hallmark of many disorders including inflammation [56]. Extracellular ROS (in particular, H2O2) cause endothelial barrier dysfunction [57,58]. Furthermore, in response to thrombin, histamine, VEGF, TNF and inflammatory cytokines, ECs generate ROS that serve as signaling molecules mediating endothelial barrier regulation (Figure 6) [59].
Figure 6. Involvement of reactive oxygen species in the regulation of endothelial barrier function.
ROS derived from activated PMNs and endothelial cells in response to permeability enhancers (thrombin and VEGF) are involved in multiple signaling pathways leading to endothelial barrier disruption. Vascular ROS stimulate intracellular Ca2+, RhoA activity and phosphorylation of MLC, resulting in stress-fiber formation and increased contractility. ROS increase several kinase activities (e.g., p38 MAP kinase, protein kinase C and tyrosine kinase Pyk2) that are implicated in cytoskeletal remodeling and destabilization of intercellular junctions.
MLC: Myosin light chain; MMP: Matrix metalloproteinase; NOX: NADPH oxidase; PMN: Polymorphonuclear leukocyte; ROS: Reactive oxygen species; ZO: Zona occludens.
A balance between EC adhesion and actin–myosin-based contractile forces within cells controls the structural integrity of endothelial barrier [60]. ROS cause cytoskeletal remodeling through signaling, including increase in cytosolic Ca2+ level, activation of Rho GTPases and enhanced phosphorylation of the myosin light chain, all of which mediate stress fiber formation and cell contractility, leading to increased vascular permeability [60–62]. Thus, through stimulation of cytoskeletal remodeling, ROS destabilize junctions between ECs, including tight junctions and adherenes junctions, which are bound to actin cytoskeleton. H2O2 exposure of ECs results in internalization of VE-cadherin, a key molecule of adherenes junctions essential for the stability of EC contacts [58,63]. In addition, vascular ROS cause phosphorylation and redistribution of the tight junction molecules [64] and activate matrix metalloproteinases, leading to disassembly of cell junctions and increased endothelial permeability [65].
The need to specifically intercept selected ROS effects
ROS signaling plays an important role in many vascular diseases including hypertension, thrombosis, inflammation and edema. In theory, ROS quenching may provide beneficial effects in management of these conditions. However, the effect of the antioxidants depends on their delivery to the sites of ROS action. For example, ROS interception in signaling endosomes is needed to alleviate endothelial pro-inflammatory activation caused by cytokines, whereas quenching extracellular ROS may alleviate tissue damage caused by activated leukocytes. Furthermore, quenching of selected ROS species may be required. For example, hypertension is mediated by superoxide depleting NO and, thus, quenching of superoxide is needed. On the other hand, in I/R injury, H2O2 seems to be the most damaging ROS and its removal may attenuate the cellular damage. AOEs are able to remove specific ROS in recurrent manner and, if delivered to the right place, might block an undesired ROS-mediated processes. In the following sections we review approaches to intercept pathological ROS and demonstrate some examples of protective properties of the delivery of AOEs to vascular endothelium.
Means to intercept ROS
AOEs & antioxidants
Cells have several systems of antioxidants that control the level of ROS. AOEs include SOD, enzymes that destroy superoxide anions [66]. Cytosolic SOD1 or Cu, Zn–SOD, controls superoxide produced by NOX, xanthine oxidase, cyclooxygenase and other sources. Mitochondrial SOD2 destroys superoxide produced by the mitochondrial respiratory chain. SOD3, or extracellular SOD secreted by cells, binds to extracellular matrix via its heparin-binding domain and destroys extracellular superoxide. Superoxide dismutation yields H2O2 that in turn gets destroyed by catalase, peroxiredoxins and peroxidases [2].
In addition to AOEs, cells contain significant amounts of nonenzymatic antioxidants including glutathione, ascorbate, tocopherols, bilirubin, uric acid and other molecules, which scavenge ROS, hydroxyl radical and other free radicals [23]. Antioxidant molecules have been extensively tested in models of oxidative stress, but most of the well-controlled studies failed to demonstrate their beneficial effects [67]. Such disappointing results may be explained in part by their relatively slow reaction with ROS and limited availability in the proper cellular compartments [20]. In the context of the latter challenge, significant progress is being reported in mitochondrial targeting of quinones for degradation of excessive ROS generated by an imbalanced respiratory chain [68]. Recent studies also demonstrated the therapeutic antioxidant potential of NOS cofactor tetrahydrobiopterin and folic acid [69]. Significant efforts are being invested in the last decade in antioxidant gene therapy. Delivery of viral and nonviral genes encoding SOD, catalase, glutathione peroxidase and other antioxidant proteins has been designed and effects of these interventions are being tested in vitro and in animal studies. Although out of the focus of this article, antioxidant gene therapy may eventually improve treatment of chronic conditions involving oxidative stress [70]. In the following sections we describe the potentials of targeted AOEs for vascular delivery.
Advanced formulations for vascular delivery
Most antioxidant agents have no specific affinity to the endothelium and, therefore, despite being exposed to circulation, ECs take up a minor fraction (in most cases <1%) of the drug. However, conjugation of drugs and their carriers (e.g., liposomes) with antibodies and antibody fragments that bind to EC surface determinants including angiotensin-converting enzyme (ACE), platelet–EC adhesion molecule (PECAM) and intercellular adhesion molecule (ICAM) permits effective delivery of 10–30% of the dose to the endothelium [8]. ECs internalize conjugates anchored to ACEs, ICAMs and PECAMs. This enables quenching of ROS in endosomes [16]. Of note, ICAM molecules delivering anchored anti-ICAM–catalase conjugates into the endosomes recycle to the cell surface, thereby allowing sustained intracellular delivery [71].
Within a few hours, internalized conjugates get degraded in the lysosomes, which limits duration of the effect [72]. To resolve this issue, methods to encapsulate AOE into polymeric and nonpolymeric nanocarriers permeable for ROS, but not proteases, have been developed [73,74]. Using PEG-catalase instead of native enzyme further enhances the encapsulation efficacy [75]. Modulating the formulations allows production of catalase-loaded nanocarriers of spherical or filamentous shape [76]. Coating of catalase-loaded nanocarriers by anti-PECAM provides endothelial targeting of the cargo in vitro and in vivo and prolonged antioxidant protection of the endothelium [77].
Of note, the PEG–poly(lactic-co-glycolic acid) polymer matrix is readily diffusible for H2O2, but not superoxide; hence encapsulation into PEG–poly(lactic-co-glycolic acid) nanocarriers obliterates enzymatic activity of SOD [77]. In contrast, encapsulation of either catalase or SOD into micelles formed by controlled precipitation of magnetic nanoparticles using calcium and oleate provides composite nanocarriers (200–300 nm diameter) containing active catalase or SOD accessible for either H2O2 of superoxide and protected from proteases [74]. Targeting this formulation to ECs using magnetic delivery or anti-PECAM conjugated to the surface of the micelles confers endothelial targeting and further boosts antioxidant protection [74].
Targeted interception of ROS signaling in vascular pathology
Antioxidants including SOD mimetics [78], mutant SOD that binds to the glycocalyx [79,80], AOE delivery using membrane-permeating peptides [81] and cell transfection by AOE genes [82] exert variable protective effects in cells and, at lesser rate of success, in animals [83,84]. PEG-based ‘stealth’ AOE delivery has been extensively studied. PEG chains (2–10 kDa) coupled to a protein, form a hydrated shell that:
-
▪
Enhances its hydrodynamic radius and water solubility;
-
▪
Inhibits its interactions with cells and proteins [85].
This prolongs circulation, enhances bioavailability and effects of PEG–proteins [86]. Several PEG–protein conjugates are in the clinical testing and in use [87]. Indeed, conjugation with PEG [88] or PEG-pluronic [89] and loading in PEG-coated carriers [90,91] prolongs AOE circulation, enhancing their bioavailability and protective effects in animal models including stroke (likely due to better diffusion in to the CNS [92,93]) and some forms of chronic oxidative stress [94–96]. Quenching of extracellular ROS (e.g., by PEG–AOE) is an important axis of management of oxidative stress.
