Abstract
Objectives
CD4+CD25+FOXP3+ regulatory T cells (Treg) inhibit the anti-tumour immune response and reduce the effect of cancer immunotherapy. Although studies have demonstrated that the number and suppressive activity of Treg increase with age, it is not clear whether these changes correlate with a higher incidence of tumours in the elderly. This study was designed to explore the relationship between increase in CD4+CD25+FOXP3+ Treg and the higher risk of lung cancer in the elderly.
Methods
Seventy lung cancer patients and 60 sex- and age-matched controls were recruited. Both groups were divided into three subgroups based on their age (young, middle-aged, or elderly). The proportion of CD4+CD25+FOXP3+ /CD4+ T cells was detected using flow cytometry, and the level of FOXP3 mRNA in the peripheral blood was examined with real-time RT-PCR.
Results
The levels of CD4+CD25+FOXP3+/CD4+ T cells and FOXP3 mRNA were significantly higher in lung cancer patients than in healthy controls (t = 7.16, P < 0.01 and t = 3.65, P < 0.01, respectively). Within the healthy groups, the elderly group had larger proportion of CD4+CD25+FOXP3+ Treg (F = 32.54, P < 0.01) and higher FOXP3 mRNA expression (F = 4.76, P < 0.01) than their younger counterparts. Among the six subgroups, the elderly lung cancer patients exhibited the highest levels of both CD4+CD25+FOXP3+ Treg (11.81 ± 2.40%) and FOXP3 mRNA (3.14 ± 1.30).
Conclusions
The accumulation of CD4+CD25+FOXP3+ Treg with age correlates well with the increasing incidence of lung cancer in the elderly.
Introduction
The International Agency for Research on Cancer has indicated that the incidence of malignancy increases with age [1]. The tumour morbidity and mortality of individuals in their 80’s were reported to be three times higher than those of individuals 55 years of age [2,3]. As for tumour type, lung cancer is the most common worldwide, and in more than 70% patients, the disease has unfortunately progressed into the intermediate or late stages upon their diagnosis. Concerning China, it is estimated that there will be more than 1 million new cases of lung cancer by 2025 [4,5]. Therefore, the relationship between age and lung cancer has emerged as a hot topic in recent years. On the other hand, recent evidence [6,7] demonstrated that the number of CD4+CD25+FOXP3+ regulatory T cells (Treg), which mediate immunosuppression and play an important role in tumour immune evasion, also increases with age. A number of studies [8–10] have reported that several types of tumours were associated with increase in Treg, while rarely focusing on the changes in proportion of Treg with age and how these changes contribute to the elevating incidence of malignancy. This study aimed to explore the relationship between age, increase in CD4+CD25+FOXP3+ Treg and the high incidence of lung cancer in the elderly. We measured the proportion of CD4+CD25+FOXP3+ Treg and the expression level of FOXP3 mRNA in peripheral blood to explore the underlying interaction among age, Treg and lung cancer susceptibility. The results might provide an experimental basis for lung cancer screening and theoretical support for Treg-targeted immunomodulatory therapy.
Materials and methods
Patients and controls
From January to December 2015, a total of 70 lung cancer patients from 34 to 90 years of age [mean age ± standard deviation (SD), 62.2 ± 18.3], including 37 male and 33 female patients, were enrolled in this study in Rushan Hospital, Binzhou Medical University, Shandong, China, a hospital with approximately 1000 beds. The lung cancer were diagnosed according to “Chinese guidelines on the diagnosis and treatment of primary lung cancer (2011)”[11]. Tumour node metastasis (TNM) stage was established based on the IASLC lung cancer staging project (seventh) [12] including 9 patients with stage I, 26 with stage II, 18 with stage III, and 17 with stage IV. Patients with hepatic or renal dysfunction, diabetes or infection, asthma, or those taking glucocorticoids, non-steroidal anti-inflammatory drugs or immunosuppressive agents within 1 month were excluded. Meanwhile, 60 age- and sex-matched healthy individuals, with ages ranging from 18 to 90 years (mean age ± SD, 58.7 ± 17.9), including 33 men and 27 women, were recruited as controls. 28 and 23 individuals were accompanied by stable chronic obstructive pulmonary disease (COPD) in lung cancer and healthy group, respectively. The diagnosis of COPD was established in accordance to the GOLD report[13]. No statistical differences existed in the sex ratio, mean age, average body weight or smoking history between the patients and controls (all P > 0.05). Both groups were divided into three subgroups according to their age: young (18–44 years), middle-aged (45–59 years), and elderly (60–90 years). The authors could not identify individual participants during the research. The study was reviewed and approved by Ethical Committee of Rushan People's Hospital, Binzhou Medical University. All participants were informed about the purpose of the study and signed the consent form prior to the experiment.
