Abstract
The goal of the current study was to characterize the immune cell types within the primate corpus luteum (CL). Luteal tissue was collected from rhesus females at discrete intervals during the luteal phase of the natural menstrual cycle. Dispersed cells were incubated with fluorescently labeled antibodies specific for the immune cell surface proteins CD11b (neutrophils and monocytes/macrophages), CD14 (monocytes/macrophages), CD16 (natural killer [NK] cells), CD20 (B-lymphocytes), and CD3epsilon (T-lymphocytes) for analysis by flow cytometry. Numbers of CD11b-positive (CD11b+) and CD14+ cells increased significantly 3 to 4 days after serum progesterone (P4) concentrations declined below 0.3 ng/ml. CD16+ cells were the most abundant immune cell type in CL during the mid and mid-late luteal phases and were 3-fold increased 3 to 4 days after serum P4 decreased to baseline levels. CD3epsilon+ cells tended to increase 3 to 4 days after P4 decline. To determine whether immune cells were upregulated by the loss of luteotropic (LH) support or through loss of LH-dependent steroid milieu, monkeys were assigned to 4 groups: control (no treatment), the GnRH antagonist Antide, Antide plus synthetic progestin (R5020), or Antide plus the estrogen receptor agonists diarylpropionitrile (DPN)/propyl-pyrazole-triol (PPT) during the mid-late luteal phase. Antide treatment increased the numbers of CD11b+ and CD14+ cells, whereas progestin, but not estrogen, replacement suppressed the numbers of CD11b+, CD14+, and CD16+ cells. Neither Antide nor steroid replacement altered numbers of CD3epsilon+ cells. These data suggest that increased numbers of innate immune cells in primate CL after P4 synthesis declines play a role in onset of structural regression of primate CL.
Keywords: corpus luteum, luteal regression, macrophage, natural killer cell, neutrophil, primate
INTRODUCTION
In nonfertile cycles, luteal regression in Old World monkeys (e.g., macaque species) and humans involves declining sensitivity to luteinizing hormone (LH) pulses, resulting in loss of progesterone (P4) secretion and onset of menses [1]. The events leading to luteal insensitivity to LH and subsequent functional and structural regression near the end of the menstrual cycle are poorly understood in primates. However, luteolysis in some species appears to be mediated, at least in part, by cells of the immune system [2]. Immune processes are conventionally considered to follow two main pathways: innate and adaptive responses [3]. Innate immune processes occur very rapidly and are associated with release of factors such as chemokines and cytokines in response to infection by microbes. Adaptive processes are slower and involve maturation of dendritic cells into antigen-presenting cells in response to inflammatory processes initiated by the innate response and production of specific antibodies to target specific pathogens [3]. Monocytes/macrophages, natural killer (NK) cells, and neutrophils are associated with innate immunity, whereas lymphatic cells (T- and B-lymphocytes) are mostly involved with adaptive immunity, although cellular responses between the two pathways are highly coordinated [3] and not necessarily distinct [4].
Several studies using domestic animal species support a role for immune cells in regulating the functional lifespan of the corpus luteum (CL) [2]. During luteal regression, macrophages, monocytes, neutrophils, and T-lymphocytes increase in number in ruminant CL [5]. Considerable interest was placed on T-lymphocyte population, with three distinct subtypes present in bovine CL that appear to modulate inflammatory processes during luteal regression [5]. In rodent CL, an increase in numbers of luteal macrophages and T-lymphocytes is associated with functional regression, whereas only T-lymphocytes are still elevated at the time of structural regression [6]. There are also reports that the numbers of neutrophils, macrophages (plus their precursor monocytes), and T-lymphocytes increase in the CL of women during luteal regression [7–10], but it is unclear whether changes occur during functional or structural luteolysis.
Elucidating the processes that regulate accumulation and activation of immune cells within reproductive tissues is an area of active investigation. Local factors such as prostaglandins (e.g., PGE2, PGF2α) have immunomodulatory properties [11], and PGF2α-induced luteal regression is associated with an increase in luteal cytokine production in ruminants [12]. Also, the vital steroid hormone P4, produced by the CL, is generally considered immunosuppressive through direct actions on immune cells [13]. For example, P4 increases the number of a subset of regulatory T-lymphocytes (TReg) within the spleen, lymph nodes, and peripheral blood of pregnant mice [14]; TReg cells suppress local inflammatory cytokines, and this process appears critical to maintaining maternal immune tolerance to the fetus during pregnancy [15]. Mice which lack the gene (Pgr knockout [PRKO]) for the nuclear P4 receptor and, thus, lack both expression of both the PR-A and PR-B receptor isoforms, have increased responses to thymus-dependent antigens, including increased antibody production and increased interferon γ production from isolated T-lymphocytes [16]. However further experiments demonstrated that most of the effects of P4 on bovine immune cells are mediated through the membrane progestin receptor PGRMC1 [17]. Thus, the mechanism of P4 modulation of lymphocyte function appears to vary by species and cell type. Nevertheless, these data support the concept that P4 directly suppresses the activity of T-lymphocytes and inflammatory responses through PR-signaling pathways.
Primate CL in the menstrual cycle also produces estrogens (e.g., estradiol [E2]) in response to LH stimulation with a pattern similar to that of P4 [18]. Effects of E2 on immune cells and inflammation are complex and vary by tissue and disease state [19]. In studies of women receiving hormone replacement therapy with E2 and P4, effects on immune system mediators are reported, but there are limited investigations of women receiving only E2 [19]. Peripheral blood mononuclear cells (PBMCs) express both isoforms of E2 receptor (ERs), ERα (ESR1) and ERβ (ESR2) [20]. PBMC preparations isolated from rats demonstrate that PBMCs are responsive to selective agonists for ESR1/2 [21]. Thus, it is possible that E2, as well as P4, could directly affect PBMC function within luteal tissue.
Our recent microarray studies identified changes in mRNA expression in rhesus macaque CL for immune regulatory components such as proinflammatory chemokines and cytokines, immune cell-associated genes, and gene signaling pathways associated with immune function during the luteal lifespan in the menstrual cycle [22]. For example, gene products associated with cytokine activity were significantly increased in CL collected during luteal regression compared to those from fully functional CL at mid luteal phase [23]. Moreover, microarray analyses of macaque CL collected during gonadotropin-releasing hormone (GnRH) antagonist treatment, which pharmacologically reduces LH secretion and induces premature luteal regression, revealed significant over-representation of gene products associated with several immune pathways including immune system development and leukocyte differentiation [24]. Collectively, these findings suggest that activation of immune pathways within the primate CL is intimately related with onset and progression through luteal regression and may be mediated by loss of luteotropic (LH) support or an LH-dependent local P4 milieu.
Presently it is unknown how prevalent innate or adaptive immune cell populations are in primate CL and whether the total numbers of each cell type change at well-defined stages of the luteal phase in normal menstrual cycles. It is also not clear if luteal immune cell populations are sensitive to the hormonal milieu (i.e., LH or steroids). Given the presence of mRNA encoding many proinflammatory cytokines at the time of regression in macaque CL [22], it is hypothesized that the numbers of many immune cell types and their activity increase within the CL and participate in luteolysis. Although similarities between ovarian processes are observed between model species [25], important species differences are known to exist in regulation and function of immune processes [26]. Thus, the objectives of the current study were to 1) quantify the numbers of several proinflammatory immune cell types present within macaque CL collected throughout the luteal phase of the natural menstrual cycle and to 2) use the model of GnRH antagonist-induced luteolysis to determine whether LH and ovarian steroids regulate the presence of immune cell types within the regressing CL.
MATERIALS AND METHODS
All procedures were performed at the Oregon National Primate Research Center (ONPRC) with approval from the Oregon Health & Science University (OHSU)/ONPRC Institutional Animal Care and Use Committee (IACUC), in compliance with American Society of Primatologists (ASP) Principles for the Ethical Treatment of Nonhuman Primates and the Animal Welfare Act (AWA; 1985) of the United States of America. A cohort of adult female rhesus macaques (n = 25) with a history of normal menstrual cycles were maintained for this study. Hormonal status of each female was monitored for detection of ovulation as previously described [27]; the first day serum E2 concentration declined at midcycle to below 100 pg/ml with a coincident rise in serum P4 was denoted as the first day of the luteal phase, in accordance with previous studies demonstrating that the LH surge occurs on the day before E2 levels decline [28]. Monkeys were under the direct care of the ONPRC Department of Comparative Medicine; all protocols requiring sterile aseptic surgical procedures were performed by trained nonhuman primate surgical veterinarians and technicians in the Department of Comparative Medicine Surgical Services Unit.
CL Collection During the Natural Menstrual Cycle
Females were randomly assigned to undergo lutectomy at various stages of luteal phase during natural menstrual cycles. Individual CL were collected from anesthetized macaques as previously described [29] during the early (Days 3–5 post-LH surge; n = 3 CL), mid (Days 6–8; n = 5), mid-late (Days 10–12 n = 7), and late (Days 15–18; n = 7) luteal phases.