However, keeping in mind that ROS act within nanometer range of the flux sites, targeting is the key requirement. For example, intratracheal AOE delivery and transfection alleviated oxidative stress in the airways, but not in lung vasculature [97,98]. However, endothelial ROS play specific and important functions in conditions including I/R, ALI and inflammation [99–101]. As discussed above, endothelial ROS produced in response to pathological factors [102] cause vascular abnormalities, including:
-
▪
Exposure of adhesion molecules facilitating white blood cell transmigration (e.g., VCAM) [103];
-
▪
Loss of thrombomodulin, which unleashes thrombin, causing thrombosis and inflammation [104];
-
▪
Endothelial barrier disruption leading to vascular leakage and edema [55].
Admittedly, our knowledge of the role of endothelial ROS in pathology remains limited [105,106], in part due to lack of site-specific antioxidant interventions [107]. Nevertheless, endothelial uptake and effects of PEG–AOE are no better than those of naked AOEs in vitro and in vivo [16]. As a result, despite high blood levels, PEG–AOE had no effect on pro-inflammatory endothelial activation [16].
Therefore, nontargeted approaches provide neither endothelial AOE delivery nor address specific cellular compartments. To achieve this goal, several groups devised ‘vascular immunotargeting’ strategies by conjugating cargoes with ligands of specific endothelial surface epitopes [108–115]. The general scheme of the AOE delivery action is depicted on Figure 7. Epitopes tested for this goal include ACE [116], other peptidases such as aminopeptidase P [117] and aminopeptidase N [118], cell adhesion and other molecules [8,110,119,120]. Thus, conjugation of AOEs with antibodies to ACE, PECAM and ICAM, and using AOE-loaded nanocarriers targeted by these antibodies, have recently been shown to provide highly effective and specific antioxidant effects [74,77,112,115,121]. Anti-ACE/AOE, anti-ICAM/AOE and anti-PECAM/AOE conjugates provided protective antioxidant effects superior to nontargeted AOE formulations such as PEG–AOE in models of acute pulmonary vascular oxidative stress [122–126]. In particular, success in protecting lungs against oxidative stress (e.g., I/R injury in lung transplantation) by anti-ICAM/AOE, anti-ACE/AOE [127] and anti-PECAM/AOE [125,126] has recently been reproduced by several laboratories in diverse animal models [128–130].
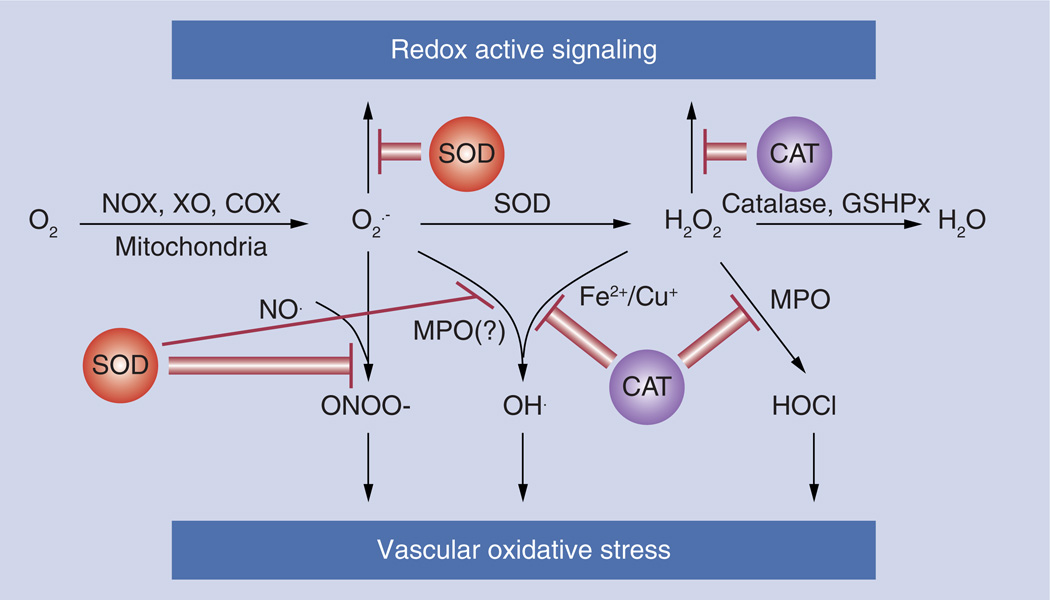
Figure 7. Targeted antioxidant interventions.
SOD or CAT conjugated with antibody to the endothelial target, specifically binds to endothelial cells and efficiently degrades the superoxide anion and hydrogen peroxide, respectively. Reactive oxygen species removal protects cells against oxidative stress or inhibits unwanted signaling.
CAT: Catalase; COX: Cycloxigenase; GSHPx: Glutathione peroxidase; MPO: Myeloperoxidase; NOX: NADPH oxidase; SOD: Superoxide dismutase; XO: Xanthine oxidase.
Antihypertensive effect of SOD delivery
Superoxide produced by ECs inactivates NO, thereby causing hypertension [131]. In addition, the peroxynitrite formed inactivates enzymes required for synthesis of vasodilator prostacyclin [132] and NO synthases [133]. Several studies demonstrated feasibility of SOD delivery to the endothelium as antihypertensive intervention. Heparin-binding fusion protein of SOD1 binds to the endothelial glycocalyx and decreases blood pressure in hypertensive rats [134]. Similar effect on blood pressure normalization was demonstrated after administration of SOD mimetic tempol [135]. SOD encapsulated into liposomes also showed hypotensive effects in Ang II-induced hypertension in mice [136]. SOD specifically targeted to the endothelium using carrier antibodies directed to endothelial adhesion molecules prevented oxidation of tetrahydrobiopterin and normalized vasoreactivity of large vessels in Ang II-treated hypertensive mice [126]. It is noteworthy that H2O2 may function as a vasodilator in some types of vessels [137] and the delivery of catalase to the endothelium did not show vasodilatory effects in Ang II-induced hypertension model [126].
Anti-inflammatory properties of targeted SOD
In theory, antioxidants can provide antiinflammatory effects [8,138]. Anti-inflammatory properties of SOD have been noticed a while ago [139] and SOD modifications having prolonged life time in the bloodstream (e.g., conjugation with PEG) have been tested in inflammation models [140,141]. SOD mimetics and superoxide quenchers inhibit VCAM expression in TNF-treated EC cultures [94,142]. Liposomal delivery of N-acetylcysteine was protective against lipopolysaccharide-mediated inflammation [143].
However, these interventions lack specific enzyme delivery and, despite three decades of intense research, the initial enthusiasm towards PEG–SOD has not been translated yet into human therapy [141]. Hybrid chimeric SOD (called SOD2/3) was genetically engineered by combination of catalytic unit of SOD2 and heparin-binding domain of SOD3. It binds to the endothelium and exerts anti-inflammatory activity [80,144]. Targeted anti-PECAM/SOD provided effective protection in oxidative stresses caused by both extracellular and intracellular superoxide radical in cell culture [115,145]. Importantly, ECs internalize these conjugates via a specific CAM-mediated endocytosis pathway [146]. This provides an ideal delivery mechanism to the key destination, the redox-active signaling endosomes [106]. As a result, anti-PECAM/SOD, but not PEG–SOD or other antioxidants, attenuated VCAM expression by ECs in response to diverse cytokines [16] and attenuated pro-inflammatory effects of lipopolysaccharide in mice [16]. Thus, specific targeting of AOEs may inhibit redox-sensitive inflammatory signaling and attenuate harmful consequences of excessive inflammatory response (Figure 7).
Normalization of pathological abnormalities of endothelial permeability
Vascular ROS play an important role in regulation of endothelial barrier function. Therefore, SOD and catalase have been tested to treat ROS-mediated endothelial barrier dysfunction [57,147]. For example, a recombinant SOD fusion protein that can bind to heparin-like proteoglycans on the EC surface exhibited promising protective effect on I/R-induced vascular permeability [144]. Furthermore, both in vivo and in vitro studies showed the alleviation of abnormal endothelial permeability by anti-PECAM/AOE formulations but not by untargeted AOEs (Figure 8) [58,126]. Endothelial targeting or expression of catalase, but not SOD, inhibited endothelial permeability in response to xanthine/xanthine oxidase-generated extracellular ROS, suggesting that extracellular H2O2 is the key disruptor of endothelial barrier function in this model [58].
Figure 8. Endothelial delivery of antioxidant enzymes alleviates reactive oxygen species-mediated endothelial barrier dysfunction.
AOEs including catalase and superoxide dismutase targeted to endothelials can quench extracellular and intracellular ROS that are generated in response to stimulation of permeability enhancers (thrombin and VEGF) or released from activated PMNs. Thus, endothelial delivery of AOEs can inhibit ROS-induced cytoskeletal remodeling and disassembly of intercellular junctions, protecting endothelial barrier function.
