Abstract
Dementia with Lewy bodies (DLB) is the second most common subtype of degenerative dementia. To our knowledge, available information about the clinical features of DLB in China remains limited. Our study therefore aimed to address this issue. Thirty-seven Chinese patients with probable DLB were recruited for this study. All subjects underwent neuropsychological assessment by trained neurologists, as well as undergoing MRI, 11C-PIB PET scans for Aβ deposition and 18F-FDG PET scans for regional cerebral glucose metabolism. Our results showed that the gender ratio of patients was 16:21 (F:M). The mean age of onset was 69.5 ± 9.0 years and the mean age at diagnosis was 71.8 ± 9.1 years. At diagnosis, the prevalence of three core clinical features of DLB was: 64.9% for fluctuating cognition, 73.0% for visual hallucinations and 62.2% for parkinsonism. The result from 11C-PiB PET and 18F-FDG PET scans confirmed Aβ deposition in the cortex and demonstrated hypometabolism in the bilateral temporoparietooccipital region, the frontal lobe, the insular lobe, and the posterior cingulate, precuneus and caudate nuclei. Our study elucidated the clinical features of Chinese DLB patients, and will improve the understanding and the early diagnosis of DLB in Chinese patients.
Introduction
Dementia with Lewy bodies (DLB), which is defined pathologically as degeneration in the central, peripheral and autonomic nervous system associated with Lewy bodies (LBs) [1], was first proposed in 1996 (Perry R, 1996). As soon as the clinical and pathological guidelines were published in the same year, the clinical diagnosis of DLB became possible [2]. Thereafter, further clinical studies showed DLB to be the second most frequent form of dementia, after Alzheimer’s disease (AD) [3, 4]. The prevalence and incidence of DLB varies widely across reported studies, and the true prevalence and incidence may be largely underestimated since DLB has remained significantly under-diagnosed [5, 6]. A recent systematic review reported that the prevalence of DLB was 0.36% in the general population and 7.5% in clinical populations; while the incidence of DLB was 3.8% of new dementia cases and 0.87 cases per 1000 person-years [7].
The clinical manifestations of DLB are cognitive fluctuations, visual hallucinations and motor parkinsonism, but the condition may also manifest with rapid eye movement sleep behavior disorder and severe sensitivity to antipsychotic medications [8, 9]. Neuroimaging studies including structural imaging and functional imaging help make the diagnosis of DLB and distinguish it from other dementias [10]. A structural imaging study found a relative preservation of the hippocampal and medial temporal lobes in DLB [11]. Molecular and functional imaging studies showed a marked reduction of dopaminergic activity in the basal ganglia of DLB and hypometabolism in the occipital lobe with the temporal lobe relatively preserved [12–14]. The characteristic pathological lesions of DLB are Lewy bodies (LBs) and Lewy neurites, caused by the aggregation of α-synuclein [15]. However, AD pathology including amyloid-beta (Aβ) and tau has also been reported in DLB patients [16, 17].
Although first described several decades ago, DLB currently remains a diagnostic challenge due to its clinical and pathological heterogenicity and its similarity in certain respects with other neurodegenerative diseases. To date, aside from some case reports, there has been limited information available about the clinical features of DLB in China[18]. This present study aimed to improve the understanding of DLB in Chinese patients by analyzing the clinical features of 37 Chinese DLB patients.
Methods
Subjects
Thirty-seven DLB patients were recruited for this study from the cognitive disorder clinic at Tianjin Huanhu Hospital, Tianjin, China, between June 2011 and March 2015. To be included in the study, patients had to be diagnosed as probable DLB, according to the diagnostic criteria defined by McKeith in 2005 [6]. The determination of Parkinsonism was according to the diagnostic criteria defined by Calne [19], and the motor features and the severity were assessed with the Motor section of Unified Parkinson disease Rating Scale (UPDRS). To distinguish DLB from Parkinson’s disease associated with dementia, we excluded patients in whom cognitive impairment had occurred more than 1 year after they were diagnosed with the extrapyramidal syndrome. The 5 controls were healthy people without a family history of neurological or psychological disorders, who were the same controls we used in our previous study [20]. All subjects underwent clinical neuropsychological assessment by trained neurologists, as well as MRI, 11C-PIB PET scans for Aβ deposition and 18F-FDG PET scans for regional cerebral glucose metabolism. Patients’ information was collected including sex, age of onset, age of diagnosis, education, and clinical symptoms and signs. All patients underwent neuropsychological assessment including MMSE, MoCA, CDT, ADL and NPI. Some of the text of our previous study was reproduced in the present study [20].
Ethics statement
Written informed consent was obtained from all subjects and/or their assigned surrogate decision-makers. The study was approved by the Tianjin Huanhu Hospital Ethics Committee.
Magnetic resonance imaging
Magnetic resonance images were acquired using a 3.0T SIEMENS Tim Trio MRI scanner. A T1-weighted coronal image was acquired using a three-dimensional spoiled gradient recalled echo inversion recovery prepped sequence (repetition time [TR] = 11 ms, echo time [TE] = 4.94 ms, flip angle [FA] = 20°, 1 mm slice thickness [zero gap], 160 slices, field of view [FOV] = 230 mm × 230 mm). All of the images from the 3T were reconstructed to a size of 256 × 256 with an isotropic resolution of 1×1×1 mm.
