Abstract
Background
Accurate clinical staging of mediastinal lymph nodes of patients with lung cancer is important in determining therapeutic options and prognoses. We aimed to compare the diagnostic performance of diffusion-weighted magnetic resonance imaging (DWI) and 18F-fluorodeoxyglucose positron emission tomography/computed tomography (18F-FDG PET/CT) in detecting mediastinal nodal metastasis of lung cancer.
Methods
Relevant studies were systematically searched in the MEDLINE, EMBASE, PUBMED, and Cochrane Library databases. Based on extracted data, the pooled sensitivity, specificity, positive and negative likelihood ratios (PLR and NLR) with individual 95% confidence intervals were calculated. In addition, the publication bias was assessed by Deek’s funnel plot of the asymmetry test. The potential heterogeneity was explored by threshold effect analysis and subgroup analyses.
Results
Forty-three studies were finally included. For PET/CT, the pooled sensitivity and specificity were 0.65 (0.63–0.67) and 0.93 (0.93–0.94), respectively. The corresponding values of DWI were 0.72 (0.68–0.76) and 0.97 (0.96–0.98), respectively. The overall PLR and NLR of DWI were 13.15 (5.98–28.89) and 0.32 (0.27–0.39), respectively. For PET/CT, the corresponding values were 8.46 (6.54–10.96) and 0.38 (0.33–0.45), respectively. The Deek’s test revealed no significant publication bias. Study design and patient enrollment were potential causes for the heterogeneity of DWI studies and the threshold was a potential source for PET/CT studies.
Conclusion
Both modalities are beneficial in detecting lymph nodes metastases in lung cancer without significant differences between them. DWI might be an alternative modality for evaluating nodal status of NSCLC.
Introduction
Lung cancer is the leading cause of all cancer-related deaths worldwide [1]. Non-small-cell cancer (NSCLC) is the main type of lung cancer, accounting for 80% of all cases. NSCLC typically metastasizes to the hilar and mediastinal lymph nodes (MLNs), and metastasis is a very important prognostic factor. The 5-year survival rates are 54.0% for patients without any metastases and 26.5% for subjects with MLNs metastases [2]. The selected treatment, such as surgery, radiotherapy and chemotherapy, is mainly dependent on the TNM staging. Therefore, accurate assessment of MLNs is necessary for TNM staging and optimal treatment selection.
Various diagnostic techniques, such as computed tomography (CT), positron emission tomography (PET), PET/CT, mediastinoscopy, and magnetic resonance imaging (MRI), are used for nodal staging assessment of NSCLC. CT is most widely used to assess the nodal status of lung cancer based on lymph node size, although lymph node size is not reliable for the evaluation of metastatic involvement [3]. FDG PET, a functional imaging modality, could detect potential tumor activity and facilitate earlier recognition of metastases [4]; however, this method has been limited by the low spatial resolution of stand-alone PET images [5]. Integrated PET/CT, which combines the anatomical detail and functional statue, is now commonly used for NSCLC staging.
Diffusion weighted imaging (DWI), an MRI technique, could detect the restricted diffusion of water molecules among tissues at the cellular level, which could be measured by apparent diffusion coefficient (ADC) value [5]. DWI and ADC values have been widely used in brain imaging for the evaluation of acute ischemic stroke, intracranial tumors and demyelinating disease [6]. However, DWI is highly sensitive to motion artifacts caused by breathing and movement of the heart and aorta, resulting in its limited application [7]. Recently, the rapid development of MRI techniques, such as echo-planar imaging sequence, multichannel coils and parallel imaging, has allowed for the application of DWI in anatomical regions prone to motion artifacts, such as the mediastinum [8]. Several studies have shown that diagnostic accuracy of DWI for nodal assessment in the mediastinum is 76–95% [9–13].
To our knowledge, the performance of DWI and FDG PET/CT in nodal staging has yet to be determined. Some studies validated the potential of DWI for N stage assessment and the characterization of mediastinal lymph nodes in patients with NSCLC with a capability similar to that of 18F-FDG PET/CT [14]. Some studies showed advantages of DWI over FDG PET/CT [4, 5], whereas other studies showed that DWI had lower capability than FDG PET/CT [8, 11]. Therefore, we performed a meta-analysis to compare the diagnostic performance of DWI and FDG PET/CT in lymph node staging in patients with NSCLC.
Materials and methods
Search strategy
An extensive search of the available literature, published from January 2001 to December 2014, was performed in the MEDLINE, EMBASE, PUBMED and Cochrane Library databases. The combination of keywords was as follows: (‘DW-MRI’ OR ‘diffusion-weighted magnetic resonance imaging’) AND (‘FDG’ OR ‘18F-FDG’ OR ‘FDG-F18’ OR ‘fluorodeoxyglucose’ OR ‘PET/CT’ OR ‘positron emission tomography/computed tomography’ OR ‘PET-CT’ OR ‘positron emission tomography-computed tomography’) AND (‘lung cancer’ OR ‘lung neoplasm’) AND (‘lymph node metastasis’ OR ‘lymphatic metastasis’) AND (‘specificity’ OR ‘sensitivity’ OR ‘false-positive’ OR ‘false-negative’ OR ‘detection’ OR ‘diagnosis’ OR ‘accuracy’).
Inclusion and exclusion criteria
The inclusion criteria were as follows: (i) the diagnostic performances of 18F-FDG PET/CT or DWI in detecting nodal metastases in lung cancer were identified in the literature; (ii) pathological analysis, surgical biopsy, mediastinoscopy or follow-up results were used as the gold standard of diagnosis; (iii) the values of true positive (TN), false positive (FP), false negative (FN) and true negative (TN) depending on the original data could be obtained in the literature; (iv) the studies were based on a per-lesion analysis; and (v) the article with the most details or the most recent article was selected when similar data appeared in more than one article.
The exclusion criteria were as follows: (i) studies that focused on the therapy response or prognosis rather than on disease diagnoses; (ii) studies regarding mediastinal tumor or pleural diseases except for lung cancer; (iii) case reports, meeting abstracts, reviews, letters, comments, animal experiments, or the studies with less than 10 samples.
Data extraction
The following information was extracted from the included studies: the first author, year of publication, study design (prospective or retrospective), country of the study, patient enrollment, technique characteristics, reference standard, and blinding method. The TP, FP, TN, and FN results were also extracted.
Two reviewers independently extracted the relevant data from each study. Any disagreements were resolved by discussion with a third reviewer.
Statistical analysis
For lesion-based analyses, we obtained the pooled sensitivities and specificities of PET/CT and DWI, as well as their 95% confidence intervals using the weighted average method. We also calculated the pooled positive and negative likelihood ratios (PLR and NLR) with their 95% confidence intervals. The data were finally summarized in receiver-operating characteristic curves (SROC), with the area under the curve (AUC) and the Q* index obtained.
We used the I2 index for heterogeneity assessment. If the I2 index was higher than 50%, a random effect model was used; otherwise, a fixed model was used. In this study, we used the random-effect model to pool estimates. To explore the sources of heterogeneity, we performed subgroup analyses based on factors such as sample size (≥ 250 vs. <250), study design (retrospective vs. prospective), country (Asia vs. non- Asia), subject enrollment (consecutive vs. nonconsecutive), and analysis method (qualitative, quantitative, or both). The threshold effect analysis was also performed, and the publication bias was examined by Deek’s funnel plot.
