Abstract
Individuals with schizophrenia demonstrate difficulties in attending to important stimuli (e.g., targets) and ignoring distractors (e.g., non-targets). We used a visual oddball task during fMRI to examine functional connectivity within and between the ventral and dorsal attention networks to determine the relative contribution of each network to detection of rare visual targets in schizophrenia. The sample comprised 25 schizophrenia patients and 27 healthy controls. Psychophysiological interaction analysis was used to examine whole-brain functional connectivity in response to targets. We used the right temporo parietal junction (TPJ) as the seed region for the ventral network and the right medial intraparietal sulcus (IPS) as the seed region for the dorsal network. We found that connectivity between right IPS and right anterior insula (AI; a component of the ventral network) was significantly greater in controls than patients. Expected patterns of within- and between-network connectivity for right TPJ were observed in controls, and not significantly different in patients. These findings indicate functional connectivity deficits between the dorsal and ventral attention networks in schizophrenia that may create problems in processing relevant versus irrelevant stimuli. Understanding the nature of network disruptions underlying cognitive deficits of schizophrenia may help shed light on the pathophysiology of this disorder.
Keywords: fMRI, psychophysiological interaction, attention, salience, oddball, target detection
1. Introduction
Individuals with schizophrenia demonstrate deficits of attention, especially in terms of control processes that guide selection of task-relevant inputs (Luck and Gold, 2008; Nuechterlein et al., 2009). Input selection may be driven by bottom-up signals based on salience or top-down biases such as expectation and behavioral goals (Corbetta et al., 2008). Impaired sustained attention has been directly linked to poor community functioning in schizophrenia (Prouteau et al., 2004), while aberrant salience processing may contribute to symptoms (Palaniyappan and Liddle, 2012; Wolf et al., 2008).
Functional neuroimaging studies have provided important information about the spatial distribution of cortical activation during attention tasks. The key regions identified can be organized into two distinct, interacting networks associated with different aspects of attentional control: a ventral network and a dorsal network (Corbetta and Shulman, 2002; Kim, 2014; Ptak, 2012; Vossel et al., 2012; Weissman and Prado, 2012).
The ventral network is thought to involve automatic alerting or reorienting of attention to novel and salient events in a “bottom-up” manner (Kucyi et al., 2012). This network tends to be right lateralized and includes the temporo-parietal junction (TPJ), anterior cingulate cortex (ACC), and anterior insula (AI). Several lines of evidence suggest that the TPJ is critical to salience detection and stimulus-driven attention (Kucyi et al., 2012) and is modulated by target search and detection (Serences et al., 2005; Shulman et al., 2003). Importantly, TPJ activity may play a key role in the interruption of ongoing cognitive activity (e.g., sustained attention) in order to facilitate the analysis of potentially behaviorally relevant stimuli, including targets and unexpected sensory events (Corbetta and Shulman, 2002; Todd et al., 2005). The dorsal network is thought to control voluntary, sustained orienting of attention, including modulation of visual cortex, in a “top-down” fashion. It includes the medial intraparietal sulcus (IPS; located in posterior parietal cortex between the superior parietal lobule and supramarginal gyrus) and inferior frontal junction (IFJ; located in posterior lateral frontal cortex, including the frontal eye fields). The IPS is thought to play a key role in the computation of an attentional priority map, integrating converging sensory information (including salience selection) with top-down signals that represent behavioral goals and expectations for the control of spatial attention (Ptak, 2012; Szczepanski et al., 2013).
A variety of tasks have been used to examine attention deficits in schizophrenia, including visual search tasks (Davenport et al., 2006; Kurachi et al., 1994), continuous performance tasks (Kurtz et al., 2001), and auditory and visual oddball tasks (Ford, 1999; Neuhaus et al., 2013; Oribe et al., 2013). During oddball tasks, subjects are instructed to detect infrequent, irregularly occurring target stimuli embedded within an otherwise repetitive stream of frequent nontarget stimuli. Accurate performance requires ongoing stimulus monitoring while attending to salient stimuli (i.e., targets) and ignoring distractors (i.e., non-targets). Oddball tasks are thus particularly useful as they implicate both top-down and bottom-up aspects of attention. Accordingly, oddball tasks tend to activate regions within both the dorsal and ventral attention networks (Ardekani et al., 2002; Calhoun et al., 2008; Clark et al., 2000; R. C. Gur et al., 2007; Kiehl et al., 2005; Stevens et al., 2000).
Prior fMRI studies of the visual oddball task in schizophrenia have revealed abnormal activity to targets in regions within both of these networks in patients (Collier et al., 2014; R. E. Gur et al., 2007; Hasenkamp et al., 2011). In the ventral network, reduced activity has been observed in cingulate cortex, insula, and superior temporal gyrus. Within the dorsal network, reduced activity has been found in the superior frontal lobe, while increased activity has been found in the inferior parietal lobule. In a recent study, we similarly found group differences in both networks when examining regional fMRI data alone (Wynn et al., 2015). Specifically, patients showed reduced activity in frontal, parietal, and occipital regions, including TPJ, as well as ACC. This study also used a specialized analysis that combined information from both fMRI and event-related potentials (ERPs) (joint independent component analysis, ICA), and found that regional group differences in activation were seen mainly in the ventral network, including ACC, AI, and TPJ (Wynn et al., 2015). These joint ICA findings for the ventral network suggest that dysfunction during target detection in schizophrenia may be linked to problems orienting to salient stimuli in the environment, aside from any difficulties in sustaining general attention to the task.
Fundamental questions remain about the way these regions functionally interact within network, and how the networks interact with each other (Calhoun et al., 2009; Hutchison et al., 2013). The auditory oddball task has been used previously to examine alterations in temporal lobe networks (e.g., (Çetin et al., 2014; Garrity et al., 2007; Yu et al., 2011). Interestingly, reduced connectivity in patients with schizophrenia in the dorsal attention network has also been shown during the auditory oddball task (Calhoun et al., 2008; Kim et al., 2009). How the two attention networks, which serve distinct and complementary purposes, communicate during visual target detection in schizophrenia is not known.
To address these questions, we used a visual oddball task to examine functional connectivity of the ventral and dorsal attention networks in patients with schizophrenia and healthy controls. Given our a priori interest in the ventral and dorsal attention networks, we used psychophysiological interaction (PPI), a seed-based approach, for functional connectivity analysis (Rogers et al., 2007). For the seed regions, we selected areas that might be considered “hubs” of their respective networks: right TPJ for the ventral network (Kucyi et al., 2012) and right IPS for the dorsal network (Ptak, 2012). Using these seed regions, we conducted whole-brain analyses to investigate how connectivity is organized within and between networks while processing rare targets during visual oddball detection.
2. Methods
2.1. Participants
Twenty-five patients with schizophrenia (4 female) and 27 healthy controls (4 female) were recruited for the study. The participants in this study were largely overlapping with those in Wynn et al. (Wynn et al., 2015) that examined regional activation and EEG, but did not consider connectivity. Patients were recruited from outpatient treatment clinics at the Greater Los Angeles VA Medical Center (GLA) and the community. Patients met diagnostic criteria for schizophrenia based on the Structured Clinical Interview for DSM-IV Axis I Disorders (SCID) (First et al., 1997b). Selection criteria were the same as previous studies from this laboratory. Patients were between 18 and 60 years of age, and were excluded from participation if they had: self-reported substance abuse in the past month or dependence in the last six months, IQ < 70 based on examination of medical records, history of loss of consciousness for more than one hour, identifiable neurological disorders, or were not sufficiently fluent in English to consent and understand procedures. Psychiatric symptoms were evaluated using the 24-item University of California, Los Angeles (UCLA) version of the Brief Psychiatric Rating Scale (BPRS) (Ventura et al., 1995) and Scale for the Assessment of Negative Symptoms (SANS) (Andreasen, 1982). For the BPRS we report means for the “positive symptom,” “negative symptom,” “agitation/mania,” and “depression/anxiety” factors (Kopelowicz et al., 2008). For the SANS we report four global scales (Blanchard and Cohen, 2006): Affective Flattening, Alogia, Avolition-Apathy, and Anhedonia-Asociality.