AOE: Antioxidant enzyme; NOX: NADPH oxidase; PMN: Polymorphonuclear leukocyte; ROS: Reactive oxygen species.
Furthermore, ECs produce ROS by NOX in response to vasoactive pro-inflammatory agents including VEGF and thrombin [148] that disrupt endothelial layer integrity and cause edema [148]. Interestingly, anti-PECAM/SOD, but not anti-PECAM/catalase, attenuated VEGF-induced endothelial barrier dysfunction, implicating O2•− in this type of pathological redox signaling [58]. It is noteworthy that nontargeted catalase and SOD including PEG-conjugated enzymes provided no effect, due to lack of delivery to the site of ROS influx and effect. Therefore, AOEs targeted to ECs provide versatile molecular tools for identifying the roles of specific ROS in vascular pathology and may be translated into remedies for these ROS-mediated vascular abnormalities.
Future perspective
In the last three decades, antioxidant interventions with optimized pharmacokinetics and specific delivery to endothelium have been devised and tested in vitro and in animal studies in early preclinical research. Some of these approaches demonstrated impressive superiority versus nontargeted interventions in animal models of human pathologies involving ROS signaling in the vasculature. The immediate goals are now:
-
▪
To refine delivery of antioxidants to selected subcellular compartments where signaling ROS are produced (specific types of endosomes and cellular vesicles, mitochondria and endoplasmic reticulum);
-
▪
To define optimal regimens of administration, therapeutic dose and time windows for the interventions in animal models;
-
▪
To translate most promising prototypes showing milestone achievements in these animal studies into a format applicable in clinical studies (i.e., replacement of targeting and enzymatic moieties by proteins and peptides that can be used in humans).
Fulfillment of these objectives will set a stage for the key steps of the subsequent industrial development of these targeted therapeutic interventions including scaling up and quality control of production, rigorous pharmacokinetic and toxicological studies in large animals and, eventually, clinical testing. Taking into account the current pace of the progress in the field, it is not overly optimistic to expect that many of these objectives will be achieved within less than a decade. These, in turn, can provide a solid basis for future clinical development and application of a new generation of targeted antioxidant interventions for treatment prevalent human maladies involving pathological ROS signaling.
Executive summary.
-
▪
Reactive oxygen species (ROS) pathological signaling in vasculature causes endothelial dysfunction. Pathological changes in the vasculature caused by excessive ROS, in particularly produced by the endothelium, are increasingly recognized as important mechanisms of human diseases, in particular, hypertension, thrombosis and inflammation.
-
▪
Means to intercept ROS include antioxidant enzymes and antioxidants. Antioxidant interventions targeting to the vascular endothelium may help to control these mechanisms. Animal studies have demonstrated superiority of targeting catalase and superoxide dismutase to endothelial markers including angiotensin-converting enzyme and cell-adhesion molecules over nontargeted formulations. These new means will help to dissect mechanisms of vascular oxidative stress and may eventually be translated into the clinical domain, thereby improving management of disease conditions involving this pathological mechanism.
-
▪
Targeted interception of ROS signaling in vascular pathology combines a diverse arsenal of targeted antioxidant formulations devised in the last decade, and shows highly promising results of specific quenching of endothelial ROS in vitro and in animal models. In addition to direct alleviation of toxic and injurious effects of excessive ROS, these novel targeted interventions provide suppression of specific pro-inflammatory mechanisms, including endothelial cytokine activation and barrier disruption.
-
▪
Advanced formulations for vascular delivery, including new generations of antioxidant nanocarriers will find use in experimental biomedicine and, perhaps, in translational medicine.
Acknowledgments
This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Key Term
- NADPH oxidases
Membrane enzyme complexes that produce superoxide and, in some cases, hydrogen peroxide when activated by agonists including cytokines, one of the major sources of signaling reactive oxygen species.
- Tight junction
Intercellular junctional complex that is particularly enriched in the brain microvasculature of the blood–brain barrier. Tight junctions (TJs) are responsible for the maintenance of functional endothelial barriers. Distinct from adhesion junctions, TJs do not form a continuous seal around the cells, but contain discontinuities or pores, allowing selective molecular sieving. Claudins, occludin and zona occludens are essential components of TJs and have been implicated in regulating the permeability properties of TJs.
- Adherenes junctions
Cellular membrane contacts formed by vascular endothelial–cadherins complexes with catenins. Adherenes junctions are critical in regulation of transendothelial migration of blood cells, as well as paracellular permeability. Disruption of vascular endothelial–cadherin distribution or the homophilic interaction leads to increased endothelial permeability and is associated with such pathological processes as inflammation and acute lung injury.
- Platelet-endothelial cell adhesion molecule
This 130-kDa glycoprotein belongs to the Ig-like superfamily of adhesion molecules. Platelet–endothelial cell adhesion molecule-1 is particularly abundant on endothelial cells, where it is localized on cell–cell borders and facilitates leukocyte endothelial transmigration. Platelet–endothelial cell adhesion molecule-1 is a good target for endothelial drug delivery, either to the cell surface or intracellularly, depending on the design of targeting system.
- Intercellular adhesion molecule
Another member of Ig-like superfamily of adhesion molecules. It serves as a receptor for leukocyte adhesion to inflamed endothelium. Intercellular cell adhesion molecule-1 is upregulated in response to inflammatory signals and participates in the recruitment of leukocytes to the sites of inflammation. Intercellular cell adhesion molecule-1 is a good candidate for endothelial targeting, in some aspects similar to platelet-endothelial cell adhesion molecule-1.
Footnotes
Financial & competing interests disclosure
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as:
▪ of interest
▪▪ of considerable interest
- 1.Forman HJ, Maiorino M, Ursini F. Signaling functions of reactive oxygen species. Biochemistry. 2010;49(5):835–842. doi: 10.1021/bi9020378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Thomas SR, Witting PK, Drummond GR. Redox control of endothelial function and dysfunction. molecular mechanisms and therapeutic opportunities. Antioxid. Redox Signal. 2008;10(10):1713–1765. doi: 10.1089/ars.2008.2027. ▪▪ Comprehensive review on mechanism of endothelial dysfunction with detailed description of reactive oxygen species (ROS) generating systems and control of endothelial function.
- 3.Ichimura H, Parthasarathi K, Quadri S, Issekutz AC, Bhattacharya J. Mechano-oxidative coupling by mitochondria induces proinflammatory responses in lung venular capillaries. J. Clin. Invest. 2003;111(5):691–699. doi: 10.1172/JCI17271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zimmerman MC, Dunlay RP, Lazartigues E, et al. Requirement for Rac1-dependent NADPH oxidase in the cardiovascular and dipsogenic actions of angiotensin II in the brain. Circ. Res. 2004;95(5):532–539. doi: 10.1161/01.RES.0000139957.22530.b9. [DOI] [PubMed] [Google Scholar]
- 5.McCord JM, Roy RS, Schaffer SW. Free radicals and myocardial ischemia. The role of xanthine oxidase. Adv. Myocardiol. 1985;5:183–189. [PubMed] [Google Scholar]
- 6.Forstermann U. Janus-faced role of endothelial NO synthase in vascular disease: uncoupling of oxygen reduction from NO synthesis and its pharmacological reversal. Biol. Chem. 2006;387(12):1521–1533. doi: 10.1515/BC.2006.190. [DOI] [PubMed] [Google Scholar]
- 7.Reddy PH. Mitochondrial medicine for aging and neurodegenerative diseases. Neuromolecular. Med. 2008;10(4):291–315. doi: 10.1007/s12017-008-8044-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Muzykantov VR. Targeting of superoxide dismutase and catalase to vascular endothelium. J. Control Release. 2001;71(1):1–21. doi: 10.1016/s0168-3659(01)00215-2. [DOI] [PubMed] [Google Scholar]
- 9.Christofidou-Solomidou M, Muzykantov VR. Antioxidant strategies in respiratory medicine. Treat Respir. Med. 2006;5(1):47–78. doi: 10.2165/00151829-200605010-00004. [DOI] [PubMed] [Google Scholar]
- 10.Matthay MA, Zimmerman GA. Acute lung injury and the acute respiratory distress syndrome: four decades of inquiry into pathogenesis and rational management. Am. J. Respir. Cell Mol. Biol. 2005;33(4):319–327. doi: 10.1165/rcmb.F305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.van der Vliet A. NADPH oxidases in lung biology and pathology: host defense enzymes, and more. Free Radic. Biol. Med. 2008;44(6):938–955. doi: 10.1016/j.freeradbiomed.2007.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gongora MC, Qin Z, Laude K, et al. Role of extracellular superoxide dismutase in hypertension. Hypertension. 2006;48(3):473–481. doi: 10.1161/01.HYP.0000235682.47673.ab. [DOI] [PubMed] [Google Scholar]
- 13.Hawkins BJ, Madesh M, Kirkpatrick CJ, Fisher AB. Superoxide flux in endothelial cells via the chloride channel-3 mediates intracellular signaling. Mol. Biol. Cell. 2007;18(6):2002–2012. doi: 10.1091/mbc.E06-09-0830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Oakley FD, Abbott D, Li Q, Engelhardt J. Signaling components of redox active endosomes: the redoxosomes. Antioxid. Redox Signal. 2009;11(6):1313–1333. doi: 10.1089/ars.2008.2363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ushio-Fukai M. Compartmentalization of redox signaling through NADPH oxidase-derived ROS. Antioxid. Redox Signal. 2009;11(6):1289–1299. doi: 10.1089/ars.2008.2333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shuvaev VV, Han J, Yu KJ, et al. PECAM-targeted delivery of SOD inhibits endothelial inflammatory response. FASEB J. 2011;25(1):348–357. doi: 10.1096/fj.10-169789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Finkel T. Signal transduction by reactive oxygen species. J. Cell. Biol. 2011;194(1):7–15. doi: 10.1083/jcb.201102095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Finkel T. Signal transduction by mitochondrial oxidants. J. Biol. Chem. 2011 doi: 10.1074/jbc.R111.271999. (Epub ahead of print) ▪ Summary of recent data on mitochondrial ROS involvement in redox signalling.