The statistical computations were performed using Stata software version 12.0 (StataCorp LP, Texas, USA) and MetaDisc version 1.4 (Unit of Clinical Biostatistics, Ramóny Cajal Hospital, Madrid, Spain). For P value, the level of statistical significance was set to 5%.
Results
Study selection and description
A total of 174 articles were screened in the primary literature search, and 43 articles (in total 48 studies, 10 studies for DWI and 38 studies for 18F-FDG PET/CT) were included based on the inclusion and exclusion criteria. A flowchart depicting the study selection is shown in Fig 1.
Fig 1. Flow chart of studies identified and included in the present meta-analysis.
The principal characteristics of the 43 selected articles [5, 9, 10, 12, 15–53] involving a total of 21,058 lymph nodes are listed in Table 1. Of these articles, 27 [15–18, 20–22, 24, 27–29, 31–35, 37, 41–43, 46, 47, 49–53] were retrospective, and 16 [5, 9, 10, 12, 19, 23, 25, 26, 30, 36, 38–40, 44, 45, 48] were prospective. Patients in 26 [5, 9, 10, 12, 15–20, 22, 23, 25, 26, 28, 29, 31, 32, 36, 38–40, 43, 44, 46, 47] articles were enrolled in a consecutive manner while the other 17 [21, 24, 27, 30, 33–35, 37, 41, 42, 45, 48–53] articles did not. In 29 articles [5, 9, 10, 12, 16–20, 22, 23, 25–28, 32, 35–38, 40, 44–50, 52], the DWI or 18F-FDG PET/CT reviewers were blinded to the histologic findings and clinical data; the remaining 14 articles [15, 21, 24, 29–31, 33, 34, 39, 41–43, 51, 53] did not specify whether the reviewers were blinded. Thirty-three articles [5, 9, 10, 12, 16, 21–26, 28–41, 43, 47–53] enrolled Asian patients. The majority of DWI studies were conducted under a magnetic field strength of 1.5 T, and the majority of PET scanning studies used an integrated PET/CT technique. The high variability regarding principal characteristics was observed between included studies.
Table 1. The principal characteristics of included studies.
| First author/year | Study design | Country | Consecutive | Mean age | No. of patients and lesions | Blind | Technique characteristics | TP | FP | FN | TN | Reference standard | Analysis method |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| DWI | |||||||||||||
| Zhang/2013 | R | China | ND | 59 | 25/78 | Y | 3.0 T SE-EPI (0,800) | 29 | 13 | 6 | 30 | HP | QN |
| He/2011 | R | China | ND | 58 | 12/56 | ND | 1.5T ASSET/STIR/SE-EPI (0,500) | 18 | 4 | 16 | 18 | HP | QN |
| Usuda/2011 | P | Japan | C | 68 | 63/319 | Y | 1.5 T SS-EPI (0,800) | 33 | 3 | 11 | 272 | HP | QN |
| Zeng/2012 | R | China | ND | 58 | 45/68 | Y | 1.5 T SE-EPI (600,800,1000) | 23 | 3 | 9 | 33 | HP | QN |
| Ohno/2011 | P | Japan | C | 73 | 250/270 | Y | 1.5 T STIR-EPI (0,1000) | 101 | 17 | 34 | 118 | HP | QN |
| Nakayama/2010 | R | Japan | ND | 68 | 70/56 | Y | 1.5 T SS-SE-EPI (50,1000) | 19 | 5 | 4 | 28 | HP | QN |
| Nomori /2008 | P | Japan | C | 70 | 88/734 | Y | 1.5 T SE-EPI (0,1000) | 24 | 5 | 12 | 693 | HP | QN |
| Xu/2014 | P | China | C | 55 | 42/119 | Y | 1.5 T SS-SE-EPI (0,1000) | 29 | 7 | 6 | 77 | HP | QN |
| Usuda/2013 | P | Japan | C | 68 | 158/705 | Y | 1.5 T SS-EPI (0,800) | 39 | 5 | 22 | 639 | HP | QN |
| Kim/2012 | P | Korea | C | 62 | 49/206 | Y | 1.5 T SS-EPI (0,100,700) | 26 | 6 | 13 | 161 | HP | QN |
| PET/CT | |||||||||||||
| Al-Sarraf, Nael/2008 | R | Ireland | C | 64.5 | 206/1145 | ND | PET-CT (Discovery ST, GE Medical systems).370MBq | 75 | 27 | 93 | 950 | HP | QN |
| An, Y. S/2008 | R | South Korea | C | 63 | 124/396 | Y | PET-CT (Discovery ST Scanner, GE Healthcare, Milwaukee, WI, USA) 370MBq | 62 | 87 | 19 | 228 | HP | QN |
| Billé, Andrea/2009 | R | Italy | C | 67 | 159/1001 | Y | PET/CT scanner (Discovery ST; GE Medical systems) 4.5–5.5 MBq/kg | 41 | 14 | 30 | 916 | HP | QL |
| Booth, K./2013 | R | England | C | 65 | 64/200 | Y | GE Discovery LS fusion PET/CT scanner 375 MBq | 7 | 8 | 11 | 174 | HP | QN/QL/ND |
| Bryant, Ayesha S/2006 | P | England | C | 67 | 143/1252 | Y | PET-CT scanner (GE Discovery LS, Milwaukee, WI). 555 MBq | 120 | 67 | 34 | 1031 | HP | QN |
| Hellwig, Dirk/2015 | R | Germany | C | 62 | 80/311 | Y | ECAT ART scanner (Siemens Medical Solutions), 250±2 MBq | 62 | 39 | 8 | 202 | HP | QL |
| Hu, M/2008 | R | China | ND | 50 | 46/584 | ND | PET-CT scanner 7.4 MBq/kg | 117 | 72 | 17 | 378 | HP | QN |
| Jeon, Tae Yeon/2010 | R | Korea | C | 65 | 168/617 | Y | PET/CT device (Discovery LS, GE Healthcare) 370MBq | 30 | 10 | 30 | 547 | HP | QL |
| Kim, Byung-Tae/2006 | P | Korea | C | 59 | 150/568 | Y | PET/CT device (Discovery LS, GE Medical Systems) 370MBq | 23 | 0 | 32 | 513 | HP | QL |
| Kim, D. W./2012 | R | Korea | ND | 68.4 | 69/268 | ND | PET/CT (Biograph Sensation 16, Siemens Medical Systems) 4.0 MBq/kg | 157 | 8 | 52 | 51 | HP+CFU | QN |
| Kim, Yoon Kyung/2007 | P | Korea | C | 61 | 674/2477 | Y | PET/CT device (Discovery LS, GE Healthcare, Milwaukee, WI) 370 MBq | 126 | 48 | 149 | 2154 | HP | QL |
| Kim, Y. N./2012 | P | Korea | C | 62 | 49/206 | Y | PET/CT device (Discovery STE, GE Healthcare, Milwaukee, WI, USA) 370 MBq | 18 | 6 | 21 | 161 | HP | QL |
| Koksal, Deniz/2013 | R | Turkey | ND | 59.8 | 81/334 | Y | PET/CT scanner (Siemens, Biograph-6- True Point) 145 μCi/kg | 14 | 86 | 8 | 226 | HP | QL |
