Abstract
Erectile dysfunction (ED) has high impact on quality of life in prostatectomy, diabetic and aging patients. An underlying mechanism is cavernous nerve (CN) injury, which causes ED in up to 80% of prostatectomy patients. We examine how sonic hedgehog (SHH) treatment with innovative peptide amphiphile nanofiber hydrogels (PA), promotes CN regeneration after injury. SHH and its receptors patched (PTCH1) and smoothened (SMO) are localized in PG neurons and glia. SMO undergoes anterograde transport to signal to down stream targets. With crush injury, PG neurons degenerate and undergo apoptosis. SHH protein decreases, SMO localization changes to the neuronal cell surface, and anterograde transport stops. With SHH treatment SHH is taken up at the injury site and undergoes retrograde transport to PG neurons, allowing SMO transport to occur, and neurons remain intact. SHH treatment prevents neuronal degeneration, maintains neuronal, glial and down stream target signaling, and is significant as a regenerative therapy.
Keywords: Peptide amphiphile nanofiber hydrogel, cavernous nerve injury (prostatectomy), Sonic hedgehog, erectile dysfunction, regeneration
Summary Sentence
Sonic hedgehog delivered by peptide amphiphile nanofiber hydrogel maintains normal signaling between pelvic ganglia neurons and glia after cavernous nerve crush, preventing neuronal degeneration and apoptosis, and erectile dysfunction.
Introduction
Erectile dysfunction (ED) is a debilitating condition that has high impact on quality of life in 52% of men aged 40 to 70 [1] and 22% of men under age 40 [2]. Men at high risk for ED development are prostate cancer patients treated by prostatectomy, diabetic patients and aging men. A significant underlying cause of ED development is injury to the cavernous nerve (CN, peripheral nerve that provides innervation to the penis) that occurs in up to 82–85% of prostatectomy patients [3–4], and with progressive peripheral neuropathy in diabetic patients (75%) [5]. Only a small portion (36%) of prostatectomy patients recover erectile function without intervention [6], and PDE5 inhibitors are ineffective in the majority (69%) of prostatectomy patients [7], thus improved treatments are needed. In response to CN injury, the down stream target of innervation, the corpora cavernosa of the penis, undergoes extensive remodeling, with abundant smooth muscle apoptosis [8] and increased collagen deposition [9–10]. This irreversible process makes the corpora cavernosal tissue unresponsive to normal signaling pathways, even when the CN undergoes limited regeneration. In order to prevent this adverse penile remodeling and ED, protection and regeneration of the CN is critical. We’ve shown in previous studies that the Sonic hedgehog (SHH) pathway is neuroprotective [11] and promotes CN regeneration by 60% at 6 weeks after CN injury when delivered by peptide amphiphile nanofiber hydrogels [12]. However the mechanism of how CN regeneration is significantly enhanced by SHH is unknown.
SHH is abundantly expressed in pelvic ganglia (PG) neurons [13]. Communication between neurons and associated glia is essential for development and maintenance of PG and CN architecture. The main components of the PG are the principal neurons (neuronal cell body and axon), sheathed by satellite glial cells (Figure 1A), which are active partners in neuronal communication [14]. Bidirectional signaling selectively occurs between specific subpopulations of glia, neurons, and synapses [15] and is important for neuronal function. Injury to peripheral nerves is common and can be caused by neuropathy, trauma, repetitive compression [16–18], or related surgery (such as prostatectomy). Regeneration begins in the axon stump distal to the site of injury within 24–36 hours. The distal nerve shows overall distortion of normal nerve anatomy, axonal swelling and axonal vacuolization which are microanatomical signs of Wallerian degeneration [19]. Local conditions influence how initial regenerative axon sprouts emerge from parent axons [20], while CN injury triggers a cascade of events in PG neurons, including changes in expression of neurotransmitters, neurotrophic factors, cytokine production [21], and SHH pathway signaling [11–12]. Following injury, the regenerative abilities of these important injured parasympathetic ganglion neurons and the factors in the environment that influence regeneration are poorly understood. [22].
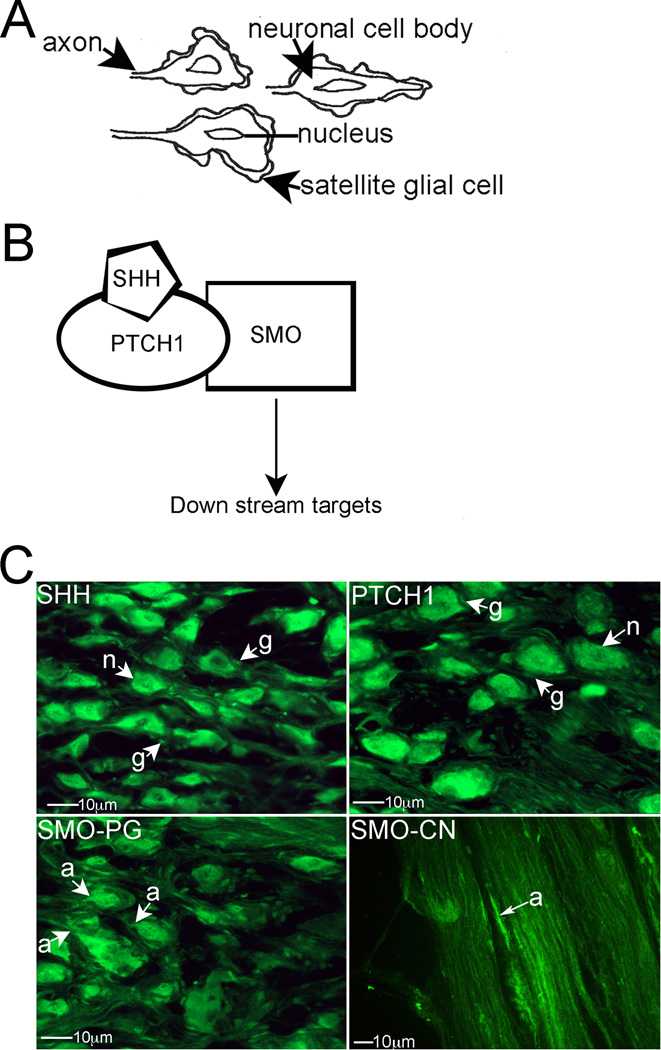
Figure 1.
