Abstract
Fenpropathrin is one of the widely used pyrethroids in agriculture and household and also reported to have neurotoxic effects in rodent models. In our Parkinson’s disease (PD) clinic, there was a unique patient with a history of daily exposure to fenpropathrin for 6 months prior to developing Parkinsonian symptoms progressively. Since whether fenpropathrin is related to any dopaminergic degeneration was unknown, we aimed in this study to evaluate the neurotoxic effects of fenpropathrin on the dopaminergic system and associated mechanisms in vitro and in vivo. In cultured SH-SY5Y cells, fenpropathrin caused cell death, reactive oxygen species generation, Lewy body-associated proteins aggregation, and Lewy body-like intracytoplasmic inclusions formation. In rodent animals, two different injections of fenpropathrin were used for administrations, intraperitoneal (i.p), or stereotaxical (ST). The rats exhibited lower number of pokes 60 days after first i.p injection, while the rats in ST group showed a significant upregulation of apomorphine-evoked rotations 60 days after first injection. Dcreased tyrosine hydroxylase (TH) and vesicular monoamine transporter 2 (VMAT2) immunoreactivity, while increased dopamine transporter (DAT) immunoreactivity were observed in rats of either i.p or ST group 60 days after the last exposure to fenpropathrin. However, the number of TH-positive cells in the substantia nigra was more reduced 120 days after the first i.p injection than those of 60 days. Our data demonstrated that exposure to fenpropathrin could mimic the pathologic and pathogenetic features of PD especially in late onset cases. These results imply fenpropathrin as a DA neurotoxin and a possible environmental risk factor for PD.
Keywords: Fenpropathrin, Parkinson’s disease, Dopamine, Dopamine transporter, Vesicular monoamine transporter 2, Tyrosine hydroxylase
Introduction
Parkinson’s disease (PD) is a common neurodegenerative disease, characterized by tremor, rigidity, bradykinesia, and postural instability. The hallmark pathology of PD includes the progressive degeneration of dopaminergic (DA) neurons and the formation of Lewy bodies in the substantia nigra [1]. Although the causes are unknown, environmental toxins especially pesticides are considered to be significant risk factors for PD. It has been proven that exposure to herbicides and insecticides increased the risk for PD with an average risk ratio of 1.62 [95 % confidence interval: 1.40, 1.88] [2].
Pyrethroid is a class of widely used pesticides, synthesized derivatives of natural insecticidal pyrethrins [3]. Pyrethroids have been increasingly used in agriculture and household due to their outstanding potency and environmental stability [4]. Pyrethroids are classified as type I and type II based on their chemical structure and neurotoxicological effects, as type I compounds have no alpha-cyano group on the phenoxybenzyl moiety and produce neurological syndrome characterized by aggressive sparring and tremors (T syndrome). However, type II has an alpha-cyano group and leads to a syndrome characterized by choreoathetosis and salivation (CS syndrome) [5]. Previous studies have indicated the toxic effects of pyrethroids on DA system. For example, deltamethrin could disrupt dopamine biosynthesis in PC12 cells by inhibition of tyrosine hydroxylase mRNA and protein expression [6]. Deltamethrin and permethrin increased DAT-mediated dopamine uptake and caused neurotransmitter release [7, 8]. In addition, exposure to cypermethrin, another pyrethroid, led to DA neurodegeneration in rats [9]. However, up to now, there is no evidence conclusively showing pyrethroids as an etiological factor of PD in epidemiology study.
Fenpropathrin (α-cyano-3-phenoxybenzyl-2, 2, 3, 3-terame-thylcyclopropanecarboxylase, (Fen)), a relatively new, synthetic pyrethroid for controlling insect pests, has not been classified into the traditional classifications of pyrethroids. Fenpropathrin is an alpha-cyano pyrethroid but exhibits neurotoxicological responses in several symptoms similar to both groups [10]. As the characteristic of quick metabolism and elimination from the body, fenpropathrin is thought to be nontoxic to mammals [11]. However, fenpropathrin displays long-lasting effects in the water and can be highly toxic to fish and be accumulated in fish [12]. It has been reported that fenpropathrin and deltamethrin decreased kynurenic acid (KYNA) concentration in rat cortical slices [13], a decreased concentration also be found in the brain tissue and cerebrospinal fluid of PD patients [14].
A 42-year-old man, farmer, was first admitted in the Department of Neurology, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology for the tremor on the left upper extremity for half a year in 2009. He has a history of eating fenpropathrin-poisoned fish for half a year, and then, the symptoms developed gradually 2 years later. About 60 days after first admission, his symptoms developed with rigidity on the left upper extremity and a slight tremor on the left lower extremity. He was diagnosed as PD according to the UK Parkinson’s Disease Society Brain Bank criteria. His Unified Parkinson’s Disease Rating Scale (UPDRS) part III (motor) score was 10 while his Hoehn and Yahr stage score was 1. His symptoms could be alleviated by madopar and sifrol, but progressed in the following 2 years. Although the fenpropathrin-mediated neurotoxicity in animals has been reported previously [5, 10], the correlation between the onset of PD and the history of fenpropathrin exposure was unclear, whether or not fenpropathrin has specific neurotoxic effects on DA cells or neurons remains unknown. Taking the possibility of fenpropathrin exposure causing lesions in DA neurons and contributing to developing PD into account, the widely usage of fenpropathrin could be a serious public health concern [15].
Thus, the present study aimed to explore the correlation between fenpropathrin and PD in vitro and in vivo DA systems. In SH-SY5Y cell model, the apoptosis, oxidative stress, and protein aggregation were assessed after fenpropathrin administration. Additionally, the animals were intraperitoneally or stereotaxically treated with fenpropathrin to investigate the effect of fenpropathrin on DA neurodegeneration and the underlying mechanisms.
Material and Methods
Chemicals and Reagents
Fenpropathrin (>92 % purity) was purchased from Zhejiang Dongyang-Xinhui Chemical Industry co., LTD, Zhejiang, China. SH-SY5Y cell line was obtained from the China Center for Type Culture Collection, China. Fetal bovine serum and DMEM/F12 medium were supplied by Gibco (Gibco BRL, NY, USA). JC-1, DCFH-DA, 3-(4, 5-dimethylthiazal-2-yl)-2, 5-diphenyl-tetrazolium bromide (MTT), Hoechst 33258, and apomorphine were purchased from Sigma-Aldrich (St. Louis, MO, USA). Dimethyl sulfoxide (DMSO), Triton-X100, and paraformaldehyde were obtained from AMRESCO (Solon, OH, USA); Annexin V/PI apoptosis detection kit from Bender MedSystems (USA); bovine serum albumin (BSA) from Invitrogen (Invitrogen, San Diego, CA, USA); rabbit polyclonal anti-alpha-synuclein antibody from Proteintech (Chicago, IL, USA); rabbit polyclonal anti-ubiquitin antibody from Abcam (Cambridge, UK); rabbit polyclonal anti-P62/SQTM1 antibody from Sigma-Aldrich (St. Louis, MO, USA); sheep polyclonal anti-TH antibodies from Abcam (Cambridge, UK); rabbit polyclonal anti-DATand rabbit polyclonal anti-VMAT2 antibodies from Santa Cruz (Santa Cruz, CA, USA); and rhodamine-conjugated goat anti-rabbit IgG and fluorescein isothiocyanate (FITC)-conjugated goat anti-rabbit IgG antibodies from Pierce (Rockford, IL, USA).
Cell Culture
SH-SY5Y cells were cultured in DMEM/F12 medium supplemented with 10 % fetal bovine serum and 5 % CO2 and 95 % air (v/v) at 37 °C. Fenpropathrin was dissolved in DMSO before diluted into the culture medium. Equal volume of DMSO was added to the culture medium in control group. The final concentration of Fen in dose-dependent study was 0.5, 1, 10, 25, 50, and 100 μM (Fen-0.5, Fen-1, Fen-10, Fen-25, Fen-50, and Fen-100 groups), respectively, treating for 24 h. For the time-dependent study, Fen (25 μM) was given for 6, 12, 24, 36, 48, and 72 h (Fen-6 h, Fen-12 h, Fen-24 h, Fen-36 h, Fen-48 h, and Fen-72 h groups), respectively, to induce cell damage.
Measurement of Cell Viability
Cell viability was examined by the 3-(4, 5-dimethylthiazal-2-yl)-2, 5-diphenyl-tetrazolium bromide (MTT) assay [16, 17]. SH-SY5Y cells were plated at a density of 1×104 cells per well in 96-well plates. After Fen treatment, the cells were incubated with 20 μl MTT (5 mg/ml; Sigma-Aldrich, St. Louis, MO, USA) at 37 °C for 4 h. After removal of the medium, 150 μl DMSO was added into each well to dissolve the formazan crystals. Then the absorbance was measured by a microplate reader at a test wavelength of 570 nM and reference wavelength of 630 nM. Cell viability was expressed as a percentage of the value in DMSO-treated control cells.
