Abstract
Background
Sarcoidosis is a systemic inflammatory disease of unknown etiology. Osteopontin (SPP1, OPN) is an extra cellular matrix glycoprotein and cytokine with a known role in granuloma formation and in autoimmune and inflammatory diseases.
Objective
To determine whether plasma OPN levels are elevated in patients with sarcoidosis and compare the frequency of four single nucleotide polymorphism (SNPs) variants in the OPN gene in sarcoidosis patients compared to healthy controls.
Methods
Demographic and clinical information, radiological studies and pulmonary function tests were evaluated in 113 patients with sarcoidosis and in 79 healthy controls. Blood samples were analyzed for SNPs of the OPN gene and for plasma OPN and CRP levels. Association between clinical features of disease and OPN levels as well as SNP frequencies was determined.
Results
Plasma OPN levels were higher in sarcoidosis patients than in healthy subjects, (median: 217 vs 122ng/ml, p<0.001). Area under the curve for receiver operator curves (ROC) was 0.798 (0.686–0.909 95% CI.) No differences were observed between sarcoidosis patients and controls in the frequency of any of the SNPs evaluated. Presence of lung parenchymal involvement was associated with SNP distribution at rs1126772 (p = 0.02). We found no correlation between SNPs distribution and plasma OPN levels.
Conclusions
Osteopontin protein levels are elevated in sarcoidosis. We found no evidence for an association between SNPs on the osteopontin gene and plasma OPN levels or the presence of sarcoidosis, however, an association between genotype and several phenotypic clinical parameters of disease was observed.
Introduction
Sarcoidosis is a systemic inflammatory disease of unknown etiology, characterised by non-caseating granuloma formation in various organs, with several recognized genetic and environmental risk factors. The prevalence of sarcoidosis varies from 4.7–64 per 100,000, with an estimated annual incidence of 1.0–35.5 in 100,000 [1]. In a study conducted in northern Israel an annual incidence of 2 in 100,000 was found [2], with a ten-fold increase in disease incidence from 1980 to 1996.
Genetic susceptibility to sarcoidosis has been found to be independently related to both HLA Class I and HLA Class II groups such as HLA-DRB1 [3], HLA-DR5 [4]. HLA groups are not only related to susceptibility for sarcoidosis, but also to its clinical course. Extra pulmonary manifestations of sarcoidosis, and specifically Löfgren's syndrome, defined by a triad of erythema nodosum (EN), arthralgia and hilar lymphadenopathy, have been associated with the human leukocyte antigen (HLA) group DRB1 in European population [5]. HLA class II alleles are associated with several phenotypes: DRB1*0401 with ocular involvement, DRB3 with bone marrow involvement in blacks, and DPB1*0101 with hypercalcemia in whites [3]. Due to linkage disequilibrium between HLA groups, it is sometimes hard to determine which is the involved genetic predisposing factor, as in the case of HLA-DRB1 and HLA-DQB1, as both were correlate to sarcoidosis, and to one another [5]. Genetic susceptibility to sarcoidosis has also been found to be related to specific genes such as Butyrophilin-like protein 2 (BTNL2), which Belongs to the immunoglobulin superfamily [6], Annexin A11 (ANAXA11), which gives rise to auto-antibodies in several inflammatory diseases, including rheumatoid arthritis, systemic lupus erythematosus and Sjögren syndrome [7], Solute carrier family 11 (Proton-coupled divalent metal ion transporter), member 1 (SLC11A1), which is associated with risk of intracellular pathogens such as tuberculosis, but also with autoimmune diseases such as rheumatoid arthritis, crohn's disease, type 1 diabetes, and primary biliary cirrhosis [8]; and to Interferon alpha (IFNA) genes polymorphisms [9], known for its involvement in Th1 diseases. TNF-β and TNF-α polymorphisms are associated with susceptibility to sarcoidosis in certain populations [10], with TNF being a key regulator of the inflammatory response. Sarcoidosis is often associated with elevated serum Angiotensin-converting enzyme (ACE) levels. An ACE Insertion/Deletion polymorphism has been tested for association with the risk of sarcoidosis. Published results are unequivocal, however, it was found that genotyping for this I/D polymorphism improves the diagnostic value of serum ACE levels measurements [11,12].
Certain phenotypes in sarcoidosis have a genetic component. Early-onset sarcoidosis, together with familial Blau syndrome is associated with mutations of Nucleotide-binding oligomerization domain-containing protein 2 (NOD2), also known as caspase recruitment domain-containing protein 15 (CARD15), that cause constitutive NF-kappa-B activation. NOD2 mutations are related to Crohn's disease as well [13]. Sarcoidosis-related-uveitis is associated with a certain SNP of Heat Shock Protein 70/Hom. Moreover, the haplotype of HSP70 can be used to discriminate it from idiopathic uveitis [14]. Several studies have described d a connection between the presence of Mycobacterium tuberculosis heat-shock proteins in sarcoidosis patients and polymorphisms of genes encoding for FC receptor γ. This suggests that reduced clearance of TB immune complex may be relevant in the pathogenesis of sarcoidosis [15]. Familial clusters have been described, but are relatively uncommon. An association between sarcoidosis and CD14, an LPS co-receptor, has been previously reported by us [16].
Osteopontin (OPN), also known as secreted phosphoprotein 1 (SPP1) and early T lymphocyte activation 1 (ETA1), is a secreted phosphoprotein, part of the small integrin-binding ligand N-linked glycoprotein (SIBLING) family [17]. It was first identified as a bone matrix protein, subsequently identified as a cytokine, and is involved in carcinogenesis, tissue formation and the immune response [18,19]. CD44 variants and several integrins serve as receptors for OPN. It interacts with most of these integrins through a central arginine-glycine-aspartate (RGD) domain, and has a heparin binding site and a thrombin cleavage site [20,21]. OPN skews the immune response towards Th1, interacting with αVβ3 integrin inducing pro-inflammatory IL-12 and suppressing IL-10 production [22]. Elevated levels of OPN are found in plasma or serum and tissue specific fluids of patients suffering from several granulomatous disorders including tuberculosis and silicosis [23,24], and from Th-1 related disorders such as inflammatory bowel disease [25,26], systemic lupus erythematosus [27], certain types of multiple sclerosis [28,29] and rheumatoid arthritis [30]. Elevated expression of OPN has been described in sarcoidosis granulomas and in the plasma from patients with sarcoidosis [31,32].
