Abstract
Background
The incidence and death rates of endometrial cancer are alarmingly increasing. The diagnosis and treatment of endometrial cancer is crucial to decreasing mortality. Cystic fibrosis transmembrane conductance regulator (CFTR) belongs to the adenosine triphosphate (ATP)-binding cassette transporter family and plays an essential role in anion regulation and tissue homeostasis of various epithelia. This study explored the expression of CFTR in endometrial carcinoma and the role of CFTR in proliferation and migration of endometrial carcinoma cells.
Material/Methods
Immunohistochemistry and real-time (RT)-PCR were used to test the expression of CFTR in normal endometrium and endometrial carcinoma. CFTR inhibitor was used to restrain the expression of CFTR on the endometrial carcinoma, the effects on the proliferation and migration of endometrial carcinoma cells were also studied. RT-PCR was performed to test the expression of mir-125b after restraining CFTR. Proliferation and migration capability of endometrial carcinoma cells were detected after transfection of endometrial carcinoma cells with mir-125b mimic.
Results
Compared with cells from normal endometrium, the expression of CFTR was significantly upregulated in endometrial carcinoma cells. After adding CFTR(inh)172, the capability for proliferation and transfer of endometrial carcinoma cells was strengthened, the expression of mir-125b was reduced, and after transfection with mir-125b mimics entering the endometrial carcinoma cells, the ability of the proliferation and transfer of endometrial carcinoma cells was also reduced.
Conclusions
The high expression of CFTR in the endometrial carcinoma cells played a pivotal role in restraining the proliferation and transfer of endometrial carcinoma cells.
MeSH Keywords: Cell Migration Inhibition, Cell Proliferation, Cystic Fibrosis Transmembrane Conductance Regulator, Endometrial Neoplasms
Background
Endometrial carcinoma (EC) is one of the most common female genital tract malignant tumors, accounting for 6% of the pathogenesis of neoplastic diseases in women [1]. Based on estrogen dependency, EC is divided into type I (dependent) and type II (nondependent). ECs develop slowly in most cases and can be diagnosed early, therefore, the 5-year survival rate is relatively high [2]. Surgery or radiotherapy have positive treatment effects in most patients suffering from EC. However, for some patients with high-grade, relapsed EC, or patients who want to retain reproductive function, these effective therapeutic methods were seldom used [3]. Furthermore some EC patients exhibited reduced effect of treatment with rapalogues [4], which might be related to the effect of the inhibitor phosphatase and tensin homologue (PTEN). Consequently, the treatment of EC needs further research.
CFTR is a special member of ATP-binding cassette transporter super family, because it affects the chloride channel that has a fairly complicated regulatory mechanism [5]. The activities of CFTR are closely linked to cell metabolism: CFTR and metabolic enzymes form a macromolecule complex, and when the cell energy needs to be increased, the activity of CFTR is restrained, hence, the cell energy is saved. CFTR is widely distributed in epithelial tissue and controls the quantity and composition of epithelial cell excreta via promotion of the transfer of water and salt. The malfunction of CFTR can seriously influence the transport of transepithelial ions, which cause or aggravate certain diseases [6].
Chan et al. found that CFTR might restrain the activity of the glandular epithelial cells sodium ion channel inside mouse endometrium and directly mediate the secretion of HCO-, which would impact the pH value of the uterine cavity fluid [7]. In addition, if the endometrial glandular epithelium lacked CFTR protein, it would obviously restrain the capacitation of sperm, and lead to sterility [7,8]. The expression of CFTR mRNA and protein are observed in endometrial glandular epithelial cells of women’s menstrual cycle, and the expression is generally thought to change with the menstrual cycle: in the later proliferative stage there is a strengthening of CFTR expression, whereas the CFTR expression and the local control in the secretory phase might participate in the regulation and control of blastocyst implantation. Using an in vitro culture model of endometrial glandular epithelial cells, the regulating effect of cultivating human endometrial glandular epithelial cells by estrogen and progesterone hormones on CFTR mRNA and protein regulation has significantly informed the research on female sterility [9].
The abnormal expression of CFTR has been associated with lung carcinoma, pancreatic carcinoma, prostatic carcinoma, and cervical carcinoma. But it is still unclear whether it plays a role in tumor cancer suppressor genes or cancer-promoting genes. Its role in endometrial carcinoma has yet to be reported [10,11]. The expression of CFTR in endometrial carcinoma and its mechanism of action are still unknown. Therefore, this study concentrates on CFTR expression and its function in endometrial carcinoma.
Material and Methods
Source of specimen
Patients treated at our hospital from October 2010 to January 2014 who were diagnosed with endometrial carcinoma (EC) via pathologic histology or who had a hysteroscopy due to benign endometrial disease were selected randomly to participate in this study. The Institutional Review Board of the First Affiliated Hospital of Nanjing Medical University Ethics Committee approved this study, and written informed consent was obtained from each participating patient or an authorized family member. There was no ethical issue regarding the experiment. After a hysterectomy or curettage procedure, 40 cases were diagnosed as EC pathologically, and 40 control group cases were identified as normal endometrium (20 cases at the proliferative stage and 20 cases at the secretory stage). The patients’ average age was 38 years old, the normal endometrium cases were not of comparability in age and other factors (p>0.05); all the cases were examined and verified via histopathology, excluding endometrial merger and other lesions. All the patients had regular menstrual cycles, and had not received hormonotherapy within three month before their surgery, and were not in gestation or lactation. Patients with adenomyosis, dysfunctional uterine bleeding, polycystic ovarian syndrome, and other hormone dependency disease were excluded.
