Abstract
Background
Haemoglobinopathies constitute the commonest recessive monogenic disorders worldwide, and the treatment of affected individuals presents a substantial global disease burden. β-thalassaemia is characterised by the reduced synthesis (β+) or absence (βo) of the β-globin chains in the HbA molecule, resulting in accumulation of excess unbound α-globin chains that precipitate in erythroid precursors in the bone marrow and in the mature erythrocytes, leading to ineffective erythropoiesis and peripheral haemolysis. Approximately 1.5% of the global population are heterozygotes (carriers) of the β-thalassemias; there is a high incidence in populations from the Mediterranean basin, throughout the Middle East, the Indian subcontinent, Southeast Asia, and Melanesia to the Pacific Islands.
Aim
The principal aim of this paper is to review, from a historical standpoint, our knowledge about an ancient disease, the β-thalassemias, and in particular, when, how and in what way β-thalassemia spread worldwide to reach such high incidences in certain populations.
Results
Mutations involving the β-globin gene are the most common cause of genetic disorders in humans. To date, more than 350 β-thalassaemia mutations have been reported. Considering the current distribution of β- thalassemia, the wide diversity of mutations and the small number of specific mutations in individual populations, it seems unlikely that β-thalassemia originated in a single place and time.
Conclusions
Various processes are known to determine the frequency of genetic disease in human populations. However, it is almost impossible to decide to what extent each process is responsible for the presence of a particular genetic disease. The wide spectrum of β-thalassemia mutations could well be explained by looking at their geographical distribution, the history of malaria, wars, invasions, mass migrations, consanguinity, and settlements. An analysis of the distribution of the molecular spectrum of haemoglobinopathies allows for the development and improvement of diagnostic tests and management of these disorders.
Keywords: Thalassemia distribution, Old World, Ancient disease
Introduction
Haemoglobinopathies constitute the commonest recessive monogenic disorders worldwide. They fall into two main groups: the thalassemia syndromes and the structural hemoglobin variants (abnormal hemoglobins). α, β, and δβ thalassemias are the main types of thalassemia with clinical importance; the most frequent and clinically important structural hemoglobin variants are HbS, HbE, HbC and HbD. The treatment of patients presents a substantial global disease burden.
Variants of thalassaemias and main abnormal haemoglobins interact to produce a wide range of clinical disorders of varying severity.1–3 Homozygotes for β-thalassemia may develop either thalassemia major or thalassemia intermedia. Individuals with thalassemia major are usually diagnosed within the first 2 years of life and require regular blood transfusions to survive.3 Patients with thalassemia intermedia are diagnosed later and as a rule, have milder anemia and do not depend on transfusion for survival.
The clinical manifestations of β-thalassemias are highly variable. They range from asymptomatic cases with mild (silent) mutations, to those with mild hypochromic anemia, to others with moderate and severe lifelong transfusion-dependent anemia and multi organ involvement.1
β-thalassemia is mainly caused by mutations resulting in a single nucleotide substitution, small deletions or insertions within the β-globin gene or its immediate flanking sequence and, rarely, by gross deletions.2,3 To date, more than 350 β-thalassaemia mutations have been reported in the IthaGenes database.4
Nearly all β-thalassemia variants are inherited in a Mendelian recessive manner, but there is a small subgroup of β-thalassemia alleles that behave in a dominant fashion.1
The principal aim of this paper is to review, from a historical standpoint, our knowledge of an ancient disease, the β-thalassemias, and to discuss when, how and in what way β-thalassemia has spread in the old world to reach such high incidences in certain populations. To this end, knowledge of the molecular defects that prevail in each country provides valuable information that can be used for population screening in prevention programmes and facilitates effective prenatal diagnosis.2,3
Epidemiology and Global Burden of the Thalassemia Disorders
The thalassemias have a high incidence in a broad area extending from the Mediterranean basin and parts of Africa, throughout the Middle East, the Indian subcontinent, Southeast Asia, and Melanesia in to the Pacific Islands.4,5
The carrier frequencies for β-thalassemia in these areas range from 1 to 20%, and rarely may be higher. The frequencies for the milder forms of α-thalassemia are much greater, varying from 10 to 20% in parts of sub- Saharan Africa, to 40% or more in some Middle Eastern and Indian populations, to as high as 80% in northern Papua New Guinea and some isolated groups in the northeast of India.6
Globally, it is estimated that there are 270 million carriers with abnormal haemoglobins and thalassemias, of which 80 million are carriers of β-thalassemia. Recent surveys suggest that between 300,000 and 400,000 babies are born with a serious hemoglobin disorder each year (23,000 with β-thalassemia major) and that up to 90% of these births occur in low- or middle-income countries.1–6
Origin, Spread and Evolutionary History of Beta-Globin Gene Mutations
In the major hemoglobinopathies, adult haemoglobin A, composed of two α-globin and two β-globin chains, is altered by genetic variants that encode single amino acid substitutions in β-globin (as in HbS, HbC, HbD and HbE) or reduced production of β-globin chains as in β-thalassaemia.1–3 Rarely do β-thalassemias result from gross β- gene deletion.3
β-thalassaemia is characterised by the partial reduction of synthesis (mild=β++, severe=β+) or total reduction (βo), of the β-globin chains in the HbA molecule. This results in accumulation of excess, unbound α-globin chains that precipitate in erythroid precursors in the bone marrow and in the mature erythrocytes, leading to ineffective erythropoiesis and peripheral haemolysis.1–3
While α- and β- thalassemias are caused by more than 450 mutations, almost 1200 mutations have been reported that change the structure of the α- and β-globin genes to create abnormal hemoglobin tetramers of HbA.1–3 Among this broad spectrum of mutations, some are very frequent and population specific.
DNA haplotypes in the β-globin gene can help to determine the date of origin, track gene flow of a particular β-globin gene mutation and detect major migrations.
The β0-39 nonsense (C→T) and the β+ IVS-1-110 (G>A) mutations are largely prevalent in the Mediterranean basin.1–3 β+ IVS-1-110 is found in the eastern part of the Mediterranean area, including Turkey,7 Lebanon,8 and Egypt.9 The β+ IVS-1-110 seems to be older than the β0-39 mutation because of its nearly exclusive association with ancestral haplotype I.10 In addition,β+ IVS-1-110, which is believed to have arisen in the Middle Orient and reaches its highest frequencies in the Eastern Mediterranean populations, may have been introduced to the other countries by a variety of settlers from the East. Its diffusion to the occident probably happened at the time of the expansion of Greek civilization, starting from the eighth century B.C.
Most Common β-Thalassemia Variants in the Mediterranean Belt and Arabic Countries
1. Italy
In the Po Delta area (northern Italy) β+ thalassemia represents approximately 50% of the total thalassemia major population. The production of β globin in a β+ homozygote and in a β +, β °39 (nonsense mutation at codon 39) double heterozygote is approximately 20% and 10%, respectively, of total non-α globin synthesis. This suggests that in the Po River Delta region the most common thalassemic genes are β °39 and β+ IVS-1-110 (G>A) mutation. It is possible that IVS-1-110 (G>A) mutation came from Greece to this area where Greek influence has been reported in the past.11
On the other hand, the β0-39 (C>T) mutation is found almost exclusively in Sardinia3. This dominating mutation seems to have undergone a significant founder effect influence on the island. It is also highly prevalent in continental Italy12 and Spain13 and is frequently encountered in Portugal14 and Tunisia.15
The exclusive presence of β0-thalassemia type in southern Sardinia, which most likely arose from a single mutational event, is not surprising since Sardinia has been free from external colonisation for a long period of time. In fact, Greek colonisation was localised to the north-east of the island while Romans and Carthaginians settled only along the southern coast.16 Moreover, other populations, such as Vandals, Goths, Saracens, Pisans, and Spanish did not stay on the island long enough or in a sufficient number to modify the genetic structure of the autochthonous population significantly. The relatively high prevalence of β+- thalassemia in the northern part of the island was probably due to the Greek colonisation.16,17
More heterogeneity is detected in Sicily, where there are a variety of mutations and where the frequency of the β0-39 mutation is only 37%.17
2. Cyprus
In Cyprus, the estimated β-thalassaemia carrier rate is around 12–15% of the population.18 The most common β-globin gene mutation is IVS-1-110 (G> A), with a percentage of 74–80%, followed by three other alleles, specifically IVS-2-745 (C> G), IVS-1-6 (T> C), IVS-1-1 (G> A), with frequencies of 5–8%.19 The presence of various haemoglobin variants in low prevalence, such as Hb S, Hb D, and Hb Lepore, is likely to be directly linked to the history of Cyprus, as archeological monuments have been found throughout the island signifying the presence for many years of the Greeks, Syrians, Persians, Arabs, Byzantines, Franks, Venetians, and Turks.20
3. Greece
Greece is a country of approximately 11 million people with a mean frequency of β-thalassaemia carriers of 7.4%.21
Extensive studies have disclosed that the distribution of β-thalassemia carriers is extremely uneven. The low fertile areas of Thessaly, Western Peloponnesus, and Western Epirus, as well as the islands of Rhodes, Lesbos, Corfu and other areas, displayed frequencies of up to 15%. In contrast, in the high altitude areas (that were free of malaria) and Macedonia, the prevalence of heterozygotes was significantly lower.22
The molecular basis of β-thalassaemia is very heterogeneous in Greece, and up to 30 β-thalassaemia mutations have been observed. The molecular characterization of β- thalassaemia was evaluated in three studies on 857 thalassemia patients (106 with thalassaemia intermedia (TI) covering more than 25% of the 3,241 patients with TM and TI registered in 2010.23
In the three studies, 27 β-thalassemia mutations were observed. Of interest are:
the rates of the prevalent β-thalassaemia mutations. In order of frequency they were: IVS-1-110(G>A) with an incidence of 43.8%; Codon 39(C>T), 18%; IVS-1-1(G>A), 12.7%; IVS-1-6(T>C), 6.9%; IVS-2-745(G>A), 4.7% and IVS- 2-1(G>A), 3%. The five mutations cover 89.1 % and the six 91.4% of the β-thal genes.