Blood collection and cell storage
A total of 5 ml anti-coagulated blood was collected from each subject by nurse and processed within 2 h. Peripheral blood mononuclear cells (PBMCs) were isolated using Ficoll lymphocyte separation medium (Chemical Reagent Co., Ltd, China). An aliquot of 100 μL (1*106 cells) suspension was separately added to the marked No. 1 (CD4 single labelled), No. 2 (CD4, CD25, FOXP3 labelled), and No. 3 (blank control) flow tubes. To the remainder of the sample, 1 mL RNAiso Plus (Shanghai Hengxin Chemical Reagent Co., Ltd, China) was added, and the samples were preserved at -80°C for the measurement of FOXP3 mRNA levels, which would be conducted within two weeks.
Flow cytometry analysis
A regulatory T cell staining kit (eBioscience, San Diego, USA) was used according to the manufacturer’s directions. Surface molecules were stained using a standard procedure, with a fluorescein isothiocyanate (FITC)-CD4-antibody and an allophycocyanin conjugated (APC)-CD25 antibody, and incubated at 4°C for 15 min. After being washed with flow cytometry staining buffer, permeabilization working solution was added, and the cells were stained with a phycoerythrin-conjugated (PE) FOXP3 antibody and incubated at 4°C for 30 min. Finally, the cells were washed, resuspended with flow cytometry staining buffer, and analysed with Gallios flow cytometry (Beckman Coulter, USA). The proportion of positive cells was determined using WinMDI version 2.8 software (The Scripps Institute, USA).
Real-time RT-PCR
DNA-free RNA templates were extracted from the PBMCs preserved at -80°C using an RNA isolation kit (ShineGene Bio-Technologies Inc., Shanghai, China). Reverse transcription was performed according to the manufacturer's instructions (Takara Biotechnology Co., LTD, China) after assessing RNA concentration and quality by A260/A280. Real-time PCR assays were carried out on an ABI 7500 Real-time PCR System with the SYBR® Premix Ex TaqTM kit (Takara Biotechnology Co., Ltd.). A 20 μl reaction system contained 10 μl SYBR Premix Ex Taq, 0.4 μl ROX Reference Dye II, 0.4 μl each primer (FOXP3, 5'-CAGCACATTCCCAGAGTTCCT-3' and 5'-AGCGTGGCGTAGGTGAAAG-3'), 2 μl cDNA, and 6.8 μl dH2O. The PCR protocol began at 94°C for 30 s, followed by 40 cycles at 94°C for 20 s and 55°C for 34 s. β-ACTIN (5'-ACCGAGCGCGGCTACAG-3' and 5'- CTTAATGTCACGCACGATTTCC-3') was used as a housekeeping gene to normalize the level of each of the gene transcripts.
Statistical analysis
Data was collected immediately after the experiments. Values were presented as the mean ± SD. Statistical analysis was performed using SAS version 8.0 software. Student’s t test was used to examine the differences between the patient and healthy groups. One-way analysis of variance was performed to compare means across multiple groups. P values < 0.05 were considered statistically significant.