| Kuo, W. H./2012 | R | Taiwan | C | 63.1 | 102/118 | Y | PET/CT scanner Discovery ST16 scanner (GE Medical Systems, Milwaukee, WI), 370 to 555 MBq | 12 | 25 | 9 | 72 | HP | QL |
| Lee, A. Y./2014 | R | Korea | C | 64.5 | 104/372 | ND | PET/CT scanner (Discovery STE, GE Healthcare, Milwaukee, WI, USA), 370 MBq | 23 | 31 | 26 | 292 | HP | QN |
| Lee, Jeong Won/2009 | P | Korea | ND | 60.7 | 182/778 | ND | a Gemini PET/CT system (Philips, Milpitas). 5.18 MBq/kg | 40 | 109 | 13 | 616 | HP | QL |
| Lee, S. M./2012 | R | Korea | C | 60.0 | 160/756 | ND | Gemini PET/CT (Philips Medical Systems, Cleveland, OH, USA) 5.2 MBq/kg | 2 | 43 | 13 | 698 | HP | QN |
| Li, Meng/2012 | R | China | C | 58 | 80/265 | Y | PET—CT device (GE Discovery ST 16), 3.70–4.44 MBq/kg | 33 | 7 | 18 | 207 | HP | QN |
| Li, Xiaolin/2011 | R | China | ND | 60 | 200/1132 | ND | PET/CT scanner (GE Discovery LS, ST, or DST) 5.55–7.40 MBq/kg | 27 | 60 | 13 | 1032 | HP | QN |
| Lin, W. Y./2012 | R | Taiwan | ND | 66 | 83/364 | ND | PET-CT scanner (Discovery VCT; GE Healthcare,Waukesha, Wisconsin, USA), 370 MBq | 18 | 50 | 20 | 276 | HP | QN |
| Liu, Bao-jun/2009 | R | China | ND | 57.5 | 39/208 | Y | PET/CT scanner (Siemens Biograph Sensation 16, Siemens, Germany) 7.4MBq/kg | 40 | 24 | 26 | 120 | HP | QN/QL |
| Morikawa, Miwa/2009 | P | Japan | C | 66.1 | 93/137 | Y | PET/CT scanner (Discovery LS; GE Healthcare). 185 MBq | 74 | 19 | 8 | 36 | HP | QN |
| Nomori, H./2008 | P | Japan | C | 70 | 88/734 | ND | PET-CT device (Discovery ST; GE Medical Systems), 3.7 MBq/kg |
26 | 18 | 10 | 680 | HP | QN |
| Ohno, Y./2007 | P | Japan | C | 68 | 115/891 | ND | PET scanner (ALLEGRO; Philips)+ CT scanner, Aquilion 16 (Toshiba Medical Systems, Ohtawara, Japan), 4.44 MBq/kg | 60 | 31 | 13 | 787 | HP | QN |
| Shim, Sung Shine/2005 | P | Korea | C | 56 | 106/393 | Y | PET/CT device (Discovery LS; GE Medical Systems, Milwaukee, Wis), 370 MBq | 28 | 58 | 5 | 302 | HP | QL |
| Sit, Alva KY/2010 | R | China | ND | 61 | 107/249 | ND | PET/CT scanner, ND | 18 | 31 | 34 | 166 | HP | QN |
| Ohno, Y./2011 | P | Japan | C | 73 | 250/270 | Y | PET/CT scanner (Discovery ST; GE Healthcare, Milwaukee, Wis). 3.3 MBq/kg | 102 | 15 | 33 | 120 | HP | QN |
| Tasci, Erdal/2010 | R | Turkey | ND | 58.2 | 127/826 | ND | on a Biograph PET/CT (Siemens/CTI) scanner, 555MBq | 41 | 50 | 24 | 711 | HP | QL |
| Toba, H./2010 | R | Japan | C | 68.0 | 42/217 | ND | PET/CT scanner Aquiduo (Toshiba Medical Systems, Tokyo, Japan) | 17 | 15 | 4 | 181 | HP | QL |
| Tournoy, KG/2007 | P | Belgium | C | 68 | 52/105 | Y | FDG-PET/CT scanner (Philips Gemini FDG-PET/CT, Philips Medical Systems, Cleveland, Ohio, USA), 4 MBq/kg | 32 | 10 | 6 | 57 | HP | QN |
| Usuda, Katsuo/2013 | P | Japan | C | 68 | 158/705 | Y | PET-CT (SIEMENS Biography Sensation 16, Erlangenm Germany), 3.7 MBq/Kg | 24 | 3 | 37 | 641 | HP | QN |
| Ventura, Elisa/2010 | R | USA | C | 66.32 | 31/90 | Y | PET (CTI Molecular Imaging, Knoxville, TN, USA)+PET/CT Siemens Molecular Imaging, Knoxville, TN, USA), 555-740MBq | 38 | 20 | 3 | 29 | HP | QL |
| Xu, N/2014 | R | China | C | 61 | 101/528 | Y | PET/CT scanner, 4.5–5.5 MBq/kg | 52 | 18 | 49 | 409 | HP | QL |
| Usuda, Katsuo/2011 | P | Japan | C | 68 | 63/319 | Y | PET/CT scanner (Siemens Biography Sensation 16), 185 MBq | 21 | 9 | 23 | 266 | HP | QN |
| Yang, Wenfeng/2009 | P | China | ND | 69 | 122/639 | Y | PET/CT system (Discovery LS; GE Healthcare), 370 MBq | 132 | 73 | 21 | 413 | HP | QL |
| Yi, Chin A/2007 | R | Korea | N | 60 | 143/453 | Y | PET/CT device (Discovery LS, GE Healthcare), 370 MBq | 22 | 4 | 28 | 399 | HP | QN |
| Vansteenkiste, Johan F/1998 | P | Belgium | ND | 62 | 56/493 | Y | PET scanner (CTI-Siemens 931/08/12), 6.5 MBq/kg | 38 | 21 | 22 | 412 | HP | QL |
| Zhou,YF/2014 | R | China | ND | 60 | 64/280 | ND | PET/CT scanner (Philips Gemini TF 16), 2.96MBq/kg | 25 | 9 | 9 | 237 | HP | QN/QL |
ND: no documented; No.: number; TP: true positive; FP: false positive; FN: false negative; TN: true negative. P: prospective; R: retrospective; Y: yes; QL: qualitative analysis; QN: quantitative analysis; HP: histopathology; C: consecutive
Quality assessment
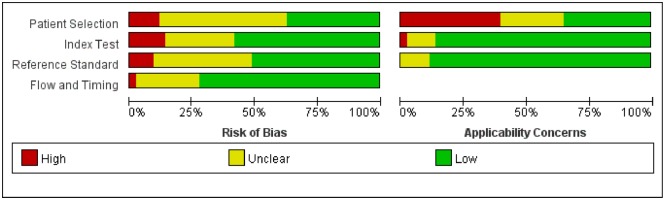
We used QUADAS-2 to analyze the quality of the studies [54]. The methodological results are displayed in Fig 2. Participant selection was judged to be at low risk of bias in 16 of the studies and at high or unclear risk of bias in the remaining 27 studies. The majority of selected studies did not provide information regarding consecutive enrollment and did not avoid a case-control design. These inclusion restrictions artificially narrowed the range of patients who would undergo PET/CT in standard practice, which gave rise to a high concern about the applicability of these studies. For the index test and reference standard, common weaknesses focused on the fact that a blinding method was not provided or used when interpreting the results. With regard to the flow and timing, 12 articles displayed unclear or high risk because they lacked an explicit description of the time interval between the index test and reference standard. In a word, a substantial amount of underreporting in the included studies resulted in “unclear” or “high” bias or concern, hampering the methodological quality.