- 19. Murphy MP, Holmgren A, Larsson NG, et al. Unraveling the biological roles of reactive oxygen species. Cell. Metab. 2011;13(4):361–366. doi: 10.1016/j.cmet.2011.03.010. ▪ Compiled expert opinion on current issues, using and misusing the terminology in area of ROS studies.
- 20.Harrison DG, Gongora MC, Guzik TJ, Widder J. Oxidative stress and hypertension. J. Am. Soc. Hypertens. 2007;1(1):30–44. doi: 10.1016/j.jash.2006.11.006. [DOI] [PubMed] [Google Scholar]
- 21.Sedeek M, Hebert RL, Kennedy CR, Burns KD, Touyz RM. Molecular mechanisms of hypertension: role of NOX family NADPH oxidases. Curr. Opin Nephrol. Hypertens. 2009;18(2):122–127. doi: 10.1097/MNH.0b013e32832923c3. [DOI] [PubMed] [Google Scholar]
- 22.Rajagopalan S, Kurz S, Munzel T, et al. Angiotensin II-mediated hypertension in the rat increases vascular superoxide production via membrane NADH/NADPH oxidase activation. Contribution to alterations of vasomotor tone. J. Clin. Invest. 1996;97(8):1916–1923. doi: 10.1172/JCI118623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Touyz RM, Briones AM. Reactive oxygen species and vascular biology: implications in human hypertension. Hypertens. Res. 2011;34(1):5–14. doi: 10.1038/hr.2010.201. [DOI] [PubMed] [Google Scholar]
- 24.Zalba G, Beaumont FJ, San Jose G, Fortuno A, Fortuno MA, Diez J. Is the balance between nitric oxide and superoxide altered in spontaneously hypertensive rats with endothelial dysfunction? Nephrol. Dial. Transplant. 2001;16(Suppl. 1):2–5. doi: 10.1093/ndt/16.suppl_1.2. [DOI] [PubMed] [Google Scholar]
- 25.Jung O, Schreiber JG, Geiger H, Pedrazzini T, Busse R, Brandes RP. gp91phox-containing NADPH oxidase mediates endothelial dysfunction in renovascular hypertension. Circulation. 2004;109(14):1795–1801. doi: 10.1161/01.CIR.0000124223.00113.A4. [DOI] [PubMed] [Google Scholar]
- 26.Landmesser U, Cai H, Dikalov S, et al. Role of p47(phox) in vascular oxidative stress and hypertension caused by angiotensin II. Hypertension. 2002;40(4):511–515. doi: 10.1161/01.hyp.0000032100.23772.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jackson SP, Mistry N, Yuan Y. Platelets and the injured vessel wall: ‘rolling into action’: focus on glycoprotein Ib/V/IX and the platelet cytoskeleton. Trends Cardiovasc. Med. 2000;10(5):192–197. doi: 10.1016/s1050-1738(00)00062-1. [DOI] [PubMed] [Google Scholar]
- 28.Yao SK, McNatt J, Cui K, et al. Combined ADP and thromboxane A2 antagonism prevents cyclic flow variations in stenosed and endothelium-injured arteries in nonhuman primates. Circulation. 1993;88(6):2888–2893. doi: 10.1161/01.cir.88.6.2888. [DOI] [PubMed] [Google Scholar]
- 29.Peire MA, Puig-Parellada P. Oxygen-free radicals and nitric oxide are involved in the thrombus growth produced by iontophoresis of ADP. Pharmacol. Res. 1998;38(5):353–356. doi: 10.1006/phrs.1998.0372. [DOI] [PubMed] [Google Scholar]
- 30.Kuwano K, Ikeda H, Oda T, et al. Xanthine oxidase mediates cyclic flow variations in a canine model of coronary arterial thrombosis. Am. J. Physiol. 1996;270(6 Pt 2):H1993–H1999. doi: 10.1152/ajpheart.1996.270.6.H1993. [DOI] [PubMed] [Google Scholar]
- 31.Krotz F, Sohn HY, Gloe T, et al. NAD(P)H oxidase-dependent platelet superoxide anion release increases platelet recruitment. Blood. 2002;100(3):917–924. doi: 10.1182/blood.v100.3.917. [DOI] [PubMed] [Google Scholar]
- 32.Salvemini D, de Nucci G, Sneddon JM, Vane JR. Superoxide anions enhance platelet adhesion and aggregation. Br. J. Pharmacol. 1989;97(4):1145–1150. doi: 10.1111/j.1476-5381.1989.tb12572.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ferroni P, Basili S, Falco A, Davi G. Oxidant stress and platelet activation in hypercholesterolemia. Antioxid. Redox Signal. 2004;6(4):747–756. doi: 10.1089/1523086041361587. [DOI] [PubMed] [Google Scholar]
- 34.Redondo PC, Salido GM, Rosado JA, Pariente JA. Effect of hydrogen peroxide on Ca2+ mobilisation in human platelets through sulphydryl oxidation dependent and independent mechanisms. Biochem. Pharmacol. 2004;67(3):491–502. doi: 10.1016/j.bcp.2003.09.031. [DOI] [PubMed] [Google Scholar]
- 35.Gregg D, de Carvalho DD, Kovacic H. Integrins and coagulation: a role for ROS/redox signaling? Antioxid. Redox Signal. 2004;6(4):757–764. doi: 10.1089/1523086041361604. [DOI] [PubMed] [Google Scholar]
- 36. Gkaliagkousi E, Ferro A. Nitric oxide signalling in the regulation of cardiovascular and platelet function. Front Biosci. 2011;16:1873–1897. doi: 10.2741/3828. ▪ Detailed review on endothelial nitric oxide synthase, its characteristics and the role of nitric oxide in the signalling.