PET imaging
Head movement was minimized using a polyurethane immobilizer molded around the head. The PET images were acquired with a GE Discovery LS PET/CT scanner in the three-dimensional scanning mode, yielding 35 slices with 4.25 mm thickness that covered the entire brain. 11C-PIB PET scans were acquired during 90-min dynamic PET acquisition (34 frames: 4 × 15s, 8 × 30s, 9 × 60s, 2 × 180s, 8 × 300s, 3 × 600s). 11C-PIB was administered into an antecubital vein as a bolus injection, with a mean dose of 370–555 MBq. The images were reconstructed to a 128 × 128 matrix (2.5 × 2.5 mm2 pixel size).
The 18F-FDG study was conducted 1 h after the 11C-PIB PET scan using the same scanner, scanning mode, positioning and reconstruction matrix. The subjects received an intravenous injection of 250 MBq 18F-FDG and remained in a darkened, quiet room. A 10-min static PET emission scan was performed 60 min after the 18F-FDG injection.
Quantification of 11C-PIB uptake
The uptake of 11C-PIB was quantified at the voxel level using the region-to-cerebellum ratio, which is identical to the standardized uptake value ratio (SUVR). This simplified quantification enables the utilization of a short 30-min image acquisition.
Automated region-of-interest analysis
Standardized regions of interest (ROIs) were defined on the MRI template image representing brain anatomy, in accordance with the Montreal Neurological Institute (MNI) space. We merged and pooled subsets from the original Automated Anatomic Labeling (AAL) atlas to form the following ROIs: middle frontal gyrus (MFG), medial prefrontal cortex (MPFC), lateral temporal cortex (LTC), hippocampus and parahippocampus (HF+), inferior parietal lobe (IP), posterior cingulate cortex and precuneus (PCCPre), striatum, thalamus, occipital lobe (OL), superior temporal gyrus (STG), and supplementary motor area (SMA).
11C-PIB PET image analysis
The preprocessing of the 11C-PIB imaging data was performed using Statistical Parametric Mapping 8 (SPM8) software and MATLAB 2010b for Windows (Mathworks, Natick, MA, USA). First, 11C-PIB integral images (data corrected for radioactive decay summed from 60 to 90 min post-injection) were created from the dynamic PET images (frames 32 to 34) and coregistered to the subject’s MRI images. Second, the MRI images were segmented into three classes (gray matter, white matter, and cerebrospinal fluid) in SPM8 using 16 non-linear iterations and 7 × 9 × 7 basis functions. Third, the PET images and gray matter magnetic resonance images were normalized using a T1-weighted MRI template that was delivered with SPM to obtain normalization parameters. The application of a 0.5 threshold to the gray matter probability map created a gray matter probability map in the MNI space. The gray matter probability map was then coregistered to the AAL template, and the PET counts were extracted from the gray matter probability map and ROIs. The mean values for all of the regions were calculated from the integral 11C-PIB image. Target-to-cerebellum ratios were subsequently calculated for 11 bilateral regions.
18F-FDG PET image analysis
Spatial preprocessing and statistical analyses of 18F-FDG PET images were also performed in all of the subjects, using SPM8 software and MATLAB 2010b for Windows. We compared cerebral glucose metabolism in the DLB group with that of the control group. First, 18F-FDG PET images were converted to the ANALYZE format and then normalized to the MNI standard proportional stereotaxic space. Second, an isotropic 10 mm full-width half-maximum Gaussian spatial smoothing filter was applied to the image. Third, all of the comparisons of brain metabolism were performed on a voxel-by-voxel basis using a two-sample t-test. Statistical significance was determined using an extent threshold of 50 voxels. Regions that reached an uncorrected P value of less than 0.001 were considered statistically significant.
Results
Patient characteristics
Detailed patients’ characteristics are showed in Table 1. Thirty-seven DLB patients were included in this study (16 females and 21 males), with a mean onset age of 69.5 ± 9.0 years (range 50–89 years) and a mean age at diagnosis of 71.8 ± 9.1 years (range 52–89 years). The mean education duration was 10.3 ± 4.4 years. The neuropsychological test scores were: 16.6 ± 7.4 for MMSE, 1.8 ± 1.5 for CDT, 40.4 ±17.4 for ADL and 9.6 ± 7.0 for MoCA, 13.9 ± 12.4 for UPDRS, respectively. Of the tested parameters, attention, calculation, delayed recall, executive function and visuospatial ability were most affected.
Table 1. The characteristics of Chinese DLB patients.
| Patients’ characteristics | |
|---|---|
| Sex (F/M) | 16/21 |
| Age of onset (years) | 69.5±9.0 |
| Age of diagnosis (years) | 71.8±9.1 |
| Education (years) | 10.3±4.4 |
| MMSE | 16.6±7.4 |
| CDT | 1.8±1.5 |
| ADL | 40.4±17.4 |
| MoCA | 9.6±7.0 |
| UPDRS | 13.9±12.4 |
Data are the mean ± SD (except gender).