Fig 2. Proportion of studies with low, high and unclear risks of bias and applicability concerns.
Review authors’ judgments about each domain presented as percentage across included studies.
Diagnostic accuracy of DWI and FDG-PET/CT
The pooled results are shown in Figs 3 and 4. Based on 10 studies, DWI had a sensitivity of 0.72 (0.68–0.76) and a specificity of 0.97 (0.96–0.98). In 33 studies, PET/CT achieved a sensitivity and specificity of 0.65 (0.63–0.67) and 0.93 (0.93–0.94), respectively. The LR syntheses gave an overall PLR of 13.15 (5.98–28.89) and NLR of 0.32 (0.27–0.39) for DWI. For 18F-FDG PET/CT, the overall PLR was 8.46 (6.54–10.96), and the NLR was 0.38 (0.33–0.45). The DOR was 46.11 (19.89–106.89) for DWI and 25.18 (18.58–34.13) for 18F-FDG PET/CT.
Fig 3. Forest plot of sensitivity and specificity for DWI.
Each solid circle represents sensitivity and specificity of individual studies, and the size of the circle indicates the study size. The diamond means the pooled sensitivity and specificity of all 10 studies.
Fig 4. Forest plot of sensitivity and specificity for PET/CT.
Each solid circle represents sensitivity and specificity of individual studies, and the size of the circle indicates the study size. The diamond means the pooled sensitivity and specificity of all 38 studies.
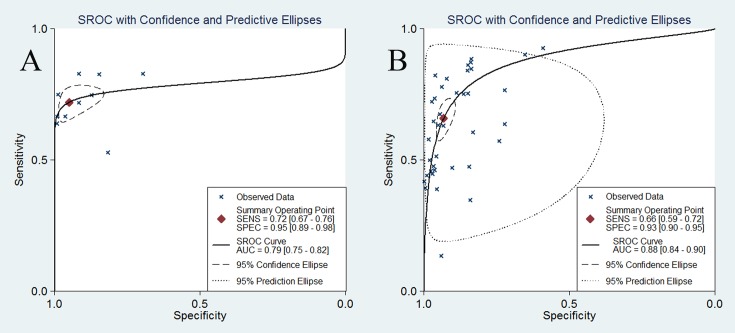
No differences were found between the pooled specificity, sensitivity, PLR and NLR between DWI and FDG-PET/CT (P > 0.05). Using a fitted SROC curve, the overall AUCs for DWI and FDG-PET/CT were 0.79 and 0.88, respectively (Fig 5). For nodal staging of NSCLC, the diagnostic capacities of these two modalities were not significantly different. However, based on the PLR and NLR, a positive finding of DWI can diagnose the malignancy while a negative DWI finding alone might not exclude the malignancy. With regard to PET/CT, it can neither rule in nor rule out the disease.
Fig 5. SROC curve of DWI (A) and 18F-FDG PET/CT (B) in detecting mediastinal nodal metastases in patients with NSCLC.
Each x represents individual study estimates. The diamond is the summary point representing the average sensitivity and specificity estimates. The ellipses around this summary point are the 95% confidence region (dashed line) and the 95% prediction region (dotted line).
Heterogeneity analysis
Our analysis revealed strong heterogeneity in sensitivity and specificity among the studies (P < 0.05, I2 > 90%). The Spearman rank correlation test indicated an absence of threshold effect in the DWI studies (coefficient = 0.364, P = 0.301) and showed a significant threshold effect in the PET/CT studies (coefficient = 0.556, P = 0.001). The threshold effect of PET/CT might arise from different cutoff values of SUV to differentiate malignant lesions from benign ones between included studies. Because of the small sample size of the DWI studies, we only performed subgroup analyses based on the sample size, study design and patient enrollment. Six studies using prospective design showed higher specificity (0.98 vs. 0.81, P < 0.05), and studies with consecutive enrollment showed higher specificity for nodal staging (0.98 vs. 0.81, P < 0.05). With regard to PET/CT studies, more factors including sample size, study design, country, patient enrollment, blinding method, and analysis method were explored in subgroup analyses; however, all these factors failed to explain the heterogeneity (P > 0.05). The results of the subgroup analyses are presented in Table 2. Deek’s funnel plot asymmetry tests indicated no significant publication bias (P = 0.277 for DWI and P = 0.098 for PET/CT) (Fig 6).
Table 2. The results of subgroup analysis for DWI and PET/CT.
| Factors | No.of studies | Sensitivity (95%CI) | Specificity (95%) |
|---|---|---|---|
| DWI | |||
| Sample size | |||
| < 250 | 6 | 0.73 (0.66–0.79) | 0.90 (0.87–0.93) |
| ≥ 250 | 4 | 0.71 (0.66–0.77) | 0.98 (0.98–0.99) |
| Study design* | |||
| Prospective | 6 | 0.72 (0.67–0.77) | 0.98 (0.97–0.98) |
| Retrospective | 4 | 0.72 (0.63–0.79) | 0.81 (0.74–0.88) |
| Consecutive enrollment* | |||
| Yes | 6 | 0.72 (0.67–0.77) | 0.98 (0.97–0.98) |
| No/Unclear | 4 | 0.72 (0.63–0.79) | 0.81 (0.74–0.88) |
| PET/CT | |||
| Sample size | |||
| < 250 | 9 | 0.68 (0.63–0.72) | 0.86 (0.84–0.88) |
| ≥ 250 | 29 | 0.64 (0.63–0.66) | 0.94 (0.93–0.94) |
| Study design | |||
| Prospective | 15 | 0.67 (0.64–0.69) | 0.94 (0.94–0.95) |
| Retrospective | 23 | 0.63 (0.61–0.66) | 0.92 (0.91–0.93) |
| Country | |||
| non-Asia | 10 | 0.66 (0.63–0.70) | 0.93 (0.92–0.94) |
| Asia | 28 | 0.64 (0.62–0.67) | 0.93 (0.93–0.94) |
| Consecutive enrollment | |||
| Yes | 26 | 0.64 (0.61–0.66) | 0.95 (0.94–0.95) |
| No/Unclear | 12 | 0.68 (0.65–0.71) | 0.90 (0.89–0.91) |
| Blind | |||
| Yes | 24 | 0.65 (0.62–0.67) | 0.93 (0.93–0.94) |
| No/Unclear | 14 | 0.65 (0.62–0.68) | 0.93 (0.92–0.93) |
| Analysis method | |||
| QN | 19 | 0.67 (0.65–0.69) | 0.93 (0.93–0.94) |
| QL | 16 | 0.62 (0.60–0.65) | 0.93 (0.92–0.94) |
| QN+QL | 3 | 0.61 (0.52–0.70) | 0.93 (0.90–0.95) |
ND: no document; No.: number; QN: quantitative; QL: qualitative.