The OPN gene resides on the long arm of chromosome 4. Crosby et al [33] showed that the SPP1 gene comprises 7 exons, 6 of which contain coding sequences. According to the Single Nucleotide Polymorphism Database (dbSNP), of OPN (SPP1) known SNPs, 404 are Single Nucleotide Variants (SNV), as opposed to deletions, insertions, copy number variations or microsatellites. Some of them are related to several autoimmune and granulomatous disorders and pulmonary diseases [34–39]. A possible relationship between Sarcoidosis and OPN gene has been suggested previously in the Slovenian population, with certain haplotype serving as a protective factor for the disease [40]. The current study focuses on four SNPs: rs1126616 is a coding synonymous and exonic splicing enhancer variant; rs1126772 and rs9138 are 3-prime untranslated region (UTR) variants and rs4754 is a coding synonymous variant. We measured plasma OPN levels as well as the frequency of 4 polymorphic sites for the OPN gene in a cohort of 113 sarcoidosis patients and compared these to a cohort of 79 healthy controls.
Methods
Study population
Patients: Patients were recruited from two large outpatient pulmonary clinics in Jerusalem, Israel between January 2011 and September 2015. All participants signed an Informed Consent Form and the study was approved by the Hadassah Medical Center Ethics Committee. Charts of patients with biopsy findings compatible with sarcoidosis as well as a compatible clinical picture as determined by symptoms, laboratory abnormalities and/or imaging and without another identified cause of granulomatous disease were included in the cohort.
Demographic and clinical information including radiological and pulmonary function tests was gathered from patient questionnaires, hospital medical records, and outpatient medical records obtained from treating physicians’ records. All data and radiological studies were reevaluated by the pulmonary physicians and radiologists at the Hadassah Medical Center. Assessment of disease extent, severity, duration of symptoms prior to diagnosis, and extent and nature of organ involvement in the disease was evaluated. For this study, we used lung function tests (LFT) that were performed prior to and as close to blood drawing as was available.
Controls: A cohort of healthy subjects with no significant medical background was recruited as a control group.
Sample collection
A single sample of 15ml venous blood into commercially available EDTA-treated tubes for DNA analysis and plasma analysis of osteopontin and CRP levels was obtained from patients and control subjects.
Evaluation of osteopontin (SPP1/OPN) levels
Plasma was separated from the blood samples and frozen at (-20)°C. OPN and CRP levels in the plasma were measured using the commercially available kits (R&D Systems Inc., MN, USA). The detection range for the OPN ELISA is 62.5pg-4000pg/ml, the detection range for CRP ELISA is 15.6-1000pg/ml. Normal range for CRP 1–3 mg/L. The samples were diluted when needed.
Genotype analysis
Genomic DNA was extracted from anticoagulated whole blood collected in EDTA from patients and controls. Isolation of DNA was done by phenol chloroform extraction [41] or using a salting out protocol, followed by alcohol precipitation [42].
Four different single nucleotide polymorphisms in the OPN gene were evaluated: rs4754, rs1126616, rs9138 and rs1126772 using commercially available primer/probe pairs: TaqMan® Predesigned SNP Genotyping Assays containing sequence-specific forward and reverse primers, and two MGB probes: VIC®-labeled probe and FAM™-labeled probe, together with TaqMan® Genotyping Master Mix according to protocol provided by the manufacturer (Applied Biosystems®). SNP genotyping was performed using StepOnePlus™ Real-Time PCR System. The results were obtained by TaqMan® Genotyper™ Software [43].
Statistical analysis
Data was analyzed using SPSS version 20.0 Software (SPSS Inc., Chicago, IL). P-value of 0.05 was considered statistically significant.
Results
Study population and patient characteristics
A total of 113 sarcoidosis patients and 79 control subjects were recruited for this study between January 2011 and September 2015. Tables 1 and 2 present clinical and demographic data of the patients group. The patients group consisted of 18% of Arab origin and 80% of Jewish origin. A family history of sarcoidosis was present in 5% of cases.
Table 1. Demographic characteristics of patients with sarcoidosis.
| % of patients (n) | ||
|---|---|---|
| Age at diagnosis (years) | All | 100 (109) |
| 20–30 | 3 (3) | |
| 31–40 | 10 (11) | |
| 41–50 | 27 (29) | |
| 51–60 | 35 (38) | |
| 61–70 | 17 (19) | |
| >70 | 8 (9) | |
| Gender | All | 100 (112) |
| Male | 37 (41) | |
| Female | 63 (71) | |
| Smoking status | All | 100 (106) |
| Active/past smoker | 23 (24) | |
| Never smoker | 77 (82) |
Table 2. Clinical characteristics of sarcoidosis patients.