Healthy controls between 18 and 60 years of age were recruited through internet postings and interviewed with the SCID-I and portions of the Structured Clinical Interview for DSM-IV Axis II Disorders (SCID-II) (First et al., 1997a). Exclusion criteria for potential controls included: an identifiable neurological disorder or head injury, a first-degree relative with schizophrenia or another psychotic disorder, insufficient fluency in English, a personal history of schizophrenia or other psychotic disorder, bipolar disorder, recurrent depression, a lifetime history of substance dependence, or any substance abuse in the last 6 months, and any of the following Axis II disorders: avoidant, paranoid, schizoid, or schizotypal.
All interviewers were trained through the Treatment Unit of the Department of Veterans Affairs VISN 22 Mental Illness Research, Education, and Clinical Center (MIRECC) to a minimum kappa of 0.75 for key psychotic and mood items. The study protocol was reviewed and approved by the Institutional Review Boards of the University of California, Los Angeles and Greater Los Angeles VA Medical Center. All participants had the capacity to give informed consent and provided written informed consent after all procedures were fully explained.
2.2. Procedures
2.2.1. Task design
Participants completed a visual oddball task, modeled after prior studies (Ardekani et al., 2002; Ford et al., 2005; Stevens et al., 2000), in which they viewed images of two letters, X and K; one letter served as a target and the other as a nontarget in a counterbalanced fashion. We used an event-related design and presented the stimuli in three separate blocks using magnet-compatible goggles (Resonance Technology, Northridge, CA). Each stimulus was displayed for 100 ms followed by an interstimulus interval (ISI) that was either 900, 1900, or 2900 ms (mean ISI = 1900 ms). The ISIs were equiprobable and randomly distributed. Participants were instructed to push a button on a MRI-compatible button box whenever they detected the target and had 3000 ms to make a response1. Within each block a total of 150 stimuli were presented: 12% were targets (n = 18) and 88% were non-targets (n = 132). Additionally, null trials were interspersed throughout each block and consisted solely of a fixation point. Duration of each block was 5 minutes. The task took approximately 20 minutes to complete.
2.2.2. fMRI data acquisition and preprocessing
Scanning was performed on a Siemens 3T (Erlangen, Germany) Trio MRI scanner. Functional runs (blocks) were acquired with 150 T2*-weighted echoplanar images (EPIs) [repetition time (TR) 2000 ms; echo time (TE) 30 ms; flip angle = 75°; 33 slices; slice thickness 4mm; matrix 64 × 64; FOV 220 mm]. The first two volumes of each functional scan are automatically discarded before data collection begins to allow for T1 equilibrium. Two sets of structural images were acquired for registration of functional data: a T1 weighted magnetization prepared rapid-acquisition gradient echo image (MPRAGE) [TR, 1900 ms; TE 3.43 ms; flip angle = 9°; 160 sagittal slices; slice thickness 1 mm; matrix 256 × 256; FOV 256 mm]; and a T2-weighted matched-bandwidth high-resolution scan with the same slice prescription as the EPI [TR, 6540 ms; TE, 13 ms; flip angle = 120°; 33 slices; slice thickness 4 mm; matrix 128 × 128; FOV 220 mm].
Preprocessing and data analysis was performed with the FMRIB Software Library (FSL v5.0; Analysis Group, Oxford, UK). To compensate for any head motion, images were realigned to the middle volume using MCFLIRT. Movement parameters calculated by MCFLIRT were also modeled in the analysis as nuisance covariates (Jenkinson et al., 2002; Jenkinson and Smith, 2001). Relative mean displacement estimates for the final sample did not exceed 0.5 mm; however, estimates for patients were significantly greater than for controls (patients: M = .18, SD = .11; controls: M = .11, SD = .01; t(49) = 2.94, p < .05). Two patients had one block (out of three) excluded for excessive head motion (relative mean displacement > 0.5 mm). One patient was excluded from the analysis due to having two out of three blocks with excessive head motion. Following these exclusions, 24 patients with schizophrenia and 27 normal controls were included in the PPI analyses.
The data were temporally filtered with a high-pass filter cutoff of 60 s and spatially smoothed with a 5 mm full width half maximum Gaussian kernel in three dimensions. Registration was carried out using FSL’s FLIRT. Each block of individual EPI data was registered first to the co-planar matched-bandwidth T2-weighted image, then to the T1-weighted MPRAGE (both using affine transformations; 6 degrees of freedom) and finally, to Montreal Neurological Institute (MNI) standard space (affine transformation, 12 degrees of freedom).
2.2.3. Behavioral data analysis
Task accuracy was recorded as percentage of correct responses during target trials as well as reaction time of correctly performed trials. Group differences in accuracy and reaction time were assessed separately using analysis of variance (ANOVA; p < .05).
2.2.4. Psychophysiological interaction (PPI) fMRI analysis
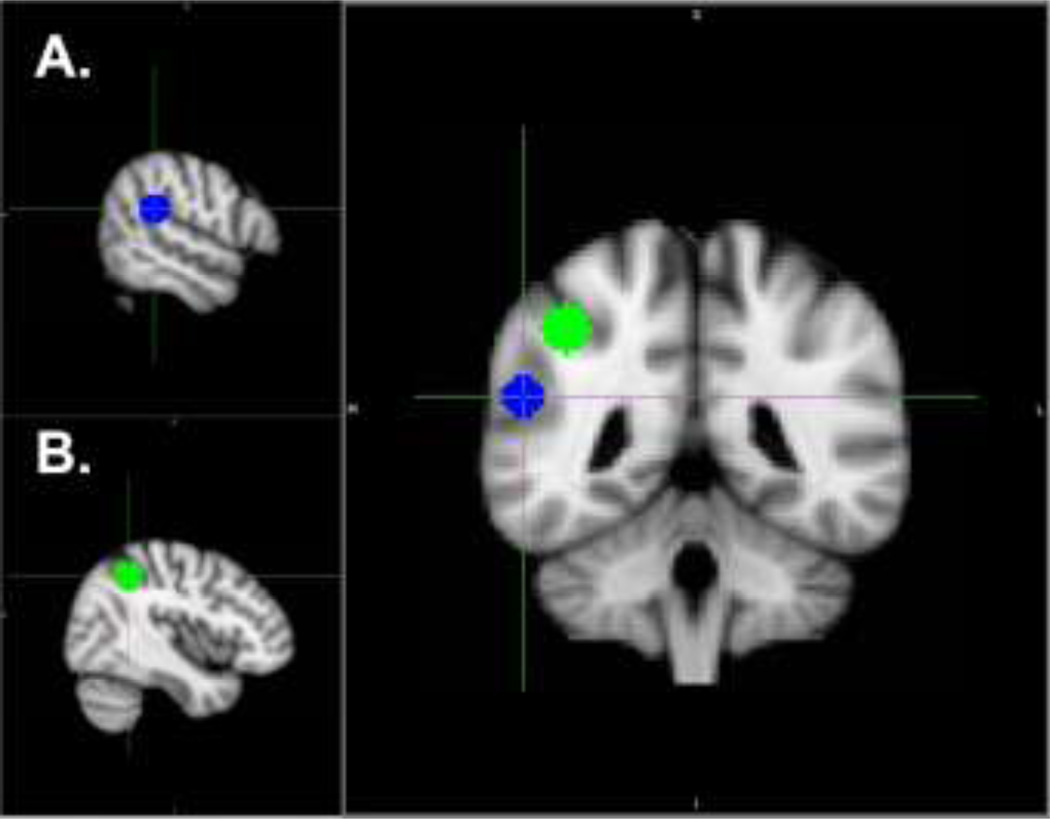
Functional connectivity within and between dorsal and ventral attention networks during target detection was examined using whole-brain PPI analyses (Friston et al., 1997). Seed regions of interest (ROI) were created by placing a 9 mm radius sphere around the coordinates listed below. Locations of ROI are illustrated in Figure 1. The seed regions for the ventral attention network (right TPJ) and the dorsal network (right IPS) were defined based on peak coordinates identified by a recent meta-analysis of visual and auditory oddball tasks in healthy controls (Kim, 2014) (right TPJ MNI coordinates: 56,-38,22; right IPS MNI coordinates: 42,-48,44). The seed ROI were defined at the group level in standard space and projected back to each individual subject’s preprocessed functional data in their native space. The time course from each seed ROI was then extracted for each subject.