Results
The percentages of CD4+CD25+FOXP3+/CD4+ T cells in the peripheral blood of the six groups measured with flow cytometry (Details available in S1 File)
As shown in Table 1, Fig 1, the proportion of CD4+CD25+FOXP3+/CD4+ T cells was significantly higher in the lung cancer patients than in their healthy counterparts by student’s t test (t = 7.16, P < 0.01). Statistical differences were also observed among the three healthy groups by one-way analysis of variance. The middle-aged group had a larger proportion of CD4+CD25+FOXP3+/CD4+ T cells compared with the younger group (F = 2.48, P < 0.01), and the elderly group had an even higher proportion of these cells (F = 12.61, P < 0.01). The elderly lung cancer patients exhibited the highest proportion of Treg cells (11.81 ± 2.4%) among the six groups.
Table 1. Proportion of CD4+CD25+FOXP3+/CD4+ cells in peripheral blood in the six study groups.
| Group | Healthy | Lung cancer | P |
|---|---|---|---|
| Young (18–44 years) | 6.23 ± 0.91 | 9.05 ± 2.14 | <0.01 |
| Middle-aged (45–59 years) | 7.81 ± 1.16a | 11.25 ± 1.84c | |
| Elderly (60–90 years) | 9.82 ± 1.19b | 11.81 ± 2.40d |
The proportion of CD4+CD25+FOXP3+/CD4+ T cells was significantly higher in the lung cancer patients than in their healthy counterparts by student’s t test (P < 0.01). Statistical differences were also observed among the three healthy groups according to one-way analysis of variance:
a P < 0.01 compared with the young healthy group,
b P < 0.01 compared with the middle-aged healthy group.
c P < 0.01 compared with the young lung cancer group,
d P < 0.01 compared with the middle-aged lung cancer group.
Fig 1. Proportion of CD4+CD25+FOXP3+/CD4+ cells in peripheral blood in the six study groups.
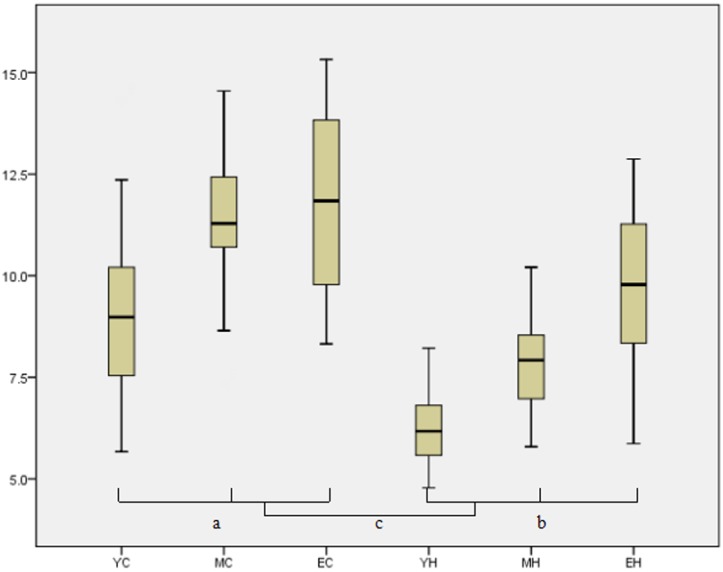
YC:Young lung cancer; MC: Middle-aged lung cancer; EC: Elderly lungcancer; YH:Young healthy; MH: Middle-aged healthy; EH: Elderly healthy; Proportion of CD4+CD25+FOXP3+/CD4+ cells increases with both age (aP<0.01, bP<0.01, one-way analysis of variance) and lung cancer (cP<0.01, student’s t test). The elderly lung cancer group displayed the highest proportion.
Furthermore, six parameters of the patients (sex, smoking status, with or without COPD, TNM staging, lymph node metastasis status, and pathological type of tumour) and their association with the CD4+CD25+FOXP3+ Treg percentage were evaluated (Table 2). The results revealed that the increasing CD4+CD25+FOXP3+ Treg percentage correlated closely with the TNM staging and lymph node metastasis status (P < 0.05), while no such relationship was observed with the sex or pathological tumour type (P > 0.05). No statistical difference was found whether the subjects smok or not, with or without COPD in both lung cancer and healthy groups.