Clinical manifestations
All patients’ symptoms of onset were recorded. At the onset of the disease, 48.6% of patients showed cognitive impairment as the initial symptom, 18.9% of patients showed visual hallucinations, 10.8% of patients showed cognitive impairment and parkinsonism, 8.1% of patients showed psychiatric symptoms (excepting visual hallucinations), 5.4% of patients showed cognitive impairment and visual hallucinations, 2.7% of patients showed parkinsonism, 2.7% of patients showed cognitive impairment and psychiatric symptoms (excepting visual hallucinations), and 2.7% of patients showed blurred vision (Table 2).
Table 2. First symptom at onset of Chinese DLB patients.
| Symptoms | Percentage |
|---|---|
| Cognitive impairment | 48.6% |
| Visual hallucinations | 18.9% |
| Cognitive impairment and parkinsonism | 10.8% |
| Psychiatric symptoms (except visual hallucinations) | 8.1% |
| Cognitive impairment and visual hallucinations | 5.4% |
| Parkinsonism | 2.7% |
| Cognitive impairment and psychiatric symptoms (except visual hallucinations) | 2.7% |
| Blurred vision | 2.7% |
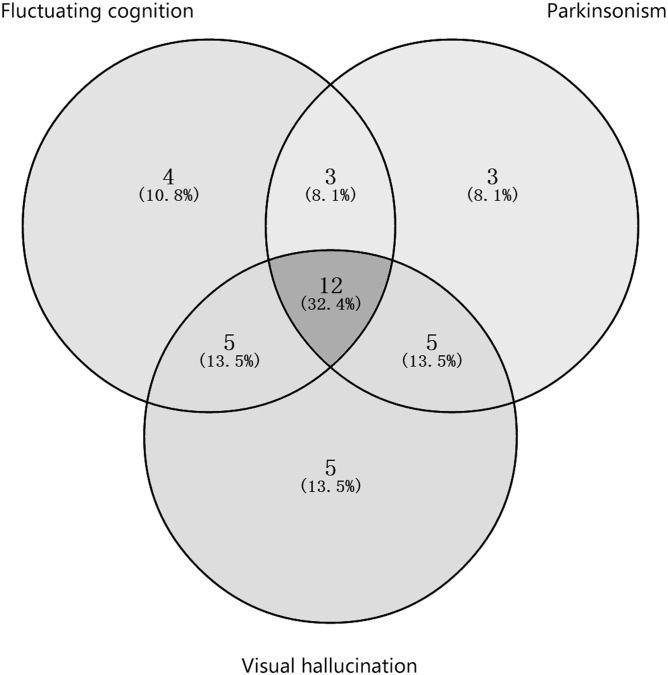
At the time of diagnosis of the disease, the core features of the Chinese DLB patients were recorded (Table 3). Of the 37 patients, 64.9% displayed fluctuating cognition. Most (83.8%) reported memory impairment, while 59.4% of patients showed visuospatial dysfunction, 24.3% of patients showed language dysfunction, 18.9% of patients showed executive dysfunction, and 5.4% of patients showed declined attention. In addition, 62.2% of the patients showed symptoms of Parkinsonism including rigidity (35.1%), postural instability and gait difficulty (29.7%), bradykinesia (21.6%), and rest tremor (18.9%). Furthermore, 73.0% of the patients manifested visual hallucinations which were recurrent, well-formed and detailed. The number and percentage of cases shared specific core features within the data set at the diagnosis were showed in Fig 1. Four patients (10.8%) displayed only fluctuating cognition, three patients (8.1%) displayed only Parkinsonism, five patients displayed (13.5%) only visual hallucinations, three patients (8.1%) displayed fluctuating cognition and Parkinsonism, five patients (13.5%) displayed fluctuating cognition and visual hallucinations, five patients (13.5%) displayed Parkinsonism and visual hallucinations, and twelve patients (32.4%) displayed all three core features.
Table 3. The core symptoms of Chinese DLB patients.
| Symptoms | Percentage |
|---|---|
| cognitive impairment | |
| Fluctuating cognition | 64.9% |
| Memory impairment | 83.8% |
| Visuospatial dysfunction | 59.4% |
| Language dysfunction | 24.3% |
| Executive dysfunction | 18.9% |
| Declined attention | 5.4% |
| Parkinsonism | 62.2% |
| Rigidity | 35.1% |
| Postural instability and gait difficulty | 29.7% |
| Bradykinesia | 21.6% |
| Rest tremor | 18.9% |
| Visual hallucinations | 73.0% |
Fig 1. The number and percentage of cases shared specific core features within the data set at the diagnosis.
In addition we analyzed the percentages of the suggestive features and the supportive features occurring in the Chinese DLB patients (Table 4). We found that 21.6% had the suggestive feature of REM sleep behavior disorder and 21.1% of the 19 patients who were treated with neuroleptics had suggestive feature of severe neuroleptic sensitivity. As for the supportive features, 75.7% of the patients showed psychiatric symptoms other than visual hallucinations, including irritability (54.1%), depression (43.2%), anxiety (40.5%), apathy (37.8%), delusions (35.1%), compulsion (24.3%), derepression (8.1%), auditory hallucinations (10.8%) and euphoria (5.4%). Moreover, 32.4% of the patients displayed autonomic dysfunction, including constipation (21.6%), syncope (18.9%), orthostatic hypotension (8.1%), frequency of urination and urgency of urination (5.4%), urinary retention (5.4%), hidrosis (5.4%) and hydrostomia (2.7%). The percentage of cases of transient, unexplained loss of consciousness was the same as that of repeated falls, at the figure of 35.1%.