(A) Diagram showing the relationship between PG neurons and associated satellite glial cells. (B) Diagram of the SHH signaling pathway. (C) Immunohistochemical analysis of the PG assaying for SHH, PTCH1 and SMO proteins, and analysis of SMO protein in the CN (400× magnification). Arrows indicate staining. n=neuron. g=glial cell. a=axon.
Our previous studies show that SHH protein is decreased in the PG and CN with CN injury [11], and when SHH is inhibited in the PG, demyelination of CN fibers and degeneration of non-myelinated fibers were observed [12], causing down stream apoptosis of penile smooth muscle, induction of ED [13], and identifying a critical role for SHH in maintaining CN architecture. SHH treatment of the CN at the time of injury by peptide amphiphile (PA) nanofiber hydrogels is both neuroprotective [11] and significantly enhances CN regeneration [12]. However the mechanism of how SHH treatment is beneficial and promotes regeneration is unknown. In other organs, Shh is important for nerve development [23–24] and in the central nervous system, SHH serves as an axon guidance molecule [25–26]. In the peripheral nervous system, adenoviral vector delivery of Shh to injured sciatic nerve, improved motor neuron survival after injury [27–28], SHH inhibition caused motor neuron death at the axotomy site [27], and there was impaired regenerative capacity in the absence of Shh [28]. Shh and its receptors, patched (PTCH1, part of the receptor SHH binds to) and smoothened (SMO, signals to down stream targets, Figure 1B), are expressed in adult dorsal root ganglia neurons and glia and knockdown of Shh in adult sensory neurons resulted in decreased regenerative axon sprouting, and branching in vitro, suggesting a role for Shh in facilitating outgrowth. Shh is necessary for maintenance of astrocyte proliferation in the optic nerve [29]. However Shh protein is not sufficient to drive proliferation of astrocytes in vitro but it was in vivo, suggesting that it’s effect on astrocyte proliferation in vivo requires cell-cell interactions that do not operate in dissociated cultures [30]. Thus we propose that SHH interaction between PG neurons and glia is important to maintain normal PG/CN signaling and down stream penile architecture.
We have developed self-assembling peptide amphiphiles for in vivo delivery of SHH protein to the CN to promote regeneration and prevent ED [12]. These innovative and broadly applicable hydrogels are composed of highly aligned monodomain nanofiber bundles [31, 32], which allow in vitro assembly of linear hydrogel with SHH protein intercalated between and along the nanofiber bundles as they form. The flexible hydrogel can be picked up with forceps and placed on top of the CN in vivo. CN preservation and regeneration are enhanced as the SHH protein is released, gradually from the gel [12, 11]. This type of PA hydrogel allows for customized, controlled protein delivery over extended periods with a biodegradable vehicle, and is easily translatable to prostatectomy patients in the clinic. In this manuscript we examine the mechanism of how SHH treatment by PA is neuroprotective and promotes CN regeneration after CN crush injury. SHH promotes CN regeneration by maintaining normal signalling between PG neurons and glia and to down stream targets and by preventing neuronal degeneration. This study is significant because understanding how regeneration occurs can provide novel avenues to further enhance regeneration and to prevent ED. We will utilize this novel PA technology for SHH delivery to the CN from a manipulable supramolecular cable (via monodomain aligned nanofibers). These PA materials have potential broad application for treatment of other peripheral neuropathies.
Materials and Methods
Animals
Sixty-four Sprague-Dawley rats postnatal day 115–120 (P115-P120) were obtained from Charles River. The study was carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. The animal care protocol was approved by the Office of Animal Care and Institutional Biosafety at the University of Illinois at Chicago and animals were given humane care in accordance with institutional OACIB approval.
Bilateral CN crush and sham surgical procedures
PG/CN were exposed and microforceps (size 0.02 X 0.06mm) were used to crush the CN bilaterally for 30 seconds. This method of CN crush has commonly been used in the literature [33–34] and the extent and reproducibility of crush injury were previously verified in our laboratory [12]. Sham surgery (control) was performed by exposing but not crushing the CN (n=9). CN crushed rats were sacrificed 4 days after injury (n=9). Non-surgery control animals were also examined (n=9). For resection injury, a 5mm portion of the CN was removed 5mm from the PG (n=7) and rats were sacrificed at 4 days after injury.
Bilateral CN crush surgery with SHH or BSA protein treatment of the CN by linear peptide amphiphile (PA)
We have previously employed self-assembling peptide amphiphiles as hydrogel carriers for controlled delivery of SHH to the CN at the time of crush injury [12]. We externally prepare V2A2E2-NH2 PA to form highly aligned, monodomain hydrogels with sufficient mechanical integrity that they can be manipulated and placed on top of an exposed CN at the time of surgery. SHH is entrapped within the PA hydrogel during cation-based assembly and crosslinking.
(C16)-V2A2E2-(NH2) PA was prepared as previously described [12]. 20mM CaCl2 was added to a glass slide and 8µl of 20mM PA plus either 2.27µg SHH or bovine serum albumin (BSA, control) proteins were pipetted onto the slide to form the linear monodomain PA hydrogel. CN crush was performed as described in the previous section. Aligned monodomain PA hydrogel containing the protein intercalated within the hydrogel, was transferred with forceps on top of the crushed CNs bilaterally so that each rat received 4.54µg SHH (n=12) or BSA (n=5) protein. The release rate of SHH protein from the PA was previously determined to be 90% by 75 hours [12]. Rats were sacrificed 4 days after injury/SHH treatment.
CN tie placement
A midline abdominal incision was made with a scalpel under direct vision through a Leica DM2500 Stereo microscope in adult Sprague-Dawley rats. The PG and CN were exposed and surgical silk (9-0) was used to tie off the CN (double knot). Three days (n=7) after the tie was placed, rats were sacrificed and the PG and CN were excised and frozen in OCT.
