Abstract
Nucleotide-binding oligomerization domain 2 (NOD2) is an intracellular pattern recognition receptor that senses bacterial peptidoglycan (PGN)-conserved motifs in cytosol and stimulates host immune response. The association of NOD2 mutations with a number of inflammatory pathologies, including Crohn disease (CD), Graft-versus-host disease (GVHD), and Blau syndrome, highlights its pivotal role in host–pathogen interactions and inflammatory response. Stimulation of NOD2 by its ligand (muramyl dipeptide) activates pro-inflammatory pathways such as nuclear factor-κB (NF-κB), mitogen-activated protein kinases (MAPKs), and Caspase-1. A loss of NOD2 function may result in a failure in the control of microbial infection, thereby initiating systemic responses and aberrant inflammation. Because the ligand of Nod2 is conserved in both gram-positive and gram-negative bacteria, NOD2 detects a wide variety of microorganisms. Furthermore, current literature evidences that NOD2 is also able to control viruses’ and parasites’ infections. In this review, we present and discuss recent developments about the role of NOD2 in shaping the gut commensal microbiota and pathogens, including bacteria, viruses, and parasites, and the mechanisms by which Nod2 mutations participate in disease occurrence.
Introduction
The mammalian intestinal tract harbors a community of trillions of bacteria, archaea, fungi, and viruses, which are collectively referred to as the microbiome. It is now well accepted that a mutualistic relationship between host and microbiome is essential for immune homeostasis [1]. The microbiome is required for the development [2] and regulation of intestinal immune responses against commensals and pathogens, thereby maintaining the intestinal homeostasis.
Initiation of the immune response depends on the recognition of microbial-associated molecular patterns (MAMPs) through special cell receptors called pattern recognition receptors (PRRs). PRRs are classified into five distinct genetic and functional clades (for review, see [3]). Most of our knowledge concerning PRRs comes from studies on toll-like receptors (TLRs), which are localized either at the cell surface or within endosomes [4,5]. By contrast, the nucleotide oligomerization domains (Nod)-like receptors (NLRs) are intracellular sensors, including 22 members in humans and 34 members in mice [6]. The activation of multiple PRRs in response to a pathogen triggers nuclear factor-κB (NF-κB), mitogen-activated protein kinases (MAPKs), Caspase-1 activation, and both interleukin 1 (IL-1) and type I interferon (IFN) secretion, inducing inflammation [3].
NOD2, also known as NLRC2, belongs to the NLR family and functions as an intracellular PRR for muramyl dipeptide (MDP) derived from peptidoglycan (PGN) of both gram-positive and gram-negative bacteria [7]. Since its identification in 2001 [8] and its association with Crohn disease (CD) [9,10], the role of NOD2 in both innate and adaptive immune responses gained increasing interest. NOD2 mutations confer highest risks for CD, but also for Graft-versus-host disease (GVHD) [11] and Blau syndrome [12]. Dysregulation of Nod2 signaling causes or contributes to increased infection risks in human and animal models. This review focuses on the role of NOD2 in the recognition and elimination of commensal and pathogenic bacteria, viruses, and parasites in the gut.
NOD2 expression, activation, structure, and signaling
In the intestine, NOD2 is expressed by numerous cell types, including hematopoietic cells [13] (such as T cells [14], B cells [15], macrophages [16], dendritic cells [17], and mast cells [18]) and nonhematopoietic cells (such as Paneth cells [19], stem cells [20], goblet cells [21], and enterocytes [22,23]). NOD2 senses MDP, which is derived from partial degradation of PGN [7]. MDP directly binds to the nucleotide-binding domain of NOD2 [24,25] from amino acids 216 to 821 [25] with an optimal efficiency within a pH ranging from 5.0 to 6.5 [24]. NOD2 is able to detect many types of PGN; however, its level of activation is dependent on the PGN’s origin [26]. Following activation, NOD2 activates NF-κB and MAPK signaling [27,28], thereby contributing to host defense via the production of inflammatory cytokines, antimicrobial molecules [29], and mucins [21].
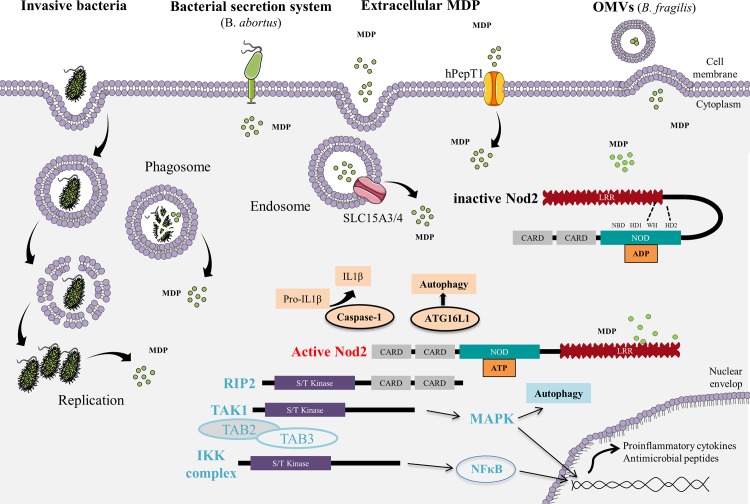
The mechanisms by which PGN enters eukaryotic cells and activates NOD2 remain poorly understood, but several routes of entry have been proposed. Host cells can internalize MDP through either phagocytosis of whole bacteria, endocytosis, uptaking of PGN fragments from outer membrane vesicle (OMVs) [30,31], or transmembrane channels such as hPepT1 [32,33]. A new way of Nod2 activation involving the entry of MDP via the apparatus secretion system of bacteria has recently been reported [34]. NOD2 activation requires its location to be in the vicinity of the site of MDP delivery, close to the plasma membrane or endosomes in which two peptide transporters, SLC15A3 and SLC15A4, may transport MDP toward the cytosolic compartment [32] (Fig 1).
Fig 1. Mechanisms by which MDP enters into cells to trigger Nod2 signaling.
Several routes of MDP entry have been evidenced. Host cells can internalize MDP through either phagocytosis of whole bacteria, endocytosis, uptaking of PGN fragments from OMVs, or transmembrane channels such as hPepT1. A new way of Nod2 activation involving the entry of MDP via the apparatus secretion system of bacteria has recently been described. NOD2 activation requires its location to be in the vicinity of the site of MDP delivery. Two peptide transporters (SLC15A3 and SLC15A4) are able to translocate MDP toward the cytosolic compartment. NOD2 protein exhibits three domains, including caspase activation and recruitment domains (CARDs), nucleotide-binding oligomerization domain (NOD), and leucine-rich repeat (LRR). The NOD module contains a nucleotide-binding domain (NBD), a winged helix (WH), and two helix domains (HD1 and HD2). The interaction between NBD and WH, important to stabilize Nod2 in an inactive form, is maintained by adenosine diphosphate (ADP)-mediated packed conformation. Upon ligand binding, HD2 mediates conformational changes of the NBD, WH, and HD1 to allow ADP-ATP exchange, self-oligomerization, and downstream signaling. The effector CARDs mediate intracellular signaling after interaction between the LRR domain and MDP. NOD2 oligomerization induces a signaling complex named nodosome. NOD2 attracts receptor-interacting serine/threonine-protein kinase 2 (RIP2) via a CARD–CARD homotypic interaction, followed by transforming growth factor beta-activated kinase 1 (TAK1) and TAK1 binding proteins 2 and 3 (TAB2 and TAB3). This complex induces the activation of both MAPKs and NF-κB pathways. The interaction of NOD2 with other partners, including Caspase-1 and ATG16L1, results in IL-1β secretion and autophagy, respectively.
NOD2 protein exhibits three domains, including caspase activation and recruitment domains (CARDs), nucleotide-binding oligomerization domain (NOD), and leucine-rich repeat (LRR). The NOD module contains a nucleotide-binding domain (NBD), a winged helix (WH), and two helix domains (HD1 and HD2). The interaction between NBD and WH, important to stabilize Nod2 in an inactive form, is maintained by ADP-mediated packed conformation [35]. In the absence of MDP binding, the LRR domain prevents NOD2 dimerization. Upon ligand binding, HD2 mediates conformational changes of the NBD, WH, and HD1 to allow ADP-ATP exchange, self-oligomerization, and downstream signaling [36]. The effector CARDs mediate intracellular signaling after interaction between the LRR domain and MDP (Fig 1). NOD2 oligomerization induces a signaling complex named nodosome [37]. The nodosome may be formed at the plasma cell membrane, where bacteria are taken in charge [37]. Among the recruited interactants, NOD2 firstly attracts RIP2 via a CARD–CARD homotypic interaction [8], followed by TAK1 and TAB2 and TAB3 [38]. The kinase activity of TAK1 induces the activation of MAPKs and NF-κB pathways [38]. The interaction of NOD2 with other partners, including Caspase-1 [39] and ATG16L1 [40], results in IL-1β secretion and autophagy, respectively (Fig 1).
NOD2 and the intestinal microbiota
Humans are colonized by a collection of microbes, the largest numbers of which reside in the distal gut. The human gut contains between 500 and 1,000 bacterial species. There is a gradual increase in bacterial populations all along the small bowel, from approximately 104 colony forming units (CFUs) per gram of luminal content in jejunum to 107 in the ileum, with a preponderance of gram-negative aerobes. By contrast, the human colon is highly colonized with anaerobic bacteria, with about 1014 per gram of luminal content. The intestinal microbiota species belong to only eight of the 55 known bacteria phyla (Firmicutes, Bacteroidetes, Actinobacteria, and Proteobacteria phyla being the most widely represented). The gut microbiota acts as a “metabolic organ” through breakdown of indigestible dietary carbohydrates and proteins and generation of fermentation end-products and vitamins. The microbiota contributes also to the intestinal barrier function, which constitutes an obstacle to pathogen invasion of the intestinal mucosa. Commensal bacterial flora is known to be affected by numerous factors, including antibiotics, genetic background, diet, parents, and siblings. Moreover, several human diseases, including inflammatory bowel diseases (IBDs), obesity and metabolic disorders, and infectious and neurological diseases, are linked to a so-called microbiota dysbiosis.
Abnormal interactions between host and microbes (either pathogen or commensal) are involved in IBDs, including CD and ulcerative colitis (UC). IBD physiopathology is associated with significant shifts in the composition of the enteric microbiota (i.e., dysbiosis), notably via an increased richness of the Bacteroidetes, Actinobacteria, and Proteobacteria phyla and a depletion of the Firmicutes phylum [41,42]. The loss of Firmicutes is mostly due to the reduction of species that belong to the bacterial order Clostridiales, particularly members of the Clostridium clusters XIVa and IV [43–45]. One member of this Clostridiales order that is drastically reduced in the ileum of patients with CD is Faecalibacterium prausnitzii [46].