Table 4. The suggestive and the supportive features of Chinese DLB patients.
| Features | Percentage | Number/Total |
|---|---|---|
| REM sleep behavior disorder | 21.6% | 8/37 |
| Severe neuroleptic sensitivity | 21.1% | 4/19 |
| Psychiatric symptoms | 75.7% | 28/37 |
| Irritability | 54.1% | 20/37 |
| Depression | 43.2% | 16/37 |
| Anxiety | 40.5% | 15/37 |
| Apathy | 37.8% | 14/37 |
| Delusion | 35.1% | 13/37 |
| Compulsion | 24.3% | 9/37 |
| Derepression | 8.1% | 3/37 |
| Auditory hallucination | 10.8% | 4/37 |
| Euphoria | 5.4% | 2/37 |
| Autonomic dysfunction | 32.4% | 12/37 |
| Constipation | 21.6% | 8/37 |
| Syncope | 18.9% | 7/37 |
| Orthostatic hypotension | 8.1% | 3/37 |
| Frequency and urgency of urination | 5.4% | 2/37 |
| Urinary retention | 5.4% | 2/37 |
| Hidrosis | 5.4% | 2/37 |
| Hydrostomia | 2.7% | 1/37 |
| Transient, unexplained loss of consciousness | 35.1% | 13/37 |
| Repeated falls | 35.1% | 13/37 |
Aβ deposition analysis with 11C-PiB PET and cerebral glucose metabolism analysis with 18F-FDG PET
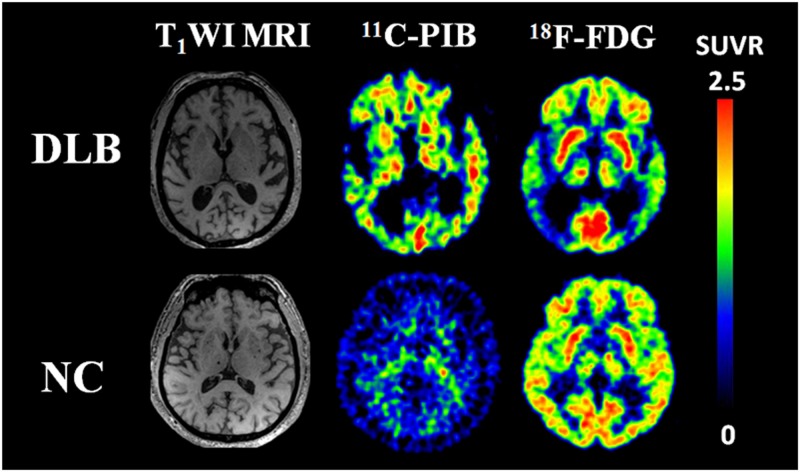
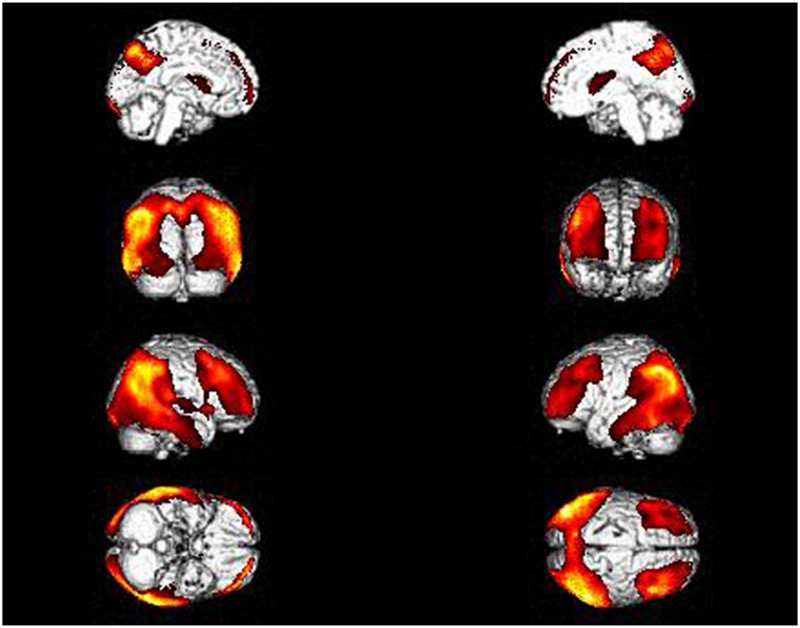
We analyzed Aβ deposition in brain with 11C-PiB PET in the Chinese DLB patients. All of the patients were Aβ positive. After 45 min of PIB injection, visual analysis showed that the clearance rate of radioactivity was slower symmetrically or asymmetrically in the cortex of the frontal lobe, parietal lobe, lateral temporal lobe, precuneus, posterior cingulate and occipital lobe (Fig 2). The SUVR of DLB was significantly higher than in controls in IP, LTC, MFG, MPFC, PCCPre, OL, SMA, STG, and striatum (S1 Table). In the same patient cohort, we also analyzed cerebral glucose metabolism with 18F-FDG PET, which showed hypometabolism in the bilateral temporoparietooccipital region, frontal lobe, insular lobe, posterior cingulate, precuneus and caudate nuclei (Fig 2). SPM analyses revealed significant hypometabolism in the left middle temporal gyrus (BA39, 21), inferior temporal gyrus (BA37, 20), superior occipital gyrus (BA19), angular gyrus (BA39), precuneus (BA7), and supramarginal gyrus (BA40), and the right middle frontal gyrus (BA8), precentral gyrus (BA9), superior temporal gyrus (BA39), middle temporal gyrus (BA37, 39), inferior temporal gyrus (BA20), inferior parietal lobule (BA40), superior occipital gyrus (BA19), precuneus (BA7,19) and supramarginal gyrus (BA40) (Fig 3 and S2 Table).