Hedgehog interacting protein (HIP) Inhibitor treatment of the PG
Affi-Gel beads (100–200 mesh, Bio-Rad Laboratories, Hercules, CA, USA) were equilibrated with HIP inhibitor (n = 3; 200 µg/mL, Santa Cruz Biotechnology, Santa Cruz, CA, USA), or Dulbecco’s phosphate buffered saline (PBS, control, n = 3) overnight at 4°C. Approximately 10–20 beads were injected under the PG bilaterally in adult Sprague-Dawley rats. Rats were sacrificed at 2 days following bead injection and PG and CN were frozen in optimal cutting temperature medium (OCT).
Immunohistochemical analysis (IHC)
IHC was performed on frozen PG/CN sections which were cut to 14µm thickness and were post fixed in acetone at 4°C for 15 minutes. OCT was removed with two washes of 1× PBS prior to blocking with 3% milk in PBS for one hour at 4°C. Sections were incubated overnight at 4°C with goat polyclonal antibody against SHH (Santa Cruz, Santa Cruz, CA, SC-1194, 1/100), PTCH1 (Santa Cruz, Santa Cruz, CA, 1/100), and rabbit smoothened (SMO, 1/100, MBL International, Woburn, MA). Secondary antibodies were 1/150 chicken anti-goat 488 and chicken anti-rabbit 594 (Molecular Probes). Sections were mounted using DPX Mounting media (Electron Microscopy Sciences, Hatfield, PA) and fluorescence was visualized using a Leica DM2500 microscope.
TUNEL analysis for apoptosis
TUNEL was performed using the Apoptag kit (Millipore) on penis tissue from rats in which surgical silk (9-0) was used to tie off the CN (double knot). Three days (n=5) after the tie was placed, rats were sacrificed and TUNEL assay was performed on frozen penis tissue. Fluorescence was visualized using a Leica DM2500 microscope
Results
SHH pathway signaling in normal pelvic ganglia
Immunohistochemical analysis was performed on PG/CN from normal, adult Sprague Dawley rats (n=9), assaying for SHH, patched (PTCH1, the SHH receptor), and SMO proteins. SHH, and its receptor PTCH1 (Figure 1B), proteins were abundant throughout the cytoplasm of PG neurons (“n”=neuron) that innervate the penis and the associated glial cells (‘g”= glial cell, Figure 1C). SMO, which also forms part of the SHH receptor, was abundant in PG neurons, in the neuronal cytoplasm (Figure 1C). SMO protein was also identified in neuronal axons (“a”=axon) in the PG and CN (Figure 1C), indicating that SMO undergoes anterograde transport to signal to SHH targets. SHH and PTCH1 do not undergo a similar anterograde transport.
SHH pathway signaling in PG with CN crush
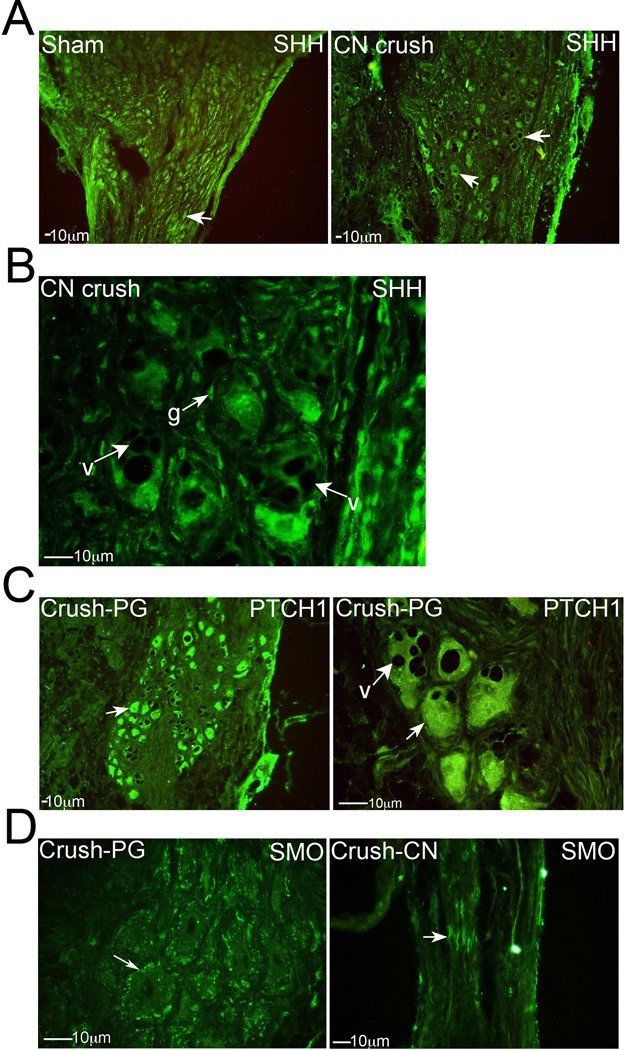
Immunohistochemical analysis was performed on sham (n=9) and 4 day CN crushed (n=9) Sprague Dawley rat PG/CN, assaying for SHH, PTCH1 and SMO proteins. When the CN is injured, such as occurs during prostatectomy, axonal degeneration results in cell death of PG neurons. The number of PG neurons that stain for SHH protein were diminished with crush injury (Figure 2A), however, associated glial cell staining remained abundant for SHH as noted in the normal PG above (Figure 1C and 2B). Vacuole formation was observed in PG neurons, indicating Wallerian degeneration of neurons and apoptosis (Figure 2B,C). PTCH1 staining remained abundant in the neuronal cytoplasm with crush injury (Figure 2C). The localization of SMO in PG neurons changes from primarily cytoplasmic to the cellular membrane (Figure 2D). Associated with this change in localization is loss of SMO transport in CN axons, and SMO protein (arrow) pools at the crush site (Figure 2D).
Figure 2.
Immunohistochemical analysis of: (A) SHH protein in PG tissue from sham and CN crushed rats, four days after CN crush (100× magnification), (B) SHH protein in PG tissue 4 days after CN crush (400× magnification), (C) PTCH1 protein in PG tissue 4 days after CN crush (100×–400×), and (D) SMO protein in PG (left) and CN (right) tissues 4 days after CN crush (200–400× magnification). Arrows indicate staining. g=glial cells. V=vacuole.