*There is significant difference between these subgroups.
Fig 6. Funnel plot of publication bias for DWI (A) and 18F-FDG PET/CT (B).
Each circle represents individual study. The dashed line means the regression line.
Discussion
Because integrated PET/CT directly combines PET data on metabolic changes with highly detailed anatomic CT information, this technique could detect lesions earlier and provide more precise location information than CT or PET alone [55]. DWI is a magnetic resonance imaging (MRI) technique based on the imaging of the molecular mobility of water [56]. Using this technique, the diagnoses of prostate cancer [57], urinary bladder cancer [58], uterine cancer [59] and rectal cancer [60] have shown promising results. Recently, some people have demonstrated that DWI could be used for the detection of mediastinal nodal metastases in lung cancer, but the diagnostic value of DWI for lung cancer has not yet been defined. The majority of the relevant meta-analyses only analyzed the diagnostic performance of PET or/and PET/CT for N staging of NSCLC [2, 61, 62]. Considering the increasing numbers of reports using DWI and the unclear diagnostic value of the method, we pooled the diagnostic performance and compared it with the diagnostic performance of 18F-FDG PET/CT. Our results in the present meta-analysis showed that the pooled sensitivity and specificity of DWI were 0.70 and 0.97 for node-based data, and the corresponding values of PET/CT were 0.69 and 0.93, respectively; these results indicated that both 18F-FDG PET/CT and DWI were beneficial in detecting mediastinal lymph nodes metastases in lung cancer without significant statistical differences in diagnostic capacity. Furthermore, the diagnostic capacity (low sensitivity and high specificity) of both modalities suggested that positive lymph nodes would be missed too often so that using individuals alone cannot make accurate evaluation of nodal status to make decisions about treatment plan, especially for those patients with potentially resectable NSCLC. Instead both modalities can help guide the next step: either mediastinoscopy with minimally invasive sampling or directly surgery.
The SROC curve and its AUC presented the relationship between the sensitivity and specificity across studies and the overall estimation of test performance. The AUC for DWI (0.93, 95% CI: 0.91–0.95) was slightly higher than the AUC for 18F-FDG PET/CT (0.89, 95% CI: 0.86–0.91), indicating that DWI might be more accurate in N staging in patients with NSCLC. By combining the sensitivity and specificity into a single number, the DOR can be regarded as a single measurement of diagnostic accuracy, and higher values indicate better discriminatory test performance [63]. The DOR of DWI is greater than that of 18F-FDG PET/CT, indicating that DWI might be more accurate in assessing mediastinal lymph nodes of NSCLC. LRs, which are more clinically meaningful estimates, are commonly used to rule in and rule out disease. A good diagnostic test might have a PLR greater than 10 and a NLR less than 0.1 [48]. In our study, the PLR of DWI was 13.15 and NLR was 0.32, meaning that DWI could be only helpful to diagnose metastatic lymph nodes, not useful to exclude metastatic lesions. PET/CT could neither diagnose metastatic lesions nor rule out metastatic lesions with the PLR of 8.46 and NLR of 0.38.
The heterogeneity between studies was notable for both PET/CT and DWI. To investigate the sources of heterogeneity, diagnostic threshold analyses and subgroup analyses were performed. The spearman correlation coefficient (0.439, P = 0.011) suggests the existence of the threshold effect for PET/CT in our meta-analysis; one possible explanation is that different diagnostic methods and thresholds were used in the individual studies. The PET/CT images were analyzed quantitatively, qualitatively or both. Although the images were all analyzed using quantitative methods, the SUV thresholds were different. Of the included PET/CT studies using quantitative methods, only 7 studies [15, 20, 21, 33, 35, 41, 48] adopted 2.5 as the SUV cutoff value, whereas the other studies used variable values. To date, the ideal cut-off value of the SUV for diagnosing malignant MLNs has not been determined. In addition, there is no standard reference for the visual interpretation. For DWI, the results of the threshold analysis showed that no significant threshold effect existed. We also conducted subgroup analyses based on factors including study design, country, sample size, analysis method, patient enrollment, and blinding. However, these factors failed to explain the heterogeneity between PET/CT studies. For the heterogeneity in DWI studies, study design and patient enrollment were potential sources. In addition, the differences in the technique characteristics of PET/CT and DWI were potential sources of heterogeneity.
In clinical practice, DWI and 18F-FDG PET/CT have satisfactory specificity, and these two highly specific techniques are suitable for confirming diseases, especially some diseases with distinctive clinical manifestations or diseases that are fatal. However, with the disappointing sensitivity, a large number of patients would be misdiagnosed because of the relatively greater false negative results. DWI appears to have several advantages over FDG PET/CT, including no radiation exposure, no fasting and short examining time [9, 38]. With comparative diagnostic capacity, the cost of DWI examination is approximately one third of PET/CT examination. Although DWI shows some advantages over PET/CT, its real value for evaluating nodal status of NSCLC in clinical practice has not been determined. There is still a long way to confirm the diagnostic value of DWI, and further confirm whether it can replace PET/CT examination for N stage of NSCLC.
The current analysis has several limitations. First and foremost, the number of DWI studies included in this meta-analysis was too small. More work is needed to enrich this field. Second, a wide variation in imaging techniques likely affected the assessment of diagnostic accuracy of DWI and PET/CT and resulted in heterogeneity. Due to limited information, these factors were not analyzed. Third, although no publication bias was found by using Deek’s funnel plot, a potential publication bias could still exist, especially with the exclusion of conference abstracts and case reports during the study selection. Finally, there was no single reference standard strategy for the histopathologic analyses, and a wide variation in patient histopathologic types was found in all studies. This factor was not analyzed because it is too mixed and difficult to classify.
Conclusion
Our meta-analysis indicated that 18F-FDG PET/CT and DWI had high specificity and low sensitivity for identifying metastatic mediastinal lymph nodes in NSCLC, and they are noninvasive imaging methods that might aid in confirming the diagnosis of metastases in clinical practice. However, the true value of DWI remains unknown in clinical practice, although DWI did show some advantages over PET/CT in some aspects. Therefore, large-scale, prospective studies are needed to further justify the diagnostic value of DWI in comparison with 18F-FDG PET/CT.
Supporting information
(DOC)
Data Availability
All relevant data are within the paper.
Funding Statement
This study was supported by National Natural Science Foundation of China, Grant No. 81571637 and 81271532).