| % of patients (n) | ||
|---|---|---|
| Pathological involvement | All | 100 (113) |
| Lung | 73 (83) | |
| Thoracic lymph nodes | 10 (11) | |
| Extra thoracic lymph nodes | 7 (8) | |
| Liver | 3 (3) | |
| Bone marrow | 1 (1) | |
| Other | 6 (7) | |
| Scadding chest radiographic class | All | 100 (104) |
| 0 | 6 (6) | |
| 1 | 17 (18) | |
| 2 | 62 (64) | |
| 3 | 10 (10) | |
| 4 | 6 (6) | |
| Radiology findings (pulmonary CT scan) | All | 100 (102) |
| Symmetric Hilar lymphadenopathy1 | 42(13/31) | |
| Hilar lymphadenopathy1 | 65 (20/31) | |
| Mediastinal lymphadenopathy1 | 77 (24/31) | |
| Interstitial non-nodular | 52(53) | |
| Nodular | 50 (51) | |
| Other imaging | 5 (5) | |
| Extent of disease)objective and/or subjective) or organ involvement | All | 100 (110) |
| Lung | 88 (97) | |
| Thoracic lymph nodes | 77 (85) | |
| Hilar lymphadenopathy1 | 68 (21/31) | |
| Parenchymal disease | 40 (44) | |
| Lymphadenopathy1,2 | 84 (26/31) | |
| Extra thoracic lymph nodes | 19 (21) | |
| Skin | 11 (12) | |
| Eyes | 14 (15) | |
| Joints | 25 (28) | |
| Hypercalcemia | 5 (6) | |
| Hypercalciuria1 | 8 (2/26) | |
| Neurosarcoid | 5 (5) | |
| Parotid/salivary gland1 | 10 (3/31) | |
| Spleen1 | 13 (4/31) | |
| Liver | 5 (5) | |
| Heart | 3 (3) | |
| Other | 5 (6) | |
| Presenting symptoms | All | 100 (34) |
| Dyspnoea | 38 (13) | |
| Cough | 44 (15) | |
| Fever/weight Loss | 26 (9) | |
| Eyes | 15 (5) | |
| Joints | 35 (12) | |
| Rash/skin manifestation | 12(4) | |
| Asymptomatic | 12(4) | |
| Fatigue | 12(4) | |
| Other | 41 (14) | |
| Symptoms | All | 100 (107) |
| Asymptomatic | 14 (15) | |
| Cough | 59 (63) | |
| Dyspnoea | 56 (60) | |
| Fever/weight loss | 17(18) | |
| Visual disturbances | 7 (7) | |
| Arthralgia | 30 (32) | |
| Rash | 12 (13) | |
| Other | 21 (23) | |
| Duration of symptoms prior to diagnosis | All | 100 (73) |
| <1 month | 14 (10) | |
| 1–3 months | 36 (26) | |
| 4–6 months | 14 (10) | |
| 7–12 months | 15(11) | |
| >12 months | 22 (16) | |
| Drugs at sampling | All | 100 (32) |
| Oral/IV Steroids | 19 (6) | |
| ICS±LABA | 3 (1) | |
| MTX | 0 (0) | |
| Other | 9 (3) | |
| Treatment3 | All | 100 (105) |
| Oral steroids | 44 (46) | |
| ICS1 | 19 (6/32) | |
| MTX | 7 (7) | |
| Other drugs | 24 (25) | |
| Abnormal PFT4 | Present | 100 (107) |
| median (IQR) | ||
| Pulmonary function tests% of predicted | FVC | 90 (77.8–103) |
| FEV1 | 87.5(78–99.5) | |
| FEV1/FVC | 84(77–91) | |
| VC | 95(81.5–105.3) | |
| FRC(TGV) | 97(81.5–114.5) | |
| TLC | 95(83–105.5) | |
| RV | 107(94–128) | |
| DLCO | 84(72.5–95) | |
| Bronchoalveolar lavage % cells | All | 100 (24) |
| BAL %lymphocyte | 10.5 (0.5–31.5) | |
| BAL %macrophage | 81.5(63.8–92.8) |
Definition of abbreviation:
1pulmonary or systemic lymphadenopathy
2Data available for limited number of patients
3Inhaled corticosteroid
4Present or past treatment
5Defined as TLC<80 or FEV/FVC<71 or DLCO<80 or as defined by a physician.
Definition of abbreviations: IQR- interquartile range LN-lymph nodes PFT- pulmonary function test, FVC- Forced vital capacity FEV1- Forced expiratory volume in 1 second FEV1/FVC- Forced vital capacity divided by forced expiratory volume in 1 second VC- vital capacity FRC (TGV (- Functional Residual Capacity, (Thoracic gas volume) TLC- total lung capacity RV- residual volume DLCO- diffusing capacity for carbon monoxide, ICS- inhaled corticosteroid MTX-methotrexate LABA- long acting beta agonists ICS±ABA- inhaled corticosteroid, with or without long acting beta agonists.
Osteopontin levels in sarcoidosis patients
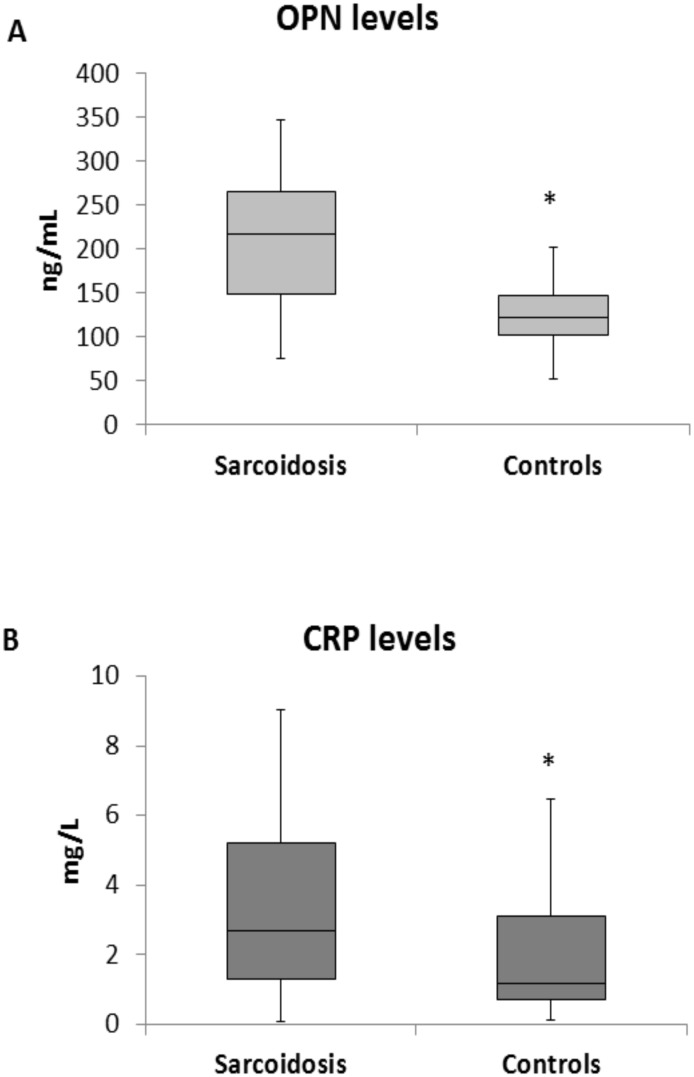
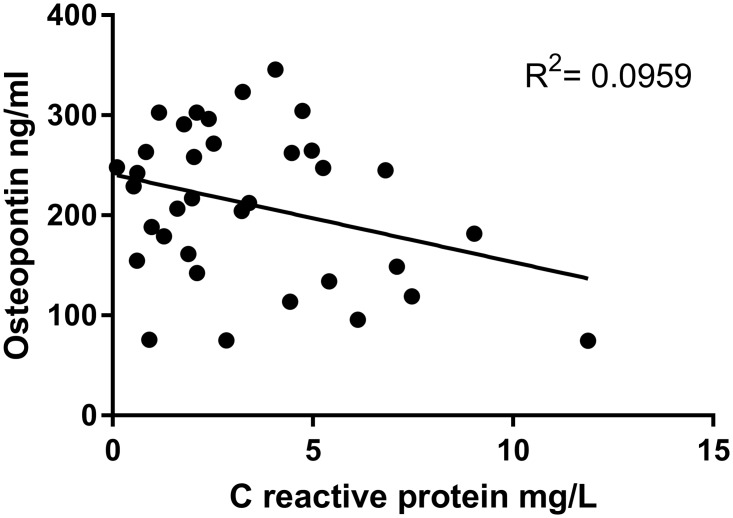
Protein levels of OPN and CRP in plasma of sarcoidosis patients and controls are presented in Fig 1. Sarcoidosis patients had significantly higher levels of OPN (patients median 217 ng/ml, 25–75% range: 149–265 vs controls median 122ng/ml, range 102–147; p<0.001), as well as CRP (patients median 2.7mg/L, 25–75% range 1.3–5.2 vs controls median 1.2mg/L, range: 0.7–3.1; p = 0.018) in comparison with healthy control subjects. We found no correlation between OPN and CRP levels (Fig 2).
