Abstract
Many studies have shown the role of agriculture in the selection and spread of resistance of Anopheles gambiae s.l. to insecticides. However, no study has directly demonstrated the presence of insecticides in breeding sources as a source of selection for this resistance. It is in this context that we investigated the presence of pesticide residues in breeding habitats and their formal involvement in vector resistance to insecticides in areas of West Africa with intensive farming. This study was carried out from June to November 2013 in Dano, southwest Burkina Faso in areas of conventional (CC) and biological cotton (BC) growing. Water and sediment samples collected from breeding sites located near BC and CC fields were submitted for chromatographic analysis to research and titrate the residual insecticide content found there. Larvae were also collected in these breeding sites and used in toxicity tests to compare their mortality to those of the susceptible strain, Anopheles gambiae Kisumu. All tested mosquitoes (living and dead) were analyzed by PCR for species identification and characterization of resistance genes. The toxicity analysis of water from breeding sites showed significantly lower mortality rates in breeding site water from biological cotton (WBC) growing sites compared to that from conventional cotton (WCC) sites respective to both An. gambiae Kisumu (WBC: 80.75% vs WCC: 92.75%) and a wild-type strain (49.75% vs 66.5%). The allele frequencies L1014F, L1014S kdr, and G116S ace -1R mutations conferring resistance, respectively, to pyrethroids and carbamates / organophosphates were 0.95, 0.4 and 0.12. Deltamethrin and lambda-cyhalothrin were identified in the water samples taken in October/November from mosquitoes breeding in the CC growing area. The concentrations obtained were respectively 0.0147ug/L and 1.49 ug/L to deltamethrin and lambdacyhalothrin. Our results provided evidence by direct analysis (biological and chromatographic tests) of the role of agriculture as a source of selection pressure on vectors to insecticides used in growing areas.
Introduction
The current control strategy of malaria is mainly based on limiting human-vector contact by the use of insecticide-treated nets (ITNs), although this last decade, indoor residual spraying (IRS) of insecticides has scaled up many African countries [1]. Pyrethroids are the class of insecticides used to impregnate mosquito nets, because they are effective at low doses and exhibit very little toxicity to humans and the environment [2]. In recent years, ITNs have shown good effectiveness in Gambia and Tanzania by reducing malaria-related morbidity and mortality among children aged 0–5 years [3], as well as in a in a stable transmission zone in Ghana [4]. However, the situation has changed significantly in Benin, where the decreased effectiveness of ITNs has recently been observed [5]. Indeed, in Africa, the first case of resistance of An. gambiae sl to pyrethroids was reported in Ivory Coast [6], and then the phenomenon spread from the Ivory Coast to Burkina Faso [7] a few years later and elsewhere in West Africa [8, 9, 10, 11] with a correlation between resistance and the involvement of the use of the insecticide in agriculture, particularly in the cotton growing regions [12, 13].
Moreover, many studies have demonstrated that mosquito larvae exposed to sub-lethal doses of pollutants, herbicides or pesticides frequently appear more tolerant to insecticides through the induction of their detoxifying system and eventually with other mechanisms, such as resistance genes (kdr and ace-1R) [14, 15]. Indeed, in Cameroon An. gambiae s.l. females were reported to frequently lay their eggs in breeding sites located around agricultural settings, suggesting that larvae may undergo a selection pressure from agricultural pesticides favoring the emergence of resistance [16]. In Khartoum (Sudan), the resistance of Anopheles arabiensis to pyrethroids through metabolic resistance was associated with pesticide usage in an intensive agriculture area [17]. More recently, another Tanzanian field study comparing urban, agricultural and low pollution areas pinpointed the elevated resistance level of An. gambiae s.s. found in proximity of intensive agriculture and identified candidate genes associated with the use of pesticides in agriculture and insecticide resistance [18]. In Burkina Faso, the frequency of resistance genes (kdr) was higher in cotton growing areas usually subjected to insecticide treatments than in rural areas where only food crops were grown without insecticides [19]. Indeed, the emergence and distribution of the resistance to insecticides of Anopheles gambiae s.l. perfectly overlaps the cotton areas where there is an increase of allelic frequencies of kdr mutations L1014F and ace-1R [20, 21] within the past five years and a very extensive geographic spread of resistance towards new cotton areas previously free of resistance [22]. These examples of agricultural-related resistance threaten the efficacy of insecticide-based vector control (LLIN) tools, particularly in West Africa [5, 22, 23].
In order to restore the efficacy of vector control while waiting for new insecticides that could replace existing tools, a resistance management plan recommended by the WHO has been put in place to improve control [24]. This plan involves a frank collaboration between agriculture and public health domains in the management of the use of insecticides.
Several studies suggest that the selection pressure of insecticide resistance is primarily of agricultural origin, although the contribution of multiple distributions of LLINs to the selection of vector resistance cannot be discounted [25]. However, no direct evidence has yet been shown that supports the direct contamination of pesticides into mosquito breeding sites that would lead to resistance selection. Our study is part of an Ecohealth concept to establish evidence of this relationship, and if so, it could serve as a point of discussion between agriculture and public health entities on this growing problem.