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65:5–29. 10.3322/caac.21254 [DOI] [PubMed] [Google Scholar]
- 2.Pak K, Park S, Cheon GJ, Kang KW, Kim IJ, Lee DS, et al. Update on nodal staging in non-small cell lung cancer with integrated positron emission tomography/computed tomography: a meta-analysis. Ann Nucl Med. 2015;29:409–419. 10.1007/s12149-015-0958-6 [DOI] [PubMed] [Google Scholar]
- 3.Wu Y, Li P, Zhang H, Shi Y, Wu H, Zhang J, et al. Diagnostic value of fluorine 18 fluorodeoxyglucose positron emission tomography/computed tomography for the detection of metastases in non-small-cell lung cancer patients. Int J Cancer. 2013;132:E37–47. 10.1002/ijc.27779 [DOI] [PubMed] [Google Scholar]
- 4.Wu LM, Xu JR, Gu HY, Hua J, Chen J, Zhang W, et al. Preoperative mediastinal and hilar nodal staging with diffusion-weighted magnetic resonance imaging and fluorodeoxyglucose positron emission tomography/computed tomography in patients with non-small-cell lung cancer: which is better? J Surg Res. 2012;178:304–314. 10.1016/j.jss.2012.03.074 [DOI] [PubMed] [Google Scholar]
- 5.Usuda K, Sagawa M, Motono N, Ueno M, Tanaka M, Machida Y, et al. Advantages of diffusion-weighted imaging over positron emission tomography-computed tomography in assessment of hilar and mediastinal lymph node in lung cancer. Ann Surg Oncol. 2013;20:1676–1683. 10.1245/s10434-012-2799-z [DOI] [PubMed] [Google Scholar]
- 6.Matoba M, Tonami H, Kondou T, Yokota H, Higashi K, Toga H, et al. Lung carcinoma: diffusion-weighted mr imaging—preliminary evaluation with apparent diffusion coefficient. Radiology. 2007;243:570–577. 10.1148/radiol.2432060131 [DOI] [PubMed] [Google Scholar]
- 7.Koh DM, Collins DJ. Diffusion-weighted MRI in the body: applications and challenges in oncology. AJR Am J Roentgenol. 2007;188:1622–1635. 10.2214/AJR.06.1403 [DOI] [PubMed] [Google Scholar]
- 8.Pauls S, Schmidt SA, Juchems MS, Klass O, Luster M, Reske SN, et al. Diffusion-weighted MR imaging in comparison to integrated 18F-FDG PET/CT for N-staging in patients with lung cancer. Eur J Radiol. 2012;81:178–182. 10.1016/j.ejrad.2010.09.001 [DOI] [PubMed] [Google Scholar]
- 9.Usuda K, Zhao X-T, Sagawa M, Matoba M, Kuginuki Y, Taniguchi M, et al. Diffusion-weighted imaging is superior to positron emission tomography in the detection and nodal assessment of lung cancers. Ann Thorac Surgery. 2011;91:1689–1695. [DOI] [PubMed] [Google Scholar]
- 10.Xu L, Tian J, Liu Y, Li C. Accuracy of diffusion-weighted (DW) MRI with background signal suppression (MR-DWIBS) in diagnosis of mediastinal lymph node metastasis of non-small-cell lung cancer (NSCLC). J Magn Reson Imaging. 2014;40:200–205. 10.1002/jmri.24343 [DOI] [PubMed] [Google Scholar]
- 11.Chen W, Jian W, Li HT, Li C, Zhang YK, Xie B, et al. Whole-body diffusion-weighted imaging vs. FDG-PET for the detection of non-small-cell lung cancer. How do they measure up? Magn Reson Imaging. 2010;28:613–620. 10.1016/j.mri.2010.02.009 [DOI] [PubMed] [Google Scholar]
- 12.Ohno Y, Koyama H, Yoshikawa T, Nishio M, Aoyama N, Onishi Y, et al. N stage disease in patients with non-small cell lung cancer: efficacy of quantitative and qualitative assessment with STIR turbo spin-echo imaging, diffusion-weighted MR imaging, and fluorodeoxyglucose PET/CT. Radiology. 2011;261:605–615. 10.1148/radiol.11110281 [DOI] [PubMed] [Google Scholar]
- 13.Mori T, Nomori H, Ikeda K, Kawanaka K, Shiraishi S, Katahira K, et al. Diffusion-weighted magnetic resonance imaging for diagnosing malignant pulmonary nodules/masses: comparison with positron emission tomography. J Thorac Oncol. 2008;3:358–364. 10.1097/JTO.0b013e318168d9ed [DOI] [PubMed] [Google Scholar]
- 14.Sommer G, Wiese M, Winter L, Lenz C, Klarhofer M, Forrer F, et al. Preoperative staging of non-small-cell lung cancer: comparison of whole-body diffusion-weighted magnetic resonance imaging and 18F-fluorodeoxyglucose-positron emission tomography/computed tomography. Eur Radiol. 2012;22:2859–2867. 10.1007/s00330-012-2542-y [DOI] [PubMed] [Google Scholar]
- 15.Al-Sarraf N, Gately K, Lucey J, Wilson L, McGovern E, Young V. Lymph node staging by means of positron emission tomography is less accurate in non-small cell lung cancer patients with enlarged lymph nodes: Analysis of 1145 lymph nodes. Lung Cancer. 2008;60:62–68. 10.1016/j.lungcan.2007.08.036 [DOI] [PubMed] [Google Scholar]
- 16.An YS, Sun JS, Park KJ, Hwang SC, Park KJ, Sheen SS, et al. Diagnostic performance of (18)F-FDG PET/CT for lymph node staging in patients with operable non-small-cell lung cancer and inflammatory lung disease. Lung. 2008;186:327–336. 10.1007/s00408-008-9109-3 [DOI] [PubMed] [Google Scholar]
- 17.Billé A, Pelosi E, Skanjeti A, Arena V, Errico L, Borasio P, et al. Preoperative intrathoracic lymph node staging in patients with non-small-cell lung cancer: accuracy of integrated positron emission tomography and computed tomography. Eur J Cardiothoracic Surg. 2009;36:440–445. [DOI] [PubMed] [Google Scholar]
- 18.Booth K, Hanna GG, McGonigle N, McManus KG, McGuigan J, O'Sullivan J, et al. The mediastinal staging accuracy of 18F-Fluorodeoxyglycose positron emission tomography/computed tomography in non-small cell lung cancer with variable time intervals to surgery. Ulster Med J. 2013;82:75–81. [PMC free article] [PubMed] [Google Scholar]
- 19.Bryant AS, Cerfolio RJ, Klemm KM, Ojha B. Maximum standard uptake value of mediastinal lymph nodes on integrated FDG-PET-CT predicts pathology in patients with non-small cell lung cancer. Ann Thorac Surg. 2006;82:417–423. 10.1016/j.athoracsur.2005.12.047 [DOI] [PubMed] [Google Scholar]
- 20.Hellwig D, Graeter TP, Ukena D, Groeschel A, Sybrecht GW, Schaefers HJ, et al. 18F-FDG PET for mediastinal staging of lung cancer: which SUV threshold makes sense? J Nucl Med. 2007;48:1761–1766. 10.2967/jnumed.107.044362 [DOI] [PubMed] [Google Scholar]
- 21.Hu M, Yu J, Liu N, Liu L, Guo H, Yang G, et al. Significance of dual-time-point 18F-FDG PET imaging in evaluation of hilar and mediastinal lymph node metastasis in non-small-cell lung cancer. Chin J Oncol. 2008;30:306–309. [PubMed] [Google Scholar]