Fig 1. Osteopontin and CRP levels.
Sarcoidosis patients had significantly higher levels of (A) plasma OPN (p<0.001) and (B) C—reactive protein (p<0.018) in comparison with healthy control subjects. Solid horizontal line indicates median. Color box indicates interquartile range. Black line indicates range of measurements. OPN- Osteopontin, CRP- c-reactive protein.
Fig 2. Correlation between osteopontin and C reactive protein.
Plasma osteopontin level is not correlated with C—reactive protein level.
We evaluated possible associations between plasma OPN levels and clinical and epidemiological parameters of disease in sarcoidosis patients. OPN levels were significantly elevated in smoking patients (p = 0.049), median levels of 247ng/ml in present and past smokers vs. 179 ng/ml in never-smokers. OPN levels were significantly decreased in patients whose presenting symptoms were rash or skin involvement (medians: 114ng/ml vs 218ng/ml, p = 0.025).
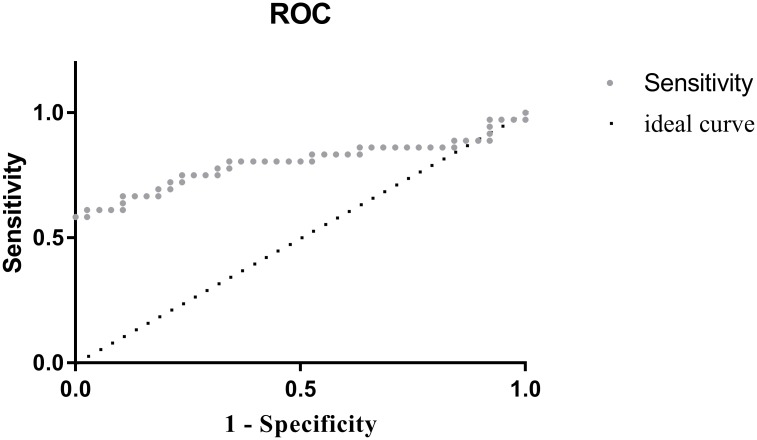
Serum OPN may serve as a diagnostic test for sarcoidosis. Using ROC curves the optimal cut-off value to differentiate sarcoidosis from healthy controls is 180ng/ml which gives a specificity of 89.5% and sensitivity of 64% (Fig 3).
Fig 3. Receiver Operating Characteristic (ROC).
Curve showing specificity and sensitivity percentages of osteopontin in patients and controls. Area under the curve is 0.798 (0.686–0.909 95% CI). 89.5% specificity and 63.9% sensitivity for cutoff value of 180ng/ml. Positive (sarcoid), Negative (control).
Single nucleotide polymorphisms
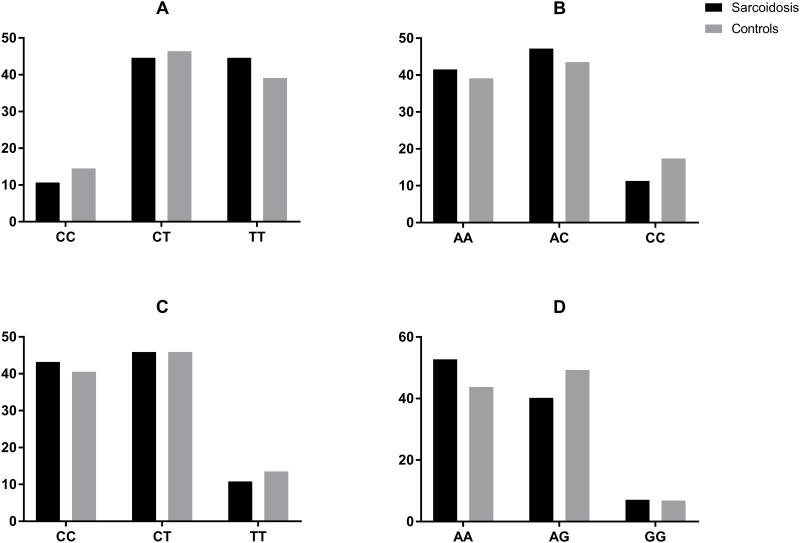
We found no differences between sarcoidosis patients and controls in the frequency of the three variations in any of the four SNPs we examined (Fig 4A–4D, Table 3). We did find some significant differences in the frequency of SNP in certain patient subgroups. Parenchymal lung involvement was found related to SNP rs1126772 (p = 0.02). Presenting symptoms of fever or significant weight loss was related to SNPs at three sites: SNP rs4754 (p = 0.01); SNP rs9138 (p = 0.027); and SNP rs11276616 (p = 0.027). Plasma OPN levels were not significantly different in any variant of any SNPs for the entire group or for only the patient group.