SHH treatment of the crushed CN by linear PA, maintains normal PG signaling and prevents neuronal degeneration and apoptosis
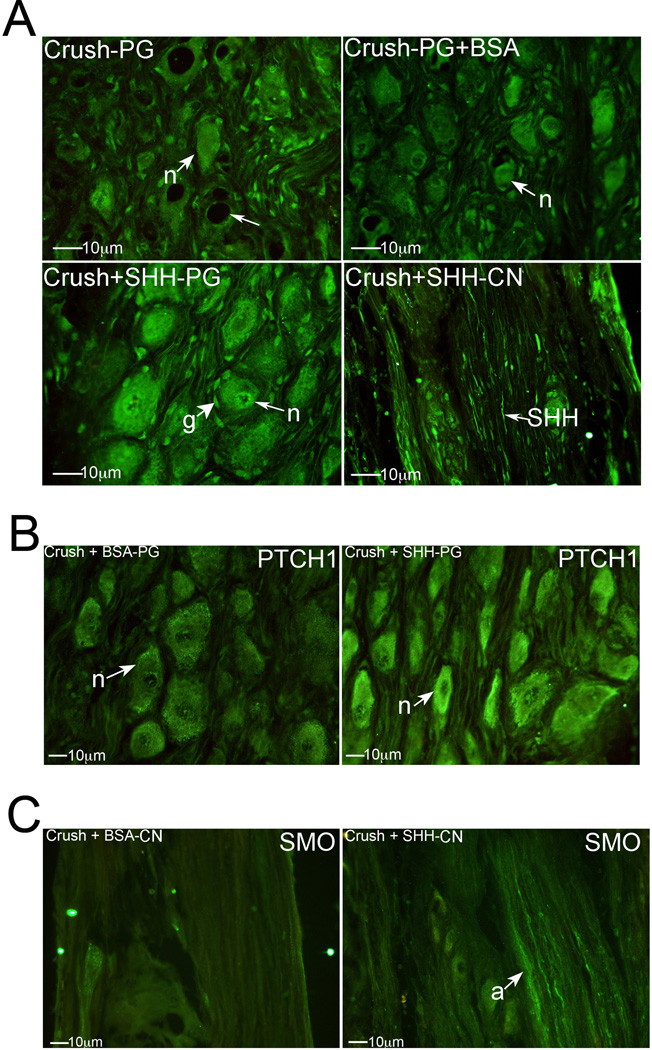
Immunohistochemical analysis was performed on PG/CN tissue assaying for SHH protein in crushed CN (n=9), crushed CN with BSA treatment by PA (control, n=5), and crushed CN with SHH treatment by PA (n=12). SHH protein treatment of the CN by PA at the time of crush injury resulted in retrograde transport of SHH (Figure 3A bottom right) to neurons in the PG (Figure 3A bottom left). This prevented PG neurons from dying off, as pictured in Figure 3A (top) after CN crush. With SHH PA treatment, the neurons and SHH staining appear normal, with no evidence of vacuolization (Figure 3A bottom left). In PG from CN crushed rats treated with BSA by PA (Figure 3A top right), overall neuronal staining for SHH was diminished in the PG, and retrograde transport of SHH was not observed.
Figure 3.
(A) Immunohistochemical analysis of SHH protein in PG tissue of rats that under went CN crush (top left), CN crush with BSA protein treatment (control, top right), or CN crush with SHH protein treatment (bottom left), and CN tissue from rats that underwent CN crush with SHH protein treatment (bottom right). SHH protein was taken up at the crush site and underwent retrograde transport (bottom right) to PG neurons to maintain normal signaling (bottom left). (B) Immunohistochemical analysis of PTCH1 protein in PG tissue from rats that underwent CN crush with BSA treatment (control) or CN crush with SHH treatment, by PA (400× magnification). (C) Immunohistochemical analysis of SMO protein in CN tissue of rats that underwent CN crush with either BSA (control) or SHH protein treatment by PA. Anterograde transport of SMO stops with CN injury (left) and is maintained with SHH PA treatment (right) of the CN (400× magnification). Arrows indicate staining. n=neuron. g=glial cell.
SHH treatment of the crushed CN maintained higher PTCH1 abundance in PG neurons in comparison to BSA treated controls (Figure 3B), and was similar to PTCH1 levels observed in normal tissue (Figure 1C). Anterograde transport of SMO in axons of the CN was maintained with crush injury in the presence of SHH treatment (Figure 3C).
SHH pathway signaling with CN cut, or tie placement on the CN
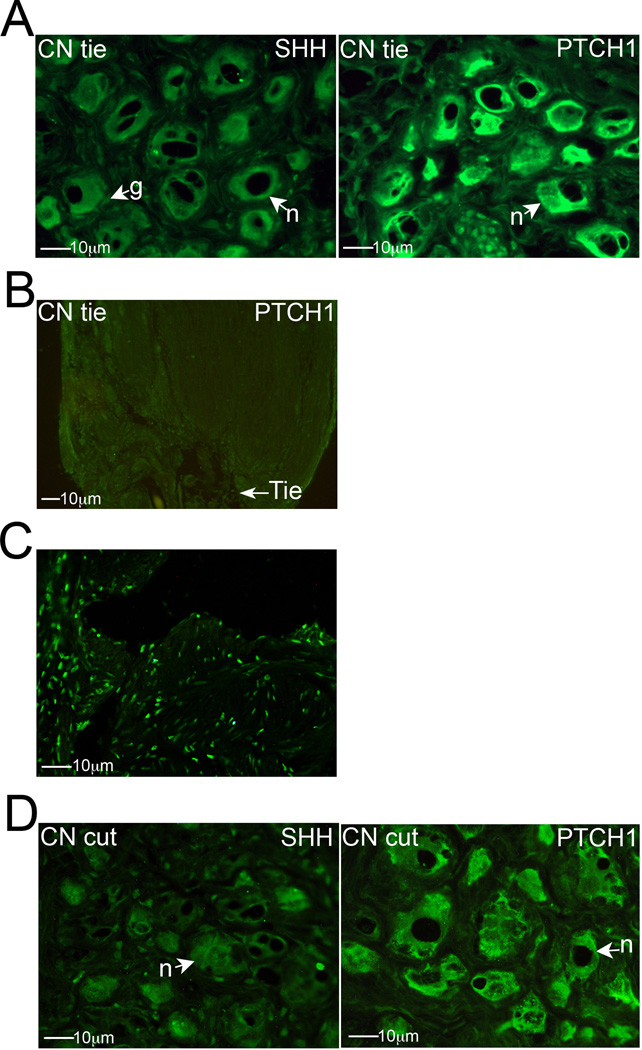
Immunohistochemical analysis of SHH pathway signaling was performed with CN resection (n=7) and tie injury (n=7). When a tie was placed around the CN for three days (mimicking a severe crush injury) and the PG/CN was stained for SHH, neurons of the PG showed reduced SHH protein. Glial cells staining remained identifiable for SHH (Figure 4A). While vacuolization of neurons was prominent with tie placement, PTCH1 staining remained abundant in the cytoplasm of PG neurons (Figure 4A). PTCH1 protein does not undergo anterograde transport, as does SMO (Figure 1C) and HIP [35], since PTCH1 protein was not observed to build up near the tie (Figure 4B). Abundant apoptosis was observed down stream in the penis by TUNEL staining, three days after tie placement, verifying crush injury with tie placement (Figure 4C).