- 22.Jeon TY, Lee KS, Yi CA, Chung MP, Kwon OJ, Kim B-T, et al. Incremental Value of PET/CT Over CT for Mediastinal Nodal Staging of Non—Small Cell Lung Cancer: Comparison Between Patients With and Without Idiopathic Pulmonary Fibrosis. Am J Roentgenol. 2010;195:370–376. [DOI] [PubMed] [Google Scholar]
- 23.Kim BT, Lee KS, Shim SS, Choi JY, Kwon OJ, Kim H, et al. Stage T1 Non—Small Cell Lung Cancer: Preoperative Mediastinal Nodal Staging with Integrated FDG PET/CT—A Prospective Study 1. Radiology. 2006;241:501–509. 10.1148/radiol.2412051173 [DOI] [PubMed] [Google Scholar]
- 24.Kim DW, Kim WH, Kim CG. Dual-time-point FDG PET/CT: Is It Useful for Lymph Node Staging in Patients with Non-Small-Cell Lung Cancer? Nucl Med Mol Imaging. 2012;46:196–200. 10.1007/s13139-012-0141-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim YK, Lee KS, Kim BT, Choi JY, Kim H, Kwon OJ, et al. Mediastinal nodal staging of nonsmall cell lung cancer using integrated 18F-FDG PET/CT in a tuberculosis-endemic country. Cancer. 2007;109:1068–1077. 10.1002/cncr.22518 [DOI] [PubMed] [Google Scholar]
- 26.Kim YN, Yi CA, Lee KS, Kwon OJ, Lee HY, Kim B-T, et al. A proposal for combined MRI and PET/CT interpretation criteria for preoperative nodal staging in non-small-cell lung cancer. Eur Radiol. 2012;22:1537–1546. 10.1007/s00330-012-2388-3 [DOI] [PubMed] [Google Scholar]
- 27.Koksal D, Demirag F, Bayiz H, Ozmen O, Tatci E, Berktas B, et al. The correlation of SUVmax with pathological characteristics of primary tumor and the value of Tumor/Lymph node SUVmax ratio for predicting metastasis to lymph nodes in resected NSCLC patients. J Cardiothorac Surg. 2013;8:63 10.1186/1749-8090-8-63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kuo WH, Wu YC, Wu CY, Ho KC, Chiu PH, Wang CW, et al. Node/aorta and node/liver SUV ratios from 18F-FDG PET/CT may improve the detection of occult mediastinal lymph node metastases in patients with non-small cell lung carcinoma. Acad Radiol. 2012;19:685–692. 10.1016/j.acra.2012.02.013 [DOI] [PubMed] [Google Scholar]
- 29.Lee AY, Choi SJ, Jung KP, Park JS, Lee SM, Bae SK. Characteristics of Metastatic Mediastinal Lymph Nodes of Non-Small Cell Lung Cancer on Preoperative F-18 FDG PET/CT. Nucl Med Mol Imaging. 2014;48:41–46. 10.1007/s13139-013-0244-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee JW, Kim BS, Lee DS, Chung JK, Lee MC, Kim S, et al. 18F-FDG PET/CT in mediastinal lymph node staging of non-small-cell lung cancer in a tuberculosis-endemic country: consideration of lymph node calcification and distribution pattern to improve specificity. Eur J Nucl Med Mol imaging. 2009;36:1794–1802. 10.1007/s00259-009-1155-4 [DOI] [PubMed] [Google Scholar]
- 31.Lee SM, Park CM, Paeng JC, Im HJ, Goo JM, Lee HJ, et al. Accuracy and predictive features of FDG-PET/CT and CT for diagnosis of lymph node metastasis of T1 non-small-cell lung cancer manifesting as a subsolid nodule. Eur Radiol. 2012;22:1556–1563. 10.1007/s00330-012-2395-4 [DOI] [PubMed] [Google Scholar]
- 32.Li M, Wu N, Liu Y, Zheng R, Liang Y, Zhang W, et al. Regional nodal staging with 18 F-FDG PET—CT in non-small cell lung cancer: Additional diagnostic value of CT attenuation and dual-time-point imaging. Eur J Radiol. 2012;81:1886–1890. 10.1016/j.ejrad.2011.03.074 [DOI] [PubMed] [Google Scholar]
- 33.Li X, Zhang H, Xing L, Ma H, Xie P, Zhang L, et al. Mediastinal lymph nodes staging by 18 F-FDG PET/CT for early stage non-small cell lung cancer: a multicenter study. Radiother Oncol. 2012;102:246–250. 10.1016/j.radonc.2011.10.016 [DOI] [PubMed] [Google Scholar]
- 34.Lin WY, Hsu WH, Lin KH, Wang SJ. Role of preoperative PET-CT in assessing mediastinal and hilar lymph node status in early stage lung cancer. J Chin Med Assoc. 2012;75:203–208. 10.1016/j.jcma.2012.04.004 [DOI] [PubMed] [Google Scholar]
- 35.Liu BJ, Dong JC, Xu CQ, Zuo CT, Le JJ, Guan YH, et al. Accuracy of 18F-FDG PET/CT for lymph node staging in non-small-cell lung cancers. Chin Med J. 2009;122:1749 [PubMed] [Google Scholar]
- 36.Morikawa M, Demura Y, Ishizaki T, Ameshima S, Miyamori I, Sasaki M, et al. The effectiveness of 18F-FDG PET/CT combined with STIR MRI for diagnosing nodal involvement in the thorax. J Nucl Med. 2009;50:81–87. 10.2967/jnumed.108.056408 [DOI] [PubMed] [Google Scholar]
- 37.Nakayama J, Miyasaka K, Omatsu T, Onodera Y, Terae S, Matsuno Y, et al. Metastases in mediastinal and hilar lymph nodes in patients with non-small cell lung cancer: quantitative assessment with diffusion-weighted magnetic resonance imaging and apparent diffusion coefficient. J Comput Assist Tomogr. 2010;34:1–8. 10.1097/RCT.0b013e3181a9cc07 [DOI] [PubMed] [Google Scholar]
- 38.Nomori H, Mori T, Ikeda K, Kawanaka K, Shiraishi S, Katahira K, et al. Diffusion-weighted magnetic resonance imaging can be used in place of positron emission tomography for N staging of non-small cell lung cancer with fewer false-positive results. J Thorac Cardiovasc Surg. 2008;135:816–822. 10.1016/j.jtcvs.2007.10.035 [DOI] [PubMed] [Google Scholar]
- 39.Ohno Y, Koyama H, Nogami M, Takenaka D, Yoshikawa T, Yoshimura M, et al. STIR turbo SE MR imaging vs. coregistered FDG-PET/CT: quantitative and qualitative assessment of N-stage in non-small-cell lung cancer patients. J Magn Reson Imaging. 2007;26:1071–1080. 10.1002/jmri.21106 [DOI] [PubMed] [Google Scholar]
- 40.Shim SS, Lee KS, Kim B-T, Chung MJ, Lee EJ, Han J, et al. Non—Small Cell Lung Cancer: Prospective Comparison of Integrated FDG PET/CT and CT Alone for Preoperative Staging 1. Radiology. 2005;236:1011–1019. 10.1148/radiol.2363041310 [DOI] [PubMed] [Google Scholar]
- 41.Sit AK, Sihoe AD, Suen WS, Cheng LC. Positron-emission tomography for lung cancer in a tuberculosis-endemic region. Asian Cardiovascular and Thoracic Annals. 2010;18:33–38. 10.1177/0218492309352119 [DOI] [PubMed] [Google Scholar]