Fig 4. Frequency of SNPs.
Frequency of SNPs in patients with sarcoidosis (clear) and controls (black) for the four allelic sites examined: each pair of columns represents percentage a possible variant of total group. (A) rs4754C/T (B) rs9138 A/C (C) rs11276616 C/T (D) rs1126772 A/G No differences were observed between groups.
Table 3. SNP frequency.
| rs1126772 | Patients % (n) | Controls %(n) | ||
|---|---|---|---|---|
| AA | 52.7 (59) | 43.8 (32) | 49.2 (91) | |
| AG | 40.2 (45) | 49.3 (36) | 43.8 (81) | |
| GG | 7.1 (8) | 6.8 (5) | 7.0 (13) | |
| Total | (112) | (73) | (185) | |
| rs4754 | ||||
| CC | 10.7 (12) | 14.5 (10) | 12.2 (22) | |
| CT | 44.6 (50) | 46.4(32) | 45.3 (82) | |
| TT | 44.6 (50) | 39.1 (27) | 42.5 (77) | |
| Total | (112) | (69) | (181) | |
| rs11276616 | ||||
| CC | 43.2 (48) | 40.5 (30) | 42.2 (78) | |
| CT | 45.9 (51) | 45.9 (34) | 45.9 (85) | |
| TT | 10.8 (12) | 13.5 (10) | 11.9 (22) | |
| Total | (111) | (74) | (185) | |
| rs9138 | ||||
| AA | 41.5 (44) | 39.1 (27) | 40.6 (71) | |
| AC | 47.2 (50) | 43.5 (30) | 45.7 (80) | |
| CC | 11.3 (12) | 17.4 (12) | 13.7 (24) | |
| Total | Count | (106) | (69) | (175) |
All individual data are available in S1 Table.
Discussion
In this study, we found elevated levels of plasma osteopontin (OPN) in patients with sarcoidosis when compared to healthy controls. We found no correlation between the presence of four single nucleotide polymorphisms (SNPs) tested and plasma OPN levels or presence of disease. However, some of the clinical parameters were found to correlate with genotype.
OPN is expressed in T- cells as an early response molecule in bacterial infections. It interacts with macrophages and induces a type-1 cytokine inflammatory response, potentiating production of IL-12 and IFN- γ. OPN has also been previously linked to “Th1-diseases” such as multiple sclerosis [44], rheumatoid arthritis [21], autoimmune and viral hepatitis [45], and to other granulomatous disorders such as Vogt–Koyanagi–Harada disease (VKH). OPN has also been found to be associated with lung diseases such as idiopathic pulmonary fibrosis (IPF) and is overexpressed in bronchoalveolar lavage of IPF patients [46]. Elevated serum OPN has been described previously in sarcoidosis patients and was also expressed in granulomas of patients with sarcoidosis [24,32]. In light of the above data, it is to be expected that an association between OPN levels and the presence of sarcoidosis will be found.
We considered whether plasma osteopontin could be of use as a biomarker to diagnose sarcoidosis or as a marker of disease activity and of response to treatment. As regards diagnosis, receiver operator curves for OPN show a diagnostic value (area under the curve–AUC) of 0.798, which is similar or slightly better than existing markers, which are being using routinely. For example, angiotensin converting enzyme (ACE), which is known to be non-specific and increased in neoplastic and chronic infectious conditions, was found to have AUC of 0.779 (0.668–0.891 confidence interval) and sIL-2R had an AUC value of 0.667 (0.539–0.795 confidence interval) [47]. Chitotriosidase has recently been reported to have an AUC of 0.98 [48]. Although OPN levels may differentiate between sarcoidosis and healthy controls, we do not know whether OPN levels would be of value to differentiate between sarcoidosis and other interstitial diseases or other diseases presenting with lymphadenopathy, particularly lymphoma, but further studies are required to clarify this matter. There may also be additional modifying factors that influence levels of OPN such as treatment with inhaled corticosteroids and smoking. The optimal value for OPN to diagnose sarcoidosis depends on the pretest probability for this diagnosis. Based on our study data, a low cutoff value of 100ng/ml will provide specificity of 21%, and sensitivity 86%. When trying to rule out sarcoidosis, a higher cutoff of 223 ng/ml is preferable, with a 100% specificity and sensitivity of 47%. Optimal cutoff value of 180ng/ml gives specificity of 89.5% and sensitivity of 64%.
Our findings do not support the use of OPN for disease monitoring. We found no correlation between OPN and CRP levels in sarcoidosis patients, despite both being elevated relative to levels found in the control group. CRP is generally considered a marker of disease activity in inflammatory diseases and OPN clearly does not simply reflect the information provided by CRP. Further studies using serial measurements pre and post treatment may clarify whether there is any correlation between OPN and disease activity.
We chose to study four of OPN's SNPs based on previously published studies that found association between these specific SNPs and different autoimmune and Th1 related diseases, such as Systemic Lupus erythematosus, type I Diabetes Mellitus and rheumatoid arthritis, or Behcet`s Disease and Vogt-Koyanagi-Harada Disease, which are also characterized by aberrant Th1 and Th17 response. The alleles distribution of rs9138, rs7687316, together with allelesrs1126616T and rs1126772A have all been associated with SLE [34,35]. A significantly increased frequency of the OPN rs4754 TT genotype was observed in VKH patients compared with healthy controls [37]. In the other hand, SNPs rs1126616, rs1126772 and rs9138 were found to be related to asthma diagnosis and clinical parameters in Puerto Rican population [36], with asthma being a Th2 disease model.
As regards studies in sarcoidosis, in the Slovenian population rs4754 was found to have different genotype frequency in sarcoidosis patients, with CC being overrepresented. This was found significant, but not after correction for multiple testing. Haplotype TT for rs11730582-C/T, rs11728697-C/T and rs4754-C/T were significantly decreased and therefore considered protective [40]. In contrast to these studies, studies in patients with Behçet's disease and with type 1 diabetes showed elevated OPN levels, but failed to show connection to SNP rs1126772 [49,50]. This is similar to what we found in our study.