Materials and methods
Study area
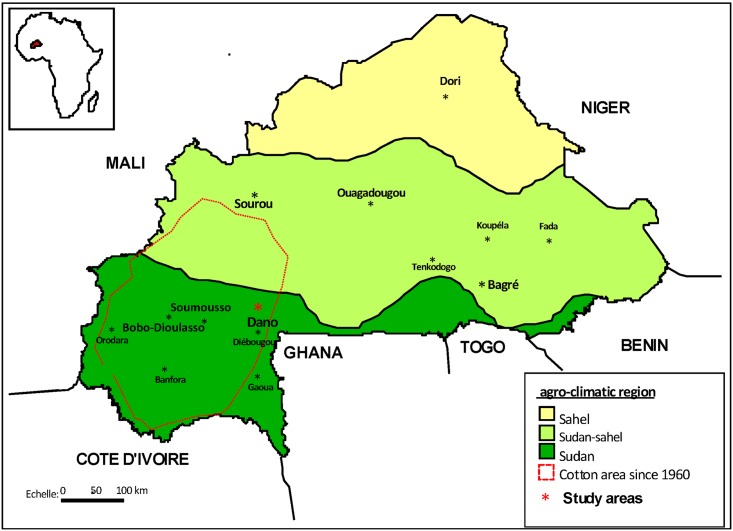
This study was performed in two villages from around the town of Dano, Burkina Faso (Fig 1). The study site was selected due to its easy accessibility and the presence of cotton agriculture that uses both conventional and biological (or organic) growing methods. Conventional and organic cotton are the same crop species, but their growing methods are different. Conventional cotton insecticide applications usually occur in six treatments over fifteen day intervals. These insecticide treatments are applied in rotation and include pyrethroids and organophosphates / carbamates. In contrast, biological cotton is grown using only biological insecticides such as neem extracts.
Fig 1. Figure showing the location of the cotton region since 1960 in Burkina Faso and the sampling sites (Dano, Soumousso and Bobo-Dioulasso).
The study took place near the town of Dano in two neighboring villages (Kompla and Dobao).
Dano (11° 08 '26 "N, 3° 03' 50" W) is located southwestern Burkina Faso and situated in woodland savannah. The mean annual rainfall is 1200 mm, which mostly falls from May to October. Malaria transmission is from June to November. An. gambiae s.l. is the most important malaria vector, followed by Anopheles funestus, which is more prevalent towards the end of the rainy season. Previous studies in this locality showed high resistance of Anopheles gambiae s.l. and particularly its S form to insecticides, owing to a high frequency (0.971 and 0.898, respectively, of the kdr L1014F mutation (knockdown resistance genes from West Africa) [26]. Two types of farms growing cotton were selected for this study: these were in the villages of Dobao (11° 06 '38 9' 'N, 3° 00'35, 9 "W) and Kompla (11° 06 '6.0' 'N; 3° 00' 2 '' W). In the village of Dobao (a new biological cotton growing area since 2004), biological cotton was produced exclusively in all fields, while in Kompla (a conventional cotton area since 1960) both types of cotton were grown. The two villages are neighbors, with no physical barrier (swamps and forest) between them.
We also studied a third site, Soumousso (11° 01'46 "N 4° 02'45" W), where conventional cotton has been exclusively produced in continuation for at least ten years. This locality is situated 30 km from the city of Bobo-Dioulasso and 150 km from Dano. This study site was chosen as a positive, unbiased control to eliminate the possibility of contamination of breeding site water when conventional and biological cotton fields occur in the same village. This study was conducted from January to December 2013 with sample collections covering the rainy season from June to November 2013.
Mosquito larva sampling and testing the toxicity of breeding site water from a conventional and biological cotton growing area
Mosquito larvae were collected during the rainy season (from June to November) corresponding to the period of insecticide treatments. The larvae of wild An. gambiae s.l. were collected in 10 breeding sites in the two farming sites. Small larvae stages (L1 and L2) were selected and used for biological tests with water taken from the breeding sites in the biological and conventional cotton fields. A susceptible strain of An. gambiae s.s. from Kisumu (from Kenya) was reared in the IRSS laboratory and used as a reference strain in this biological test.
The biological test is an indirect bioassay focused on the study of factors capable of inhibiting the normal growth of mosquito larvae in breeding sites. In our study, it was intended to show that in these larval waters there would be toxic compounds in water and sediment samples from breeding sites in the study sites which could affect the normal development of the larval stages of wild and susceptible An. gambiae s.l. populations. The protocol used in this evaluation is mainly based on comparison of the growth of larvae in test breeding sites (water and soil samples from agricultural areas under pesticide pressure) and in control breeding sites (water and soil samples from similar areas, but not under pesticide pressure). About 2,400 An. gambiae larvae were used in this test. 1200 larvae of each strain (wild and susceptible) were distributed into three treatment groups: one treatment group served as a negative control (using spring water free of any pesticide that is used in the IRSS insectary for the maintenance of colony mosquitoes) while the other treatment groups used water from the biological cotton breeding sites (Dobao) and from the conventional cotton breeding sites (Soumousso and Kompla). About 100 first and second stage wild An. gambiae s.l. larvae were exposed into 1L containers with breeding site water or spring water and reared to the adult stage. Each container was covered with mosquito netting and housed in a room where the relative humidity was between 70 to 80% and the temperature between 25–30°C. The larvae were washed and fed every two days. At emergence, adults were fed with glucose (5%). All emerging adults were counted to calculate the mortality rates per treatment group and older females (2 to 5 days post emergence) were used in tests of insecticide susceptibility and the efficacy of LLINs.
