Abstract
Neuroblastoma arises from the neural crest, the precursor cells of the sympathoadrenal axis, and differentiation status is a key prognostic factor used for clinical risk group stratification and treatment strategies. Neuroblastoma tumor-initiating cells have been successfully isolated from patient tumor samples and bone marrow using sphere culture, which is well established to promote growth of neural crest stem cells. However, accurate quantification of sphere-forming frequency of commonly used neuroblastoma cell lines has not been reported. Here, we show that MYCN-amplified neuroblastoma cell lines form spheres more frequently than non-MYCN-amplified cell lines. We also show that sphere formation is directly sensitive to cellular differentiation status. 13-cis-retinoic acid is a clinically used differentiating agent that induces a neuronal phenotype in neuroblastoma cells. Induced differentiation nearly completely blocked sphere formation. Furthermore, sphere formation was specifically FGF-responsive and did not respond to increasing doses of EGF. Taken together, these data suggest that sphere formation is an accurate method of quantifying the stemness phenotype in neuroblastoma.
Keywords: Sphere assay, limiting dilution analysis, neuroblastoma, cancer stem cell, retinoic acid
INTRODUCTION
Neuroblastoma arises from the neural crest, the precursor cells of the sympathoadrenal axis, and is the most common extracranial solid tumor in children [1]. High-risk disease accounts for the vast majority of deaths and is characterized by MYCN gene amplification, lack of tumor stroma, and poor or undifferentiated tumor cell histology [1]. Identifying those tumor cell subsets with undifferentiated features and developing a better understanding of the molecular mechanisms that maintain this cellular state are key challenges in the process of developing novel therapeutics to alter the devastating clinical course of high-risk neuroblastoma [2].
Cancer stem cells are tumor cells that demonstrate stem cell features and that can be prospectively identified by cell surface marker expression [3, 4]. Tumor-initiating cells are those subsets that form in vivo xenografts at higher frequency than control or parental cell populations, and must be able to faithfully recapitulate the genetic and histologic features of the parental tumor [3]. Neuroblastoma tumor-initiating cells have been successfully isolated from patient tumor samples and bone marrow using sphere culture [5, 6], which is well established to promote growth of neural crest stem cells [7]. However, the mechanisms that are responsible for sphere formation remain poorly understood.
A major limitation of current studies involving neuroblastoma sphere formation has been the lack of a reliable quantification method that can be easily repeated. The predominant approach is to count the number of spheres that are over a certain size after a period of incubation [8] and to report the number of spheres formed as a percentage of cells plated. This method is highly dependent on the number of cells plated and culture area, for which there are no standard parameters. In this study, we apply limiting dilution analysis to quantitatively assess the frequency of intrinsic sphere-forming ability for a variety of neuroblastoma cell lines, and use induced differentiation with retinoic acid to validate this approach. Retinoic acid is a clinical therapeutic that is used as differentiating maintenance therapy after induction and consolidation chemotherapy in children with high-risk neuroblastoma [9]. Retinoic acid is well established to induce differentiation in vitro across multiple neuroblastoma cell lines [10–12] and is also shown to regulate stem cell differentiation [13]. We further show that limiting dilution analysis can be used to assess the contribution of signaling pathways to sphere formation.
MATERIALS AND METHODS
Adherent cell culture and sphere culture conditions
Human neuroblastoma cell lines SK-N-AS, SK-N-SH, BE(2)-C and LAN-1, were purchased from American Type Culture Collection (Manassas, VA). Cells were cultured on tissue-culture treated flasks and plates in RMPI 1640 media (Cellgro Mediatech, Herndon, VA) supplemented with fetal bovine serum at 10% v/v (Sigma-Aldrich, St. Louis, MO) in a humidified atmosphere of 5% CO2 at 37°C. Spheres were cultured in a 1:1 mixture of DMEM and Ham’s F12, with B27 supplement (50×), penicillin/streptomycin (5% v/v), epidermal growth factor (EGF, 20 ng/ml) and fibroblast growth factor (FGF, 40 ng/ml) on polystyrene-coated, ultra-low attachment plates (Corning Inc., Corning, NY).