CL Collection Following Induced Luteal Regression
Beginning on Day 9 of the luteal phase (mid-late), macaques (n = 14) were randomly assigned to 4 treatment groups: control (no treatment, n = 4); treatment with the GnRH antagonist Antide to induce early onset of luteal regression, as previously reported (3 mg/kg/day; n = 4; The Salk Institute, La Jolla, CA) [24]; treatment with Antide plus progestin replacement with the synthetic progestin R5020 (2.5 mg/day; n = 3; Perkin-Elmer Inc.); or treatment with Antide plus estrogen replacement with two specific estrogen receptor (ER) agonists, ER1 propyl-pyrazole-triol (PPT) and ER2 diarylpropionitrile (DPN; both 0.15 mg/kg/day, n = 3; Tocris Bioscience) [30]. CL was collected from anesthetized females on Day 12 of the luteal phase, after 3 days of treatment.
Quantification of Immune Cell Populations by Flow Cytometric Analyses
Each CL was weighed and enzymatically dispersed as previously reported [31]. Cells, except for any remaining red blood cells, were then counted using a hemocytometer and assessed for viability by Trypan blue dye exclusion test (Sigma-Aldrich). Equal aliquots of dispersed cells (225 416 ± 22 647 cells/aliquot) from each CL were incubated with fluorescently labeled antibodies (BD Biosciences, Inc.) specific for macaque immune cell surface proteins (1 aliquot/antibody) according to the manufacture's protocol (Supplemental Table S1A; all Supplemental Data are available online at www.biolreprod.org). All antibodies were previously validated for identification of rhesus macaque leukocytes (BD Biosciences) [32, 33]. In addition, each antibody was validated using PBMCs (100 000 cells/aliquot) isolated from potassium/EDTA-treated blood collected from rhesus females as part of this study. Numbers of antibody-labeled cells within a total of 10 000 cells from each aliquot of dispersed cell preparations were assessed using an LSR II model flow cytometer (BD Bioscience) in the ONPRC Flow Cytometry Core. All data were analyzed using FCS Express version 3 software (De Novo Software). A nonimmune, isotype-matched antibody was used as a negative control to define the parameters for quantifying positive cells within each sample (Supplemental Table S1B). Results are reported as the number of marker-positive (+) cells/10 000 cells analyzed.
Statistics
The number of positive cells/10 000 cells recorded for each immune cell type, as well as CL wet weight, total numbers of cells recovered/CL, viability of recovered cells, and total viable cells/CL collected at defined stages of the natural luteal phase of menstrual cycles and after Antide treatment and/or steroid replacement, were analyzed by one-way ANOVA (using generalized liner model [GLM] procedure of SAS version 9.3 software; SAS Institute Inc.). Multiple comparison tests were performed to interrogate differences between CL collected at different stages of the luteal phase, using least square difference (LSD; SAS), which controlled for type I error rate. Serum E2 and P4 levels were analyzed by either one-way ANOVA (individual CL luteal phase) or repeated measures ANOVA (Antide-treated samples) when appropriate (SAS).
RESULTS
Experiment 1: CL of the Menstrual Cycle
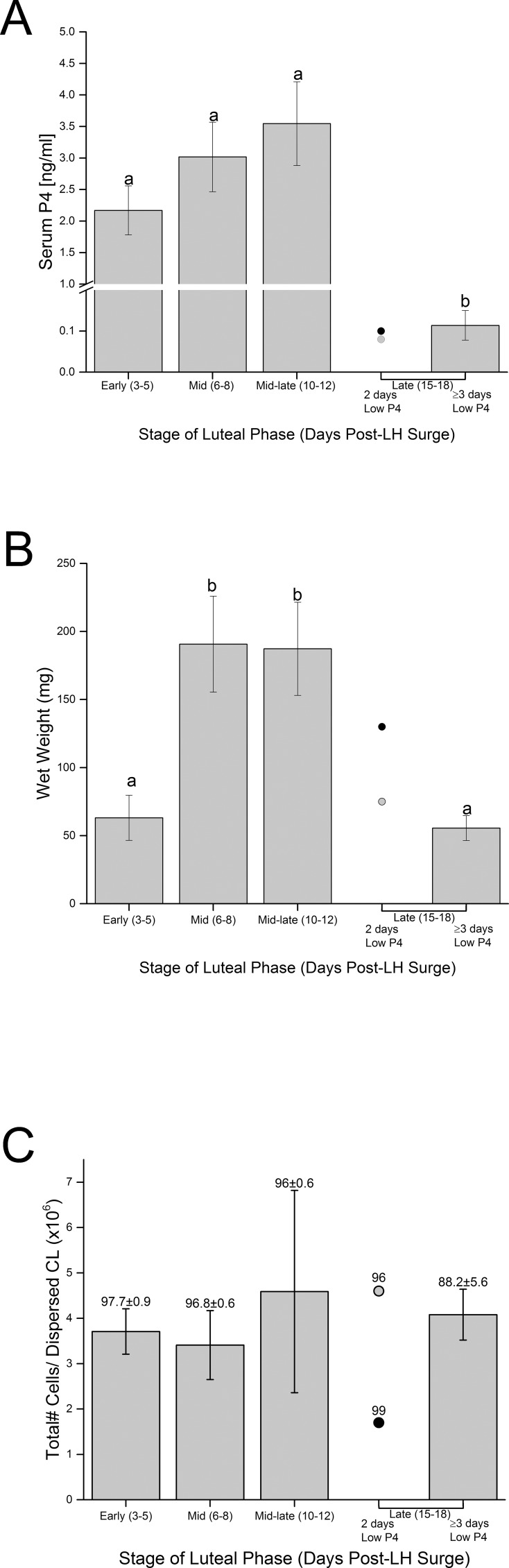
Initially, CL collection was based on previously defined stages of luteal development, function and regression (post-LH surge) in female macaques [22]. However, initial flow cytometric analyses discovered wide variation in immune cell numbers in CL collected during the late luteal phase, corresponding to Days 15 to 18 post-LH surge. Further analyses revealed lower numbers of each cell type in CL collected at late luteal phase when serum P4 concentrations were at baseline (0.3 ng/ml) for up to 2 days (functionally regressed; before onset of menses), whereas increased numbers were observed in most CL collected 3 to 4 days after P4 declined (near onset of structural regression, late stage; 1.2 ± 0.4 days post-onset of menses [Day 0]) (Supplemental Fig. S1). Therefore, CL collected during the late luteal phase were separated into these two classifications based on luteal function: early functional regressed stage (P4 < 0.03 ng/ml for 2 days; n = 2) and late stage CL (P4 < 0.03 ng/ml for 3–4 days; n = 5). Subsequent statistical analyses focused on changes in the immune cell populations in the late stage CL compared to those from earlier stages of the luteal phase.
Serum P4 levels were elevated on the day of CL collection at early through mid-late luteal phase but were low in early functional regressed and late stage CL (P < 0.004) (Fig. 1A). Luteal weight of early stage CL was low and increased to a maximum in CL collected at mid and mid-late luteal phases (P < 0.03) (Fig. 1B), corresponding to the interval of peak P4 production. Weight of early functional regressed CL was variable, whereas that of late stage CL was significantly less than CL collected at mid and mid-late luteal phase. Viability of cells recovered did not vary by stage of CL (P > 0.15; overall mean ± SEM 94.4 ± 1.6% viability) (Fig. 1C). Furthermore, total numbers of cells recovered from enzymatic dispersal of CL did not vary significantly by luteal stage (P > 0.9; mean ± SEM 4.1 ± 0.8 million cells/CL) (Fig. 1C). Thus, the total number of viable cells dispersed from CL did not differ between stages of the luteal phase (P > 0.08; mean ± SEM 3.8 ± 0.7 million cells/CL collected; data not shown).
FIG. 1.
A) Serum P4 concentrations of female macaques (mean ± SEM, n = 3–7/group; except for those in early regression, which are represented as individual values indicated by gray and black dots, n = 2) on the day of CL collection by stage of luteal phase. Different letters indicate significant differences in serum P4 levels (P < 0.05). B) Wet weight of CL collected at discrete stages of the normal luteal phase. Different letters indicate significant differences in weight by stage of luteal phase (P < 0.05). C) Total number of cells recovered from enzymatic dispersal of luteal tissue from each CL. Numbers above the columns indicate percentage of viability (mean ± SEM) of cell preparations. There were no significant differences between stages of luteal phase for either total number of cells recovered (P > 0.9) or percentage of viability of dispersed cells (P > 0.15).