Figure 4.
(A) Immunohistochemical analysis of SHH and PTCH1 proteins in PG tissue that had undergone CN tie placement (400× magnification). (B) PTCH1 protein does not build up near the CN tie, indicating that PTCH1 does not undergo anterograde transport (100× magnification). (C) TUNEL staining for apoptosis in the corpora cavernosa of the penis in the presence of a CN tie (400× magnification). (D) SHH and PTCH1 proteins in the PG after CN resection (400× magnification). Arrows indicate neuronal staining. n=neuron. g=glial cell.
SHH protein staining was also reduced in PG neurons with resection injury and associated glial cell staining remained identifiable (Figure 4D). PTCH1 staining remained abundant in neuronal cytoplasm with resection injury (Figure 4D) and its localization was unchanged in comparison to sham (Figure 1C).
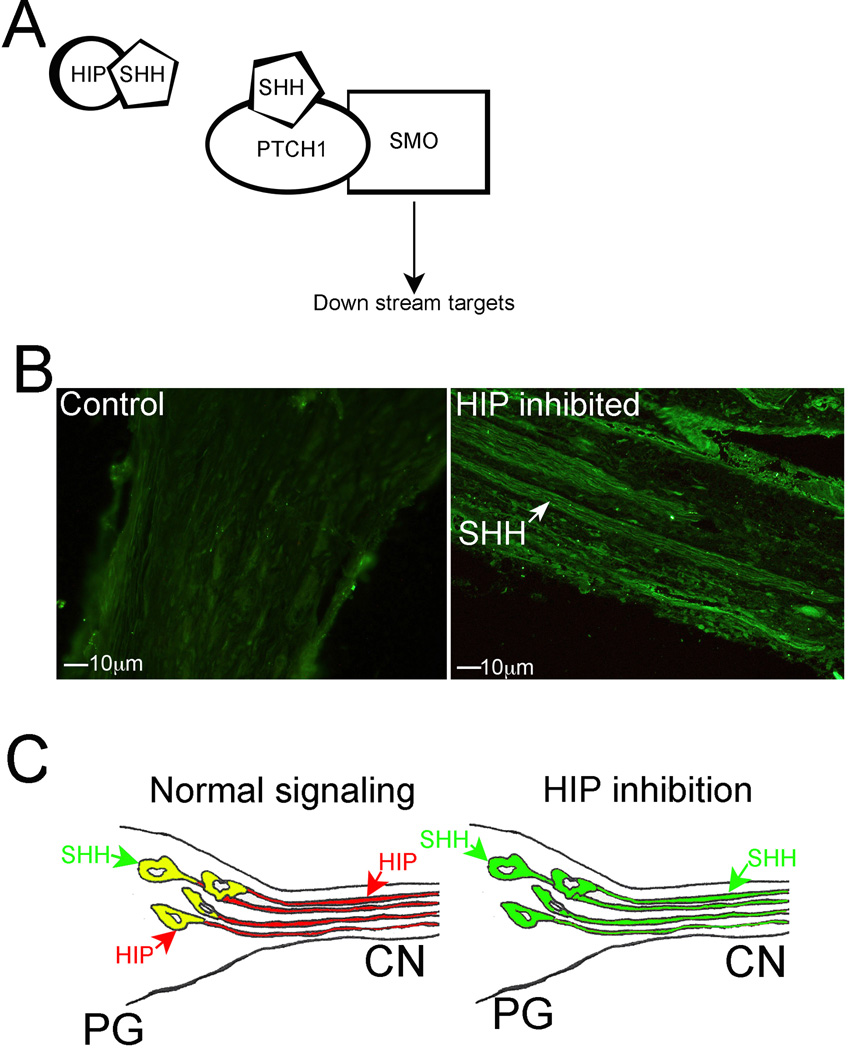
Changes in SHH localization with perturbation of hedgehog interacting protein in the PG/CN
Hedgehog interacting protein (HIP) is a type I membrane glycoprotein that acts as a negative regulator of the SHH pathway by competing with PTCH1 for SHH ligand binding (Figure 5A, [35]). HIP is one of only two SHH pathway members that undergo anterograde transport in neurons of the PG and CN [35]. We examined how HIP affects SHH signaling in the PG/CN by inhibiting HIP in the PG and CN and examining SHH signaling by immunohistochemical analysis for SHH protein in HIP inhibited (n=3) and control (PBS, n=3) PG/CN, delivered via Affi-Gel beads placed under the PG. SHH protein does not undergo anterograde transport under normal signaling conditions in control PG/CN (Figure 5B left). However when HIP signaling is inhibited, SHH protein undergoes anterograde transport by axons in the CN (Figure 5B right), indicating that a function of HIP is to restrict SHH localization and signaling in PG/CN neurons (Figure 5C).
Figure 5.
(A) Diagram of SHH pathway showing HIP protein competing with PTCH1 for SHH binding. (B) Immunohistochemical analysis of the CN assayed for SHH protein in control (left) and HIP inhibited (right) PG tissue (200× magnification). SHH protein undergoes anterograde transport in CN axons (arrow) with HIP inhibition that is not present under normal signaling conditions. (C) Diagram showing restriction of SHH protein to PG neuronal cell bodies (soma) under normal signaling conditions (left) and anterograde transport of SHH protein to neuronal axons with HIP inhibition (right).