the three very mild mutations (+1480 (C>T), -101 (C>T) and + 33 C>G) that were found only in TI patients covering 22% of TI β-thalassaemia mutations.21–23
4. Turkey
The overall incidence of β-thalassemia trait in Turkey is 2.1% (range: 0.6–13%). At present, the Turkish population is about 80 million, with 1.6 million people with thalassemia trait and about 5500 patients with homozygous thalassemia and other hemoglobinopathies.24
By April 1999, the total number of β-thalassemia alleles described in the Turkish population was ~40, and this number can be considered as a testimony of past settlements in Asia Minor.23 The most frequent thalassemia allele in the Turkish population is IVS-I-110 (G>A) mutation (40%), being the most common thalassemia mutation in the majority of the high risk regions of the Mediterranean area. The other common mutations in Turkey are: IVS-1-6(T>C), FSC-8(-AA), IVS-1-1(G>A), IVS-2-745(G>A), Cd39 (C>T), -30 (T>A) and FSC-5 (-CT). Malarial selection for the oldest β-thalassemia allele in Anatolia (i.e., IVS-1-110 G>A) may have occurred between 6,500 and 2,000 BC. Since then, Arab, Byzantine, Sumerian, Hittite and Turkish societies have played an important role in the history of the country, leading to a complex ethnic structure.
Currently, the minority of the population living in Adana and Mersin, in southern Turkey, is of Arab and African origin, and are known as “Eti-Turks”, whose ancestors immigrated from Syria and Egypt centuries ago. From that date on, most of the common β-thalassemia mutations in Turkey were established, and by the 13th century A.D., most of them were present at frequencies close to those7% observed today.25,26 Turkey’s large molecular heterogeneity can be explained by its unique geographical position and rich history, an important crossroad between cultures, civilizations, and continents for several centuries.
The three most prevalent mutations include: IVS-1-110 (G>A): 39.5%, IVS -1-6 (T>C):10.1% and FSC -8 (-AA): 5.5%.
5. Arab populations
The standard definition of the Arab world includes the 22 states and territories from the Atlantic Ocean in the west to the Arabian Sea in the east, and from the Mediterranean Sea in the north to the Horn of Africa and the Indian Ocean in the southeast. It has a combined population of around 350 million people, one third of who are under 15 years of age. β-thalassemia is encountered in polymorphic frequencies in almost all Arab countries with carrier rates ranging from 1 to 11%.27
Various papers reporting the spectrum of β-thalassemia mutations in different Arab countries have been published.28–46 However, data for some countries is still lacking or only preliminary.
The spectrum of β-thalassemia mutations in the Arab populations has been extensively reported by Zahed in 2001,41 Tadmouri in 200342 Haj Khelil in 201040 and Hamamy in 2013.27
The number of mutations detected in each population varies depending on its origin and interaction with other populations as well as the methods used for characterisation.27–43
Among Arabs, the heterogeneity of these mutations varies from 44 different mutations in UAE to 10 in Eastern Saudi Arabia. The most widespread and common mutation among Arabs is IVS-1-110 (G>A). The latter mutation has its highest prevalence in Cyprus and Greece suggesting that it may be of Greek origin. In the Eastern Arabian Peninsula, the Asian Indian mutations (IVS-1-5 (G>C), codons 8/9 (+G) and IVS-1 (−25 bp del) are more common.27
Although β-thalassemia mutations reported are mainly of Mediterranean and Asian origin, some countries have unique mutations, e.g. Hb Dhofar in Oman.29 Codon 39 (C>T) has been found in all Arab countries without exception. IVSI-110 (G>A), the most common mutation, has rates of 12%–38% in Arab countries of the eastern Mediterranean but reaches lower frequencies in countries around the Gulf (0%–2%). IVS-2-1 (G>A) has been detected in all countries except Tunisia and Algeria. The highest frequency has been reported in North Jordan (20%) (IVS-2-1)43 and in Kuwait.44
The most widespread and common mutations are presumably the oldest. Codon 39 (C>T) is believed to be of Roman origin, and IVS-1-110 is believed to have arisen in Turkey. It reaches its highest frequency in the Eastern Mediterranean Arab countries and may have been introduced to other countries by a variety of settlers from the East, including Turks, Greeks, or Phoenicians.41
a. Egypt
Egypt is divided into three main geographical regions: the Nile Valley, the Eastern desert and the Western desert. The Nile Valley represents 4% of the area of Egypt and is divided into Upper Egypt, Lower Egypt, Suez Canal, and Northern coast lakes regions.
Cairo’s population has risen to more than 18 million (the highest population density in Egypt).
Egyptians are mainly descended from ancient Egyptians (94%). Ethnic minorities in Egypt include Nubians, Berbers, Bedouin Arabs, Beja and Dome (4%) and others (2%). The position of Egypt in the center of the Middle East, contiguous with the Mediterranean countries, has facilitated genetic admixture of Egyptians with several populations of diverse geographic and ethnic origins.35
El-Hashemite et al. reported that of 1.5 million annual live births, approximately 1000 babies are born with β-thalassemia major. The most common mutations in Egyptian children with β-thalassemia are IVS-1-110(G>A) 48%, IVS-1-6 (T>C) 40%, IVS-1-1(G>A) 24%, IVS-1-5(G>C) 10%, IVS-2-848 (C>A) 9%, IVS-2-745(C>G) 8%, IVS-2-1(G>A) 7%.35
b. Oman
The nature of the β- thalassaemia mutations that have been determined in the Omani population suggests that the majority have been introduced by gene flow. There was active commerce between Oman and the area around the Indus Valley for centuries. Burial sites and pottery dating to around 3,000 B.C. have also been unearthed in the surrounding Gulf countries. Using archaeological data from all these various sites it has been possible to establish that there was trade between Mesopotamia (present day Iraq), the lands bordering the Arabian Gulf and the Indus Valley peoples. Historically, Oman was a major maritime nation with links to the Far East and India on the one hand and East Africa and Egypt on the other, as well as trade in the Arabian Gulf. IVS-1-5 (G>C), a severe β+ allele, is the most prevalent of the mutations thus far described in Oman. A detailed analysis of the distribution of β-thalassaemia mutations in Pakistan shows that the IVS I-5 (G>C) mutation is widespread in that country, reaching a peak incidence in the Baluchis (76.2%) and having a high prevalence among the Sindhis (43.9%). It is also found at a high frequency in India.27,29
Other common β-thalassemia alleles common in Oman are the codon 44 (-C) (frequent in Iraq),37 IVS-1-3′ –25bp (epicentre Bahrain),34 IVS-2-1 (G>A) (Indian subcontinent)47 mutations, and the 619bp deletion at the 3′ end of the beta globin gene (Indian subcontinent),47 all of which almost certainly were introduced to Oman through gene flow. The only mutation unique to Oman is Hb Dhofar, which has originated in the southern region of the Sultanate and thus far has only been described in Omanis.29
c. Qatar
Qatar is a peninsula bordering the Arabian Gulf and Saudi Arabia. The country’s population has been roughly split with 20% native Qatari, largely tribal, and 25% other Arabs from Egypt, Syria, Iraq, Lebanon, Yemen, Palestine, and Jordan. The rest of the population (55%) consists of expatriate workers from the East and the West.
Qatar’s history is very rich indicating the various phases that led to the ultimate development of the present State. The first trace of human settlements was found in the Qatar peninsula around 4,000 B.C. At the beginning of the 16th century, Qatar fell under the control of the Portuguese who were successful in establishing their control in many parts of the Arabian Peninsula. Later, the Ottomans ruled Qatar for four centuries.
The frequency of β-thalassemia heterozygotes is estimated to be 2–3%. Al-Obaidli et al. analyzed the molecular basis of β-thalassemia in Qatar. They found the most common mutant alleles were IVS-1-5 (G>C) and codon 8/9 (+G), representing 35.4% and 26.1% of the total, respectively. Most of these two mutations are homozygous, probably because of the high rate of consanguinity. The frequencies of three other common mutant alleles, IVS-2-1 (G>A), 25bp deletion and IVS-1-110 (G>A) are higher in surrounding locations, such as Southern Iran, Kuwait, eastern Saudi Arabia and Bahrain.38
Recently, a novel β-thalassemia deletional variant allele in an ethnic Qatari patient was reported. The deletion spans exon 1, the entire intron 1 and the first two bases of exon 2 causing a frameshift and the premature appearance of a stop codon. The presence of this novel deletional allele in a compound heterozygous state with a non-deletional allele is alarming in a diagnostic setting, especially in the absence of family studies.39
6. Iran
Iran, centrally located in the Asian continent, has served for centuries as a gateway for movement of human populations across diverse spheres of Asia and Europe. The Iranian population consists of diverse ethnic and linguistic groups namely Arabs, Armenians, Assyrians, Azeris, Baluchis, Gilaks, Mazandarani, Kurds, Lurs, Persian, Turkmen, and Zoroastrians.
In the eastern Mediterranean region, Iran is one of the major centers for the prevalence of β-thalassemia. Due to the high consanguinity in the population, it is estimated that there are more than three million β-thalassemia carriers (4–8%) and 20,000 patients.