Apoptosis Detection in SH-SY5Y Cells
Apoptosis was analyzed by flow cytometry (BD, Franklin Lakes, NJ, USA) according to manufacturer’s instructions of the Annexin V/PI apoptosis detection kit [17, 16]. After fenpropathrin treatment, 2×106 SH-SY5Y cells were harvested by treatment with 0.25 % trypsin, washed with ice-cold phosphate-buffered saline (PBS) for three times, and incubated for 10 min with a combination of 5 μl of Annexin V-FITC and 10 μl propidium iodide (PI) working solution at 37 °C in the darkness. The specific fluorescence of 10,000 cells was analyzed by the FACSCalibur (BD) within 1 h after the FITC-conjugated Annexin V was added. Data were analyzed by FlowJo 7.6.5. The apoptosis rate=[Annexin V(+) PI(−) cells+ Annexin(+) PI(+) cells]/total cell×100 %.
Detection of MMP and ROS in SH-SY5Y Cells
The method for the analysis of mitochondrial membrane potential (MMP) and reactive oxygen species (ROS) was referred to a previously study [18]. Cells were harvested, resuspended in PBS, and immediately stained with JC-1 (1 mg/ml in DMSO) or DCFH-DA (10 μM), and incubated in darkness at 37 °C for 30 min. After washing twice with ice-cold PBS, the samples were subject to FACScan flow Cytometry (BD). Data were analyzed by FlowJo 7.6.5.
Histopathological Analysis of SH-SY5Y Cells
SH-SY5Y cells grown on cover slips were fixed with 4 % paraformaldehyde at 4 °C for 30 min, washed by PBS and then stained with hematoxylin and eosin (H&E), and examined under a light microscope to examine Lewy body-like structure.
Immunocytochemistry Detection in SH-SY5Y Cells
SH-SY5Y cells were fixed with 4 % paraformaldehyde at 4 °C for 30 min, washed with 0.1 M PBS for three times, and permeabilized with 0.1 % Triton-X100 for 20 min. The slips were blocked with 5 % BSA in 0.1 M PBS for 1 h [19], then incubated with anti-alpha-synuclein (1:50 dilution), anti-ubiquitin (1:1000 dilution), or anti-P62/SQTM1 (1:1000 dilution) at 4 °C overnight and then rhodamine-conjugated goat anti-rabbit IgG (1:200 dilution) or FITC-conjugated goat anti-rabbit IgG (1:200 dilution) at 37 °C for 1 h. After washed by PBS, the cells were incubated with Hoechst 33258 (10 μg/ml) at room temperature for 10 min. The slides were observed under a Zeiss confocal microscope (Carl Zeiss; Jena, Germany), and the pictures were analyzed by Image-Pro Plus 6.0 (Bethesda, MD, USA).
Animals and Treatment
Adult Sprague-Dawley rats were obtained from the Wuhan University Center for Animal experiment/A3-Lab. Rats were maintained under standard conditions with a 12-h light/dark cycle and at temperature 22±2 °C and relative humidity at 50±10 %. Food and water were provided ad libitum. Animal handling and procedures were approved by the Ethical Committee on Animal Experimentation of Tongji Medical College, Huazhong University of Science and Technology.
Rats were randomly assigned to fenpropathrin-treated and control groups. Fenpropathrin was dissolved in corn oil or DMSO and administered to rats by intraperitoneal injection (i.p) or stereotaxical injection (ST) (Supplementary Fig. 1). Some of the rats were continuously treated intraperitoneally with fenpropathrin (15 mg/kg/day) while the controls were treated with an equal volume of corn oil for 60 days (1 day for rest in every 7 days). Furthermore, half of the fenpropathrin-treated rats were left untreated under normal conditions for 60 days after the last injection. Half of the rats were sacrificed 24 h after the last treatment of fenpropathrin.
For ST infusion, the method was following our previous report [20]. Fenpropathrin dissolved in DMSO (6 μg/μL, 1 μL) was infused into the right ventral tegmental area (VTA; coordinates: AP −5.0 mm, ML 1.0 mm, DV −7.8 mm) and substantia nigra pars compacta (SNc) (coordinates: AP −5.0 mm, ML 2.0 mm, DV −8.0 mm) at a flow rate of 0.2 μL/min. After the injection, the needle was left in place for additional 5 min for complete diffusion of the drug before being slowly withdrawn from the brain. The animals received proper care after surgery until completely recovered, then were fed under normal conditions for 60 days.
Behavioral Studies
Behavioral studies were performed using the adjusting steps test, hole and board test, and apomorphine (APO)-induced rotations. The adjusting steps test is an assessment of rigidity extent [21]. The experimenter placed one hand along the side of the rat and with the other hand fixing the forelimb that was not to be monitored. The rats were pushed to move slowly sideways. The number of adjusting steps for both directions and both forelimbs was counted. The hole and board test was used to assess exploratory behavior of the rats [22]. The rats were put on a wooden plank with many holes. The tendency of the rats to insert their head into the holes was observed. The number of exploring the holes per 5 min was recorded for each animal. APO-induced rotations were used to evaluate the lesions of DA neurons in the ST infusion rats every 14 days after fenpropathrin exposure. The rats were placed on a table (1×1 m) with a railing and allowed to acclimate to the environment for 10 min. And then, the rats received APO (1.5 mg/kg) intraperitoneally [20]. The rotations were recorded for 30 min after APO exposure.
TH immunoreactivity and TH-positive Neuron Count
Tissue staining and cell counts followed the previously reported method with slight modifications [20]. Rats were deeply anesthetized with 10 % chloral hydrate (3.5 ml/kg, i.p) at the time of sacrifice and perfused with ice-cold 0.9 % sodium chloride and fixated with 4 % paraformaldehyde in 0.1 M phosphate-buffered saline (PBS). Brains were quickly removed and fixed in 4 % paraformaldehyde at 4 °C for 24 h. Then the brains were mounted on gelatin-coated slides and air-dried overnight. Coronal sections (5 μm) were cut through the striatum and the substantia nigra by using a sledge microtome (Wetzlar, Germany). The sections were de-waxed, hydrated in descending grades of ethanol, and then quenched with 0.3 % H2O2 for 30 min to consume endogenous peroxidase. After antigen retrieval by citrate buffer, the slides were treated with 0.1 % Triton-X100 for 30 min and 5 % bovine serum albumin (BSA) at room temperature for 2 h. The sections were first incubated with anti-TH antibody (1:500) at 4 °C overnight. After washing with PBS, the sections were incubated with a biotinylated goat anti-sheep secondary antibody at room temperature for 1 h. After incubating with peroxidase-conjugated streptavidin for 45 min, the sections were visualized by 3, 3′-diaminobenzidine (DAB) for 15–20 min. The sections were visualized under the light microscope, and the TH-positive cells were counted according to the method described previously [23].
For immunofluorescence assay, sections of the SNc and striatum were incubated with TH antibody (1:500) at 4 °C overnight and then with the secondary FITC-conjugated donkey anti-sheep IgG antibody (1:200) diluted by 10 μg/ml Hoechst 33258 at room temperature for 1 h. The slides were then observed under a confocal fluorescence microscope, and the pictures were analyzed by Image-Pro plus 6.0.
Measurements of Dopamine, DOPAC, and HVA
The animals were sacrificed via cervical dislocation. The striatum was isolated from the brain, immediately frozen and stored at −80 °C and preceded for analyses. Dopamine, 3,4-dihydroxyphenylacetic acid (DOPAC), and homovanillic acid (HVA) were measured as described previously [20]. The striatum was sonicated in ice-cold 0.01 M HClO4 solution containing 0.01 % EDTA. The homogenate was centrifuged at 12,000g at 4°C for 10 min, and the supernatant was filtered through a syringe filter (0.22 μm). The filtrate (20 μL) was injected into reverse phase C-18 high-performance liquid chromatography (HPLC) column equipped with a fluorescence detector (Waters, MA). The mobile phase consisted of trisodium citrate (0.02 M), sodium dihydrogen phosphate (0.05 M), methanol (40 %), EDTA (0.028 g/L), and SDS (0.15 g/L). The solution was adjusted to PH 3.5 with 98 % H2SO4, filtered through a 0.45-μm membrane filter. The separation was achieved at a flow rate of 1.0 ml/min, and the column temperature was set at 40 °C. After separation, DA, DOPAC, and HVA were detected at the excitation wavelength of 280 nm and an emission wavelength of 315 nm.
DAT and VMAT2 Immunoreactivity
For the dopamine transporter (DAT) or vesicular monoamine transporter 2 (VMAT2) staining, the brain tissues were sectioned into 15 μm-thick slices with a cryostat. The sections were fixed with 4 % paraformaldehyde for 30 min and then treated with 0.1 % Triton-X100 for 30 min and 5 % bovine serum albumin (BSA) at room temperature for 2 h. The sections were incubated with anti-DAT antibody (1:50 dilution) or anti-VMAT2 antibody (1:50 dilution) at 4 °C overnight. Primary antibodies were visualized by the FITC-conjugated donkey anti-rabbit IgG (1:100 dilution), and the nuclei were stained with Hoechst 33258 (10 μg/ml) at room temperature for 1 h. The slides were then observed under a confocal fluorescence microscope, and the pictures were analyzed by Image-Pro plus 6.0.