Discussion
When SHH is delivered by PA to the CN crush site, it is neuroprotective [11], enhances CN regeneration, and significantly improves erectile function as measured by increased intracavernosal pressure [12]. The mechanism by which SHH affords neuroprotection and promotes regeneration, was previously unknown until now. The SHH pathway is part of normal signaling in the PG and CN [13, 11]. SHH and its receptor PTCH1 are localized in both neurons and associated glia (Figure 1). In the peripheral nervous system there are several types of glial cells, the most commonly observed are the satellite glial cells which form a thin cellular sheath surrounding individual neuronal cell bodies, and Schwann cells, which envelop the neuronal axons. Satellite glial cells are believed to have a similar role in the peripheral nervous system as astrocytes in the central nervous system. They control the microenvironment of ganglia, supply nutrients to the surrounding neurons and provide structure, acting as protective and cushioning cells for the neurons [36]. Satellite glial cells do not have synapses, as do neurons, however they have a variety of receptors that affect neuronal cell physiology, including those for the neurotransmitters acetylcholine, GABA, glutamate, ATP, noradrenaline and substance P [37–38]. Glial cell function in injury repair mechanisms is not fully understood.
SHH and PTCH1 proteins are localized in both neurons and satellite glial cells (Figure 1). SHH is also abundant in Schwann cells during CN regeneration [13]. Previous studies from our group by in situ hybridization have shown that Shh is synthesized in PG neurons but not satellite glial cells, suggesting that the glial cells are targets of SHH signaling, given the presence of the SHH receptor. Since PTCH1 is present in both PG neurons and glial cells, this supports the idea of SHH pathway involvement in communication between neurons and associated glial cells. In the central nervous system, bidirectional communication between astrocytes and neurons is an important element of synaptic transmission [39–41], and it has been suggested in the optic nerve that Shh helps stimulate proliferation of astrocytes. Ptch1 is expressed by astrocytes and their precursors in the developing rodent optic nerve as a result of signaling by axon-derived Shh, and astrocyte proliferation in the developing nerve is reduced by treatment with anti-Shh antibodies, suggesting that Shh stimulates this proliferation [30]. Astrocytes are similar in function to satellite glial cells in the peripheral nervous system. It appears quite possible that the glial cells and PG neuronal cells are involved in a similar bidirectional communication. SMO is normally abundant in the cytoplasm of PG neurons and is one of only two members of the SHH pathway that we’ve identified anterograde transport from neuronal cell bodies in the PG to axons in the CN. The down stream target of SMO in the axon terminals is unknown.
When the CN is injured, as occurs during prostatectomy, SHH protein decreases in PG neurons (Figure 2). This is supported by previous western analysis of PG/CN tissue, which showed decreased precursor and active SHH protein with CN crush [11]. SHH remains abundant in satellite glial cells even as the associated neuron degenerates. This was also observed with CN tie (Figure 4), mimicking a severe, sustained crush injury, and in the more severe resection model. As SHH protein decreases, vacuolization of PG neurons is observed (Figures 2B, 4A, 4D), indicating Wallerian degeneration and apoptosis. This change in morphology is identical to what happens to the CN when SHH is inhibited in the PG, with resulting demyelination and axonal degeneration of CN fibers [12]. With crush injury, SMO localization becomes restricted to the membrane of PG neurons and anterograde transport in CN axons stops, indicating loss of signaling to down stream targets in the axonal terminus. Retrograde effects of the injury have been suggested beyond the injured neurons in other peripheral nerves since preganglionic terminals showed a marked loss of synaptic proteins [22].
The success of functional reinnervation of target organs depends on the capacity of the neurons to survive [42]. With SHH protein treatment of the CN by PA at the time of CN injury, SHH signaling in PG neurons and satellite glial cells remains abundant with no evidence of interrupted signaling, neuronal vacuolization, Wallerian degeneration or apoptosis (Figure 3). Anterograde transport of SMO by neuronal axons in the CN continues as under normal CN conditions. This maintenance of normal signaling between neurons and satellite glial cells occurs due to uptake of SHH protein by axons at the crush site, which then undergo retrograde transport to neuronal cell bodies in the PG (Figure 3).
Vertebrates express an additional factor in the SHH signaling pathway, HIP. HIP is a transmembrane protein that has been shown to bind directly to all vertebrate hedgehog proteins [43]. We’ve shown in a previous study that HIP is synthesized and the protein expressed in the cytoplasm of PG neurons [11] and HIP protein is one of the few proteins we have observed to undergo anterograde transport by axons of the CN [35]. HIP competes with PTCH1 for SHH binding in other organs, and thereby affects signal transduction by preventing SHH binding to PTCH1 (Figure 5A). HIP inhibition in the PG causes axonal degradation and demyelination of CN fibers in a manner similar to SHH inhibition [35]. We show that when HIP signaling is inhibited in the PG, this allows SHH protein to undergo anterograde transport by axons in the CN (Figure 5B and 5C), suggesting that a function of HIP is to restrict the localization and signaling of SHH to the neuronal cell body, and thereby limit its signaling and localization.
SHH treatment of the CN at the time of injury by peptide amphiphile is neuroprotective and promotes CN regeneration by maintaining normal signaling between PG neurons, associated glial cells, and down stream targets. SHH treatment prevents vacuolization of PG neurons, Wallerian degeneration and subsequent apoptosis. This study is significant because understanding how regeneration occurs can provide novel avenues to further enhance regeneration and to prevent ED.
Acknowledgments
This project was supported by NIH/NIDDK Award numbers R01DK101536 and R01DK079184. Peptide synthesis, purification, and characterization were performed by staff in the Peptide Synthesis Core Facility of the Simpson Querrey Institute at Northwestern University. The U.S. Army Research Office, the U.S. Army Medical Research and Material Command, and Northwestern University provided funding to develop this facility.