Fig 2. MRI (left), PiB (middle) and FDG (right) images of a Chinese DLB patient and a healthy control.
PiB and FDG images are quantified by SUVR with the displayed color scales. In DLB patient, the clearance rate of radioactivity was slower symmetrically or asymmetrically in the cortex of the frontal lobe, parietal lobe, lateral temporal lobe, precuneus, posterior cingulate and occipital lobe.
Fig 3. Topography of hypometabolism in Chinese DLB patients.
Discussion
The present study is the first to explore, in a single clinic center, the clinical features of Chinese DLB patients, as well as the pattern of cerebral Aβ deposition and glucose metabolism in these patients. Our results showed that amongst these patients, the gender ratio was 16:21 (F:M), the mean onset age was 69.5 ± 9.0 years and the mean diagnosed age was 71.8 ± 9.1 years. Furthermore, the results of neuropsychological tests indicated that both the cognitive functioning and the daily life capacity of DLB patients had declined by the time they visited our clinic. The disease usually initially presents with core clinical features; the percentage of patients with the three individual core clinical features at time of diagnosis was 64.9% for fluctuating cognition, 73.0% for visual hallucinations and 62.2% for parkinsonism. The result from 11C-PiB PET and 18F-FDG PET scans confirmed Aβ deposition and revealed hypometabolism in the cortex. Our study provides a better understanding of DLB in Chinese patients and will help to improve the identification and the early diagnosis of patients with this condition.
In the present study, we found a slight preponderance of males in our Chinese DLB patients. However, past studies of gender differences in DLB patients have shown inconsistent results [7]. Some researchers have reported disproportionately more females with the disease, while others have reported disproportionately more males [21, 22]. The condition is thought to first occur in the later years of life, generally at ages 60 to 90 [2, 23], with a peak incidence reported in the 7th decade of life [24]. In our study the age of onset of the condition was around the 7th decade of life, which is consistent with previous reports.
Our study confirmed that cognitive decline is the most common symptom at the onset of the disease, with visual hallucinations and parkinsonism less likely to be manifest [18]. However, importantly, DLB can also begin with psychiatric symptoms other than visual hallucinations [8, 25]. Although a previous study has reported that fluctuating cognition occurred in 80% or more of individuals with DLB [26], we found a slightly lower frequency of fluctuating cognition was evident in the Chinese DLB patients in our study. We believe our result was probably a more accurate reflection of the real prevalence of fluctuating cognition in Chinese DLB patients. Moreover, our study showed that memory deficits is the most common component of cognitive impairment in Chinese DLB patients. The results from MMSE, MoCA and CDT tests indicated that, besides memory, visuospatial function, language, executive function and attention were also affected. Previous studies have suggested that in the early stages of the disease, DLB patients tend to exhibit pronounced visual-perceptual, attentional and frontal executive impairments, whereas memory impairment may not necessarily be evident [6, 27–29]. Nevertheless in our study, most of the patients complained of memory decline when first affected by the disorder. This phenomenon might have resulted from the small size of the sample or it may represent an unique characteristic feature in Chinese DLB, which needs to be further studied with a bigger sample size. In our study, the frequency of Parkinsonism in DLB, another core clinical feature of the condition, was also consistent with that reported in the literature [30, 31]. The features of parkinsonism in DLB, which may include tremor, rigidity, bradykinesia, diminishing facial expressions and impaired fine motor performance, may differ from those found in idiopathic PD [32]. In line with previous studies, which have reported the prevalence rate of visual hallucinations to be in excess of 60% [33], we found in our study that 73.0% of Chinese DLB patients showed visual hallucinations. These seem to be associated with the impairment of anterior and posterior regions (secondary visual areas, orbitofrontal cortex and anterior cingulate cortex) involved in a top-down (impaired attentional binding) and a bottom-up (perceptual processes) mechanism, respectively.