Figure 1. Location of seed regions within A) ventral and B) dorsal attention networks.
Each seed region (9mm radius sphere) centered on the following coordinates (x, y, z) in Montreal Neurological Institute (MNI) standard space:
A). Right temporo-parietal junction (right TPJ): 56_-38_22 (Kim, 2014).
B). Right medial intraparietal sulcus (right IPS): 42,-48,44 (Kim, 2014).
A lower-level general linear model (GLM) was constructed for each subject using FSL’s fMRI Expert Analysis Tool (FEAT) with four main regressors. A psychological regressor represented the experimental task variable of interest (“targets” vs “non-targets”), modeled by convolution with a double-gamma canonical hemodynamic response function. A physiological regressor represented task-related BOLD activation in a priori seed regions (right TPJ for the ventral network and right IPS for the dorsal network). An interaction regressor was used to model the interaction between the “targets” vs “non-targets” psychological regressor and the physiological regressor. An additional regressor of non-interest modeled shared variance between targets and non-targets (i.e., “targets” + “non-targets”) to improve overall model fit (O’Reilly et al., 2012). Six parameters for motion correction were included as regressors of non-interest. From the lower-level parameter estimate maps, a second-level fixed-effects analysis was performed to average across the three task blocks for each participant. The resulting contrast images were entered into a group-level random-effects analysis using the FLAME (FMRIB’s Local Analysis of Mixed Effects) stage 1 module (Beckmann et al., 2003). At the group level, participant task performance was included to ensure that group effects were not attributable to group differences in accuracy and reaction time. Results were subjected to pre-threshold masking to constrain the search to areas activated by the target vs non-target contrast from the regional analysis of data (using a threshold of Z > 3.1, p < .05). This masking served to mitigate effects of a large amount of de-activation present in all subjects during non-target stimuli. Finally, resulting within group activation maps were thresholded at Z > 2.3, p < 0.05, cluster-corrected for whole-brain multiple comparisons to control for family wise Type I error rate (Eklund et al., 2016; Friston et al., 1994).
3. Results
3.1. Demographic and clinical characteristics
Table 1 lists group demographics and patient clinical and antipsychotic medication information. There were no differences between groups in terms of age, sex, ethnicity, or parental education. As most patient participants were recruited from VA clinics, the sample is predominantly male. Patients were clinically stable and exhibited mild to moderate clinical symptoms. All patients were receiving second generation antipsychotic medication. Mean daily doses were calculated in chlorpromazine equivalents (Andreasen et al., 2010). Behavioral task performance is also listed in Table 1. Patients were significantly less accurate and had higher reaction times than controls.
Table 1.
Demographic, clinical, and behavioral data.
| Characteristic | Patients n = 25 |
Controls n = 27 |
Statistic | df | P value |
|---|---|---|---|---|---|
| Mean (SD) | Mean (SD) | ||||
| Age | 44.04 (11.7) | 41.52 (9.0) | F =.77 | 1,50 | .39 |
| Education (years) | 12.56 (1.1) | 14.41 (1.8) | F =20.01 | 1,50 | <.001 |
| Average parental education (years)a | 13.83 (3.2) | 14.37 (3.0) | F =.39 | 1,49 | .54 |
| No. | No. | ||||
| Gender | 4F 21M | 4F 23M | χ 2 = .01 | 1 | .91 |
| Clinical Data | Mean (SD) | Mean (SD) | |||
|
Medication dosage (chlorpromazine equivalent in mg/day) |
322.2 (161.3) | n/a | |||
| BPRS | |||||
| Positive symptoms | 1.93 (.8) | n/a | |||
| Negative symptoms | 1.65 (.8) | n/a | |||
| Agitation/mania | 1.27 (.4) | n/a | |||
| Depression/anxiety | 1.85 (.8) | n/a | |||
| SANS | |||||
| Affective Flattening | 1.48 (1.3) | n/a | |||
| Alogia | 0.92 (1.3) | ||||
| Avolition | 2.68 (1.3) | ||||
| Anhedonia | 2.40 (1.3) | ||||
| Task Performanceb | |||||
| Accuracy (%) | 82.36 (19.7) | 94.81 (9.0) | F =8.00 | 1,44 | <.01 |
| Reaction time (ms) | 592.26 (125.7) | 503.91 (66.0) | F =9.33 | 1,44 | <.005 |
Data available for 24 patients, 27 controls
Data available for 21 patients, 25 controls; Abbreviations: SD, standard deviation; No., number; BPRS, Brief Psychiatric Rating Scale; SANS, Scale for the Assessment of Negative Symptoms.
3.2. PPI findings
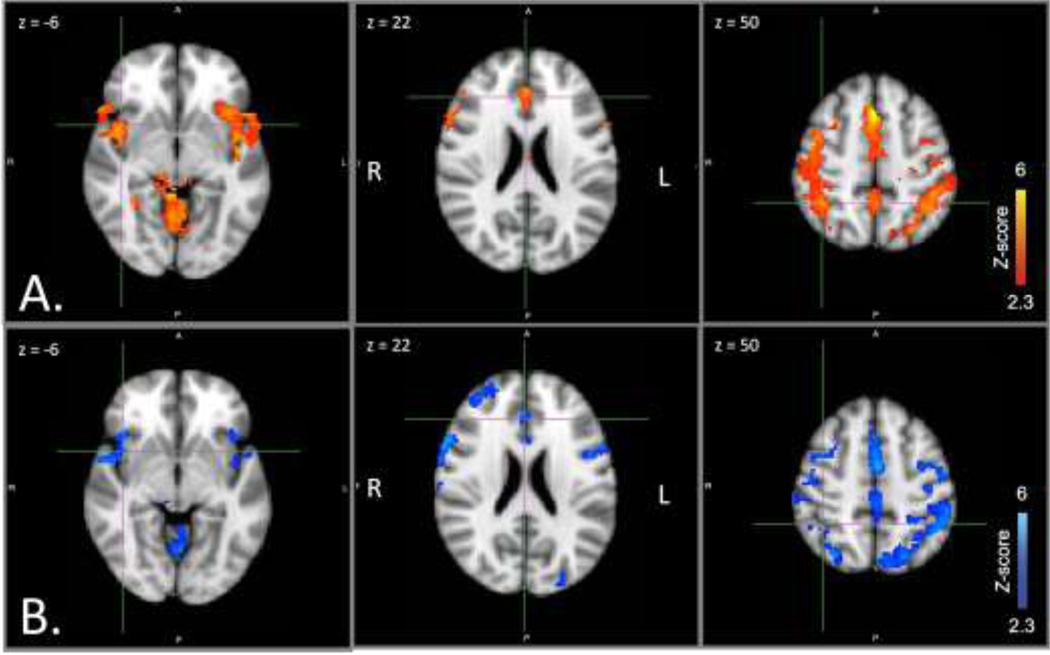
Table 2 lists all regions of significant activation within and between groups for both seed regions. For the ventral network seed, controlling for effects of reaction time and accuracy, both controls and patients showed positive functional connectivity between right TPJ (ventral network seed region) and bilateral AI, ACC, thalamus, middle and inferior frontal gyrus, superior parietal lobule, and precuneus (Figure 2). There were no significant group differences in positive connectivity for right TPJ that survived correction for multiple comparisons. There were no areas of significant negative connectivity with right TPJ for either group.