Since Nod2 is an intracellular microbial sensor for gram-positive and gram-negative bacteria, it has been proposed that Nod2 deficiency or mutations can contribute to the modification of microbial composition, and then disease development. In humans, an increased load of Bacteriodetes was observed in the ileal mucosa of CD patients with homozygosity in NOD2 mutations [47]. NOD2 mutations have also been associated with an increased load of Escherichia coli (Proteobacteria) and a reduced load of F. prausnitzii (Firmicutes) [45,47–49]. In mice, numerous studies have reported the key role played by Nod2 in the maintenance of the gut microbiota [21,47,50–55]. Compared to control mice, Nod2KO mice display an increased frequency of the Bacteriodetes phylum and a decrease in the Firmicutes phylum in intestine and feces [21,47,50–55]. As the modifications of the microbiota linked to Nod2 deficiency at genus level is dependent on the conditions of animal housing, the identification of bacterial species impacted by Nod2 remains difficult to establish. Although microbial dysbiosis in Nod2KO mice have been reported by several groups, two studies failed to show significant differences in the gut microbiota when Nod2KO and wild-type (WT) mice were cohoused [56,57]. Indeed, if the cage effect, drift in independent lines, coprophagia, and genetic background have not all been taken into consideration, studies investigating microbiota communities in genetically altered mice are often misleading. Cohousing seems to be a very rigorous strategy, but the absence of any difference between WT and Nod2KO mice [56,57] may result from coprophagia and the subsequent homogenization of mouse microbiota [57]. Indeed, Nod2KO mice obtained by embryo transfer into WT mice exhibit an intestinal microbiota different from their mothers but similar to that of single-housed Nod2KO mice [53]. Thus, the use of embryo transfer strategy, which reduces the impact of environmental and mother parameters, points out the role of Nod2 deficiency in the active acquisition of dysbiosis [53]. WT and Nod2KO mice obtained by embryo transfer into WT mother mice exhibit the same microbiota when housed in the same cage, confirming the homogenization of the gut microbiota between cohoused mice (likely through coprophagia). Moreover, the difference in intestinal flora between WT and Nod2KO offspring and their WT mothers shows that microbial dysbiosis linked to Nod2 deletion is transmissible and dominant [53]. Moreover, microbiota dysbiosis, which occurs in Nod2KO but also in RIP2KO mice, may enhance sensitivity to both colitis and colonic adenocarcinoma. Sensitivity to colitis is transmissible to WT mice via the microbiota after cohousing. Since diet dominates host genotype in shaping the gut microbiota [58], a common dysbiosis shared by people in close contact might explain development of CD in spouses of CD patients and the nonrandom distribution of CD within multiplex sibships [59].
The mechanisms by which Nod2 regulates microbiota communities in the gut are still unclear, even though it is commonly admitted that Nod2 in intestinal epithelial cells plays a major role by promoting the production of antibacterial compounds, including defensins, by Paneth cells [19,29,48,54,60–62]. The impact of the genetic background in the effect of Nod2 deficiency on the expression of defensins is, however, matter of debate [29,57]. Goblet cell abnormalities, including decrease in number and mucins secretion [21], have also been reported to be linked to Nod2 deficiency. The failure in goblet cell function was associated with an overproduction of IFN-γ by intraepithelial lymphocytes and the expansion of Bacteroides vulgatus.
Nod2 not only regulates the bacterial load and microbiota composition but also plays a key role in shaping bacterial translocation and attachment on gut epithelium. Indeed, Nod2KO mice exhibit an increased bacterial translocation of both gram-positive and gram-negative bacteria and the yeast Saccharomyces cerevisiae. This barrier defect is specifically located at Peyer’s patches in the ileum [63]. Although commensal E. coli may attach at all intestinal segments [64], adherent-invasive E. coli (AIEC), known to be associated with CD, has an excessive capacity to attach at the surface of Peyer’s patches in Nod2KO mice [65]. The infiltration of T helper type 1 (Th1) lymphocytes (secreting TNF-α and IFN-γ) resulting in an overexpression of the myosin light chain kinase (MLCK) in epithelial cells was proposed as a mechanism for bacteria translocation across the Peyer’s patches [66]. Similarly, a bacteria-induced overactivation of the MLCK may increase the number of TGF-β-producing regulatory CD4+ T cells in the colonic lamina propria of Nod2KO mice through the induction of an excessive permeability [67]. This reciprocal link between immune cells, intestinal permeability, and microbiota is further evidenced by the fact that endocytosis of commensal bacteria in epithelial cells is dependent on MLCK-activated brush border fanning triggered by IFNγ [68,69]. Thus, Nod2, by regulating the load and the composition of the microbiota, the passage of the intestinal barrier, and the immune response against the intestinal flora (including innate but also Th1, Th2, and Th17 adaptive immunity), acts as a primordial barrier guard [70–73].
NOD2 and pathogens
In addition to its role in the regulation of gut microbiota in normal conditions, NOD2 is involved in the host response against infectious pathogens, including bacteria, viruses, and parasites. A large literature reported that TLR stimulation, required to initiate innate and adaptive immunity upon infection, is modulated by NOD2 [74]. However, as pathogens are sensed by multiple PRRs, Nod2 deficiency has only modest effects on pathogen clearance in vivo [75]. In addition, as exemplified in Brucella abortus infection, Nod2 may also induce inflammation via endoplasmic reticulum stress/Nod2/RIP2 pathway [34].
Bacteria
Since NOD2 is expressed in hematopoietic and nonhematopoietic cells and is able to recognize a fragment of PGN from gram-positive and gram-negative bacteria, it is involved in the control of a large panel of pathogenic bacteria. Over the last 10 years, Nod2 has emerged as a key player in the control of pathogenic bacteria like Campylobacter, Citrobacter, Escherichia, Helicobacter, Listeria, Mycobacteria, Pseudomonas, Staphylococcus, Yersinia, and other species. The variety of the cellular and animal models, as well as the large spectrum of bacterial strains, has led to the identification of many signaling pathways involving Nod2, which sometimes may be contradictory for the same pathogenic bacteria genus. However, the recruitment of RIP2/TAK1 complexes by Nod2 is consistently required to control bacterial infection and related inflammation (Table 1).
Table 1. Role of Nod2 in the host response toward pathogenic bacteria.
| Bacteria | Bacterial susceptibility in Nod2KO | Intestinal inflammation in Nod2KO | Cytokines/ chemokines in Nod2KO | Intestinal permeability | Nod2 and TLR synergy | RIP2 mediated | Activation of Caspase-1 and IL-1β | Refs |
|---|---|---|---|---|---|---|---|---|
| Yersinia pseudotuberculosis, Y. enterocolitica | Decreased | Exacerbated | IL-1β decreased | Increased in WT mice. Unchanged in Nod2KO mice. | Yes (TLR2) | Yes | Yes | [39,87,88] |
| Listeria monocytogenes | Increased | Not studied | IL-6, IL-12 & TNF-α decreased | Increased in WT mice. Not studied in Nod2KO mice. | Not studied | Yes | Not studied | [29,98] |
| Pseudomonas fluorescens | Decreased | Absent | IL-1β & TNF-α decreased | Increased in WT mice. Unchanged in Nod2KO mice. | Not studied | Yes | Yes | [105,108] |
| E. coli | Increased (attachment to M-cells enhanced) | Absent | TNF-α decreased | Increased in WT mice. | Not studied | Not studied | Not studied | [65,122] |
| Citrobacter rodentium | Increased | Reduced at day 12 Increased at day 22 | IFN-γ, IL-17 α CCL2 decreased | Not studied | Not studied | Yes | Not studied | [123, 124, 125] |
| Campylobacter jejuni | Unchanged in Nod2KO mice. Increased in IL10/Nod2KO mice | Absent in Nod2KO mice. Exacerbated in IL10/Nod2KO mice. | Unchanged in Nod2KO mice. IL-1β, TNF-α & CxCL1 decreased in IL10/Nod2KO mice. | Not studied | Not studied | Not studied | Yes | [135,136] |
| Heliobacter hepaticus | Increased | Exacerbated | IFN-γ increased | Not studied | Not studied | Yes | Not studied | [72,110] |
Yersinia
Yersinia genus, a gram-negative rod-shaped bacteria, contains about ten species. Three species are pathogenic for humans and rodents: Y. enterocolitica, Y. pestis, and Y. pseudotuberculosis. Y. enterocolitica and Y. pseudotuberculosis are enteropathogens, able to invade the host through Peyer’s patches [76–78]. Y. pestis is the causative agent of the systemic invasive infectious disease known as plague [79]. All of them cause a wide range of symptoms and pathologies, including diarrhea, gastroenteritis, and mesenteric adenolymphitis, in both humans and rodents [80,81]. These infections are usually acquired by ingestion of contaminated food or water. In mice, oral inoculation with enteropathogenic Yersinia results in translocation of bacteria from the intestine to the spleen and liver and leads to animal death [82]. In some cases, especially in patients with a compromised immune system, enteric Yersinia may disseminate systemically [83,84].
Initial reports on humans suggested that Nod2 is involved in the recognition of pathogenic Yersinia species [85,86]. Peripheral blood mononuclear cells (PBMCs) from homozygous carriers of the NOD23020insC mutation display lower production of anti-inflammatory cytokines in response to Y. enterocolitica, Y. pestis, or Y. pseudotuberculosis [85]. IL-6 production induced by Y. enterocolitica was also impaired in PBMCs from a patient with NOD2 mutations and chronic yersiniosis [86]. When orally inoculated, Y. pseudotuberculosis induces an ileal inflammation associated with an altered permeability of the intestinal barrier mediated by TLR2 [87] and Nod2 signaling [39,88]. Yersinia virulent factor YopJ exacerbates this effect by blocking the NOD2/RIP2/TAK1 signaling pathway and thus facilitating Nod2/Caspase-1 interaction with a subsequent production of IL-1β. In case of Nod2 deficiency, YopJ is no more able to activate the Nod2-dependant Caspase-1 signaling pathway, limiting the ileal inflammation at the beginning of enteral infection [39]. This effect is sufficient to reduce the mortality rate of Nod2KO mice orally inoculated with Y. pseudotuberculosis. By contrast, in naive bone-marrow-derived macrophages (BMDMs), NOD2 [89] and RIP2 [90,91] are dispensable for innate immune response against Y. enterocolitica. The production of cytokines and nitric oxide, the activation of NF-κB and MAPK, and the phagocytic activity remain unchanged in Yersinia-infected BMDMs from Nod2KO mice [91]. In agreement, Meinzer et al. showed that Nod2 was critical in case of infection by Y. pseudotuberculosis via the oral (but not systemic) route in mice [88].