Quantification of sphere formation
Cells were trypsinized with 0.25% trypsin (Cellgro Mediatech, Herndon, VA) for 1–5 minutes at 37°C and reaction was quenched with 10% FBS-containing RPMI 1650 media. Cells were stained with Trypan Blue to determine viability and manually counted in 8 replicates on a light microscope. Separate aliquots of 5 × 105 cells constituted each biological replicate for analysis, and were centrifuged at 1,000 rpm × 5 min and supernatant removed. Cells were resuspended in 500 μl of sphere media as described above. Two 10× dilutions followed by seven 2× dilutions were serially performed. Cells were plated on polystyrene-coated, ultra-low attachment 96-well plates (Corning Inc., Corning, NY) at final cell doses of 500, 250, 125, 62, 31, 16, 8 and 4 cells/well in 100 μl of sphere media in replicates of 6 per dose. After 4 d incubation in a humidified atmosphere of 5% CO2 at 37°C, each well was score as “positive” or “negative” for sphere formation, determined by the presence of ≥1 sphere consisting of ≥10 cells. The Eliza-Hall online calculator was used to generate a point estimate of 1/× cells with sphere-forming frequency (SFF) from the tested population [14]. Each biological replicate was repeated for a minimum of 6 replicates per group on different days to limit the potential impact of non-experimental variability such as cell counts or aliquots on the final estimates of sphere-forming frequency, and values were averaged and analyzed by Student’s t test or one-way ANOVA as appropriate.
Statistical analysis
All results are shown as mean ± SEM. Data were analyzed with Student’s t test or one-way ANOVA with Tukey correction for two or ≥3 group experiments, respectively. In all instances, a p value <0.05 was considered significant.
RESULTS
Sphere formation in neuroblastoma cells correlates with MYCN status
All human neuroblastoma cell lines tested formed spheres after variable periods in culture, indicating a general capacity for neuroblastoma cells to proliferate in conditions that favor the growth of neural crest stem cells [7]. To assess the basal SFF of neuroblastoma cells, four human neuroblastoma cell lines (SK-N-AS, SK-N-SH, BE(2)-C, LAN-1) were cultured in sphere culture conditions for 4 d (Figure 1A). After 4 d, formed spheres had a tightly packed morphology even when plated at low cell density (Figure 1B). The two cell lines that contain only a single copy of the MYCN oncogene demonstrated a low “background” rate of sphere formation (SK-N-AS: 0.7±0.09%, SK-N-SH: 1.7±0.18%, Figure 1C). Cell lines with MYCN amplification, however, showed high SFF at 4 d of incubation (BE(2)-C: 4.3±0.41%, LAN-1: 6.1±0.74%, Figure 1C). BE(2)-C and LAN-1 sphere formation was significantly higher than SK-N-AS or SK-N-SH (p < 0.001). These data suggest that sphere formation is correlated with other known parameters of cell behavior, as the highly aggressive cells lines were are able to form spheres at a much higher rate compared to less aggressive, slower growing, MYCN-single copy cell lines [15].
Figure 1.
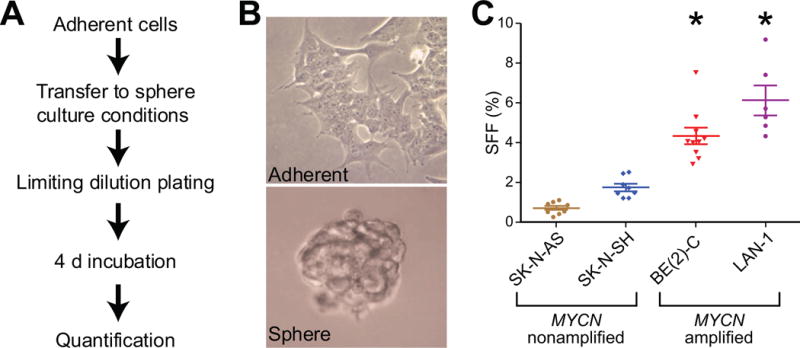
MYCN-amplified neuroblastoma cell lines have higher intrinsic sphere-forming frequency (SFF) measured by limiting dilution analysis. (A) Cells were plated in serum-free, EGF (20 ng/ml) and buff (40 ng/ml) supplemented media and cultured on low-attachment polystyrene-coated 96-well plates. Frequency of sphere formation was assessed after 4 d in culture. (B) BE(2)-C human neuroblastoma cells grown in typical adherent conditions with 10% fetal bovine serum (top panel) and in sphere conditions (bottom panel), 40× magnification. (C) SFF at 4 d by limiting dilution analysis. * = p<0.01 for BE(2)-C and LAN-1 compared to both SK-N-AS and SK-N-SH.