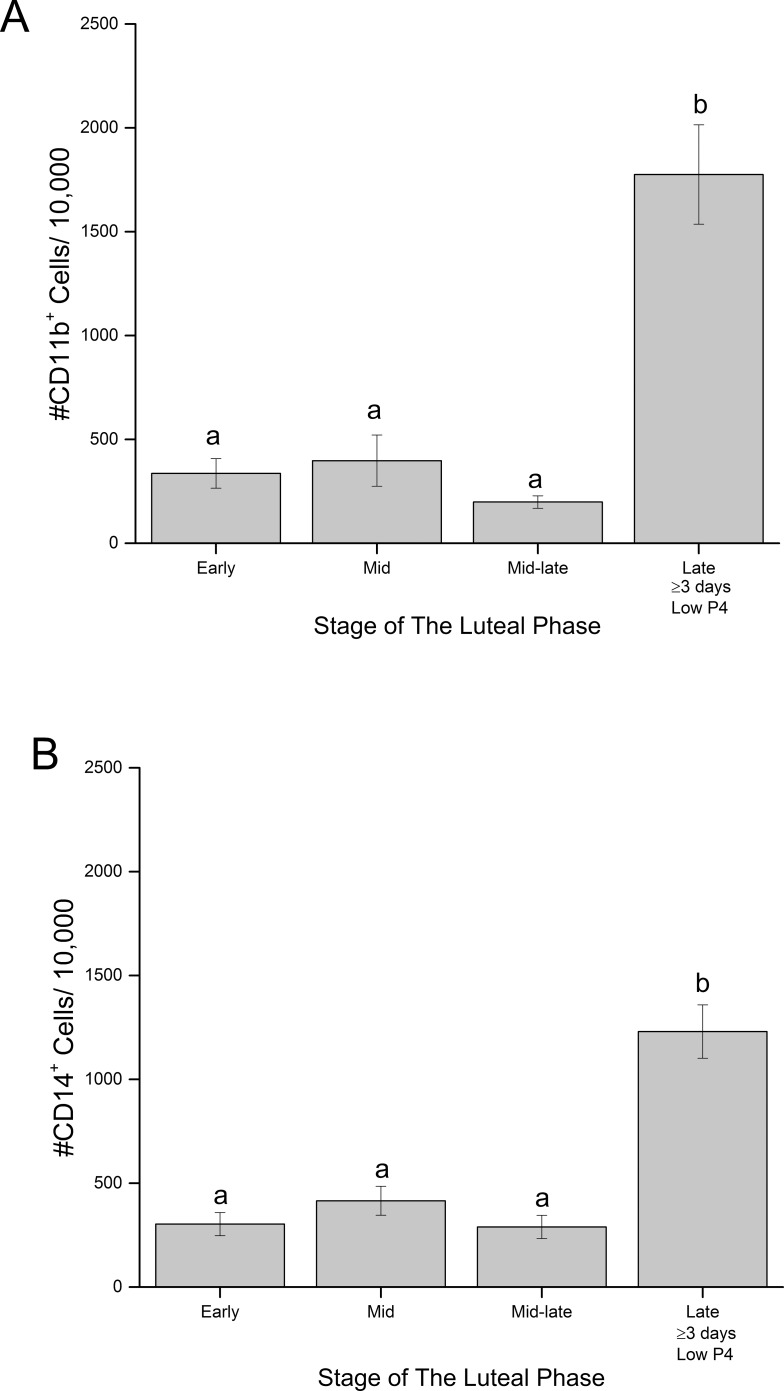
Flow cytometry analyses of dispersed cells from the macaque CL detected cell surface markers corresponding to neutrophils/ monocytes/macrophages (CD11b+), monocytes/macrophages (CD14+), and NK cells (CD16+) (Supplemental Table S1). Both CD11b+ and CD14+ cells (Fig. 2) are present at low levels in macaque CL during the developing (early) and functional (mid) luteal phases as well as at the mid-late luteal phase (near the onset of functional regression). Numbers of CD11b+ and CD14+ cells remained low but detectable in CL collected at early regression (Supplemental Fig. S1, A and B). However, CD11b+ and CD14+ cells were 7.5- and 6.2-fold increased, respectively, in dispersed luteal preparations collected during late regression (P < 0.0001) (Fig. 2, A and B). The number of CD16+ cells were 2.7-fold increased in dispersed cell preparations between early and mid-luteal phase (P < 0.03) (Fig. 3A), and were 1.8-fold further increased in late stage versus that in mid-late stage CL (P < 0.05) (Fig. 3A). CD16+ cells were the most abundant immune cell type in CL from early through mid-late luteal phase, but by late luteal stage, the numbers of CD11b+, CD14+, and CD16+ cells in CL were similar.
FIG. 2.
Quantification of cells positive for the cell surface markers CDllb (A) and CD14 (B) in dispersed cell preparations of macaque CL collected at discrete stages of normal luteal phase (mean ± SEM, n = 3–7/group [see Materials and Methods for details]). Different letters indicate significant differences (P < 0.05) in numbers of CD11b+ or CD14+ cells within dispersed cell preparations of CL at each stage of the luteal phase.
FIG. 3.
Quantification of numbers of CD16+ (A) and CD3ε+ (B) cells in dispersed cell preparations of CL collected at discrete stages of luteal phase (mean ± SEM, n = 3–7/group [see Materials and Methods for details]). Different letters indicate significant (P < 0.05) differences in numbers of CD16+ cells at each stage of the luteal phase. See Results section for details of CD3ε+ cell analyses.
Low numbers of CD3ε+ cells (T-lymphocytes) were detected in dispersed cells from CL collected at all stages of luteal phase and were typically fewer in number than CD11b+, CD14+, and CD16+ cells. Although overall changes in numbers of CD3ε+ cells analyzed by one-way ANOVA from all CL collected throughout the luteal phase were not significant (P = 0.07) (Fig. 3B), the numbers of CD3ε+ cells did increase in CL collected at late compared to those collected at mid-late luteal phase (LSD mean comparison test; P = 0.05). Numbers of CD20+ cells (B-lymphocytes) in macaque CL were extremely low when present (<30 positive cells/10 000 in 6 of 23 CL). However, CD20+ cells were reliably detected in PBMC preparations (data not shown).
Experiment 2: GnRH Antagonist-Induced Luteal Regression
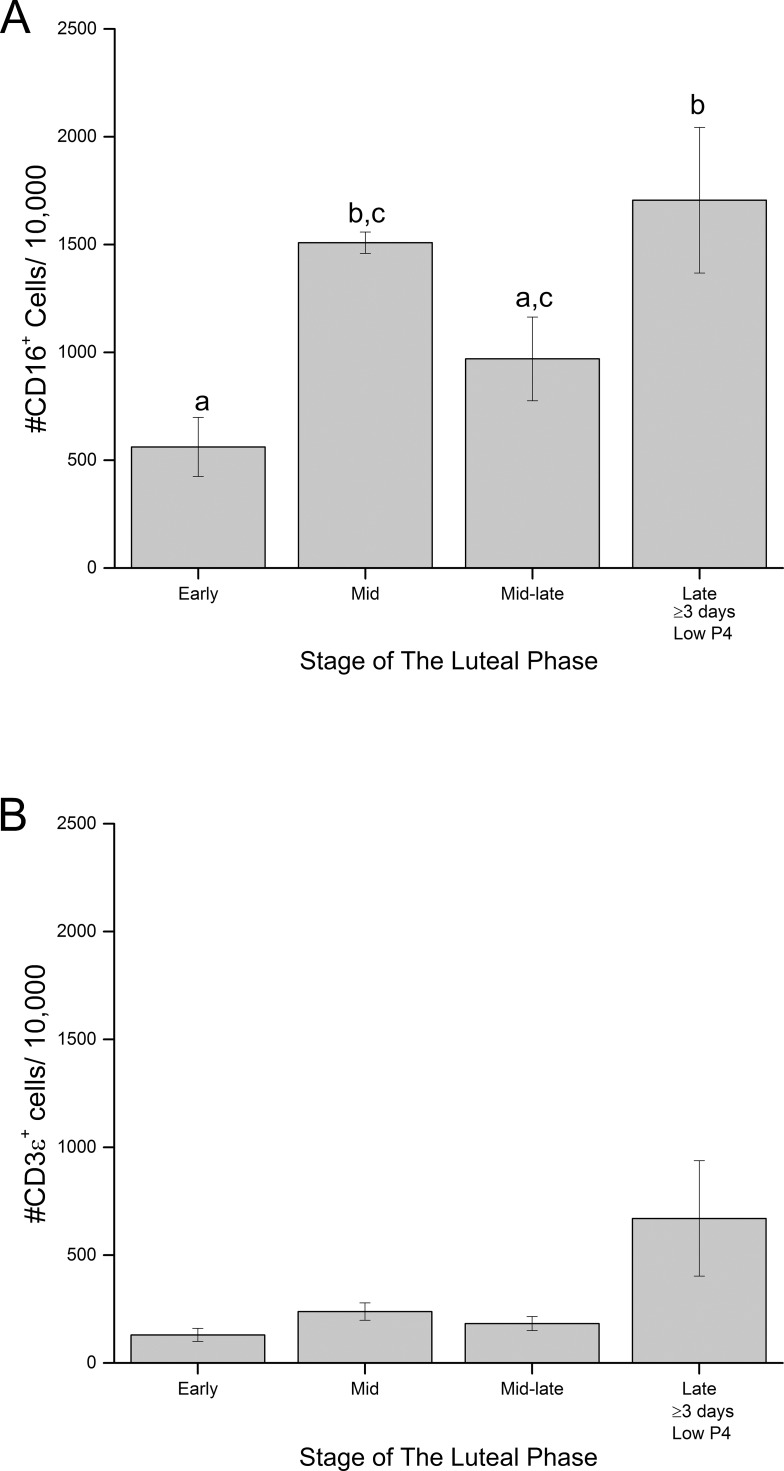
Administration of the GnRH antagonist Antide at mid-luteal phase of the menstrual cycle significantly reduced serum P4 levels by 24 h (effect of Antide; P < 0.001) (Fig. 4A) and E2 levels by 48 h (P < 0.001, data not shown). Steroid levels remained low throughout the remainder of the treatment interval until CL collection at 72 h. Concomitant treatment with progestin (R5020) or estrogen agonists (DPN/PPT; ERα/ERβ agonists) did not increase serum levels of endogenous P4 or E2 compared to that of Antide alone (Antide vs R5020 or DPN/PPT, P > 0.05; it is important to note that the radioimmunoassays used in this study for measuring both P4 and E2 used antibodies which do not cross-react with R5020 or DPN/PPT, respectively). Previous studies in rhesus females using the same dose of R5020 reported serum concentrations of progestin at between 6 and 7 ng/ml [34], similar to physiologic concentrations of P4 in functional CL. Treatment with Antide caused premature menstruation in all females at the time of CL collection (Day 12 post-LH surge). In contrast, none of the females treated with both Antide and R5020 displayed menses until at least 5 days after luteal collection. Menses occurred on the day of CL collection or one day later in the Antide plus DPN/PPT-treated monkeys. Three days of Antide exposure reduced CL wet weight compared to that of untreated control CL (P < 0.02) (Fig. 4B), and CL weight was unaffected by coadministration of R5020 or DPN/PPT relative to that with Antide alone. The total number of cells recovered by enzymatic dispersal of CL was unaffected by treatments (P > 0.3; mean ± SEM 3.2 ± 0.4 million cells/CL) (Fig. 4C). Also, percentage of viability of recovered cells was unaffected by treatments (P > 0.17; mean ± SEM 92 ± 1%) (Fig. 4C). Thus, the number of viable cells in dispersed cell preparations were not affected by any treatment (3.0 ± 0.4 million viable cells/CL; data not shown; P > 0.3).