Histopathological Analysis of Brains
After being de-waxed, hydrated in descending grades of ethanol, the SNc sections (5 μm) were stained with hematoxylin and eosin (H&E) and then examined under a light microscope.
Pathology of the Peripheral Organs
The pathologic changes of the peripheral organs including the heart, lung, kidney, and liver were examined on four rats from each group. The peripheral organs were dissected and fixed in 4 % paraformaldehyde before being embedded in paraffin. The tissue was sectioned into 5 μm pieces, stained with hematoxylin and eosin (H&E), and then examined under a light microscope.
Statistical Analysis
Statistical analyses were carried out using SPSS version 12.0 for Windows (SPSS, Chicago, IL, USA). Given a normal distribution in all groups, the intergroup differences were assessed by one-way analysis of variance (ANOVA) and/or Student t tests. The results are presented as the means±SD, with P value<0.05 as statistically significant.
Results
Fenpropathrin Inhibited the Cell Proliferation of SH-SY5Y in a Dose-Dependent Fashion
We first analyzed the neurotoxic effect of fenpropathrin in SH-SY5Y cells. By the MTT test, fenpropathrin inhibited SH-SY5Y cell viability in a dose-dependent manner (Fig. 1). Compared to the control group, SH-SY5Y cell viability was decreased to 56.09±4.61, 51.77±3.17, 42.83±3.45, 28.01± 1.42, and 36.86±1.83 % in Fen-1, Fen-10, Fen-25, Fen-50, and Fen-100 groups, respectively, after a 24 h exposure to Fen. Fenpropathrin at a dose range from 1 to 100 μM significantly inhibited the cell viability (Fig. 1g). Furthermore, fenpropathrin altered the morphology of the SH-SY5Y cells, causing shorter apophysis and shrunken bodies compared to the control cells (Fig. 1a–f).
Fig. 1.
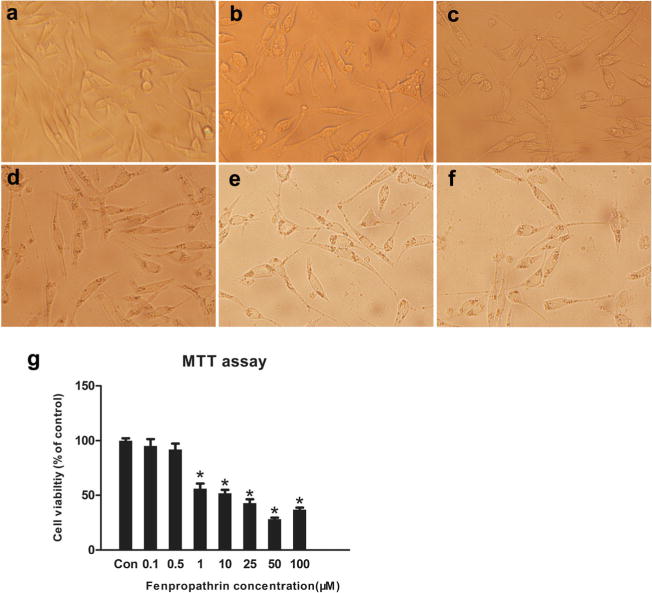
Fenpropathrin induced morphological changes and decreased viability in SH-SY5Y .a–f Morphological changes in cells after 24 h treatment with fenpropathrin in Con, Fen-1, Fen-10, Fen-25, Fen-50, and Fen-100 groups; g Dose-dependent effect of fenpropathrin treatment for 24 h on cell viability in Con, Fen-0.5, Fen-1, Fen-10, Fen-25, Fen-50, and Fen-100 groups. Results are expressed as percentage of values in untreated control culture and are calculated as mean ± standard error of three replicate values in three independent experiments. Significant changes are expressed as *P<0.05, compared to the Con group
Fenpropathrin Induced SH-SY5Y Cell Apoptosis in a Dose- and Time-Dependent Manner
After fenpropathrin exposure, cells were harvested and incubated with Annexin V-FITC/PI before submitted to a flow cytometry. Our data showed that fenpropathrin evoked dose-and time-dependent apoptosis in SH-SY5Y. The apoptosis rate of Fen-10, Fen-25, Fen-50, and Fen-100 groups was significantly different from that in the Con group (Fig. 2b). When exposed to fenpropathrin (25 μM), the apoptosis rate of Fen-24 h, Fen-36 h, Fen-48 h, and Fen-72 h groups was significant higher than that in the Con group (Fig. 2a, c).
Fig. 2.
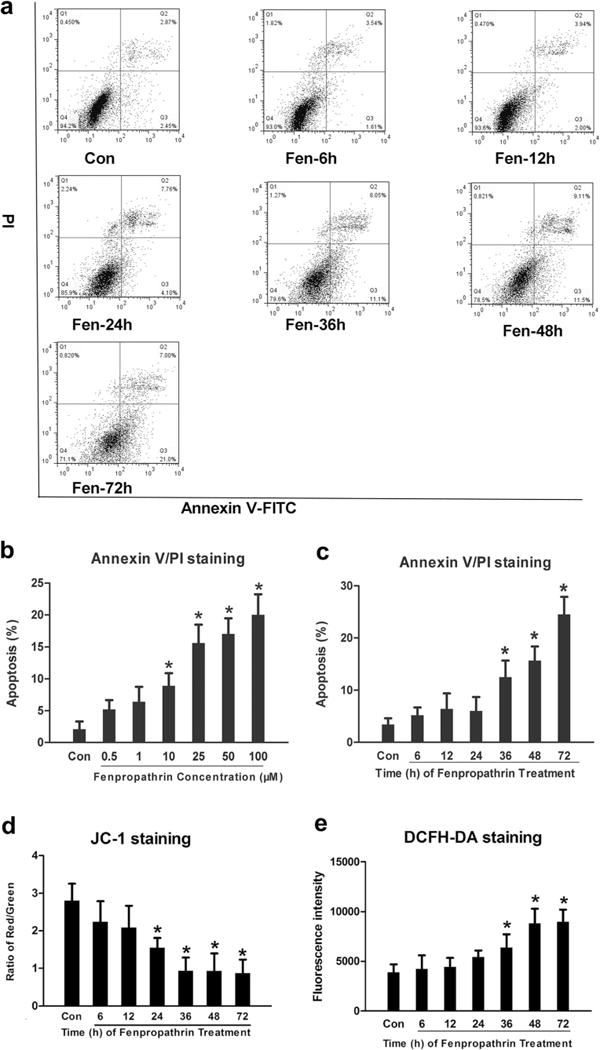
Fenpropathrin induced dose- and time-dependently apoptosis, time-dependently MMP decrease, and ROS generation in SH-SY5Y. a Scatter diagram of propidium iodide (PI)/Annexin V gating from fenpropathrin (25 μM) treatment in Con, Fen-6 h, Fen-12 h, Fen-24 h, Fen-36 h, Fen-48 h, and Fen-72 h groups (the apoptosis rates=[Annexin V(+)/PI(+) cells+Annexin V(+)/PI(−) cells]/total cells×100 %). b, c Statistical analysis of apoptosis rates in dose-dependent groups or time-dependent groups (*P<0.05, compared to the Con group). d Relative percentage of red/green immunofluorescence stained cells in Con, Fen-6 h, Fen-12 h, Fen-24 h, Fen-36 h, Fen-48 h, and Fen-72 h groups. e Fluorescence intensity of DCFH in Con, Fen-6 h, Fen-12 h, Fen-24 h, Fen-36 h, Fen-48 h, and Fen-72 h groups (*P<0.05, compared to the Con group)
Fenpropathrin Induced MMP Reduction and ROS Generation in SH-SY5Y Cells
The loss of MMP is a hallmark of early apoptosis. During apoptosis, JC-1 aggregates in the mitochondrial membrane change to JC-1 monomeric form, resulting in red fluorescence changing to green fluorescence. Our results showed that fenpropathrin treatment induced the JC-1 monomer formation in a time-dependent manner. The ratio of JC-1 monomers of Fen-24 h, Fen-36 h, Fen-48 h, and Fen-72 h groups was significantly different from that in the Con group (Fig. 2d).
Probe DCFH-DA was used to detect ROS in SH-SY5Y cells after exposure to Fen. When DCFH-DA diffuses into the cell, it forms a nonfluorescent compound DCFH and then could be oxidized into fluorescent compound DCF by ROS. Our data showed that fenpropathrin caused ROS generation in a time-dependent manner. The fluorescence intensity of DCF of Fen-36 h, Fen-48 h, and Fen-72 h groups was significantly higher than that in the Con group (Fig. 2e).
Fenpropathrin Caused Lewy Body-Like Inclusion Formation
SH-SY5Y cells were exposed to fenpropathrin for 48 h in a dose-dependent manner, and the H&E staining was employed to observe the pathological changes in SH-SY5Y cells. Lewy body-like eosinophilic intracytoplasmic inclusions could be observed in fenpropathrin-treated SH-SY5Y cells at a concentration higher than 10 μM, especially in Fen-25 and Fen-50 groups (Fig. 3a).
Fig. 3.
