Abstract
We determined whether sensorimotor peripheral nerve (PN) function was associated with physical activity (PA) in older men. The Osteoporotic Fractures in Men Study Pittsburgh, PA, site (n=328, age 78.8±4.7 years), conducted PN testing, including: peroneal motor and sural sensory nerve conduction (latencies, amplitudes: CMAP and SNAP for motor and sensory amplitude, respectively), 1.4g/10g monofilament (dorsum of the great toe), and neuropathy symptoms. ANOVA and multivariate linear regression modeled PN associations with PA (Physical Activity Scale for the Elderly (PASE) and SenseWear Armband). After multivariable adjustment, better motor latency was associated with higher PASE scores (160.5±4.8 vs 135.6±6.7, p<0.01). Those without vs. with neuropathy symptoms had higher PASE scores (157.6±5.3 vs 132.9±7.1, p<0.01). Better vs. worse SNAP was associated with slightly more daily vigorous activity (9.5±0.8 vs. 7.3±0.7, p=0.05). Other PN measures were not associated with PA. Certain PN measures were associated with lower PA, suggesting a potential pathway for disability.
INTRODUCTION
Sensorimotor peripheral nerve (PN) dysfunction negatively impacts mobility in older adults—a cornerstone for maintaining independence. Worse sensorimotor PN function in older adults is associated with poorer lower extremity function (Cimbiz & Cakir, 2005; McDermott et al., 2004; Resnick et al., 2002; Strotmeyer et al., 2008), strength (Strotmeyer et al., 2009; Ward et al., 2015), and power (Ward et al., 2014a), as well as higher risk of falls (Ferrucci et al., 2004; Lalli et al., 2013; Richardson, Ching, & Hurvitz, 1992; Schwartz et al., 2008) and mobility disability (Ward et al., 2014b). Deficits in sensorimotor nerve function may result in pain or loss of sensation in the extremities, which could greatly influence the ability to be physically active. A major gap in the literature exists regarding the relationship between PN function and PA in older adults. Whether worse PN function is related to lower levels of PA is unclear. PA could potentially be in the pathway from PN impairments to mobility disability.
Few studies have examined the association between clinical peripheral neuropathy and physical activity (PA). Existing work has focused on adults with diabetes (Loprinzi, Hager, & Ramulu, 2013; van Sloten et al., 2011) rather than more general populations of community-dwelling older adults. Diabetes is a common contributor, PN impairments are common in older adults in the absence of diabetes, and this prevalence increases with age (Baldereschi et al., 2007; Gregg et al., 2004). Moreover, worse peripheral nerve function is associated with poor lower extremity outcomes in non-diabetic older adults even without diagnosed clinical peripheral neuropathy (Strotmeyer et al., 2008), highlighting the importance of examining a full range of peripheral nerve function in older adults. The purpose of this study was to determine whether PN function is associated PA in older men. We hypothesized that better PN function will be associated with higher levels of self-reported and objectively measured PA.
METHODS
Participants
Participants at the Monongahela Valley, PA (rural area near Pittsburgh, PA), site of the Osteoporotic Fractures in Men Study (MrOS) underwent peripheral nerve function assessments at the 2007–09 clinic visit. MrOS is a multicenter longitudinal cohort study of healthy aging focusing on the risk factors for fractures in older men (n=5,994; mean age 73.7 ± 5.9 years at baseline) (Orwoll et al., 2005). Ambulatory men age 65 and older were recruited and completed the baseline visit between March 2000 and April 2002 from six U.S. clinical sites (n=1,005 in Pittsburgh at the baseline visit) (Blank et al., 2005). Eligibility criteria for the main study at the baseline visit included the ability to walk without assistance from another person or walking aid, ability to provide self-reported data, capacity to understand and provide informed consent, absence of bilateral hip replacement, absence of any severe disease or condition that would results in imminent death, and anticipated residence near a clinical site for the duration of the study period. In total, 425 men from this clinical site had data from the 2007–09 clinic visit PN function ancillary (≥1 measure of PN function), and objective physical activity data was available for 328 participants. Reasons for not having objective physical activity data included: n=11 refused armband, n=44 did not receive armband due to armband exclusion, n=26 with <90% wear time, n=14 had wear time issues, and n=3 were missing for unknown reasons. The study protocol was approved by the University of Pittsburgh Institutional Review Board, and all participants provided written informed consent before testing.
Peripheral Nerve Function Examination
Nerve conduction testing was performed bilaterally on the deep peroneal motor nerve and the sural sensory nerve using the NC-stat System (NeuroMetrix, Inc.), an automated, non-invasive nerve conduction study device)—a valid and reliable method for assessing nerve function in older adults (Fisher, 2005). Before testing, participants’ feet were warmed using a heating pad to 30°C if they were <30°C (measured using an infra-red thermometer). Peroneal motor nerve parameters included: amplitude of the compound muscle action potential (CMAP, millivolts); distal motor latency (milliseconds); and mean F-wave latency (milliseconds). Sural sensory nerve measures included amplitude of the sural nerve action potential (SNAP, microvolts) and distal sensory latency (milliseconds). These measures of nerve function were split into poor function (lowest tertile) and higher function (middle and highest tertiles) to approximate cut-points indicating poor nerve function. Sensory PN function was also assessed through monofilament testing using light (1.4-g) and standard (10-g) monofilament touches at the dorsum of the left great toe, with detection defined as feeling at least 3 out of 4 touches. The standard monofilament was only used if the participant had light monofilament insensitivity. These two measures were combined in a single insensitivity variable as follows: 1.4g detection, 1.4g insensitivity, and 10g insensitivity. Sensory testing was performed on the non-dominant side while motor testing was performed on both sides except in the case of testing difficulty (including participant issues of flaky skin, swelling in the legs, metal objects in the legs, or technical difficulty due to sensor positioning issues). Only the non-dominant side was used for analysis.