The failure to identify correlation between genotype and the presence of sarcoidosis in our cohort was surprising and somewhat disappointing. Although unlikely, this may be due to a type II error related to the size of our patient cohort. Another possible explanation is that we did not check for haplotypes, but for isolated SNPs, and the only correlation previously found between the genotype of OPN and the presence of sarcoidosis, after correction for multiple testing, was for the whole haplotype. The lack of correlation could also be reflecting perhaps another, non-genetic, epigenetic or other mechanism in which OPN contributes to Th1 related diseases, in a way similar to previous studies in Behçet's disease and type-1 diabetes. Lastly, correlation studies regarding SNP's are invariably population related and therefore may be different in our cohort of Israeli patients, especially bearing in mind that our patients have heterogeneous ethnic origins.
Some clinical parameters were found to correlate with the frequency of SNP's we have examined, with the presence of constitutional symptoms showing the strongest correlation. These findings could be explained in numerous ways. They could be reflecting a subset of sarcoidosis with genetic predisposition which is affected by the OPN genotype and which manifests with a prominent systemic reaction rather than as disease limited to the chest. In patients presenting with fever or weight loss, all four SNPs examined were found to have the same distribution, suggesting a similar haplotype for this presentation.
From our study it is not clear whether OPN is involved in the pathogenesis of sarcoidosis or merely a marker of the disease. If OPN is found to be relevant to the pathogenesis, it may serve as a target for future therapy. There is increasing interest in OPN and its ligands as a therapeutic target in multiple sclerosis [51], and in various cancers [52].
Conclusion
In conclusion, our study demonstrates that there is an association between the protein osteopontin and sarcoidosis. Plasma osteopontin may be a suitable marker for the presence of sarcoidosis, but control groups with other lung diseases need to be studied. Surprisingly, no genetic association was found between osteopontin and the presence of sarcoidosis, however, a genetic association was found with some clinical subtypes of disease.
Supporting information
(XLSX)
Data Availability
All underlying data are available within the manuscript and supporting information file.
Funding Statement
The authors received no specific funding for this work.
References
- 1.Valeyre D, Prasse A, Nunes H, Uzunhan Y, Brillet PY, Müller-Quernheim J. Sarcoidosis. Lancet. 2014; 383:1155–67. 10.1016/S0140-6736(13)60680-7 [DOI] [PubMed] [Google Scholar]
- 2.Yigla M, Badarna-Abu-Ria N, Goralnik L, Rubin AH, Weiler-Ravell D. Sarcoidosis in residents of northern Israel of Arabic and Jewish origin: a comparative study. Respirology. 2006; 11: 586–91. 10.1111/j.1440-1843.2006.00891.x [DOI] [PubMed] [Google Scholar]
- 3.Rossman MD, Thompson B, Frederick M, Maliarik M, Iannuzzi MC, Rybicki BA et al. HLA-DRB1*1101: a significant risk factor for sarcoidosis in blacks and whites. Am. J Hum. Genet. 2003; 73: 720–735. 10.1086/378097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nowack C, Goebel KM. Genetic aspects of sarcoidosis: class II histocompatibility antigens and a family study. Arch. Intern. Med. 1987; 147: 481–483. [DOI] [PubMed] [Google Scholar]
- 5.Grunewald J, Eklund A, Olerup O. Human Leukocyte Antigen Class I Alleles and the Disease Course in Sarcoidosis Patients. Am J Respir Crit Care Med 2004; 169(6):696–702. 10.1164/rccm.200303-459OC [DOI] [PubMed] [Google Scholar]
- 6.Valentonyte R, Hampe J, Huse K, Rosenstiel P, Albrecht M, Stenzel A et al. Sarcoidosis is associated with a truncating splice site mutation in BTNL2. Nat. Genet. 2005; 37: 357–64. 10.1038/ng1519 [DOI] [PubMed] [Google Scholar]
- 7.Hofmann S, Franke A, Fischer A, Jacobs G, Nothnagel M, Gaede KI et al. Genome-wide association study identifies ANXA11 as a new susceptibility locus for sarcoidosis. Nat. Genet. 2008; 40: 1103–6. 10.1038/ng.198 [DOI] [PubMed] [Google Scholar]
- 8.Dubaniewicz A, Jamieson SE, Dubaniewicz-Wybieralska M, Fakiola M, Miller EN, Blackwell J M. Association between SLC11A1 (formerly NRAMP1) and the risk of sarcoidosis in Poland. Europ. J. Hum. Genet. 2005; 13: 829–834. 10.1038/sj.ejhg.5201370 [DOI] [PubMed] [Google Scholar]
- 9.Akahoshi M, Ishihara M, Remus N, Uno K, Miyake K, Hirota T et al. Association between IFNA genotype and the risk of sarcoidosis. Hum. Genet. 2004; 114: 503–509. 10.1007/s00439-004-1099-5 [DOI] [PubMed] [Google Scholar]
- 10.Feng Y, Zhou J, Gu C, Ding Y, Wan H, Ni L et al. Association of six well-characterized polymorphisms in TNF-α and TNF-β genes with sarcoidosis: a meta-analysis. PLoS. One. 2013; 8: e80150 10.1371/journal.pone.0080150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fløe A, Hoffmann HJ, Nissen PH, Møller HJ, Hilberg O. Genotyping increases the yield of angiotensin-converting enzyme in sarcoidosis—a systematic review. Dan. Med. J. 2014; 61: A4815 [PubMed] [Google Scholar]
- 12.Schürmann M. Angiotensin-converting enzyme gene polymorphisms in patients with pulmonary sarcoidosis: impact on disease severity. Am. J. Pharmacogenomics. 2003;3: 233–43. [DOI] [PubMed] [Google Scholar]