Listeria monocytogenes
Listeria monocytogenes is a causative agent for human listeriosis, a potentially fatal foodborne infection. L. monocytogenes is an intracellular pathogen phagocytosed by monocytes/macrophages that escape from the phagosome into the host cell cytosol via its pore-forming toxin listeriolysin O (LLO) [92]. L. monocytogenes also invades nonphagocytic cells, such as enterocytes and M cells. This process is critical for bacterial translocation through the intestinal epithelium [93–95]. The role of Nod2 in the response against L. monocytogenes is controversial. In an earlier study, Kobayashi and collaborators reported that Nod2KO mice challenged with L. monocytogenes via the intragastrical route are more susceptible to infection, with higher translocation rates from the intestine to the liver and spleen [29,96]. This phenotype is lost in the case of systemic infection. In a later study, Rip2KO mice were shown to be highly susceptible to systemic Listeria infection [97]. In infected Nod2KO mice, the number of L. monocytogenes was not increased in Peyer's patches, suggesting an M cell-independent route of bacterial invasion [29]. To explain the hypersensitivity to Listeria infection, the authors reported a decrease in the production of defensin-related cryptdin 4 (Defcr4) and Defcr-related sequence 10 (Defcr-rs10) by Paneth cells in Nod2KO mice [29]. However, the Sartor group recently reported that WT and Nod2KO mice produced similar levels of a large number of cryptins/α-defensins but do not express Defcr4 [57].
Contradictory results about the role of NOD2 in the induction of pro-inflammatory cytokines by macrophages in response to infection by L. monocytogenes were also reported in vitro [98,99]. RNA interference and other Nod2 inhibition experiments in human PBMCs, as well as experiments using BMDMs from NLRP3 or RIP2KO mice, demonstrated that Listeria-induced IL-1β release was dependent on apoptosis-associated speck-like protein containing a CARD (ASC), Caspase-1, and NLRP3, whereas NOD2, RIP2, NLRP1, NLRP6, NLRP12, NLRC4, and absent in melanoma 2 (AIM2) appeared to be dispensable [100]. Furthermore, in murine BMDMs, Nod1 and Nod2 seem to have redundant functions with regards to Listeria infection. Nod1 or Nod2 deficiency alone does not result in a significant alteration in cytokine response to Listeria infection, while cytokine production is downregulated in Rip2KO and Nod1-Nod2DKO macrophages [101]. Attachment of bacteria to the cell surface is sufficient to activate macrophages [102]. This finding is consistent with the observation that Nod2 and RIP2 cooperate with TLR signaling for optimal responses to TLR ligands [101].
P. fluorescens
P. fluorescens is present at low numbers in the intestinal lumen and in many ecological niches, including soil, water, and refrigerated food [103]. Although P. fluorescens has long been considered a psychotrophic microorganism, some clinical strains have been able to adapt at a growth temperature of 37 °C [104]. Clinical strains of P. fluorescens were shown to increase the paracellular permeability, cell cytotoxicity, and cytokine response in human enterocyte cells lines [105–107]. In vivo, P. fluorescens increases the paracellular permeability of the intestinal mucosa via the release of IL-1β by immune cells and the activation of MLCK in the epithelial cells in a Nod2-dependent way [108].
H. hepaticus
H. hepaticus is the best studied member of the enterohepatic Helicobacter species. This gram-negative microaerophilic bacterium is an opportunistic pathogen [109] that induces colitis in immunodeficient mice. In both Nod2KO and Rip2KO mice, Helicobacter has been associated with the development of colitis (resembling human IBD) and cancer [110]. Nod2KO and Rip2KO mice were reported to be unable to regulate the H. hepaticus load in ileum [72]. Both of them develop a granulomatous ileitis and enlarged Peyer’s patches and mesenteric lymph nodes, with an expansion of IFNγ-producing CD4 and CD8 T cells [72]. Inflammatory Th1 response is associated with Nod2 expression in the crypts of the small intestine, suggesting a role for Paneth cells [72].
Mycobacteria
Mycobacteria are an important group of pathological microorganisms. Worldwide, 2,000,000,000 people are infected with M. tuberculosis, and 2 million people die from tuberculosis each year [111]. Other mycobacterial species, such as M. leprae, are endemic in developing countries and are responsible for high morbidity and disability rates [112]. In patients with a compromised immune system, nonpathogenic mycobacteria may also cause disease. M. avium paratuberculosis (MAP) has been suggested to be associated with CD. This suggestion is controversial, but some findings support a causative role of MAP in the pathogenesis of CD [113]. In cattle, MAP causes Johne disease, which clinically resembles CD [114]. Furthermore, MAP has been identified by PCR and sometimes by culture in gut biopsies from CD patients [115].
As M. paratuberculosis and NOD2 have been involved in CD, the role of NOD2 in the regulation of host susceptibility to M. paratuberculosis has been investigated [116]. NF-κB activation in NOD2-transfected HEK293 cells was found to be dose-dependent on MAP exposure [116]. Moreover, MAP-infected PBMCs from CD patients synthetize less inflammatory cytokines in case of NOD2 mutations [116]. Of note, genomewide association studies have evidenced an association between NOD2 and RIP2 polymorphisms and leprosy caused by M. leprae [117]. Recently, synthesis of characteristic Mycobacterium PGN fragments has been shown to modulate the innate immune responses of Nod1 and Nod2 [118].
E. coli
E. coli is widely spread in many ecological systems, including the human gut, where most bacteria are friendly commensal but a few strains are well-known pathogens [119]. Pathogenic E. coli strains are divided into two major groups: extra-intestinal pathogenic E. coli (ExPEC) and intestinal pathogenic E. coli (InPEC). Among the InPEC strains causing diarrheagenic infections, several well-defined pathotypes have been identified, including enteropathogenic E. coli (EPEC), enterotoxigenic E. coli (ETEC), enterohemorrhagic E. coli (EHEC), enteroaggregative E. coli (EAEC), enteroinvasive E. coli (EIEC), and AIEC [119]. AIEC interact with mouse and human Peyer’s patches via long polar fimbriae (LPF) and translocate across the M cells at the surface of Peyer’s patches[65]. AIEC are abnormally present in chronic ileal lesions of CD [120,121], and they frequently exhibit the LPF operon [65]. Although Nod2KO mice do not develop macroscopic lesions of colitis, gut colonization by AIEC does not require antibiotics as for WT mice [65,122].
C. rodentium
C. rodentium is a mouse-restricted pathogen. It colonizes intestinal mucosa and shares several pathogenic mechanisms with EPEC and EHEC, which are two clinically important human gastrointestinal pathogens [123]. C. rodentium induces a marked infiltration of inflammatory cells ten days after infection, and the colonization is resolved three weeks later [124]. The development of a humoral response against C. rodentium is required for this clearance [125]. Nod2 regulates the bacterial clearance by controlling the production of CCL2 and the subsequent influx of circulating inflammatory monocytes at the site of infection [126]. The regulation of CCL2 by Nod2 is mediated by hematopoietic and nonhematopoietic cells [126]. Colonic stromal cells producing CCL2 and pro-inflammatory CCR2-expressing Ly6Chi monocytes are required for the clearance of C. rodentium [126]. Signaling pathways involved in Nod2-mediated clearance of C. rodentium include activation of NF-κB, MAPKs, and inflammasome, as well as autophagy [127–130].
C. jejuni
C. jejuni is a gram-negative spiral-shaped bacteria that colonizes and survives as a commensal in the gastrointestinal tract of many animals and humans [128]. It is the foremost cause of bacterial foodborne diarrheal diseases worldwide, with up to 2.4 million cases annually in the United States alone. The main sources of transmission to humans are the consumption and handling of contaminated poultry. The “invasive” nature of C. jejuni led to investigation of the contribution of cytoplasmic PRRs as Nod1 and Nod2 in initiating the host response. Zilbauer et al. suggested that NOD1 (but not NOD2) is a potential PRR for C. jejuni in intestinal epithelial cells in vitro [131]. In agreement, although C. jejuni products elicit an inflammatory response from intestinal epithelial cells through the activation of NF-κB and the release of CXCL8 [132,133], Nod2KO mice failed to develop colitis [134,135]. However, NOD2 signaling seems critical to control campylobacteriosis in IL-10KO mice by improving nitric-oxide-dependent bactericidal activity [135].
Viruses
During infection with viruses, TLR activation induces the production of type I IFN, which plays an important role in antiviral defense [136,137]. TLR-recognizing viral motifs include TLR3 for viral double stranded RNA [138], TLR7 and TLR8 for viral single stranded RNA [139], TLR9 for DNA containing unmethylated CpG motifs present in numerous viral pathogens, and TLR13 for bacterial ribosomal RNA. The regulatory role of Nod2 in viral infections is related to its capacity to sense microbiota-derived MDP and to modulate the TLR pathways activated by RNA and DNA viruses, including respiratory syncytial virus (RSV), influenza A virus (IAV), human immunodeficiency virus type-1 (HIV-1), norovirus (NV), and human enterovirus species B (HEV-B) (Table 2). MDP upregulates the production of IFN-β in PBMCs infected by RSV [140], a response that is lost when NOD2 is mutated [140]. In agreement with the role of Nod2 in antiviral response, Nod2KO and RIP2KO mice are hypersensitive to infection with RSV. This hypersensitivity is associated with a failure in mitochondria autophagy and superoxide overproduction, resulting in mitochondrial damage and activation of the NLRP3 inflammasome and subsequent IL-18 release [141]. Nod2 also regulates the innate anti-RSV response via its interaction with the adaptor protein MAVS (mitochondrial antiviral signaling) [142].