Insecticide susceptibility assays
Adult susceptibility assays were carried out with two insecticides representative of those available for use in public health applications, using insecticide-treated filter papers at the diagnostic dose as recommended by the WHO [27] including one type II pyrethroid (0.05% deltamethrin), and one carbamate (0.1% bendiocarb). These molecules were selected because they are used directly or represent families of public health insecticides used for the impregnation of mosquito nets or in indoor residual spraying campaigns. Bioassays were conducted with 2 to 5 days old, non-blood fed adult female mosquitoes. Batches of 20–25 test mosquitoes were exposed for one hour to insecticide impregnated papers. Control mosquitoes (20–25 Kisumu strain females per test) were exposed for the same amount of time to untreated filter papers. After exposure, mosquitoes were transferred into insecticide-free observation tubes and maintained on 5% sucrose solution at a temperature ranging from 25 to 28°C. Final mortality in test and control mosquitoes was recorded 24 h after exposure. The threshold of susceptibility was fixed at 98% mortality rate for the two active molecules according to the WHO’s protocol [27]. When the mortality rates were between 98 and 80%, the population was considered as less susceptible while the population was considered resistant to the tested insecticides when mortality rates were below 80%.
Efficacy tests of mosquito nets
In addition, the efficacy of nets was assessed using the WHO cone test. The nets tested were the PermaNet® 3.0 and Interceptor® which are nets used in the study area for the prevention of malaria. Susceptible and resistant An. gambiae s.s strains were tested separately. Batches of 10 unfed, 2–5 day old female An. gambiae s.s., including the standard susceptible Kisumu strain and wild insecticide-resistant adult mosquitoes, were placed inside the WHO cone and exposed simultaneously for three minutes before being transferred from cones to holding containers. The number of mosquitoes knocked down was then recorded 60 minutes after exposure and the final mortality was recorded 24 hours post-exposure. Survivors were maintained on 5% glucose solution. An untreated net was used as a negative control. Living and dead specimens from this test were separated and retained in silica gel for analysis of molecular forms and identification of resistance genes (kdr and ace-1R).
Species identification and genotyping (kdr and ace-1R)
Genomic DNA from mosquitoes was extracted with 2% cetyl trimethyl ammonium bromide (2% CTAB). Species and molecular forms of An. gambiae s.l. were identified and characterized, following previously published protocol of Santolamazza et al., [28]. Detection of mutations involved in insecticide resistance was also performed by PCR using the protocol of Martinez-Torres et al., [29] for the kdr L1014F mutation and of Weill et al., [30] for the ace-1R G119S mutation.
Chromatographic analysis of water and sediment samples from study sites
A part of each sample of breeding water was sent to a reference laboratory for identification and quantification of possible pesticide residues. The analysis by chromatographic methods aimed to determine the nature of these toxic compounds in water and sediments of breeding sites for Anopheles gambiae s.l. Two rounds of sampling were performed in this study. The first in late July / early August corresponding to the period before insecticide treatment and the second in late October / early November which corresponded to the period after treatment but before the cotton harvest. The quantity of samples required for pesticide residue analysis, were 1000 mL of water and 500 g of sediment / soil. Water and sediment samples from breeding sites were collected at random from different plots of the two comparative (biological and conventional) sites, around and within the cotton fields and following the vertical runoff of rainwater. Each identified plot was sampled in duplicate, mixed, and an amount of at least 1L was taken. Samples of breeding site water were collected using a dipper and put in beakers, whereas soil samples were collected using a soil auger. The breeding site water samples were placed in brown or clear glass bottles that were sterilized in advance and then wrapped with aluminum foil to protect the samples from light. These samples were then placed in coolers containing cold packs and delivered to the laboratory where they were analyzed within 24 hours. The pH value was checked in all samples (required value between 5 and 7.5). Storage of sediment samples (soil) was in plastic bags, which were kept away from moisture. All bottles and plastic bags were mark with an identifying code using permanent ink. All sampling points were geo-referenced. A total of twenty (20) water and sediment samples were randomly collected during the first and second sampling phase per site and sent to the reference laboratory for the detection and quantification of pesticide residue content.
Analytical methods aimed at identifying and quantifying pesticides in different compartments of the environment can be highly differentiated. The best methods are chromatographic methods. Chromatography can separate chemical compounds in a mixture of components by analyzing the mobile and stationary phases (contained in a column). When the mobile phase is a gas, gas chromatography separates the compounds according to their specific affinity for the stationary phase. When the mobile phase is a liquid (typically water-methanol mixtures, water-acetonitrile, etc.) liquid chromatography such as HPLC (High Performance Liquid Chromatography) is used. The pesticides were extracted from the samples of water and sediment (soil) successively by dichloro-methane and hexane. The extracts were then concentrated and purified. The compounds were identified and quantified by gas chromatography with the multi-residue analysis method [31] with a detection limit of 10 ppb. This is a method of detecting pesticides in water, soil and food, among other things. All the families of pesticides were tested according to their sensitivity to different detector chromatographs (GPC / HPLC).
Ethics statement
The study did not involve vertebrates. Field studies did not involve endangered or protected species. For any locations/activities the authors state clearly that no specific permissions were required for these locations/activities. The permission has been obtained from local authorities of the town of Dano (Union of Cotton Producers of Dano, the traditional authorities of the two villages, the Chief Medical Officer of Dano) before the start of the study.