Symptoms of peripheral neuropathy within the past 12 months were assessed via self-report from a modified Michigan Neuropathic Screening Instrument (Feldman et al., 1994) and included: numbness or tingling; sudden stabbing, burning, or aches; an open persistent sore or gangrene on either foot or leg in the past 12 months. Symptoms were analyzed using a composite variable (any symptoms vs. none) and individually.
Physical Activity Assessment
Self-report physical activity was assessed using the Physical Activity Scale for the Elderly (PASE) at the same clinic visit (Washburn, Smith, Jette, & Janney, 1993). Briefly, the PASE includes questions about the intensity, frequency, and duration of various physical activities over the past seven days. Activities include walking, strenuous (e.g. jogging, swimming, singles tennis), moderate (e.g. golf without a cart, doubles tennis) and light activities (e.g. golf with a cart, shuffleboard), muscle strengthening exercises, lawn work and gardening, occupational activities that include walking or standing, caring for another person, home repairs, and housework. The frequency and duration of participation in the various categories are multiplied by activity weights based on intensity and summed in order to give a total PASE score. The PASE score is a unitless, relative measure, with higher scores indicating higher levels of physical activity. The PASE has been previously validated against energy expenditure measured via doubly labeled water (Bonnefoy et al., 2001; Schuit, Schouten, Westerterp, & Saris, 1997) and objectively measured PA (Harada, V, King, & Stewart, 2001; Washburn & Ficker, 1999), and it is correlated with physiologic and performance characteristics in older adults (Washburn, McAuley, Katula, Mihalko, & Boileau, 1999). The PASE has high test-retest reliability (Dinger, Oman, Taylor, Vesely, & Able, 2004; Washburn et al., 1993).
Objective physical activity was measured using the SenseWear Armband (SWA; Body Media, Inc., Pittsburgh, PA), which was worn for 7 days after the clinic visit. Participants were instructed to wear the monitor at all times, removing the monitor only for brief periods for bathing or water activities. The SWA includes heat flux, galvanic skin response, skin temperature, and near body temperature sensors, as well as a two-axis accelerometer. Data were sampled in 1-minute epochs and used to estimate energy expenditure in kilocalories per day (Jakicic et al., 2004). Data collected by the sensors along with age, height, weight, handedness, and smoking status were used in propriety algorithms (Innerview Professional 5.1 software) to estimate energy expenditure, metabolic equivalents (METS), and sleep time. Energy expenditure measured by SWA has been validated using doubly labeled water in older adults (Mackey et al., 2011). Average minutes per day spent in each level of PA were calculated using MET cut-points, with categories being defined as follows: light/lifestyle activity = 1.6 to <3.0 METS, moderate activity 3.0–<6.0 METS, vigorous activity 6.0+ METS, and sedentary time <1.6 METS (excluding sleep time). Total time spent in each of these categories during the wearing period was averaged over all days in order to limit variability and reflect usual activity patterns. As described previously by Cawthon and colleagues (Cawthon et al., 2013), if a participant wore the monitor < 90% of the time during any 24-hour period, that period was not used in calculating energy expenditure. Only participants with at least five 24-hour periods were included in analyses.
Covariates
We considered several factors potentially related to PA or PN function as covariates. Height and weight were measured using a stadiometer and calibrated balance beam scale, respectively, and were used to calculate body mass index (BMI). Diabetes was defined by self-reported physician diagnosis, hypoglycemic medication use, or fasting glucose of ≥126mg/dL (American Diabetes Association, 2013). Peripheral arterial disease was defined as ankle-brachial index (ABI) <0.9 and arterial stiffening as an ABI>1.3 (Aboyans et al., 2012). The Teng Modified Mini-Mental State Exam (3MSE) was used to assess cognition (Teng & Chui, 1987) and the Geriatric Depression Scale (GDS) measured depressive symptoms (Yesavage et al., 1982). History of cigarette smoking (never, current, former), current alcohol consumption (drinks/week), and health status (excellent, good, fair, poor, very poor) were assessed through self-report. Chronic health conditions included self-report physician diagnosis of hypertension and prior heart attack.
Statistical Analyses
Descriptive statistics were expressed using mean ± standard deviation or median and interquartile range where appropriate for continuous variables and frequencies for categorical variables. Participants were grouped based upon tertile of mean daily minutes spent in moderate and vigorous physical activity (3+ METS) from the SenseWear Armband. Tests of trend were used to assess differences in participant characteristics between the three ordered groups. PN measures and minutes spent in the four levels of activity were also compared between groups. ANOVA models compared the adjusted mean PASE score between PA groups.
Certain measures of PN measures were correlated (correlation coefficients ranging from 0.17 for sensory amplitude and latency and 0.34 between motor and sensory amplitude, p<0.05 each), each measure of PN was modeled separately. Multivariate linear regression was used to model the association between PN and outcome of objectively measured PA as categorized as four levels, which allowed a simultaneous examination of the effect at each level of activity (sedentary, light, moderate, and vigorous) in one model. This method used the correlations between the components of the multi-dimensional outcome to identify differences between the groups. Minutes spent in each level of activity were right skewed, thus natural logged versions of these variables were used for analysis. Least squared means were calculated, and results were back transformed in order to report minutes spent in each level of activity.
All models (ANOVA models for PASE; multivariate regression models for SWA outcomes) were built stepwise, with covariates with p<0.10 being considered for a multivariable model. Diabetes was forced into the final model regardless of significance due to its association with PN. Factors that could influence PA or PN function were considered as covariates until a final, parsimonious model with only factors reaching p<0.05 was determined for each PN predictor and PA outcome. Models excluding diabetic participants were considered in sensitivity analyses. LS Means and standard errors were reported from these models. All data analyses were performed using STATA version 12.1 (StataCorp, College Station, TX) and SAS 9.4. (SAS Institute, Inc., Cary, NC).