Table 2. Role of Nod2 in the host response toward viruses.
| Bacteria | Viral susceptibility in Nod2KO | Nod2 expression | Viruses replication/ reactivation Nod2KO | Viral clearance in Nod2ko | Nod2 and TLR synergy | Enhanced inflammatory cytokines/ chemokines | RIP2 mediated | Refs |
|---|---|---|---|---|---|---|---|---|
| RNA viruses | ||||||||
| RSV | Increased | Enhanced | Reduced | Enhanced | Yes (TLR3) | IL-1β & TNF-α | Yes | [141,142] |
| IAV | Increased | Not studied | Reduced | Enhanced | Not studied | IFN-γ | Not studied | [143] |
| HIV-1 | Not studied | Enhanced | Enhanced | Not studied | CXCL8 | Not studied | [144] | |
| NV | Decreased | Enhanced | Not studied | Not studied | Yes | TNF-α | Yes | [145] |
| RNA viruses | ||||||||
| Human cytomegalovirus (HCMV) | Not studied | Yes | Increased replication | Not studied | Not studied | CXCL8 | Yes | [146] |
| Human herpes viruses (HVs) | Not studied | No | Increased reactivation in case of NOD2 mutation | Not studied | Not studied | Not studied | Not studied | [147] |
Although the innate immune system is able to trigger an inflammatory response to viruses, efficient clearance requires the combined efforts of both innate and adaptive immunity. Indeed, Nod2KO mice infected IAV exhibit reduced IFN-β levels, fewer activated dendritic cells, and virus-specific CD8+ T cells that produce low levels of IFN-γ. Nod2KO dendritic cells have a lower costimulatory capacity and are more prone to cell death [143]. Similarly, some RNA viruses, such as HIV-1, may impact adaptive T cell response via the activation of dectin-1/TLR2 and NOD2 in dendritic cells [144]. Moreover, infection by RNA viruses, including RSV, NV, and HIV-1, is commonly associated with Nod2 upregulation, which results in the overproduction of TNF-α [145].
Nod2 is also involved in the control of the replication or reactivation of DNA viruses, including HCMV and HVs. Similar to RNA viruses, HCMV upregulates NOD2 as early as two hours post-infection and for up to 24 hours afterward [146]. As shown in HCMV-infected cells, the overexpression of NOD2 or its downstream kinase RIP2 leads to the production of both IFN-β and pro-inflammatory cytokines/chemokines [146]. Conversely, NOD2 deficiency, as well as NOD23020insC mutation, downregulates both IFN-β and CXCL8, thereby favoring HCMV replication [146]. In contrast to HCMV, HV is not able to upregulate NOD2. However, the NOD2 mutation SNP8 (2104C>T) has been associated with HV reactivation and bacteremia, with both occurring after allogeneic hematopoietic stem cell transplantation [147].
Parasites and yeasts
Little is known about the role of NOD2 in parasitic or fungal infections. Over the last ten years, growing evidence has reported that Nod2 could be instrumental in controlling Toxoplasma gondii infection, while its role in Leishmania, Trypanosoma cruzia, and Candida albicans infections remains minor (Table 3). T. gondii is an obligate intracellular protozoan pathogen able to infect various animal species, leading to severe diseases, including pneumonia and encephalitis, in immunocompromised hosts. The outcome of T. gondii infection is dependent on the ability of the host to elicit a robust cellular immune response, particularly the production of IFN-γ by natural killer cells and Th1 lymphocytes [148]. The role of Nod2 in the protection of the host is supported by the demonstration that the administration of T. gondii orally induces a more severe ileitis in Nod2KO mice than in WT mice [149]. Infected Nod2KO mice display an increase in the parasitic load in the small intestine and the brain and a higher translocation of bacteria from the gut to the liver, spleen, and kidneys [149]. Reconstitution of T cell-deficient mice with Nod2KO T cells followed by T. gondii infection demonstrated an intrinsic defect of Nod2KO T lymphocytes to produce IL-2 and differentiate into Th1 lymphocytes [14]. Based on an inverse correlation between Nod2 transcript levels and the intracellular survival of Leishmania infantum in macrophages [150], it has been proposed that Nod2 might also play a role in host defense against Leishmania. By contrast, Nod2 has virtually no impact on the outcome of the infections with T. cruzi [151] and C. albicans [152], although chitin particles from the commensal yeast C. albicans induce IL-10 through Nod2 and TLR9 pathways [153]. Finally, a positive association between NOD2 mutations linked to CD and elevated levels of anti-Saccharomyces cerevisiae antibodies in the serum of CD patients has been described [154].
Table 3. Role of Nod2 in the host response toward parasites and yeasts.
| Parasites/yeasts | Intestinal inflammation in Nod2KO | Association between NOD2 mutations and parasite infection | Parasitic load in Nod2KO | Cytokines in Nod2KO | Refs |
|---|---|---|---|---|---|
| T. gondii | Exacerbated | Not studied | Increased | IFNγ & IL-12 decreased | [14,148, 149] |
| Leishmania spp. | Not studied | Not studied | Increased | Not studied | [150] |
| T. cruzi | Not studied | Not studied | Unchanged | Unchanged | [151] |
| C. albicans | Not studied | None | Not studied | Unchanged or IL-10 increased | [152, 153, 154] |
Concluding remarks
The mucosal surfaces of the intestinal tract are constantly exposed to complex microbial communities containing commensal microorganisms and sometimes pathogens. Hosts harbor multiple mechanisms to maintain intestinal barrier integrity and immune tolerance toward commensal bacteria while reacting against pathogens. In this context, NOD2 plays a key role in gut–microbe homeostasis by sensing both commensal and pathogenic microbes and modulating TLR signaling pathways.
CD and UC result from a chronic, uncontrolled immune response against components of the intestinal microbiome in genetically susceptible hosts. Initiation and/or relapse of IBDs are often associated with pathogenic microbes, including bacteria, viruses, and parasites. In genetically predisposed individuals, IBDs occur due to an alteration of the subtle interplay between resident microbiota and the immune system, which often originates from intestinal barrier dysfunction. Over the last 20 years, a large number of studies reported that pathogens (such as Y. pseudotuberculosis, Y. enterocolitica, P. fluorescence, AEIC, and L. monocytogenes) and/or an altered microbiota are often involved in the physiopathology of IBDs. All these bacterial strains may alter paracellular permeability and favor bacterial translocation, but their detrimental effects on host intestinal mucosa are downmodulated by Nod2. CD has also been associated with CMV infection and, to a lesser degree, HV, rotavirus, NV, and adenovirus, all of which alter intestinal permeability. Replication and/or reactivation of most of these viruses, as well as the cycles of parasites and/or yeasts (known to alter intestinal permeability), are regulated by NOD2. Furthermore, Nod2 deficiency is often associated with exacerbated immune responses against pathogens as diverse as bacteria (Y. pseudotuberculosis, H. hepaticus), parasites (T gondii), and viruses (Norovirus). Although the regulatory role of NOD2 in the response of the host against pathogens is largely admitted, its impact on microbiota composition is still a matter of debate. The main difficulty is controlling the environmental parameters known to influence microbiota composition, such as coprophagia, which homogenizes microbiota upon cohousing. The transfer of Nod2KO embryos into WT mothers, which represents an experimental alternative to overcome misleading results, has shown that Nod2 deficiency results in dominant and transmissible microbial dysbiosis. The mechanisms involved and the role of bacterial dysbiosis in the development and/or aggravation of IBD remain unclear, however. Indeed, Nod2KO mice display high numbers of CD4+ T cells in Peyer’s patches and an increased intestinal permeability, but the transfer of microbiota from Nod2KO mice to WT mice alters neither CD4+ T lymphocyte count nor permeability. By contrast, the decreased production of both antimicrobial peptides and mucins by nonhematopoietic cells, as well as susceptibility to colitis, may be acquired by transferring Nod2KO-associated dysbiosis. However, the impact of bacterial dysbiosis on pathogen implantation and vice versa, as well as the contribution of pathogens to the effects of dysbiosis on intestinal inflammation, still remains to be determined.
Funding Statement
Financial support was provided by INSERM, Université Paris Diderot, Assistance Publique Hopitaux de Paris, and Association François Aupetit. We acknowledge the financial support of the Investissements d’Avenir programme ANR-11-IDEX-0005-02, Sorbonne Paris Cite, Laboratoire d’excellence INFLAMEX. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Hooper L V, Littman DR, Macpherson AJ. Interactions between the microbiota and the immune system. Science. 2012;336: 1268–73. 10.1126/science.1223490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Min YW, Rhee P-L. The Role of Microbiota on the Gut Immunology. Clin Ther. 2015;37: 968–75. 10.1016/j.clinthera.2015.03.009 [DOI] [PubMed] [Google Scholar]
- 3.Sellge G, Kufer TA. PRR-signaling pathways–Learning from microbial tactics. Semin Immunol. 2015;27: 75–84. 10.1016/j.smim.2015.03.009 [DOI] [PubMed] [Google Scholar]
- 4.Uematsu S, Akira S. Toll-like receptors and Type I interferons. J Biol Chem. 2007;282: 15319–23. 10.1074/jbc.R700009200 [DOI] [PubMed] [Google Scholar]
- 5.Takeda K, Akira S. Toll-like receptors in innate immunity. Int Immunol. 2005;17: 1–14. 10.1093/intimm/dxh186 [DOI] [PubMed] [Google Scholar]