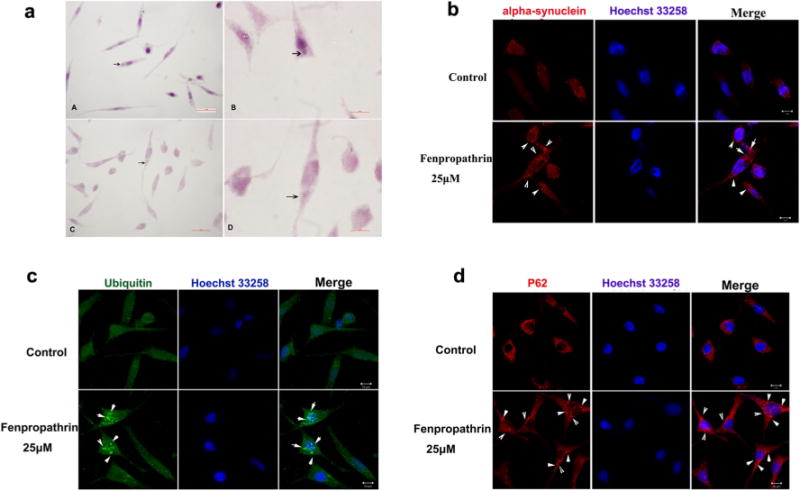
Fenpropathrin induced intracytoplasmic eosinophilic inclusion formation and α-synuclein, ubiquitin, and P62 aggregation in SH-SY5Y. a Intracytoplasmic eosinophilic aggregations (arrows) in some of fenpropathrin-treated (25 μM (3A–A, B) and 50 μM (3A–C, D) for 48 h) groups. b α-synuclein immunofluorescence staining positive aggregations (arrows) in fenpropathrin (25 μM) treatment group. c Ubiquitin immunofluorescence staining positive aggregations (arrows) in fenpropathrin (25 μM) treatment group. d P62 immunofluorescence staining positive aggregations (arrows) in fenpropathrin (25 μM) treatment group
Fenpropathrin Induced α-Synuclein, Ubiquitin, P62 Aggregates in SH-SY5Y Cells
Fenpropathrin-treated SH-SY5Y cells (25 μM, for 48 h) were stained with antibodies for α-synuclein, ubiquitin, and P62/SQTM 1 (Fig. 3). Lewy body is the hallmark of PD, and it has been reported that α-synuclein, ubiquitin, and P62 aggregations are immunoreactively positive in Lewy body [24]. Our data indicated that cell body swelling could be observed in fenpropathrin-administrated SH-SY5Y cells (Fig. 3). Meanwhile, the α-synuclein (Fig. 3b) and ubiquitin (Fig. 3c) were concentrated in the cytosol; P62 (Fig. 3d) aggregation increased in fenpropathrin group compared to that in the Con group, indicating that fenpropathrin could induce protein accumulation which is a main feature of Lewy body.
Fenpropathrin Caused Locomotor Activity Reduction
Continuously, fenpropathrin treatment by i.p induced restlessness and hyperexcitability during the initial 2 weeks, followed by a gradual onset of symptoms, including hypo-excitability, hypokinesia, and hair loss about 4 weeks later. The rats showed less locomotor activities, less climbing, weaken behavior of resisting arrest, and slow movement. Two of the i.p group rats exhibited tremor in unilateral limbs after 60 days exposure to fenpropathrin. One hundred twenty days after the first exposure, four of the rats in i.p group showed back hunching and whole body tremor. All rats were submitted to adjusting steps test to assess rigidity and akinesia 1 day before the sacrifice [25, 21]; however, no significant difference was observed among the fenpropathrin ST group and fenpropathrin i.p group (Fig. 4b). While the hole and board test demonstrated that i.p fenpropathrin-treated rats showed deteriorated exploratory ability 120 days after the first exposure, ST infusion group and control group showed no changes (Fig. 4c), suggesting that chronic fenpropathrin treatment may decrease motor skills gradually. ST infusion group rats were submitted to APO-induced rotations to assess the lesions of DA neuron. The ST infusion rats showed typical contralateral rotations after APO injections at all time points (Fig. 4d), indicating that DA neurons were damaged by fenpropathrin. But the rotational speed was different among the rats in ST group, ranged from 198 to 452 times per 30 min. Collectively, the behavioral results showed the loss of motor ability after fenpropathrin exposure. Moreover, we found that i.p infusion group exhibited slower growth in body weight than ST infusion group and control group (Fig. 4a).
Fig. 4.
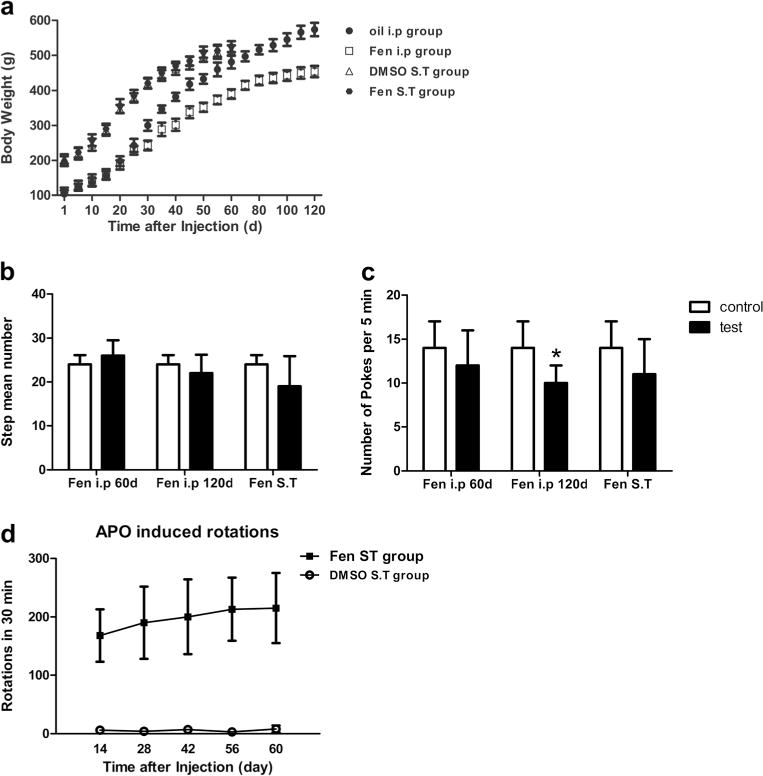
Fenpropathrin induced body weight and behavioral changes in rats. a Body weight of animals after i.p or ST infusion of fenpropathrin. I.p administration of fenpropathrin decreased body weight of these animals after 30 days exposure continuously, while no significant difference was found between the fenpropathrin ST group and DMSO-ST control group animals in whole period. b Step adjusting test and c Poke test were carried out 60 and 120 days after fenpropathrin first treatment by i.p and 60 days after fenpropathrin ST infusion (*P<0.05, compared to the Con group). d Effects of fenpropathrin on APO-evoked rotations 14, 28, 42, 56, and 60 days after ST surgery (Fen i.p 60 day, fenpropathrin treatment for 60 days by i.p group; Fen i.p 120 day, 120 days after the first exposure to fenpropathrin by i.p infusion group; Fen ST, 60 days after the last exposure to fenpropathrin by ST infusion)
Fenpropathrin Caused Decreases in TH-positive Neuron Counts and TH immunoreactivity in the CPu and SNc
Sixty days after the last exposure to i.p or ST infusion of fenpropathrin, the rats exhibited a significant reduction in number of TH-positive neurons compared to control groups (Fig. 5a, b). The rats in i.p group exhibited a slightly reduced number of TH-positive neurons 60 days after first injection, without reaching statistical significance.
Fig. 5.