References
- 1.Feldman HA, Goldstein I, Hatzichristou DG, Krane RJ, McKinlay JB. Impotence and its medical and psychosocial correlates: results of the Massachusetts Male Aging Study. J Urol. 1994;151:54–61. doi: 10.1016/s0022-5347(17)34871-1. [DOI] [PubMed] [Google Scholar]
- 2.Heruti R, Shochat T, Tekes-Manova D, Ashkenazi I, Justo D. Prevalence of erectile dysfunction among young adults: results of a large-scale survey. J Sex Med. 2004;1:284–291. doi: 10.1111/j.1743-6109.04041.x. [DOI] [PubMed] [Google Scholar]
- 3.Kendirci M, Hellstrom WJ. Current concepts in the management of erectile dysfunction in men with prostate cancer. Clin Prostate Cancer. 2004;3:87–92. doi: 10.3816/cgc.2004.n.017. [DOI] [PubMed] [Google Scholar]
- 4.Emanu JC, Avildsen IK, Nelson CJ. Erectile dysfunction after radical prostatectomy: prevalence, medical treatments, and psychosocial interventions. Curr Opin Support Palliat Care. 2016;10:102–107. doi: 10.1097/SPC.0000000000000195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hakim LS, Goldstein I. Diabetic sexual dysfunction. Endocrinol Metab Clin North AM. 1996;24:379–400. doi: 10.1016/s0889-8529(05)70329-7. [DOI] [PubMed] [Google Scholar]
- 6.Gallina A, Ferrari M, Suardi N, Capitanio U, Abdollah F, Tutolo M, Bianchi M, Sacca A, Salonia A, Rigatti P, Montorsi F, Briganti A. Erectile function outcome after bilateral nerve sparing radical prostatectomy: Which patients may be left untreated? J Sex Med. 2012;9:903–908. doi: 10.1111/j.1743-6109.2011.02622.x. [DOI] [PubMed] [Google Scholar]
- 7.Pace G, Del Rosso A, Vicentini C. Penile rehabilitation therapy following radical prostatectomy. Disabil Rehabil. 2010;32:1204–1208. doi: 10.3109/09638280903511594. [DOI] [PubMed] [Google Scholar]
- 8.User HM, Hairston JH, Zelner DJ, McKenna KE, McVary KT. Penile weight and cell subtype specific changes in a postradical prostatectomy model of erectile dysfunction. J Urol. 2003;169:1175–1179. doi: 10.1097/01.ju.0000048974.47461.50. [DOI] [PubMed] [Google Scholar]
- 9.Leungwattanakij S, Bivalacqua TJ, Usta MF, Yang D-Y, Hyun J-S, Champion HC, Abdel-Madeed AB, Hellstrom WJG. Cavernous neurotomy causes hypoxia and fibrosis in rat corpus cavernosum. J Androl. 2003;24:239–245. doi: 10.1002/j.1939-4640.2003.tb02668.x. [DOI] [PubMed] [Google Scholar]
- 10.Choe S, Veliceasa D, Bond CW, Harrington DA, Stupp SI, McVary KT, Podlasek CA. Sonic hedgehog delivery from self-assembled nanofiber hydrogels reduces the fibrotic response in models of erectile dysfunction. Acta Biomateralia. 2016;16:30014–30019. doi: 10.1016/j.actbio.2016.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Angeloni N, Bond CW, Harrington D, Stupp S, Podlasek CA. Sonic hedgehog is neuroprotective in the cavernous nerve with crush injury. J Sex Med. 2013;10:1240–1250. doi: 10.1111/j.1743-6109.2012.02930.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Angeloni NL, Bond CW, Tang Y, Harrington DA, Zhang S, Stupp SI, McKenna KE, Podlasek CA. Regeneration of the cavernous nerve by Sonic hedgehog using aligned peptide amphiphile nanofibers. Biomaterials. 2011;32:1091–1101. doi: 10.1016/j.biomaterials.2010.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bond C, Tang Y, Podlasek CA. Neural influences on Sonic hedgehog and apoptosis in the rat penis. Biol Reprod. 2008;78:947–956. doi: 10.1095/biolreprod.107.064766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Matthias K, Kirchhoff F, Seifert G, Hüttmann K, Matyash M, Kettenmann H, Steinhäuser C. Segregated expression of AMPA-type glutamate receptors and glutamate transporters defines distinct astrocyte populations in the mouse hippocampus. J Neurosci. 2003;23:1750–1758. doi: 10.1523/JNEUROSCI.23-05-01750.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Martin R, Bajo-Graneras R, Moratalla R, Perea G, Araque A. Circuit-specific signaling in astrocyte-neruon networks in basal ganglia pathways. Science. 2015;349:730–734. doi: 10.1126/science.aaa7945. [DOI] [PubMed] [Google Scholar]
- 16.Pfister BJ, Gordon T, Loverde JR, Kochar AS, Mackinnon SE, Cullen DK. Biomedical engineering strategies for peripheral nerve repair: surgical applications, state of the art, and future challenges. Crit Rev Biomed Eng. 2011;39:81–124. doi: 10.1615/critrevbiomedeng.v39.i2.20. [DOI] [PubMed] [Google Scholar]
- 17.Griffin MF, Malahias M, Hindocha S, Kahn WS. Peripheral nerve injury: principles for repair and regeneration. Open Orthopaed J. 2014;8 Suppl 1:199–203. doi: 10.2174/1874325001408010199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Evans GRD. Peripheral nerve injury: a review and approach to tissue engineered constructs. Anat Rec. 2001;263:396–404. doi: 10.1002/ar.1120. [DOI] [PubMed] [Google Scholar]
- 19.Albersen M, Fandel TM, Zhang H, Banie L, Lin G, et al. Pentoxifylline promotes recovery of erectile function in a rat model of postprostatectomy erectile dysfunction. Eur Urol. 2011;59:286–296. doi: 10.1016/j.eururo.2010.10.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xu QG, Midha R, Martinez JA, Guo GF, Zochodne DW. Facilitated sprouting in a peripheral nerve injury. Neuroscience. 2008;152:877–887. doi: 10.1016/j.neuroscience.2008.01.060. [DOI] [PubMed] [Google Scholar]