Results
Effects of toxic compounds present in breeding sites on development of An. gambiae larvae
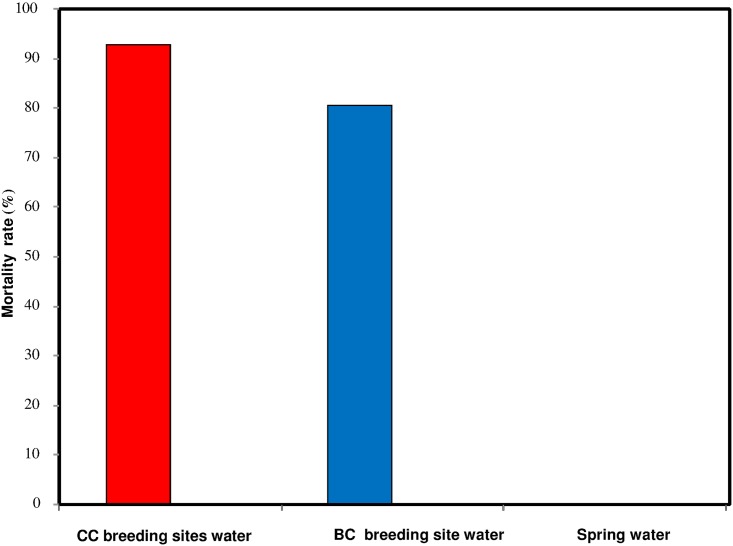
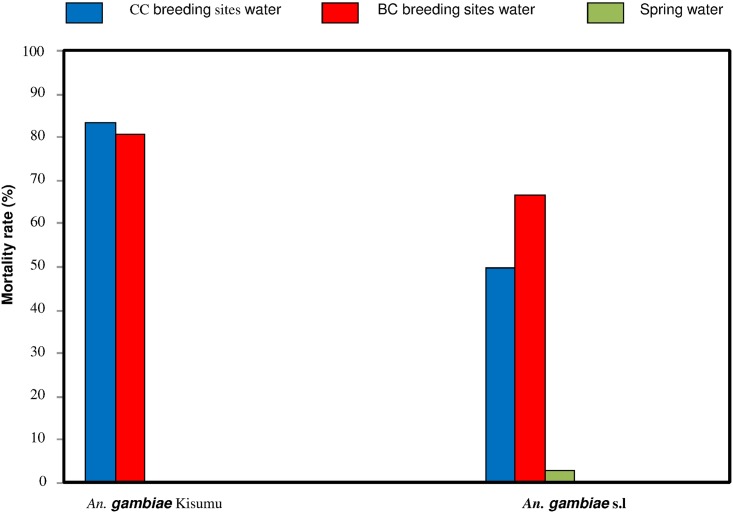
The development of larvae was estimated by quantifying the proportion of first instar larvae reaching the adult stage. This is a good indicator to assess the toxicity of breeding site water in which larva may develop. This indicator takes into consideration the developmental cycle of larvae from first instars to adults. This cycle is relatively long and involves many interactions between larvae and constituents of breeding site waters which allows expressions of inhibiting factors in simulated breeding sites. The first results obtained with the susceptible Kisumu strain of An. gambiae exposed to the spring water from the region of Nasso and to breeding site water from a conventional cotton site (Soumousso) showed that with spring water (negative control), the larval mortality was very low (0.3%) whereas this mortality was very high (92.75%) in water from breeding sites in the conventional cotton area (Fig 2). The difference in mortality rates was highly significant (n = 400 P-Kisumu <0.0001, χ = 573, df = 1). Mortality rates obtained with spring water (negative control) were below 5% whatever the strain used, which confirms its non-contamination by insecticide residues or other pollutants. In addition, the mortality rate obtained with the susceptible strain exposed to breeding site waters from Dobao (80.75%), Kompla (83.5%) showed no significant difference (n = 400; P-Kisumu (Dobao vs Kompla) = 0.31, χ = 1.03, df = 1) (Fig 2). But, comparing the susceptible strain mortality in water collected at breeding sites from Soumousso (an exclusive conventional cotton area) and Dobao (an exclusive biological cotton area), we found a highly significant difference (n = 400, P-Kisumu (Dobao vs Soumousso) < 0.0001, χ = 30.83, df = 1) (Fig 2). Furthermore, mortality rates obtained with wild populations of An. gambiae s.l. exposed to breeding site waters from Dobao (49.75%) or Kompla (66.5%) and spring water (3%) were low compared to the mortality rates obtained with the susceptible strain (Fig 3). The statistical difference between the mortality rates obtained with wild populations was highly significant (n = 400, P-wild population (Dobao vs Kompla) < 0.0001; χ = 23.05, df = 1).
Fig 2. Rate of 1st instar An. gambiae Kisumu strain larvae reaching adult stages after exposure to breeding site waters from Soumousso (exclusive conventional cotton area), Dobao (exclusive biological cotton area), or Nasso (spring water control).
Fig 3. Rate of 1st instar An. gambiae s.l strain larvae reaching adult stages after exposure to breeding site waters from Kompla (conventional cotton area), Dobao (exclusive biological cotton area), or Nasso (spring water control).
