Abstract
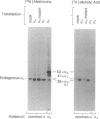
Myristoylation of seven different alpha subunits of guanine nucleotide-binding regulatory proteins (G proteins) was examined by expressing these proteins in monkey kidney COS cells. Metabolic labeling studies of cells transfected with cytomegalovirus-based expression vectors indicated that [3H]myristate was incorporated into alpha i1, alpha i2, alpha i3, alpha 0, alpha t, and alpha z but not alpha s subunits. The role of myristoylation in the association of alpha subunits with membranes was analyzed by site-directed mutagenesis and by substitution of myristate with a less hydrophobic analog, 10-(propoxy)decanoate (11-oxamyristate). Myristoylation of alpha 0 was blocked when an alanine residue was substituted for its amino-terminal glycine, as was association of the protein with membranes. Substitution of the myristoyl group with 11-oxamyristate affected the cellular distribution of a subset of acylated alpha subunits. The results are consistent with a model wherein the hydrophobic interaction of myristate with the bilayer permits continued association of the protein with the plasma membrane when G-protein alpha subunits dissociate from beta gamma.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersson S., Davis D. L., Dahlbäck H., Jörnvall H., Russell D. W. Cloning, structure, and expression of the mitochondrial cytochrome P-450 sterol 26-hydroxylase, a bile acid biosynthetic enzyme. J Biol Chem. 1989 May 15;264(14):8222–8229. [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Buss J. E., Der C. J., Solski P. A. The six amino-terminal amino acids of p60src are sufficient to cause myristylation of p21v-ras. Mol Cell Biol. 1988 Sep;8(9):3960–3963. doi: 10.1128/mcb.8.9.3960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buss J. E., Mumby S. M., Casey P. J., Gilman A. G., Sefton B. M. Myristoylated alpha subunits of guanine nucleotide-binding regulatory proteins. Proc Natl Acad Sci U S A. 1987 Nov;84(21):7493–7497. doi: 10.1073/pnas.84.21.7493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eide B., Gierschik P., Milligan G., Mullaney I., Unson C., Goldsmith P., Spiegel A. GTP-binding proteins in brain and neutrophil are tethered to the plasma membrane via their amino termini. Biochem Biophys Res Commun. 1987 Nov 13;148(3):1398–1405. doi: 10.1016/s0006-291x(87)80287-5. [DOI] [PubMed] [Google Scholar]
- Fong H. K., Yoshimoto K. K., Eversole-Cire P., Simon M. I. Identification of a GTP-binding protein alpha subunit that lacks an apparent ADP-ribosylation site for pertussis toxin. Proc Natl Acad Sci U S A. 1988 May;85(9):3066–3070. doi: 10.1073/pnas.85.9.3066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilman A. G. G proteins: transducers of receptor-generated signals. Annu Rev Biochem. 1987;56:615–649. doi: 10.1146/annurev.bi.56.070187.003151. [DOI] [PubMed] [Google Scholar]
- Hedo J. A., Collier E., Watkinson A. Myristyl and palmityl acylation of the insulin receptor. J Biol Chem. 1987 Jan 25;262(3):954–957. [PubMed] [Google Scholar]
- Heuckeroth R. O., Glaser L., Gordon J. I. Heteroatom-substituted fatty acid analogs as substrates for N-myristoyltransferase: an approach for studying both the enzymology and function of protein acylation. Proc Natl Acad Sci U S A. 1988 Dec;85(23):8795–8799. doi: 10.1073/pnas.85.23.8795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heuckeroth R. O., Gordon J. I. Altered membrane association of p60v-src and a murine 63-kDa N-myristoyl protein after incorporation of an oxygen-substituted analog of myristic acid. Proc Natl Acad Sci U S A. 1989 Jul;86(14):5262–5266. doi: 10.1073/pnas.86.14.5262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurley J. B., Simon M. I., Teplow D. B., Robishaw J. D., Gilman A. G. Homologies between signal transducing G proteins and ras gene products. Science. 1984 Nov 16;226(4676):860–862. doi: 10.1126/science.6436980. [DOI] [PubMed] [Google Scholar]
- Jones D. T., Reed R. R. Molecular cloning of five GTP-binding protein cDNA species from rat olfactory neuroepithelium. J Biol Chem. 1987 Oct 15;262(29):14241–14249. [PubMed] [Google Scholar]