FIG. 4.
A) Changes in serum P4 levels before (Day 9) and during 3 days of treatment with Antide alone or with the synthetic progestin R5020 (A+R5020) or E2 receptor agonists DPN/PPT (A+DPN/PPT) during the mid-late luteal phase (mean ± SEM; n = 3–4/group). Note that the radioimmunoassay used to detect endogenous P4 levels does not recognize synthetic progestin R5020. Different lowercase letters indicate significant differences within treatment group by day (P < 0.05). B) Wet weight of CL collected from each treatment group before dispersal into cell preparations. Different uppercase letters indicate significant differences in weight by treatment (P < 0.05). C) Total numbers of cells recovered from enzymatic dispersal of luteal tissue from CL of each treatment group. Numbers above the columns indicate percentage of viability of dispersed cells (mean ± SEM). There were no significant differences in number of cells recovered (P > 0.3) or percentage of viability (P > 0.17) by treatment.
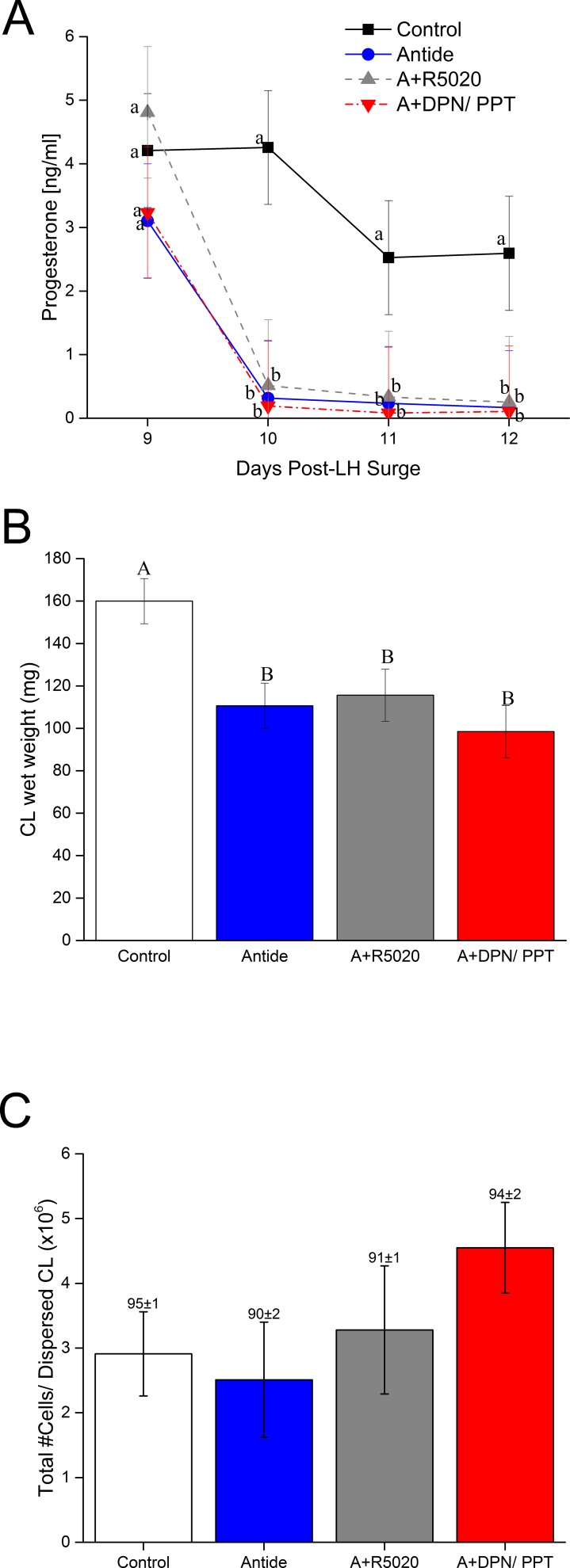
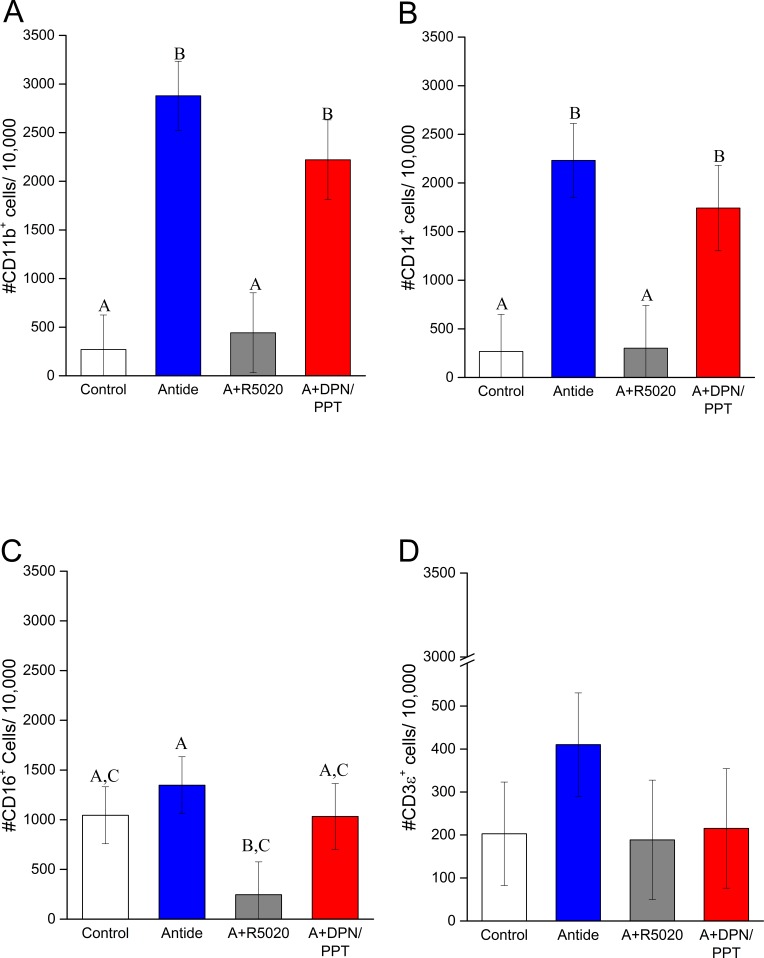
Three days of Antide treatment markedly increased the numbers of CD11b+ and CD14+ cells (10.7- and 8.3-fold, respectively) in dispersed cell preparations of CL compared to that in cells from untreated controls (P < 0.0004 and 0.005, respectively) (Fig. 5, A and B). Concurrent treatment with R5020 prevented Antide-induced increase in CD11b+ and CD14+ cells (P < 0.002 and 0.008, respectively; Antide vs. Antide plus R5020) (Fig. 5, A and B). However, the increase in CD11b+ and CD14+ cells caused by Antide treatment was not affected by coadministration of E2 agonists (P > 0.5) (Fig. 5, A and B). Antide alone did not alter the numbers of CD16+ cells (Fig. 5C), but concurrent treatment with R5020 suppressed their numbers in CL (Antide vs. Antide plus R5020; P < 0.03) (Fig. 5C). However, addition of E2 agonists did not significantly alter the numbers of CD16+ cells compared to Antide treatment alone. The numbers of CD3ε+ cells in dispersed cell preparations were not significantly influenced by Antide alone (P > 0.3), Antide and R5020 (P > 0.9), or Antide and DPN/PPT (P > 0.3) (Fig. 5D).