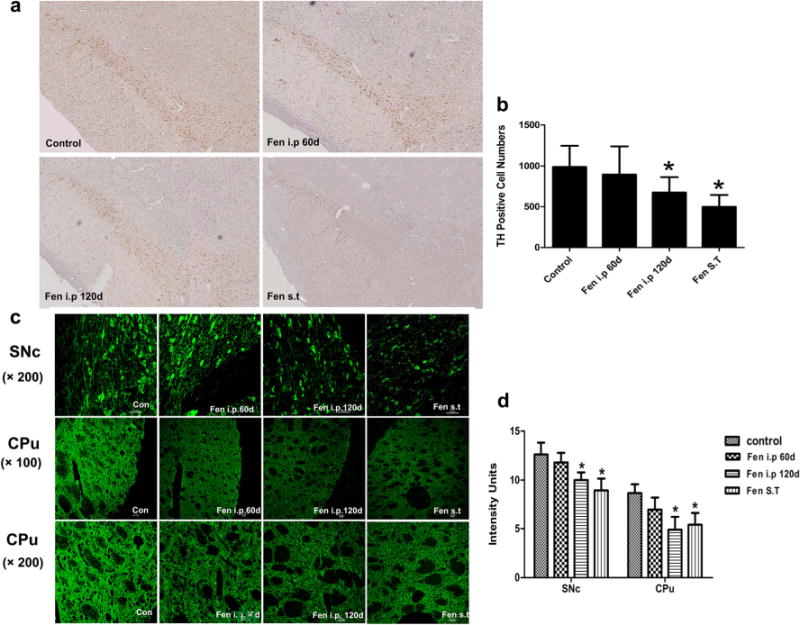
Fenpropathrin induced loss of TH immunoreactivity in the CPu and SNc. a TH-positive cells in the SNc of the rats exposed to fenpropathrin by i.p infusion or ST infusion stained by TH immunohistology, and was observed by bright-field microscopy at 4× magnification. c TH-positive cells in the SNc and the TH immunoreactivity in the CPu of the rats exposed to fenpropathrin by i.p infusion or ST infusion. Quantitative analysis of the number of TH-positive cells in the SNc b and the TH immunoreactivity d in the CPu and SNc (*P<0.05, compared to the Con group)
Meanwhile, for the immunofluorescence staining of the SNc and striatum tissues, TH immunoreactivity in the striatum was lower in the animals treated with fenpropathrin by i.p or ST group than that in the untreated group (Fig. 5c, d). Specifically, the animals exhibited a more pronounced decrease in striatal TH immunoreactivity of either i.p group or ST group 60 days after the last exposure to fenpropathrin, comparing to the rats exposed to the oil or DMSO. In consistency with the striatal immunostaining, compared with control group, the SNc TH immunoreactivity was lower in both i.p and ST rats 60 days after the last exposure to fenpropathrin. However, TH immunoreactivity did not display any significant change between the rats in i.p group 60 days after first injection with the control group.
Fenpropathrin Decreased the Levels of Dopamine, DOPCA, and HVA in Treated Animals
The striatum levels of dopamine and its metabolites in Fentreated animals were assessed (Fig. 6). The levels of dopamine, DOPAC, and HVA in the striatum were significantly reduced in the rats of either 120 days after the first injection of the i.p group or 60 days after the injection of the ST group comparing with their control groups.
Fig. 6.
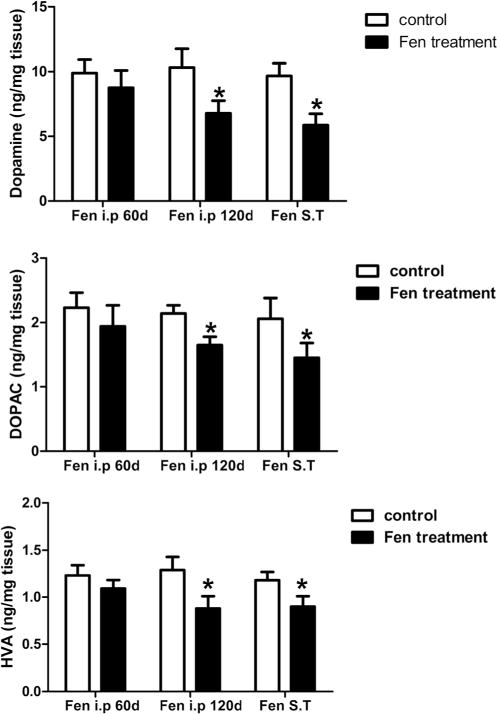
Fenpropathrin decreased dopamine, DOPAC, and HVA. High-performance liquid chromatography (HLPC) analyses of dopamine (a), DOPAC (b), and HVA (c) were performed in the striatum of control and fenpropathrin-treated rats. The values are calculated as mean ± standard error of the mean (n=3 to 4) (*P<0.05, compared to the Con group)
Fenpropathrin Induced Upregulation of DAT and Downregulation of VMAT2 Expression
The effect of fenpropathrin on DAT or VMAT2 immunoreactivity of the neurons in the SNc was assessed (Fig. 7). Fenpropathrin significantly increased DAT immunoreactivity, especially in ST infusion group (Fig. 7a, b). The VMAT2 immunoreactivity was decreased in the rats during 60 days after the last exposure to fenpropathrin by either i.p infusion or ST infusion comparing with control rats (Fig. 7c, d). Moreover, the i.p infusion group exhibited higher DAT immunoreactivity and lower VMAT2 immunoreactivity 120 days after the first exposure than that of 60 days after the first exposure.
Fig. 7.
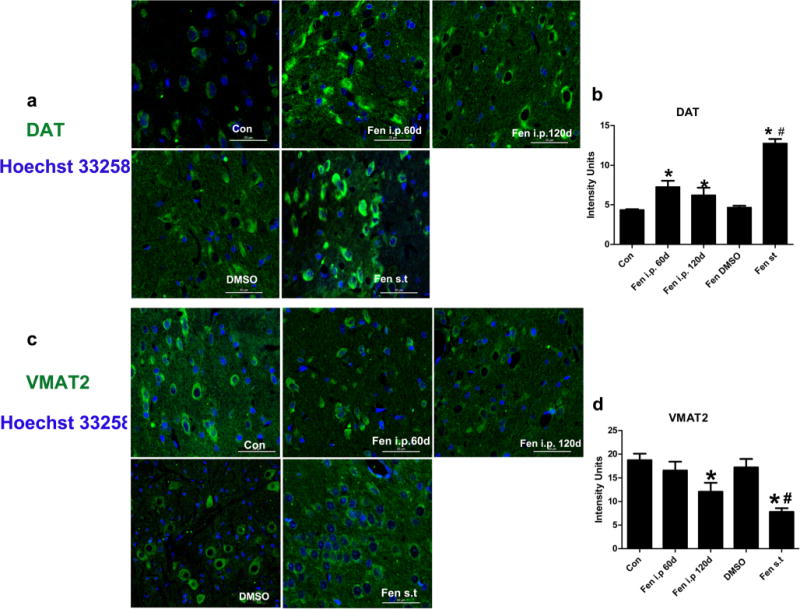
Fenpropathrin increased DAT but decreased VMAT2 immunoreactivities in the SNc. a DAT immunoreactivity in the SNc of Con, Fen-60d, Fen-120d, DMSO, and Fen-ST groups. b Quantitative analysis of DAT immunoreactivity in the SNc. c VMAT2 immunoreactivity in the SNc of Con, Fen-60d, Fen-120d, DMSO-, and Fen-ST groups. d Quantitative analysis of VMAT2 immunoreactivity in the SNc (*P<0.05, compared to the Con group; #P<0.05, compared to DMSO group)
Fenpropathrin Caused Distorted Neuronal Morphology in the SNc
H&E staining revealed distorted neuronal morphology in the SNc of fenpropathrin-treated rats comparing to controls. However, no eosinophilic inclusions were found in the SNc neurons (Fig. 8a, b).
Fig. 8.
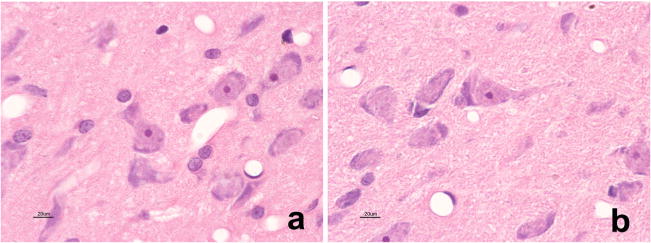
Fenpropathrin caused distorted neuronal morphology in the SNc. H&E staining was used to observe the morphological changes of the SNc neurons in fenpropathrin-treated rats. a and b showed the morphological changes of the neurons in the SNc of control group and fenpropathrin (ST) group, respectively
I.p Infusion of Fenpropathrin Induced Peripheral Organ Toxicity in the Animals
In i.p infusion animals, significant changes were observed in the lung and liver, while no obvious changes could be found in the kidney and heart (Fig. 9). For the ST infusion animals, no changes were found in the peripheral organs 60 days after the first injection. In i.p animals, alveolar ectasia and alveolar wall fragmentation were detected in the lung. Meanwhile, hepatic sinus expansion around the central tube was observed in the liver. No mortality was associated with i.p-infused animals within 60 days of the fenpropathrin administration.
Fig. 9.