Immunohistochemical method
Common paraffin embedding included the thickness of tissue slices at 5 μm for hematoxylin and eosin (H&E) staining (Sigma-Aldrich, Shanghai, China). The mounted slides were observed under the microscope and representative endometrial organizers were selected in group of specimens. The immunohistochemical steps were performed according to the specification of immunohistochemical S-P kit (Beijing Bioss Biotechnology Co., Ltd, Beijing, China.): sample slides were dewaxed and hydrated, and antigen retrieval for 20 minutes by microwave in citrate buffer solution (pH 6.0, Sigma-Aldrich, Shanghai, China). The slides were cooled to room temperature (RT) naturally. Slides were rinsed with PBS for 3 minutes, 3 times, followed by 3% H202 incubation for 15 minutes at RT. Afterwards, slides were rinsed with PBS for 3 minutes, 3 times, followed by 5% donkey serum (ab7475, Abcam Company, Cambridge, UK) added to block unspecific antigens and incubated at 37°C for 30 minutes. Mouse-anti-human CFTR monoclonal antibody (1:100, ab2784, Abcam Company, Cambridge, UK) was added and incubated overnight at 4°C. In the negative controls, PBS was used to replace the primary antibody. The second day, after rinsing in PBS for 3 minutes, 3 times, horseradish peroxidase labeled goat anti mouse IgG (1: 2000, bs-0296G-HRP, Beijing Bioss Biotechnology Co., Ltd. Beijing, China) was added at RT for 1 hour. Then the slides were rinsed in PBS for 3 minutes, 3 times, followed by DAB (Vector, CA, US) solution added to activate the immunological signals. In the negative controls, PBS was used to replace the primary antibodies. The positive result criterion inlcuded: all slices were observed under the light microscope independently by three doctors from the pathology department using high power fields (10×40 magnification), the proportion of each specimen’s positive cell number was counted, and the positive results were determined per the method described by De Falco M, et al. [12]: 0 score (tpositive cell less than 1%); 1 score (positive cell 1% to 20%); 2 scores (positive cell 21% to 40%); 3 scores (positive cell 41% to 60%); 4 scores (positive cell over 61%). Interest area optical density (IOD) was also calculated by Image Pro Plus 6.0 software by randomly selecting different 3, 3′-diaminobenzidine (DAB) positive areas. All values were obtained by mean values ± standard error (mean ±SEM).
Real-time RT PCR
The expression of CFTR and mir-125b were detected by fluorogenic quantitative PCR (Bio-Rad Laboratories, IQ5, CA, USA). Total RNA of tissue was extracted with Trizol (Life Technology, NY, USA) followed by reverse transcription. CFTR was amplified by PCR; and β-actin was the internal control. The reverse transcription kit and RNA extraction reagent were purchased from Guangzhou GeneCopoeia Co., Ltd (Guangzhou, China). CFTR and mir-125b primer and real-time quantitative (qRT) PCR kit were purchased from Guangzhou GeneCopoeia Co., Ltd. (Guangzhou, China). Reaction conditions were: initial denaturation 95°C for 10 minutes, 95°C for 10 seconds, 60°C for 20 seconds, 72°C for 10 seconds, repeated 40 times. As for the negative control, RNAse-free water was used to replace the cDNA template; in each specimen and control, a set of 3 parallel repeated wells was used to get the average value. After finishing the reaction, the software showed automatically the amplification efficiency of the fluorescence reaction curve and each specimen reaction system and Ct values; which were repeated three times at different experimental times.
The hsa-mir-125b mimics and β-actin siRNAs were synthesized by Shanghai Gene Pharmaceutical Technology Co., Ltd (Shanghai, China). Has-mir-125b sequence was 5′-ACCAGACUUUUCCUAGU CCCUGAGACCCU AACUUGUGAGGUAUUUUAGUAACAUCAC AAGUCAGGCUCUUGGGACCU AGGCGGAGGGGA-3′. β-actin siRNAs sequence was 5′-UGA AGA UCA AGA UCA UUG CdTdT-3′ and 5′-GCA AUG AUC UUG AUC UUC AdTdT-3′. After 0.25% trypsin digestion, RPMI 1640 medium plus 10% fetal calf serum was used to make single-cell suspension into 6-well culture plates in a concentration of 1.0×105 cells/100 μL. When the cells proliferated to ~50% fusion, miR-125b was transfected, mixed with RNA-mate which had a final concentration of 5 nmol/L. After 48 hours of transfection, total RNA and total protein from the same specimen were extracted.
Cell proliferation detected by Edu test
Ishikawa endometrial carcinoma cell line was purchased from Shanghai Institute for Biological Sciences, Chinese Academy of Sciences Institute (Shanghai, China). The cells were cultured in RPMI 1640 plus 10% FBS at 37°C with 5% CO2. CFTR(inh)-172 was purchased from Sigma-Aldrich (Shanghai, China). Each of the experimental group cells were obtained after 72 hours of culture with CFTR(inh)-172, using 104 per well, respectively inoculated in 96-well culture plates, with 100 μL of RPMI 1640 (Gibco, CA, USA) of culture solution with 10% fetal calf serum (Gibco, CA, USA) added to each well. The edu was purchased from Guangzhou RiboBio Co., Ltd. (Guangzhou, China), and the test was carried out in line with the protocols by He et al. [13]. Each experimental group had 3 parallel specimen sets; the mean values for each group represented the cells’ proliferation condition.