Table 2.
Regions of positive functional connectivity for each PPI seed region. Maximum z-score and MNI coordinates for within and between group effects. PPI contrast: targets minus non-targets. Covariates included in the model: reaction time and accuracy.
| Region | P value | Voxels | Max z- score |
Max X (mm) |
Max Y (mm) |
Max Z (mm) |
|---|---|---|---|---|---|---|
| PPI seed: right TPJ | ||||||
| Controls | ||||||
| Bilateral anterior cingulate cortex | 1.67E-37 | 11277 | 5.36 | 0 | 18 | 50 |
| Bilateral thalamus | 3.96E-16 | 3363 | 4.88 | 12 | 0 | 14 |
| Left inferior frontal gyrus | 2.27E-08 | 1371 | 5.21 | −56 | 12 | 28 |
| Patients | ||||||
| Bilateral anterior cingulate cortex | 4.61E-36 | 10652 | 4.67 | 0 | 26 | 42 |
| Right middle frontal gyrus/frontal pole | 0.00031 | 580 | 4.16 | 30 | 44 | 30 |
| Left anterior insula | 0.0131 | 336 | 3.42 | −42 | 18 | −12 |
| Lingual gyrus | 0.0158 | 325 | 3.63 | 2 | −66 | −8 |
| Left inferior frontal gyrus | 0.0215 | 307 | 3.83 | −48 | 8 | 20 |
| Bilateral thalamus | 0.0391 | 273 | 4.64 | 0 | −24 | 4 |
| Controls > Patients | ||||||
| None | ||||||
| Patients > Controls | ||||||
| None | ||||||
| PPI seed: right IPS | ||||||
| Controls | ||||||
| Bilateral superior parietal lobule, precuneus, paracingulate gyrus, anterior cingulate gyrus |
0 | 30341 | 5.62 | 26 | −42 | 62 |
| Right frontal pole/middle frontal gyrus | 0.00679 | 395 | 3.81 | 32 | 44 | 32 |
| Patients | ||||||
| Right superior frontal gyrus, paracingulate gyrus, anterior cingulate gyrus |
6.01E-37 | 11701 | 6.03 | 10 | 8 | 68 |
| Bilateral thalamus | 2.51E-05 | 809 | 4.13 | 10 | 6 | 10 |
| Cerebellum | 0.00146 | 499 | 4.06 | −2 | −72 | −16 |
| Controls > Patients | ||||||
| Insula | 0.000732 | 548 | 3.8 | 38 | −22 | −10 |
| Left fusiform gyrus/lingual gyrus | 0.00411 | 428 | 4.17 | −18 | −52 | −12 |
| Right fusiform gyrus/lingual gyrus | 0.0045 | 422 | 3.65 | 24 | −64 | −12 |
| Right precuneus/intracalcarine cortex | 0.0332 | 296 | 3.12 | 14 | −72 | 14 |
| Patients > Controls |
Figure 2. Ventral network positive functional connectivity by group.
Seed region: right TPJ. Axial slices at MNI x coordinates shown. A. Controls (red). B. Patients (blue). From left, crosshairs centered on right AI, ACC, right SPL. Abbreviations: ACC, anterior cingulate cortex; AI, anterior insula; MNI, Montreal Neurological Institute; SPL, superior parietal lobule; TPJ, temporo-parietal junction.
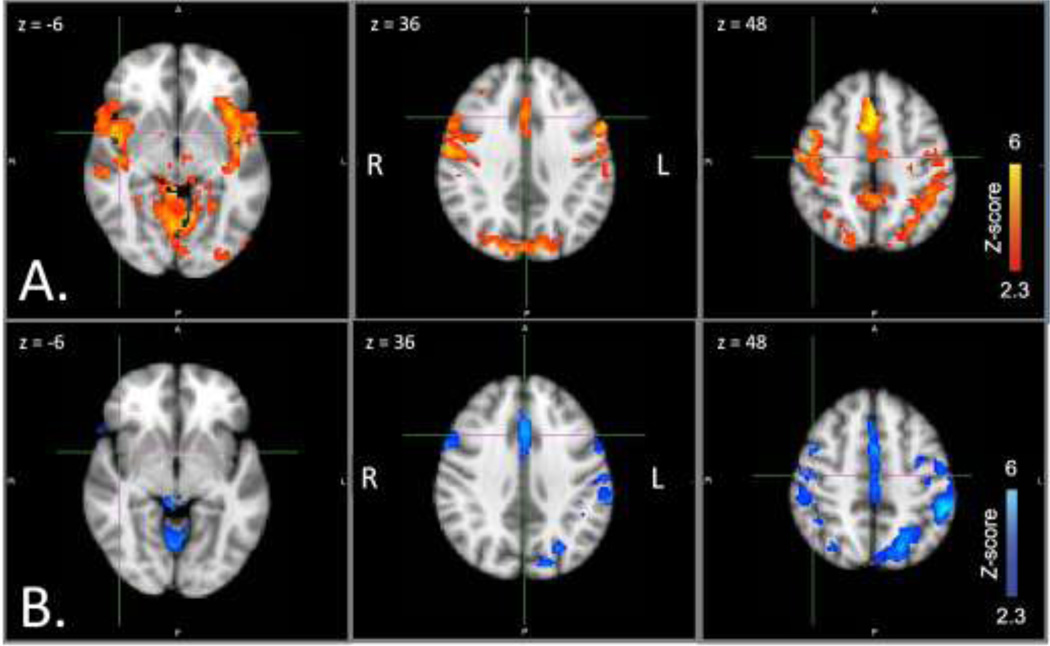
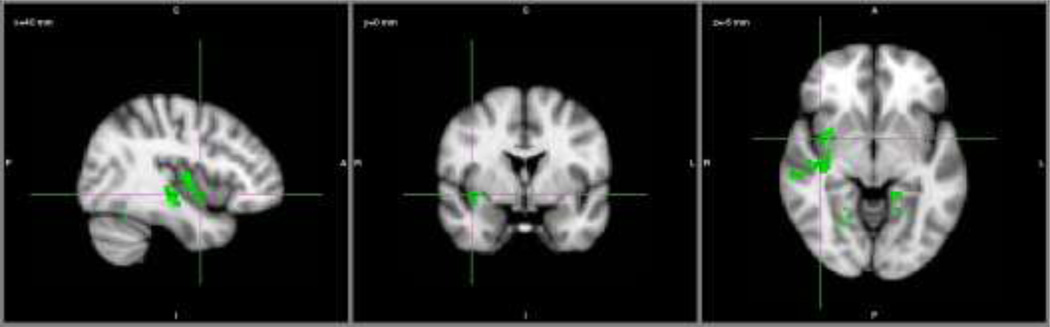
For the dorsal network seed, controlling for effects of reaction time and accuracy, controls showed positive connectivity between right IPS and frontal regions corresponding to left IFJ (including portions of middle frontal gyrus and frontal eye fields), bilateral thalamus, ACC, and superior parietal lobule. Controls also showed positive connectivity between right IPS and bilateral AI (Figure 3A). In contrast, patients showed positive functional connectivity between right IPS and left insula, bilateral thalamus, ACC, and supramarginal gyrus (Figure 3B). Direct group comparisons were significant: controls showed greater positive connectivity than patients between right IPS and right AI (a dorsal to ventral network connection), extending into inferior frontal gyrus and superior temporal gyrus, as well as between right IPS and bilateral fusiform gyrus (Figure 4). There were no areas of significant negative connectivity with right IPS for either group.