- 6.Motta V, Soares F, Sun T, Philpott DJ. NOD-like receptors: versatile cytosolic sentinels. Physiol Rev. 2015;95: 149–78. 10.1152/physrev.00009.2014 [DOI] [PubMed] [Google Scholar]
- 7.Girardin SE, Boneca IG, Viala J, Chamaillard M, Labigne A, Thomas G, et al. Nod2 is a general sensor of peptidoglycan through muramyl dipeptide (MDP) detection. J Biol Chem. 2003;278: 8869–72. 10.1074/jbc.C200651200 [DOI] [PubMed] [Google Scholar]
- 8.Ogura Y, Inohara N, Benito A, Chen FF, Yamaoka S, Nunez G. Nod2, a Nod1/Apaf-1 family member that is restricted to monocytes and activates NF-kappaB. J Biol Chem. 2001;276: 4812–8. 10.1074/jbc.M008072200 [DOI] [PubMed] [Google Scholar]
- 9.Hugot JP, Chamaillard M, Zouali H, Lesage S, Cézard JP, Belaiche J, et al. Association of NOD2 leucine-rich repeat variants with susceptibility to Crohn’s disease. Nature. 2001;411: 599–603. 10.1038/35079107 [DOI] [PubMed] [Google Scholar]
- 10.Ogura Y, Bonen DK, Inohara N, Nicolae DL, Chen FF, Ramos R, et al. A frameshift mutation in NOD2 associated with susceptibility to Crohn’s disease. Nature. 2001;411: 603–6. 10.1038/35079114 [DOI] [PubMed] [Google Scholar]
- 11.Holler E, Rogler G, Herfarth H, Brenmoehl J, Wild PJ, Hahn J, et al. Both donor and recipient NOD2/CARD15 mutations associate with transplant-related mortality and GvHD following allogeneic stem cell transplantation. Blood. 2004;104: 889–94. 10.1182/blood-2003-10-3543 [DOI] [PubMed] [Google Scholar]
- 12.Miceli-Richard C, Lesage S, Rybojad M, Prieur AM, Manouvrier-Hanu S, Häfner R, et al. CARD15 mutations in Blau syndrome. Nat Genet. 2001;29: 19–20. 10.1038/ng720 [DOI] [PubMed] [Google Scholar]
- 13.Penack O, Smith OM, Cunningham-Bussel A, Liu X, Rao U, Yim N, et al. NOD2 regulates hematopoietic cell function during graft-versus-host disease. J Exp Med. 2009;206: 2101–10. 10.1084/jem.20090623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shaw MH, Reimer T, Sánchez-Valdepeñas C, Warner N, Kim Y-G, Fresno M, et al. T cell-intrinsic role of Nod2 in promoting type 1 immunity to Toxoplasma gondii. Nat Immunol. 2009;10: 1267–74. 10.1038/ni.1816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Petterson T, Jendholm J, Månsson A, Bjartell A, Riesbeck K, Cardell L-O. Effects of NOD-like receptors in human B lymphocytes and crosstalk between NOD1/NOD2 and Toll-like receptors. J Leukoc Biol. 2011;89: 177–87. 10.1189/jlb.0210061 [DOI] [PubMed] [Google Scholar]
- 16.Hedl M, Li J, Cho JH, Abraham C. Chronic stimulation of Nod2 mediates tolerance to bacterial products. Proc Natl Acad Sci U S A. 2007;104: 19440–5. 10.1073/pnas.0706097104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cooney R, Baker J, Brain O, Danis B, Pichulik T, Allan P, et al. NOD2 stimulation induces autophagy in dendritic cells influencing bacterial handling and antigen presentation. Nat Med. 2010;16: 90–7. 10.1038/nm.2069 [DOI] [PubMed] [Google Scholar]
- 18.Okumura S, Yuki K, Kobayashi R, Okamura S, Ohmori K, Saito H, et al. Hyperexpression of NOD2 in intestinal mast cells of Crohn’s disease patients: preferential expression of inflammatory cell-recruiting molecules via NOD2 in mast cells. Clin Immunol. 2009;130: 175–85. 10.1016/j.clim.2008.08.027 [DOI] [PubMed] [Google Scholar]
- 19.Ogura Y, Lala S, Xin W, Smith E, Dowds TA, Chen FF, et al. Expression of NOD2 in Paneth cells: a possible link to Crohn’s ileitis. Gut. 2003;52: 1591–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nigro G, Rossi R, Commere P-H, Jay P, Sansonetti PJ. The cytosolic bacterial peptidoglycan sensor Nod2 affords stem cell protection and links microbes to gut epithelial regeneration. Cell Host Microbe. 2014;15: 792–8. 10.1016/j.chom.2014.05.003 [DOI] [PubMed] [Google Scholar]
- 21.Ramanan D, Tang MS, Bowcutt R, Loke P, Cadwell K. Bacterial sensor Nod2 prevents inflammation of the small intestine by restricting the expansion of the commensal Bacteroides vulgatus. Immunity. 2014;41: 311–24. 10.1016/j.immuni.2014.06.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hisamatsu T, Suzuki M, Reinecker H-C, Nadeau WJ, McCormick BA, Podolsky DK. CARD15/NOD2 functions as an antibacterial factor in human intestinal epithelial cells. Gastroenterology. 2003;124: 993–1000. 10.1053/gast.2003.50153 [DOI] [PubMed] [Google Scholar]
- 23.Rosenstiel P, Fantini M, Bräutigam K, Kühbacher T, Waetzig GH, Seegert D, et al. TNF-alpha and IFN-gamma regulate the expression of the NOD2 (CARD15) gene in human intestinal epithelial cells. Gastroenterology. 2003;124: 1001–9. 10.1053/gast.2003.50157 [DOI] [PubMed] [Google Scholar]
- 24.Grimes CL, Ariyananda LDZ, Melnyk JE, O’Shea EK. The Innate Immune Protein Nod2 Binds Directly to MDP, a Bacterial Cell Wall Fragment. J Am Chem Soc. 2012;134: 13535–13537. 10.1021/ja303883c [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mo J, Boyle JP, Howard CB, Monie TP, Davis BK, Duncan JA. Pathogen Sensing by Nucleotide-binding Oligomerization Domain-containing Protein 2 (NOD2) Is Mediated by Direct Binding to Muramyl Dipeptide and ATP. J Biol Chem. 2012;287: 23057–23067. 10.1074/jbc.M112.344283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hasegawa M, Yang K, Hashimoto M, Park J-H, Kim Y-G, Fujimoto Y, et al. Differential release and distribution of Nod1 and Nod2 immunostimulatory molecules among bacterial species and environments. J Biol Chem. 2006;281: 29054–63. 10.1074/jbc.M602638200 [DOI] [PubMed] [Google Scholar]
- 27.Opitz B, Püschel A, Schmeck B, Hocke AC, Rosseau S, Hammerschmidt S, et al. Nucleotide-binding oligomerization domain proteins are innate immune receptors for internalized Streptococcus pneumoniae. J Biol Chem. 2004;279: 36426–32. 10.1074/jbc.M403861200 [DOI] [PubMed] [Google Scholar]
- 28.Theivanthiran B, Batra S, Balamayooran G, Cai S, Kobayashi K, Flavell RA, et al. NOD2 signaling contributes to host defense in the lungs against Escherichia coli infection. Infect Immun. 2012;80: 2558–69. 10.1128/IAI.06230-11 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 29.Kobayashi KS, Chamaillard M, Ogura Y, Henegariu O, Inohara N, Nuñez G, et al. Nod2-dependent regulation of innate and adaptive immunity in the intestinal tract. Science. 2005;307: 731–4. 10.1126/science.1104911 [DOI] [PubMed] [Google Scholar]
- 30.Thay B, Damm A, Kufer TA, Wai SN, Oscarsson J. Aggregatibacter actinomycetemcomitans outer membrane vesicles are internalized in human host cells and trigger NOD1- and NOD2-dependent NF-κB activation. Infect Immun. 2014;82: 4034–46. 10.1128/IAI.01980-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chu H, Khosravi A, Kusumawardhani IP, Kwon AHK, Vasconcelos AC, Cunha LD, et al. Gene-microbiota interactions contribute to the pathogenesis of inflammatory bowel disease. Science (80-). 2016;352: 1116–1120. 10.1126/science.aad9948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nakamura N, Lill JR, Phung Q, Jiang Z, Bakalarski C, de Mazière A, et al. Endosomes are specialized platforms for bacterial sensing and NOD2 signalling. Nature. 2014;509: 240–4. 10.1038/nature13133 [DOI] [PubMed] [Google Scholar]
- 33.Vavricka SR, Musch MW, Chang JE, Nakagawa Y, Phanvijhitsiri K, Waypa TS, et al. hPepT1 transports muramyl dipeptide, activating NF-kappaB and stimulating IL-8 secretion in human colonic Caco2/bbe cells. Gastroenterology. 2004;127: 1401–9. [DOI] [PubMed] [Google Scholar]
- 34.Keestra-Gounder AM, Byndloss MX, Seyffert N, Young BM, Chávez-Arroyo A, Tsai AY, et al. NOD1 and NOD2 signalling links ER stress with inflammation. Nature. 2016;532: 394–397. 10.1038/nature17631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Maekawa S, Ohto U, Shibata T, Miyake K, Shimizu T. Crystal structure of NOD2 and its implications in human disease. Nat Commun. 2016;7: 11813 10.1038/ncomms11813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lechtenberg BC, Mace PD, Riedl SJ. Structural mechanisms in NLR inflammasome signaling. Curr Opin Struct Biol. 2014;29: 17–25. 10.1016/j.sbi.2014.08.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tattoli I, Travassos LH, Carneiro LA, Magalhaes JG, Girardin SE. The Nodosome: Nod1 and Nod2 control bacterial infections and inflammation. Semin Immunopathol. 2007;29: 289–301. 10.1007/s00281-007-0083-2 [DOI] [PubMed] [Google Scholar]
- 38.Zhong Y, Kinio A, Saleh M. Functions of NOD-Like Receptors in Human Diseases. Front Immunol. 2013;4: 333 10.3389/fimmu.2013.00333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Meinzer U, Barreau F, Esmiol-Welterlin S, Jung C, Villard C, Léger T, et al. Yersinia pseudotuberculosis effector YopJ subverts the Nod2/RICK/TAK1 pathway and activates caspase-1 to induce intestinal barrier dysfunction. Cell Host Microbe. 2012;11: 337–51. 10.1016/j.chom.2012.02.009 [DOI] [PubMed] [Google Scholar]
- 40.Travassos LH, Carneiro LAM, Ramjeet M, Hussey S, Kim Y-G, Magalhães JG, et al. Nod1 and Nod2 direct autophagy by recruiting ATG16L1 to the plasma membrane at the site of bacterial entry. Nat Immunol. 2010;11: 55–62. 10.1038/ni.1823 [DOI] [PubMed] [Google Scholar]
- 41.Øyri SF, Műzes G, Sipos F. Dysbiotic gut microbiome: A key element of Crohn’s disease. Comp Immunol Microbiol Infect Dis. 2015;43: 36–49. 10.1016/j.cimid.2015.10.005 [DOI] [PubMed] [Google Scholar]
- 42.Ohkusa T, Koido S. Intestinal microbiota and ulcerative colitis. J Infect Chemother. 2015;21: 761–768. 10.1016/j.jiac.2015.07.010 [DOI] [PubMed] [Google Scholar]