The gene frequency of β-thalassemia is high and varies considerably between areas, with double the country average rate in Mazandaran, Sistan and Baluchistan, Fars, Hormozgan, and Kerman provinces and half the average country rate in provinces of Tehran, East Azerbaijan, Khorasan, Hamedan, Yazd and West Azerbaijan. There are more than 47 different β-globin gene mutations. Among them, the most common is IVS-2-1 (G > A; beta0) mutation, followed in decreasing order of frequency by IVS-1-5 (G > C, beta+), codons 8/9 (+G, beta0), IVS-1-110 (G > A, beta+), IVS-1-1(G > A, beta0), 25 bp deletion (beta0), IVS-I-6 (T > C, beta+), codon 5 (-CT, beta0), and codon 39 (C >T, beta0) mutations.46 It seems that codons 8/9 (+G) might reflect two historical events: one connected to the secular trade along the great Silk Road which extends from Xian in China through the Indian subcontinent to Iran and the Eastern Mediterranean and the other to the invasion of the Mongols (1,220 A.D.) and the Tatars (1,380–87 A.D.). The prevalence of codon 8/9 (+G) in the Northeast of Iran, and toward the center and northwest, may be related to the Oghuz Turks who migrated there from central Asia.48
From extensive studies on the geographical distribution of the molecular basis of β-thalassemia, it has been shown that, despite the wide spectrum of mutations, the number of prevalent molecular defects for each population is limited.
The information provided in Table 1 is derived from published reports. The reported data often do not represent the entire population. Therefore, each single publication should be judged and used according to its merits, including sample size, the population group studied, geographical location within a country, and methodology used. A complete updated list of β-thalassemia variants is available through the Globin Gene Server Web Site (http://www.globin.cse.psu.edu).
Table 1.
The distribution of β-thalassemia variants in other countries.
| Country | Mutations | References |
|---|---|---|
| Eastern Province of Saudi Arabia | IVS-2-1 (G>A) (27.5%); IVS-1-5 (G>C) (23.2%); codon 39 (C>T) (20.3%); IVS-1-1 (G> A) (5.8%); IVS-1-25 bp (4.4%) and codon 44(-C) | Al-Ali AK et al. J Biomed Biotechnol. 2005:4:322–5 |
| Iraq | IVS-2-1 (G>A) (28.7%); IVS-1-1(G>A) (17.7%); codon 8 (–AA) (9.1%); codon 8/9 (9.1%); codon 39 (C>T) (9.1%); codon 44 (-C) (8.3%) and codon 5 (-C) (6.3%) | Al-Allawi NA et al. Mol Biol Int. 2010; Article ID 479282 |
| Jordan | IVS-1-110 (G>A) (25%), IVS-2-1 (G>A) (15%), IVS2-745 (C>G) (14.2%), IVS-1-1 (G>A) (10%), IVS-1-6 (T>C) (8.3%), codon 37 (G>A) (6.3%), codon 39 (C>T) (4.6%), and codon 5 (-C) (3.8%) | Sadiq MF et al. Am J Hematol. 2001;68:16–22. |
| Kuwait | IVS-2-1 G>A and IVS-1-6 T>C accounted for 63.9% of all mutations. | Adekile A et al. Med Princ Pract 2005;14(suppl 1):69–72 |
| Morocco | β0 39 (C>T); β0 Fs CD 8 (–AA); β+ IVS-1-6 (T>C) and β0 IVS-1-1(G>A); β0 FsCD6 (–A) and β+ 29 (A>G) cap site account for 75% of the 86 independent β thal chromosomes studied | Lemsaddek W et al. Am J Hematol. 2003; 73:161–8 |
| Syria | IVS-1-110 (G>A) (17.0%), IVS-1-1 (G>A) (14.7%), codon 39 (C>T) (14.4%), IVS-2-1 (G>A) (9.8%), codon 8 (-AA) (6.2%), IVS-1-6 (T>C) (5.2%), IVS-1-5 (G>C) (4.9%) | Jarjour RA et al. Hemoglobin. 2014;38:272–6 |
| Azerbaijan | Three mutations (codon 8-AA, IVS-2-1(G>A) and IVS-1-110 (G>A) account for over 80% of thalassaemia genes | Kuliev AM et al. J Med Genet.1994;31: 209–12. |
| Bulgaria | The codon 39 (C>T) and IVS-1-110 (G>A) mutations occur most frequently, and seven additional mutations are observed with a frequency from 2.4% to 14.2% | Petkov GH and Efremov GD. Hemoglobin. 2007;31:225–32. |
| Lebanon | IVS-1-110 (G>A) (34.2%); IVS-1-1 (G>A) (15%); IVS-1-6 (T>C) (14.4%); cd 29 (C>T) (9.6%); IVS-2-1 (G>A) (8.6%) and cd 5 (–CT) (5%) | Makhoul NJ et al. Ann Hum Genet.2005; 69:55–66 |
| Romania | IVS-1-110 (G>A) (31.2%); cd 39 (C>T) (25%); IVS-2-745 (C>G) (15.6%); IVS-1-1 (G>A) (12.5%) | Talnaci R et al. J. Cell Mol Med. 2004; 8: 232–40 |
| Serbia and Montenegro | Codon 39 (C>T), IVS-1-110 (G>A), IVS-2-745 (C>G), codon 44 (-C), -87 (C>G), IVS-2-1 (G>A), IVS-1-6 (T>C), IVS-1-1 (G>A) | Pavlovic S et al. Acta Haematol. 2005;113:175–80 |
| Spain | IVS-1-1 (G>A), IVS-1-6 (T>C), IVS-1-110 (G>A), codon 39 (C>T), codons 8/9 (+G) | Villegas A et al. Hemoglobin. 2001; 25: 273–83. |
The Phylogeography and Phytogeographic Brief History of People in Ancient Times
Genetic data on living human populations have been used to reconstruct the evolutionary history of the human species by considering how global patterns of human variation could be produced, given different evolutionary scenarios. Various processes (selection, mutation, migration and genetic drift) are known to determine the frequency of genetic diseases in human populations, but so far it has proved almost impossible to identify to what extent each process is responsible for the presence of a particular genetic disease.49–54
The most controversial issue at present is exactly when and how these anatomically and genetically modern populations first spread from Africa to other parts of Asia and Europe. There are two main possibilities. The first is that the initial expansion occurred via North Africa and the Nile valley, with subsequent dispersals to the west into Europe and to the east into Asia.50–54 The second is that the initial dispersal was from Ethiopia, across the mouth of the Red Sea, and then either northward through Arabia or eastward along the south Asian coastline to Australasia,.55,56 The strongest evidence at present for the second hypothesis is provided by the mtDNA lineage-analysis patterns.50,51 This model would mean that modern populations in southwest Asia and Europe must have reached these areas substantially later, via western or central Asia.51
Why did modern human populations disperse from Africa ca. 60,000 years ago?
Childe57 suggested that, after the glaciations, North Africa and South-west Asia became drier and humans began to aggregate in areas where water was available.
The hypothesis of Childe on the role of the Younger Dryas (a geological period from c. 12,900 to c. 11,700 calendar years ago, characterized by a cool and dry climate) which was contemporary with the beginning of cultivation (c. 10,000 B.C.) in the region, is supported by recent studies from ancient sites of the Middle Euphrates region.58–61
These suggest that the world’s first civilization emerged in the late fourth millennium B.C. in southern Iraq, developing urban centers situated along the banks of the Tigris and Euphrates Rivers (Mesopotamia). This area, a small region of southeast Turkey and north-east Syria around the Middle Euphrates, might be the cradle of wheat agricultural innovation.62–66
After domestication, free-threshing wheat spread west through the Mediterranean basin, reaching its western edge by 5,000 B.C.67,68. Between 2,000 B.C. and 1,100 A.D., Anatolia served as the ground for several civilizations (Hittite, Persian, Greek, Roman, Byzantine, and Seljuk), resulting in an intensive flow of mutations into and out of present-day Turkey. Before reaching the Western Europe, agriculture spread in Greece during 4th millennium B.C.; it is possible that during that period Greece was one of the most malarious regions of the Mediterranean.69,70
During the 8th century B.C. the Greeks arrived in Italy. They came from Euboea, Argolis, Locris, Crete and the Aegean islands, settling on the southern coasts (from Campania to Apulia) and eastern and southern Sicily. They founded many prosperous colonies whose economy was generally based on agriculture and commerce.
It is possible that thalassemia existed among Sicily’s native populations (Lymians, Sicanians, and Sicels) before that time, as it is postulated that some of these earlier inhabitants migrated to Sicily from the eastern Mediterranean, albeit probably before 4,000 B.C. A further historical influx of the thalassemia genes may be associated with mass immigration of the Saracens (Arabs) in the ninth and tenth centuries, as this population had a strong Middle-eastern Semitic component.