- 37.Coleman LG, Jr, Polanowska-Grabowska RK, Marcinkiewicz M, Gear AR. LDL oxidized by hypochlorous acid causes irreversible platelet aggregation when combined with low levels of ADP, thrombin, epinephrine, or macrophage-derived chemokine (CCL22) Blood. 2004;104(2):380–389. doi: 10.1182/blood-2003-08-2961. [DOI] [PubMed] [Google Scholar]
- 38.Badimon L, Storey RF, Vilahur G. Update on lipids, inflammation and atherothrombosis. Thromb. Haemost. 2011;105(Suppl. 1):S34–S42. doi: 10.1160/THS10-11-0717. [DOI] [PubMed] [Google Scholar]
- 39.Hasunuma Y, Matsuura E, Makita Z, Katahira T, Nishi S, Koike T. Involvement of β 2-glycoprotein I and anticardiolipin antibodies in oxidatively modified low-density lipoprotein uptake by macrophages. Clin. Exp. Immunol. 1997;107(3):569–573. doi: 10.1046/j.1365-2249.1997.d01-948.x. [DOI] [PubMed] [Google Scholar]
- 40.Lopez D, Garcia-Valladares I, Palafox-Sanchez CA, et al. Oxidized low-density lipoprotein/beta2-glycoprotein I complexes and autoantibodies to oxLig-1/beta-glycoprotein I in patients with systemic lupus erythematosus and antiphospholipid syndrome. Am. J. Clin. Pathol. 2004;121(3):426–436. doi: 10.1309/2AUE-6HD4-W6TL-EUU5. [DOI] [PubMed] [Google Scholar]
- 41.Bouma B, de Groot PG, van den Elsen JM, et al. Adhesion mechanism of human b(2)-glycoprotein I to phospholipids based on its crystal structure. EMBO J. 1999;18(19):5166–5174. doi: 10.1093/emboj/18.19.5166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Matsuura E, Kobayashi K, Hurley BL, Lopez LR. Atherogenic oxidized low-density lipoprotein/beta2-glycoprotein I (oxLDL/β2GPI) complexes in patients with systemic lupus erythematosus and antiphospholipid syndrome. Lupus. 2006;15(7):478–483. doi: 10.1191/0961203306lu2337oa. [DOI] [PubMed] [Google Scholar]
- 43.Ishikawa M, Stokes KY, Zhang JH, Nanda A, Granger DN. Cerebral microvascular responses to hypercholesterolemia: roles of NADPH oxidase and P-selectin. Circ. Res. 2004;94(2):239–244. doi: 10.1161/01.RES.0000111524.05779.60. [DOI] [PubMed] [Google Scholar]
- 44.Simon F, Stutzin A. Protein kinase C-mediated phosphorylation of p47 phox modulates platelet-derived growth factor-induced H2O2 generation and cell proliferation in human umbilical vein endothelial cells. Endothelium. 2008;15(4):175–188. doi: 10.1080/10623320802174480. [DOI] [PubMed] [Google Scholar]
- 45.Sturrock A, Cahill B, Norman K, et al. Transforming growth factor-beta1 induces NOX4 NAD(P)H oxidase and reactive oxygen species-dependent proliferation in human pulmonary artery smooth muscle cells. Am. J. Physiol. Lung Cell Mol. Physiol. 2006;290(4):L661–L673. doi: 10.1152/ajplung.00269.2005. [DOI] [PubMed] [Google Scholar]
- 46.Abe H, Okajima K, Okabe H, Takatsuki K, Binder BR. Granulocyte proteases and hydrogen peroxide synergistically inactivate thrombomodulin of endothelial cells in vitro. J. Lab. Clin. Med. 1994;123(6):874–881. [PubMed] [Google Scholar]
- 47.Herkert O, Djordjevic T, BelAiba RS, Gorlach A. Insights into the redox control of blood coagulation: role of vascular NADPH oxidase-derived reactive oxygen species in the thrombogenic cycle. Antioxid. Redox Signal. 2004;6(4):765–776. doi: 10.1089/1523086041361695. [DOI] [PubMed] [Google Scholar]
- 48.Sanguigni V, Ferro D, Pignatelli P, et al. CD40 ligand enhances monocyte tissue factor expression and thrombin generation via oxidative stress in patients with hypercholesterolemia. J. Am. Coll. Cardiol. 2005;45(1):35–42. doi: 10.1016/j.jacc.2004.09.047. [DOI] [PubMed] [Google Scholar]
- 49.Couzin-Frankel J. Inflammation bares a dark side. Science. 2010;330(6011):1621. doi: 10.1126/science.330.6011.1621. [DOI] [PubMed] [Google Scholar]
- 50.Lonkar P, Dedon PC. Reactive species and DNA damage in chronic inflammation: reconciling chemical mechanisms and biological fates. Int. J. Cancer. 2011;128(9):1999–2009. doi: 10.1002/ijc.25815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Roederer M, Staal FJ, Raju PA, Ela SW, Herzenberg LA. Cytokine-stimulated human immunodeficiency virus replication is inhibited by N-acetyl-l-cysteine. Proc. Natl Acad. Sci. USA. 1990;87(12):4884–4888. doi: 10.1073/pnas.87.12.4884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rhee SG. Cell signaling. H2O2, a necessary evil for cell signaling. Science. 2006;312(5782):1882–1883. doi: 10.1126/science.1130481. [DOI] [PubMed] [Google Scholar]
- 53.Morgan MJ, Liu ZG. Crosstalk of reactive oxygen species and NF-κB signaling. Cell Res. 2011;21(1):103–115. doi: 10.1038/cr.2010.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Reynaert NL, van der Vliet A, Guala AS, et al. Dynamic redox control of NF-kappaB through glutaredoxin-regulated S-glutathionylation of inhibitory kappaB kinase beta. Proc. Natl Acad. Sci. USA. 2006;103(35):13086–13091. doi: 10.1073/pnas.0603290103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Boueiz A, Hassoun PM. Regulation of endothelial barrier function by reactive oxygen and nitrogen species. Microvasc. Res. 2009;77(1):26–34. doi: 10.1016/j.mvr.2008.10.005. ▪ Detailed review on ROS involvement in endothelial permeability.
- 56.Lucas R, Verin AD, Black SM, Catravas JD. Regulators of endothelial and epithelial barrier integrity and function in acute lung injury. Biochem. Pharmacol. 2009;77(12):1763–1772. doi: 10.1016/j.bcp.2009.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Barnard ML, Matalon S. Mechanisms of extracellular reactive oxygen species injury to the pulmonary microvasculature. J. Appl. Physiol. 1992;72(5):1724–1729. doi: 10.1152/jappl.1992.72.5.1724. [DOI] [PubMed] [Google Scholar]
- 58.Han J, Shuvaev VV, Muzykantov VR. Catalase and superoxide dismutase conjugated with platelet-endothelial cell adhesion molecule antibody distinctly alleviate abnormal endothelial permeability caused by exogenous reactive oxygen species and vascular endothelial growth factor. J. Pharmacol. Exp. Ther. 2011;338(1):82–91. doi: 10.1124/jpet.111.180620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Alom-Ruiz SP, Anilkumar N, Shah AM. Reactive oxygen species and endothelial activation. Antioxid. Redox Signal. 2008;10(6):1089–1100. doi: 10.1089/ars.2007.2007. [DOI] [PubMed] [Google Scholar]
- 60.Dudek SM, Garcia JG. Cytoskeletal regulation of pulmonary vascular permeability. J. Appl. Physiol. 2001;91(4):1487–1500. doi: 10.1152/jappl.2001.91.4.1487. [DOI] [PubMed] [Google Scholar]
- 61.Lum H, Roebuck KA. Oxidant stress and endothelial cell dysfunction. Am. J. Physiol. Cell Physiol. 2001;280(4):C719–C741. doi: 10.1152/ajpcell.2001.280.4.C719. [DOI] [PubMed] [Google Scholar]
- 62.Wojciak-Stothard B, Tsang LY, Paleolog E, Hall SM, Haworth SG. Rac1 and RhoA as regulators of endothelial phenotype and barrier function in hypoxia-induced neonatal pulmonary hypertension. Am. J. Physiol. Lung Cell Mol. Physiol. 2006;290(6):L1173–L1182. doi: 10.1152/ajplung.00309.2005. [DOI] [PubMed] [Google Scholar]
- 63.Alexander JS, Alexander BC, Eppihimer LA, et al. Inflammatory mediators induce sequestration of VE-cadherin in cultured human endothelial cells. Inflammation. 2000;24(2):99–113. doi: 10.1023/a:1007025325451. [DOI] [PubMed] [Google Scholar]
- 64.Kevil CG, Oshima T, Alexander B, Coe LL, Alexander JS. H2O2-mediated permeability: role of MAPK and occludin. Am. J. Physiol. Cell Physiol. 2000;279(1):C21–C30. doi: 10.1152/ajpcell.2000.279.1.C21. [DOI] [PubMed] [Google Scholar]
- 65.Haorah J, Ramirez SH, Schall K, Smith D, Pandya R, Persidsky Y. Oxidative stress activates protein tyrosine kinase and matrix metalloproteinases leading to blood–brain barrier dysfunction. J. Neurochem. 2007;101(2):566–576. doi: 10.1111/j.1471-4159.2006.04393.x. [DOI] [PubMed] [Google Scholar]
- 66.McCord JM, Edeas MA. SOD, oxidative stress and human pathologies: a brief history and a future vision. Biomed. Pharmacother. 2005;59(4):139–142. doi: 10.1016/j.biopha.2005.03.005. [DOI] [PubMed] [Google Scholar]
- 67.Guzik TJ, Harrison DG. Vascular NADPH oxidases as drug targets for novel antioxidant strategies. Drug Discov. Today. 2006;11(11–12):524–533. doi: 10.1016/j.drudis.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 68.Smith RA, Murphy MP. Animal and human studies with the mitochondria-targeted antioxidant MitoQ. Ann. NY Acad. Sci. 2010;1201:96–103. doi: 10.1111/j.1749-6632.2010.05627.x. [DOI] [PubMed] [Google Scholar]
- 69.Moens AL, Kass DA. Therapeutic potential of tetrahydrobiopterin for treating vascular and cardiac disease. J. Cardiovasc. Pharmacol. 2007;50(3):238–246. doi: 10.1097/FJC.0b013e318123f854. [DOI] [PubMed] [Google Scholar]
- 70. Van Assche T, Huygelen V, Crabtree MJ. Targeting vascular redox biology through antioxidant gene delivery: a historical view and current perspectives. Recent Pat. Cardiovasc. Drug Discov. 2011;6(2):89–102. doi: 10.2174/157489011795933873. ▪ Review on development of gene delivery of antioxidants, approach that otuside the scope of the present review.