FIG. 5.
Quantification of CD11b+ ([A] monocytes, macrophages, and neutrophils), CD14+ ([B] monocytes or macrophages), CD16+ ([C] NK cells), and CD3ε+ ([D] T lymphocytes) cells in dispersed cell preparations of macaque CL collected 3 days after Antide treatment alone or with progestin (A+R5020) or estrogen (A+DPN/PPT) replacement (mean ± SEM, n = 3–4/group). Different letters indicate significant differences in numbers of CD11b+, CD14+, and CD16+ cells between treatment groups (P < 0.05). There were no significant differences in CD3ε+ cells between treatments.
DISCUSSION
Binding of the CD11b antibody to a subpopulation of dispersed cells from the macaque CL strongly suggests that macrophages and/or neutrophils are present in primate luteal tissue. The cell surface antigen CD11b, also known as Mac-1 or integrin, alpha M (complement component 3 receptor 3 subunit; ITGAM, National Center for Biotechnology Information (NCBI) gene ID: 3684) is part of an alpha-M/beta 2 integrin involved in immune cell migration and is expressed primarily by macrophages/neutrophils [35]. The low numbers of neutrophils/macrophages in dispersed cell preparations from macaque CL collected during the functional (early to mid-late) luteal phase versus the large numbers present at the late luteal phase, at onset of structural regression, is consistent with previous reports on CL of women [36, 37], and domestic animals [5]. Macrophages and neutrophils secrete many similar cytokines and express several similar cell surface receptors and, thus, have overlapping and complementary local actions [35]. Macrophages are associated with tissue remodeling and are reported to destroy neoplastic cells in tumors (termed tumor-associated macrophages [TAMs]) [38]. Furthermore, these TAMs secrete proteases which can degrade extracellular matrix proteins and liberate fragments of fibronectin/fibrinogen, which serve as chemoattractants to other immune cell types [39]. Luteal macrophages may have similar functions. Neutrophils are recruited to tissues by cytokine gradients produced by local macrophages in response to injury or infection, where these cells secrete proinflammatory chemokines and cytokines to modulate the local immune responses [40], which may be involved in progression of luteolytic processes.
Because CD11b is expressed on both macrophages and neutrophils, an antibody to the CD14 cell surface protein (NCBI gene ID: 697482) was used to confirm the presence of macrophages in the cells populating the macaque CL. This cell surface protein has higher expression on macrophages than on neutrophils and functions to enable these cells to coordinate local immune responses [41]. The proportion of CD14+ cells can be compared to CD11b+ cells to estimate the local population of neutrophils within cell preparations. Patterns for numbers of CD11b+ and CD14+ cells during stages of the luteal phase were identical. In cell preparations from the early luteal phase, the number of CD14+ cells (macrophages) is 90% of that for CD11b+ cells (total neutrophils/macrophages), whereas the number of CD14+ cells is 65% of CD11b+ cells in CL during late regression (when P4 levels were at baseline for 3–4 days). This suggests most neutrophils migrate into the CL in late luteal phase, possibly following gradients of chemokines produced by the increased numbers of macrophages.
The presence of NK cells within monkey CL was detected by antibody binding to the cell surface receptor CD16 (FCGR3; NCBI cene ID: 720006); they were the most numerous immune cell type within dispersed cell preparations of macaque CL during the functional luteal phase and remained elevated during late luteal regression. Although some macrophages coexpress CD11b and CD16, the number of CD11b+ cells are much lower than the CD16+ cells in CL during the functional luteal phase (i.e., early through mid-late stages), indicating a distinct population of NK cells in macaque CL. Our data are consistent with those of one report [10] of immunostaining for NK cells within the human CL and provide evidence of their dynamics during the primate luteal lifespan. Primate NK cells are divided into two subtypes: immature (5% of total population) and mature (95% of population) NK cells [42, 43]. The immature subtype secretes more cytokines than mature NK cells, whereas mature NK cells function mainly to lyse target cells through a major histocompatibility complex (MHC)-independent mechanism (as reviewed by Caligiuri [43]). One function of neutrophils is to induce NK cell maturation [40]. Most research to date on NK cell function in the reproductive tract focuses on their role during embryo implantation and placentation within the uterus [44]. It is important to mention that most primate uterine NK cells are CD56bright, and increased numbers of CD56dim NK cells in the uterus are associated with reproductive failure [45], notably, a subpopulation of primate NK cells is CD16+ CD56dim [46]. This could explain why some previous investigations failed to identify NK cells in primate luteal tissue (e.g., in the human ovary) [47]. The function of NK cells has yet to be studied in the non-neoplastic ovary, but there are some reports on the use of peripheral cytotoxic NK cells as therapy for ovarian cancer [48, 49]. Because NK cells appear to promote placentation and uterine vascular remodeling [44], they may have a similar role in promoting vessel growth, maturation and stability during formation (early) and function (mid- to mid-late) of the CL, before assuming degenerative roles during luteal regression. NK cells may be considered the innate counterparts of cytotoxic T cells [4] and may switch from tropic to lytic roles during the lifespan of primate CL much as proposed for T cells in the CL of domestic animals [2, 50]. The dual functions of both NK and T cells contribute to the overlap in cells participating in innate and adaptive immune responses [4], and thus further investigation into cytokine/chemokine production by ovarian NK cells are warranted.
Unexpectedly, immune cells conventionally associated with adaptive immune responses, including T as well as B cells, only sparsely populated the macaque CL. Because CD20+ cells were largely undetectable in dispersed CL preparations collected throughout the luteal phase, the small number of B-lymphocytes likely reflects contamination from circulating blood cells. Our findings support immunohistological evaluations [47], which also report lack of CD20 immunostaining in CL of women. The presence of T-lymphocytes in macaque CL was noted by detection of cells binding antibody to the cell surface receptor CD3ε (CD3E; NCBI gene ID: 699467), part of the T-cell receptor complex that is present on all T-cell populations. The T-cell population was fewer in number than macrophages, neutrophils, and NK cells, and the numbers of T-lymphocytes only modestly increased in CL isolated during the late luteal phase at onset of menses (Fig. 3B). Lower numbers of CD3ε+ cells were also observed in the CL of women by immunohistological methods, with an increase in abundance in regressing CL near onset of menses [47]. Our findings contrast with several reports using domestic animal models wherein large increases in CD4+ and CD8+ T-lymphocytes occurred in CL at the onset of regression [5, 50]. CD3ε antibody was chosen as a marker of T-lymphocytes in the current study because the CD3ε receptor is expressed selectively by all T-lymphocytes, unlike CD8 which is typically coexpressed with CD16 in primate NK cells [51]. Studies in bovine CL focused on a particular subtype of T-cell, CD8+γδ T-cells. This subtype increases during luteolysis in ruminants, whereas other T-cell subtypes, including TReg, decrease [50]. Changes in T-cell subtypes may not have been detected in macaque luteal tissue in the current flow cytometry analyses due to use of a pan T-lymphocyte marker. Future analyses could reveal more remarkable dynamics and possible roles for T-lymphocytes subtypes in primate CL that are not captured by changes in total T cells.
In the current study, premature luteal regression, induced by treatment with the GnRH-antagonist Antide, resulted in changes in immune cell numbers in dispersed CL preparations that generally equated with those observed during natural luteolysis. Both CD11b+ and CD14+ cells (and to a lesser extent CD3ε+) increased to levels observed in CL during the late luteal phase of the natural menstrual cycle. The numbers of CD16+ cells may not have increased due to their higher level in the functional CL, as well as the structurally regressing CL. Although Antide treatment resulted in loss of both LH and LH-induced steroid hormones, data strongly suggest that the numbers of neutrophils, macrophages, and NK cells within macaque luteal tissue are regulated by P4 but not by E2. Concurrent treatment with the progestin R5020 suppressed numbers of macrophages, neutrophils, and NK cells. Thus, high levels of P4 from/within the functional CL may limit the numbers of these immune cells, whereas the loss of P4 in the regressing CL permits their numbers to increase. The mechanism(s) of P4 action awaits investigation; whether these effects are mediated by progestin receptor(s) in luteal cells and/or directly on immune cells is unknown. Human peripheral blood macrophages express the nuclear PGR [52], and P4 decreases the inflammatory response from peripheral primary monocytes [53]. Apoptosis of peripheral blood NK cells can be induced by P4 in a dose-dependent manner; this effect was only detected in NK cells coexpressing PGR [54]. In contrast, CD56bright NK cells in the uterus reportedly lack PGR [55], but it is unknown whether the CD16+ NK cell population in primate CL possess PGR or other progestin receptors. Although bovine T-lymphocytes express different types of membrane progestin receptors [13], there were no effects of either Antide-induced LH withdrawal or progestin replacement on the total number of CD3ε+ T-lymphocytes in macaque cell preparations in the current study. Because reports suggest a direct role for P4 through progestin receptors [16, 17], and species-specific differences in regulation and function of immune cell types [26], further investigation of steroid regulation of immune cells in primate CL is warranted.