Cell migration test
24-well Boyden chemotaxis chamber was used (8 m, Becton Dickinson, NJ, USA), and the migration test was carried out according to the manufacturer’s manual. Cells in each experimental group were obtained after 72 hours of culture. Then 0.2 mL cell suspension (1×104) was added to the upper chamber, the Roswell Park Memorial Institute (RPMI) 1640 (Gibco, NY, USA) culture medium 600 μL with 10% fetal calf serum (Gibco, NY, USA) was added to the lower chamber, and three repeat wells were used for each group, and incubated at 37°C, using 5% CO2 for 24 hours. Then the transwell membrane was taken out, and matrigel on the upper membrane was wiped off to eliminate the uninvaded cells. The cells on the other side of the membrane were fixed by methyl alcohol for 20 minutes at 4°C followed by a PBS rinse and H&E (Sigma-Aldrich, Shanghai, China) staining. Under the optical microscope, the number of cells migrating through the membrane was observed under an optical microscope; and the number of cells in five high power fields (hpf, ×100) in each well were counted.
CFTR protein detection
25 μg cell lysate was collected from each well followed by polyacrylamide gel electrophoresis. Proteins were electrotransfered to the polyvinylidene fluoride film (PVDF, Millipore, MA, USA). Mouse-anti-human CFTR polyclonal antibody (1:1000, ab2784, Abcam Company, Cambridge, UK) and horseradish peroxidase labeled goat anti-mouse IgG (ab6789, 1:5000, Abcam, Cambridge, UK) were added to the samples followed by electrogenerated chemiluminescence (ECL, Millipore, MA, USA). The experiment was carried out according to the company’s instructions. Gel-imaging system (Gel Doc XR+, Bio-Rad, CA, USA) was used to record the results followed by gray level scanning and analysis. The relative quantization was calculated by the ratio between CFTR gray level area integral and internal reference.
Statistical analysis
SPSS 11.5 software was used for statistical analysis. The experimental data was normally distributed, all the data variance were similar in nature, and the comparison between the two groups of data used independent-sample t test for comparative analysis; as for three groups, one-way ANOVA was used for the analysis. A p value <0.05 was considered of statistical significance.
Results
CFTR protein was expressed in endometrial carcinoma
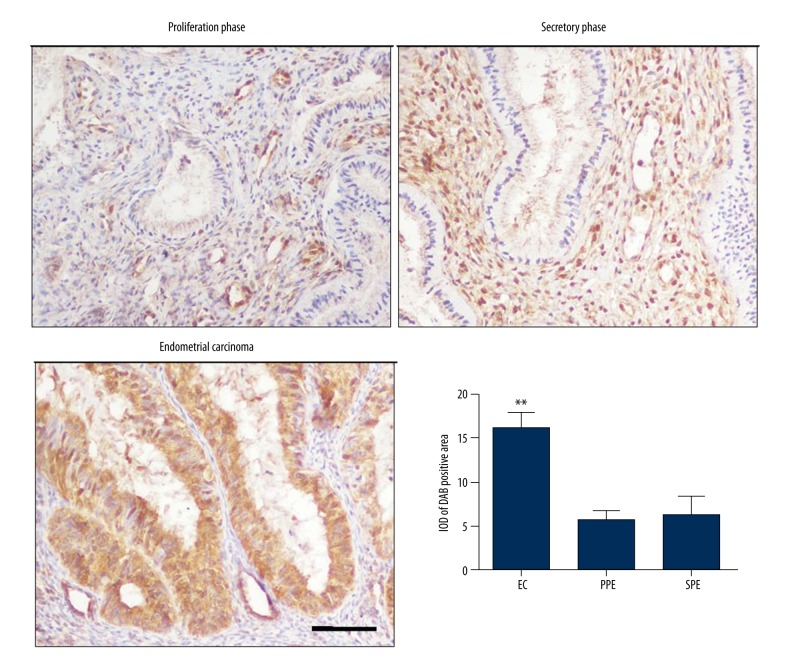
In both the observation group and control group, the CFTR protein was expressed in the glandular epithelial cells and cytoplasm, and the cell nucleus of mesenchymal cells. When comparing the secretory phase (SPE) and proliferation phase (PPE) in the endometrium specimens, the expression of CFTR was increased in endometrial carcinoma specimens (** p<0.05 vs. PPE and SPE, n=4, Figure 1).
Figure 1.
The expression of CFTR in endometrium tissue at proliferation phase, and secretory phase and in endometrial carcinoma tissue (scale bar=50 μm). The expression of CFTR was increased in endometrial carcinoma tissue when compared with tissue from endometrium of secretory phase and proliferation phase by interest area optical density (IOD) analysis. (** p<0.05, n=4). PPE – proliferation phase; SPE – secretory phase, EC – endometrial carcinoma.
CFTR mRNA was upregulated in endometrial carcinoma
Real-time RT PCR (qRT-PCR) was used to test the expression of CFTR mRNA in the endometrium at the proliferation phase, in the endometrium at the secretory phase and in the endometrial carcinoma tissue. It was found that CFTR mRNA was increased more obviously in the endometrial carcinoma tissue than in the proliferation phase and the secretory phase tissue. (** p<0.05 vs. PPE and SPE, n=3, Figure 2).
Figure 2.