RESULTS
This cohort of older men (n=328, age 78.8±4.7 years, BMI 28.2±3.9 kg/m2) had a mean PASE score of 148.1 ± 68.5 and spent a median (interquartile range) number of 832.0 (762.1–906.3), 63.7 (43.8–85.0), 69.7 (41.5–78.80), and 9.0 (4.8–17.0) minutes per day in sedentary, light, moderate, and vigorous activities, respectively. Those with fewer mean daily minutes of moderate and vigorous activity tended to be older, have a higher BMI, were more likely to have fair/poor health, and reported more depressive symptoms (Table 1; p≤0.001 for all). Men with lower levels of PA were also less likely to consume three or more alcoholic drinks per week (p=0.02). Differences across other chronic conditions and diseases were not significant, though these conditions were generally less prevalent in the higher tertile of activity. As expected, those in the lower tertile of daily minutes of moderate and vigorous activity had lower scores on the PASE, spent fewer minutes per day in light activities, and they spent more minutes per day in sedentary behaviors compared to those in the higher tertiles of daily minutes in moderate and vigorous activates (Table 1; p≤0.001 for all). Participants in the lower tertile of daily minutes of moderate and vigorous activity had lower motor amplitude, lower sensory amplitude, were more likely to have an undetectable sensory amplitude, were more likely to have 1.4 or 10-g monofilament insensitivity, and were more likely to self-report numbness (Table 2).
Table 1.
Descriptive Characteristics of Participants by Tertile of Average Daily Minutes of Moderate and Vigorous Activity
| Tertile 1 N=108 |
Tertile 2 N=110 |
Tertile 3 N=110 |
P for Trend | |
|---|---|---|---|---|
| Mean Minutes of Combined Moderate and Vigorous Activity | 32.3 ± 14.1 | 74.5 ± 14.2 | 155.1 ± 50.0 | |
|
| ||||
| Age, Mean ± SD | 79.7 ± 5.1 | 78.9 ± 4.7 | 77.7 ± 4.2 | <0.01 |
| BMI, kg/m2 | 29.1 ± 4.5 | 28.3 ± 3.4 | 27.3 ± 3.6 | <0.01 |
| Health Fair/Poor | 20 (18.5) | 11 (10.0) | 6 (5.4) | <0.01 |
| Health Habits | ||||
| Consume ≥3 Drinks/Week | 27 (25.0) | 33 (30.6) | 43 (39.5) | 0.02 |
| Former Smoker, N (%) | 60 (55.6) | 70 (63.6) | 66 (60) | 0.98 |
| Current Smoker | 5 (4.6) | 2 (1.8) | 2 (1.8) | |
| Chronic Health Conditions | ||||
| Diabetes, N (%) | 28 (26.2) | 21 (19.3) | 19 (17.3) | 0.11 |
| Hypertension | 64 (59.3) | 57 (51.8) | 60 (54.5) | 0.49 |
| Heart Attack | 19 (17.6) | 13 (11.8) | 11 (10) | 0.10 |
| Peripheral Arterial Disease | 15 (15.5) | 9 (8.7) | 9 (8.7) | 0.80 |
| Arterial Stiffening | 1 (1.0) | 5 (4.9) | 6 (5.8) | |
| Cognition and Mental Health | ||||
| 3MSE Score | 93.0 ± 5.5 | 93.7 ± 4.8 | 92.9 ± 6.3 | 0.74 |
| GDS Score | 1.9 ± 1.7 | 1.7 ± 2 | 1.4 ± 1.5 | <0.01 |
| Physical Activity | ||||
| PASE Score | 121.8 ± 61.6 | 154.5 ± 69.9 | 167.5 ± 66.2 | <0.01 |
| * Sedentary, <1.6 METS | 898.1 ± 80.7 | 853.5 ± 83.5 | 746.1 ± 90.1 | <0.01 |
| * Light, 1.6 to <3.0 METS | 42.1 ± 19.1 | 67.1 ± 25.4 | 91.7 ± 29 | <0.01 |
| * Moderate, 3.0–<6.0 METS | 31.6 ± 13.8 | 69.2 ± 14.1 | 139.9 ± 43.5 | <0.01 |
| * Vigorous, ≥ 6.0 METS | 5.9 ± 6.0 | 11.5 ± 8.8 | 21.7 ± 17.5 | <0.01 |
Mean minutes spent in each level of physical activity as measured by the SenseWear Armband. Sedentary time excludes sleep time.
Table 2.
Peripheral Nerve Function by Tertile of Average Daily Minutes of Moderate and Vigorous Activity
| Tertile 1 N=108 |
Tertile 2 N=110 |
Tertile 3 N=110 |
P for Trend | |
|---|---|---|---|---|
| Mean Minutes of Combined Moderate and Vigorous Activity | 32.3 ± 14.1 | 74.5 ± 14.2 | 155.1 ± 50.0 | |
|
| ||||
| Motor Nerve Function | ||||
| Motor Amplitude (CMAP), mV | 2.0 ±1.4 | 2.6 ± 1.5 | 2.4 1.4 | 0.03 |
| CMAP = 0, N (%) | 7 (6.7) | 7 (6.5) | 2 (1.9) | 0.11 |
| Distal Motor Latency*, ms | 4.7 ± 0.9 | 4.5 ± 0.7 | 4.5 ± 0.8 | 0.30 |
| Mean F-Wave Latency, ms | 62.3 ± 6.4 | 60.4 ±5.4 | 61.2 ± 5.4 | 0.28 |
| Sensory Nerve Function | ||||
| Sensory Amplitude (SNAP), uV | 4.1 ± 1.7 | 4.8 ± 2.3 | 5.4 ± 2.4 | <0.01 |
| SNAP = 0, N (%) | 44 (46.3) | 29 (29.3) | 26 (26.8) | <0.01 |
| Distal Sensory Latency*, ms | 3.1 ± 0.2 | 3.2 ± 0.3 | 3.2 ± 0.3 | 0.53 |
| 1.4-g Monofilament Insensitivity | 24 (27.4) | 26 (30.6) | 21 (22.1) | 0.14 |
| 10-g Monofilament Insensitivity | 15 (17.9) | 9 (10.6) | 12 (12.6) | |
| Neuropathic Symptoms | ||||
| Numbness | 30 (36.1) | 28 (32.9) | 20 (21.1) | 0.03 |
| Stabbing Pain | 10 (11.9) | 14 (16.5) | 11 (11.7) | 0.91 |
| Open Sore | 1 (1.2) | 1 (1.2) | 0 (0.0) | 0.35 |
| Any symptoms | 34 (40.5) | 36 (42.4) | 25 (26.6) | 0.06 |
Motor and sensory latencies could only be determined for participants with detectable amplitude values (>0 mV).