Patients were also investigated for the presence of suggestive features of Chinese DLB. We found that neither REM sleep behavior disorder nor severe neuroleptic sensitivity were common in Chinese DLB patients. In our study, the percentage of those patients with the suggestive features was lower than in the previous study [25]. It has been reported that at least 80% of patients with DLB experience some form of neuropsychiatric symptoms [33]. Our data produced results similar to the previous study. Moreover, in addition to visual hallucinations, patients with DLB can also experience other psychiatric symptoms such as irritability, depression, anxiety, apathy, delusion, compulsion, derepression, auditory hallucination and euphoria, as was demonstrated in our study. In addition, a small number of patients also showed supportive features including autonomic dysfunction, transient, unexplained loss of consciousness and repeated falls. It has been reported that in DLB some noncognitive symptoms of the condition such as constipation, hyposmia and postural dizziness can predate the onset of memory impairment by years [34]. Therefore, the presence of these suggestive and supportive features may help to improve the recognition of DLB in clinical practice.
Although the main pathologic feature of DLB is the Lewy bodies and Lewy neurites, most patients with DLB also have the pathology typically seen in AD patients [35, 36]. Using 11C-PiB PET, we analyzed amyloid-beta deposition in the Chinese DLB patients. The results confirmed the presence of the deposition of Aβ in the cortex of the Chinese DLB patients, which is similar to that seen in AD using visual analysis. Indeed, the deposition of Aβ into neuritic and diffuse plaques is present in approximately 85% of cases of DLB [37]. Some studies have reported similarities in Aβ deposition in DLB and AD cases, while other studies have reported lower mean cortical Aβ ligand binding in DLB patients [38]. Some studies have established a distinctive pattern of hypometabolism in the occipital cortex and visual association cortices [37, 39, 40]. However, though the finding is not consistent with previous studies, the data from 18F-FDG PET indicated hypometabolism in the bilateral temporoparietooccipital region, frontal lobe, insular lobe, posterior cingulate, precuneus and caudate nuclei.
Conclusions
In conclusion, our study identified and elucidated the clinical features of Chinese DLB patients. Our results indicated that DLB in the Chinese population is a late-onset dementia with a slight male preponderance. Cognitive impairment is the most common symptom at the onset of the disease and memory deficits are the most common component of cognitive impairment in Chinese DLB patients. The prevalence of three core clinical features at diagnosis was 64.9% for fluctuating cognition, 73.0% for visual hallucinations and 62.0% for parkinsonism. The result from 11C-PiB PET and 18F-FDG PET scans confirmed Aβ deposition and hypometabolism in the cortex. Our findings provide a comprehensive view of DLB in Chinese patients.
Supporting information
(DOCX)
(DOCX)
Data Availability
All data are stored in the Tianjin Dementia Institute, however these data cannot be publicly deposited due to patient privacy. Future interested researchers may request access to confidential data here: Mengyuan Liu, E-mail: flylmy2008@126.com.
Funding Statement
Support was provided by National Natural Science Foundation of China to YJ (funding numbers: 81571057) and X-DW (funding numbers: 81300947) [http://www.nsfc.gov.cn/]; Tianjin Science and Technology Support Program to YJ (funding numbers: 12ZCZDSY02900) [http://www.tstc.gov.cn/]; Science and Technology Foundation of Tianjin Municipal Health Bureau to YJ (funding number: 2014KR10) and X-DW (funding number: 2013KY15) [http://www.tjwsj.gov.cn/html/WSJn/portal/index/index.htm]; Key Project of Tianjin Municipal Health Bureau to YJ (funding number: 14KG117) [http://www.tjwsj.gov.cn/html/WSJn/portal/index/index.htm]. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Dickson DW, Fujishiro H, Orr C, DelleDonne A, Josephs KA, Frigerio R, et al. Neuropathology of non-motor features of Parkinson disease. Parkinsonism Relat Disord. 2009;15 Suppl 3:S1–5. [DOI] [PubMed] [Google Scholar]
- 2.McKeith IG, Galasko D, Kosaka K, Perry EK, Dickson DW, Hansen LA, et al. Consensus guidelines for the clinical and pathologic diagnosis of dementia with Lewy bodies (DLB): report of the consortium on DLB international workshop. Neurology. 1996;47(5):1113–24. [DOI] [PubMed] [Google Scholar]