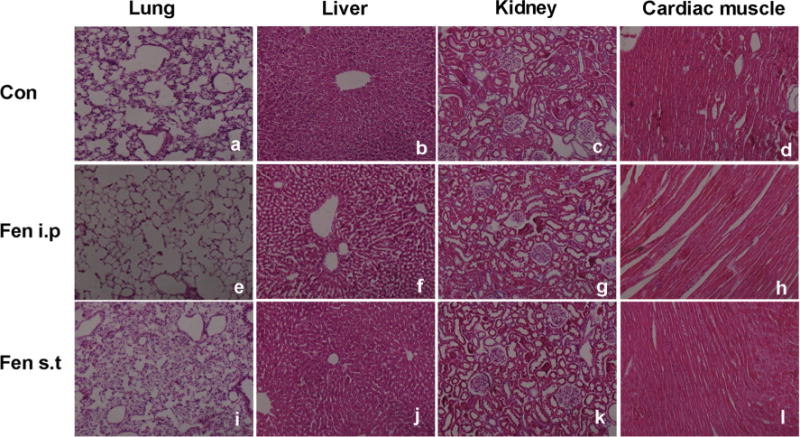
Pathological changes in peripheral organs of rats exposed to fenpropathrin by i.p or ST infusion, based on H&E staining. a–d Pathological morphology of the lung, liver, kidney, and cardiac muscle in the Con group. e–h Pathological morphology of the lung, liver, kidney, and cardiac muscle in fenpropathrin i.p group. i–l Pathological morphology of the lung, liver, kidney, and cardiac muscle in fenpropathrin ST group
Discussion
In this study, we present solid and consistent evidences for the specifically neurotoxic effects of fenpropathrin on DA neurons in both in vitro and in vivo models. First, fenpropathrin decreased SH-SY5Y cell viability in a dose-dependent manner. Second, fenpropathrin induced cell apoptosis in a dose- and time-dependent fashion, MMP reduction, and ROS generation in time-dependent way. Third, fenpropathrin caused eosinophilic Lewy body-like inclusion formation and increased alpha-synuclein, ubiquitin, and P62 aggregations in SH-SY5Y cell cytoplasm. Fourth, fenpropathrin decreased the number of TH-positive neurons in the SNc and the TH immunoreactivity in both the SNc and striatum, especially in the ST group which was consistent with APO-evoked rotation changes. Finally, fenpropathrin increased the expression of DAT, but decreased the expression of VMAT2 in vivo. These data suggest that fenpropathrin could model pathogenesis features in vitro and in vivo for PD.
Oxidative damage may be an important mechanism of fenpropathrin-induced apoptosis, as the ROS level was significantly increased in fenpropathrin-treated groups compared to that in the Con group. SH-SY5Y cell line is a widely used DA neuronal model for PD research [26, 27], because these cells possess many characteristics of DA neurons, such as the ability to synthesize dopamine (DA) or norepinephrine and express tyrosine hydroxylase and dopamine-β-hydroxylases [28]. SH-SY5Y cells are susceptible to damage by ROS just like dopamine neurons [29, 30]. Over-generation of ROS by fenpropathrin may aggravate oxidative damage to SH-SY5Y cells. Consistent with previous observations, other pyrethroids such as deltamethrin and cypermethrin have been reported to produce oxidative stress in cells as well [31, 6]. However, the underlying mechanism by which pyrethroids induced oxidative stress is still unknown. It was reported that decreased activity of mitochondria complex I and cytochrome P450 [32], and mitochondrial dysfunction was involved in pyrethroid-induced oxidative stress [33].
Protein aggregates could be another harmful factor in fenpropathrin-treated SH-SY5Y cells. Eosinophilic intracytoplasmic inclusions with increased α-synuclein, ubiquitin, and P62 protein aggregates could be found in our fenpropathrin-treated SH-SY5Y cells. Lewy body is one of the histopathological hallmarks of PD and appears in neuronal somata as eosinophilic, rounded inclusions. It is known that Lewy body is intracellular deposit of proteins and lipids [34], including PD-linked gene products (alpha-synuclein, parkin, LRRK2, DJ-1), molecules implicated in ubiquitin-proteasome system (ubiquitin, P62), and mitochondria-related proteins [24, 35]. According to the character of Lewy bodies, we concluded that fenpropathrin induced Lewy body-like structural formation and Lewy body-associated protein aggregates in SH-SY5Y cells. However, the underling mechanism and consequence of the protein aggregates need further investigation.
Fenpropathrin could easily cross the blood brain barrier because of its lipophilic nature, as we have found fenpropathrin in the brain tissue of mice exposed to fenpropathrin by intraperitoneal administration (Supplementary Fig. 2). In preliminary experiment, 7.5 mg/kg/day (1/30 LD50), 11.25 mg/kg/day (1/20 LD50), 15 mg/kg/day (1/15 LD50), and 22.5 mg/kg/day (1/10 LD50) fenpropathrin (LD50 225 mg/kg for male rats) were used in i.p administration for rat model. During the study, we found that 22.5 mg/kg/day fenpropathrin was lethal to some rats after 2 days exposure, while the 7.5 mg/kg/day fenpropathrin did not induced any behavior changes after 1 month exposure. Thus, we chose 15 mg/kg/day (1/15 LD50) fenpropathrin as the experimental dose for the i.p injection. Nevertheless, i.p administration of fenpropathrin could cause peripheral toxicity and disrupt general health so that i.p administration of fenpropathrin could not reflex the central neural system-specific deficit characteristic of PD caused by fenpropathrin. In order to observe the direct role of fenpropathrin on the substantia nigra-striatum, fenpropathrin was infused stereotaxically into the right VTA and SNc of the rats. Pyrethroids, such as cypermethrin, were reported to induce DA neurodegeneration in rats after prolonged exposure, as well as other pesticides such as paraquat [36] and rotenone [37]. Therefore, in this study, the effect of fenpropathrin was evaluated on 60 days after the first exposure to fenpropathrin by i.p or ST and 120 days after the first exposure to fenpropathrin by i.p. However, peripheral organs showed slight alterations 60 days after the first exposure to fenpropathrin (15 mg/kg) by i.p. The rats with i.p administration of fenpropathrin could partly mimic the exposure route of fenpropathrin through the alimentary tract in the patient.
The i.p animals did not exhibit reductions in motor behavioral indices 60 days after the first exposure to fenpropathrin. However, 120 days after the first exposure, the animals exhibited deteriorated exploratory ability. This result demonstrated that fenpropathrin-induced motor responses were not acute but progressive and delayed, similar to cypermethrin-induced Parkinsonism motor deficits [9]. It was in accordance with the disease history of that patient who exhibited Parkinsonian symptoms 2 years after eating fenpropathrin-poisoned fish. However, there was no epidemiological observation supporting the correlation between PD and fenpropathrin exposure yet. Our findings for the first time suggest that long-term repeated exposure to fenpropathrin may be relevant to the onset of Parkinsonian symptoms.
Due to the PD symptom in the patient instead of 5-HT, NA or GABA symptoms were typical, and the PD representation in the animal models exposure to fenpropathrin was obviously observed in our research, we determined to exclusively focus on the effect of fenpropathrin on DA neurons. DA neuron degeneration could lead to reduced DA levels in the striatum. The loss of TH, a DA biosynthesis enzyme, could result in decreased DA production, manifesting abnormal dopamine system function [38]. In this study, both loss of TH-positive neurons in the SNc and decrease in the intensity of TH expression in the SNc and striatum were found in all i.p infusion and ST infusion groups. The levels of DA and its metabolism in striatum were reduced by fenpropathrin treatment, as observed with cypermethrin [9]. The results showed that exposure to fenpropathrin could target the TH-positive DA neurons. However, the specificity and mechanism of DA neurons damage caused by fenpropathrin need to be performed in our continuous research.
DAT and VMAT2 are known to be main regulators of the cytosolic DA concentration. DAT takes up extracellular dopamine into the cytosol, and VMAT2 sequesters cytosolic dopamine into intracellular vesicles. Dopamine is known to be an endogenous cytotoxin to dopamine neurons. Therefore, higher level of DAT and lower VMAT2 could lead to exacerbated neurotoxicity of dopamine neurons. In this study, higher level of DAT may be a result of DAT gene upregulation by the toxin, similar to upregulation by other small chemicals [39, 40]. Meanwhile, DAT belongs to a family of Na+/Cl−-dependent transporter [41], and fenpropathrin could interfere the curves of Na+ channel [42]. So fenpropathrin could damage the function of DAT. VMAT2 was known to protect against DA neurons through vesicular sequestration of toxic metabolites so that reduced VMAT2 expression could induce dopamine neurons’ loss in PD [43] and enhance MPTP toxicity to dopamine neurons [44]. The decreased VMAT2 level after prolonged fenpropathrin exposure could exacerbate fenpropathrin-induced neurotoxicity, consistent with cypermethrin-mediated decrease in VMAT2 expression [45].
In summary, this is the first report that both in vitro and in vivo exposure to fenpropathrin cause DA neurotoxicity. In vitro, fenpropathrin mimics the pathogenesis characters of PD including apoptosis, oxidative stress, Lewy body-like structural formation and protein aggregation. In vivo, fenpropathrin could induce a late onset of TH-positive neurons loss in the SNc after a long-term exposure. We suggest that the increased level of DAT and decreased level of VMAT2 by fenpropathrin increase the cytosolic concentration of dopamine, resulting in increased damage to the dopamine neurons. These findings provide further consideration of fenpropathrin as a potential DA neurotoxin and an environmental risk factor for PD.
Supplementary Material
Acknowledgments
This work was supported by grants 30870866, 81071021, and 31171211 from the National Natural Science Foundation of China (to TW); grant 81200983 from the National Natural Science Foundation of China (to NX); grant 81100958 from the National Natural Science Foundation of China (to ZTZ); grant 81301082 from the National Natural Science Foundation of China (to JSH); grant 2012B09 from China Medical Foundation (to NX); and grant 0203201343 from Hubei Molecular Imaging Key Laboratory (to NX). The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Abbreviations
- Fen
Fenpropathrin
- PD
Parkinson’s disease
- i.p
Intraperitoneal injection
- ST
Stereotaxical injection
- DAT
Dopamine transporter
- VMAT2
Vesicular monoamine transporter 2
- DA
Dopamine
Footnotes
Electronic supplementary material The online version of this article (doi:10.1007/s12035-014-9057-2) contains supplementary material, which is available to authorized users.