13-cis-retinoic acid induces cellular differentiation and blocks sphere formation
Sphere culture is known to promote proliferation of neural crest stem cells [7]. We therefore hypothesized that sphere formation in neuroblastoma would be sensitive to the differentiation status of the cells being exposed to the sphere stimulus. The differences in SFF between cell lines (Figure 1C) suggests this, as BE(2)-C are I-type cells that retain the ability to differentiate along the neuronal or the Schwannian cell lineages after retinoic acid or bromodeoxyuridine stimulation, respectively [16]. SK-N-AS and SK-N-SH cells are both neuronal-type [15], indicating a more differentiated basal state. To directly test our hypothesis, we used 13-cis-retinoic acid (RA), an agent that is utilized clinically as a differentiating maintenance therapy after induction and consolidation chemotherapy and radiation [9]. Incubation of BE(2)-C cells with RA (5 μM) for 4 d in sphere culture conditions nearly completely blocked sphere formation (Figure 2A). Sphere formation could be rescued by simultaneous co-treatment with the pan-retinoic acid receptor inhibitor BMS493 (1 μM, Figure 2A).
Figure 2.
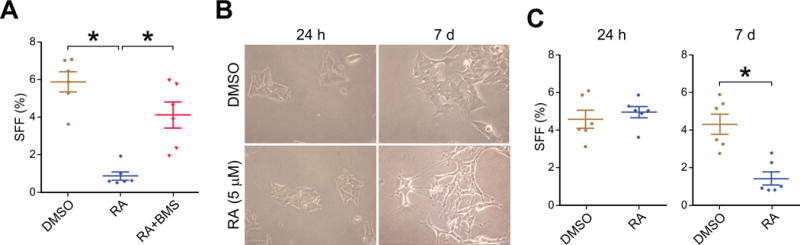
Induced differentiation with 13-cis-retinoic acid (RA) inhibits sphere formation by BE(2)-C neuroblastoma cells. (A) Adding RA (5 μM) to sphere culture media for the 4 d incubation period (Figure 1A) blocks sphere formation. Addition of BMS493 (1 μM), a specific pan-retinoic acid receptor inhibitor, to the sphere culture media is sufficient to reverse the RA effect on sphere formation. (B) Pre-treatment of adherent BE(2)-C cells with RA (5 μM) or vehicle control (DMSO) for 7 d induced neurite outgrowth and elongation of cell bodies (bottom right panel), indicative of neuronal differentiation, while 24 h of pre-treatment had no effect (left column). (C) 7 d pre-treatment with RA (5 μM) inhibited sphere formation (bottom panel) while 24 h pre-treatment did not (top panel). * = p<0.01 for each individual comparison, with multiple comparison adjustment for (A).
To further test the impact of differentiation status on sphere formation, we pre-treated BE(2)-C cells with RA (5 μM) for 24 h or 7 d prior to exposure to the sphere culture conditions. Seven days of RA treatment, but not 24 h, was able to induce neurite outgrowth and elongation of cell bodies indicating differentiation along a neuronal lineage (Figure 2B) [10–12]. Pretreatment with RA for only 24 h had no effect on sphere formation compared to vehicle-treated controls (Figure 2C). After successful differentiation with 7 d of RA, however, sphere formation was dramatically reduced (4.3±1.3% vs. 1.4±0.8%, p<0.001, Figure 2C). Taken together, these data suggest that sphere formation is critically regulated by differentiation status. In addition to inducing differentiation, RA could be exerting a direct effect on sphere formation given its central role in regulating differentiation-related behaviors, and we next sought to address this possibility directly.
FGF, but not EGF, promotes sphere formation
There are three main factors that are fundamental to sphere growth: serum-free media, FGF and EGF supplementation and non-attachment. We hypothesized that activation of downstream signaling pathways in response to FGF and EGF stimuli would be a site of potential regulation by RA. To test this hypothesis, sphere formation was assessed in response to each individual growth factor. Increasing doses of FGF led to increased SFF (Figure 3B, p < 0.05 for 80 ng/ml vs. 40 ng/ml). Increasing doses of EGF had no effect on SFF over the same dose range (Figure 3A, p = NS). These data suggest that sphere formation is a phenotypic response to activated signaling pathways rather than a fixed property of a subset of cells in a population. FGF signaling via the FGF receptor 1 is predominantly transduced through the MAPK signaling pathway [17]. PD98059 is a specific MAPK inhibitor and was able to inhibit SFF (Figure 3C). These data suggest that activation of canonical downstream FGF signaling via the MAPK pathway is critical for sphere formation.