The transition from early to late regressive events is associated with an increase in the percentage of most immune cell types in rhesus CL. Early regression, although not investigated in depth here, appears to correspond to the time of functional regression in which steroidogenic activities of the CL cease [23, 56]; steroidogenic luteal cells appear to shrink in size [57] as cholesterol stores are actively shuttled out of the cells [58]. This is distinct from onset of structural regression; the late luteal phase of the menstrual cycle, when P4 levels are at baseline for at least 3 days, appears to correspond to the time of onset of structural regression. Structural luteal regression is a time of major tissue remodeling and destruction, based on immunohistochemical analyses of increased collagen fiber deposition [59] and decreased numbers of vascular endothelial cells [60]. Collectively, these result in changes in gene products for proteases, extracellular matrix components, and angiogenic/angiolytic factors in the CL [23, 61] allowing for the formation of the remnant structure, the corpus albicans. Morphological studies of marmoset monkey ovaries containing luteal tissue suggest that this process can last into the late follicular phase of the subsequent menstrual cycle, indicating a lengthy duration of luteal structural regression in primate species compared to others [62]. This lengthy delay suggests that multiple processes might occur in luteal tissue between a loss of sensitivity to LH (manifested by lower P4 secretion) and increases in luteal immune cell numbers.
The number of macrophages/monocytes and neutrophils (CD11b+ cells) present in dispersed CL collected at the late stage comprises ∼18% of the total population analyzed. These data are consistent with previous flow cytometry studies of dispersed luteal tissue from women. Castro et al. [9] reported that the total leukocyte population within the luteal preparations was between 20% and 50% of all cells analyzed, but the percentage of CD14+ macrophages did not differ in CL staged among early, mid-late, and late phases (10–14 days following ovulation) of natural menstrual cycles [9]; however, serum P4 levels were not reported for these women. Whether this represents invasion of luteal tissue by immune cells or an increase in proportion of immune cells as a function of total cell population is not known. Similar percentages of viability of dispersed CL are reported for women (>90% [9]) and for macaques by this analysis. It is possible that degenerating/apoptotic cells (either luteal, endothelial, or other types) were lost during enzymatic dispersal of the luteal tissue or structural degeneration at time of luteolysis, leading to an enrichment of immune cells at later stages of the luteal phase. Increased numbers of immune cells, coupled with loss of nonimmune cell types would result in constant numbers of total cells in the CL, independent of cycle stage, as observed in this study. Recent immunohistochemical analyses of regressing CL from women at menses found putative macrophages to be the predominant cell type within cross-sections of luteal tissue, confined to strict regions adjacent to steroidogenic (luteal) cells [63]. It is reported in rhesus monkeys that the large-to-small cell ratio in CL decreases in latter stages of the luteal phase, but some of these small cells retain the ability to secrete P4 if stimulated by LH [57]. Given these data, it is unlikely that the large increase in immune cell populations found in late regression is an artifact. Further investigations into the processes activated during this transitional period are needed.
It is important to recognize, however, there are low numbers of macrophages present in macaque luteal tissue immediately prior to and at the time of functional regression (Fig. 2 and Supplemental Fig. S1). Although low in number, these immune cells may be secreting cytokines to facilitate luteal remodeling during structural regression and secreting chemoattractants to recruit greater numbers of monocytes/macrophages, as well as neutrophils and NK cells and, to some extent, T-lymphocytes to luteal tissue to promote further regressive events. One major product of proinflammatory macrophages, which also has chemoattractant activity, is monocyte chemoattractant protein 1 (MCP1 [also known as CCL2]) [64]; Affymetrix rhesus macaque microarray studies reveal CCL2 mRNA is present at low levels throughout the macaque luteal phase, increasing 2-fold in very late CL staged by days post-LH surge (GEO dataset Series GSE10367; [22]). Furthermore, studies in women report increased CCL2 protein within CL collected at menses with concurrent increases in cells staining positive for the macrophage marker CD68 [63]. Intriguingly, CCL2 is expressed by granulosa- and theca-lutein cells, suggesting that steroidogenic cells of the CL are producing chemotactic signals, resulting in increased numbers of luteal immune cells during luteal regression [63]. Our microarray studies of LH/steroid ablation and progestin replacement (dataset series GSE12281; GEO) [24] revealed a large number of gene products associated with immune system processes that are regulated in primate CL directly by LH or indirectly through LH-stimulated P4 production. Of note, mRNA for chemokine (C-C motif) receptor 1 (CCR1) increases 13.6-fold in CL collected from rhesus females following exposure to Antide, as well as mRNA for the CCR1 ligand chemokine (C-C motif) ligand 3 (CCL3; 10.5-fold increase, data not shown). CCR1 protein is expressed by monocytes/macrophages, neutrophils, and T-cells [65], and CCL3/CCR1 signaling is reported to induce macrophage migration/infiltration [66]. Further studies will investigate the functional potential of NK cells, neutrophils, and macrophages during the functional and late (structurally regressing) stages of the luteal phase.
In summary, these data demonstrate a significant increase in several types of immune cells (i.e., macrophages, neutrophils, and NK cells) in primate CL during the late luteal phase, corresponding to the onset of structural luteolysis. Data presented here suggest, for the first time, that NK cells have a major role in promoting the structure and function of primate CL during mid and mid-late luteal phases. We report that NK cells are the most prevalent immune cell type during the functional luteal phase. Following a decline in P4 levels to baseline for at least 3 days, there is a remarkable increase in neutrophils, monocytes/macrophages, NK cells, and to some extent, T-lymphocytes, suggesting that activation of multiple signaling pathways within primate CL facilitates this event. The increase in CD11b+/CD14+ macrophages/neutrophils in luteal tissue after removal of gonadotropic support is dependent on the loss of LH-dependent P4 synthesis. Replacement of progestin following Antide exposure suppressed the numbers of luteal CD16+ NK cells, possibly through the immune-suppressive actions of P4 unrelated to LH. The presence of CD3ε+ T-lymphocytes in primate CL is not dependent on either LH or LH-dependent P4 secretion, and CD3ε+ T-lymphocytes are the least abundant cell type in luteal tissue quantified during the functional luteal phase and during regressive events. The results are consistent with the concept that signaling processes activated during early functional regression, marked by a decline in P4 secretion and local action, promote processes that induce changes in numbers of luteal immune cell populations during the latter stages of functional regression. Further experiments are needed into the signaling cascades activated during this transitional period in primate CL.
ACKNOWLEDGMENT
The authors would like to recognize assistance by Jonah Sacha, PhD, and Ilhem Messaoudi, PhD, and associates in the ONPRC Division of Pathobiology and Immunology for assistance in selecting the nonhuman primate antibodies used in this study. The authors are grateful to the ONPRC Flow Cytometry Support Core and Endocrine Technology Support Core Lab for assistance with these studies. In addition, we thank the OHSU Flow Cytometry Shared Resource for providing software and guidance with data analyses. These studies would not be possible without the outstanding animal care and support provided by the ONPRC Department of Comparative Medicine and Surgical Support Unit.
Footnotes
Supported by grants R01HD020869 to R.L.S. and P51OD011092 to the Oregon National Primate Research Center. These data were presented in part at the 45th Annual Meeting of the Society for the Study of Reproduction, August 12–15, 2012, State College, Pennsylvania, and at the 46th Annual Meeting of the Society for the Study of Reproduction, July 22–26, 2013, Montréal, Québec, Canada.