Resistance to deltamethrin and reduced efficacy of Long Lasting Impregnated Nets (LLINs)
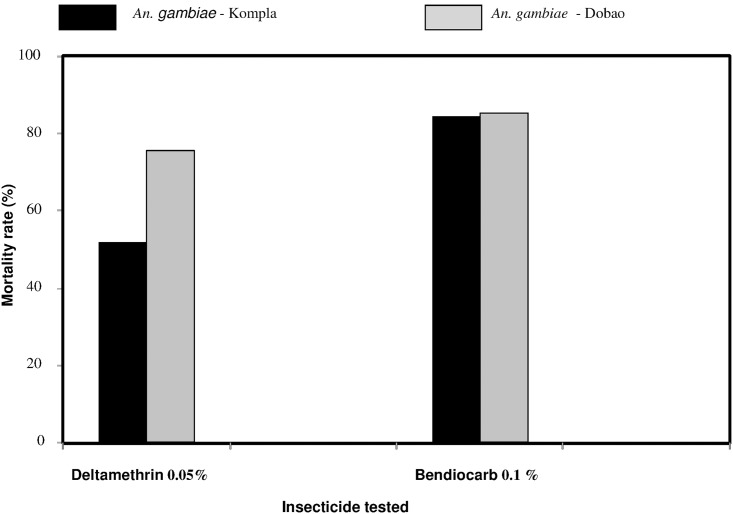
Mortality rates varied according to the insecticide tested. The mortality observed with the susceptible Kisumu strain was 100% with both 0.05% deltamethrin and 0.1% bendiocarb. Mortalities observed with wild populations of An. gambiae s.l. in both localities showed intermediate resistance to bendiocarb, with 85.32% and 84.54%, respectively, from Dobao and Kompla, but the difference was not significant (P>0.05). Wild strains from both Dobao and Kompla were resistant to 0.05% deltamethrin with respective mortality rates of 75.96% and 52.04% (Dobao vs. Kompla, P = 0.48) (Fig 4).
Fig 4. Mortality rates of Anopheles gambiae s.l. from two study sites observed after exposure to 0.05% deltamethrin and 0.1% bendiocarb.
Allele frequency of the kdr gene was not different between the two localities (0.95–0.98) and 0.4–0.33, respectively, for L1014F and L1014S (P> 0.05) and that of the ace-1Rgene frequency did not vary between the two sites (0.12 for both sites).
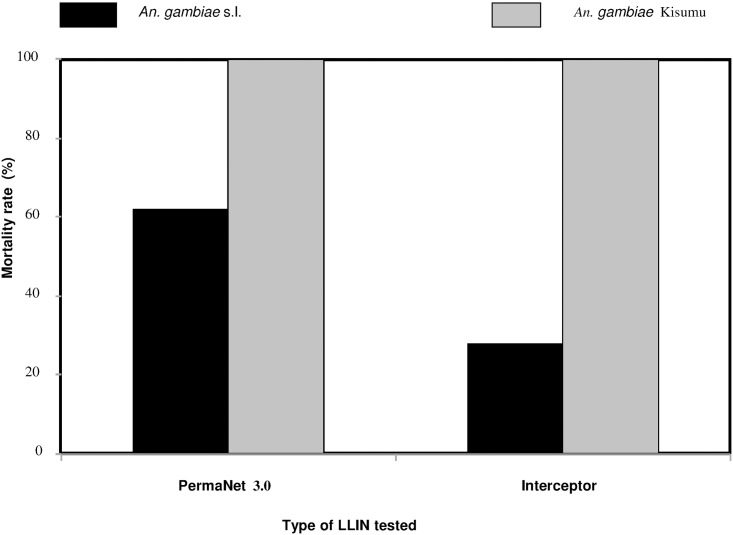
Furthermore, the results of the efficacy test performed on nets (PermaNet® 3.0) used in the two study sites showed a low mortality rate of 62% with the local strain of An. gambiae s.l. (Fig 5). But this net caused 100% mortality in the susceptible reference Kisumu strain. The susceptible strain mortality was very low in experiments with the Interceptor® net (28%).
Fig 5. Efficacy of PermaNet® 3.0 and Interceptor® nets tested against susceptible and wild populations of An. gambiae s.l. study sites.
Pesticide residues detected in the water and sediment from larval breeding study sites using chromatographic analysis
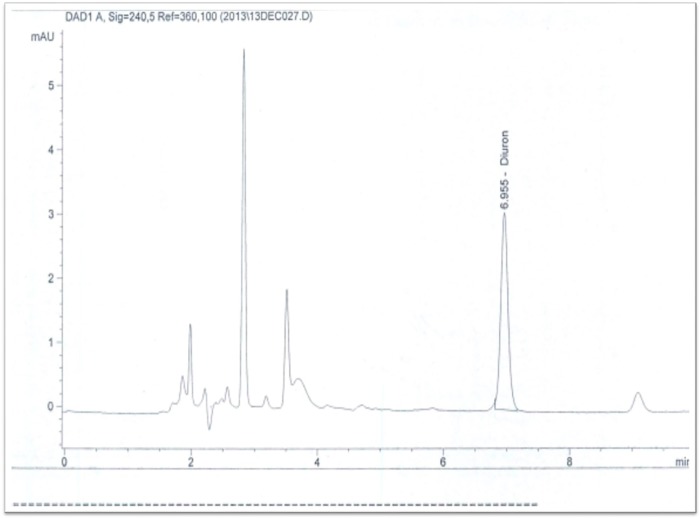
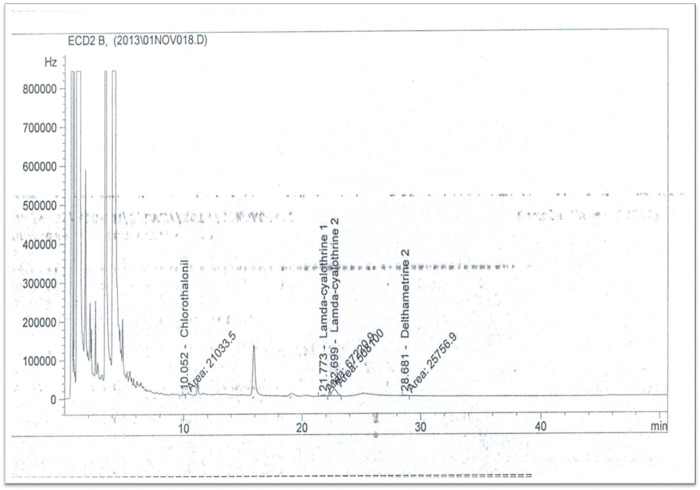
Chromatographic analysis of pesticide residues in the sediments of larval breeding sites located in Dobao and Kompla were performed in late July to early August (before treatment of fields with pesticides) showed the presence of diuron (organophosphate herbicide) at a concentration of 52.39 mg / kg of soil (Fig 6). This contamination was greater in the conventional cotton zone, where a maximum concentration of 105.5 mg diuron / kg of soil were found in Kompla (CC) compared to 29 mg diuron / kg of soil from Dobao (OC). In addition to diuron, other chemicals such as benzoyprop-ethyl, the fungicide chloroneb, pyridate, allethrin and bromacil were also identified in very low concentrations from soil residues in the collected samples. No pesticide residue was detected in any breeding site water at this earlier, pre-treatment collection time. But in late October through early November, corresponding to the period after treatment, the analysis revealed the presence of two pyrethroids in samples of breeding waters from the conventional cotton growing area. These pesticides were deltamethrin and lambda-cyhalothrin at concentrations, respectively, of 0.0164 μg / L and 1.49 μg / L (Fig 7).
Fig 6. GC-μECD chromatogram of a sediment sample taken from a conventional cotton field (Kompla) before insecticide treatment showing the presence of diuron at a concentration of 105mm / kg soil.
Fig 7. GC-μECD chromatograms of a water sample from a conventional cotton growing area after insecticide treatment of cotton fields showing the presence of deltamethrin and lambdacyhalothrin.
Discussion
The high mortality of the Kisumu reference strain larvae observed in the breeding site water from a conventional cotton growing area compared to spring water indicates the presence of toxic compounds in the water of the conventional cotton site. In our study, these compounds can be assumed to be pesticides, especially as the analysis of our water and soil samples revealed their presence in varying doses. Indeed, after treatments, these pesticides could be leached by rain water and could be found in the runoff from the fields that constitute the water found in many breeding sites. The insecticide residues in these deposits would exert a selection pressure on larval mosquito populations that are there. The analysis of the toxicity test results performed with wild populations of An. gambiae s.l. from the two study sites showed a