Conflict of Interest There are no actual or potential conflicts of interest.
Authors’ Contributions JX, XZ, JH, CC, ZC, LL, JY, ZZ, NX, and TW contributed to the conception and design. JX, XZ, JH, and CC took care of the cell culture studies. JX, XZ, ZC, LL, and JY took care of the animal studies. JX, XZ, JH, CC, ZC, and NX analyzed and interoperated the data. JX, XZ, JH, NX, and TW coordinated all the experiments and helped to draft the manuscript. All authors read, revised, and approved the final manuscript.
Contributor Information
Jing Xiong, Department of Neurology, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, 1277 Jiefang Road, Wuhan 430022, Hubei, China; Department of Neurology, Renmin Hospital of Wuhan University, Wuhan 430060, Hubei Province, China.
Xiaowei Zhang, Department of Neurology, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, 1277 Jiefang Road, Wuhan 430022, Hubei, China.
Jinsha Huang, Department of Neurology, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, 1277 Jiefang Road, Wuhan 430022, Hubei, China.
Chunnuan Chen, Department of Neurology, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, 1277 Jiefang Road, Wuhan 430022, Hubei, China; Department of Neurology, Second Affiliated Hospital of Fujian Medical University, Quanzhou 362000, Fujian, China.
Zhenzhen Chen, Department of Neurology, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, 1277 Jiefang Road, Wuhan 430022, Hubei, China.
Ling Liu, Department of Neurology, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, 1277 Jiefang Road, Wuhan 430022, Hubei, China.
Guoxin Zhang, Department of Neurology, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, 1277 Jiefang Road, Wuhan 430022, Hubei, China.
Jiaolong Yang, Department of Neurology, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, 1277 Jiefang Road, Wuhan 430022, Hubei, China.
Zhentao Zhang, Department of Neurology, Renmin Hospital of Wuhan University, Wuhan 430060, Hubei Province, China.
Zhaohui Zhang, Department of Neurology, Renmin Hospital of Wuhan University, Wuhan 430060, Hubei Province, China.
Zhicheng Lin, Department of Psychiatry, Harvard Medical School; Division of Alcohol and Drug Abuse, and Mailman Neuroscience Research Center, McLean Hospital, Belmont, MA 02478, USA; Harvard NeuroDiscovery Center, Boston, MA 02114, USA.
Nian Xiong, Department of Neurology, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, 1277 Jiefang Road, Wuhan 430022, Hubei, China.
Tao Wang, Department of Neurology, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, 1277 Jiefang Road, Wuhan 430022, Hubei, China.
References
- 1.Lees AJ, Hardy J, Revesz T. Parkinson’s disease. Lancet. 2009;373(9680):2055–2066. doi: 10.1016/S0140-6736(09)60492-X. [DOI] [PubMed] [Google Scholar]
- 2.van der Mark M, Brouwer M, Kromhout H, Nijssen P, Huss A, Vermeulen R. Is pesticide use related to Parkinson disease? Some clues to heterogeneity in study results. Environ Health Perspect. 2012;120(3):340–347. doi: 10.1289/ehp.1103881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Casida JE. Pyrethrum flowers and pyrethroid insecticides. Environ Health Perspect. 1980;34:189–202. doi: 10.1289/ehp.8034189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Amweg EL, Weston DP, Ureda NM. Use and toxicity of pyrethroid pesticides in the Central Valley, California, USA. Environ Toxicol Chem / SETAC. 2005;24(4):966–972. doi: 10.1897/04-146r1.1. [DOI] [PubMed] [Google Scholar]
- 5.Wolansky MJ, Harrill JA. Neurobehavioral toxicology of pyrethroid insecticides in adult animals: a critical review. Neurotoxicol Teratol. 2008;30(2):55–78. doi: 10.1016/j.ntt.2007.10.005. [DOI] [PubMed] [Google Scholar]
- 6.Liu GP, Shi N. The inhibitory effects of deltamethrin on dopamine biosynthesis in rat PC12 cells. Toxicol Lett. 2006;161(3):195–199. doi: 10.1016/j.toxlet.2005.09.011. [DOI] [PubMed] [Google Scholar]
- 7.Gillette JS, Bloomquist JR. Differential up-regulation of striatal dopamine transporter and alpha-synuclein by the pyrethroid insecticide permethrin. Toxicol Appl Pharmacol. 2003;192(3):287–293. doi: 10.1016/s0041-008x(03)00326-0. [DOI] [PubMed] [Google Scholar]
- 8.Elwan MA, Richardson JR, Guillot TS, Caudle WM, Miller GW. Pyrethroid pesticide-induced alterations in dopamine transporter function. Toxicol Appl Pharmacol. 2006;211(3):188–197. doi: 10.1016/j.taap.2005.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Singh AK, Tiwari MN, Upadhyay G, Patel DK, Singh D, Prakash O, Singh MP. Long term exposure to cypermethrin induces nigrostriatal dopaminergic neurodegeneration in adult rats: postnatal exposure enhances the susceptibility during adulthood. Neurobiol Aging. 2012;33(2):404–415. doi: 10.1016/j.neurobiolaging.2010.02.018. [DOI] [PubMed] [Google Scholar]
- 10.Weiner ML, Nemec M, Sheets L, Sargent D, Breckenridge C. Comparative functional observational battery study of twelve commercial pyrethroid insecticides in male rats following acute oral exposure. Neurotoxicology. 2009;30(Suppl 1):S1–S16. doi: 10.1016/j.neuro.2009.08.014. [DOI] [PubMed] [Google Scholar]
- 11.Bradberry SM, Cage SA, Proudfoot AT, Vale JA. Poisoning due to pyrethroids. Toxicol Rev. 2005;24(2):93–106. doi: 10.2165/00139709-200524020-00003. [DOI] [PubMed] [Google Scholar]
- 12.Al-Makkawy HK, Madbouly MD. Persistence and accumulation of some organic insecticides in Nile water and fish. Resour Conserv Recycl. 1999;27(1–2):105–115. doi: 10.1016/s0921-3449(98)00090-1. [DOI] [Google Scholar]
- 13.Zielinska E, Kocki T, Saran T, Borbely S, Kuc D, Vilagi I, Urbanska EM, Turski WA. Effect of pesticides on kynurenic acid production in rat brain slices. Ann Agric Environ Med: AAEM. 2005;12(2):177–179. [PubMed] [Google Scholar]
- 14.Ogawa T, Matson WR, Beal MF, Myers RH, Bird ED, Milbury P, Saso S. Kynurenine pathway abnormalities in Parkinson’s disease. Neurology. 1992;42(9):1702–1706. doi: 10.1212/wnl.42.9.1702. [DOI] [PubMed] [Google Scholar]
- 15.Kanawi E, Budd R, Tjeerdema RS. Environmental fate and ecotoxicology of fenpropathrin. Rev Environ Contam Toxicol. 2013;225:77–93. doi: 10.1007/978-1-4614-6470-9_3. [DOI] [PubMed] [Google Scholar]
- 16.Zhang Z, Cao X, Xiong N, Wang H, Huang J, Sun S, Liang Z, Wang T. DNA polymerase-beta is required for 1-methyl-4-phenylpyridinium-induced apoptotic death in neurons. Apoptosis: Int J Programmed Cell Death. 2010;15(1):105–115. doi: 10.1007/s10495-009-0425-8. [DOI] [PubMed] [Google Scholar]
- 17.Xiong N, Huang J, Chen C, Zhao Y, Zhang Z, Jia M, Hou L, Yang H, Cao X, Liang Z, Zhang Y, Sun S, Lin Z, Wang T. Dl-3-n-butylphthalide, a natural antioxidant, protects dopamine neurons in rotenone models for Parkinson’s disease. Neurobiol Aging. 2012;33(8):1777–1791. doi: 10.1016/j.neurobiolaging.2011.03.007. [DOI] [PubMed] [Google Scholar]
- 18.Salvioli S, Ardizzoni A, Franceschi C, Cossarizza A. JC-1, but not DiOC6(3) or rhodamine 123, is a reliable fluorescent probe to assess delta psi changes in intact cells: implications for studies on mitochondrial functionality during apoptosis. FEBS Lett. 1997;411(1):77–82. doi: 10.1016/s0014-5793(97)00669-8. [DOI] [PubMed] [Google Scholar]
- 19.Huang J, Hao L, Xiong N, Cao X, Liang Z, Sun S, Wang T. Involvement of glyceraldehyde-3-phosphate dehydrogenase in rotenone-induced cell apoptosis: relevance to protein misfolding and aggregation. Brain Res. 2009;1279:1–8. doi: 10.1016/j.brainres.2009.05.011. [DOI] [PubMed] [Google Scholar]