Figure 3. Dorsal network positive functional connectivity by group.
Seed region: right IPS. Axial slices at MNI x coordinates shown. A. Controls (red). B. Patients (blue). From left, crosshairs centered on right AI, ACC, right IFJ. Abbreviations: ACC, anterior cingulate cortex; AI, anterior insula; IFJ, inferior frontal junction; IPS, medial intraparietal sulcus; MNI, Montreal Neurological Institute.
Figure 4. Dorsal network positive functional connectivity direct group comparison.
Seed region: right IPS. Controls > Patients (green). Crosshairs centered on MNI coordinates x=40, y=0, z=−6 (right AI). Abbreviations: AI, anterior insula; IPS, medial intraparietal sulcus; MNI, Montreal Neurological Institute.
In post hoc analyses we explored the relationship between task performance, medication dose equivalents, and clinical symptom ratings with significant PPI results (right IPS seed region) in patients. We extracted estimates of functional connectivity (betas) for regions within the two largest clusters that were significantly different between groups; namely, the cluster that included right AI extending into inferior frontal gyrus and superior temporal gyrus, and the cluster that included left fusiform gyrus (see Table 2). Nonparametric analyses (Spearman correlations) were chosen to minimize potential effects of data outliers and of a non-Gaussian distribution of the data. We found no significant correlations between right IPS functional connectivity and accuracy or reaction time by group, or medication dosage (chlorpromazine equivalents) or either symptom scale (BPRS factor scores or SANS global scales) among patients that survived correction for multiple comparisons.
4. Discussion
In the current study, we utilized a visual oddball task to examine functional connectivity of the ventral and dorsal attention networks in schizophrenia. We found evidence for disrupted connectivity in patients primarily between the ventral and dorsal networks when processing targets. Specifically, we found reduced positive connectivity between the dorsal network seed (i.e. right IPS) and right AI (i.e., a key region of the ventral network), in patients compared to controls.
In general, target detection during an oddball task reflects an array of lower and higher level cognitive operations, including arousal, salience coding, goal-directed selective attention, vigilance, and working memory (Polich, 2007). Successful target detection requires higher and lower level processes to interact at the moment when ongoing monitoring of frequent events is interrupted with a rare, but important, target (Vossel et al., 2014). In previous work (Wynn et al., 2015) we isolated regional activation deficits during target detection in schizophrenia to the ventral attention network, implicating lower, rather than higher level processes. However, when examining functional connectivity, it was coordination with a ventral system node by a dorsal system node that was specifically altered. That is, in healthy controls, the dorsal network region right IPS coactivated with the ventral network region right AI; this functional relationship was deficient in patients.
Recent studies have highlighted the importance of flexible interactions between dorsal and ventral attention systems. During top-down visual search, dorsal regions can suppress activation in ventral areas when sustaining attention over time (Shulman et al., 2007). However, dorsal regions also activate ventral regions when salient stimuli are encountered (DiQuattro and Geng, 2011; Vossel et al., 2012). Our finding of reduced positive connectivity between right IPS and right AI in patients during rare target detection could reflect difficulty with switching from suppression to activation modes of interaction.
We did not find significant between-group differences when using right TPJ as the seed for our PPI analysis, either within the ventral network or between the right TPJ and regions of the dorsal network. We chose the right TPJ as the ventral network seed region due to its putative importance in re-orienting attention away from ongoing processes toward unexpected or important events (Corbetta and Shulman, 2002; Kucyi et al., 2012; Todd et al., 2005). However, in the current study, right TPJ did not discriminate between patients and controls in terms of functional connectivity. On the other hand, in line with our findings, right AI has increasingly been the focus of investigations into aberrant salience processing in schizophrenia, and this region has been suggested as a critical “outflow hub” of the ventral network (Chand and Dhamala, 2016; Menon and Uddin, 2010).
It is potentially noteworthy that we found deficient connectivity localized to anterior insula within the right hemisphere in patients with schizophrenia. Although in prior studies abnormalities within the ventral attention network in schizophrenia were not limited to the right hemisphere (Laurens et al., 2005; Tregellas et al., 2012), right hemisphere dominance of attention processing in healthy controls is well-established, and has been observed in multiple species (Corbetta and Shulman, 2011). Not only does processing of spatial relations occur in the dorsal regions of the right hemisphere, but more ventral regions of the right hemisphere are the seat of emotional arousal, critical for detecting and responding to unexpected, salient environmental stimuli. Similar findings across species suggests this may be a longstanding phenomenon in our evolutionary history (Corbetta and Shulman, 2011). Our findings of reduced connectivity in schizophrenia in the right AI could point to core deficits in arousal, reorienting, and detection.
Overall, the current findings suggest inefficient neural responsiveness to rare targets, extending established findings in the literature indicating aberrant salience processing in schizophrenia. Indeed, some theories propose that delusions and hallucinations may result from the inaccurate assignment of salience to irrelevant signals (both internal and external) due to aberrant activation of AI (Palaniyappan and Liddle, 2012; White et al., 2013; Wylie and Tregellas, 2010). While previous work, including our own, indicated that abnormal ventral network activity could underlie aberrant salience processing, the current findings suggest a more nuanced picture, such that faulty dorsal-ventral connectivity could also contribute to this problem. That is, functional connectivity deficits between the networks could create unmodulated processing of salience that would, in turn, make it difficult to separate relevant from irrelevant stimuli and lead to impaired target detection (Moran et al., 2013; Uddin, 2015). Other possible mechanisms for impaired recruitment of attention networks in schizophrenia have also been investigated. For example, hippocampal hyperactivity and loss of inhibitory signaling in the hippocampus may relate to impaired ability to inhibit distraction during selective attention processing (Smucny et al., 2015; Tregellas et al., 2012).
We assessed the behavioral relevance of network connectivity deficits in two ways. First, we included performance metrics (accuracy and reaction time) in the PPI analyses for both the ventral and dorsal seeds. Second, we directly assessed the relationship between function connectivity and behavioral performance in regions significantly different between groups (i.e., right IPS connectivity in right AI and right FFG). Within the PPI analyses, differences in functional connectivity between groups were not attributable to group differences in performance. Further, behavioral performance was not significantly correlated with PPI findings. We suggest that this lack of correlation may be a function of limited variability in the data, rather than evidence against potential functional relevance of our findings. The version of visual oddball task chosen for the current study was intended to be simple and easily performed by our patients. Although their performance was reduced relative to controls, patient performance was still quite high (i.e., > 80% accuracy). Ceiling effects in controls might have similarly impacted our ability to detect significant correlations in that group. We also examined the relationship between functional connectivity and symptom severity. We did not find any correlation between PPI findings and clinical measures (symptom ratings) in those post hoc analyses. It is worth noting that our patients were generally stable, with mild to moderate symptom profiles. This lack of variability in the clinical symptom data could have similarly limited our ability to detect significant correlations. Alternatively, the relatively small sample size could have limited our power to detect effects in this type of analysis.