Note: Percentages were calculated using the number of participants who completed the test rather than the entire sample. (N=309 completed motor amplitude testing; N=282 F-Wave latency testing; N=289 sensory nerve testing, N=263 monofilament testing.)
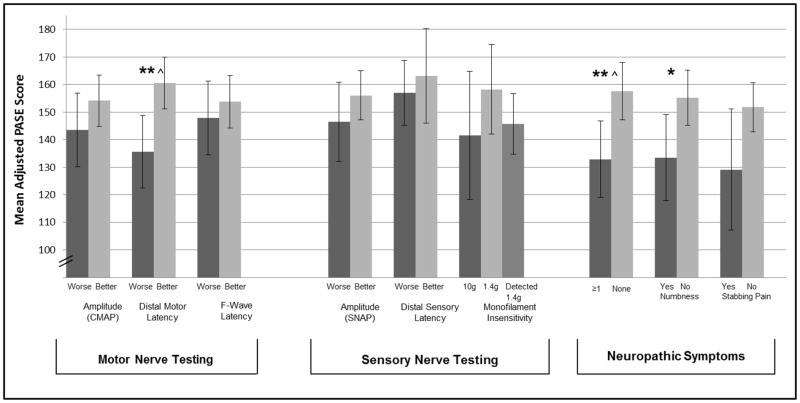
Unadjusted PASE scores differed by better and worse CMAP (155.7±4.6 vs. 134.8±6.6, p≤0.01), distal motor latency (158.5±4.7 vs. 134.3±6.6, p≤0.01), SNAP (156.1±5.1 vs. 137.9±6.9, p<0.05) and presence of symptoms (156.7±5.2 vs. 128.8±6.9 for no vs. any symptoms, p≤0.01) (data not shown). Figure 1 displays the differences in adjusted mean PASE score (adjusting for age, BMI, self-reported health, diabetes, peripheral arterial disease, and arterial stiffening) by PN function. Those with better distal motor latency had significantly higher total PASE scores compared to those with worse (160.5±4.8 vs 135.6±6.7, p≤0.01. Those without symptoms of peripheral neuropathy had higher PASE scores compared to those reporting any symptoms (157.6±5.3 vs 132.9±7.1, p≤0.01). Specifically, those without numbness had higher PASE scores compared to those with numbness (153.2±65.2 vs 132.7±67.0, p<0.05). While trends remained that those with better PN function had better PASE scores, relationships between CMAP and SNAP with PASE score were attenuated when adjusting by the above covariates. Neither f-wave latency, distal sensory latency, monofilament detection, symptoms of stabbing pain nor open sores were associated with PASE score.
Figure 1. Adjusted Means of Total Physical Activity Scale for the Elderly (PASE) Score by Peripheral Nerve Function.
Means adjusted for age, BMI, self-reported health, diabetes, peripheral arterial disease, arterial and stiffening. Continuous measures of nerve function were split into tertiles and those with worse function (lowest tertile of amplitude, highest tertile of latency) were compared to those with better function (combined highest and middle tertile of amplitude, combined lowest and middle tertile of latency). Error bars indicate 95% confidence intervals.
*P<0.05 and **P<0.001 for difference between groups, ^statically significant after correcting for 9 comparisons.
In unadjusted regression models examining minutes spent in objectively measured activity, men with better CMAP participated in more mean daily minutes of light (63.8±2.2 vs 52.5±2.6, p≤0.01), moderate (68.3±3.5 vs. 55.6±4.1, p<0.05), and vigorous physical activity (9.9±0.7 vs. 6.4±0.7, p<0.001) and fewer minutes of sedentary behaviors (816.0±7.4 vs 844.8±10.9, p<0.05). Likewise, better SNAP was associated with more daily average minutes of participation in light (62.9±2.3 vs. 52.6±2.7, p≤0.01), moderate (69.3±3.9 vs. 53.9±4.2, p≤0.001), and vigorous activities (9.7±0.7 vs. 6.0±0.7, p≤0.001), though sedentary time did not differ significantly (821.0±7.8 vs 837.3±11.0, p=0.22). Adjusting for age, BMI, self-reported health, diabetes, peripheral arterial disease, and arterial stiffening attenuated the relationships between CMAP and SNAP with light, moderate, and sedentary time to non-significance. The majority of attenuation was due to differences in age and BMI. In final adjusted models, those with better SNAP participated in slightly more minutes of vigorous activity (9.5±0.8 vs. 7.3±0.7; Table 3) though this reached borderline statistical significance, p=0.05. Distal motor latency, f-wave latency, distal sensory latency, monofilament detection, and symptoms of peripheral neuropathy were not associated with objectively assessed physical activity, though generally trended in a consistent direction (results not shown). In sensitivity analyses, results remained consistent when excluding participants with diabetes.
Table 3.
Mean Minutes (95% Confidence Interval) Spent in Light, Moderate, and Vigorous Activity per Day by Motor and Sensory Amplitude
| Minutes of Sedentary Time | Minutes of Light Activity | Minutes of Moderate Activity | Minutes of Vigorous Activity | |
|---|---|---|---|---|
| CMAP | ||||
| Better | 816.8 (802.5–831.4) | 62.9 (58.9–67.1) | 67.3 (61.0–71.6) | 9.6 (8.4–11.0) |
| Worse | 829.8 (808.8–851.4) | 57.9 (52.7–63.6) | 62.1 (53.9–71.7) | 7.7 (6.3–9.4) |
| P-Value for Difference | 0.33 | 0.16 | 0.37 | 0.07 |
| SNAP | ||||
| Better | 821.0 (805.9–836.3) | 61.7 (57.6–66.1) | 68.4 (61.5–76.1) | 9.5 (8.2–10.9) |
| Worse | 837.3 (816.0–859.1) | 58.4 (52.9–64.4) | 59.9 (51.4–69.7) | 7.3 (5.8–9.0) |
| P-Value for Difference | 0.22 | 0.38 | 0.17 | 0.05 |
Means adjusted for age, BMI, self-reported health, diabetes, peripheral arterial disease, and arterial stiffening. Activity Definitions; Light: 1.6 to <3.0 METS; Moderate: 3.0 to < 6.0 METS; Vigorous: ≥ 6 METS.