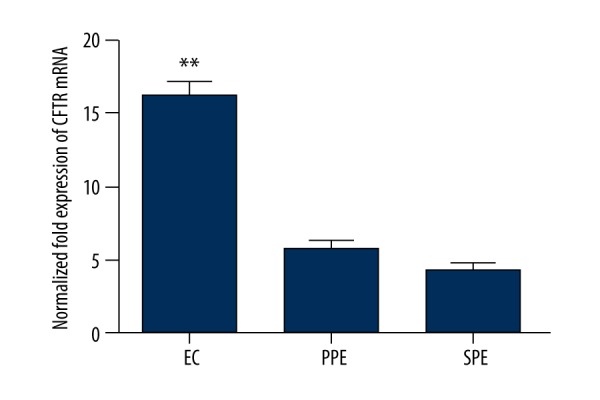
The relative expression of CFTR in endometrium tissue at proliferation phase (PPE), secretory phase (SPE) and endometrial carcinoma (EC) tissue (** p<0.05, n=3).
CFTR(inh)-172 inhibits the expression of CFTR mRNA in endometrial carcinoma
To explore the role of CFTR in endometrial carcinoma, CFTR specificity inhibitor CFTR(inh)-172 was used to inhibit the expression and function of CFTR: 0, 2.5, 10 and 20 μmol concentration of CFTR(inh)-172 were added respectively into the cell cultures, then RNA was extracted after 48-hour culture. After adding CFTR inhibitor, it was found that CFTR(inh)-172 inhibited CFTR mRNA expression gradually depending on concentration (i.e., 0, 2.5 and 10 μmol), but the expression of CFTR mRNA increased when CFTR(inh)-172 concentration was 20 μmol. We speculated that this might be related to CFTR receptor saturation and negative feedback. Therefore, the concentration of CFTR(inh)-172 was selected as 10 μmol in the following Ishikawa experiments (** p<0.05 vs. 2.5 and 10 μM, n=3, Figure 3).
Figure 3.
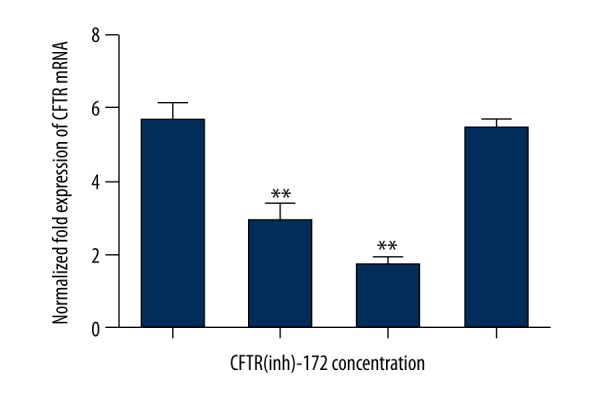
CFTR(inh)-172 obviously inhibited the expression of CFTR mRNA in different concentrations of inhibitor treatment (** p<0.05, n=3).
CFTR(inh)-172 promoted the endometrial carcinoma cell strain Ishikawa proliferation
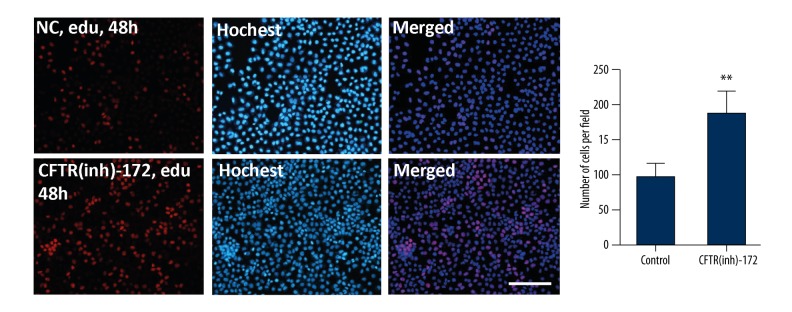
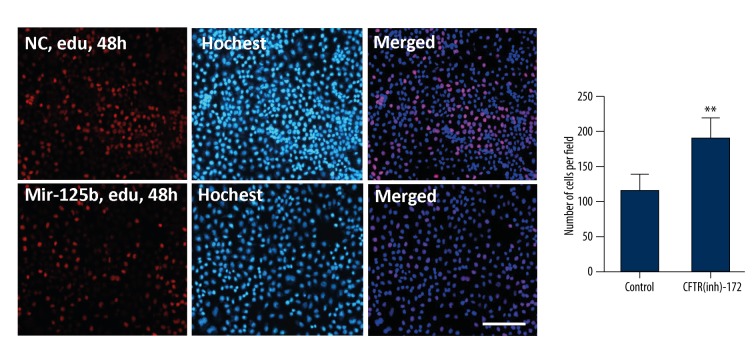
After 10 μmol of CFTR(inh)-172 was added for 48 hours, edu was used to mark the endometrial carcinoma cells’ multiplication capacity. The concentration of edu was 10 μmol, and was added to endometrial carcinoma cells and left for 48 hours. Salic A et al. method was used [14]. It was found that, after adding CFTR(inh)-172 at 10 μmol to the endometrial carcinoma cells for 48 hours, the cell proliferation capability was enhanced (** p<0.05, Figure 4).
Figure 4.
CFTR(inh)-172 promotes proliferation of the endometrial carcinoma cell line Ishikawa. The cells were labeled by edu, and counterstained by Hoechst (** p<0.05, scale bar=50 μm).