The present study has several limitations. First, the patients in our study were all receiving second generation antipsychotic medication. The effects of long-term medication use on response to targets within this task have not been systematically studied, either in humans or animal models. However, a recent longitudinal study of first-episode schizophrenia patients using a visual oddball task paradigm during EEG found no association with medication (chlorpromazine equivalent scores) at either baseline or one year follow-up, indicating those results were not confounded by effects of antipsychotic medication (Oribe et al., 2015). Furthermore, a recent prospective longitudinal study of individuals at clinical high-risk for psychosis found that cognitive functioning was unrelated to medication status at an eight month follow-up, whether or not an individual converted to psychosis during that time period (Carrion et al., 2015). In the current study of individuals with chronic schizophrenia, chlorpromazine equivalents were not significantly associated with performance or estimates of functional connectivity, suggesting that our findings are at least partially independent of medication status. Second, we used a visual, rather than the more common auditory, oddball task. EEG and fMRI studies have generally used the auditory modality, which some suggest may have more extensive abnormalities in schizophrenia ((Collier et al., 2014; Kiehl et al., 2005) but see also (Kim, 2014)). The generalizability of our findings to other modalities and types of visual target detection tasks requires further investigation. Third, our study included targets and non-targets only, whereas some studies include novel non-targets to serve as distractors (e.g., (R. E. Gur et al., 2007; Kiehl et al., 2001)). It would be informative to understand whether our findings of disrupted connectivity to targets extend to non-target, but nevertheless salient, distractors as well. Finally, we found that patients had significantly greater movement parameter estimates compared to controls. We sought to minimize these effects by excluding blocks corrupted by large motion (>0.5mm mean relative displacement) and taking steps during pre-processing to model the effects of motion out of the data. Still, given the fact that functional connectivity analyses may be particularly susceptible to confounding effects of motion (Power et al., 2012), we acknowledge these group differences as a limitation of the data, and results should thus be interpreted with caution.
Despite the limitations, the current findings add to a growing body of literature highlighting disrupted connectivity as a core feature of schizophrenia (Calhoun et al., 2009; Friston and Frith, 1995; Karlsgodt et al., 2008; Pettersson-Yeo et al., 2011). In particular, our findings of disrupted connectivity between the ventral attention network and dorsal attention network help elucidate the neural underpinnings of poor attentional control, a fundamental impairment in schizophrenia (e.g., (Nuechterlein et al., 2009)). Understanding the nature of network mechanisms underlying cognitive deficits of schizophrenia and how regional organization within and between networks is disrupted in terms of connectivity will aid in the development of novel therapeutic strategies (Hwang et al., 2010; Smucny et al., 2016a, 2016b, 2015) and may help shed light on the pathophysiology of this disorder.
Highlights.
Attention deficits in schizophrenia involve salience and sustained control processes
These processes are associated with ventral and dorsal brain networks, respectively
We examined functional connectivity of these networks during a visual oddball task
Patients showed reduced connectivity between the ventral and dorsal networks
The findings may explain difficulties processing relevant versus irrelevant stimuli
Acknowledgments
Funding:
This research was supported by a VA Career Development Award to Jonathan K. Wynn, Ph.D. and NIMH Grants MH43292 and MH065707 (PI: Michael F. Green, Ph.D). Writing of this manuscript was supported by the Office of Academic Affiliations, Advanced Fellowship Program in Mental Illness Research and Treatment, Department of Veterans Affairs. For generous support, we also thank the Brain Mapping Medical Research Organization, Brain Mapping Support Foundation, Pierson-Lovelace Foundation, The Ahmanson Foundation, William M. and Linda R. Dietel Philanthropic Fund at the Northern Piedmont Community Foundation, Tamkin Foundation, Jennifer Jones-Simon Foundation, Capital Group Companies Charitable Foundation, Robson Family, and Northstar Fund. The authors thank Poorang Nori and Crystal Gibson for assistance in data collection. The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript. These data were presented, in part, as a poster at the 2015 Annual Meeting of the Society for Research in Psychopathology.
Footnotes
Note that although the response window to targets (3000ms) overlapped with the onset of non-targets, the vast majority of participants responded faster than 1000ms and responded almost exclusively to the target. See Wynn et al. (Wynn et al., 2015) for further comment on the justification for this design.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributors
AJ took the lead on data analysis, interpretation, and manuscript writing, including management of edits and revisions based on co-author feedback. JW and MG formulated the study concept and design and managed data collection. JL, MG, and JW assisted with data analysis planning, data interpretation, manuscript drafting, and editing.
Conflict of interest:
Dr. Green has been a consultant to AbbVie, DSP, Forum, and Roche, and he is on the scientific advisory board of Mnemosyne. He has received research funds from Amgen. The rest of the authors report no biomedical financial interests or potential conflicts of interest.
References
- Andreasen NC. Negative symptoms in schizophrenia: Definition and reliability. Arch. Gen. Psychiatry. 1982;39:784–788. doi: 10.1001/archpsyc.1982.04290070020005. [DOI] [PubMed] [Google Scholar]
- Andreasen NC, Pressler M, Nopoulos P, et al. Antipsychotic dose equivalents and dose-years: A standardized method for comparing exposure to different drugs. Biol. Psychiatry. 2010;67:255–262. doi: 10.1016/j.biopsych.2009.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ardekani BA, Choi SJ, Hossein-Zadeh GA, et al. Functional magnetic resonance imaging of brain activity in the visual oddball task. Cogn. Brain Res. 2002;14:347–356. doi: 10.1016/s0926-6410(02)00137-4. [DOI] [PubMed] [Google Scholar]
- Beckmann CF, Jenkinson M, Smith SM. General multilevel linear modeling for group analysis in FMRI. Neuroimage. 2003;20:1052–1063. doi: 10.1016/S1053-8119(03)00435-X. [DOI] [PubMed] [Google Scholar]
- Blanchard JJ, Cohen AS. The structure of negative symptoms within schizophrenia: Implications for assessment. Schizophr. Bull. 2006;32:238–245. doi: 10.1093/schbul/sbj013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calhoun VD, Eichele T, Pearlson G. Functional brain networks in schizophrenia: A review. Front. Hum. Neurosci. 2009;3:1–12. doi: 10.3389/neuro.09.017.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calhoun VD, Kiehl KA, Pearlson GD. Modulation of temporally coherent brain networks estimated using ICA at rest and during cognitive tasks. Hum. Brain Mapp. 2008;29:828–838. doi: 10.1002/hbm.20581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrion R, McLaughlin D, Auther AM, et al. The impact of psychosis on the course of cognition: a prospective, nested case-control study in individuals at clinical high-risk for psychosis. Psychol. Med. 2015;45:3341–3354. doi: 10.1017/S0033291715001233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Çetin MS, Christensen F, Abbott CC, et al. Thalamus and posterior temporal lobe show greater inter-network connectivity at rest and across sensory paradigms in schizophrenia. Neuroimage. 2014;97:117–126. doi: 10.1016/j.neuroimage.2014.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chand GB, Dhamala M. Interactions among brain default-mode, salience, and central-executive networks during perceptual decision-making of moving dots. Brain Connect. 2016;6:249–254. doi: 10.1089/brain.2015.0379. [DOI] [PubMed] [Google Scholar]