Table 2. Comparison of CD4+CD25+FOXP3+/CD4+ T cells in lung cancer patients.
| Parameter | Case Number | Treg proporation (%) | P values |
|---|---|---|---|
| Sex | |||
| Male | 37 | 11.17 ± 0.98 | 0.12 |
| Female | 33 | 10.53 ± 1.03 | |
| Smoking | |||
| Yes | 38 | 10.92±2.6 | 0.59 |
| No | 32 | 10.61±2.19 | |
| COPD | |||
| With | 28 | 10.70±2.31 | 0.82 |
| Without | 42 | 10.83±2.50 | |
| Stage | |||
| I | 9 | 7.36 ± 1.26 | <0.05 |
| II | 26 | 9.82 ± 2.05 | |
| III | 18 | 11.74 ± 1.70 | |
| IV | 17 | 13.49 ± 2.07 | |
| Lymph node metastasis | |||
| Yes | 43 | 11.71 ± 2.12 | <0.05 |
| No | 27 | 8.47 ± 2.01 | |
| Pathologic type | |||
| Squamous carcinoma | 34 | 10.81 ± 2.33 | 0.98 |
| Adenocarcinoma | 26 | 10.86 ± 2.78 | |
| Small-cell cancer | 10 | 10.69 ± 1.92 |
FOXP3 mRNA expression level in the six groups
(Details available in S2 File).
Similar to CD4+CD25+FOXP3+ Treg counts, we found that the expression level of FOXP3 mRNA was considerably higher in patients than in controls by student’s t test (t = 3.65, P < 0.01, shown in Table 3, Fig 2). Among the healthy groups, FOXP3 mRNA levels in the peripheral blood also increased with age according to one-way analysis of variance (middle-aged vs. young, F = 5.51, P < 0.01; elderly vs. middle-aged, F = 30.49, P < 0.01). The elderly lung cancer group displayed the highest expression level of FOXP3 mRNA (3.14 ± 1.30), suggesting that FOXP3 mRNA increases with both age and lung cancer.
Table 3. FOXP3 mRNA expression levels in the six study groups.
| Group | Healthy | Lung cancer | P |
|---|---|---|---|
| Young (18–44 years) | 1.17 ± 0.51 | 2.28 ± 1.21 | <0.01 |
| Middle-aged (45–59 years) | 1.76 ± 0.80a | 2.53 ± 1.16c | |
| Elderly (60–90 years) | 2.67 ± 0.99b | 3.14 ± 1.30d |
The expression level of FOXP3 mRNA in the peripheral blood was considerably higher in patients than in controls by student’s t test (P < 0.01). FOXP3 mRNA levels also increased with age according to one-way analysis of variance:
a P < 0.01 compared with the young healthy group,
b P < 0.01 compared with the middle-aged healthy group,
c P < 0.01 compared with the young lung cancer group,
d P < 0.01 compared with the middle-aged lung cancer group.
Fig 2. Comparison of FOXP3 mRNA expression levels in the six groups.
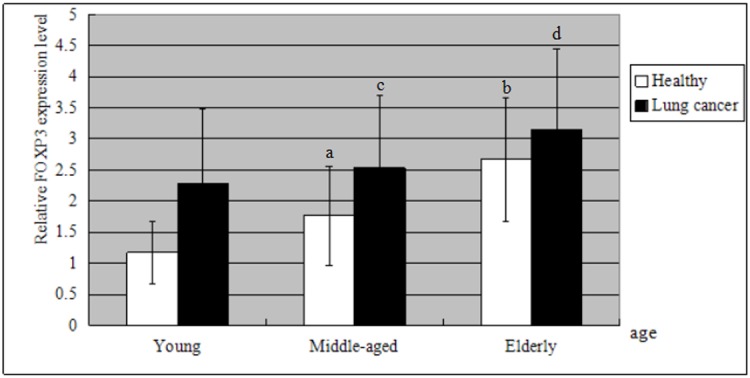
The expression level of FOXP3 mRNA was higher in patients than in healthy group by student’s t test (P < 0.01). It also increased with age according to one-way analysis of variance: aP < 0.01 compared with the young healthy group, bP < 0.01 compared with the middle-aged healthy group, cP < 0.01 compared with the young lung cancer group, dP < 0.01 compared with the middle-aged lung cancer group. FOXP3 mRNA increases with both age and lung cancer. The elderly lung cancer group displayed the highest expression level of FOXP3 mRNA (3.14 ± 1.30).