Figure 3.
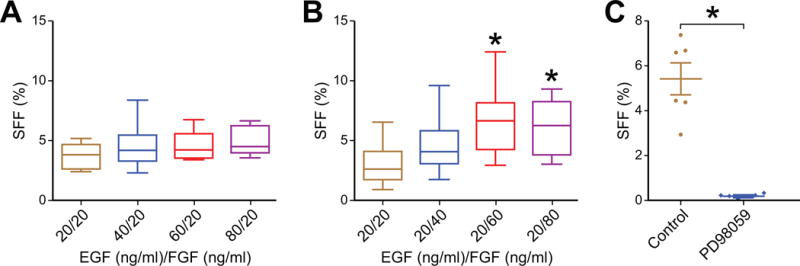
Sphere formation in human neuroblastoma BE(2)-C cells is FGF-responsive. (A) Increasing doses of EGF did not affect SFF (p = NS). (B) 60 ng/ml and 80 ng/ml of FGF increased sphere formation compared to 20 ng/ml. (C) Inhibition of MAPK signaling with PD98059 (100 μM) blocked sphere formation compared to vehicle control (DMSO). * = p<0.01.
DISCUSSION
Neuroblastoma is the prototypical embryonal cancer, generally held to originate from arrested differentiation of neural crest progenitor cells [1], thus explaining the distribution of neuroblastoma primary tumors along the entire sympathetic chain. It is likely that a component of the oncogenic transformation for neuroblastoma involves the pathologic maintenance of a stem cell state, with continued activation of stem cell maintenance programs and use of stem cell behavior to avoid death and promote proliferation and metastasis [4]. Therefore, it is a high research priority to identify assays that can assess the stemness of a particular sample, compound or treatment or signaling pathway manipulation. Sphere formation is one such assay. Sphere formation is a hallmark of neural stem cell behavior [18]. Accurate quantification of sphere formation in neuroblastoma has remained elusive, often relying on sphere size or counting the number of spheres present [18]. In this study, we applied the robust method of limiting dilution analysis to reliably quantify the frequency of sphere formation from parental cell lines and after experimental treatments.
Limiting dilution analysis is well suited to the study of binary events such as sphere formation, since each individual cell forms a sphere or does not. This method insulates against two important factors: first, sphere size might not be an accurate measure of sphere formation, because the mechanisms that enable a particular cell (or even a few cells) to form a sphere may be distinct from those that promote proliferation of the group of cells in a sphere once formed, and, second, adequately separately a tumor cell population into single cells and plating individual cells in individual wells on a plate (true clonal conditions) is technically very challenging and thus is not always practical. Furthermore, adequately generating a true single cell suspension with highly adherent tumor cells will frequently produce a lot of cell death, thus causing a selection bias for any results that come from that cell population. Previous studies have used sphere size as an indicator of stemness. Sphere size is an inaccurate measure of the process of interest, which is sphere formation, not proliferation after spheres are formed. Utilizing multiple replicates per cell dose and scoring each well as only “positive” or “negative” for any sphere formation ensures that results are not biased to favor cell types with strong paracrine signaling capabilities that may aid in subsequent proliferation and prevents counting spheres that have merged into one as false positives. It is our opinion that limiting dilution analysis should be the standard for all future studies that evaluate sphere formation in neuroblastoma.
This is the first report to utilize limiting dilution analysis to assess stemness in neuroblastoma and to validate it with retinoic acid. Retinoic acid is a clinically active agent used to maintain remission by inducing differentiation of any minimal residual disease after induction and consolidation chemotherapy, surgical resection and adjuvant radiation. In our study, either co-culture with retinoic acid or induced differentiation by pre-treatment was capable of significantly inhibiting sphere-forming frequency in a cell line that is known to respond to retinoic acid by differentiating [10, 12]. This is consistent with a previous report that retinoic acid inhibited neuroblastoma sphere formation [8]. The pan-retinoic acid receptor inhibitor BMS493 was able to reverse this effect. These experiments are significant, in that they confirm that sphere formation is directly sensitive to differentiation status, rather than simply correlated with it.