- Clark VP, Fannon S, Lai S, et al. Responses to rare visual target and distractor stimuli using event-related fMRI. J. Neurophysiol. 2000;83:3133–3139. doi: 10.1152/jn.2000.83.5.3133. [DOI] [PubMed] [Google Scholar]
- Collier AK, Wolf DH, Valdez JN, et al. Comparison of auditory and visual oddball fMRI in schizophrenia. Schizophr. Res. 2014;158:183–188. doi: 10.1016/j.schres.2014.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbetta M, Patel G, Shulman GL. The reorienting system of the human brain: From environment to theory of mind. Neuron. 2008;58:306–324. doi: 10.1016/j.neuron.2008.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbetta M, Shulman GL. Spatial neglect and attention networks. Annu. Rev. Neurosci. 2011;34:569–599. doi: 10.1146/annurev-neuro-061010-113731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nat. Rev. Neurosci. 2002;3:201–215. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- Davenport ND, Sponheim SR, Stanwyck JJ. Neural anomalies during visual search in schizophrenia patients and unaffected siblings of schizophrenia patients. Schizophr. Res. 2006;82:15–26. doi: 10.1016/j.schres.2005.09.011. [DOI] [PubMed] [Google Scholar]
- DiQuattro NE, Geng JJ. Contextual knowledge configures attentional control networks. J. Neurosci. 2011;31:18026–18035. doi: 10.1523/JNEUROSCI.4040-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eklund A, Nichols TE, Knutsson H. Cluster failure: Why fMRI inferences for spatial extent have inflated false-positive rates. Proc. Natl. Acad. Sci. 2016:201602413. doi: 10.1073/pnas.1602413113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First M, Gibbon M, Spitzer R. Structured Clinical Interview for DSM-IV Axis II Personality Disorders (SCID-II) Am. Psychiatr. Press. Inc. 1997a [Google Scholar]
- First M, Spitzer R, Gibbon M, et al. Structured Clinical Interview for DSM-IV Axis I Disorders-Patient Edition (SCID-I/P) Am. Psychiatr. Press. Inc. 1997b [Google Scholar]
- Ford JM. Schizophrenia: the broken P300 and beyond. Psychophysiology. 1999;36:667–682. [PubMed] [Google Scholar]
- Ford JM, Johnson MB, Whitfield SL, et al. Delayed hemodynamic responses in schizophrenia. Neuroimage. 2005;26:922–931. doi: 10.1016/j.neuroimage.2005.03.001. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Buechel C, Fink GR, et al. Psychophysiological and modulatory interactions in neuroimaging. Neuroimage. 1997;6:218–229. doi: 10.1006/nimg.1997.0291. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Frith CD. Schizophrenia: A disconnection syndrome? Clin. Neurosci. 1995;3:89–97. [PubMed] [Google Scholar]
- Friston KJ, Worsley KJ, Frackowiak RS, et al. Assessing the significance of focal activations using their spatial extent. Hum. Brain Mapp. 1994;1:210–20. doi: 10.1002/hbm.460010306. [DOI] [PubMed] [Google Scholar]
- Garrity AG, Pearlson GD, McKiernan KA, et al. Aberrant “default mode” functional connectivity in schizophrenia. Am J Psychiatry. 2007;164:450–457. doi: 10.1176/ajp.2007.164.3.450. [DOI] [PubMed] [Google Scholar]
- Gur RC, Turetsky BI, Loughead J, et al. Hemodynamic responses in neural circuitries for detection of visual target and novelty: An event-related fMRI study. Hum. Brain Mapp. 2007;28:263–274. doi: 10.1002/hbm.20319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gur RE, Turetsky BI, Loughead J, et al. Visual attention circuitry in schizophrenia investigated with oddball event-related functional magnetic resonance imaging. Am. J. Psychiatry. 2007;164:442–449. doi: 10.1176/ajp.2007.164.3.442. [DOI] [PubMed] [Google Scholar]
- Hasenkamp W, James GA, Boshoven W, et al. Altered engagement of attention and default networks during target detection in schizophrenia. Schizophr. Res. 2011;125:169–173. doi: 10.1016/j.schres.2010.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchison RM, Womelsdorf T, Allen EA, et al. Dynamic functional connectivity: Promise , issues , and interpretations. Neuroimage. 2013;80:360–378. doi: 10.1016/j.neuroimage.2013.05.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang JH, Kim SH, Park CS, et al. Acute high-frequency rTMS of the left dorsolateral prefrontal cortex and attentional control in healthy young men. Brain Res. 2010;1329:152–158. doi: 10.1016/j.brainres.2010.03.013. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Bannister P, Brady M, et al. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage. 2002;17:825–841. doi: 10.1016/s1053-8119(02)91132-8. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Smith S. A global optimisation method for robust affine registration of brain images. Med. Image Anal. 2001;5:143–156. doi: 10.1016/s1361-8415(01)00036-6. [DOI] [PubMed] [Google Scholar]
- Karlsgodt KH, Sun D, Jimenez AM, et al. Developmental disruptions in neural connectivity in the pathophysiology of schizophrenia. Dev. Psychopathol. 2008;20:1297–1327. doi: 10.1017/S095457940800062X. [DOI] [PubMed] [Google Scholar]
- Kiehl KA, Stevens MC, Celone K, et al. Abnormal hemodynamics in schizophrenia during an auditory oddball task. Biol. Psychiatry. 2005;57:1029–1040. doi: 10.1016/j.biopsych.2005.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiehl K, Laurens KR, Duty TL, et al. Neural sources involved in auditory target detection and novelty processing: an event-related fMRI study. Psychophysiology. 2001;38:133–142. [PubMed] [Google Scholar]
- Kim H. Involvement of the dorsal and ventral attention networks in oddball stimulus processing: A meta-analysis. Hum. Brain Mapp. 2014;35:2265–84. doi: 10.1002/hbm.22326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim DIl, Mathalon DH, Ford JM, et al. Auditory oddball deficits in schizophrenia: An independent component analysis of the fMRI multisite function BIRN study. Schizophr. Bull. 2009;35:67–81. doi: 10.1093/schbul/sbn133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopelowicz A, Ventura J, Liberman RP, et al. Consistency of brief psychiatric rating scale factor structure across a broad spectrum of schizophrenia patients. Psychopathology. 2008;41:77–84. doi: 10.1159/000111551. [DOI] [PubMed] [Google Scholar]
- Kucyi A, Hodaie M, Davis KD. Lateralization in intrinsic functional connectivity of the temporoparietal junction with salience- and attention-related brain networks. J. Neurophysiol. 2012;108:3382–3392. doi: 10.1152/jn.00674.2012. [DOI] [PubMed] [Google Scholar]
- Kurachi M, Matsui M, Kiba K, et al. Limited visual search on the WAIS Picture Completion test in patients with schizophrenia. Schizophr. Res. 1994;12:75–80. doi: 10.1016/0920-9964(94)90086-8. [DOI] [PubMed] [Google Scholar]
- Kurtz MM, Ragland JD, Bilker W, et al. Comparison of the continuous performance test with and without working memory demands in healthy controls and patients with schizophrenia. Schizophr. Res. 2001;48:307–316. doi: 10.1016/s0920-9964(00)00060-8. [DOI] [PubMed] [Google Scholar]
- Laurens KR, Kiehl KA, Ngan ETC, et al. Attention orienting dysfunction during salient novel stimulus processing in schizophrenia. Schizophr. Res. 2005;75:159–171. doi: 10.1016/j.schres.2004.12.010. [DOI] [PubMed] [Google Scholar]