- 71.Muro S, Gajewski C, Koval M, Muzykantov VR. ICAM-1 recycling in endothelial cells: a novel pathway for sustained intracellular delivery and prolonged effects of drugs. Blood. 2005;105(2):650–658. doi: 10.1182/blood-2004-05-1714. [DOI] [PubMed] [Google Scholar]
- 72.Muro S, Mateescu M, Gajewski C, Robinson M, Muzykantov VR, Koval M. Control of intracellular trafficking of ICAM-1-targeted nanocarriers by endothelial Na+/H+ exchanger proteins. Am. J. Physiol. Lung Cell Mol. Physiol. 2006;290(5):L809–L817. doi: 10.1152/ajplung.00311.2005. [DOI] [PubMed] [Google Scholar]
- 73.Dziubla TD, Karim A, Muzykantov VR. Polymer nanocarriers protecting active enzyme cargo against proteolysis. J. Control Release. 2005;102(2):427–439. doi: 10.1016/j.jconrel.2004.10.017. [DOI] [PubMed] [Google Scholar]
- 74.Chorny M, Hood E, Levy RJ, Muzykantov VR. Endothelial delivery of antioxidant enzymes loaded into nonpolymeric magnetic nanoparticles. J. Control Release. 2010;146(1):144–151. doi: 10.1016/j.jconrel.2010.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Simone EA, Dziubla TD, Arguiri E, et al. Loading PEG-catalase into filamentous and spherical polymer nanocarriers. Pharm. Res. 2009;26(1):250–260. doi: 10.1007/s11095-008-9744-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Simone EA, Dziubla TD, Discher DE, Muzykantov VR. Filamentous polymer nanocarriers of tunable stiffness that encapsulate the therapeutic enzyme catalase. Biomacromolecules. 2009;10(6):1324–1330. doi: 10.1021/bm900189x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Dziubla TD, Shuvaev VV, Hong NK, et al. Endothelial targeting of semi-permeable polymer nanocarriers for enzyme therapies. Biomaterials. 2008;29(2):215–227. doi: 10.1016/j.biomaterials.2007.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Vujaskovic Z, Batinic-Haberle I, Rabbani ZN, et al. A small molecular weight catalytic metalloporphyrin antioxidant with superoxide dismutase (SOD) mimetic properties protects lungs from radiation-induced injury. Free Radic. Biol. Med. 2002;33(6):857–863. doi: 10.1016/s0891-5849(02)00980-2. [DOI] [PubMed] [Google Scholar]
- 79.Boissinot M, Kuhn LA, Lee P, et al. Rational design and expression of a heparin-targeted human superoxide dismutase. Biochem. Biophys. Res. Commun. 1993;190(1):250–256. doi: 10.1006/bbrc.1993.1038. [DOI] [PubMed] [Google Scholar]
- 80.Hernandez-Saavedra D, Zhou H, McCord JM. Anti-inflammatory properties of a chimeric recombinant superoxide dismutase: SOD2/3. Biomed. Pharmacother. 2005;59(4):204–208. doi: 10.1016/j.biopha.2005.03.001. [DOI] [PubMed] [Google Scholar]
- 81.Watanabe N, Iwamoto T, Bowen KD, Dickinson DA, Torres M, Forman HJ. Bio-effectiveness of Tat-catalase conjugate: a potential tool for the identification of H2O2-dependent cellular signal transduction pathways. Biochem. Biophys. Res. Commun. 2003;303(1):287–293. doi: 10.1016/s0006-291x(03)00335-8. [DOI] [PubMed] [Google Scholar]
- 82.Nagata K, Iwasaki Y, Yamada T, et al. Overexpression of manganese superoxide dismutase by N-acetylcysteine in hyperoxic lung injury. Respir. Med. 2007;101(4):800–807. doi: 10.1016/j.rmed.2006.07.017. [DOI] [PubMed] [Google Scholar]
- 83.Epperly MW, Sikora CA, DeFilippi SJ, et al. Pulmonary irradiation-induced expression of VCAM-I and ICAM-I is decreased by manganese superoxide dismutase-plasmid/liposome (MnSOD-PL) gene therapy. Biol. Blood Marrow Transplant. 2002;8(4):175–187. doi: 10.1053/bbmt.2002.v8.pm12014807. [DOI] [PubMed] [Google Scholar]
- 84.Supinski GS, Callahan LA. Polyethylene glycol-superoxide dismutase prevents endotoxin-induced cardiac dysfunction. Am. J. Respir. Crit. Care Med. 2006;173(11):1240–1247. doi: 10.1164/rccm.200410-1346OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Joralemon MJ, McRae S, Emrick T. PEGylated polymers for medicine: from conjugation to self-assembled systems. Chem. Commun. 2010;46(9):1377–1393. doi: 10.1039/b920570p. [DOI] [PubMed] [Google Scholar]
- 86.Wen J, Jiang X, Dai Y, et al. Adenosine deaminase enzyme therapy prevents and reverses the heightened cavernosal relaxation in priapism. J. Sex. Med. 2009;7(9):3011–3022. doi: 10.1111/j.1743-6109.2009.01552.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Payne RW, Murphy BM, Manning MC. Product development issues for PEGylated proteins. Pharm. Dev. Technol. 2011;16(5):423–440. doi: 10.3109/10837450.2010.513990. [DOI] [PubMed] [Google Scholar]
- 88.White CW, Jackson JH, Abuchowski A, et al. Polyethylene glycol-attached antioxidant enzymes decrease pulmonary oxygen toxicity in rats. J. Appl. Physiol. 1989;66(2):584–590. doi: 10.1152/jappl.1989.66.2.584. [DOI] [PubMed] [Google Scholar]
- 89.Yi X, Zimmerman MC, Yang R, Tong J, Vinogradov S, Kabanov AV. Pluronic-modified superoxide dismutase 1 attenuates angiotensin II-induced increase in intracellular superoxide in neurons. Free Radic. Biol. Med. 2010;49(4):548–558. doi: 10.1016/j.freeradbiomed.2010.04.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lee S, Yang SC, Heffernan MJ, Taylor WR, Murthy N. Polyketal microparticles: a new delivery vehicle for superoxide dismutase. Bioconjug. Chem. 2007;18(1):4–7. doi: 10.1021/bc060259s. [DOI] [PubMed] [Google Scholar]
- 91.Reddy MK, Labhasetwar V. Nanoparticle-mediated delivery of superoxide dismutase to the brain: an effective strategy to reduce ischemia-reperfusion injury. FASEB J. 2009;23(5):1384–1395. doi: 10.1096/fj.08-116947. [DOI] [PubMed] [Google Scholar]
- 92.Rosenbaugh EG, Roat JW, Gao L, et al. The attenuation of central angiotensin II-dependent pressor response and intraneuronal signaling by intracarotid injection of nanoformulated copper/zinc superoxide dismutase. Biomaterials. 2010;31(19):5218–5226. doi: 10.1016/j.biomaterials.2010.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Klyachko NL, Manickam DS, Brynskikh AM, et al. Cross-linked antioxidant nanozymes for improved delivery to CNS. Nanomedicine. 2011 doi: 10.1016/j.nano.2011.05.010. (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Yamagishi S, Nakamura K, Matsui T. Role of oxidative stress in the development of vascular injury and its therapeutic intervention by nifedipine. Curr. Med. Chem. 2008;15(2):172–177. doi: 10.2174/092986708783330557. [DOI] [PubMed] [Google Scholar]
- 95.Lin SJ, Shyue SK, Shih MC, et al. Superoxide dismutase and catalase inhibit oxidized low-density lipoprotein-induced human aortic smooth muscle cell proliferation: role of cell-cycle regulation, mitogen-activated protein kinases, and transcription factors. Atherosclerosis. 2007;190(1):124–134. doi: 10.1016/j.atherosclerosis.2006.02.044. [DOI] [PubMed] [Google Scholar]