- 43.Frank DN, St. Amand AL, Feldman RA, Boedeker EC, Harpaz N, Pace NR. Molecular-phylogenetic characterization of microbial community imbalances in human inflammatory bowel diseases. Proc Natl Acad Sci. 2007;104: 13780–13785. 10.1073/pnas.0706625104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Collins MD, Lawson PA, Willems A, Cordoba JJ, Fernandez-Garayzabal J, Garcia P, et al. The phylogeny of the genus Clostridium: proposal of five new genera and eleven new species combinations. Int J Syst Bacteriol. 1994;44: 812–26. 10.1099/00207713-44-4-812 [DOI] [PubMed] [Google Scholar]
- 45.Frank DN, Robertson CE, Hamm CM, Kpadeh Z, Zhang T, Chen H, et al. Disease phenotype and genotype are associated with shifts in intestinal-associated microbiota in inflammatory bowel diseases. Inflamm Bowel Dis. 2011;17: 179–84. 10.1002/ibd.21339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sokol H, Pigneur B, Watterlot L, Lakhdari O, Bermudez-Humaran LG, Gratadoux J-J, et al. Faecalibacterium prausnitzii is an anti-inflammatory commensal bacterium identified by gut microbiota analysis of Crohn disease patients. Proc Natl Acad Sci. 2008;105: 16731–16736. 10.1073/pnas.0804812105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rehman A, Sina C, Gavrilova O, Häsler R, Ott S, Baines JF, et al. Nod2 is essential for temporal development of intestinal microbial communities. Gut. 2011;60: 1354–62. 10.1136/gut.2010.216259 [DOI] [PubMed] [Google Scholar]
- 48.Li E, Hamm CM, Gulati AS, Sartor RB, Chen H, Wu X, et al. Inflammatory Bowel Diseases Phenotype, C. difficile and NOD2 Genotype Are Associated with Shifts in Human Ileum Associated Microbial Composition. Bereswill S, editor. PLoS ONE. 2012;7: e26284 10.1371/journal.pone.0026284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Knights D, Silverberg MS, Weersma RK, Gevers D, Dijkstra G, Huang H, et al. Complex host genetics influence the microbiome in inflammatory bowel disease. Genome Med. 2014;6: 107 10.1186/s13073-014-0107-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Petnicki-Ocwieja T, Hrncir T, Liu Y-J, Biswas A, Hudcovic T, Tlaskalova-Hogenova H, et al. Nod2 is required for the regulation of commensal microbiota in the intestine. Proc Natl Acad Sci. 2009;106: 15813–15818. 10.1073/pnas.0907722106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mondot S, Barreau F, Al Nabhani Z, Dussaillant M, Le Roux K, Doré J, et al. Altered gut microbiota composition in immune-impaired Nod2(-/-) mice. Gut. 2012;61: 634–5. 10.1136/gutjnl-2011-300478 [DOI] [PubMed] [Google Scholar]
- 52.Couturier-Maillard A, Secher T, Rehman A, Normand S, De Arcangelis A, Haesler R, et al. NOD2-mediated dysbiosis predisposes mice to transmissible colitis and colorectal cancer. J Clin Invest. 2013;123: 700–11. 10.1172/JCI62236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Al Nabhani Z, Lepage P, Mauny P, Montcuquet N, Roy M, Le Roux K, et al. Nod2 deficiency leads to a specific and transmissible mucosa-associated microbial dysbiosis which is independent of the mucosal barrier defect. J Crohns Colitis. 2016; [DOI] [PubMed] [Google Scholar]
- 54.Alnabhani Z, Hugot J-P, Montcuquet N, Le Roux K, Dussaillant M, Roy M, et al. Respective Roles of Hematopoietic and Nonhematopoietic Nod2 on the Gut Microbiota and Mucosal Homeostasis. Inflamm Bowel Dis. 2016;22: 763–73. 10.1097/MIB.0000000000000749 [DOI] [PubMed] [Google Scholar]
- 55.Ramanan D, Bowcutt R, Lee SC, Tang MS, Kurtz ZD, Ding Y, et al. Helminth infection promotes colonization resistance via type 2 immunity. Science (80-). 2016;352: 608–612. 10.1126/science.aaf3229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Robertson SJ, Zhou JY, Geddes K, Rubino SJ, Cho JH, Girardin SE, et al. Nod1 and Nod2 signaling does not alter the composition of intestinal bacterial communities at homeostasis. Gut Microbes. 2013;4: 222–31. 10.4161/gmic.24373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shanahan MT, Carroll IM, Grossniklaus E, White A, von Furstenberg RJ, Barner R, et al. Mouse Paneth cell antimicrobial function is independent of Nod2. Gut. 2014;63: 903–10. 10.1136/gutjnl-2012-304190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Carmody RN, Gerber GK, Luevano JM, Gatti DM, Somes L, Svenson KL, et al. Diet Dominates Host Genotype in Shaping the Murine Gut Microbiota. Cell Host Microbe. 2015;17: 72–84. 10.1016/j.chom.2014.11.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hugot J-P, Zouali H, Lesage S. Lessons to be learned from the NOD2 gene in Crohn’s disease. Eur J Gastroenterol Hepatol. 2003;15: 593–7. 10.1097/01.meg.0000059147.68845.ba [DOI] [PubMed] [Google Scholar]
- 60.Bevins CL, Stange EF, Wehkamp J. Decreased Paneth cell defensin expression in ileal Crohn’s disease is independent of inflammation, but linked to the NOD2 1007fs genotype. Gut. 2009;58: 882-3-4. [PubMed] [Google Scholar]
- 61.Wehkamp J, Stange EF. NOD2 mutation and mice: no Crohn’s disease but many lessons to learn. Trends Mol Med. 2005;11: 307–309. 10.1016/j.molmed.2005.06.003 [DOI] [PubMed] [Google Scholar]
- 62.Wehkamp J, Wang G, Kubler I, Nuding S, Gregorieff A, Schnabel A, et al. The Paneth Cell -Defensin Deficiency of Ileal Crohn’s Disease Is Linked to Wnt/Tcf-4. J Immunol. 2007;179: 3109–3118. [DOI] [PubMed] [Google Scholar]
- 63.Barreau F, Meinzer U, Chareyre F, Berrebi D, Niwa-Kawakita M, Dussaillant M, et al. CARD15/NOD2 is required for Peyer’s patches homeostasis in mice. PLoS ONE. 2007;2: e523 10.1371/journal.pone.0000523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Denou E, Lolmède K, Garidou L, Pomie C, Chabo C, Lau TC, et al. Defective NOD2 peptidoglycan sensing promotes diet-induced inflammation, dysbiosis, and insulin resistance. EMBO Mol Med. 2015;7: 259–74. 10.15252/emmm.201404169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chassaing B, Rolhion N, de Vallée A, Salim SY, Prorok-Hamon M, Neut C, et al. Crohn disease—associated adherent-invasive E. coli bacteria target mouse and human Peyer’s patches via long polar fimbriae. J Clin Invest. 2011;121: 966–75. 10.1172/JCI44632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Barreau F, Madre C, Meinzer U, Berrebi D, Dussaillant M, Merlin F, et al. Nod2 regulates the host response towards microflora by modulating T cell function and epithelial permeability in mouse Peyer’s patches. Gut. 2010;59: 207–17. 10.1136/gut.2008.171546 [DOI] [PubMed] [Google Scholar]
- 67.Amendola A, Butera A, Sanchez M, Strober W, Boirivant M. Nod2 deficiency is associated with an increased mucosal immunoregulatory response to commensal microorganisms. Mucosal Immunol. 2014;7: 391–404. 10.1038/mi.2013.58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wu L-L, Peng W-H, Kuo W-T, Huang C-Y, Ni Y-H, Lu K-S, et al. Commensal Bacterial Endocytosis in Epithelial Cells Is Dependent on Myosin Light Chain Kinase–Activated Brush Border Fanning by Interferon-γ. Am J Pathol. 2014;184: 2260–2274. 10.1016/j.ajpath.2014.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kim D, Kim Y-G, Seo S-U, Kim D-J, Kamada N, Prescott D, et al. Corrigendum: Nod2-mediated recognition of the microbiota is critical for mucosal adjuvant activity of cholera toxin. Nat Med. 2016;22: 961 10.1038/nm0816-961 [DOI] [PubMed] [Google Scholar]
- 70.Su L, Shen L, Clayburgh DR, Nalle SC, Sullivan EA, Meddings JB, et al. Targeted Epithelial Tight Junction Dysfunction Causes Immune Activation and Contributes to Development of Experimental Colitis. Gastroenterology. 2009;136: 551–563. 10.1053/j.gastro.2008.10.081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Caruso R, Warner N, Inohara N, Núñez G. NOD1 and NOD2: signaling, host defense, and inflammatory disease. Immunity. 2014;41: 898–908. 10.1016/j.immuni.2014.12.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Biswas A, Liu Y-J, Hao L, Mizoguchi A, Salzman NH, Bevins CL, et al. Induction and rescue of Nod2-dependent Th1-driven granulomatous inflammation of the ileum. Proc Natl Acad Sci U S A. 2010;107: 14739–44. 10.1073/pnas.1003363107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Geddes K, Rubino SJ, Magalhaes JG, Streutker C, Le Bourhis L, Cho JH, et al. Identification of an innate T helper type 17 response to intestinal bacterial pathogens. Nat Med. 2011;17: 837–44. 10.1038/nm.2391 [DOI] [PubMed] [Google Scholar]
- 74.Watanabe T, Kitani A, Strober W. NOD2 regulation of Toll-like receptor responses and the pathogenesis of Crohn’s disease. Gut. 2005;54: 1515–8. 10.1136/gut.2005.071795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Philpott DJ, Sorbara MT, Robertson SJ, Croitoru K, Girardin SE. NOD proteins: regulators of inflammation in health and disease. Nat Rev Immunol. 2014;14: 9–23. 10.1038/nri3565 [DOI] [PubMed] [Google Scholar]
- 76.Autenrieth IB, Firsching R. Penetration of M cells and destruction of Peyer’s patches by Yersinia enterocolitica: an ultrastructural and histological study. J Med Microbiol. 1996;44: 285–94. 10.1099/00222615-44-4-285 [DOI] [PubMed] [Google Scholar]
- 77.Clark MA, Hirst BH, Jepson MA. M-cell surface beta1 integrin expression and invasin-mediated targeting of Yersinia pseudotuberculosis to mouse Peyer’s patch M cells. Infect Immun. 1998;66: 1237–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Handley SA, Dube PH, Revell PA, Miller VL. Characterization of oral Yersinia enterocolitica infection in three different strains of inbred mice. Infect Immun. 2004;72: 1645–56. 10.1128/IAI.72.3.1645-1656.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Perry RD, Fetherston JD. Yersinia pestis—etiologic agent of plague. Clin Microbiol Rev. 1997;10: 35–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bottone EJ. Yersinia enterocolitica: overview and epidemiologic correlates. Microbes Infect. 1999;1: 323–33. [DOI] [PubMed] [Google Scholar]