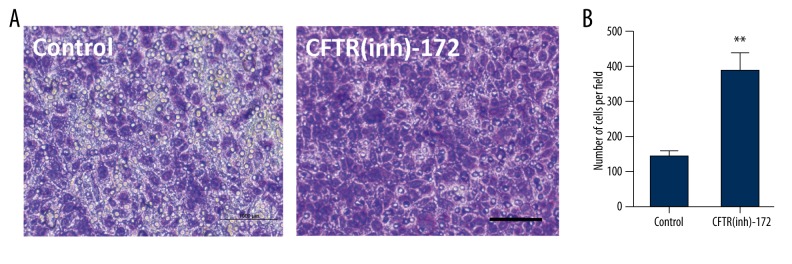
After the inhibitor was added for 72 hours, the transwell chamber was used to test the transfer ability of endometrial carcinoma cells. It was found that the migration ability of endometrial carcinoma cells was obviously strengthened after the inhibitor was added (** p<0.05, Figure 5).
Figure 5.
(A, B) Endometrial carcinoma cells were transfected with CFTR(inh)-172 at 10 μmol for 72 hours; transwell experiment tested the migration of the endometrial carcinoma cells (** p<0.05, n=3, scale bar=50 μm).
Transfection of mir-125b mimics restrained the proliferation and migration of endometrial carcinoma cells
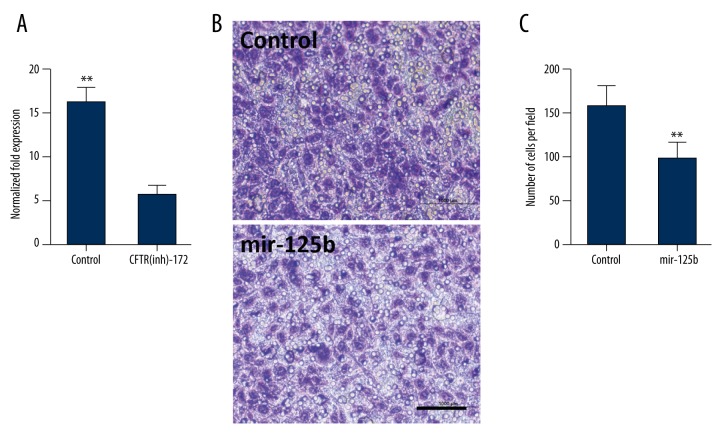
After adding inhibitor for 48 hours, it was found by qRT-PCR testing that the expression of mir-125b in the inhibitor group was reduced by about 2 times that of the control group (p<0.05, n=3, Figure 6A). After transfecting mir-125b into the endometrial carcinoma cells, 48 hours later, the proliferation and invasion ability of endometrial carcinoma cells were tested (Figure 6B). It was found that after transfecting with mir-125b, the proliferation and transfer abilities of the endometrial carcinoma cells were evidently reduced (** p<0.05, Figures 6C, 7).
Figure 6.
Transfection of mir-125b mimics can restrain the proliferation and transfer of endometrial carcinoma cells. (A) The expression of mir-125b was induced in the CFTR-in172 group compared to the control group (** p<0.05, n=3). (B) After the endometrial carcinoma cells were transfect with mir-125b at 5 nmol for 48 hours, transwell tested the transfer ability of the endometrial carcinoma cells (** p<0.05, n=3, scale bar=50 μm). (C) The number of endometrial carcinoma cells was reduced significantly after mir-125b transfection (** p<0.05, n=3).
Figure 7.
CFTR(in)-172 mimics can restrain the proliferation and migration of endometrial carcinoma cells. The cells were labeled by edu and counterstained by Hoechst (** p<0.05, n=3, scale bar=50 μm).
Western blot testing of the impact of mir-125b on the expression of MMP11 and VEGF-A
After transfecting the mir-125b at 5 nmol into the endometrial carcinoma cells for 48 hours, cell protein was extracted to test for the expression of MMP11 and VEGF-A. It was found that after transfecting the mir-125b, the expression of MMP11 and VEGF-A in the endometrial carcinoma cells was obviously reduced (** p<0.05, n=3, Figure 8).
Figure 8.
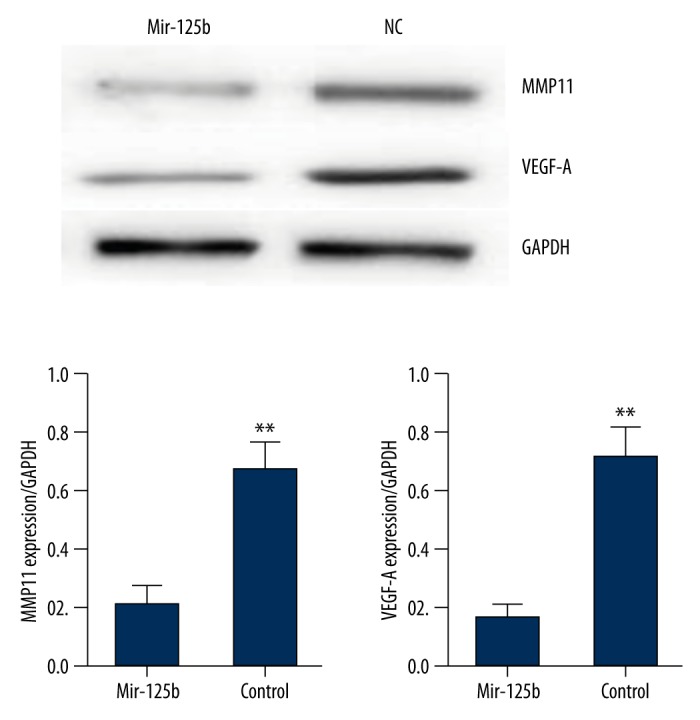
The expression of MMP11 and VEGF-A in the endometrial carcinoma cells was obviously reduced after mir-125b transfection (** p<0.05, n=3).