- 3.Aarsland D, Rongve A, Nore SP, Skogseth R, Skulstad S, Ehrt U, et al. Frequency and case identification of dementia with Lewy bodies using the revised consensus criteria. Dement Geriatr Cogn Disord. 2008;26(5):445–52. 10.1159/000165917 [DOI] [PubMed] [Google Scholar]
- 4.McKeith I, Mintzer J, Aarsland D, Burn D, Chiu H, Cohen-Mansfield J, et al. Dementia with Lewy bodies. Lancet Neurol. 2004;3(1):19–28. [DOI] [PubMed] [Google Scholar]
- 5.Palmqvist S, Hansson O, Minthon L, Londos E. Practical suggestions on how to differentiate dementia with Lewy bodies from Alzheimer's disease with common cognitive tests. Int J Geriatr Psychiatry. 2009;24(12):1405–12. 10.1002/gps.2277 [DOI] [PubMed] [Google Scholar]
- 6.McKeith IG, Dickson DW, Lowe J, Emre M, O'Brien JT, Feldman H, et al. Diagnosis and management of dementia with Lewy bodies: third report of the DLB Consortium. Neurology. 2005;65(12):1863–72. 10.1212/01.wnl.0000187889.17253.b1 [DOI] [PubMed] [Google Scholar]
- 7.Vann Jones SA, O'Brien JT. The prevalence and incidence of dementia with Lewy bodies: a systematic review of population and clinical studies. Psychol Med. 2014;44(4):673–83. 10.1017/S0033291713000494 [DOI] [PubMed] [Google Scholar]
- 8.Donaghy PC, McKeith IG. The clinical characteristics of dementia with Lewy bodies and a consideration of prodromal diagnosis. Alzheimers Res Ther. 2014;6(4):46 10.1186/alzrt274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chiba Y, Fujishiro H, Ota K, Kasanuki K, Arai H, Hirayasu Y, et al. Clinical profiles of dementia with Lewy bodies with and without Alzheimer's disease-like hypometabolism. Int J Geriatr Psychiatry. 2015;30(3):316–23. 10.1002/gps.4144 [DOI] [PubMed] [Google Scholar]
- 10.Magierski R, Sobow T. Magnetic resonance spectroscopy in the diagnosis of dementia with Lewy bodies. Biomed Res Int. 2014;2014:809503 10.1155/2014/809503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Watson R, Blamire AM, O'Brien JT. Magnetic resonance imaging in lewy body dementias. Dement Geriatr Cogn Disord. 2009;28(6):493–506. 10.1159/000264614 [DOI] [PubMed] [Google Scholar]
- 12.Lobotesis K, Fenwick JD, Phipps A, Ryman A, Swann A, Ballard C, et al. Occipital hypoperfusion on SPECT in dementia with Lewy bodies but not AD. Neurology. 2001;56(5):643–9. [DOI] [PubMed] [Google Scholar]
- 13.Sinha N, Firbank M, O'Brien JT. Biomarkers in dementia with Lewy bodies: a review. Int J Geriatr Psychiatry. 2012;27(5):443–53. 10.1002/gps.2749 [DOI] [PubMed] [Google Scholar]
- 14.Warr L, Walker Z. Identification of biomarkers in Lewy-body disorders. Q J Nucl Med Mol Imaging. 2012;56(1):39–54. [PubMed] [Google Scholar]
- 15.Perry RH, Irving D, Blessed G, Fairbairn A, Perry EK. Senile dementia of Lewy body type. A clinically and neuropathologically distinct form of Lewy body dementia in the elderly. J Neurol Sci. 1990;95(2):119–39. [DOI] [PubMed] [Google Scholar]
- 16.Lopez OL, Becker JT, Kaufer DI, Hamilton RL, Sweet RA, Klunk W, et al. Research evaluation and prospective diagnosis of dementia with Lewy bodies. Arch Neurol. 2002;59(1):43–6. [DOI] [PubMed] [Google Scholar]
- 17.Ballard C, Ziabreva I, Perry R, Larsen JP, O'Brien J, McKeith I, et al. Differences in neuropathologic characteristics across the Lewy body dementia spectrum. Neurology. 2006;67(11):1931–4. 10.1212/01.wnl.0000249130.63615.cc [DOI] [PubMed] [Google Scholar]
- 18.Han D, Wang Q, Gao Z, Chen T, Wang Z. Clinical features of dementia with lewy bodies in 35 Chinese patients. Transl Neurodegener. 2014;3(1):1 10.1186/2047-9158-3-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Calne DB, Snow BJ, Lee C. Criteria for diagnosing Parkinson's disease. Ann Neurol. 1992;32 Suppl:S125–7. [DOI] [PubMed] [Google Scholar]
- 20.Wang XD, Lu H, Shi Z, Cai L, Liu S, Han T, et al. A Pilot Study on Clinical and Neuroimaging Characteristics of Chinese Posterior Cortical Atrophy: Comparison with Typical Alzheimer's Disease. PLoS One. 2015;10(8):e0134956 10.1371/journal.pone.0134956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Alladi S, Mekala S, Chadalawada SK, Jala S, Mridula R, Kaul S. Subtypes of dementia: a study from a memory clinic in India. Dement Geriatr Cogn Disord. 2011;32(1):32–8. 10.1159/000329862 [DOI] [PubMed] [Google Scholar]
- 22.Yoshida H, Terada S, Honda H, Ata T, Takeda N, Kishimoto Y, et al. Validation of Addenbrooke's cognitive examination for detecting early dementia in a Japanese population. Psychiatry Res. 2011;185(1–2):211–4. 10.1016/j.psychres.2009.06.012 [DOI] [PubMed] [Google Scholar]
- 23.Jellinger KA, Wenning GK, Seppi K. Predictors of survival in dementia with lewy bodies and Parkinson dementia. Neurodegener Dis. 2007;4(6):428–30. 10.1159/000107703 [DOI] [PubMed] [Google Scholar]