Silvestroni and Bianco recorded a very high percentage of β-thalassemia trait in people originating from the area of the Po River’s delta (Upper Adriatic): 10% in Ferrara, 16% in Codigoro and 12% in Pomposa. These towns in the past were in direct connection to the important Greek emporium of Spina through an ancient branch of the Po River.71 Spina was a commercial port founded at the beginning of the 6th century B.C. by Etruscans. Because of its location, along the Po River delta, the port became an ideal community to facilitate trade with the Greeks. The port was abandoned towards the end of the 3rd century B.C.71
The expansion of the Ottoman Empire (15th and 16th centuries) towards Eastern Europe, Northern Africa, and Central Asia permitted further spread of β-thalassemia mutations in and out of Anatolia making it a melting-pot of a large number of alleles. The migration of many Muslim groups living in former Turkish territories in Southeastern Europe during the decline of the Ottoman Empire (starting from 1914 AD) contributed more to the racial mixture (i.e., the introduction of IVS-2-745, -87, IVS-2-654).24–26
Factors Influencing the Global Distribution of Thalassemias
Several other factors have contributed to the global distribution of thalassemias. These factors include:
1. The resistance to malaria
World-wide, the distribution of malaria and the common haemoglobinopathies have largely confirmed the close relationship between malaria and high prevalence of hemoglobinopathy traits (HbS and β-thalassemia) and G-6PD deficiency in populations living in highly malarious areas. Micro-population epidemiological surveys have established this association.72,73
In 1948, Haldane suggested that the heterozygote state in β-thalassaemia provided a selective advantage against attack by the malarial parasite.74 This theory was based on the fact that homozygous beta thalassaemia results in a lethal condition, whereas the heterozygote only has a mild microcytic anaemia. If the carrier had no selective advantage over the normal or was less fit, then a very high mutation rate for beta thalassaemia would be required to secure equilibrium. Haldane felt that the heterozygote was fitter than normal, and he suggested that this might be due to the resistance of the microcytic erythrocytes to attack by the malaria parasite.74,75
It is not possible at this point to say exactly when malarial parasites evolved. Although there is no direct evidence as to the antiquity of malaria, there are many historical references to intermittent fevers and splenomegaly. Most authors believe that malaria was established in pre-historic times; it may have originated in tropical areas of the Old World, but the Pleistocene glaciations (130,000-10,000 years ago) delayed its spread in the Northern Hemisphere.76,77
Until about 3,000 – 4,000 years ago population groups were small and isolated and infection could not spread easily. In addition, the vector for malaria, Anopheles gambiae, breeds in stagnant water that does not generally occur in the unbroken tropical rain forest. It is only when forest is cut down to make way for agriculture that these stagnant pools are found. The spread of malaria probably evolved with the development of tools during the Neolithic period (~6,000 B.C.–1,000 B.C.), which has traditionally been associated with the origins of farming and more settled human groups. It is possible that the infection then spread to most of the tropical and much of the temperate regions of the world.
Genetic variants that affect the structure and production of the β- or α-chains of haemoglobin are variously associated with protection from a range of clinical manifestations of P. falciparum infection. The degree of protection varies between haemoglobinopathies, but in general is greatest against severe malaria, moderate against uncomplicated malaria, and absent against asymptomatic P. falciparum parasitaemia.78–83 HbS and to a lesser extent HbC protect from malaria but not from parasitaemia, suggesting that these haemoglobin variants prevent the transition from asymptomatic parasitaemia to malaria. This protective effect may derive from the abnormal display of parasite virulence factors on the surface of parasitized HbC and HbS erythrocytes,78,79 possibly owing to the disruption of the parasite’s remodeling of erythrocyte’s intracellular trafficking network by HbS and HbC.80 Additionally, the age-dependent nature of malaria protection due to HbAS81,82 and α-thalassaemia83 among children in recent reports support a protective mechanism based upon an enhanced acquisition of malaria immunity.
Additional possible mechanisms for protection due to haemoglobinopathies include an enhanced clearance of parasitized erythrocytes,84 impaired parasite growth,85 or the induction of protective immunomodulatory mechanisms by parasitized erythrocytes.86
2. Consanguinity
The roots of Western attitudes toward consanguinity extend back over 1,500 years. Consanguineous marriage is especially common throughout the Eastern Mediterranean, Anatolia, North Africa and the Indian subcontinent, where 25–70% of unions involve related family members. Religious, cultural and economic factors are commonly perceived reasons for such marriage. As a consequence, at least 8.4% of the world’s children have related parents. The practice is also accepted in South America and parts of sub-Saharan Africa. The highest overall prevalence of consanguineous unions is in poor rural communities, which are typified by low levels of maternal education, early age at marriage and first birth, short birth intervals, and longer reproductive spans.87–89
3. Nutrition and infections
Another important factor is the diseases’ epidemiological transition. Because social and public health standards have gradually improved in developing countries, nutritional and infectious diseases are less frequent causes of mortality compared to the past.1,90,91
Furthermore, despite premarital screening and prenatal diagnosis, sociocultural challenges (traditional customs, religious beliefs or superstition) especially in countries with high thalassemia trait populations, still impact on β-thalassaemia major birth rates and global distribution of thalassemia.91
4. Migration
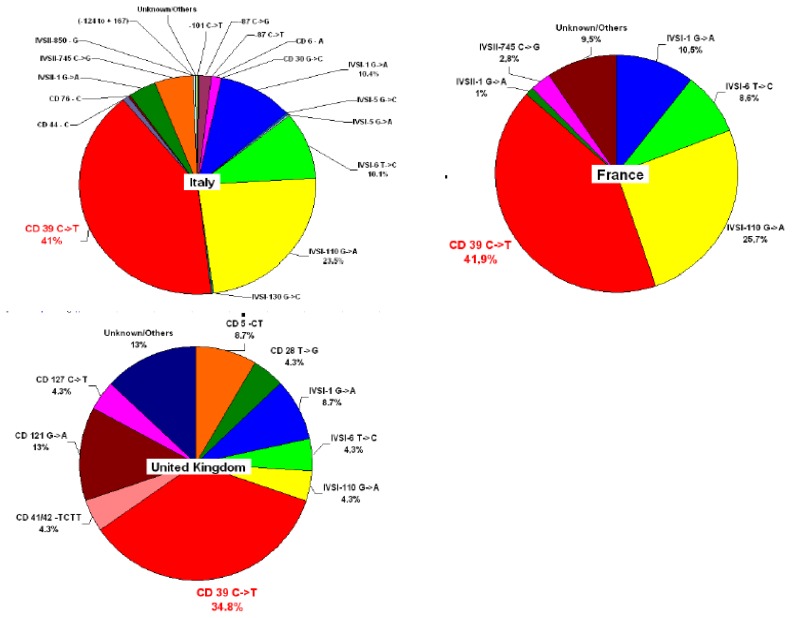
The consistent multi-ethnic migrations of the last decades have considerably changed the epidemiology of hemoglobinopathies (Figure 1). Healthy carriers of these conditions are living today in many nonendemic countries, and severely affected children are now born in areas where these diseases were previously rare or unknown. In view of population migration, the distribution of the thalassemia gene appears to be worldwide affecting all ethnic groups except those originating from western, central and north Europe. The number of patients with haemoglobinopathies has been estimated to be almost 44,000 in the 10 EU countries, varying from 150 cases in Sweden to 10,500 in France.92
Figure 1.
Heterogeneity of β–thalassemia mutations related to recent migration in France and the United Kingdom compared to Italy. The prevalence of the most common mutation in the country is shown in red (Based on Galanello R, Eleftheriou A, Trager-Synodinos J, Old J, Petrou M, Angastiniotis M. Prevention of thalassaemias and other haemoglobin disorders. Thalassemia International Federation [TIF] Publications. Vol 1, 2003; by courtesy of TIF)
Since β-thalassemia major has increased markedly in some European countries due to immigration from Africa and Asia, therefore national programmes of care and prevention are needed. Furthermore, major health organizations and funding agencies must support these initiatives.
5. Prevention
In the late 1970s, pilot programs directed to prevent β-thalassemia major started in several at-risk populations in the Mediterranean area. At present, several countries have set up comprehensive national prevention programs, which include public awareness and education, carrier screening, and counseling, as well as information on prenatal diagnosis and preimplantation diagnosis.3 The efficacy of the prevention programmes in Italy, Cyprus, and Greece is reflected by the fact that the number of babies with thalassemia major has decreased substantially in the last two decades. In Sardinia, for instance, the number of thalassemia major children born per year predicted on the basis of the carrier rate, assuming random mating, shows a reduction from 1:250 live births to 1:1660 in 2009 with an effective prevention of 85% of the cases.3 In Greece, the expected number of 160 annual births of affected newborns per 100,000 live birth decreased to 8 during 2005 to 2009.5
Archeological Remains Suggestive of Hereditary Anemias
Besides malaria, the evolution of β-thalassemia has been studied from two other perspectives: macroscopic examination of archeological skeletal remains on the basis of bone pathology, and analysis of distribution of mutations in various living populations.
Angel analysed 2334 skeletal specimens coming from several archaeological sites in the Eastern Mediterranean (in particular from Turkey, Greece, Cyprus, and Morocco), belonging to the Upper Paleolithic period to Middle Ages. He used bone modifications, in particular, the porotic hyperostosis of the skull, as a marker of thalassemia trait and on this basis, started researching the appearance and diffusion of malaria and thalassemia. Porotic hyperostosis was classified as a thickening of the cranial vault caused by expansion of the diploë due to anaemia usually accompanied by porosity on the external cranial vault (cribra cranii) and often on the orbital roof (cribra orbitalia).93 Changes can be observed in both adults and older children and may occur in an active or healed condition. Healed and activecribra orbitalia differ by size of the affected bone tissue, size of the perforating lesions and thickness of the porous bone.
An infant skull (2–4 months old) with porotic hyperostosis was found in a cemetery in the old city of Nicosia between two churches of the late Byzantine and early Frankish period (around 1,200 A.D.) (Angastiniotis et al., unpublished data). The researchers tried to isolate DNA and to identify mutations but even though fragmented DNA was isolated no mutations were found.