- Magee T., Hanley M. Protein modification. Sticky fingers and CAAX boxes. Nature. 1988 Sep 8;335(6186):114–115. doi: 10.1038/335114a0. [DOI] [PubMed] [Google Scholar]
- Matsuoka M., Itoh H., Kozasa T., Kaziro Y. Sequence analysis of cDNA and genomic DNA for a putative pertussis toxin-insensitive guanine nucleotide-binding regulatory protein alpha subunit. Proc Natl Acad Sci U S A. 1988 Aug;85(15):5384–5388. doi: 10.1073/pnas.85.15.5384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McArdle H., Mullaney I., Magee A., Unson C., Milligan G. GTP analogues cause release of the alpha subunit of the GTP binding protein, GO, from the plasma membrane of NG108-15 cells. Biochem Biophys Res Commun. 1988 Apr 15;152(1):243–251. doi: 10.1016/s0006-291x(88)80706-x. [DOI] [PubMed] [Google Scholar]
- Milligan G., Mullaney I., Unson C. G., Marshall L., Spiegel A. M., McArdle H. GTP analogues promote release of the alpha subunit of the guanine nucleotide binding protein, Gi2, from membranes of rat glioma C6 BU1 cells. Biochem J. 1988 Sep 1;254(2):391–396. doi: 10.1042/bj2540391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mumby S. M., Kahn R. A., Manning D. R., Gilman A. G. Antisera of designed specificity for subunits of guanine nucleotide-binding regulatory proteins. Proc Natl Acad Sci U S A. 1986 Jan;83(2):265–269. doi: 10.1073/pnas.83.2.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mumby S., Pang I. H., Gilman A. G., Sternweis P. C. Chromatographic resolution and immunologic identification of the alpha 40 and alpha 41 subunits of guanine nucleotide-binding regulatory proteins from bovine brain. J Biol Chem. 1988 Feb 5;263(4):2020–2026. [PubMed] [Google Scholar]
- Resh M. D. Specific and saturable binding of pp60v-src to plasma membranes: evidence for a myristyl-src receptor. Cell. 1989 Jul 28;58(2):281–286. doi: 10.1016/0092-8674(89)90842-8. [DOI] [PubMed] [Google Scholar]
- Robishaw J. D., Russell D. W., Harris B. A., Smigel M. D., Gilman A. G. Deduced primary structure of the alpha subunit of the GTP-binding stimulatory protein of adenylate cyclase. Proc Natl Acad Sci U S A. 1986 Mar;83(5):1251–1255. doi: 10.1073/pnas.83.5.1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robishaw J. D., Smigel M. D., Gilman A. G. Molecular basis for two forms of the G protein that stimulates adenylate cyclase. J Biol Chem. 1986 Jul 25;261(21):9587–9590. [PubMed] [Google Scholar]
- Schultz A. M., Tsai S. C., Kung H. F., Oroszlan S., Moss J., Vaughan M. Hydroxylamine-stable covalent linkage of myristic acid in G0 alpha, a guanine nucleotide-binding protein of bovine brain. Biochem Biophys Res Commun. 1987 Aug 14;146(3):1234–1239. doi: 10.1016/0006-291x(87)90780-7. [DOI] [PubMed] [Google Scholar]
- Sternweis P. C. The purified alpha subunits of Go and Gi from bovine brain require beta gamma for association with phospholipid vesicles. J Biol Chem. 1986 Jan 15;261(2):631–637. [PubMed] [Google Scholar]
- Stryer L., Bourne H. R. G proteins: a family of signal transducers. Annu Rev Cell Biol. 1986;2:391–419. doi: 10.1146/annurev.cb.02.110186.002135. [DOI] [PubMed] [Google Scholar]
- Sullivan K. A., Liao Y. C., Alborzi A., Beiderman B., Chang F. H., Masters S. B., Levinson A. D., Bourne H. R. Inhibitory and stimulatory G proteins of adenylate cyclase: cDNA and amino acid sequences of the alpha chains. Proc Natl Acad Sci U S A. 1986 Sep;83(18):6687–6691. doi: 10.1073/pnas.83.18.6687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towler D. A., Adams S. P., Eubanks S. R., Towery D. S., Jackson-Machelski E., Glaser L., Gordon J. I. Purification and characterization of yeast myristoyl CoA:protein N-myristoyltransferase. Proc Natl Acad Sci U S A. 1987 May;84(9):2708–2712. doi: 10.1073/pnas.84.9.2708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towler D. A., Gordon J. I., Adams S. P., Glaser L. The biology and enzymology of eukaryotic protein acylation. Annu Rev Biochem. 1988;57:69–99. doi: 10.1146/annurev.bi.57.070188.000441. [DOI] [PubMed] [Google Scholar]
- Zoller M. J., Smith M. Oligonucleotide-directed mutagenesis: a simple method using two oligonucleotide primers and a single-stranded DNA template. DNA. 1984 Dec;3(6):479–488. doi: 10.1089/dna.1.1984.3.479. [DOI] [PubMed] [Google Scholar]