statistically significant difference. These results are consistent with our initial hypothesis that the presence of pesticide residues in breeding sites around conventional cotton cultivation plots could be the basis for the selection and spread of insecticide-resistant An. gambiae sl. The failure to observe a significant difference in allele frequencies of the kdr gene in mosquitoes collected from the two different locations may be explained by the lack of a physical barrier between the conventional and organic cotton fields. Indeed, the distance between some fields of organic and conventional growing areas is not large, with an average of one kilometer in some localities where both types of fields overlap. Therefore runoff rainwater from conventional cotton growing areas containing pesticide residues could easily fill breeding sites around organic cotton fields that have not received pesticide treatment. Moreover, the mortality rates in mosquito bioassays and the resistance status of wild An. gambiae s.l. are similar between these two areas. Indeed, the results of WHO tube tests confirmed intermediate resistance to bendiocarb and high resistance to deltamethrin in the two sites that were previously described by Dabire et al., [12], which also showed expanded vector resistance to carbamates and organophosphates in most cotton growing areas in Burkina Faso. These results reinforce those of our bioassays and the data suggest that vector control tools (LLINs) based on pyrethroids in the two study sites are likely to be very compromised. The resistance observed with deltamethrin may be primarily due to the intensive use of this insecticide [13, 19] for the treatment of cotton fields. However, the use of LLINs, especially during the free distribution of insecticide-treated nets in 2010 and 2013, could be a second source of resistance selection pressure because most of these LLINs are impregnated with deltamethrin. Furthermore, the results of tests with commercial nets are in agreement with the results of our mosquito bioassays as evidenced by the level of resistance of An. gambiae s.l. in these communities and the high frequency of the kdr mutation L1014F. These results clearly confirm the existence of selection pressure for resistance exerted by the intensive use of insecticides in agriculture on larval populations of An. gambiae s.l. in agricultural areas in general, and with cotton farming in particular.
Two insecticides have been found in the water in the conventional cotton breeding zones of Kompla. Deltamethrin and lambdacyhalothrin (S1 Table) were found in samples collected during the month of October, 2013, while those collected from late July through early August showed no traces of pesticides in the samples analyzed. The detection of insecticide molecules in this time period reflects the treatments carried out for crop protection from insect pests between September and October following the phenology of cotton plants and the cotton treatment window adopted by producers. The identification of pesticide residues in water and soil collections reinforces all the studies that have suggested a role of agriculture in the selection of resistance of malaria vectors to insecticides [19, 32] and especially in cotton growing areas [13, 19]. In view of these results, we can conclude that the intense use of insecticides in conventional cotton growing areas is highly toxic to the susceptible Kisumu strain of An. gambiae. Even bioassays using insecticide-impregnated papers have shown similar results with significantly higher mortality in mosquitoes collected from the organic cotton growing area. The large-scale promotion of this agriculture that is free from chemical insecticides was probably an advantage that allowed for the presence of insecticide-sensitive of malaria vectors in these localities. We can conclude from this study that it is necessary, more than ever, to undertake farming practices that use little or no chemical insecticides (organic horticulture), which is expected to restore long-term insecticide-sensitive populations of Anopheles gambiae s.l. and which will contribute to the effectiveness of our control tools based on pyrethroids (LLINs and IRS) in these areas of high insecticide use.