Several other documented skeletal remains with bone pathology suggestive of β-thalassemia were discovered in various prehistoric sites of the world in areas such as Greece, Albania, Australia,94 the Middle East95 and Southeast Asia.96
It is now it is known that porotic hyperostosis is not a characteristic mark of β-thalassemia, as Angel thought, but is an unspecific indicator of several diseases such as megaloblastic anaemia, deficiency of vitamin C and B12 and chronic iron-deficiency anemia. These difficulties are characteristic of all morphological skeletal diagnosis of ancient diseases.97–99
Rabino Massa et al. examined samples from the Marro Collection, belonging to the Anthropological and Ethnographic Museum of Turin, to determine the presence of malaria antigens. They assessed the specimens belonging to predynastic mummies (3,200 B.C.) from Gebelen excluding only the poorly conserved mummies. They analyzed about fifty of the 85 individuals of this collection. In these Egyptian mummies, the authors detected the presence of malaria.100
The existence of a hemolytic disorder was observed in histological preparations of one individual, indicating pathological variations of hemoglobin. Radiographic examination confirmed the existence of hemoglobinopathy: in some mummified heads, there was the classical presence of “brush skulls”.101
Although skeletal changes are important additional findings for some clinical assessments, they cannot be used alone for diagnosis. Many other criteria have to be used for accurate diagnosis. Most recently, a variety of chemical methods has been used to study human bones at the molecular level including DNA analysis.
In 1995, Filon et al. presented the first direct proof of the occurrence of an inherited anemia in the archeological remains of a child, eight years old, with severe bone pathology consistent with β-thalassemia major. The remains came from an Israeli archaeological site, Akhziv, thought to date to the Ottoman period, sometime between the 16th and the 19th centuries. DNA analysis has shown that the child was homozygous for the frameshift mutation at codon 8 (-AA) as well as the variant (C-T) in the second codon of the β-globin gene.102
Unfortunately, quantitation of aDNA templates was not carried out in this analysis. Quantitation of aDNA should be carried out in aDNA studies to estimate the authenticity of aDNA sequences and the risk of contamination with modern DNA.103,104 If amounts of the aDNA templates are insufficient, then it is possible that PCR amplifications may have preferentially amplified one specific allele (mutant codon 8) over another (normal codon 8), resulting in homozygous sequences.
Conclusions
Ancient literature alludes to conditions which appear to have been caused by the thalassemias, but infant mortality was so high (from this and other causes), and so little was known about hemolytic disorders that the true causes of the thalassemic syndromes were only determined in the second half of the 20th century, with a better knowledge of genetics.
Before the advent of agriculture, roughly 5,000 years ago, it is unlikely that humans were exposed to large malaria outbreaks and that this stage was only reached whenever Neolithic (early) farmers settled in mosquito-infested soft and marshy soil near standing water. In these surroundings young carriers of the thalassemias (α and β) had better chances than normal to survive malaria during infancy, and were able to reproduce and pass the trait to the next generation, thus increasing the gene frequency. The higher and varying distribution of certain hemoglobin disorders in different populations may also reflect the high frequency of consanguineous marriages,105 mass migration, improvement of nutritional status, prevention and treatment of infectious diseases, settlements and the strong constitutions of inhabitants.96–98
Considering the current distribution of β-thalassemia and the diversity of mutation patterns, it is unlikely that β-thalassemia originated from a single place and time and then spread throughout the rest of the malaria belt. In fact, the contiguous distribution of β-thalassemia, as well as other β-globin gene mutations in localized areas of the world, had led to the conclusion that these mutations had emerged even before malaria became an important selective factor.
Because hemoglobinopathies are endemic or have expanded following mobility flows, they are present in all European countries creating an important impact on health services99 that has not yet been effectively addressed by Member States Health authorities. Recent surveys suggest that between 300,000 and 400,000 babies are born with a serious hemoglobin disorder each year (23,000 babies with β-thalassemia major) and that up to 90% of these births occur in low- or middle-income countries.1–6
The global distribution of the various globin gene mutations poses a number of interesting and important conundrums. Nowadays, a suitable strategy for identifying the β-globin mutations calls for the knowledge of the frequency of mutations, common or rare, in the ethnic group of the individuals.
The chronicity of the disease and the high cost of life-long treatment make prevention strategies crucial in the management of this disease. This requires intervention in high risk/targeted populations. Thanks to the implementation of massive prevention programs, mainly for thalassemia, in several countries the incidence of the disease has been reduced substantially, and in some areas, the annual rate of affected births has decreased by more than 90%.105–107
Footnotes
Competing interests: The authors have declared that no competing interests exist.
References
- 1.Weatherall DJ, Clegg JB. The thalassaemia syndromes. 4th Edition. Oxford: Blackwell Science Ltd; 2001. 2001. https://doi.org/10.1002/9780470696705. [Google Scholar]
- 2.Thein SL. The molecular basis of β-thalassemia. Cold Spring Harb Perspect Med. 2013 May 1;3(5):a011700. doi: 10.1101/cshperspect.a011700. https://doi.org/10.1101/cshperspect.a011700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cao A, Kan YW. The prevention of thalassemia. Cold Spring Harb Perspect Med. 2013 Feb 1;3(2):a011775. doi: 10.1101/cshperspect.a011775. https://doi.org/10.1101/cshperspect.a011775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kountouris P, Lederer CW, Fanis P, Feleki X, Old J, Kleanthous M. IthaGenes: an interactive database for haemoglobin variations and epidemiology. PLoS One. 2014 Jul 24;9(7):e103020. doi: 10.1371/journal.pone.0103020. eCollection 2014 https://doi.org/10.1371/journal.pone.0103020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ladis V, Kaagiorga–Langana M, Chouliaras Tsiarta I. Thirty-year experience in preventing hemoglobinopathies in Greece: achievements and potentials for optimisations. Eur J Haematol. 2013;90:313–322. doi: 10.1111/ejh.12076. https://doi.org/10.1111/ejh.12076. [DOI] [PubMed] [Google Scholar]
- 6.Williams TN, Weatherall DJ. World distribution, population genetics, and health burden of the hemoglobinopathies. Cold Spring Harb Perspect Med. 2012 Sep 1;2(9):a011692. doi: 10.1101/cshperspect.a011692. https://doi.org/10.1101/cshperspect.a011692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Basak AN. The molecular pathology of beta-thalassemia in Turkey: the Bogaziçi University experience. Hemoglobin. 2007;31:233–241. doi: 10.1080/03630260701296735. https://doi.org/10.1080/03630260701296735. [DOI] [PubMed] [Google Scholar]
- 8.Chehab FF, Der Kaloustian V, Khouri FP, Deeb SS, Kan YW. The molecular basis of beta-thalassemia in Lebanon: application to prenatal diagnosis. Blood. 1987;69:1141–1145. [PubMed] [Google Scholar]
- 9.El-Hazmi MA, al-Swailem AR, Warsy AS. Molecular defects in beta-thalassaemias in the population of Saudi Arabia. Hum Hered. 1995;45:278–285. doi: 10.1159/000154314. https://doi.org/10.1159/000154314. [DOI] [PubMed] [Google Scholar]
- 10.Zhong Chen L, Easteal S, Board PG. Evolution of the β-globin haplotypes in human populations. Mol Biol Evol. 1990;7:423–437. doi: 10.1093/oxfordjournals.molbev.a040613. [DOI] [PubMed] [Google Scholar]