REFERENCES
- Stouffer RL. Progesterone as a mediator of gonadotrophin action in the corpus luteum: beyond steroidogenesis. Hum Reprod Update. 2003;9:99–117. doi: 10.1093/humupd/dmg016. [DOI] [PubMed] [Google Scholar]
- Pate JL, Toyokawa K, Walusimbi S, Brzezicka E. The interface of the immune and reproductive systems in the ovary: lessons learned from the corpus luteum of domestic animal models. Am J Reprod Immunol. 2010;64:275–286. doi: 10.1111/j.1600-0897.2010.00906.x. [DOI] [PubMed] [Google Scholar]
- Janeway CJ, Travers P, Walport M, Shlomchik M. Principles of Innate and Adaptive Immunity. Immunobiology: the Immune System in Health and Disease, 5th ed New York: Garland Science; 2001. [Google Scholar]
- Eberl G, Colonna M, Di Santo JP, McKenzie AN. Innate lymphoid cells. Innate lymphoid cells: a new paradigm in immunology Science 2015. 348:aaa6566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walusimbi SS, Pate JL. Physiology and Endocrinology Symposium: role of immune cells in the corpus luteum. J Anim Sci. 2013;91:1650–1659. doi: 10.2527/jas.2012-6179. [DOI] [PubMed] [Google Scholar]
- Komatsu K, Manabe N, Kiso M, Shimabe M, Miyamoto H. Changes in localization of immune cells and cytokines in corpora lutea during luteolysis in murine ovaries. J Exp Zool Comp Exp Biol. 2003;296:152–159. doi: 10.1002/jez.a.10246. [DOI] [PubMed] [Google Scholar]
- Wu R, Van der Hoek KH, Ryan NK, Norman RJ, Robker RL. Macrophage contributions to ovarian function. Hum Reprod Update. 2004;10:119–133. doi: 10.1093/humupd/dmh011. [DOI] [PubMed] [Google Scholar]
- Suzuki T, Sasano H, Takaya R, Fukaya T, Yajima A, Date F, Nagura H. Leukocytes in normal-cycling human ovaries: immunohistochemical distribution and characterization. Hum Reprod. 1998;13:2186–2191. doi: 10.1093/humrep/13.8.2186. [DOI] [PubMed] [Google Scholar]
- Castro A, Castro O, Troncoso JL, Kohen P, Simon C, Vega M, Devoto L. Luteal leukocytes are modulators of the steroidogenic process of human mid-luteal cells. Hum Reprod. 1998;13:1584–1589. doi: 10.1093/humrep/13.6.1584. [DOI] [PubMed] [Google Scholar]
- Wang LJ, Pascoe V, Petrucco OM, Norman RJ. Distribution of leukocyte subpopulations in the human corpus luteum. Hum Reprod. 1992;7:197–202. doi: 10.1093/oxfordjournals.humrep.a137616. [DOI] [PubMed] [Google Scholar]
- Goodwin JS, Ceuppens J. Regulation of the immune response by prostaglandins. J Clin Immunol. 1983;3:295–315. doi: 10.1007/BF00915791. [DOI] [PubMed] [Google Scholar]
- Miyamoto A, KShirasuna T, Bollwein H, Schams D. Regulation of corpus luteum development and maintenance: specific roles of angiogenesis and action of prostaglandin F2α. Soc Reprod Fertil Suppl. 2010;67:289–304. doi: 10.7313/upo9781907284991.024. [DOI] [PubMed] [Google Scholar]
- Ndiaye K, Poole DH, Walusimbi S, Cannon MJ, Toyokawa K, Maalouf SW, Dong J, Thomas P, Pate JL. Progesterone effects on lymphocytes may be mediated by membrane progesterone receptors. J Reprod Immunol. 2012;95:15–26. doi: 10.1016/j.jri.2012.04.004. [DOI] [PubMed] [Google Scholar]
- Mao G, Wang J, Kang Y, Tai P, Wen J, Zou Q, Li G, Ouyang H, Xia G, Wang B. Progesterone increases systemic and local uterine proportions of CD4+CD25+ Treg cells during midterm pregnancy in mice. Endocrinology. 2010;151:5477–5488. doi: 10.1210/en.2010-0426. [DOI] [PubMed] [Google Scholar]
- Guerin LR, Prins JR, Robertson SA. Regulatory T-cells and immune tolerance in pregnancy: a new target for infertility treatment? Hum Reprod Update. 2009;15:517–535. doi: 10.1093/humupd/dmp004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes GC, Clark EA, Wong AH. The intracellular progesterone receptor regulates CD4+ T cells and T cell-dependent antibody responses. J Leukoc Biol. 2013;93:369–375. doi: 10.1189/jlb.1012491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda Y, Ohtsuka H, Tomioka M, Oikawa M. Effect of progesterone on Th1/Th2/Th17 and regulatory T cell-related genes in peripheral blood mononuclear cells during pregnancy in cows. Vet Res Commun. 2013;37:43–49. doi: 10.1007/s11259-012-9545-7. [DOI] [PubMed] [Google Scholar]
- Atkinson LE, Hotchkiss J, Fritz GR, Surve AH, Neill JD, Knobil E. Circulating levels of steroids and chorionic gonadotropin during pregnancy in the rhesus monkey, with special attention to the rescue of the corpus luteum in early pregnancy. Biol Reprod. 1975;12:335–345. doi: 10.1095/biolreprod12.3.335. [DOI] [PubMed] [Google Scholar]
- Straub RH. The complex role of estrogens in inflammation. Endocr Rev. 2007;28:521–574. doi: 10.1210/er.2007-0001. [DOI] [PubMed] [Google Scholar]
- Phiel KL, Henderson RA, Adelman SJ, Elloso MM. Differential estrogen receptor gene expression in human peripheral blood mononuclear cell populations. Immunol Lett. 2005;97:107–113. doi: 10.1016/j.imlet.2004.10.007. [DOI] [PubMed] [Google Scholar]
- Stygar D, Masironi B, Eriksson H, Sahlin L. Studies on estrogen receptor (ER) alpha and beta responses on gene regulation in peripheral blood leukocytes in vivo using selective ER agonists. J Endocrinol. 2007;194:101–119. doi: 10.1677/JOE-06-0060. [DOI] [PubMed] [Google Scholar]
- Bogan RL, Murphy MJ, Stouffer RL, Hennebold JD. Systematic determination of differential gene expression in the primate corpus luteum during the luteal phase of the menstrual cycle. Mol Endocrinol. 2008;22:1260–1273. doi: 10.1210/me.2007-0484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogan RL, Murphy MJ, Hennebold JD. Dynamic changes in gene expression that occur during the period of spontaneous functional regression in the rhesus macaque corpus luteum. Endocrinology. 2009;150:1521–1529. doi: 10.1210/en.2008-1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop CV, Hennebold JD, Stouffer RL. The effects of luteinizing hormone ablation/replacement versus steroid ablation/replacement on gene expression in the primate corpus luteum. Mol Hum Reprod. 2009;15:181–193. doi: 10.1093/molehr/gap005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaffin CL, Vandevoort CA. Follicle growth, ovulation, and luteal formation in primates and rodents: a comparative perspective. Exp Biol Med (Maywood) 2013;238:539–548. doi: 10.1177/1535370213489437. [DOI] [PubMed] [Google Scholar]
- Mestas J, Hughes CC. Of mice and not men: differences between mouse and human immunology. J Immunol. 2004;172:2731–2738. doi: 10.4049/jimmunol.172.5.2731. [DOI] [PubMed] [Google Scholar]
- Bishop CV, Satterwhite S, Xu L, Hennebold JD, Stouffer RL. Microarray analysis of the primate luteal transcriptome during chorionic gonadotrophin administration simulating early pregnancy. Mol Hum Reprod. 2011;18:216–227. doi: 10.1093/molehr/gar073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffy DM, Stewart DR, Stouffer RL. Titrating luteinizing hormone replacement to sustain the structure and function of the corpus luteum after gonadotropin-releasing hormone antagonist treatment in rhesus monkeys. J Clin Endocrinol Metab. 1999;84:342–349. doi: 10.1210/jcem.84.1.5362. [DOI] [PubMed] [Google Scholar]
- Duffy DM, Chaffin CL, Stouffer RL. Expression of estrogen receptor alpha and beta in the rhesus monkey corpus luteum during the menstrual cycle: regulation by luteinizing hormone and progesterone. Endocrinology. 2000;141:1711–1717. doi: 10.1210/endo.141.5.7477. [DOI] [PubMed] [Google Scholar]
- Lacreuse A, Wilson ME, Herndon JG. No effect of different estrogen receptor ligands on cognition in adult female monkeys. Physiol Behav. 2009;96:448–456. doi: 10.1016/j.physbeh.2008.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brannian JD, Stouffer RL. Progesterone production by monkey luteal cell subpopulations at different stages of the menstrual cycle: changes in agonist responsiveness. Biol Reprod. 1991;44:141–149. doi: 10.1095/biolreprod44.1.141. [DOI] [PubMed] [Google Scholar]
- Elbim C, Monceaux V, Francois S, Hurtrel B, Gougerot-Pocidalo MA, Estaquier J. Increased neutrophil apoptosis in chronically SIV-infected macaques. Retrovirology. 2009;6:29. doi: 10.1186/1742-4690-6-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster RL, Johnson RP. Delineation of multiple subpopulations of natural killer cells in rhesus macaques. Immunology. 2005;115:206–214. doi: 10.1111/j.1365-2567.2005.02147.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young KA, Stouffer RL. Gonadotropin and steroid regulation of matrix metalloproteinases and their endogenous tissue inhibitors in the developed corpus luteum of the rhesus monkey during the menstrual cycle. Biol Reprod. 2004;70:244–252. doi: 10.1095/biolreprod.103.022053. [DOI] [PubMed] [Google Scholar]