- 81.Naktin J, Beavis KG. Yersinia enterocolitica and Yersinia pseudotuberculosis. Clin Lab Med. 1999;19: 523–36, vi. [PubMed] [Google Scholar]
- 82.Heesemann J, Gaede K, Autenrieth IB. Experimental Yersinia enterocolitica infection in rodents: a model for human yersiniosis. APMIS. 1993;101: 417–29. [PubMed] [Google Scholar]
- 83.Abbott M, Galloway A, Cunningham JL. Haemochromatosis presenting with a double Yersinia infection. J Infect. 1986;13: 143–5. [DOI] [PubMed] [Google Scholar]
- 84.Bockemühl J, Roggentin P. [Intestinal yersiniosis. Clinical importance, epidemiology, diagnosis, and prevention]. Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz. 2004;47: 685–91. 10.1007/s00103-004-0865-9 [DOI] [PubMed] [Google Scholar]
- 85.Ferwerda B, McCall MBB, de Vries MC, Hopman J, Maiga B, Dolo A, et al. Caspase-12 and the inflammatory response to Yersinia pestis. PLoS ONE. 2009;4: e6870 10.1371/journal.pone.0006870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Netea MG, van der Leij F, Drenth JPH, Joosten LAB, te Morsche R, Verweij P, et al. Chronic yersiniosis due to defects in the TLR5 and NOD2 recognition pathways. Neth J Med. 2010;68: 310–5. [PubMed] [Google Scholar]
- 87.Jung C, Meinzer U, Montcuquet N, Thachil E, Château D, Thiébaut R, et al. Yersinia pseudotuberculosis disrupts intestinal barrier integrity through hematopoietic TLR-2 signaling. J Clin Invest. 2012;122: 2239–51. 10.1172/JCI58147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Meinzer U, Esmiol-Welterlin S, Barreau F, Berrebi D, Dussaillant M, Bonacorsi S, et al. Nod2 mediates susceptibility to Yersinia pseudotuberculosis in mice. PLoS ONE. 2008;3: e2769 10.1371/journal.pone.0002769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kim Y-G, Park J-H, Daignault S, Fukase K, Núñez G. Cross-tolerization between Nod1 and Nod2 signaling results in reduced refractoriness to bacterial infection in Nod2-deficient macrophages. J Immunol. 2008;181: 4340–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Jeong Y-J, Kim C-H, Kim J-C, Oh S-M, Lee K-B, Park J-H, et al. RIP2/RICK-dependent cytokine production upon Yersinia enterocolitica infection in macrophages with TLR4 deficiency. Scand J Immunol. 2013;78: 401–7. 10.1111/sji.12100 [DOI] [PubMed] [Google Scholar]
- 91.Jeong Y-J, Kim C-H, Song E-J, Kang M-J, Kim J-C, Oh S-M, et al. Nucleotide-binding oligomerization domain 2 (Nod2) is dispensable for the innate immune responses of macrophages against Yersinia enterocolitica. J Microbiol. 2012;50: 489–95. 10.1007/s12275-012-1534-6 [DOI] [PubMed] [Google Scholar]
- 92.Bielecki J, Youngman P, Connelly P, Portnoy DA. Bacillus subtilis expressing a haemolysin gene from Listeria monocytogenes can grow in mammalian cells. Nature. 1990;345: 175–6. 10.1038/345175a0 [DOI] [PubMed] [Google Scholar]
- 93.Corr S, Hill C, Gahan CGM. An in vitro cell-culture model demonstrates internalin- and hemolysin-independent translocation of Listeria monocytogenes across M cells. Microb Pathog. 2006;41: 241–50. 10.1016/j.micpath.2006.08.003 [DOI] [PubMed] [Google Scholar]
- 94.Daniels JJ, Autenrieth IB, Goebel W. Interaction of Listeria monocytogenes with the intestinal epithelium. FEMS Microbiol Lett. 2000;190: 323–8. [DOI] [PubMed] [Google Scholar]
- 95.Pron B, Boumaila C, Jaubert F, Sarnacki S, Monnet JP, Berche P, et al. Comprehensive study of the intestinal stage of listeriosis in a rat ligated ileal loop system. Infect Immun. 1998;66: 747–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kim Y-G, Park J-H, Shaw MH, Franchi L, Inohara N, Núñez G. The cytosolic sensors Nod1 and Nod2 are critical for bacterial recognition and host defense after exposure to Toll-like receptor ligands. Immunity. 2008;28: 246–57. 10.1016/j.immuni.2007.12.012 [DOI] [PubMed] [Google Scholar]
- 97.Chin AI, Dempsey PW, Bruhn K, Miller JF, Xu Y, Cheng G. Involvement of receptor-interacting protein 2 in innate and adaptive immune responses. Nature. 2002;416: 190–4. 10.1038/416190a [DOI] [PubMed] [Google Scholar]
- 98.Leber JH, Crimmins GT, Raghavan S, Meyer-Morse NP, Cox JS, Portnoy DA. Distinct TLR- and NLR-mediated transcriptional responses to an intracellular pathogen. PLoS Pathog. 2008;4: e6 10.1371/journal.ppat.0040006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Stockinger S, Reutterer B, Schaljo B, Schellack C, Brunner S, Materna T, et al. IFN regulatory factor 3-dependent induction of type I IFNs by intracellular bacteria is mediated by a TLR- and Nod2-independent mechanism. J Immunol. 2004;173: 7416–25. [DOI] [PubMed] [Google Scholar]
- 100.Meixenberger K, Pache F, Eitel J, Schmeck B, Hippenstiel S, Slevogt H, et al. Listeria monocytogenes-infected human peripheral blood mononuclear cells produce IL-1beta, depending on listeriolysin O and NLRP3. J Immunol. 2010;184: 922–30. 10.4049/jimmunol.0901346 [DOI] [PubMed] [Google Scholar]
- 101.Park J-H, Kim Y-G, McDonald C, Kanneganti T-D, Hasegawa M, Body-Malapel M, et al. RICK/RIP2 mediates innate immune responses induced through Nod1 and Nod2 but not TLRs. J Immunol. 2007;178: 2380–6. [DOI] [PubMed] [Google Scholar]
- 102.Kobayashi K, Inohara N, Hernandez LD, Galán JE, Núñez G, Janeway CA, et al. RICK/Rip2/CARDIAK mediates signalling for receptors of the innate and adaptive immune systems. Nature. 2002;416: 194–9. 10.1038/416194a [DOI] [PubMed] [Google Scholar]
- 103.Rajmohan S, Dodd CER, Waites WM. Enzymes from isolates of Pseudomonas fluorescens involved in food spoilage. J Appl Microbiol. 2002;93: 205–13. [DOI] [PubMed] [Google Scholar]
- 104.Chapalain A, Rossignol G, Lesouhaitier O, Merieau A, Gruffaz C, Guerillon J, et al. Comparative study of 7 fluorescent pseudomonad clinical isolates. Can J Microbiol. 2008;54: 19–27. 10.1139/w07-110 [DOI] [PubMed] [Google Scholar]
- 105.Madi A, Svinareff P, Orange N, Feuilloley MG, Connil N. Pseudomonas fluorescens alters epithelial permeability and translocates across Caco-2/TC7 intestinal cells. Gut Pathog. 2010;2: 16 10.1186/1757-4749-2-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Sperandio D, Rossignol G, Guerillon J, Connil N, Orange N, Feuilloley MGJ, et al. Cell-associated hemolysis activity in the clinical strain of Pseudomonas fluorescens MFN1032. BMC Microbiol. 2010;10: 124 10.1186/1471-2180-10-124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Madi A, Alnabhani Z, Leneveu C, Mijouin L, Feuilloley M, Connil N. Pseudomonas fluorescens can induce and divert the human β-defensin-2 secretion in intestinal epithelial cells to enhance its virulence. Arch Microbiol. 2013;195: 189–95. 10.1007/s00203-012-0865-3 [DOI] [PubMed] [Google Scholar]
- 108.Alnabhani Z, Montcuquet N, Biaggini K, Dussaillant M, Roy M, Ogier-Denis E, et al. Pseudomonas fluorescens alters the intestinal barrier function by modulating IL-1β expression through hematopoietic NOD2 signaling. Inflamm Bowel Dis. 2015;21: 543–55. 10.1097/MIB.0000000000000291 [DOI] [PubMed] [Google Scholar]
- 109.Solnick J V, Schauer DB. Emergence of diverse Helicobacter species in the pathogenesis of gastric and enterohepatic diseases. Clin Microbiol Rev. 2001;14: 59–97. 10.1128/CMR.14.1.59-97.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Fox JG, Ge Z, Whary MT, Erdman SE, Horwitz BH. Helicobacter hepaticus infection in mice: models for understanding lower bowel inflammation and cancer. Mucosal Immunol. 2011;4: 22–30. 10.1038/mi.2010.61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Frieden TR, Sterling TR, Munsiff SS, Watt CJ, Dye C. T uberculosis. Lancet (London, England). 2003;362: 887–99. [DOI] [PubMed] [Google Scholar]
- 112.Engers H, Morel CM. Leprosy. Nat Rev Microbiol. 2003;1: 94–5. 10.1038/nrmicro764 [DOI] [PubMed] [Google Scholar]
- 113.Shanahan F, O’Mahony J. The mycobacteria story in Crohn’s disease. Am J Gastroenterol. 2005;100: 1537–8. 10.1111/j.1572-0241.2005.50358.x [DOI] [PubMed] [Google Scholar]
- 114.Chacon O, Bermudez LE, Barletta RG. Johne’s disease, inflammatory bowel disease, and Mycobacterium paratuberculosis. Annu Rev Microbiol. 2004;58: 329–63. 10.1146/annurev.micro.58.030603.123726 [DOI] [PubMed] [Google Scholar]
- 115.Naser SA, Shafran I, Schwartz D, El-Zaatari F, Biggerstaff J. In situ identification of mycobacteria in Crohn’s disease patient tissue using confocal scanning laser microscopy. Mol Cell Probes. 2002;16: 41–8. 10.1006/mcpr.2001.0395 [DOI] [PubMed] [Google Scholar]
- 116.Ferwerda G, Kullberg BJ, de Jong DJ, Girardin SE, Langenberg DML, van Crevel R, et al. Mycobacterium paratuberculosis is recognized by Toll-like receptors and NOD2. J Leukoc Biol. 2007;82: 1011–8. 10.1189/jlb.0307147 [DOI] [PubMed] [Google Scholar]
- 117.Zhang F-R, Huang W, Chen S-M, Sun L-D, Liu H, Li Y, et al. Genomewide association study of leprosy. N Engl J Med. 2009;361: 2609–18. 10.1056/NEJMoa0903753 [DOI] [PubMed] [Google Scholar]
- 118.Wang Q, Matsuo Y, Pradipta AR, Inohara N, Fujimoto Y, Fukase K. Synthesis of characteristic Mycobacterium peptidoglycan (PGN) fragments utilizing with chemoenzymatic preparation of meso-diaminopimelic acid (DAP), and their modulation of innate immune responses. Org Biomol Chem. 2016;14: 1013–23. 10.1039/c5ob02145f [DOI] [PubMed] [Google Scholar]
- 119.Moriel DG, Rosini R, Seib KL, Serino L, Pizza M, Rappuoli R. Escherichia coli: great diversity around a common core. MBio. 2012;3: e00118–12-. 10.1128/mBio.00118-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Boudeau J, Glasser AL, Masseret E, Joly B, Darfeuille-Michaud A. Invasive ability of an Escherichia coli strain isolated from the ileal mucosa of a patient with Crohn’s disease. Infect Immun. 1999;67: 4499–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Darfeuille-Michaud A, Boudeau J, Bulois P, Neut C, Glasser A-L, Barnich N, et al. High prevalence of adherent-invasive Escherichia coli associated with ileal mucosa in Crohn’s disease. Gastroenterology. 2004;127: 412–421. [DOI] [PubMed] [Google Scholar]