- 11.Del Senno L, Pirastu M, Barbieri R, Bernardi F, Buzzoni D, Marchetti G, Perrotta C, Vullo C, Kan YW, Conconi F. beta (+)-Thalassaemia in the Po River Delta region (northern Italy): genotype and beta globin synthesis. J Med Genet. 1985;22:54–58. doi: 10.1136/jmg.22.1.54. https://doi.org/10.1136/jmg.22.1.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pirastu M, Saglio G, Camaschella C, Loi A, Serra A, Bertero T, Gabutti W, Cao A. Delineation of specific beta-thalassemia mutations in high-risk areas of Italy: a prerequisite for prenatal diagnosis. Blood. 1987;71:983–988. [PubMed] [Google Scholar]
- 13.Amselem S, Nunes V, Vidaud M, Estivill X, Wong C, d’Auriol L, Vidaud D, Galibert F, Baiget M, Goossens M. Determination of the spectrum of beta-thalassemia genes in Spain by use of dot-blot analysis of amplified beta-globin DNA. Am J Hum Genet. 1988;43:95–100. [PMC free article] [PubMed] [Google Scholar]
- 14.Faustino P, Pacheco P, Loureiro P, Nogueira PJ, Lavinha J. The geographic pattern of beta-thalassaemia mutations in the Portuguese population. Br J Haematol. 1999;107:903–904. doi: 10.1046/j.1365-2141.1999.01821.x. https://doi.org/10.1046/j.1365-2141.1999.01821.x. [DOI] [PubMed] [Google Scholar]
- 15.Fattoum S, Guemira F, Oner C, Li HW, Kutlar F, Huisman TH. Beta-thalassemia, HB S-beta-thalassemia and sickle cell anemia among Tunisians. Hemoglobin. 1991;15:11–21. doi: 10.3109/03630269109072481. https://doi.org/10.3109/03630269109072481. [DOI] [PubMed] [Google Scholar]
- 16.Falchi A, Giovannoni L, Vacca L, Latini V, Vona G, Varesi L. β-globin gene cluster haplotypes associated with beta-thalassemia on Corsica island. Am J Hematol. 2005;78:27–32. doi: 10.1002/ajh.20199. https://doi.org/10.1002/ajh.20199. [DOI] [PubMed] [Google Scholar]
- 17.Maggio A, Giambona A, Cai SP, Wall J, Kan YW, Chehab FF. Rapid and simultaneous typing of hemoglobin S, hemoglobin C, and seven Mediterranean beta-thalassemia mutations by covalent reverse dot-blot analysis: application to prenatal diagnosis in Sicily. Blood. 1993;81:239–242. [PubMed] [Google Scholar]
- 18.Kountouris P, Kousiappa I, Papasavva T, Christopoulos G, Pavlou E, Petrou M, Feleki X, Karitzie E, Phylactides M, Fanis P, Lederer CW, Kyrri AR, Kalogerou E, Makariou C, Ioannou C, Kythreotis L, Hadjilambi G, Andreou N, Pangalou E, Savvidou I, Angastiniotis M, Hadjigavriel M, Sitarou M, Kolnagou A, Kleanthous M, Christou S. The molecular spectrum and distribution of haemoglobinopathies in Cyprus: a 20-year retrospective study. Sci Rep. 2016 May 20;6:26371. doi: 10.1038/srep26371. https://doi.org/10.1038/srep26371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Baysal E, Indrak K, Bozkurt G, Berkalp A, Aritkan E, Old J, Ioannou P, Angastiniotis M, Droushiotou A, Yüregir GT. The beta thalassaemia mutations in the population of Cyprus. Br J Haematol. 1992;81:607–609. doi: 10.1111/j.1365-2141.1992.tb03000.x. https://doi.org/10.1111/j.1365-2141.1992.tb03000.x. [DOI] [PubMed] [Google Scholar]
- 20.Kyrri AR, Felekis X, Kalogerou E, Wild BJ, Kythreotis L, Phylactides M, Kleanthous M. Hemoglobin variants in Cyprus. Hemoglobin. 2009;33:81–94. doi: 10.1080/03630260902813502. https://doi.org/10.1080/03630260902813502. [DOI] [PubMed] [Google Scholar]
- 21.Malamos B, Fessas Ph, Stamatoyiannopoulos G. Types of thalassemia trait carriers, as revealed by a study of their incidence in Greece. Br J Haemat. 1962;8:5–14. doi: 10.1111/j.1365-2141.1962.tb06489.x. https://doi.org/10.1111/j.1365-2141.1962.tb06489.x. [DOI] [PubMed] [Google Scholar]
- 22.Loukopoulos D. Current status of thalassemia and sickle cell syndromes in Greece. Sem Hematol. 1996;33:76–86. [PubMed] [Google Scholar]
- 23.Kattamis C, Hu H, Cheng G, Reese J, Gonzalez-Redondo M, Kutlar A, Kutlar F, Huisman THJ. Molecular characterization of β–thalassemia in 174 Greek patients with thalassemia major. Br J Haem. 1990;74:342–346. doi: 10.1111/j.1365-2141.1990.tb02593.x. https://doi.org/10.1111/j.1365-2141.1990.tb02593.x. [DOI] [PubMed] [Google Scholar]
- 24.Canatan D. Thalassemias and hemoglobinopathies in Turkey. Hemoglobin. 2014;38:305–307. doi: 10.3109/03630269.2014.938163. https://doi.org/10.3109/03630269.2014.938163. [DOI] [PubMed] [Google Scholar]
- 25.Tadmouri GO, Garguier N, Demont J, Perrin P, Basak AN. History and origin of beta-thalassemia in Turkey: sequence haplotype diversity of beta-globin genes. Hum Biol. 2001;73:661–674. doi: 10.1353/hub.2001.0075. https://doi.org/10.1353/hub.2001.0075. [DOI] [PubMed] [Google Scholar]
- 26.Aksoy M, Ikin EW, Mourant AE, Lehmann H. Blood groups, haemoglobins, and thalassaemia in Turks in southern Turkey and Eti-Turks. Br Med J. 1958;2:937–939. doi: 10.1136/bmj.2.5102.937. https://doi.org/10.1136/bmj.2.5102.937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hamamy HA, Al-Allawi NA. Epidemiological profile of common haemoglobinopathies in Arab countries. J Community Genet. 2013;4:147–167. doi: 10.1007/s12687-012-0127-8. https://doi.org/10.1007/s12687-012-0127-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Agouti I, Badens C, Abouyoub A, Levy N, Bennani M. Molecular basis of beta-thalassemia in Morocco: possible origins of the molecular heterogeneity. Genet Test. 2008;12:563–568. doi: 10.1089/gte.2008.0058. https://doi.org/10.1089/gte.2008.0058. [DOI] [PubMed] [Google Scholar]
- 29.Daar S, Gravell D, Hussein HM, Pathare AV, Wali Y, Krishnamoorthy R. Haematological and clinical features of beta-thalassaemia associated with Hb Dhofar. Eur J Haematol. 2008;80:67–70. doi: 10.1111/j.1600-0609.2007.00989.x. https://doi.org/10.1111/j.1600-0609.2007.00989.x. [DOI] [PubMed] [Google Scholar]
- 30.De Leo R, Deidda G, Novelletto A, El-Kalla S, Mathews AR, Felicetti L. Analysis of β-thalassemia mutations in the United Arab Emirates provides evidence for recurrent origin of the IVSI nt 5 (G-C) mutation. Hum Mutat. 1995;5:327–328. doi: 10.1002/humu.1380050409. https://doi.org/10.1002/humu.1380050409. [DOI] [PubMed] [Google Scholar]
- 31.Kyriacou K, Al-Quobaili F, Pavlou E, Christopoulos G, Ioannou P, Kleanthous M. Molecular characterization of β-thalassemia in Syria. Hemoglobin. 2000;24:1–13. doi: 10.3109/03630260009002268. https://doi.org/10.3109/03630260009002268. [DOI] [PubMed] [Google Scholar]
- 32.Zahed L, Qatanani M, Nabulsi M, Taher A. β- thalassemia mutations and haplotype analysis in Lebanon. Hemoglobin. 2000;24:269–276. doi: 10.3109/03630260008993133. https://doi.org/10.3109/03630260008993133. [DOI] [PubMed] [Google Scholar]
- 33.Filon D, Oron V, Shawa R, Shawa R, Elborno E, Najjar K, Tulchinsky T, Rachmilewitz E, Rund D, Oppenheim A. Spectrum of β- thalassemia mutations in the Gaza area. Hum Mutat. 1995;5:351–353. doi: 10.1002/humu.1380050416. https://doi.org/10.1002/humu.1380050416. [DOI] [PubMed] [Google Scholar]
- 34.Jassim N, Merghoub T, Pascaud O, al Mukharraq H, Ducrocq R, Labie D, Elion J, Krishnamoorthy R, Arrayed SA. Molecular basis of β-thalassemia in Bahrain. Ann NY Acad Sci. 1998;850:407–409. doi: 10.1111/j.1749-6632.1998.tb10505.x. https://doi.org/10.1111/j.1749-6632.1998.tb10505.x. [DOI] [PubMed] [Google Scholar]
- 35.El-Hashemite N, Petrou M, Khalifa AS, Heshmat NM, Rady MS, Delhanty JD. Identification of novel Asian Indian and Japanese mutations causing β-thalassemia in Egyptian population. Hum Genet. 1997;99:271–274. doi: 10.1007/s004390050352. https://doi.org/10.1007/s004390050352. [DOI] [PubMed] [Google Scholar]
- 36.El-Shanshory M, Hagag A, Shebl S, Badria I, Abd Elhameed A, Abd El-Bar E, Al-Tonbary Y, Mansour A, Hassab H, Hamdy M, Alfy M, Sherief L, Sharaf E. Spectrum of beta globin gene mutations in Egyptian children with β-Thalassemia. Mediterr J Hematol Infect Dis. 2014 Nov 1;6(1):e2014071. doi: 10.4084/MJHID.2014.071. https://doi.org/10.4084/mjhid.2014.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Al-Allawi NA, Al-Mousawi BM, Badi AI, Jalal SD. The spectrum of β-thalassemia mutations in Baghdad, Central Iraq. Hemoglobin. 2013;37:444–453. doi: 10.3109/03630269.2013.810641. https://doi.org/10.3109/03630269.2013.810641. [DOI] [PubMed] [Google Scholar]
- 38.Al-Obaidli A, Hamodat M, Fawzi Z, Abu-Laban M, Gerard N, Krishnamoorthy R. Molecular basis of thalassemia in Qatar. Hemoglobin. 2007;31:121–127. doi: 10.1080/03630260701288815. https://doi.org/10.1080/03630260701288815. [DOI] [PubMed] [Google Scholar]
- 39.Al-Obaidli A, Gerard N, Al Zadjali S, Fawzi Z, Pravin S, Pathare A, Krishnamoorthy R. A novel deletional betathalassemic variant in an ethnic Qatari patient. Hemoglobin. 2009;33:214–219. doi: 10.1080/03630260903081398. https://doi.org/10.1080/03630260903081398. [DOI] [PubMed] [Google Scholar]