- 96.Epperly MW, Guo HL, Jefferson M, et al. Cell phenotype specific kinetics of expression of intratracheally injected manganese superoxide dismutase-plasmid/liposomes (MnSOD-PL) during lung radioprotective gene therapy. Gene Ther. 2003;10(2):163–171. doi: 10.1038/sj.gt.3301852. [DOI] [PubMed] [Google Scholar]
- 97.Danel C, Erzurum SC, Prayssac P, et al. Gene therapy for oxidant injury-related diseases: adenovirus-mediated transfer of superoxide dismutase and catalase cDNAs protects against hyperoxia but not against ischemia-reperfusion lung injury. Hum. Gene Ther. 1998;9(10):1487–1496. doi: 10.1089/hum.1998.9.10-1487. [DOI] [PubMed] [Google Scholar]
- 98.Folz RJ, Abushamaa AM, Suliman HB. Extracellular superoxide dismutase in the airways of transgenic mice reduces inflammation and attenuates lung toxicity following hyperoxia. J. Clin. Invest. 1999;103(7):1055–1066. doi: 10.1172/JCI3816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Segal BH, Han W, Bushey JJ, et al. NADPH oxidase limits innate immune responses in the lungs in mice. PLoS One. 2010;5(3):e9631. doi: 10.1371/journal.pone.0009631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Finigan JH. The coagulation system and pulmonary endothelial function in acute lung injury. Microvasc. Res. 2009;77(1):35–38. doi: 10.1016/j.mvr.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 101.Varani J, Ward PA. Mechanisms of endothelial cell injury in acute inflammation. Shock. 1994;2(5):311–319. doi: 10.1097/00024382-199411000-00001. [DOI] [PubMed] [Google Scholar]
- 102.Fruehauf JP, Meyskens FL., Jr Reactive oxygen species: a breath of life or death? Clin. Cancer Res. 2007;13(3):789–794. doi: 10.1158/1078-0432.CCR-06-2082. [DOI] [PubMed] [Google Scholar]
- 103.Doerschuk CM, Quinlan WM, Doyle NA, et al. The role of P-selectin and ICAM-1 in acute lung injury as determined using blocking antibodies and mutant mice. J. Immunol. 1996;157(10):4609–4614. [PubMed] [Google Scholar]
- 104.Esmon CT. The interactions between inflammation and coagulation. Br. J. Haematol. 2005;131(4):417–430. doi: 10.1111/j.1365-2141.2005.05753.x. [DOI] [PubMed] [Google Scholar]
- 105.Yao H, Yang SR, Kode A, et al. Redox regulation of lung inflammation: role of NADPH oxidase and NF-kappaB signalling. Biochem. Soc. Trans. 2007;35(Pt 5):1151–1155. doi: 10.1042/BST0351151. [DOI] [PubMed] [Google Scholar]
- 106.Oakley FD, Abbott D, Li Q, Engelhardt JF. Signaling components of redox active endosomes: the redoxosomes. Antioxid. Redox Signal. 2009;11(6):1313–1333. doi: 10.1089/ars.2008.2363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kim DW, Kim SY, Lee SH, et al. Protein transduction of an antioxidant enzyme: subcellular localization of superoxide dismutase fusion protein in cells. BMB Rep. 2008;41(2):170–175. doi: 10.5483/bmbrep.2008.41.2.170. [DOI] [PubMed] [Google Scholar]
- 108.Muzykantov VR. Biomedical aspects of targeted delivery of drugs to pulmonary endothelium. Expert Opin. Drug Deliv. 2005;2(5):909–926. doi: 10.1517/17425247.2.5.909. [DOI] [PubMed] [Google Scholar]
- 109.McIntosh DP, Tan XY, Oh P, Schnitzer JE. Targeting endothelium and its dynamic caveolae for tissue-specific transcytosis in vivo: a pathway to overcome cell barriers to drug and gene delivery. Proc. Natl Acad. Sci. USA. 2002;99(4):1996–2001. doi: 10.1073/pnas.251662398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Pasqualini R, McDonald DM, Arap W. Vascular targeting and antigen presentation. Nat. Immunol. 2001;2(7):567–568. doi: 10.1038/89704. [DOI] [PubMed] [Google Scholar]
- 111.Schnitzer JE. Vascular targeting as a strategy for cancer therapy. N. Engl. J. Med. 1998;339(7):472–474. doi: 10.1056/NEJM199808133390711. [DOI] [PubMed] [Google Scholar]
- 112.Simone E, Ding BS, Muzykantov V. Targeted delivery of therapeutics to endothelium. Cell Tissue Res. 2009;335(1):283–300. doi: 10.1007/s00441-008-0676-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Stan RV, Kubitza M, Palade GE. PV-1 is a component of the fenestral and stomatal diaphragms in fenestrated endothelia. Proc. Natl Acad. Sci. USA. 1999;96(23):13203–13207. doi: 10.1073/pnas.96.23.13203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Wilson A, Zhou W, Champion HC, et al. Targeted delivery of oligodeoxynucleotides to mouse lung endothelial cells in vitro and in vivo. Mol. Ther. 2005;12(3):510–518. doi: 10.1016/j.ymthe.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 115.Shuvaev VV, Muzykantov VR. Targeted modulation of reactive oxygen species in the vascular endothelium. J. Control Release. 2011;153(1):56–63. doi: 10.1016/j.jconrel.2011.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Danilov SM, Gavrilyuk VD, Franke FE, et al. Lung uptake of antibodies to endothelial antigens: key determinants of vascular immunotargeting. Am. J. Physiol. Lung Cell Mol. Physiol. 2001;280(6):L1335–L1347. doi: 10.1152/ajplung.2001.280.6.L1335. [DOI] [PubMed] [Google Scholar]
- 117.Oh P, Li Y, Yu J, et al. Subtractive proteomic mapping of the endothelial surface in lung and solid tumours for tissue-specific therapy. Nature. 2004;429(6992):629–635. doi: 10.1038/nature02580. [DOI] [PubMed] [Google Scholar]
- 118.Pasqualini R, Koivunen E, Kain R, et al. Aminopeptidase N is a receptor for tumor-homing peptides and a target for inhibiting angiogenesis. Cancer Res. 2000;60(3):722–727. [PMC free article] [PubMed] [Google Scholar]
- 119.Harari OA, Wickham TJ, Stocker CJ, et al. Targeting an adenoviral gene vector to cytokine-activated vascular endothelium via E-selectin. Gene Ther. 1999;6(5):801–807. doi: 10.1038/sj.gt.3300898. [DOI] [PubMed] [Google Scholar]
- 120.Lindner JR, Song J, Christiansen J, Klibanov AL, Xu F, Ley K. Ultrasound assessment of inflammation and renal tissue injury with microbubbles targeted to P-selectin. Circulation. 2001;104(17):2107–2112. doi: 10.1161/hc4201.097061. [DOI] [PubMed] [Google Scholar]
- 121.Shuvaev VV, Tliba S, Pick J, et al. Modulation of endothelial targeting by size of antibody–antioxidant enzyme conjugates. J. Control Release. 2011;149(3):236–241. doi: 10.1016/j.jconrel.2010.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Muzykantov VR, Atochina EN, Ischiropoulos H, Danilov SM, Fisher AB. Immunotargeting of antioxidant enzyme to the pulmonary endothelium. Proc. Natl Acad. Sci. USA. 1996;93(11):5213–5218. doi: 10.1073/pnas.93.11.5213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Atochina EN, Balyasnikova IV, Danilov SM, Granger DN, Fisher AB, Muzykantov VR. Immunotargeting of catalase to ACE or ICAM-1 protects perfused rat lungs against oxidative stress. Am. J. Physiol. 1998;275(4 Pt 1):L806–L817. doi: 10.1152/ajplung.1998.275.4.L806. [DOI] [PubMed] [Google Scholar]