Discussion
In this study, it was found that the expression of CFTR in endometrial carcinoma tissue was more than in normal endometrial tissue. The expression of CFTR was increased more in the proliferation phase than the secretory phase, which might be caused by the induction of estrogen [15]. After CFTR specificity was added to restrain CFTR(inh)-172, the expression of CFTR mRNA was downregulated, and the proliferation and transfer abilities of endometrial carcinoma cells were increased. At the same time, it was found that after lowering CFTR, the expression of mir-125b was decreased, and after transfecting mir-125b mimics, the proliferation and transfer abilities of endometrial carcinoma cells were reduced. It has been shown that miR-125b is a tumor suppressor that influences prostate cancer progression [16]. Our result are consistence with previous results that showed CFTR reduced miR-125b expression in mouse embryos. It has been shown that knockdown of miR-125b exerts the effect of CFTR inhibition, however, injection of miR-125b precursor reverses the effect [17].
The matrix metalloproteinase (MMP) 11 is one member of the MMP family and found to be an important protein in the occurrence, development, and transfer of tumor cells. In cervical carcinoma, MMP11 participated in the occurrence, development, and transfer of cervical carcinoma and endometrial carcinoma cells [18,19]. Furthermore MMP11 is one of the 31 candidate genes found to be upregulated in EECs related genes, which are hormonally regulated during the menstrual cycle and known to be important in endometrial homeostasis [20]. Knockdown of MMP11 expression could reduce the proliferation and invasion of human gastric adenocarcinoma cells [21]. Vascular endothelial growth factor (VEGF) was found to be the key moderator in the development of tumor vessels, which participated in the angiogenesis of endometrial carcinoma; in addition, its expression had been associated with endometrial carcinoma prognosis [22]. Preoperative serum concentration of VEGF correlates with the presence of metastases in endometrioid endometrial carcinoma, also 63% of endometrial cancers expressed VEGF-A, which was significantly correlated with microvessel density [23,24]. In our study it was found that after transfecting mir-125b, the expression of MMP11 and VEGF-A was reduced; which was in accordance with the report of Qian Bi et al. [25]. It also showed that CFTR may play the regulatory effect via mir-125b in endometrial carcinoma.
Although research on CFTRs effects on tumor cells continues, the specific mechanism is still not clear. In pancreatic carcinoma, the CFTR gene mutation increased the probability of pancreatic carcinoma [26]. However, in prostate carcinoma and lung carcinoma, it played a protective role [27,28]. In prostate carcinoma, CFTR expression was reduced, and it was speculated that it played the role of a cancer suppressor gene [29]. In cervical carcinoma, when compared with the normal cervix uteri and precancerous lesions of uterine cervix, the expression of CFTR was higher in tumor tissue, and was related to the development of cervical carcinoma [11]. In our study, CFTR expression in endometrial carcinoma tissue was higher than in normal tissue. At the same time, it was found that the restraining of CFTR expression might promote the proliferation and invasion of cervical carcinoma cells, which might then play a role or function as cancer suppressor gene in endometrial carcinoma.
MicroRNAs (miRNAs) are a type of endogenic non-coding RNA found in the eukaryons, which have a regulatory function, and its size is about 20~25 nucleotides. Mature miRNAs are processed and produced by a longer primary transcript via a series of nuclease, and then assembled into a RNA-induced silencing complex; mRNA was identified using the method of base complementary pairing, and the silencing complex was guided to degrade mRNA or stop the interpretation of mRNA according to the different complementation degrees. It plays an important role in cell proliferation, differentiation, cell cycle, and tumor invasion [30]. Recently, it was shown that the expression of miR-125b was low in breast carcinoma [31], prostate carcinoma [32], thyroid carcinoma [33], oral carcinoma [34], ovarian carcinoma [35] and endometrial carcinoma [36]. Scott et al. [31] found that transfecting miR-125b in a breast carcinoma cell line could obviously reduce ERBB2 and ERBB3, thus, restraining the growth of breast cancer cells. It was found by Le et al. [37] that miR-125b restrained the cell apoptosis via reducing p53 in human neuroblastoma and lung fibroblast. Shi et al. found that miR-125b had high expression in most prostate carcinoma tissues; and in the androgen receptor positive prostate carcinoma cell line LNCAP promoted cell proliferation and transfer via up regulating miR-125b. And that after restraining the expression of CFTR, the expression of miR-125b was reduced, meanwhile, after transfecting the mir-125b, the proliferation and transfer of endometrial carcinoma cells were restrained, which was in accordance with the functions of other tumors and mir-125b.
Conclusions
We found that the expression of CFTR was increased in endometrial carcinoma tissue, and the role of CFTR in endometrial carcinoma might act via the regulating of mir-125b. Our research was not an in vivo experiment; so in future research our focused will be on how to construct a model of endometrial carcinoma transplantation tumor to study the role and mechanism of CFTR in endometrial carcinoma.