Discussion
CD4+CD25+FOXP3+ Treg mediate immunosuppression and play an important role in tumour immune evasion [6]. The forkhead transcription factor FOXP3 has also been verified as a key player in Treg function [14,15]. Our experiment confirmed that, within the same age group, the proportion of CD4+CD25+FOXP3+/CD4+ T cells and the expression level of FOXP3 mRNA in the peripheral blood were significantly higher in lung cancer patients than in healthy controls. Miyara et.al [16] found that human FOXP3+CD4+T cells were composed of three phenotypically and functionally distinct subpopulations: CD45RA+FoxP3lo resting Treg cells (rTreg cells) and CD45RA-FoxP3 hi activated Treg cells (aTreg cells), both of which were suppressive in vitro, and cytokine-secreting CD45RA-FoxP3lo nonsuppressive T cells. Which subpopulation the increasing Treg cells belong to and whether these cells have suppressive function need further research.
The increasing CD4+CD25+FOXP3+ Treg percentage was also closely in accordance with the TNM staging and lymph node metastasis status, which is consistent with previous studies [17,18]. However, no significant difference is found between COPD and non-COPD group, which is in accordance with DB Tan’ research[19], but different from J. Domagała-Kulawik’s[20]. It maybe concerned with clinical stage and stability of COPD. Chen et al. [21] reported that the TGF-β and IL-10 factors secreted by tumour cells may directly or indirectly induce Treg proliferation in the local tumour environment as well as in peripheral blood. The increasing Treg cells further suppress antitumor immunity and lead to tumour growth. Thus, appropriate interventions to inhibit CD4+CD25+FOXP3+ Treg elevation could aid in preventing tumour progression, and regular monitoring of Treg cells in clinical patients may help to establish their prognosis.
Our data also revealed that CD4+CD25+FOXP3+/CD4+ proportion and FOXP3 mRNA expression levels increased with age in healthy individuals, which was in agreement with the research of Rosenkranz et al. [22]. Miyara et al [16] further reported that aged donors had high proportions of aTreg cells and low but still detectable proportions of rTreg cells. And aTreg cells were the main effectors of suppression. The mechanism of this age-related change remains unclear. Peterson et al. [23] demonstrated the change was linked to two subsets of CD4+CD25+FOXP3+ Treg, the CD44high phenotype and CD44int phenotype, which separately feature rapid proliferation rates and long-term survival without division, respectively. Another explanation stated that some cytokines such as IL-10 and TGF-β accumulated with age and also induce CD4+CD25-FOXP3- T cells to transform into CD4+CD25+FOXP3+ Treg, which highly express functional FOXP3 and suppress the activation and proliferation of CD4+ and CD8+ T cells [24]. The reduced T-cell-mediated antitumor immunity contributes to immune evasion at the early stage and finally leads to the higher morbidity and mortality associated with tumours in the elderly. Therefore, developing approaches to prevent CD4+CD25+FOXP3+ Treg elevation with age may enhance T-cell-mediated immune response and further reduce the risk of cancer. In addition, it is of interest to explore whether normal reference ranges of CD4+CD25+FOXP3+ Treg could be established according to different age groups, which may make it possible to evaluate immune-activity against a tumour through regular monitoring of Treg populations.
In conclusion, we have demonstrated that the proportion of CD4+CD25+FOXP3+/CD4+ correlates well with both lung cancer disease and the ageing process. Our findings may provide new prospects for designing Treg-targeted immunomodulatory strategies for the prevention and treatment of lung cancer.