Supporting information
(PDF)
(PDF)
Acknowledgments
The authors are grateful to the villagers of all localities for their assistance during the sampling sessions and to the households that facilitated data collections. We are particularly grateful to the provincial cotton union of Dano, National Cotton Producer Union of Burkina Faso (NCPU-B) that facilitated the meeting with farmers of study sites, IDRC Project and National Malaria Control Program-Burkina Faso.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This study was financially supported by the PRIPA project 104263-009 of IDRC granted to DKR. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Guimaraes RM, Asmus CIRF, Meyer A. DDT reintroduction for malaria control: the cost-benefit debate for public health. Cad SaudePublica 2007; 23: 2835–2844. [DOI] [PubMed] [Google Scholar]
- 2.Elliot M. The pyrethroids: early discovery, recent advance and the future. Pesticide Science 1989; 27: 337–351. [Google Scholar]
- 3.D’Alessandro U, Olaleye BO, McGuire W, Langerock P, Bennett S, Aikins MK, et al. Mortality and morbidity from malaria in gambian children after introduction of an impregnated bednets programme. Lancet 1995; 345: 479–483. [DOI] [PubMed] [Google Scholar]
- 4.Binka FN, Kubaje A, Adjuik M, Williams LA, Lengler C, Maude GH, et al. Impact of permethrin treaded bednets on chlid mortality in kassena-Nankana district, Ghana: a ramdomized trial. Trop Med Int Health 1996; 1: 147–154. [DOI] [PubMed] [Google Scholar]
- 5.N'Guessan R, Corbel V, Akogbeto M, Rowland M. Reduced efficacy of insecticide treated nets and indoor residual spraying for malaria control in pyrethroid resistance area, Benin. Emerg Infect Dis 2007; 13: 199–206. 10.3201/eid1302.060631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Elissa N, Mouchet J, Rivière F, Meunier J-Y, Yao K. Resistance of Anopheles gambiae s.s. to pyrethroïds in Côte-d’Ivoire. Ann Soc Belg Méd Trop 1993; 73: 291–294. [PubMed] [Google Scholar]
- 7.Chandre F, Darrier F, Manga L, Akogbeto M, Faye O, Mouchet J, et al. Status of pyrethroid resistance in Anopheles gambiae sensu lato. Bull World Health Organ 1999; 77: 230–234. [PMC free article] [PubMed] [Google Scholar]
- 8.Fanello C, Petrarca V, della Torre A, Santolamazza F, Dolo G, Coulibaly M, et al. The pyrethroid knock-down resistance gene in the Anopheles gambiae complex in Mali and further indication of incipient speciation within An. gambiae ss. Insect Mol Biol 2003; 12: 241–245. [DOI] [PubMed] [Google Scholar]
- 9.Awolola TS, Oyewole IO, Amajoh CN, Idowu ET, Ajayi MB, Oduola A, et al. Distribution of the molecular forms of Anopheles gambiae and pyrethroid knock down resistance gene in Nigeria. Acta Trop 2005; 95: 204–209. 10.1016/j.actatropica.2005.06.002 [DOI] [PubMed] [Google Scholar]
- 10.Czeher C, Labbo R, Arzika I, Duchemin JB. Evidence of increasing Leu-Phe knockdown resistance mutation in Anopheles gambiae from Niger following a nationwide long-lasting insecticide-treated nets implementation. Malar J 2008; 7:189 10.1186/1475-2875-7-189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yadouleton AW, Padonou G, Asidi A, Moiroux N, Banganna S, Corbel V, et al. Insecticide resistance status in Anopheles gambiae in southern Benin. Malar J 2010; 9: 83 10.1186/1475-2875-9-83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dabiré K.R., Diabaté A., Namountougou M., Djogbenou L., Wondji C., Chandre F, et al. Trends in Insecticide Resistance in Natural Populations of Malaria Vectors in Burkina Faso, West Africa: 10 Years’ Surveys. In: Perveen F, editors. Insecticides—Pest Engineering 2012; 22:479–502. [Google Scholar]
- 13.Yadouleton A., Martin T., Padonou G., Chandre F., Asidi A., Djogbenou L, et al. Cotton pest management practices and the selection of pyrethroid resistance in Anopheles gambiae population in northern Benin. Parasite and Vectors 2011; 4: 60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Akogbeto MC, Djouaka RF, Kinde-Gazard DA. Screening of pesticide residues in soil and water samples from agricultural settings. Malar J 2006; 5:22 10.1186/1475-2875-5-22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.David JP, Coissac E., Melodelima C., Poupardin R., Riaz M. A., Chandor-Proust A et al. Transcriptome response to pollutants and insecticides in the dengue vector Aedes aegypti using next-generation sequencing technology. BMC Genomics, 2010; 11:216 10.1186/1471-2164-11-216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Antonio-Nkondjio C, Fossog BT, Ndo C, Djantio BM, Togouet SZ, Awono-Ambene P,et al. Anopheles gambiae distribution and insecticide resistance in the cities of Douala