- Luck SJ, Gold JM. The construct of attention in schizophrenia. Biol. Psychiatry. 2008;64:34–39. doi: 10.1016/j.biopsych.2008.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon V, Uddin LQ. Saliency, switching, attention and control: A network model of insula function. Brain Struct. Funct. 2010;214:655–667. doi: 10.1007/s00429-010-0262-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran LV, Tagamets MA, Sampath H, et al. Disruption of anterior insula modulation. Biol. Psychiatry. 2013;74:467–474. doi: 10.1016/j.biopsych.2013.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuhaus AH, Brandt ESL, Goldberg TE, et al. Evidence for impaired visual prediction error in schizophrenia. Schizophr. Res. 2013;147:326–330. doi: 10.1016/j.schres.2013.04.004. [DOI] [PubMed] [Google Scholar]
- Nuechterlein KH, Luck SJ, Lustig C, et al. CNTRICS final task selection: control of attention. Schizophr. Bull. 2009;35:182–196. doi: 10.1093/schbul/sbn158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Reilly JX, Woolrich MW, Behrens TEJ, et al. Tools of the trade: Psychophysiological interactions and functional connectivity. Soc. Cogn. Affect. Neurosci. 2012;7:604–9. doi: 10.1093/scan/nss055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oribe N, Hirano Y, Kanba S, et al. Progressive reduction of visual P300 amplitude in patients with first-episode schizophrenia: An ERP study. Schizophr. Bull. 2015;41:460–470. doi: 10.1093/schbul/sbu083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oribe N, Hirano Y, Kanba S, et al. Early and late stages of visual processing in individuals in prodromal state and first episode schizophrenia: An ERP study. Schizophr. Res. 2013;146:95–102. doi: 10.1016/j.schres.2013.01.015. [DOI] [PubMed] [Google Scholar]
- Palaniyappan L, Liddle PF. Does the salience network play a cardinal role in psychosis? An emerging hypothesis of insular dysfunction. J. Psychiatry Neurosci. 2012;37:17–27. doi: 10.1503/jpn.100176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettersson-Yeo W, Allen P, Benetti S, et al. Dysconnectivity in schizophrenia: Where are we now? Neurosci. Biobehav. Rev. 2011;35:1110–24. doi: 10.1016/j.neubiorev.2010.11.004. [DOI] [PubMed] [Google Scholar]
- Polich J. Updating P300: An integrative theory of P3a and P3b. Clin. Neurophysiol. 2007;118:2128–2148. doi: 10.1016/j.clinph.2007.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power JD, Barnes KA, Snyder AZ, et al. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motio.n. Neuroimage. 2012;59:2142–2154. doi: 10.1016/j.neuroimage.2011.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prouteau A, Briand C, Lesage A, et al. The crucial role of sustained attention in community functioning in outpatients with schizophrenia. Psychiatry Res. 2004;129:171–177. doi: 10.1016/j.psychres.2004.07.005. [DOI] [PubMed] [Google Scholar]
- Ptak R. The frontoparietal attention network of the human brain: Action, saliency, and a priority map of the environment. Neurosci. 2012;18:502–515. doi: 10.1177/1073858411409051. [DOI] [PubMed] [Google Scholar]
- Rogers BP, Morgan VL, Newton AT, et al. Assessing functional connectivity in the human brain by fMRI. Magn. Reson. Imaging. 2007;25:1347–1357. doi: 10.1016/j.mri.2007.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serences JT, Shomstein S, Leber AB, et al. Coordination of voluntary and stimulus-driven attentional control in human cortex. Psychol. Sci. 2005;16:114–122. doi: 10.1111/j.0956-7976.2005.00791.x. [DOI] [PubMed] [Google Scholar]
- Shulman GL, Astafiev SV, McAvoy MP, et al. Right TPJ deactivation during visual search: Functional significance and support for a filter hypothesis. Cereb. Cortex. 2007;17:2625–2633. doi: 10.1093/cercor/bhl170. [DOI] [PubMed] [Google Scholar]
- Shulman GL, Mcavoy MP, Cowan MC, et al. Quantitative analysis of attention and detection signals during visual search. J. Neurophysiol. 2003;90:3384–3397. doi: 10.1152/jn.00343.2003. [DOI] [PubMed] [Google Scholar]
- Smucny J, Olincy A, Rojas DC, et al. Neuronal effects of nicotine during auditory selective attention in schizophrenia. Hum. Brain Mapp. 2016a;37:410–421. doi: 10.1002/hbm.23040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smucny J, Olincy A, Tregellas JR. Nicotine restores functional connectivity of the ventral attention network in schizophrenia. Neuropharmacology. 2016b;108:144–151. doi: 10.1016/j.neuropharm.2016.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smucny J, Visani A, Tregellas JR. Could vagus nerve stimulation target hippocampal hyperactivity to improve cognition in schizophrenia? Front. Psychiatry. 2015;6:1–5. doi: 10.3389/fpsyt.2015.00043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens AA, Skudlarski P, Gatenby JC, et al. Event-related fMRI of auditory and visual oddball tasks. Magn. Reson. Imaging. 2000;18:495–502. doi: 10.1016/s0730-725x(00)00128-4. [DOI] [PubMed] [Google Scholar]
- Szczepanski SM, Pinsk MA, Douglas MM, et al. Functional and structural architecture of the human dorsal frontoparietal attention network 2013. 2013 doi: 10.1073/pnas.1313903110. doi:10.1073/pnas.1313903110/-/DCSupplemental. www.pnas.org/cgi/doi/10.1073/pnas.1313903110. [DOI] [PMC free article] [PubMed]
- Todd JJ, Fougnie D, Marois R. Visual short-term memory load suppresses temporo-parietal junction activity and induces inattentional blindness. Psychol. Sci. 2005;16:965–72. doi: 10.1111/j.1467-9280.2005.01645.x. [DOI] [PubMed] [Google Scholar]
- Tregellas JR, Smucny J, Eichman L, et al. The effect of distracting noise on the neuronal mechanisms of attention in schizophrenia. Schizophr. Res. 2012;142:230–236. doi: 10.1016/j.schres.2012.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uddin LQ. Salience processing and insular cortical function and dysfunction. Nat. Rev. Neurosci. 2015;16:55–61. doi: 10.1038/nrn3857. [DOI] [PubMed] [Google Scholar]
- Ventura J, Nuechterlein KH, Subotnik K, et al. Symptom dimensions in recent-onset schizophrenia: The 24-item expanded BPRS. Schizophr. Res. 1995;15:22. [Google Scholar]
- Vossel S, Geng JJ, Fink GR. Dorsal and ventral attention systems: Distinct neural circuits but collaborative roles. Neurosci. 2014;20:150–159. doi: 10.1177/1073858413494269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vossel S, Weidner R, Driver J, et al. Deconstructing the architecture of dorsal and ventral attention systems with dynamic causal modelling. J. Neurosci. 2012;32:10637–10648. doi: 10.1523/JNEUROSCI.0414-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissman DH, Prado J. Heightened activity in a key region of the ventral attention network is linked to reduced activity in a key region of the dorsal attention network during unexpected shifts of covert visual spatial attention. Neuroimage. 2012;61:798–804. doi: 10.1016/j.neuroimage.2012.03.032. [DOI] [PubMed] [Google Scholar]
- White TP, Gilleen J, Shergill SS. Dysregulated but not decreased salience network activity in schizophrenia. Front. Hum. Neurosci. 2013;7:1–12. doi: 10.3389/fnhum.2013.00065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf DH, Turetsky BI, Loughead J, et al. Auditory oddball fMRI in schizophrenia: Association of negative symptoms with regional hypoactivation to novel distractors. Brain Imaging Behav. 2008;2:132–145. doi: 10.1007/s11682-008-9022-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wylie KP, Tregellas JR. The role of the insula in schizophrenia. Schizophr. Res. 2010;123:93–104. doi: 10.1016/j.schres.2010.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wynn JK, Jimenez AM, Roach BJ, et al. Impaired target detection in schizophrenia and the ventral attentional network: Findings from a joint event-related potential - functional MRI analysis. NeuroImage. Clin. 2015;9:95–102. doi: 10.1016/j.nicl.2015.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Q, Sui J, Rachakonda S, et al. Altered small-world brain networks in temporal lobe in patients with schizophrenia performing an auditory oddball task. Front. Syst. Neurosci. 2011;5:1–13. doi: 10.3389/fnsys.2011.00007. [DOI] [PMC free article] [PubMed] [Google Scholar]