Supporting information
(XLS)
(XLS)
Acknowledgments
This study was supported by Shandong Province Natural Science Foundation (ZR2014HP061) and the projects of medical and health technology development program in Shandong province (2013WS0066).
Data Availability
All relevant data are included within the manuscript and its supporting information files.
Funding Statement
Support was provided by Shandong Province Natural Science Foundation, grant ZR2014HP061 [http://www.sdnsf.gov.cn/portal/] to Pan-Fei Hou and the projects of medical and health technology development program in Shandong province, grant 2013WS0066 [http://www.sdwskj.cn/] to Li-Jing Zhu. The funders played a role in data collection and analysis.
References
- 1.The Ministry of Health of the People's Republic of China. Third national retrospect spot-check of death-causation. Beijing: Peking Union Medical College Press 2008;2(5): 344–345.
- 2.Burkle A, Caselli G, Franceschi C, Mariani E, Sansoni P. Santoni A, et al. Pathophysiology of ageing, longevity and age related diseases. Immunity & Ageing 2007; 4: 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anisimov VN. Carcinogenesis and ageing 20 years after: escaping horizon Mechanisms of ageing and Development 2009; 130: 105–121. 10.1016/j.mad.2008.02.004 [DOI] [PubMed] [Google Scholar]
- 4.Chen Wan-Qing, Zhang Si-Wei, Zou Xiao-Nong. Estimation and Projection of Lung Cancer Incidence and Mortality in China. Chin J Lung Cancer 2010; 13 (5). 488–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, et al. Cancer statistics in China, 2015. CA Cancer J Clin, 2016, 66(2):115–32. 10.3322/caac.21338 [DOI] [PubMed] [Google Scholar]
- 6.Nishikawa H, Sakaguchi S. Regulatory T cells in cancer immunotherapy. Curr Opin Immunol 2014; 27: 1–7. 10.1016/j.coi.2013.12.005 [DOI] [PubMed] [Google Scholar]
- 7.Zhu Li-Jing, Hou Pan-Fei, Wang Ling, Zhang Guang-Bo, Xie Yan, Pan Xu-Dong, et al. Changes in CD4+CD25+Foxp3+ Regulatory T Cells in Relation to ageing and Lung Tumor Incidence. International Journal of Gerontology 2012; 6(3):187–191. [Google Scholar]
- 8.Zhang X, Kelaria S, Kerstetter J, Wang J. The functional and prognostic implications of regulatory T cells in colorectal carcinoma. J Gastrointest Oncol. 2015, 6(3):307–313. 10.3978/j.issn.2078-6891.2015.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li F, Guo Z, Lizee G, Yu H, Wang H, Si T. Clinical prognostic value of CD4+CD25+FOXP3+ regulatory T cells in peripheral blood of Barcelona Clinic Liver Cancer (BCLC) stage B hepatocellular carcinoma patients. Clin Chem Lab Med. 2014;52(9):1357–65. 10.1515/cclm-2013-0878 [DOI] [PubMed] [Google Scholar]
- 10.Li J, Feng G, Liu J, Rong R, Luo F, Guo L, et al. Renal cell carcinoma may evade the immune system by converting CD4+Foxp3- T cells into CD4+CD25+Foxp3+ regulatory T cells: Role of tumor COX-2-derived PGE2. Mol Med Rep. 2010, 3(6):959–63. 10.3892/mmr.2010.374 [DOI] [PubMed] [Google Scholar]
- 11.Zhi X, Wu Y, Bu H, Cheng G, Cheng Y, Du X, et al. Chinese guidelines on the diagnosis and treatment of primary lung cancer (2011). Journal of Thoracic Disease, 2012, 4(1):88–101. 10.3978/j.issn.2072-1439.2010.08.01 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Groome P A, Bolejack V, Crowley J J, Kennedy C, Krasnik M, Sobin L H, et al. The IASLC lung cancer staging project: validation of the proposals for revision of the T, N, and M descriptors and consequent stage groupings in the forthcoming (seventh) edition of the TNM classification of malignant tumours. J Thorac Oncol, 2007, 2(8): 694–705. 10.1097/JTO.0b013e31812d05d5 [DOI] [PubMed] [Google Scholar]