- 20.Xiong N, Huang J, Zhang Z, Xiong J, Liu X, Jia M, Wang F, Chen C, Cao X, Liang Z, Sun S, Lin Z, Wang T. Stereotaxical infusion of rotenone: a reliable rodent model for Parkinson’s disease. PLoS One. 2009;4(11):e7878. doi: 10.1371/journal.pone.0007878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Deumens R, Blokland A, Prickaerts J. Modeling Parkinson’s disease in rats: an evaluation of 6-OHDA lesions of the nigrostriatal pathway. Exp Neurol. 2002;175(2):303–317. doi: 10.1006/exnr.2002.7891. [DOI] [PubMed] [Google Scholar]
- 22.Sonia Angeline M, Chaterjee P, Anand K, Ambasta RK, Kumar P. Rotenone-induced parkinsonism elicits behavioral impairments and differential expression of parkin, heat shock proteins and caspases in the rat. Neuroscience. 2012;220:291–301. doi: 10.1016/j.neuroscience.2012.06.021. [DOI] [PubMed] [Google Scholar]
- 23.Singh S, Singh K, Patel DK, Singh C, Nath C, Singh VK, Singh RK, Singh MP. The expression of CYP2D22, an ortholog of human CYP2D6, in mouse striatum and its modulation in 1-methyl 4-phenyl-1,2,3,6-tetrahydropyridine-induced Parkinson’s disease phenotype and nicotine-mediated neuroprotection. Rejuvenation Res. 2009;12(3):185–197. doi: 10.1089/rej.2009.0850. [DOI] [PubMed] [Google Scholar]
- 24.Kuusisto E, Parkkinen L, Alafuzoff I. Morphogenesis of Lewy bodies: dissimilar incorporation of alpha-synuclein, ubiquitin, and p62. J Neuropathol Exp Neurol. 2003;62(12):1241–1253. doi: 10.1093/jnen/62.12.1241. [DOI] [PubMed] [Google Scholar]
- 25.Olsson M, Nikkhah G, Bentlage C, Bjorklund A. Forelimb akinesia in the rat Parkinson model: differential effects of dopamine agonists and nigral transplants as assessed by a new stepping test. J Neurosci: Off J Soc Neurosci. 1995;15(5 Pt 2):3863–3875. doi: 10.1523/JNEUROSCI.15-05-03863.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xiong N, Jia M, Chen C, Xiong J, Zhang Z, Huang J, Hou L, Yang H, Cao X, Liang Z, Sun S, Lin Z, Wang T. Potential autophagy enhancers attenuate rotenone-induced toxicity in SH-SY5Y. Neuroscience. 2011;199:292–302. doi: 10.1016/j.neuroscience.2011.10.031. [DOI] [PubMed] [Google Scholar]
- 27.Watabe M, Nakaki T. Mitochondrial complex I inhibitor rotenone inhibits and redistributes vesicular monoamine transporter 2 via nitration in human dopaminergic SH-SY5Y cells. Mol Pharmacol. 2008;74(4):933–940. doi: 10.1124/mol.108.048546. [DOI] [PubMed] [Google Scholar]
- 28.Xie HR, Hu LS, Li GY. SH-SY5Y human neuroblastoma cell line: in vitro cell model of dopaminergic neurons in Parkinson’s disease. Chin Med J. 2010;123(8):1086–1092. [PubMed] [Google Scholar]
- 29.Jana S, Sinha M, Chanda D, Roy T, Banerjee K, Munshi S, Patro BS, Chakrabarti S. Mitochondrial dysfunction mediated by quinone oxidation products of dopamine: implications in dopamine cytotoxicity and pathogenesis of Parkinson’s disease. Biochim Biophys Acta. 2011;1812(6):663–673. doi: 10.1016/j.bbadis.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 30.Hastings TG, Lewis DA, Zigmond MJ. Role of oxidation in the neurotoxic effects of intrastriatal dopamine injections. Proc Natl Acad Sci U S A. 1996;93(5):1956–1961. doi: 10.1073/pnas.93.5.1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Giray B, Gurbay A, Hincal F. Cypermethrin-induced oxidative stress in rat brain and liver is prevented by vitamin E or allopurinol. Toxicol Lett. 2001;118(3):139–146. doi: 10.1016/s0378-4274(00)00277-0. [DOI] [PubMed] [Google Scholar]
- 32.Abdou R, Sasaki K, Khalil W, Shah S, Murasawa Y, Shimoda M. Effects of several pyrethroids on hepatic cytochrome P450 activities in rats. J Vet Med Sci / Jpn Soc Vet Sci. 2010;72(4):425–433. doi: 10.1292/jvms.09-0347. [DOI] [PubMed] [Google Scholar]
- 33.Gassner B, Wuthrich A, Scholtysik G, Solioz M. The pyrethroids permethrin and cyhalothrin are potent inhibitors of the mitochondrial complex I. J Pharmacol Exp Ther. 1997;281(2):855–860. [PubMed] [Google Scholar]
- 34.Gai WP, Yuan HX, Li XQ, Power JT, Blumbergs PC, Jensen PH. In situ and in vitro study of colocalization and segregation of alpha-synuclein, ubiquitin, and lipids in Lewy bodies. Exp Neurol. 2000;166(2):324–333. doi: 10.1006/exnr.2000.7527. [DOI] [PubMed] [Google Scholar]
- 35.Wakabayashi K, Tanji K, Odagiri S, Miki Y, Mori F, Takahashi H. The Lewy body in Parkinson’s disease and related neurodegenerative disorders. Mol Neurobiol. 2013;47(2):495–508. doi: 10.1007/s12035-012-8280-y. [DOI] [PubMed] [Google Scholar]
- 36.Patel S, Singh V, Kumar A, Gupta YK, Singh MP. Status of antioxidant defense system and expression of toxicant responsive genes in striatum of maneb- and paraquat-induced Parkinson’s disease phenotype in mouse: mechanism of neurodegeneration. Brain Res. 2006;1081(1):9–18. doi: 10.1016/j.brainres.2006.01.060. [DOI] [PubMed] [Google Scholar]
- 37.Xiong N, Long X, Xiong J, Jia M, Chen C, Huang J, Ghoorah D, Kong X, Lin Z, Wang T. Mitochondrial complex I inhibitor rotenone-induced toxicity and its potential mechanisms in Parkinson’s disease models. Crit Rev Toxicol. 2012;42(7):613–632. doi: 10.3109/10408444.2012.680431. [DOI] [PubMed] [Google Scholar]
- 38.Pardridge WM. Tyrosine hydroxylase replacement in experimental Parkinson’s disease with transvascular gene therapy. NeuroRx: J Am Soc Exp Neurother. 2005;2(1):129–138. doi: 10.1602/neurorx.2.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Richardson JR, Caudle WM, Wang MZ, Dean ED, Pennell KD, Miller GW. Developmental heptachlor exposure increases susceptibility of dopamine neurons to N-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)in a gender-specific manner. Neurotoxicology. 2008;29(5):855–863. doi: 10.1016/j.neuro.2008.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rodriguez VM, Limon-Pacheco JH, Mendoza-Trejo MS, Gonzalez-Gallardo A, Hernandez-Plata I, Giordano M. Repeated exposure to the herbicide atrazine alters locomotor activity and the nigrostriatal dopaminergic system of the albino rat. Neurotoxicology. 2013;34:82–94. doi: 10.1016/j.neuro.2012.10.012. [DOI] [PubMed] [Google Scholar]
- 41.Khoshbouei H, Wang H, Lechleiter JD, Javitch JA, Galli A. Amphetamine-induced dopamine efflux. A voltage-sensitive and intracellular Na+-dependent mechanism. J Biol Chem. 2003;278(14):12070–12077. doi: 10.1074/jbc.M212815200. [DOI] [PubMed] [Google Scholar]
- 42.Yoon JY, Ahn SH, Oh H, Kim YS, Ryu SY, Ho WK, Lee SH. A novel Na+ channel agonist, dimethyl lithospermate B, slows Na+ current inactivation and increases action potential duration in isolated rat ventricular myocytes. Br J Pharmacol. 2004;143(6):765–773. doi: 10.1038/sj.bjp.0705969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Caudle WM, Richardson JR, Wang MZ, Taylor TN, Guillot TS, McCormack AL, Colebrooke RE, Di Monte DA, Emson PC, Miller GW. Reduced vesicular storage of dopamine causes progressive nigrostriatal neurodegeneration. J Neurosci: Off J Soc Neurosci. 2007;27(30):8138–8148. doi: 10.1523/JNEUROSCI.0319-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Takahashi N, Miner LL, Sora I, Ujike H, Revay RS, Kostic V, Jackson-Lewis V, Przedborski S, Uhl GR. VMAT2 knockout mice: heterozygotes display reduced amphetamine-conditioned reward, enhanced amphetamine locomotion, and enhanced MPTP toxicity. Proc Natl Acad Sci U S A. 1997;94(18):9938–9943. doi: 10.1073/pnas.94.18.9938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tiwari MN, Singh AK, Ahmad I, Upadhyay G, Singh D, Patel DK, Singh C, Prakash O, Singh MP. Effects of cypermethrin on monoamine transporters, xenobiotic metabolizing enzymes and lipid peroxidation in the rat nigrostriatal system. Free Radic Res. 2010;44(12):1416–1424. doi: 10.3109/10715762.2010.512041. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