- 40.Haj Khelil A, Denden S, Leban N, Daimi H, Lakhdhar R, Lefranc G, Ben Chibani J, Perrin P. Hemoglobinopathies in North Africa: a review. Hemoglobin. 2010;34:1–23. doi: 10.3109/03630260903571286. https://doi.org/10.3109/03630260903571286. [DOI] [PubMed] [Google Scholar]
- 41.Zahed L. The spectrum of beta-thalassemia mutations in the Arab populations. J Biomed Biotechnol. 2001;1:129–132. doi: 10.1155/S1110724301000298. https://doi.org/10.1155/S1110724301000298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tadmouri GO, Gulen RI. Deniz: the electronic database for beta-thalassemia mutations in the Arab world. Saudi Med J. 2003;24:1192–1198. [PubMed] [Google Scholar]
- 43.Sadiq MFG, Huisman THJ. Molecular characterization of β-thalassemia in North Jordan. Hemoglobin. 1994;18:325–332. doi: 10.3109/03630269408996198. https://doi.org/10.3109/03630269408996198. [DOI] [PubMed] [Google Scholar]
- 44.Adekile AD, Gu LH, Baysal E, Haider MZ, al-Fuzae L, Aboobacker KC, al-Rashied A, Huisman TH. Molecular characterization of alpha thalassemia determinants, beta thalassemia alleles and beta S haplotypes among Kuwaiti Arabs. Acta Haematol. 1994;92:176–181. doi: 10.1159/000204216. https://doi.org/10.1159/000204216. [DOI] [PubMed] [Google Scholar]
- 45.Fattoum S, Guemira F, Oner C, Li HW, Kutlar F, Huisman TH. Beta-thalassemia, HbS-beta-thalassemia and sickle cell anemia among Tunisians. Hemoglobin. 1991;15:11–21. doi: 10.3109/03630269109072481. https://doi.org/10.3109/03630269109072481. [DOI] [PubMed] [Google Scholar]
- 46.Maryami F, Azarkeivan A, Fallah MS, Zeinali S. A large cohort study of genotype and phenotype correlations of beta-thalassemia in Iranian population. Int J Hematol Oncol Stem Cell Res. 2015;9:198–202. [PMC free article] [PubMed] [Google Scholar]
- 47.Varawalla NY, Fitches AC, Old JM. Analysis of beta-globin gene haplotypes in Asian Indians: origin and spread of beta-thalassaemia on the Indian subcontinent. Hum Genet. 1992;90:443–449. doi: 10.1007/BF00220475. https://doi.org/10.1007/BF00220475. [DOI] [PubMed] [Google Scholar]
- 48.Rezaee AR, Banoei MM, Khalili E, Houshmand M. Beta-Thalassemia in Iran: new insight into the role of genetic admixture and migration. Sc World J. 2012;2012:635183. doi: 10.1100/2012/635183. Epub 2012 Dec 18 https://doi.org/10.1100/2012/635183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Harcourt AH. Human phylogeography and diversity. Proc Natl Acad Sci USA. 2016;113:8072–8078. doi: 10.1073/pnas.1601068113. https://doi.org/10.1073/pnas.1601068113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lahr MM, Foley RA. Towards a theory of modern human origins: geography, demography, and diversity in recent human evolution. Am J Phys Anthropol. 1998;(Suppl 27):137–176. doi: 10.1002/(sici)1096-8644(1998)107:27+<137::aid-ajpa6>3.0.co;2-q. https://doi.org/10.1002/(SICI)1096-8644(1998)107:27+<137::AID-AJPA6>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 51.Liu H, Prugnolle F, Manica A, Balloux F. A geographically explicit genetic model of worldwide human-settlement history. Am J Hum Genet. 2006;79:230–237. doi: 10.1086/505436. https://doi.org/10.1086/505436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mellars P. Why did modern human populations disperse from Africa ca. 60,000 years ago? A new model. Proc Natl Acad Sci USA. 2006;103:9381–9386. doi: 10.1073/pnas.0510792103. https://doi.org/10.1073/pnas.0510792103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.O’Connell J, Allen J. Dating the colonization of the Sahul (Pleistocene Australia-New Guinea): A review of recent research. J Archaeol Sc USA. 2004;31:835–853. [Google Scholar]
- 54.Armitage SJ, Jasim SA, Marks AE, Parker AG, Usik VI, Uerpmann HP. The southern route “out of Africa”: evidence for an early expansion of modern humans into Arabia. Science. 2011;331:453–456. doi: 10.1126/science.1199113. https://doi.org/10.1126/science.1199113. [DOI] [PubMed] [Google Scholar]
- 55.Forster P, Matsumura S. Evolution. Did early humans go north or south? Science. 2005;308:965–966. doi: 10.1126/science.1113261. https://doi.org/10.1126/science.1113261. [DOI] [PubMed] [Google Scholar]
- 56.Stringer C. Palaeoanthropology. Coasting out of Africa. Nature. 2000;405:24–25. doi: 10.1038/35011166. https://doi.org/10.1038/35011166. [DOI] [PubMed] [Google Scholar]
- 57.Childe VG. New light on the most ancient east. 4th ed. London: Routledge and Paul; 1952. [Google Scholar]
- 58.Bar-Yosef O. On the nature of transitions: the middle to upper Palaeolithic and the Neolithic revolution. Cambridge Archaeol J. 1998;8:141–163. https://doi.org/10.1017/S0959774300001815. [Google Scholar]
- 59.Bar-Yosef O, Kislev M. Early farming communities in the Jordan Valley. In: Harris DR, Hillman GC, editors. Foraging and farming The evolution of plant exploitation. London: Unwin Hyman; 1989. pp. 633–642. [Google Scholar]
- 60.Zohary D, Hopf M, Weiss R. Domestication of plants in the old world. 4th ed. New York: Oxford University Press; 2012. pp. 1–264. https://doi.org/10.1093/acprof:osobl/9780199549061.003.0001. [Google Scholar]
- 61.Araus JL, Ferrio JP, Buxó R, Voltas J. The historical perspective of dryland agriculture: lessons learned from 10,000 years of wheat cultivation. J Exp Bot. 2007;58:131–145. doi: 10.1093/jxb/erl133. https://doi.org/10.1093/jxb/erl133. [DOI] [PubMed] [Google Scholar]
- 62.Salamini F, Ozkan H, Brandolini A, Schäfer-Pregl R, Martin W. Genetics and geography of wild cereal domestication in the near east. Nat Rev Genet. 2002;3:429–441. doi: 10.1038/nrg817. [DOI] [PubMed] [Google Scholar]
- 63.Kenoyer JM, Price TD, Burton JH. A new approach to tracking connections between the Indus Valley and Mesopotamia: initial results of strontium isotope analyses from Harappa and Ur. J Archaeol Sci. 2013;40:2286–2297. https://doi.org/10.1016/j.jas.2012.12.040. [Google Scholar]
- 64.Crawford HEW. Sumer and the Sumerians. Cambridge University Press; 2004. pp. 1–11. [Google Scholar]
- 65.Wiercinski A. Anthropology of ancient Mesopotamia. In: Braun PWN, editor. Mesopotamia. National Scientific Publishing; 1997. pp. 42–45. [Google Scholar]
- 66.Araus JL, Buxó R. Changes in carbon isotope discrimination in grain cereals from the north-western Mediterranean Basin during the past seven millenia. Aust J Plant Physiol. 1993;20:117–128. https://doi.org/10.1071/PP9930117. [Google Scholar]
- 67.Buxó R. Arqueologi’a de las plantas: la explotacion economica de las semillas y los frutos en el marco mediterraneo de la Peninsula Iberica. Barcelona: Critica, Grijalbo; 1998. pp. 1–367. [Google Scholar]
- 68.Feldman M. Origin of cultivated wheat. In: Bonjean AP, Angus WJ, editors. The world wheat book a history of wheat breeding. Paris: Lavoisier Publishing; 2001. pp. 3–57. [Google Scholar]
- 69.Iandola F. Tesi di Laurea. Fondazione De Marchi; La thalassemia dall’alba della civiltà occidentale ad oggi. www.demarchi.org/thalassemia.htm. [Google Scholar]
- 70.Grmek MD. Malaria in the Eastern Mediterranean in prehistory and antiquity. Parassitologia. 1994;36:1–6. [PubMed] [Google Scholar]
- 71.Silvestroni E, Bianco I, Alfieri N. Sulle origini della microcitemia in Italia e nelle altre regioni della terra. Medicina. 1952;2:187–216. [Google Scholar]
- 72.Choremis C, Fessas Ph, Kattamis C, Stamatoyiannopoulos G, Zannos-Mariolea L, Karaklis A, Belios G. Three inherited red-cell abnormalities in a district of Greece. Thalassemia, sickling and glucose-6-phosphate-dehydrogenase deficiency. Lancet. 1963;1:907–909. doi: 10.1016/s0140-6736(63)91688-x. https://doi.org/10.1016/S0140-6736(63)91688-X. [DOI] [PubMed] [Google Scholar]
- 73.Flint J, Harding RM, Boyce AJ, Clegg JB. The population genetics of the haemoglobinopathies. Baillieres Clin Haematol. 1998;11:1–51. doi: 10.1016/s0950-3536(98)80069-3. https://doi.org/10.1016/S0950-3536(98)80069-3. [DOI] [PubMed] [Google Scholar]
- 74.Haldane JBS. Disease and evolution. La Ricerca Scientifica. 1949;19:68–76. [Google Scholar]
- 75.Canali S. Researches on thalassemia and malaria in Italy and the origins of the “Haldane hypothesis”. Med Secoli. 2008;20:827–846. [PubMed] [Google Scholar]
- 76.Ratcliffe AW. The historical background of malaria; a reconsideration. J Indiana State Med Assoc. 1946;39:339–347. [PubMed] [Google Scholar]
- 77.Bruce-Chwatt LJ. Aleogenesis and paleo-epidemiology of primate malaria. Bull World Health Organ. 1965;32:363–387. [PMC free article] [PubMed] [Google Scholar]