- 13.Kanazawa N, Okafuji I, Kambe N, Nishikomori R, Nakata-Hizume M, Nagai S et al. Early-onset sarcoidosis and CARD15 mutations with constitutive nuclear factor-kappaB activation: common genetic etiology with Blau syndrome. Blood 2005; 105: 1195–7. 10.1182/blood-2004-07-2972 [DOI] [PubMed] [Google Scholar]
- 14.Spagnolo P, Sato H, Marshall SE, Antoniou KM, Ahmad T, Wells AU et al. Association between heat shock protein 70/Hom genetic polymorphisms and uveitis in patients with sarcoidosis. Invest. Ophthalmol. Vis. Sci. 2007; 48: 3019–25. 10.1167/iovs.06-1485 [DOI] [PubMed] [Google Scholar]
- 15.Typiak M, Rębała K, Dudziak M, Słomiński JM, Dubaniewicz A. Polymorphism of FCGR2A, FCGR2C, and FCGR3B Genes in the Pathogenesis of Sarcoidosis. Adv Exp Med Biol 2016; 905:57–68. 10.1007/5584_2015_193 [DOI] [PubMed] [Google Scholar]
- 16.Fridlender ZG, Schwartz A, Kohan M, Amir G, Glazer M, Berkman N. Association between CD14 gene polymorphisms and disease phenotype in sarcoidosis: Respir. Med. 2010; 104:1336–43. 10.1016/j.rmed.2010.03.029 [DOI] [PubMed] [Google Scholar]
- 17.Ogbureke KU, Fisher LW. SIBLING expression patterns in duct epithelia reflect the degree of metabolic activity. J. Histochem. Cytochem. 2007; 55: 403–409. 10.1369/jhc.6A7075.2007 [DOI] [PubMed] [Google Scholar]
- 18.Denhardt DT, Noda M, O'Regan AW, Pavlin D, Berman JS. Osteopontin as a means to cope with environmental insults: regulation of inflammation, tissue remodeling, and cell survival.J. Clin. Invest. 2001; 107: 1055–61. 10.1172/JCI12980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang KX, Denhardt DT. Osteopontin: role in immune regulation and stress responses. Cytokine Growth Factor Rev. 2008; 19: 333–45. 10.1016/j.cytogfr.2008.08.001 [DOI] [PubMed] [Google Scholar]
- 20.Weber GF, Ashkar S, Glimcher MJ, Cantor H. Receptor-ligand interaction between CD44 and osteopontin (Eta-1).Science 1996; 271: 509–12. [DOI] [PubMed] [Google Scholar]
- 21.Yamamoto N, Sakai F, Kon S, Morimoto J, Kimura C, Yamazaki H et al. Essential role of the cryptic epitope SLAYGLR within osteopontin in a murine model of rheumatoid arthritis. J. Clin. Invest. 2003; 112: 181–188. 10.1172/JCI17778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ashkar S, Weber GF, Panoutsakopoulou V, Sanchirico ME, Jansson M, Zawaideh S et al. Eta-1 (osteopontin): an early component of type-1 (cell-mediated) immunity. Science 2000; 287: 860–864. [DOI] [PubMed] [Google Scholar]
- 23.Nau GJ, Guilfoile P, Chupp GL, Berman JS, Kim SJ, Kornfeld H et al. A chemoattractant cytokine associated with granulomas in tuberculosis and silicosis. Proc. Natl. Acad. Sci. U S A 1997; 94: 6414–6419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Carlson I, Tognazzi K, Manseau EJ, Dvorak HF, Brown LF. Osteopontin is strongly expressed by histiocytes in granulomas of diverse etiology. Lab. Invest. 1997; 77: 103–108. [PubMed] [Google Scholar]
- 25.Sato T, Nakai T, Tamura N, Okamoto S, Matsuoka K, Sakuraba A et al. Osteopontin/Eta-1 upregulated in Crohn's disease regulates the Th1 immune response. Gut 2005; 54: 1254–1262. 10.1136/gut.2004.048298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mishima R, Takeshima F, Sawai T, Ohba K, Ohnita K, Isomoto H et al. High plasma osteopontin levels in patients with inflammatory bowel disease. J. Clin. Gastroenterol. 2007; 41:167–172. 10.1097/MCG.0b013e31802d6268 [DOI] [PubMed] [Google Scholar]
- 27.Wong CK, Lit LC, Tam LS, Li EK, Lam CW. Elevation of plasma osteopontin concentration is correlated with disease activity in patients with systemic lupus erythematosus. Rheumatology (Oxford) 2005; 44: 602–606. [DOI] [PubMed] [Google Scholar]
- 28.Vogt MH, Lopatinskaya L, Smits M, Polman CH, Nagelkerken L. Elevated osteopontin levels in active relapsing-remitting multiple sclerosis. Ann. Neurol. 2003; 53: 819–822. 10.1002/ana.10606 [DOI] [PubMed] [Google Scholar]
- 29.Comabella M, Pericot I, Goertsches R, Nos C, Castillo M, Blas Navarro J et al. Plasma osteopontin levels in multiple sclerosis. J. Neuroimmunol. 2005; 158: 231–239. 10.1016/j.jneuroim.2004.09.004 [DOI] [PubMed] [Google Scholar]
- 30.Petrow PK, Hummel KM, Schedel J, Franz JK, Klein CL, Müller-Ladner U et al. Expression of osteopontin messenger RNA and protein in rheumatoid arthritis: effects of osteopontin on the release of collagenase 1 from articular chondrocytes and synovial fibroblasts. Arthritis. Rheum. 2000; 43: 1597–1605 [DOI] [PubMed] [Google Scholar]
- 31.Su R, Nguyen ML, Agarwal MR, Kirby C, Nguyen CP, Ramstein J et al. Interferon-inducible chemokines reflect severity and progression in sarcoidosis. Respir. Res. 2013; 14:121 10.1186/1465-9921-14-121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Maeda K, Takahashi K, Takahashi F, Tamura N, Maeda M, Kon S et al. Distinct roles of osteopontin fragments in the development of the pulmonary involvement in sarcoidosis. Lung 2001; 179: 279–291. 10.1007/s004080000068 [DOI] [PubMed] [Google Scholar]
- 33.Crosby AH, Edwards SJ, Murray JC, Dixon MJ. Genomic organization of the human osteopontin gene: exclusion of the locus from a causative role in the pathogenesis of dentinogenesis imperfecta type II. Genomics 1995; 27: 155–160. 10.1006/geno.1995.1018 [DOI] [PubMed] [Google Scholar]