- 42.Tascı E, Tezel C, Orki A, Akın O, Falay O, Kutlu CA. The role of integrated positron emission tomography and computed tomography in the assessment of nodal spread in cases with non-small cell lung cancer. Interact Cardiovasc Thorac Surg. 2010;10:200–203. 10.1510/icvts.2009.220392 [DOI] [PubMed] [Google Scholar]
- 43.Toba H, Kondo K, Otsuka H, Takizawa H, Kenzaki K, Sakiyama S, et al. Diagnosis of the presence of lymph node metastasis and decision of operative indication using fluorodeoxyglucose-positron emission tomography and computed tomography in patients with primary lung cancer. J Med Invest. 2010;57:305–313. [DOI] [PubMed] [Google Scholar]
- 44.Tournoy K, Maddens S, Gosselin R, Van Maele G, Van Meerbeeck J, Kelles A. Integrated FDG-PET/CT does not make invasive staging of the intrathoracic lymph nodes in non-small cell lung cancer redundant: a prospective study. Thorax. 2007;62:696–701. 10.1136/thx.2006.072959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vansteenkiste JF, Stroobants SG, Dupont PJ, De Leyn PR, De Wever WF, Verbeken EK, et al. FDG-PET scan in potentially operable non-small cell lung cancer: do anatometabolic PET-CT fusion images improve the localisation of regional lymph node metastases? Eur J Nucl Med. 1998;25:1495–1501. [DOI] [PubMed] [Google Scholar]
- 46.Ventura E, Islam T, Gee MS, Mahmood U, Braschi M, Harisinghani MG. Detection of nodal metastatic disease in patients with non-small cell lung cancer: comparison of positron emission tomography (PET), contrast-enhanced computed tomography (CT), and combined PET-CT. Clin Imaging. 2010;34:20–28. 10.1016/j.clinimag.2009.03.012 [DOI] [PubMed] [Google Scholar]
- 47.Xu N, Wang M, Zhu Z, Zhang Y, Jiao Y, Fang W. Integrated positron emission tomography and computed tomography in preoperative lymph node staging of non-small cell lung cancer. Chin Med J (Engl). 2014;127:607–613. [PubMed] [Google Scholar]
- 48.Yang W, Fu Z, Yu J, Yuan S, Zhang B, Li D, et al. Value of PET/CT versus enhanced CT for locoregional lymph nodes in non-small cell lung cancer. Lung Cancer. 2008;61:35–43. 10.1016/j.lungcan.2007.11.007 [DOI] [PubMed] [Google Scholar]
- 49.Yi CA, Lee KS, Kim B-T, Shim SS, Chung MJ, Sung YM, et al. Efficacy of helical dynamic CT versus integrated PET/CT for detection of mediastinal nodal metastasis in non-small cell lung cancer. Am J Roentgenol. 2007;188:318–325. [DOI] [PubMed] [Google Scholar]
- 50.Zeng Z, Liao Q, Cai J, Liu A. Diffusion-weighted imaging and apparent diffusion coefficient values in the differential diagnosis of hilar and mediastinal lymph nodes of non-small cell lung cancer. Chin J Clin Oncol. 2012;39:706–710. [Google Scholar]
- 51.He W, Zhou X, He W, Xu J, Guo L. Value of diffusion weighted imaging in diagnosis of metastatic lymph nodes in lung cancer. Chin J Med Imaging Technol. 2011;27:2013–2016. [Google Scholar]
- 52.Zhang X, Xing W, Chen J, Ding J, Gao X, Shen N. Application of DWI in differentail dignosis of lymph nodes in patietns with lung cancer. Chin Compu Med Imaging. 2013;19:213–216. [Google Scholar]
- 53.Zhou Y, Xia J. Clinical value of 18F-FDG PET-CT imaging in the preoperative diagnosis and staging of regional lymph nodes in non-small cell lung cancer. Chin J CT& MRI. 2014;12:70–74. [Google Scholar]
- 54.Whiting PF, Rutjes AW, Westwood ME, Mallett S, Deeks JJ, Reitsma JB, et al. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med. 2011;155:529–536. 10.7326/0003-4819-155-8-201110180-00009 [DOI] [PubMed] [Google Scholar]
- 55.Lv YL, Yuan DM, Wang K, Miao XH, Qian Q, Wei SZ, et al. Diagnostic performance of integrated positron emission tomography/computed tomography for mediastinal lymph node staging in non-small cell lung cancer: a bivariate systematic review and meta-analysis. J Thorac Oncol. 2011;6:1350–1358. 10.1097/JTO.0b013e31821d4384 [DOI] [PubMed] [Google Scholar]
- 56.Wu LM, Hu JN, Hua J, Liu MJ, Chen J, Xu JR. Diagnostic value of diffusion-weighted magnetic resonance imaging compared with fluorodeoxyglucose positron emission tomography/computed tomography for pancreatic malignancy: a meta-analysis using a hierarchical regression model. J Gastroenterol Hepatol. 2012;27:1027–1035. 10.1111/j.1440-1746.2012.07112.x [DOI] [PubMed] [Google Scholar]
- 57.Yamamura J, Salomon G, Buchert R, Hohenstein A, Graessner J, Huland H, et al. Magnetic resonance imaging of prostate cancer: diffusion-weighted imaging in comparison with sextant biopsy. J Comput Assist Tomogr. 2011;35:223–228. 10.1097/RCT.0b013e3181fc5409 [DOI] [PubMed] [Google Scholar]
- 58.Matsuki M, Inada Y, Tatsugami F, Tanikake M, Narabayashi I, Katsuoka Y. Diffusion-weighted MR imaging for urinary bladder carcinoma: initial results. Eur Radiol. 2007;17:201–204. 10.1007/s00330-006-0281-7 [DOI] [PubMed] [Google Scholar]
- 59.Busard MP, Mijatovic V, van Kuijk C, Pieters-van den Bos IC, Hompes PG, van Waesberghe JH. Magnetic resonance imaging in the evaluation of (deep infiltrating) endometriosis: the value of diffusion-weighted imaging. J Magn Reson Imaging. 2010;31:1117–1123. 10.1002/jmri.22139 [DOI] [PubMed] [Google Scholar]
- 60.Ichikawa T, Erturk SM, Motosugi U, Sou H, Iino H, Araki T, et al. High-B-value diffusion-weighted MRI in colorectal cancer. AJR Am J Roentgenol. 2006;187:181–184. 10.2214/AJR.05.1005 [DOI] [PubMed] [Google Scholar]
- 61.Schmidt-Hansen M, Baldwin DR, Hasler E, Zamora J, Abraira V, Roque IFM. PET-CT for assessing mediastinal lymph node involvement in patients with suspected resectable non-small cell lung cancer. Cochrane Database Syst Rev. 2014;11:CD009519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wang Z, Wang Y, Sui X, Zhang W, Shi R, Zhang Y, et al. Performance of FLT-PET for pulmonary lesion diagnosis compared with traditional FDG-PET: A meta-analysis. Eur J Radiol. 2015;84:1371–1377. 10.1016/j.ejrad.2015.03.025 [DOI] [PubMed] [Google Scholar]
- 63.Glas AS, Lijmer JG, Prins MH, Bonsel GJ, Bossuyt PM. The diagnostic odds ratio: a single indicator of test performance. J Clin Epidemiol. 2003;56:1129–1135. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOC)
Data Availability Statement
All relevant data are within the paper.