DISCUSSION
In this cohort of community dwelling older men, certain, measures of better PN were associated with higher levels of self-reported PA and more daily minutes of objectively measured vigorous activity. Only sensory amplitude (SNAP) was associated with objective vigorous PA, while only distal motor latency and presence of neuropathy symptoms were associated with self-report PA. We are uncertain as to why these relationships varied,; still, they were consistent in indicating modest relationships between better motor nerve function and higher levels of PA. Importantly, PN function – particularly motor function - is rarely measured in studies of PA in older adults, and future work should aim to understand the mechanisms of how PN influences PA.
Very little work has been done in investigating the relationship between PN function and PA, and existing work has focused on diabetic populations rather than older adults in general. In diabetic participants in the in 2003–2004 cycle of the National Health and Nutrition Examination Survey (NHANES), no direct association was found between minutes per day of moderate to vigorous PA and peripheral neuropathy (Loprinzi et al., 2013). However, those with better diabetic control and more daily PA were less likely to have peripheral neuropathy compared to what would be expected from the individual effects of PA and diabetes control. On average, the NHANES population participated in very few minutes of objectively measured PA per day (measured using hip-worn accelerometers; mean = 11.7 minutes of moderate to vigorous PA per day, 95% CI = 9.1–14.4 minutes), potentially limiting the ability to examine the relationship between PA and peripheral neuropathy in this population of diabetic adults. Additionally, differences in cut-points used to define levels of activity may have contributed to the varying results.
In diabetic populations, peripheral neuropathy—particularly symptoms of peripheral neuropathy—have been acknowledged to potentially limit PA (Colberg et al., 2010). In a joint position statement, the American College of Sports Medicine and American Diabetes Association recommended that those with peripheral neuropathy can safely participate in weight-bearing exercises as long as they do not have any open sores (Colberg et al., 2010). Because of the relationship between poor sensory PN and worse lower extremity outcomes in general populations of older adults, (Resnick et al., 2000; Strotmeyer et al., 2008; Strotmeyer et al., 2009; Ward et al., 2014a; Ward et al., 2015; Ward et al., 2014b) the consideration of the effects of PN impairments on PA may be relevant for non-diabetic older adults as well. However, PA recommendations have not been well-evaluated for older adults with PN impairments, and clearly more work is needed in this area.
Prior work in the InCHIANTI study—a population based cohort study of older adults in Italy—showed that higher CMAP measured at the peroneal motor nerve was associated with higher calf-muscle density in older adults (Lauretani et al., 2006). Worse muscle quality and function could make activity more difficult, and PA is also known to influence body composition and muscle function. Determining the timing of these neuromuscular changes and whether they are modifiable by PA is important in order to understand the pathway between PN impairments mobility limitations, and ultimately develop interventions to reduce the burden of lower extremity outcomes.
In our study, amplitude but not latency was associated with objective PA, though these relationships were largely attenuated to non-significance in adjusted models. Ultimately, only relationships between amplitude and vigorous activity remained borderline significant. However, motor latency and the presence of symptoms of neuropathy were associated with self-reported PA, with these relationships remaining statistically significant in final adjusted models. Amplitude and latency are indicators of different types of PN damage, with worse amplitude indicative of axonal degeneration, while latency, a component of conduction velocity, is a sign of demyelination (Mallik & Weir, 2005). The PASE and objective measure may have picked up different types of activity, potentially explaining why the associations were different across PA measurement types, and indicating that both may be useful in older or impaired populations. Using both methods allows for the detection of activities not assessed by monitors (i.e. swimming), and was also particularly helpful for assessing light intensity activities and sedentary time, which are difficult to assess via self-report. The majority of energy expenditure for older adults comes from sedentary and light activities (Colbert, Matthews, Schoeller, Havinghurst, & Kim, 2013; Copeland & Esliger, 2009), which makes it especially important to measure the lower end of intensity in order to capture the full extent of activity in this population.
We considered the cross-sectional relationship between PN function and PA; still, a bidirectional relationship between PN function and PA is possible. PN impairments—particularly sensory impairments—may make activity more difficult, while PA may be beneficial for improving PN function, though it is not clear which types of activities may be best. Small studies of participants with diabetes have indicated that exercise training may help reduce symptoms in those with peripheral neuropathy (Kluding et al., 2012). A long-term exercise intervention (4 years) reduced the incidence of peripheral neuropathy (Balducci et al., 2006), and short-term interventions may produce beneficial changes in gait performance in those with diabetic peripheral neuropathy (Mueller et al., 2013). Improving functional outcomes via PA is important for helping those with PN impairments to maintain independence.
Strengths and Limitations
A major strength of this study is the inclusion of comprehensive PN function measures, which allowed us to examine a range of PN function and also examine potential specific pathways of PN function. Limitations to this work should be considered. The use of an automated neurodiagnostic instrument allowed nerve conduction testing to be done in a non-invasive, efficient manner, but clinical cut-points established using traditional methods cannot be applied. Instead, we approximated clinical thresholds by comparing those in the worst tertile to those in the middle and best tertiles. Although nerve conduction testing is considered a gold standard assessment, many older adults—including our population—have very low amplitudes at an undetectable level. Future work is also needed in order to determine whether these results are applicable to other populations, including those with disabilities, women and non-white older adults.