- 122.Drouet M, Vignal C, Singer E, Djouina M, Dubreuil L, Cortot A, et al. AIEC colonization and pathogenicity: influence of previous antibiotic treatment and preexisting inflammation. Inflamm Bowel Dis. 2012;18: 1923–31. 10.1002/ibd.22908 [DOI] [PubMed] [Google Scholar]
- 123.Collins JW, Keeney KM, Crepin VF, Rathinam VAK, Fitzgerald KA, Finlay BB, et al. Citrobacter rodentium: infection, inflammation and the microbiota. Nat Rev Microbiol. 2014;12: 612–23. 10.1038/nrmicro3315 [DOI] [PubMed] [Google Scholar]
- 124.Mundy R, MacDonald TT, Dougan G, Frankel G, Wiles S. Citrobacter rodentium of mice and man. Cell Microbiol. 2005;7: 1697–706. 10.1111/j.1462-5822.2005.00625.x [DOI] [PubMed] [Google Scholar]
- 125.Simmons CP, Clare S, Ghaem-Maghami M, Uren TK, Rankin J, Huett A, et al. Central role for B lymphocytes and CD4+ T cells in immunity to infection by the attaching and effacing pathogen Citrobacter rodentium. Infect Immun. 2003;71: 5077–86. 10.1128/IAI.71.9.5077-5086.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Kim Y-G, Kamada N, Shaw MH, Warner N, Chen GY, Franchi L, et al. The Nod2 sensor promotes intestinal pathogen eradication via the chemokine CCL2-dependent recruitment of inflammatory monocytes. Immunity. 2011;34: 769–80. 10.1016/j.immuni.2011.04.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Marchiando AM, Ramanan D, Ding Y, Gomez LE, Hubbard-Lucey VM, Maurer K, et al. A deficiency in the autophagy gene Atg16L1 enhances resistance to enteric bacterial infection. Cell Host Microbe. 2013;14: 216–24. 10.1016/j.chom.2013.07.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Park SF. The physiology of Campylobacter species and its relevance to their role as foodborne pathogens. Int J Food Microbiol. 2002;74: 177–88. [DOI] [PubMed] [Google Scholar]
- 129.Yang S, Wang B, Humphries F, Jackson R, Healy ME, Bergin R, et al. Pellino3 ubiquitinates RIP2 and mediates Nod2-induced signaling and protective effects in colitis. Nat Immunol. Nature Publishing Group, a division of Macmillan Publishers Limited. All Rights Reserved.; 2013;14: 927–36. 10.1038/ni.2669 [DOI] [PubMed] [Google Scholar]
- 130.Lupfer CR, Anand PK, Liu Z, Stokes KL, Vogel P, Lamkanfi M, et al. Reactive Oxygen Species Regulate Caspase-11 Expression and Activation of the Non-canonical NLRP3 Inflammasome during Enteric Pathogen Infection. Monack DM, editor. PLoS Pathog. 2014;10: e1004410 10.1371/journal.ppat.1004410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Zilbauer M, Dorrell N, Elmi A, Lindley KJ, Schüller S, Jones HE, et al. A major role for intestinal epithelial nucleotide oligomerization domain 1 (NOD1) in eliciting host bactericidal immune responses to Campylobacter jejuni. Cell Microbiol. 2007;9: 2404–16. 10.1111/j.1462-5822.2007.00969.x [DOI] [PubMed] [Google Scholar]
- 132.Mellits KH, Mullen J, Wand M, Armbruster G, Patel A, Connerton PL, et al. Activation of the transcription factor NF-kappaB by Campylobacter jejuni. Microbiology. 2002;148: 2753–63. 10.1099/00221287-148-9-2753 [DOI] [PubMed] [Google Scholar]
- 133.Zheng J, Meng J, Zhao S, Singh R, Song W. Campylobacter-induced interleukin-8 secretion in polarized human intestinal epithelial cells requires Campylobacter-secreted cytolethal distending toxin- and Toll-like receptor-mediated activation of NF-kappaB. Infect Immun. 2008;76: 4498–508. 10.1128/IAI.01317-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Lippert E, Karrasch T, Sun X, Allard B, Herfarth HH, Threadgill D, et al. Gnotobiotic IL-10; NF-kappaB mice develop rapid and severe colitis following Campylobacter jejuni infection. PLoS ONE. 2009;4: e7413 10.1371/journal.pone.0007413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Sun X, Jobin C. Nucleotide-binding oligomerization domain-containing protein 2 controls host response to Campylobacter jejuni in Il10-/- mice. J Infect Dis. 2014;210: 1145–54. 10.1093/infdis/jiu148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Doughty L, Nguyen K, Durbin J, Biron C. A role for IFN-alpha beta in virus infection-induced sensitization to endotoxin. J Immunol. 2001;166: 2658–64. [DOI] [PubMed] [Google Scholar]
- 137.Nansen A, Randrup Thomsen A. Viral infection causes rapid sensitization to lipopolysaccharide: central role of IFN-alpha beta. J Immunol. 2001;166: 982–8. [DOI] [PubMed] [Google Scholar]
- 138.Alexopoulou L, Holt AC, Medzhitov R, Flavell RA. Recognition of double-stranded RNA and activation of NF-kappaB by Toll-like receptor 3. Nature. 2001;413: 732–8. 10.1038/35099560 [DOI] [PubMed] [Google Scholar]
- 139.Diebold SS, Kaisho T, Hemmi H, Akira S, Reis e Sousa C. Innate antiviral responses by means of TLR7-mediated recognition of single-stranded RNA. Science. 2004;303: 1529–31. 10.1126/science.1093616 [DOI] [PubMed] [Google Scholar]
- 140.Vissers M, Remijn T, Oosting M, de Jong DJ, Diavatopoulos DA, Hermans PWM, et al. Respiratory syncytial virus infection augments NOD2 signaling in an IFN-β-dependent manner in human primary cells. Eur J Immunol. 2012;42: 2727–35. 10.1002/eji.201242396 [DOI] [PubMed] [Google Scholar]
- 141.Lupfer C, Thomas PG, Anand PK, Vogel P, Milasta S, Martinez J, et al. Receptor interacting protein kinase 2-mediated mitophagy regulates inflammasome activation during virus infection. Nat Immunol. 2013;14: 480–8. 10.1038/ni.2563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Sabbah A, Chang TH, Harnack R, Frohlich V, Tominaga K, Dube PH, et al. Activation of innate immune antiviral responses by Nod2. Nat Immunol. 2009;10: 1073–80. 10.1038/ni.1782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Lupfer C, Thomas PG, Kanneganti T-D. Nucleotide oligomerization and binding domain 2-dependent dendritic cell activation is necessary for innate immunity and optimal CD8+ T Cell responses to influenza A virus infection. J Virol. 2014;88: 8946–55. 10.1128/JVI.01110-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Côté SC, Plante A, Tardif MR, Tremblay MJ. Dectin-1/TLR2 and NOD2 agonists render dendritic cells susceptible to infection by X4-using HIV-1 and promote cis-infection of CD4(+) T cells. PLoS ONE. 2013;8: e67735 10.1371/journal.pone.0067735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Kim Y-G, Park J-H, Reimer T, Baker DP, Kawai T, Kumar H, et al. Viral infection augments Nod1/2 signaling to potentiate lethality associated with secondary bacterial infections. Cell Host Microbe. 2011;9: 496–507. 10.1016/j.chom.2011.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Kapoor A, Forman M, Arav-Boger R. Activation of nucleotide oligomerization domain 2 (NOD2) by human cytomegalovirus initiates innate immune responses and restricts virus replication. PLoS ONE. 2014;9: e92704 10.1371/journal.pone.0092704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Jaskula E, Lange A, Kyrcz-Krzemien S, Markiewicz M, Dzierzak-Mietla M, Jedrzejczak WW, et al. NOD2/CARD15 single nucleotide polymorphism 13 (3020insC) is associated with risk of sepsis and single nucleotide polymorphism 8 (2104C>T) with herpes viruses reactivation in patients after allogeneic hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2014;20: 409–14. 10.1016/j.bbmt.2013.12.558 [DOI] [PubMed] [Google Scholar]
- 148.Denkers EY, Gazzinelli RT. Regulation and function of T-cell-mediated immunity during Toxoplasma gondii infection. Clin Microbiol Rev. 1998;11: 569–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Heimesaat MM, Dunay IR, Alutis M, Fischer A, Möhle L, Göbel UB, et al. Nucleotide-Oligomerization-Domain-2 Affects Commensal Gut Microbiota Composition and Intracerebral Immunopathology in Acute Toxoplasma gondii Induced Murine Ileitis. Blader IJ, editor. PLoS ONE. 2014;9: e105120 10.1371/journal.pone.0105120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Turchetti AP, da Costa LF, Romão E de L, Fujiwara RT, da Paixão TA, Santos RL. Transcription of innate immunity genes and cytokine secretion by canine macrophages resistant or susceptible to intracellular survival of Leishmania infantum. Vet Immunol Immunopathol. 2015;163: 67–76. 10.1016/j.vetimm.2014.11.010 [DOI] [PubMed] [Google Scholar]
- 151.Silva GK, Gutierrez FRS, Guedes PMM, Horta C V, Cunha LD, Mineo TWP, et al. Cutting edge: nucleotide-binding oligomerization domain 1-dependent responses account for murine resistance against Trypanosoma cruzi infection. J Immunol. 2010;184: 1148–52. 10.4049/jimmunol.0902254 [DOI] [PubMed] [Google Scholar]
- 152.van der Graaf CAA, Netea MG, Franke B, Girardin SE, van der Meer JWM, Kullberg BJ. Nucleotide oligomerization domain 2 (Nod2) is not involved in the pattern recognition of Candida albicans. Clin Vaccine Immunol. 2006;13: 423–5. 10.1128/CVI.13.3.423-425.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Wagener J, Malireddi RKS, Lenardon MD, Köberle M, Vautier S, MacCallum DM, et al. Fungal Chitin Dampens Inflammation through IL-10 Induction Mediated by NOD2 and TLR9 Activation. Sil A, editor. PLoS Pathog. 2014;10: e1004050 10.1371/journal.ppat.1004050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Vasseur F, Sendid B, Jouault T, Standaert-Vitse A, Dubuquoy L, Francois N, et al. Variants of NOD1 and NOD2 genes display opposite associations with familial risk of Crohn’s disease and anti-saccharomyces cerevisiae antibody levels. Inflamm Bowel Dis. 2012;18: 430–8. 10.1002/ibd.21817 [DOI] [PubMed] [Google Scholar]