Abbreviations
- CFTR
cystic fibrosis transmembrane conductance regulator
- MMP
matrix metalloproteinase
- VEGF-A
vascular endothelial growth factor
- MTT
4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
- DMSO
dimethylsulfoxide
- H&E
hematoxylin and eosin
- PVDF
polyvinylidene fluoride
Footnotes
Source of support: This work was supported by grants from the National Natural Science Foundation of China (Grant No. 81502853), the Natural Science Foundation of Jiangsu Province of China (Grant No. BK20151026)
Conflict of interest
There is no conflict of interest.
References
- 1.Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2009. Cancer J Clin. 2009;59(4):225–49. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 2.Amant F, Moerman P, Neven P, et al. Endometrial cancer. Lancet. 2005;366(9484):491–505. doi: 10.1016/S0140-6736(05)67063-8. [DOI] [PubMed] [Google Scholar]
- 3.Bakkum-Gamez JN, Gonzalez-Bosquet J, Laack NN, et al. Current issues in the management of endometrial cancer. Mayo Clin Proc. 2008;83(1):97–112. doi: 10.4065/83.1.97. [DOI] [PubMed] [Google Scholar]
- 4.Fong P, Meng LR. Effect of mTOR inhibitors in nude mice with endometrial carcinoma and variable PTEN expression status. Med Sci Monit Basic Res. 2014;20:146–52. doi: 10.12659/MSMBR.892514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Anderson MP, Gregory RJ, Thompson S, et al. Demonstration that CFTR is a chloride channel by alteration of its anion selectivity. Science. 1991;253(5016):202–5. doi: 10.1126/science.1712984. [DOI] [PubMed] [Google Scholar]
- 6.Itokazu Y, Pagano RE, Schroeder AS, et al. Reduced GM1 ganglioside in CFTR-deficient human airway cells results in decreased beta1-integrin signaling and delayed wound repair. Am J Physiol Cell Physiol. 2014;306(9):C819–30. doi: 10.1152/ajpcell.00168.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chan LN, Wang XF, Tsang LL, et al. Inhibition of amiloride-sensitive Na(+) absorption by activation of CFTR in mouse endometrial epithelium. Pflugers Arch. 2001;443(Suppl 1):S132–36. doi: 10.1007/s004240100660. [DOI] [PubMed] [Google Scholar]
- 8.Wang XF, Zhou CX, Shi QX, et al. Involvement of CFTR in uterine bicarbonate secretion and the fertilizing capacity of sperm. Nat Cell Biol. 2003;5(10):902–6. doi: 10.1038/ncb1047. [DOI] [PubMed] [Google Scholar]
- 9.Song Y, Wang Q, Huang W, et al. NF kappaB expression increases and CFTR and MUC1 expression decreases in the endometrium of infertile patients with hydrosalpinx: A comparative study. Reprod Biol Endocrinol. 2012;10:86. doi: 10.1186/1477-7827-10-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cafferata EG, González-Guerrico AM, Giordano L, et al. Interleukin-1beta regulates CFTR expression in human intestinal T84 cells. Biochim Biophys Acta. 2000;1500(2):241–48. doi: 10.1016/s0925-4439(99)00105-2. [DOI] [PubMed] [Google Scholar]
- 11.Peng X, Wu Z, Yu L, et al. Overexpression of cystic fibrosis transmembrane conductance regulator (CFTR) is associated with human cervical cancer malignancy, progression and prognosis. Gynecol Oncol. 2012;125(2):470–76. doi: 10.1016/j.ygyno.2012.02.015. [DOI] [PubMed] [Google Scholar]
- 12.De Falco M, Fedele V, Cobellis L, et al. Pattern of expression of cyclin D1/CDK4 complex in human placenta during gestation. Cell Tissue Res. 2004;317(2):187–94. doi: 10.1007/s00441-004-0880-z. [DOI] [PubMed] [Google Scholar]
- 13.He Q, Man L, Ji Y, Ding F. Comparison in the biological characteristics between primary cultured sensory and motor Schwann cells. Neurosci Lett. 2012;521(1):57–61. doi: 10.1016/j.neulet.2012.05.059. [DOI] [PubMed] [Google Scholar]
- 14.Salic A, Mitchison TJ. A chemical method for fast and sensitive detection of DNA synthesis in vivo. Proc Natl Acad Sci USA. 2008;105(7):2415–20. doi: 10.1073/pnas.0712168105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang JZ, Ajonuma LC, Tsang LL, et al. Differential expression and localization of CFTR and ENaC in mouse endometrium during pre-implantation. Cell Biol Int. 2004;28(6):433–39. doi: 10.1016/j.cellbi.2004.03.011. [DOI] [PubMed] [Google Scholar]
- 16.Budd WT, Seashols-Williams SJ, Clark GC, et al. Dual action of miR-125b as a tumor suppressor and OncomiR-22 promotes prostate cancer tumorigenesis. PLoS One. 2015;10(11):e0142373. doi: 10.1371/journal.pone.0142373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lu YC, Chen H, Fok KL, et al. CFTR mediates bicarbonate-dependent activation of miR-125b in preimplantation embryo development. Cell Res. 2012;22(10):1453–66. doi: 10.1038/cr.2012.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Valdivia A, Peralta R, Matute-González M, et al. Co-expression of metalloproteinases 11 and 12 in cervical scrapes cells from cervical precursor lesions. Int J Clin Exp Pathol. 2011;4(7):674–82. [PMC free article] [PubMed] [Google Scholar]