In our analyses examining objective activity, we used MET cut-points to define intensity levels of physical activity. These categories allowed us to examine a range of activity intensities, however, cut-points can have limitations when applied to populations of older adults. Physical activity guidelines include recommendations for specific intensity levels, but relative intensity is important for prescribing physical activity for older adults (Nelson et al., 2007). Because many studies originally validating cut-points from accelerometer output were conducted in younger, healthy populations, alternate activity count cut-points and novel analysis methods have been suggested for assessing physical activity for older adults using accelerometers (Evenson, Buchner, & Morland, 2012; Gorman et al., 2014; Pruitt et al., 2008; Schrack et al., 2014). Many of these suggestions were made for traditional accelerometers, not necessarily for multi-sensor devices which process data using proprietary algorithms as we have used. Additionally, specific activity types could not be evaluated using these algorithms, but advances in data processing and analysis may allow for this in the future.
Conclusions
In conclusion, certain measures of better PN function were modestly associated with more daily minutes of vigorous PA per day and self-reported activity in older men. Lower levels of physical activity may be in the pathway between worse PN function and lower extremity disability, though future work is needed in order to fully understand the causal direction of this relationship. This potential pathway warrants further investigation in diverse populations of older men and women, and also in longitudinal work to elicit temporal relationships.
Acknowledgments
The Osteoporotic Fractures in Men (MrOS) Study is supported by National Institutes of Health funding. The following institutes provide support: the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS), the National Institute on Aging (NIA), the National Center for Research Resources (NCRR), and NIH Roadmap for Medical Research under the following grant numbers: U01 AR45580, U01 AR45614, U01 AR45632, U01 AR45647, U01 AR45654, U01 AR45583, U01 AG18197, U01 AG027810, and UL1 TR000128. This work was also supported by the American Diabetes Association (1-04-JF-46 to E.S.S.) Brittney S. Lange-Maia is funded by a National Institute on Aging Training Grant T32- AG000181 (to A.B.N.).
Accepted author manuscript version reprinted, by permission, from Journal of Aging and Physical Activity.
References
- Aboyans V, Criqui MH, Abraham P, Allison MA, Creager MA, Diehm C, … Treat-Jacobson D. Measurement and interpretation of the ankle-brachial index: A scientific statement from the American Heart Association. Circulation. 2012;126(24):2890–2909. doi: 10.1161/CIR.0b013e318276fbcb. [DOI] [PubMed] [Google Scholar]
- American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2013;36(Suppl 1):S67–74. doi: 10.2337/dc13-S067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldereschi M, Inzitari M, Di Carlo A, Farchi G, Scafato E, Inzitari D. Epidemiology of distal symmetrical neuropathies in the Italian elderly. Neurology. 2007;68(18):1460–1467. doi: 10.1212/01.wnl.0000260606.36443.29. [DOI] [PubMed] [Google Scholar]
- Balducci S, Iacobellis G, Parisi L, Di Biase N, Calandriello E, Leonetti F, Fallucca F. Exercise training can modify the natural history of peripheral neuropathy. Journal of Diabetes and its Complications. 2006;20:216–223. doi: 10.1016/j.jdiacomp.2005.07.005. [DOI] [PubMed] [Google Scholar]
- Blank JB, Cawthon PM, Carrion-Petersen ML, Harper L, Johnson JP, Mitson E, Delay RR. Overview of recruitment for the osteoporotic fractures in men study (MrOS) Contemporary Clinical Trials. 2005;26(5):557–568. doi: 10.1016/j.cct.2005.05.005. http://dx.doi.org/10.1016/j.cct.2005.05.005. [DOI] [PubMed] [Google Scholar]
- Bonnefoy M, Normand S, Pachiaudi C, Lacour JR, Laville M, Kostka T. Simultaneous validation of ten physical activity questionnaires in older men: A doubly labeled water study. Journal of the American Geriatrics Society. 2001;49(1):28–35. doi: 10.1046/j.1532-5415.2001.49006.x. [DOI] [PubMed] [Google Scholar]
- Cawthon P, Blackwell T, Cauley J, Ensrud K, Dam T, Harrison S, … Mackey D. Objective assessment of activity, energy expenditure, and functional limitations in older men: The osteoporotic fractures in men study. Journals of Gerontology, Series A, Biological Sciences and Medical Sciences. 2013;68(12):1518–24. doi: 10.1093/gerona/glt054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cimbiz A, Cakir O. Evaluation of balance and physical fitness in diabetic neuropathic patients. Journal of Diabetes and its Complications. 2005;19(3):160–164. doi: 10.1016/j.jdiacomp.2004.06.005. [DOI] [PubMed] [Google Scholar]
- Colberg SR, Sigal RJ, Fernhall B, Regensteiner JG, Blissmer BJ, Rubin RR, … Braun B. Exercise and type 2 diabetes: The American College of Sports Medicine and the American Diabetes Association: Joint position statement executive summary. Medicine and Science in Sports and Exercise. 2010;42(12):2282–2303. doi: 10.1249/MSS.0b013e3181eeb61c. [DOI] [PubMed] [Google Scholar]
- Colbert L, Matthews C, Schoeller D, Havinghurst T, Kim K. Intensity of physical activity in the energy expenditure of older adults. Journal of Aging and Physical Activity. 2013;22(4):571–577. doi: 10.1123/japa.2012-0257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copeland J, Esliger D. Accelerometer assessment of physical activity in active, healthy older adults. Journal of Aging and Physical Activity. 2009;17(1):17–30. doi: 10.1123/japa.17.1.17. [DOI] [PubMed] [Google Scholar]