- 24.Savica R, Grossardt BR, Bower JH, Boeve BF, Ahlskog JE, Rocca WA. Incidence of dementia with Lewy bodies and Parkinson disease dementia. JAMA Neurol. 2013;70(11):1396–402. 10.1001/jamaneurol.2013.3579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Auning E, Rongve A, Fladby T, Booij J, Hortobagyi T, Siepel FJ, et al. Early and presenting symptoms of dementia with lewy bodies. Dement Geriatr Cogn Disord. 2011;32(3):202–8. 10.1159/000333072 [DOI] [PubMed] [Google Scholar]
- 26.Walker MP, Ayre GA, Cummings JL, Wesnes K, McKeith IG, O'Brien JT, et al. The Clinician Assessment of Fluctuation and the One Day Fluctuation Assessment Scale. Two methods to assess fluctuating confusion in dementia. Br J Psychiatry. 2000;177:252–6. [DOI] [PubMed] [Google Scholar]
- 27.Collerton D, Burn D, McKeith I, O'Brien J. Systematic review and meta-analysis show that dementia with Lewy bodies is a visual-perceptual and attentional-executive dementia. Dement Geriatr Cogn Disord. 2003;16(4):229–37. [DOI] [PubMed] [Google Scholar]
- 28.Dalrymple-Alford J. Comparative neuropsychology of Lewy body and Alzheimer's dementia. J Neurol Neurosurg Psychiatry. 2001;70(2):148 10.1136/jnnp.70.2.148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Noe E, Marder K, Bell KL, Jacobs DM, Manly JJ, Stern Y. Comparison of dementia with Lewy bodies to Alzheimer's disease and Parkinson's disease with dementia. Mov Disord. 2004;19(1):60–7. 10.1002/mds.10633 [DOI] [PubMed] [Google Scholar]
- 30.Galasko D, Katzman R, Salmon DP, Hansen L. Clinical and neuropathological findings in Lewy body dementias. Brain Cogn. 1996;31(2):166–75. 10.1006/brcg.1996.0040 [DOI] [PubMed] [Google Scholar]
- 31.Ballard C, O'Brien J, Swann A, Neill D, Lantos P, Holmes C, et al. One year follow-up of parkinsonism in dementia with Lewy bodies. Dement Geriatr Cogn Disord. 2000;11(4):219–22. [DOI] [PubMed] [Google Scholar]
- 32.Gnanalingham KK, Byrne EJ, Thornton A, Sambrook MA, Bannister P. Motor and cognitive function in Lewy body dementia: comparison with Alzheimer's and Parkinson's diseases. J Neurol Neurosurg Psychiatry. 1997;62(3):243–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ballard C, Holmes C, McKeith I, Neill D, O'Brien J, Cairns N, et al. Psychiatric morbidity in dementia with Lewy bodies: a prospective clinical and neuropathological comparative study with Alzheimer's disease. Am J Psychiatry. 1999;156(7):1039–45. [DOI] [PubMed] [Google Scholar]
- 34.Chiba Y, Fujishiro H, Iseki E, Ota K, Kasanuki K, Hirayasu Y, et al. Retrospective survey of prodromal symptoms in dementia with Lewy bodies: comparison with Alzheimer's disease. Dement Geriatr Cogn Disord. 2012;33(4):273–81. 10.1159/000339363 [DOI] [PubMed] [Google Scholar]
- 35.Jellinger KA, Attems J. Prevalence and impact of vascular and Alzheimer pathologies in Lewy body disease. Acta Neuropathol. 2008;115(4):427–36. 10.1007/s00401-008-0347-5 [DOI] [PubMed] [Google Scholar]
- 36.Fujishiro H, Iseki E, Higashi S, Kasanuki K, Murayama N, Togo T, et al. Distribution of cerebral amyloid deposition and its relevance to clinical phenotype in Lewy body dementia. Neurosci Lett. 2010;486(1):19–23. 10.1016/j.neulet.2010.09.036 [DOI] [PubMed] [Google Scholar]
- 37.Edison P, Rowe CC, Rinne JO, Ng S, Ahmed I, Kemppainen N, et al. Amyloid load in Parkinson's disease dementia and Lewy body dementia measured with [11C]PIB positron emission tomography. J Neurol Neurosurg Psychiatry. 2008;79(12):1331–8. 10.1136/jnnp.2007.127878 [DOI] [PubMed] [Google Scholar]
- 38.Donaghy P, Thomas AJ, O'Brien JT. Amyloid PET Imaging in Lewy body disorders. Am J Geriatr Psychiatry. 2015;23(1):23–37. 10.1016/j.jagp.2013.03.001 [DOI] [PubMed] [Google Scholar]
- 39.Klein JC, Eggers C, Kalbe E, Weisenbach S, Hohmann C, Vollmar S, et al. Neurotransmitter changes in dementia with Lewy bodies and Parkinson disease dementia in vivo. Neurology. 2010;74(11):885–92. 10.1212/WNL.0b013e3181d55f61 [DOI] [PubMed] [Google Scholar]
- 40.Perneczky R, Drzezga A, Boecker H, Forstl H, Kurz A, Haussermann P. Cerebral metabolic dysfunction in patients with dementia with Lewy bodies and visual hallucinations. Dement Geriatr Cogn Disord. 2008;25(6):531–8. 10.1159/000132084 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
(DOCX)
Data Availability Statement
All data are stored in the Tianjin Dementia Institute, however these data cannot be publicly deposited due to patient privacy. Future interested researchers may request access to confidential data here: Mengyuan Liu, E-mail: flylmy2008@126.com.