- 19.Obokata A, Watanabe J, Nishimura Y, et al. Significance of matrix metalloproteinase-7 [correction of matrix metalloproteinase-2], -11 and tissue inhibitor of metalloproteinase-1 expression in normal, hyperplastic and neoplastic endometrium. Anticancer Res. 2007;27(1A):95–105. [PubMed] [Google Scholar]
- 20.Moreno-Bueno G1, Sánchez-Estévez C, Cassia R, et al. Differential gene expression profile in endometrioid and nonendometrioid endometrial carcinoma: STK15 is frequently overexpressed and amplified in nonendometrioid carcinomas. Cancer Res. 2003;63(18):5697–702. [PubMed] [Google Scholar]
- 21.Kou YB, Zhang SY, Zhao BL, et al. Knockdown of MMP11 inhibits proliferation and invasion of gastric cancer cells. Int J Immunopathol Pharmacol. 2013;26(2):361–70. doi: 10.1177/039463201302600209. [DOI] [PubMed] [Google Scholar]
- 22.Dobrzycka B, Terlikowski SJ, Kwiatkowski M, et al. Prognostic significance of VEGF and its receptors in endometrioid endometrial cancer. Ginekol Pol. 2010;81(6):422–25. [PubMed] [Google Scholar]
- 23.Saarelainen SK, Staff S, Peltonen N, et al. Endoglin, VEGF, and its receptors in predicting metastases in endometrial carcinoma. Tumour Biol. 2014;35(5):4651–57. doi: 10.1007/s13277-014-1609-6. [DOI] [PubMed] [Google Scholar]
- 24.Wang J, Taylor A, Showeil R, et al. Expression profiling and significance of VEGF-A, VEGFR2, VEGFR3 and related proteins in endometrial carcinoma. Cytokine. 2014;68(2):94–100. doi: 10.1016/j.cyto.2014.04.005. [DOI] [PubMed] [Google Scholar]
- 25.Bi Q, Tang S, Xia L, et al. Ectopic expression of MiR-125a inhibits the proliferation and metastasis of hepatocellular carcinoma by targeting MMP11 and VEGF. PLoS One. 2012;7(6):e40169. doi: 10.1371/journal.pone.0040169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McWilliams RR, Petersen GM, Rabe KG, et al. Cystic fibrosis transmembrane conductance regulator (CFTR) gene mutations and risk for pancreatic adenocarcinoma. Cancer. 2010;116(1):203–9. doi: 10.1002/cncr.24697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li Y, Sun Z, Wu Y, et al. Cystic fibrosis transmembrane conductance regulator gene mutation and lung cancer risk. Lung Cancer. 2010;70(1):14–21. doi: 10.1016/j.lungcan.2010.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Qiao D, Yi L, Hua L, et al. Cystic fibrosis transmembrane conductance regulator (CFTR) gene 5T allele may protect against prostate cancer: A case-control study in Chinese Han population. J Cyst Fibros. 2008;7(3):210–14. doi: 10.1016/j.jcf.2007.07.011. [DOI] [PubMed] [Google Scholar]
- 29.Xie C, Jiang XH, Zhang JT, et al. CFTR suppresses tumor progression through miR-193b targeting urokinase plasminogen activator (uPA) in prostate cancer. Oncogene. 2013;32(18):2282–91. 2291e1–7. doi: 10.1038/onc.2012.251. [DOI] [PubMed] [Google Scholar]
- 30.Xu X, Yang X, Xing C, et al. miRNA: The nemesis of gastric cancer (Review) Oncol Lett. 2013;6(3):631–41. doi: 10.3892/ol.2013.1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Scott GK, Goga A, Bhaumik D, et al. Coordinate suppression of ERBB2 and ERBB3 by enforced expression of micro-RNA miR-125a or miR-125b. J Biol Chem. 2007;282(2):1479–86. doi: 10.1074/jbc.M609383200. [DOI] [PubMed] [Google Scholar]
- 32.Ozen M, Creighton CJ, Ozdemir M, Ittmann M, et al. Widespread deregulation of microRNA expression in human prostate cancer. Oncogene. 2008;27(12):1788–93. doi: 10.1038/sj.onc.1210809. [DOI] [PubMed] [Google Scholar]
- 33.Visone R, Pallante P, Vecchione A, et al. Specific microRNAs are downregulated in human thyroid anaplastic carcinomas. Oncogene. 2007;26(54):7590–95. doi: 10.1038/sj.onc.1210564. [DOI] [PubMed] [Google Scholar]
- 34.Henson BJ, Bhattacharjee S, O’Dee DM, et al. Decreased expression of miR-125b and miR-100 in oral cancer cells contributes to malignancy. Genes Chromosomes Cancer. 2009;48(7):569–82. doi: 10.1002/gcc.20666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nam EJ, Yoon H, Kim SW, et al. MicroRNA expression profiles in serous ovarian carcinoma. Clin Cancer Res. 2008;14(9):2690–95. doi: 10.1158/1078-0432.CCR-07-1731. [DOI] [PubMed] [Google Scholar]
- 36.Shang C, Lu YM, Meng LR. MicroRNA-125b down-regulation mediates endometrial cancer invasion by targeting ERBB2. Med Sci Monit. 2012;18(4):BR149–55. doi: 10.12659/MSM.882617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Le MT, Teh C, Shyh-Chang N, et al. MicroRNA-125b is a novel negative regulator of p53. Genes Dev. 2009;23(7):862–76. doi: 10.1101/gad.1767609. [DOI] [PMC free article] [PubMed] [Google Scholar]