- Dinger MK, Oman RF, Taylor EL, Vesely SK, Able J. Stability and convergent validity of the physical activity scale for the elderly (PASE) Journal of Sports Medicine and Physical Fitness. 2004;44(2):186–192. [PubMed] [Google Scholar]
- Evenson KR, Buchner DM, Morland KB. Objective measurement of physical activity and sedentary behavior among US adults aged 60 years or older. Preventing Chronic Disease. 2012;9:E26. [PMC free article] [PubMed] [Google Scholar]
- Feldman EL, Stevens MJ, Thomas PK, Brown MB, Canal N, Greene DA. A practical two-step quantitative clinical and electrophysiological assessment for the diagnosis and staging of diabetic neuropathy. Diabetes Care. 1994;17(11):1281–9. doi: 10.2337/diacare.17.11.1281. [DOI] [PubMed] [Google Scholar]
- Ferrucci L, Bandinelli S, Cavazzini C, Lauretani F, Corsi A, Bartali B, … Guralnik J. Neurological examination findings to predict limitations in mobility and falls in older persons without a history of neurological disease. American Journal of Medicine. 2004;116(12):807–815. doi: 10.1016/j.amjmed.2004.01.010. http://dx.doi.org/10.1016/j.amjmed.2004.01.010. [DOI] [PubMed] [Google Scholar]
- Fisher MA. Comparison of automated and manual F-wave latency measurements. Clinical Neurophysiology: Official Journal of the International Federation of Clinical Neurophysiology. 2005;116(2):264–9. doi: 10.1016/j.clinph.2004.08.016. [DOI] [PubMed] [Google Scholar]
- Gorman E, Hanson HM, Yang PH, Khan KM, Liu-Ambrose T, Ashe MC. Accelerometry analysis of physical activity and sedentary behavior in older adults: A systematic review and data analysis. European Review of Aging and Physical Activity. 2014;11(1):35–49. doi: 10.1007/s11556-013-0132-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregg E, Sorlie P, Pulose-Ram R, Eberhardt M, Wolz M, VB, … Geiss L. Prevalence of lower-extremity disease in the U.S. adult population 40+ years of age with and without diabetes. Diabetes Care. 2004;27(27):1591–1597. doi: 10.2337/diacare.27.7.1591. [DOI] [PubMed] [Google Scholar]
- Harada ND, Chiu V, King AC, Stewart AL. An evaluation of three self-report physical activity instruments for older adults. Medicine and Science in Sport and Exercise. 2001;33(6):962–70. doi: 10.1097/00005768-200106000-00016. [DOI] [PubMed] [Google Scholar]
- Jakicic JM, Marcus M, Gallagher KI, Randall C, Thomas E, Goss FL, Robertson RJ. Evaluation of the SenseWear pro armband to assess energy expenditure during exercise. Medicine and Science in Sport and Exercise. 2004;36(5):897–904. doi: 10.1249/01.mss.0000126805.32659.43. [DOI] [PubMed] [Google Scholar]
- Kluding P, Pasnoor M, Singh R, Jernigan S, KFarmer K, Rucker J, … Wright D. The effect of exercise on neuropathic symptoms, nerve function, and cutaneous innervation in people with diabetic peripheral neuropathy. Journal of Diabetes and its Complications. 2012;26:424–429. doi: 10.1016/j.jdiacomp.2012.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lalli P, Chan A, Garven A, Midha N, Chan C, Brady S, … Toth C. Increased gait variability in diabetes mellitus patients with neuropathic pain. Journal of Diabetes and its Complications. 2013;27(3):248–54. doi: 10.1016/j.jdiacomp.2012.10.013. [DOI] [PubMed] [Google Scholar]
- Lauretani F, Bandinelli S, Bartali B, Di Iorio A, Giacomini V, Corsi AM, … Ferrucci L. Axonal degeneration affects muscle density in older men and women. Neurobiology of Aging. 2006;27(8):1145–54. doi: 10.1016/j.neurobiolaging.2005.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loprinzi PD, Hager KK, Ramulu PY. Physical activity, glycemic control, and diabetic peripheral neuropathy: A national sample. Journal of Diabetes and its Complications. 2014;28(1):17–21. doi: 10.1016/j.jdiacomp.2013.08.008. http://dx.doi.org/10.1016/j.jdiacomp.2013.08.008. [DOI] [PubMed] [Google Scholar]
- Mackey DC, Manini TM, Schoeller DA, Koster A, Glynn NW, Goodpaster BH, … Cummings SR. Validation of an armband to measure daily energy expenditure in older adults. Journals of Gerontology, Series A, Biological Sciences and Medical Sciences. 2011;66(10):1108–1113. doi: 10.1093/gerona/glr101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallik A, Weir AI. Nerve conduction studies: Essentials and pitfalls in practice. Journal of Neurology, Neurosurgery, and Psychiatry. 2005;76(Suppl 2):ii23–31. doi: 10.1136/jnnp.2005.069138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDermott MM, Guralnik JM, Albay M, Bandinelli S, Miniati B, Ferrucci L. Impairments of muscles and nerves associated with peripheral arterial disease and their relationship with lower extremity functioning: The InCHIANTI Study. Journal of the American Geriatrics Society. 2004;52(3):405–410. doi: 10.1111/j.1532-5415.2004.52113.x. [DOI] [PubMed] [Google Scholar]
- Mueller MJ, Tuttle LJ, LeMaster JW, Strube MJ, McGill JB, Hastings MK, Sinacore DR. Weight-bearing versus nonweight-bearing exercise for persons with diabetes and peripheral neuropathy: A randomized controlled trial. Archives of Physical Medicine and Rehabilitation. 2013;94(5):829–838. doi: 10.1016/j.apmr.2012.12.015. http://dx.doi.org/10.1016/j.apmr.2012.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson ME, Rejeski WJ, Blair SN, Duncan PW, Judge JO, King AC, … Castaneda-Sceppa C. Physical activity and public health in older adults: Recommendation from the American College of Sports Medicine and the American Heart Association. Medicine and Science in Sports and Exercise. 2007;39(8):1435–45. doi: 10.1249/mss.0b013e3180616aa2. [DOI] [PubMed] [Google Scholar]