Sphere culture is defined by culture in serum-free media supplemented with EGF and FGF. In this study, sphere formation in neuroblastoma was specifically dependent upon activation of FGF signaling, and not to EGF, as sphere formation in BE(2)-C cells was inhibited by blockage of MAPK signaling, a key downstream pathway of FGF signal transduction. It was recently shown [19] that the majority of relapsed neuroblastomas have activated RAS-MAPK signaling, either by mutation or by clonal evolution and predominance. In addition, a majority of cell lines tested (11/18) also demonstrated aberrant MAPK signaling activation and this predicted sensitivity to MEK inhibition [19]. Here we demonstrate that MAPK activation is a critical mechanism of sphere formation in an I-type neuroblastoma cell line. Sphere culture may mediate its selection for a stemness phenotype by activating the key downstream signaling pathways associated with relapsed neuroblastoma. More work is necessary to determine whether MAPK pathway activation in relapse is specific to MYCN-amplified cells or whether it is a more general mechanism. Our data would suggest it to be associated with MYCN amplification as these cell lines exhibited significantly higher intrinsic sphere formation than the non-MYCN-amplified cells.
In conclusion, our data show that sphere formation in neuroblastoma is directly sensitive to differentiation status and is a signaling-mediated phenotypic behavior. Limiting dilution analysis provides a robust and reliable quantification method for the precise assessment of sphere-forming frequency. We propose that limiting dilution analysis of sphere formation be adopted as an in vitro test of differentiation effect for future studies seeking to address these and related questions. Whether this in vitro behavior correlates with translation ally significant endpoints such as therapeutic resistance will need to be the subject of future studies.
HIGHLIGHTS.
Limiting dilution analysis reliably quantifies sphere-forming frequency
Sphere-forming frequency of neuroblastoma cells is associated with MYCN status
13-cis-retinoic acid inhibits sphere formation in neuroblastoma cells
Activation of FGF signaling critically regulates sphere-forming frequency
Acknowledgments
The authors would like to thank Karen Martin for her assistance with figure and manuscript preparation.
FUNDING
This work was supported with funding from the NIH RO1 DK61470 (PI: Chung) and a Rally Foundation for Cancer Research Pediatric Oncology Fellowship Award (Craig).
ABBREVIATIONS
- RA
13-cis-retinoic acid
- SFF
sphere-forming frequency
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Louis CU, Shohet JM. Neuroblastoma: Molecular Pathogenesis and Therapy. Annual Review of Medicine. 2015;66:49–63. doi: 10.1146/annurev-med-011514-023121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schleiermacher G, Janoueix-Lerosey I, Delattre O. Recent insights into the biology of neuroblastoma. International Journal of Cancer. 2014;135:2249–2261. doi: 10.1002/ijc.29077. [DOI] [PubMed] [Google Scholar]
- 3.Clarke MF, Dick JE, Dirks PB, Eaves CJ, Jamieson CHM, Jones DL, Visvader J, Weissman IL, Wahl GM. Cancer Stem Cells–Perspectives on Current Status and Future Directions: AACR Workshop on Cancer Stem Cells. Cancer Research. 2006;66:9339–9344. doi: 10.1158/0008-5472.CAN-06-3126. [DOI] [PubMed] [Google Scholar]
- 4.Kreso A, Dick JE. Evolution of the Cancer Stem Cell Model. Stem Cell. 2014;14:275–291. doi: 10.1016/j.stem.2014.02.006. [DOI] [PubMed] [Google Scholar]
- 5.Hansford LM, McKee AE, Zhang L, George RE, Gerstle JT, Thorner PS, Smith KM, Look AT, Yeger H, Miller FD, Irwin MS, Thiele CJ, Kaplan DR. Neuroblastoma Cells Isolated from Bone Marrow Metastases Contain a Naturally Enriched Tumor-Initiating Cell. Cancer Research. 2007;67:11234–11243. doi: 10.1158/0008-5472.CAN-07-0718. [DOI] [PubMed] [Google Scholar]