- 78.Fairhurst RM, Baruch DI, Brittain NJ, Ostera GR, Wallach JS, Hoang HL, Hayton K, Guindo A, Makobongo MO, Schwartz OM, Tounkara A, Doumbo OK, Diallo DA, Fujioka H, Ho M, Wellems TE. Abnormal display of PfEMP-1 on erythrocytes carrying haemoglobin C may protect against malaria. Nature. 2005;435:1117–1121. doi: 10.1038/nature03631. https://doi.org/10.1038/nature03631. [DOI] [PubMed] [Google Scholar]
- 79.Cholera R, Brittain NJ, Gillrie MR, Lopera-Mesa TM, Diakité SA, Arie T, Krause MA, Guindo A, Tubman A, Fujioka H, Diallo DA, Doumbo OK, Ho M, Wellems TE, Fairhurst RM. Impaired cytoadherence of Plasmodium falciparum-infected erythrocytes containing sickle hemoglobin. Proc Natl Acad Sci U S A. 2008;105:991–996. doi: 10.1073/pnas.0711401105. https://doi.org/10.1073/pnas.0711401105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Cyrklaff M, Sanchez CP, Kilian N, Bisseye C, Simpore J, Frischknecht F, Lanzer M. Hemoglobins S and C Interfere with Actin Remodeling in Plasmodium falciparum Infected Erythrocytes. Science. 2011;334:1283–1286. doi: 10.1126/science.1213775. https://doi.org/10.1126/science.1213775. [DOI] [PubMed] [Google Scholar]
- 81.Williams TN, Mwangi TW, Roberts DJ, Alexander ND, Weatherall DJ, Wambua S, Kortok M, Snow RW, Marsh K. An immune basis for malaria protection by the sickle cell trait. PLoS Med. 2005 May;2(5):e128. doi: 10.1371/journal.pmed.0020128. https://doi.org/10.1371/journal.pmed.0020128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Gong L, Maiteki-Sebuguzi C, Rosenthal PJ, Hubbard AE, Drakeley CJ, Dorsey G, Greenhouse B. Evidence for both innate and acquired mechanisms of protection from Plasmodium falciparum in children with sickle cell trait. Blood. 2012 Apr 19;119(16):3808–14. doi: 10.1182/blood-2011-08-371062. https://doi.org/10.1182/blood-2011-08-371062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Veenemans J, Jansen EJ, Baidjoe AY, Mbugi EV, Demir AY, Kraaijenhagen RJ, Savelkoul HF, Verhoef H. Effect of alpha (+) -thalassaemia on episodes of fever due to malaria and other causes: a community-based cohort study in Tanzania. Malar J. 2011 Sep 22;10:280. doi: 10.1186/1475-2875-10-280. https://doi.org/10.1186/1475-2875-10-280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Tan X, Traore B, Kayentao K, Ongoiba A, Doumbo S, Waisberg M, Doumbo OK, Felgner PL, Fairhurst RM, Crompton PD. Hemoglobin S and C heterozygosity enhances neither the magnitude nor breadth of antibody responses to a diverse array of Plasmodium falciparum antigens. J Infect Dis. 2011;204:1750–1761. doi: 10.1093/infdis/jir638. https://doi.org/10.1093/infdis/jir638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Cabrera G, Cot M, Migot-Nabias F, Kremsner PG, Deloron P, Luty AJ. The sickle cell trait is associated with enhanced immunoglobulin G antibody responses to Plasmodium falciparum variant surface antigens. J Infect Dis. 2005;191:1631–1638. doi: 10.1086/429832. https://doi.org/10.1086/429832. [DOI] [PubMed] [Google Scholar]
- 86.Ayi K, Turrini F, Piga A, Arese P. Enhanced phagocytosis of ring-parasitized mutant erythrocytes: a common mechanism that may explain protection against falciparum malaria in sickle trait and beta-thalassemia trait. Blood. 2004;104:3364–3371. doi: 10.1182/blood-2003-11-3820. https://doi.org/10.1182/blood-2003-11-3820. [DOI] [PubMed] [Google Scholar]
- 87.Christianson A, Howson CP, Modell B. March of Dimes global report on birth defects. March of Dimes Birth Defects Foundation; New York: 2009. pp. 21–23. [Google Scholar]
- 88.Rudra S, Chakrabarty P, Hossain MA, Ripon MJ, Rudra M, Mirza TT. Awareness among parents of β-Thalassemia major patients regarding prenatal diagnosis and premarital screening in day care centre of Transfusion Medicine Department. Mymensingh Med J. 2016;25:12–17. [PubMed] [Google Scholar]
- 89.Canatan D, Karadogan C, Oguz N, Balta N, Cosan R. The frequency of consanguineous marriages in patients with hereditary blood disorders in South of Turkey. Community Genet. 2003;6:58. doi: 10.1159/000069542. [DOI] [PubMed] [Google Scholar]
- 90.Moghadam M, Karimi M, Dehghani SJ, Dehbozorgian J, Montazeri S, Javanmardi E, Asadzade R, Amiri A, Saghatoleslam Z, Sotodegan F, Morshedi N, Imanifard J, Afrasiabi A. Effectiveness of β-thalassemia prenatal diagnosis in Southern Iran: a cohort study. Prenat Diagn. 2015;35:1238–1242. doi: 10.1002/pd.4684. https://doi.org/10.1002/pd.4684. [DOI] [PubMed] [Google Scholar]
- 91.Weatherall DJ. Thalassemia as a global health problem: Recent progress towards its control in the developing countries. Ann NY Acad Sci. 2010;1202:17–23. doi: 10.1111/j.1749-6632.2010.05546.x. https://doi.org/10.1111/j.1749-6632.2010.05546.x. [DOI] [PubMed] [Google Scholar]
- 92.Weatherall DJ. The inherited diseases of hemoglobin are an emerging global health burden. Blood. 2010;115:4331–4336. doi: 10.1182/blood-2010-01-251348. https://doi.org/10.1182/blood-2010-01-251348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Angel JL. Porotic hyperostosis, anemias, malarias, and marshes in the prehistoric eastern Mediterranean. Science. 1966;153:760–763. doi: 10.1126/science.153.3737.760. https://doi.org/10.1126/science.153.3737.760. [DOI] [PubMed] [Google Scholar]
- 94.Webb S. Cranial thickening in an Australian hominid as a possible palaeoepidemiological indicator. Am J Phys Anthropol. 1990;82:403–411. doi: 10.1002/ajpa.1330820402. https://doi.org/10.1002/ajpa.1330820402. [DOI] [PubMed] [Google Scholar]
- 95.Hershkovitz I, Ring B, Speirs M, Galili E, Kislev M, Edelson G, Hershkovitz A. Possible congenital hemolytic anemia in prehistoric coastal inhabitants of Israel. Am J Phys Anthropol. 1991;85:7–13. doi: 10.1002/ajpa.1330850103. https://doi.org/10.1002/ajpa.1330850103. [DOI] [PubMed] [Google Scholar]
- 96.Tayles N. Anemia, genetic diseases, and malaria in prehistoric mainland Southeast Asia. Am J Phys Anthropol. 1996;101:11–27. doi: 10.1002/(SICI)1096-8644(199609)101:1<11::AID-AJPA2>3.0.CO;2-G. https://doi.org/10.1002/(SICI)1096-8644(199609)101:1<11::AID-AJPA2>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 97.Aufderheide AC. Progress in paleopathology. Biomedical studies of human mummies. Minn Med. 1998;81:28–31. [PubMed] [Google Scholar]
- 98.Stuart-Macadam P. Porotic hyperostosis: new evidence to support the anemia theory. Am J Phys Anthropol. 1987;74:521–526. doi: 10.1002/ajpa.1330740410. https://doi.org/10.1002/ajpa.1330740410. [DOI] [PubMed] [Google Scholar]
- 99.Schultz M. Paleohistopathology of bone: a new approach to the study of ancient diseases. Am J Phys Anthropol. 2001;(Suppl 33):106–147. doi: 10.1002/ajpa.10024.abs. https://doi.org/10.1002/ajpa.10024. [DOI] [PubMed] [Google Scholar]
- 100.Rabino Massa E. Conservazione dei Globuli in Tessuti di Mummie Egiziane. Archivio per L’Antropologia e L’Etnologia. La Nuova Italia. 1967;3:181–182. [Google Scholar]
- 101.Rabino Massa E. Presence of Thalassemia in Egyptian Mummies. J Hum Evol. 1977;6:223–225. [Google Scholar]
- 102.Filon D, Faerman M, Smith P, Oppenheim A. Sequence analysis reveals a beta-thalassaemia mutation in the DNA of skeletal remains from the archaeological site of Akhziv, Israel. Nat Genet. 1995;9:365–368. doi: 10.1038/ng0495-365. https://doi.org/10.1038/ng0495-365. [DOI] [PubMed] [Google Scholar]
- 103.Handt O, Krings M, Ward RH, Pääbo S. The retrieval of ancient human DNA sequences. Am J Hum Genet. 1996;59:368–376. [PMC free article] [PubMed] [Google Scholar]
- 104.Paabo S. Ancient DNA: extraction, characterization, molecular cloning, and enzymatic amplification. Proc Natl Acad Sci USA. 1989;1989;86:1939–1943. doi: 10.1073/pnas.86.6.1939. https://doi.org/10.1073/pnas.86.6.1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Christianson A, Howson CP, Modell B. March of Dimes global report on birth defects. March of Dimes Birth Defects Foundation; New York: 2006. pp. 18–23. [Google Scholar]
- 106.Aguilar Martinez P, Angastiniotis M, Eleftheriou A, Gulbis B, del Maú Pereira MM, Petrova-Benedict R, Corrons JL. Haemoglobinopathies in Europe: health & migration policy perspectives. Orphanet J Rare Dis. 2014 Jul 1;9:97. doi: 10.1186/1750-1172-9-97. https://doi.org/10.1186/1750-1172-9-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Giordano PC, Harteveld CL, Bakker E. Genetic epidemiology and preventive healthcare in multiethnic societies: the hemoglobinopathies. Int J Environ Res Public Health. 2014;11:6136–6146. doi: 10.3390/ijerph110606136. https://doi.org/10.3390/ijerph110606136. [DOI] [PMC free article] [PubMed] [Google Scholar]