- Orwoll E, Blank JB, Barrett-Connor E, Cauley J, Cummings S, Ensrud K, … Stone K. Design and baseline characteristics of the osteoporotic fractures in men (MrOS) study — A large observational study of the determinants of fracture in older men. Contemporary Clinical Trials. 2005;26(5):569–585. doi: 10.1016/j.cct.2005.05.006. http://dx.doi.org/10.1016/j.cct.2005.05.006. [DOI] [PubMed] [Google Scholar]
- Pruitt LA, Glynn NW, King AC, Guralnik JM, Aiken EK, Miller G, Haskell WL. Use of accelerometry to measure physical activity in older adults at risk for mobility disability. Journal of Aging and Physical Activity. 2008;16(4):416–34. doi: 10.1123/japa.16.4.416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resnick HE, Stansberry KB, Harris TB, Tirivedi M, Smith K, Morgan P, Vinik AI. Diabetes, peripheral neuropathy, and old age disability. Muscle & Nerve. 2002;25(1):43–50. doi: 10.1002/mus.1217. [DOI] [PubMed] [Google Scholar]
- Resnick HE, Vinik AI, Schwartz AV, Leveille SG, Brancati FL, Balfour J, Guralnik JM. Independent effects of peripheral nerve dysfunction on lower-extremity physical function in old age: The Women’s Health and Aging Study. Diabetes Care. 2000;23(11):1642–1647. doi: 10.2337/diacare.23.11.1642. [DOI] [PubMed] [Google Scholar]
- Richardson JK, Ching C, Hurvitz EA. The relationship between electromyographically documented peripheral neuropathy and falls. Journal of the American Geriatrics Society. 1992;40(10):1008–1012. doi: 10.1111/j.1532-5415.1992.tb04477.x. [DOI] [PubMed] [Google Scholar]
- Schrack JA, Zipunnikov V, Goldsmith J, Bai J, Simonsick EM, Crainiceanu C, Ferrucci L. Assessing the “physical cliff”: Detailed quantification of age-related differences in daily patterns of physical activity. Journals of Gerontology, Series A, Biological Sciences and Medical Sciences. 2014;69(8):973–9. doi: 10.1093/gerona/glt199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuit AJ, Schouten EG, Westerterp KR, Saris WH. Validity of the physical activity scale for the elderly (PASE): According to energy expenditure assessed by the doubly labeled water method. Journal of Clinical Epidemiology. 1997;50(5):541–546. doi: 10.1016/s0895-4356(97)00010-3. [DOI] [PubMed] [Google Scholar]
- Schwartz AV, Vittinghoff E, Sellmeyer DE, Feingold KR, de Rekeneire N, Strotmeyer ES, … Harris TB. Diabetes-related complications, glycemic control, and falls in older adults. Diabetes Care. 2008;31(3):391–396. doi: 10.2337/dc07-1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strotmeyer ES, de Rekeneire N, Schwartz AV, Resnick HE, Goodpaster BH, Faulkner KA, … Newman AB. Sensory and motor peripheral nerve function and lower-extremity quadriceps strength: The health, aging and body composition study. Journal of the American Geriatrics Society. 2009;57(11):2004–2010. doi: 10.1111/j.1532-5415.2009.02487.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strotmeyer ES, De Rekeneire N, Schwartz AV, Faulkner KA, Resnick HE, Goodpaster BH, … Newman AB. The relationship of reduced peripheral nerve function and diabetes with physical performance in older white and black adults. Diabetes Care. 2008;31(9):17671–1772. doi: 10.2337/dc08-0433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teng E, Chui H. The modified mini-mental state (3MS) examination. Journal of Clinical Psychiatry. 1987;48(8):314–318. [PubMed] [Google Scholar]
- van Sloten TT, Savelberg HH, Duimel-Peeters IG, Meijer K, Henry RM, Stehouwer CD, Schaper NC. Peripheral neuropathy, decreased muscle strength and obesity are strongly associated with walking in persons with type 2 diabetes without manifest mobility limitations. Diabetes Research and Clinical Practice. 2011;91(1):32–9. doi: 10.1016/j.diabres.2010.09.030. [DOI] [PubMed] [Google Scholar]
- Ward RE, Boudreau RM, Caserotti P, Harris TB, Zivkovic S, Goodpaster BH, … Strotmeyer ES. Sensory and motor peripheral nerve function and longitudinal changes in quadriceps strength. Journals of Gerontology, Series A, Biological Sciences and Medical Sciences. 2015;70(4):464–70. doi: 10.1093/gerona/glu183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward RE, Caserotti P, Faulkner KA, Boudreau RM, Zivkovic S, Lee C, … Strotmeyer ES. Peripheral nerve function and lower extremity muscle power in older men. Archives of Physical Medicine and Rehabilitation. 2014a;95(4):726–33. doi: 10.1016/j.apmr.2013.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward RE, Boudreau RM, Caserotti P, Harris TB, Zivkovic S, Goodpaster BH, … Strotmeyer ES. Sensory and motor peripheral nerve function and incident mobility disability. Journal of the American Geriatrics Society. 2014b;62(12):2273–9. doi: 10.1111/jgs.13152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Washburn RA, McAuley E, Katula J, Mihalko SL, Boileau RA. The physical activity scale for the elderly (PASE): Evidence for validity. Journal of Clinical Epidemiology. 1999;52(7):643–651. doi: 10.1016/s0895-4356(99)00049-9. http://dx.doi.org/10.1016/S0895-4356(99)00049-9. [DOI] [PubMed] [Google Scholar]
- Washburn RA, Ficker JL. Physical activity scale for the elderly (PASE): The relationship with activity measured by a portable accelerometer. Journal of Sports Medicine and Physical Fitness. 1999;39(4):336–40. [PubMed] [Google Scholar]
- Washburn RA, Smith KW, Jette AM, Janney CA. The physical activity scale for the elderly (PASE): Development and evaluation. Journal of Clinical Epidemiology. 1993;46(2):153–162. doi: 10.1016/0895-4356(93)90053-4. http://dx.doi.org/10.1016/0895-4356(93)90053-4. [DOI] [PubMed] [Google Scholar]
- Yesavage JA, Brink TL, Rose TL, Lum O, VH, Adey M, Leirer VO. Development and validation of a geriatric depression screening scale: A preliminary report. Journal of Psychiatric Research. 1982;17(1):37–49. doi: 10.1016/0022-3956(82)90033-4. http://dx.doi.org/10.1016/0022-3956(82)90033-4. [DOI] [PubMed] [Google Scholar]