- Futosi K, Fodor S, Mocsai A. Reprint of Neutrophil cell surface receptors and their intracellular signal transduction pathways. Int Immunopharmacol. 2013;17:1185–1197. doi: 10.1016/j.intimp.2013.11.010. [DOI] [PubMed] [Google Scholar]
- Brannstrom M, Pascoe V, Norman RJ, McClure N. Localization of leukocyte subsets in the follicle wall and in the corpus luteum throughout the human menstrual cycle. Fertil Steril. 1994;61:488–495. [PubMed] [Google Scholar]
- Duncan WC, Rodger FE, Illingworth PJ. The human corpus luteum: reduction in macrophages during simulated maternal recognition of pregnancy. Hum Reprod. 1998;13:2435–2442. doi: 10.1093/humrep/13.9.2435. [DOI] [PubMed] [Google Scholar]
- Mantovani A, Bottazzi B, Colotta F, Sozzani S, Ruco L. The origin and function of tumor-associated macrophages. Immunol Today. 1992;13:265–270. doi: 10.1016/0167-5699(92)90008-U. [DOI] [PubMed] [Google Scholar]
- Allavena P, Sica A, Solinas G, Porta C, Mantovani A. The inflammatory micro-environment in tumor progression: the role of tumor-associated macrophages. Crit Rev Oncol. 2008;66:1–9. doi: 10.1016/j.critrevonc.2007.07.004. [DOI] [PubMed] [Google Scholar]
- Nauseef WM, Borregaard N. Neutrophils at work. Nat Immunol. 2014;15:602–611. doi: 10.1038/ni.2921. [DOI] [PubMed] [Google Scholar]
- Metkar S, Kim KS, Silver J, Goyert SM. Differential expression of CD14-dependent and independent pathways for chemokine induction regulates neutrophil trafficking in infection. J Leukoc Biol. 2012;92:389–396. doi: 10.1189/jlb.0112011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F, Zhou Y, Fu B, Wu Y, Zhang R, Sun R, Tian Z, Wei H. Molecular signatures and transcriptional regulatory networks of human immature decidual NK and mature peripheral NK cells. Eur J Immunol. 2014;44:2771–2784. doi: 10.1002/eji.201344183. [DOI] [PubMed] [Google Scholar]
- Caligiuri MA. Human natural killer cells. Blood. 2008;112:461–469. doi: 10.1182/blood-2007-09-077438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tessier DR, Yockell-Lelievre J, Gruslin A. Uterine spiral artery remodeling: the role of uterine natural killer cells and extravillous trophoblasts in normal and high-risk human pregnancies. Am J Reprod Immunol. 2015;74:1–11. doi: 10.1111/aji.12345. [DOI] [PubMed] [Google Scholar]
- Russell P, Sacks G, Tremellen K, Gee A. The distribution of immune cells and macrophages in the endometrium of women with recurrent reproductive failure. III: Further observations and reference ranges. Pathology. 2013;45:393–401. doi: 10.1097/PAT.0b013e328361429b. [DOI] [PubMed] [Google Scholar]
- Cichocki F, Miller JS, Anderson SK, Bryceson YT. Epigenetic regulation of NK cell differentiation and effector functions. Front Immunol. 2013;4:55. doi: 10.3389/fimmu.2013.00055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Best CL, Pudney J, Welch WR, Burger N, Hill JA. Localization and characterization of white blood cell populations within the human ovary throughout the menstrual cycle and menopause. Hum Reprod. 1996;11:790–797. doi: 10.1093/oxfordjournals.humrep.a019256. [DOI] [PubMed] [Google Scholar]
- Carlsten M, Bjorkstrom NK, Norell H, Bryceson Y, van Hall T, Baumann BC, Hanson M, Schedvins K, Kiessling R, Ljunggren HG, Malmberg KJ. DNAX accessory molecule-1 mediated recognition of freshly isolated ovarian carcinoma by resting natural killer cells. Cancer Res. 2007;67:1317–1325. doi: 10.1158/0008-5472.CAN-06-2264. [DOI] [PubMed] [Google Scholar]
- Lutgendorf SK, Sood AK, Anderson B, McGinn S, Maiseri H, Dao M, Sorosky JI, De Geest K, Ritchie J, Lubaroff DM. Social support, psychological distress, and natural killer cell activity in ovarian cancer. J Clin Oncol. 2005;23:7105–7113. doi: 10.1200/JCO.2005.10.015. [DOI] [PubMed] [Google Scholar]
- Poole DH, Pate JL. Luteal microenvironment directs resident T lymphocyte function in cows. Biol Reprod. 2012;86:29. doi: 10.1095/biolreprod.111.092296. [DOI] [PubMed] [Google Scholar]
- Freeman CM, Stolberg VR, Crudgington S, Martinez FJ, Han MK, Chensue SW, Arenberg DA, Meldrum CA, McCloskey L, Curtis JL. Human CD56+ cytotoxic lung lymphocytes kill autologous lung cells in chronic obstructive pulmonary disease. PLoS One. 2014;9:e103840. doi: 10.1371/journal.pone.0103840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan KN, Masuzaki H, Fujishita A, Kitajima M, Sekine I, Matsuyama T, Ishimaru T. Estrogen and progesterone receptor expression in macrophages and regulation of hepatocyte growth factor by ovarian steroids in women with endometriosis. Hum Reprod. 2005;20:2004–2013. doi: 10.1093/humrep/deh897. [DOI] [PubMed] [Google Scholar]
- Pergola C, Schaible AM, Nikels F, Dodt G, Northoff H, Werz O. Progesterone rapidly down-regulates the biosynthesis of 5-lipoxygenase products in human primary monocytes. Pharmacol Res. 2015;94:42–50. doi: 10.1016/j.phrs.2015.01.007. [DOI] [PubMed] [Google Scholar]
- Arruvito L, Giulianelli S, Flores AC, Paladino N, Barboza M, Lanari C, Fainboim L. NK cells expressing a progesterone receptor are susceptible to progesterone-induced apoptosis. J Immunol. 2008;180:5746–5753. doi: 10.4049/jimmunol.180.8.5746. [DOI] [PubMed] [Google Scholar]
- Henderson TA, Saunders PT, Moffett-King A, Groome NP, Critchley HO. Steroid receptor expression in uterine natural killer cells. J Clin Endocrinol Metab. 2003;88:440–449. doi: 10.1210/jc.2002-021174. [DOI] [PubMed] [Google Scholar]
- Stouffer RL. Structure, function, and regulation of the corpus luteum In: Knobil E, Neill JD (eds.), The Physiology of Reproduction, New York: Raven; 2006. 475 526 [Google Scholar]
- Stouffer R, Brannian J. Adashi EY and Leung PCK (eds.), The Ovary, 2nd ed. New York: Raven Press;; 1993. The function and regulation of cell populations composing the corpus luteum of the ovarian cycle; pp. 245–259. In. [Google Scholar]
- Bogan RL, Hennebold JD. The reverse cholesterol transport system as a potential mediator of luteolysis in the primate corpus luteum. Reproduction. 2010;139:163–176. doi: 10.1530/REP-09-0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irving-Rodgers HF, Friden BE, Morris SE, Mason HD, Brannstrom M, Sekiguchi K, Sanzen N, Sorokin LM, Sado Y, Ninomiya Y, Rodgers RJ. Extracellular matrix of the human cyclic corpus luteum. Mol Hum Reprod. 2006;12:525–534. doi: 10.1093/molehr/gal060. [DOI] [PubMed] [Google Scholar]
- Christenson LK, Stouffer RL. Proliferation of microvascular endothelial cells in the primate corpus luteum during the menstrual cycle and simulated early pregnancy. Endocrinology. 1996;137:367–374. doi: 10.1210/endo.137.1.8536637. [DOI] [PubMed] [Google Scholar]
- Young KA, Hennebold JD, Stouffer RL. Dynamic expression of mRNAs and proteins for matrix metalloproteinases and their tissue inhibitors in the primate corpus luteum during the menstrual cycle. Mol Hum Reprod. 2002;8:833–840. doi: 10.1093/molehr/8.9.833. [DOI] [PubMed] [Google Scholar]
- Fraser HM, Lunn SF, Harrison DJ, Kerr JB. Luteal regression in the primate: different forms of cell death during naturaland gonadotropin-releasing hormone antagonist or prostaglandin analogue-induced luteolysis. Biol Reprod. 1999;61:1468–1479. doi: 10.1095/biolreprod61.6.1468. [DOI] [PubMed] [Google Scholar]
- Nio-Kobayashi J, Kudo M, Sakuragi N, Kimura S, Iwanaga T, Duncan WC. Regulated CC motif ligand 2 (CCL2) in luteal cells contributes to macrophage infiltration into the human corpus luteum during luteolysis Mol Hum Reprod 2015. gav028. [DOI] [PubMed] [Google Scholar]
- Penny LA. Monocyte chemoattractant protein 1 in luteolysis. Rev Reprod. 2000;5:63–66. doi: 10.1530/ror.0.0050063. [DOI] [PubMed] [Google Scholar]
- White GE, Iqbal AJ, Greaves DR. CC chemokine receptors and chronic inflammation–therapeutic opportunities and pharmacological challenges. Pharmacol Rev. 2013;65:47–89. doi: 10.1124/pr.111.005074. [DOI] [PubMed] [Google Scholar]
- Wang J, Tian Y, Phillips KL, Chiverton N, Haddock G, Bunning RA, Cross AK, Shapiro IM, Le Maitre CL, Risbud MV. Tumor necrosis factor alpha- and interleukin-1beta-dependent induction of CCL3 expression by nucleus pulposus cells promotes macrophage migration through CCR1. Arthritis Rheum. 2013;65:832–842. doi: 10.1002/art.37819. [DOI] [PMC free article] [PubMed] [Google Scholar]