and Yaounde (Cameroon): influence of urban agriculture and pollution. Malar J. 2011; 10:154 10.1186/1475-2875-10-154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Abuelmaali SA, Elaagip AH, Basheer MA, Frah EA, Ahmed FTA, Elhaj HFA, et al. Impacts of agricultural practices on insecticide resistance in the malaria vector Anopheles arabiensis in Khartoum State, Sudan. PLoS One. 2013; 18; 8(11):e80549 10.1371/journal.pone.0080549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nkya T, Akhouayri I, Poupardin R, Batengana B, Mosha F, Magesa S, et al. Insecticide resistance mechanisms associated with different environments in the malaria vector Anopheles gambiae: a case study in Tanzania. Malar J 2014; 13:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Diabate A, Baldet T, Chandre F, Akogbeto M, Guiguemde TR, Darriet F, et al. The role of agricultural use of insecticides in resistance to pyrethroids in Anopheles gambiae s.l. in Burkina Faso. Am J Trop Med Hyg 2002; 67:617–622. [DOI] [PubMed] [Google Scholar]
- 20.Dabiré KR, Diabaté A, Namountougou M, Djogbenou L, Kengne P, Simard F, et al. Distribution of insensitive acetylcholinesterase (ace-1R) in Anopheles gambiae s.l. populations from Burkina Faso (West Africa). Trop Med Int Health 2009; 14: 396–403(a). 10.1111/j.1365-3156.2009.02243.x [DOI] [PubMed] [Google Scholar]
- 21.Dabiré KR, Diabaté A, Namountougou M, Toe KH, Ouari A, Kengne P, et al. Distribution of pyrethroid and DDT resistance and the L1014F kdr mutation in Anopheles gambiae s.l. from Burkina Faso (West Africa). Trans R Soc Trop Med Hyg 2009; 103: 1113–1120(b). 10.1016/j.trstmh.2009.01.008 [DOI] [PubMed] [Google Scholar]
- 22.Dabiré RK, Namountougou M, Diabaté A, Soma DD, Bado J, Toé HK, et al. Distribution and Frequency of kdr Mutations within Anopheles gambiae s.l. Populations and First Report of the Ace.1G119S Mutation in Anopheles arabiensis from Burkina Faso (West Africa). PLoS One. 2015; 10(11):e0141645 10.1371/journal.pone.0141645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hemingway J, Ranson H, Magill A, Kolaczinski J, Fornadel C, Gimnig J, et al. Averting a malaria disaster: will insecticide resistance derail malaria control? Lancet 2016; 387(10029):1785–8. 10.1016/S0140-6736(15)00417-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.WHO. World Malaria Report. www.who.int/malaria/publications/world_malaria…2014/en/ISBN: 978 92 4 1564830.p 242.2014 December 9.
- 25.Protopopoff N, Verhaeghen K, Van Bortel W, Roelants P, Marcotty T, Baza D, et al. Significant increase in kdr in Anopheles gambiae is associated with an intensive vector control intervention in Burundi highlands. Trop Med Int Health 2008; 13(12):1479–87. 10.1111/j.1365-3156.2008.02164.x [DOI] [PubMed] [Google Scholar]
- 26.Namountougou M, Diabaté A, Etang J, Bass C, Sawadogo SP, Gnankiné O, et al. First report of the L1014S kdr mutation in wild populations of Anopheles gambiae M and S molecular forms in Burkina Faso (West Africa). Acta Trop 2012; 125: 123–127. 10.1016/j.actatropica.2012.10.012 [DOI] [PubMed] [Google Scholar]
- 27.World Health Organization. Report of the WHO informal consultation on test procedures for insecticides on treated surfaces. WHO/CDS/CPC/ MAL/1998.
- 28.Santolamazza F, Mancini E, Simard F, Qi Y, Tu Z, della Torre A. Insertion polymorphisms of SINE200 retrotransposons within speciation islands of Anopheles gambiae molecular forms. Malar J 2008; 7:163 10.1186/1475-2875-7-163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Martinez-Torres D, Chandre F, Williamson MS, Darriet F, Berge JB, Devonchire AL, et al. Molecular characterization of pyrethroid knockdown resistance (kdr) in the major malaria vector Anopheles gambiae s.s. Insect Mol Bio 1998; l7: 179–184. [DOI] [PubMed] [Google Scholar]
- 30.Weill M, Malcolm C, Chandre F, Mogensen K, Berthomieu A, Marquine M, et al. The unique mutation in ace-1 giving high insecticide resistance is easily detectable in mosquito vectors. Insect Mol Biol 2004; 13: 1–7. [DOI] [PubMed] [Google Scholar]
- 31.DFG. Manual of pesticide residue analysis pesticide commission In Hans-Peter Thier and Hans Zeumer (editors). Published by VCH Pesticides Commission, Weinheim, 1987. 28p. [Google Scholar]
- 32.Chouaibou M, Etang J, Brevault T, Nwane P, Hinzoumbe CK, Mimpfoundi R, et al. Dynamics of insecticide resistance in the malaria vector Anopheles gambiaes.l. from an area of extensive cotton cultivation in Northern Cameroon. Trop Med Int Health 2008; 13: 476–486. 10.1111/j.1365-3156.2008.02025.x [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF)
(PDF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.