- 13.Celli BR, MacNee W. Standards for the diagnosis and treatment of patients with COPD: a summary of the ATS/ERS position paper. Eur Respir J, 2004,23:932–46. [DOI] [PubMed] [Google Scholar]
- 14.Walecki M, Eisel F, Klug J, Baal N, Paradowskadogan A, Wahle E, et al. Androgen receptor modulates Foxp3 expression in CD4+CD25+Foxp3+regulatory T-cells. Mol Biol Cell 2015;26(15):2845–2857. 10.1091/mbc.E14-08-1323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science,2003,299(5609):1057–1061. 10.1126/science.1079490 [DOI] [PubMed] [Google Scholar]
- 16.Miyara M, Yoshioka Y, Kitoh A, Shima T, Wing K, Niwa A, et al. Functional delineation and differentiation dynamics of human CD4+ T cells expressing the FoxP3 transcription factor. Immunity, 2009, 30(6): 899 10.1016/j.immuni.2009.03.019 [DOI] [PubMed] [Google Scholar]
- 17.Wei T, Zhang J, Qin Y, Wu Y, Zhu L, Lu L, et al. Increased expression of immunosuppressive molecules on intratumoral and circulating regulatory T cells in non-small-cell lung cancer patients. Am J Cancer Res 2015;5(7):2190–2201. [PMC free article] [PubMed] [Google Scholar]
- 18.Shen X, Li N, Li H, et al. Increased prevalence of regulatory T cells in the tumor microenvironment and its correlation with TNM stage of hepatocellular carcinoma. J Cancer Res Clin Oncol. 2010;136(11):1745–54. 10.1007/s00432-010-0833-8 [DOI] [PubMed] [Google Scholar]
- 19.Tan DB, Fernandez S, Price P, French M A, Thompson P J, Moodley YP. Impaired function of regulatory T-cells in patients with chronic obstructive pulmonary disease (COPD). Immunobiology, 2014,219(12), 975–979. 10.1016/j.imbio.2014.07.005 [DOI] [PubMed] [Google Scholar]
- 20.Domagała-Kulawik J., Hoser G., Dabrowska M., et al. CD4+/CD25+ Cells in Systemic Inflammation in COPD. Scandinavian Journal of Immunology, 2011, 73: 59–65. 10.1111/j.1365-3083.2010.02474.x [DOI] [PubMed] [Google Scholar]
- 21.Chen W, Jin W, Hardegen N, Zhang T, Wang F, Li Q, et al. Conversion of peripheral CD4+CD25- naive T cells to CD4+CD25+ regulatory T cells by TGF-beta induction of transcription factor Foxp3. J Exp Med 2003;198:1875–1886. 10.1084/jem.20030152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rosenkranz D, Weyer S, Tolosa E, Gaenslen A, Berg D, Leyhe T, et al. Higher frequency of regulatory T cells in the elderly and increased suppressive activity in neurodegeneration. J Neuroimmunol 2007; 188 (1/2): 117–127. [DOI] [PubMed] [Google Scholar]
- 23.Peterson RA. Regulatory T-cells: diverse phenotypes integral to immune homeostasis and suppression. Toxicol Pathol 2012;40(2): 186–204. 10.1177/0192623311430693 [DOI] [PubMed] [Google Scholar]
- 24.Lee Kang Mi, Stott Ryan T, Zhao Gaoping, SooHoo Julie, Xiong Wei, Lian Moh Moh, et al. TGF-β-producing regulatory B cells induce regulatory T cells and promote transplantation tolerance. Eur J Immunol 2014;44(6): 1728–1736. 10.1002/eji.201344062 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(XLS)
(XLS)
Data Availability Statement
All relevant data are included within the manuscript and its supporting information files.