- 21.Hlaing SM, Garcia LA, Kovanecz I, Martinez RA, Shah S, Artaza JN, Ferrini MG. Sildenafil promotes neuroprotection of the pelvic ganglia neurons after bilateral cavernosal nerve resection in the rat. BLU International. 2012;111:159–170. doi: 10.1111/j.1464-410X.2012.11278.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Palma CA, Keast JR. Structural effects and potential changes in growth factor signaling in penis-projecting autonomic neurons after axotomy. BMC Neuroscience. 2006;7:41. doi: 10.1186/1471-2202-7-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hammerschmidt M, Brook A, McMahon AP. The world according to hedgehog. Trends Genet. 1997;13:14–21. doi: 10.1016/s0168-9525(96)10051-2. [DOI] [PubMed] [Google Scholar]
- 24.Ingham PW. Transducing hedgehog: the story so far. EMBO J. 1998;17:3505–3511. doi: 10.1093/emboj/17.13.3505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Charron F, Stein E, Jeong J, McMahon AP, Tessier-Lavigne M. The morphogen sonic hedgehog is an axonal chemoattractant that collaborates with netrin-1 in midline axon guidance. Cell. 2003;113:11–23. doi: 10.1016/s0092-8674(03)00199-5. [DOI] [PubMed] [Google Scholar]
- 26.Kolodkin AL, Tessier-Lavigne M. Mechamisms and molecules of neuronal wiring: A primer. Cold Springs Harbor Perspect Biol. 2011;3:a:001727. doi: 10.1101/cshperspect.a001727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Akazawa C, Kohsaka S. In vivo characterization of sonic hedgehog in the peripheral nerve regeneration. Brain Nerve. 2007;59:1341–1346. [PubMed] [Google Scholar]
- 28.Martinez JA, Kobayashi M, Krishnan A, Webber C, Christie K, Guo G, Singh V, Zochodne DW. Intrinsic facilitation of adult peripheral nerve regeneration by the Sonic hedgehog morphogen. Experimental Neurology. 2015;271:493–505. doi: 10.1016/j.expneurol.2015.07.018. [DOI] [PubMed] [Google Scholar]
- 29.Dakubo GD, Beug ST, Mazerolle CJ, Thurig S, Wang Y, Wallace VA. Control of glial precursor cell development in the mouse optic nerve by sonic hedgehog from retinal ganglion cells. Brain Res. 2008;1228:27–42. doi: 10.1016/j.brainres.2008.06.058. [DOI] [PubMed] [Google Scholar]
- 30.Wallace CA, Raff MC. A role for Sonic hedgehog in axon-to-astrocyte signaling in the rodent optic nerve. Development. 1999;126:2901–2909. doi: 10.1242/dev.126.13.2901. [DOI] [PubMed] [Google Scholar]
- 31.Zhang S, Greenfield MA, Mata A, Palmer LC, Bitton R, Mantei JR, Aparicio C, de la Cruz MO, Stupp SI. A self-assembly pathway to aligned monodomain gels. Nat Mater. 2010;9:594–601. doi: 10.1038/nmat2778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Berns EJ, Sur S, Pan L, Goldberger JE, Suresh S, Zhang S, Kessler JA, Stupp SI. Aligned neurite outgrowth and directed cell migration in self assembled monodomain gels. Biomaterials. 2014;35:185–195. doi: 10.1016/j.biomaterials.2013.09.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mullerad M, Donohue JF, Li PS, Scardino PT, Mulhall JP. Functional Sequelae of Cavernous Nerve Injury in the Rat: is There Model Dependency. J Sex Med. 2006;3:77–83. doi: 10.1111/j.1743-6109.2005.00158.x. [DOI] [PubMed] [Google Scholar]
- 34.Nangle MR, Keast JR. Reduced Efficacy of Nitrergic Neurotransmission Exacerbates Erectile Dysfunction After Penile Nerve Injury Despite Axonal Regeneration. Exp Neurol. 2007;207:30–41. doi: 10.1016/j.expneurol.2007.05.011. [DOI] [PubMed] [Google Scholar]
- 35.Angeloni NL, Bond CW, Monsivais D, Tang Y, Podlasek CA. The role of Hedgehog-interacting protein in maintaining cavernous nerve integrity and adult penile morphology. J Sex Med. 2009;6:2480–2493. doi: 10.1111/j.1743-6109.2009.01349.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hanani M. Satellite glial cells in sympathetic and parasympathetic ganglia: in search of function. Brain Res Rev. 2010;64:304–327. doi: 10.1016/j.brainresrev.2010.04.009. [DOI] [PubMed] [Google Scholar]
- 37.Shinder V, Devor M. Structural basis of neuron-to-neuron cross-excitation in dorsal root ganglia. J. Neurocytol. 1994;23:515–531. doi: 10.1007/BF01262054. [DOI] [PubMed] [Google Scholar]
- 38.Julius D, Basbaum AI. Molecular mechanisms of nociception. Nature. 2001;413:203–210. doi: 10.1038/35093019. [DOI] [PubMed] [Google Scholar]
- 39.Zhang JM, Wang HK, Ye CQ, Ge W, Chen Y, Jiang ZL, Wu CP, Poo MM, Duan S. ATP released by astrocytes mediates glutamatergic activity-dependent heterosynaptic suppression. Neuron. 2003;40:971–982. doi: 10.1016/s0896-6273(03)00717-7. [DOI] [PubMed] [Google Scholar]
- 40.Araque A. Astrocyte process synaptic information. Neuron Glia Biol. 2008;4:3–10. doi: 10.1017/S1740925X09000064. [DOI] [PubMed] [Google Scholar]
- 41.Garcia ADR, Petrova R, Eng L, Joyner AL. Sonic hedgehog regulates discrete populations of astrocytes in the adult mouse forebrain. Journal of Neuroscience. 2010;30:13597–13608. doi: 10.1523/JNEUROSCI.0830-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Navarro X, Vivó M, Valero-Cabré A. Neural plasticity after peripheral nerve injury and regeneration. Prog Neurobiol. 2007;82:163–201. doi: 10.1016/j.pneurobio.2007.06.005. [DOI] [PubMed] [Google Scholar]
- 43.Chuang PT, McMahon AP. Vertebrate Hedgehog signaling modulated by induction of a Hedgehog-binding protein. Nature. 1999;397:617–621. doi: 10.1038/17611. [DOI] [PubMed] [Google Scholar]