- 124.Scherpereel A, Wiewrodt R, Christofidou-Solomidou M, et al. Cell-selective intracellular delivery of a foreign enzyme to endothelium in vivo using vascular immunotargeting. FASEB J. 2001;15(2):416–426. doi: 10.1096/fj.00-0022com. [DOI] [PubMed] [Google Scholar]
- 125.Kozower BD, Christofidou-Solomidou M, Sweitzer TD, et al. Immunotargeting of catalase to the pulmonary endothelium alleviates oxidative stress and reduces acute lung transplantation injury. Nat. Biotechnol. 2003;21(4):392–398. doi: 10.1038/nbt806. [DOI] [PubMed] [Google Scholar]
- 126.Shuvaev VV, Christofidou-Solomidou M, Bhora F, et al. Targeted detoxification of selected reactive oxygen species in the vascular endothelium. J. Pharmacol. Exp. Ther. 2009;331(2):404–411. doi: 10.1124/jpet.109.156877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Muzykantov VR, Atochina EN, Kuo A, et al. Endothelial cells internalize monoclonal antibody to angiotensin-converting enzyme. Am. J. Physiol. 1996;270(5 Pt 1):L704–L713. doi: 10.1152/ajplung.1996.270.5.L704. [DOI] [PubMed] [Google Scholar]
- 128.Nowak K, Hanusch C, Nicksch K, et al. Pre-ischaemic conditioning of the pulmonary endothelium by immunotargeting of catalase via angiotensin-converting-enzyme antibodies. Eur. J. Cardiothorac. Surg. 2010;37(4):859–863. doi: 10.1016/j.ejcts.2009.10.029. [DOI] [PubMed] [Google Scholar]
- 129.Nowak K, Weih S, Metzger R, et al. Immunotargeting of catalase to lung endothelium via anti-angiotensin-converting enzyme antibodies attenuates ischemia-reperfusion injury of the lung in vivo. Am. J. Physiol. Lung Cell Mol. Physiol. 2007;293(1):L162–L169. doi: 10.1152/ajplung.00001.2007. [DOI] [PubMed] [Google Scholar]
- 130.Preissler G, Loehe F, Huff IV, et al. Targeted endothelial delivery of nanosized catalase immunoconjugates protects lung grafts donated after cardiac death. Transplantation. 2011;92(4):380–387. doi: 10.1097/TP.0b013e318226bc6b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Cai H, Griendling KK, Harrison DG. The vascular NAD(P)H oxidases as therapeutic targets in cardiovascular diseases. Trends Pharmacol. Sci. 2003;24(9):471–478. doi: 10.1016/S0165-6147(03)00233-5. [DOI] [PubMed] [Google Scholar]
- 132.Zou MH. Peroxynitrite and protein tyrosine nitration of prostacyclin synthase. Prostaglandins Other Lipid Mediat. 2007;82(1–4):119–127. doi: 10.1016/j.prostaglandins.2006.05.005. [DOI] [PubMed] [Google Scholar]
- 133.Kuzkaya N, Weissmann N, Harrison DG, Dikalov S. Interactions of peroxynitrite, tetrahydrobiopterin, ascorbic acid and thiols: implications for uncoupling endothelial nitric-oxide synthase. J. Biol. Chem. 2003;278(25):22546–22554. doi: 10.1074/jbc.M302227200. [DOI] [PubMed] [Google Scholar]
- 134.Nakazono K, Watanabe N, Matsuno K, Sasaki J, Sato T, Inoue M. Does superoxide underlie the pathogenesis of hypertension? Proc. Natl Acad. Sci. USA. 1991;88(22):10045–10048. doi: 10.1073/pnas.88.22.10045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Schnackenberg CG, Welch WJ, Wilcox CS. Normalization of blood pressure and renal vascular resistance in SHR with a membrane-permeable superoxide dismutase mimetic: role of nitric oxide. Hypertension. 1998;32(1):59–64. doi: 10.1161/01.hyp.32.1.59. [DOI] [PubMed] [Google Scholar]
- 136.Laursen JB, Rajagopalan S, Galis Z, Tarpey M, Freeman BA, Harrison DG. Role of superoxide in angiotensin II-induced but not catecholamine-induced hypertension. Circulation. 1997;95(3):588–593. doi: 10.1161/01.cir.95.3.588. [DOI] [PubMed] [Google Scholar]
- 137.Cai H. Hydrogen peroxide regulation of endothelial function: origins, mechanisms, and consequences. Cardiovasc. Res. 2005;68(1):26–36. doi: 10.1016/j.cardiores.2005.06.021. [DOI] [PubMed] [Google Scholar]
- 138.Fang J, Seki T, Maeda H. Therapeutic strategies by modulating oxygen stress in cancer and inflammation. Adv. Drug Deliv. Rev. 2009;61(4):290–302. doi: 10.1016/j.addr.2009.02.005. [DOI] [PubMed] [Google Scholar]
- 139.McCord JM, Wong K, Stokes SH, Petrone WF, English D. Superoxide and inflammation: a mechanism for the anti-inflammatory activity of superoxide dismutase. Acta Physiol. Scand. Suppl. 1980;492:25–30. [PubMed] [Google Scholar]
- 140.Valdivia A, Perez Y, Dominguez A, et al. Improved anti-inflammatory and pharmacokinetic properties for superoxide dismutase by chemical glycosidation with carboxymethylchitin. Macro Mol. Biosci. 2005;5(2):118–123. doi: 10.1002/mabi.200400114. [DOI] [PubMed] [Google Scholar]
- 141.Veronese FM, Caliceti P, Schiavon O, Sergi M. Polyethylene glycol-superoxide dismutase, a conjugate in search of exploitation. Adv. Drug Deliv. Rev. 2002;54(4):587–606. doi: 10.1016/s0169-409x(02)00029-7. [DOI] [PubMed] [Google Scholar]
- 142.Matsui T, Yamagishi S, Nakamura K, Inoue H. Bay w 9798, a dihydropyridine structurally related to nifedipine with no calcium channel-blocking properties, inhibits tumour necrosis factor-alpha-induced vascular cell adhesion molecule-1 expression in endothelial cells by suppressing reactive oxygen species generation. J. Int. Med. Res. 2007;35(6):886–891. doi: 10.1177/147323000703500617. [DOI] [PubMed] [Google Scholar]
- 143.Mitsopoulos P, Omri A, Alipour M, Vermeulen N, Smith MG, Suntres ZE. Effectiveness of liposomal-N-acetylcysteine against LPS-induced lung injuries in rodents. Int. J. Pharm. 2008;363(1–2):106–111. doi: 10.1016/j.ijpharm.2008.07.015. [DOI] [PubMed] [Google Scholar]
- 144.Bonder CS, Knight D, Hernandez-Saavedra D, McCord JM, Kubes P. Chimeric SOD2/3 inhibits at the endothelial-neutrophil interface to limit vascular dysfunction in ischemia-reperfusion. Am. J. Physiol. Gastrointest. Liver Physiol. 2004;287(3):G676–G684. doi: 10.1152/ajpgi.00049.2004. [DOI] [PubMed] [Google Scholar]
- 145.Shuvaev VV, Tliba S, Nakada M, Albelda SM, Muzykantov VR. Platelet-endothelial cell adhesion molecule-1-directed endothelial targeting of superoxide dismutase alleviates oxidative stress caused by either. extracellular or intracellular superoxide. J. Pharmacol. Exp. Ther. 2007;323(2):450–457. doi: 10.1124/jpet.107.127126. [DOI] [PubMed] [Google Scholar]
- 146.Muro S, Wiewrodt R, Thomas A, et al. A novel endocytic pathway induced by clustering endothelial ICAM-1 or PECAM-1. J. Cell Sci. 2003;116(Pt 8):1599–1609. doi: 10.1242/jcs.00367. [DOI] [PubMed] [Google Scholar]
- 147.Horgan MJ, Lum H, Malik AB. Pulmonary edema after pulmonary artery occlusion and reperfusion. Am. Rev. Respir. Dis. 1989;140(5):1421–1428. doi: 10.1164/ajrccm/140.5.1421. [DOI] [PubMed] [Google Scholar]
- 148.Birukov KG. Cyclic stretch, reactive oxygen species, and vascular remodeling. Antioxid. Redox Signal. 2009;11(7):1651–1667. doi: 10.1089/ars.2008.2390. [DOI] [PMC free article] [PubMed] [Google Scholar]