- 34.D'Alfonso S, Barizzone N, Giordano M, Chiocchetti A, Magnani C, Castelli L et al. Two single-nucleotide polymorphisms in the 5' and 3' ends of the osteopontin gene contribute to susceptibility to systemic lupus erythematosus. Arthritis. Rheum. 2005; 52: 539–547. 10.1002/art.20808 [DOI] [PubMed] [Google Scholar]
- 35.Kariuki SN, Moore JG, Kirou KA, Crow MK, Utset TO, Niewold TB. Age- and gender-specific modulation of serum osteopontin and interferon-alpha by osteopontin genotype in systemic lupus erythematosus. Genes Immun. 2009; 10: 487–94. 10.1038/gene.2009.15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Arjomandi M, Galanter JM, Choudhry S, Eng C, Hu D, Beckman K et al. Polymorphism in Osteopontin Gene (SPP1) Is Associated with Asthma and Related Phenotypes in a Puerto Rican Population. Pediatr. Allergy Immunol. Pulmonol. 2011; 24: 207–214. 10.1089/ped.2011.0095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chu M, Yang P, Hu R, Hou S, Li F, Chen Y, Kijlstra A. Elevated serum osteopontin levels and genetic polymorphisms of osteopontin are associated with Vogt-Koyanagi-Harada disease. Invest. Ophthalmol. Vis. Sci. 2011; 52:7084–7089. [DOI] [PubMed] [Google Scholar]
- 38.Chiocchetti A, Comi C, Indelicato M, Castelli L, Mesturini R, Bensi T et al. Osteopontin gene haplotypes correlate with multiple sclerosis development and progression. J. Neuroimmunol. 2005; 163: 172–178. 10.1016/j.jneuroim.2005.02.020 [DOI] [PubMed] [Google Scholar]
- 39.Glas J, Seiderer J, Bayrle C, Wetzke M, Fries C, Tillack C et al. The role of osteopontin (OPN/SPP1) haplotypes in the susceptibility to Crohn's disease. PloS. one 2011; 6: e29309 10.1371/journal.pone.0029309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Maver A, Medica I, Salobir B, Tercelj M, Peterlin B. Genetic variation in osteopontin gene is associated with susceptibility to sarcoidosis in Slovenian population. Dis. Markers 2009; 27: 295–302. 10.3233/DMA-2009-0675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kochl S, Niederstatter H, Parson W. DNA extraction and quantitation of forensic samples using the phenol-chloroform method and real-time PCR. Methods in molecular biology (Clifton, NJ) 2005; 297:13–30. [DOI] [PubMed] [Google Scholar]
- 42.Miller SA, Dykes DD, Polesky HF. A simple salting out procedure for extracting DNA from human nucleated cells. Nucleic acids research 1988; 16: 1215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Life Technologies Corporation. TaqMan® SNP Genotyping Assays TaqMan® Predesigned SNP Genotyping Assays, TaqMan® Custom SNP Genotyping Assays, and TaqMan® Drug Metabolism Enzyme Genotyping Assays Catalog Number 4351379, 4351384, 4351376, 4351382, 4351374, 4351380, 4362691, 4331349, 4332077, 4332072, 4332075, 4332073, and 4332076 Publication Number MAN0009593 Revision A.0, 2014 https://tools.lifetechnologies.com/content/sfs/manuals/TaqMan_SNP_Genotyping_Assays_man.pdf accessed 17/3/2015
- 44.Chabas D, Baranzini SE, Mitchell D, Bernard CC, Rittling SR, Denhardt DT et al. The influence of the proinflammatory cytokine, osteopontin, on autoimmune demyelinating disease. Science 2001; 294: 1731–1735. 10.1126/science.1062960 [DOI] [PubMed] [Google Scholar]
- 45.Diao H, Kon S, Iwabuchi K, Kimura C, Morimoto J, Ito D et al. Osteopontin as a mediator of NKT cell function in T cell-mediated liver diseases. Immunity 2004; 21: 539–550. 10.1016/j.immuni.2004.08.012 [DOI] [PubMed] [Google Scholar]
- 46.Pardo A, Gibson K, Cisneros J, Richards TJ, Yang Y, Becerril C et al. Up-regulation and profibrotic role of osteopontin in human idiopathic pulmonary fibrosis. PLoS medicine 2005; 2: e251 10.1371/journal.pmed.0020251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bons JA, Drent M, Bouwman FG, Mariman EC, van Dieijen-Visser MP, Wodzig WK. Potential biomarkers for diagnosis of sarcoidosis using proteomics in serum. Respir. Med. 2007; 101: 1687–1695. 10.1016/j.rmed.2007.03.002 [DOI] [PubMed] [Google Scholar]
- 48.Bargagli E, Bennett D, Maggiorelli C, Di Sipio P, Margollicci M, Bianchi N et al. Human chitotriosidase: a sensitive biomarker of sarcoidosis. J. Clin. Immunol. 2013; 33: 264–270. 10.1007/s10875-012-9754-4 [DOI] [PubMed] [Google Scholar]
- 49.Chu M, Yang P, Hou S, Li F, Chen Y, Kijlstra A. Behcet's disease exhibits an increased osteopontin serum level in active stage but no association with osteopontin and its receptor gene polymorphisms. Hum. Immunol. 2011; 6: 525–529. [DOI] [PubMed] [Google Scholar]
- 50.Karamizadeh Z, Kamali Sarvestani E, Saki F, Karamifar H, Amirhakimi GH, Namavar Shooshtarian MH et al. Investigation of osteopontin levels and genomic variation of osteopontin and its receptors in Type 1 diabetes mellitus. J. Endocrinol. Invest. 2013; 36: 1090–1093. 10.3275/9098 [DOI] [PubMed] [Google Scholar]
- 51.Steinman L. A molecular trio in relapse and remission in multiple sclerosis. Nat. Rev. Immunol. 2009; 9: 440–447. 10.1038/nri2548 [DOI] [PubMed] [Google Scholar]
- 52.Bandopadhyay M, Bulbule A, Butti R, Chakraborty G, Ghorpade P, Ghosh P et al. Osteopontin as a therapeutic target for cancer. Expert. Opin. Ther. Targets. 2014; 18: 883–895. 10.1517/14728222.2014.925447 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(XLSX)
Data Availability Statement
All underlying data are available within the manuscript and supporting information file.