- 6.Bate-Eya LT, Ebus ME, Koster J, den Hartog IJM, Zwijnenburg DA, Schild L, van der Ploeg I, Dolman MEM, Caron HN, Versteeg R, Molenaar JJ. Newly-derived neuroblastoma cell lines propagated in serum-free media recapitulate the genotype and phenotype of primary neuroblastoma tumours. European journal of cancer (Oxford, England : 1990) 2014;50:628–637. doi: 10.1016/j.ejca.2013.11.015. [DOI] [PubMed] [Google Scholar]
- 7.Chung KF, Sicard F, Vukicevic V, Hermann A, Storch A, Huttner WB, Bornstein SR, Ehrhart-Bornstein M. Isolation of Neural Crest Derived Chromaffin Progenitors from Adult Adrenal Medulla. Stem cells (Dayton, Ohio) 2009;27:2602–2613. doi: 10.1002/stem.180. [DOI] [PubMed] [Google Scholar]
- 8.Hammerle B, Yanez Y, Palanca S, Canete A, Burks DJ, Castel V, Mora J Font de. Targeting neuroblastoma stem cells with retinoic acid and proteasome inhibitor. PLoS One. 2013;8:e76761. doi: 10.1371/journal.pone.0076761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Reynolds CP, Matthay KK, Villablanca JG, Maurer BJ. Retinoid therapy of high-risk neuroblastoma. Cancer Letters. 2003;197:185–192. doi: 10.1016/s0304-3835(03)00108-3. [DOI] [PubMed] [Google Scholar]
- 10.Sidell N, Altman A, Haussler MR, Seeger RC. Effects of retinoic acid (RA) on the growth and phenotypic expression of several human neuroblastoma cell lines. Experimental cell research. 1983;148:21–30. doi: 10.1016/0014-4827(83)90184-2. [DOI] [PubMed] [Google Scholar]
- 11.Thiele CJ, Reynolds CP, Israel MA. Decreased expression of N-myc precedes retinoic acid-induced morphological differentiation of human neuroblastoma. Nature. 1985;313:404–406. doi: 10.1038/313404a0. [DOI] [PubMed] [Google Scholar]
- 12.Abemayor E, Chang B, Sidell N. Effects of retinoic acid on the in vivo growth of human neuroblastoma cells. Cancer Letters. 1990;55:1–5. doi: 10.1016/0304-3835(90)90057-5. [DOI] [PubMed] [Google Scholar]
- 13.Gudas LJ. Retinoids induce stem cell differentiation via epigenetic changes. Seminars in Cell and Developmental Biology. 2013;24:701–705. doi: 10.1016/j.semcdb.2013.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hu Y, Smyth GK. ELDA: Extreme limiting dilution analysis for comparing depleted and enriched populations in stem cell and other assays. Journal of Immunological Methods. 2009;347:70–78. doi: 10.1016/j.jim.2009.06.008. [DOI] [PubMed] [Google Scholar]
- 15.Thiele CJ. Neuroblastoma Cell Lines. Human Cell Culture. 1998:1–35. [Google Scholar]
- 16.Ross RA, Spengler BA, Domènech C, Porubcin M, Rettig WJ, Biedler JL. Human neuroblastoma I-type cells are malignant neural crest stem cells. Cell growth & differentiation : the molecular biology journal of the American Association for Cancer Research. 1995;6:449–456. [PubMed] [Google Scholar]
- 17.Eswarakumar VP, Lax I, Schlessinger J. Cellular signaling by fibroblast growth factor receptors. Cytokine & Growth Factor Reviews. 2005;16:139–149. doi: 10.1016/j.cytogfr.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 18.Pastrana E, Silva-Vargas V, Doetsch F. Eyes Wide Open: A Critical Review of Sphere-Formation as an Assay for Stem Cells. Stem Cell. 2011;8:486–498. doi: 10.1016/j.stem.2011.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Eleveld TF, Oldridge DA, Bernard V, Koster J, Daage LC, Diskin SJ, Schild L, Bentahar NB, Bellini A, Chicard M, Lapouble E, Combaret V, Legoix-Ne P, Michon J, Pugh TJ, Hart LS, Rader J, Attiyeh EF, Wei JS, Zhang S, Naranjo A, Gastier-Foster JM, Hogarty MD, Asgharzadeh S, Smith MA, Auvil JM Guidry, Watkins TB, Zwijnenburg DA, Ebus ME, Sluis P van, Hakkert A, Wezel E van, Schoot CE van der, Westerhout EM, Schulte JH, Tytgat GA, Dolman ME, Janoueix-Lerosey I, Gerhard DS, Caron HN, Delattre O, Khan J, Versteeg R, Schleiermacher G, Molenaar JJ, Maris JM. Relapsed neuroblastomas show frequent RAS-MAPK pathway mutations. Nat Genet. 2015;47:864–871. doi: 10.1038/ng.3333. [DOI] [PMC free article] [PubMed] [Google Scholar]


