Abstract
The spotted snakehead Channa punctatus is a seasonally breeding teleost widely distributed in the Indian subcontinent and economically important due to high nutritional value. The declining population of C. punctatus prompted us to focus on genetic regulation of its reproduction. The present study carried out de novo testicular transcriptome sequencing during the four reproductive phases and correlated differential expression of transcripts with various testicular events in C. punctatus. The Illumina paired-end sequencing of testicular transcriptome from resting, preparatory, spawning and postspawning phases generated 41.94, 47.51, 61.81 and 44.45 million reads, and 105526, 105169, 122964 and 106544 transcripts, respectively. Transcripts annotated using Rattus norvegicus reference protein sequences and classified under various subcategories of biological process, molecular function and cellular component showed that the majority of the subcategories had highest number of transcripts during spawning phase. In addition, analysis of transcripts exhibiting differential expression during the four phases revealed an appreciable increase in upregulated transcripts of biological processes such as cell proliferation and differentiation, cytoskeleton organization, response to vitamin A, transcription and translation, regulation of angiogenesis and response to hypoxia during spermatogenically active phases. The study also identified significant differential expression of transcripts relevant to spermatogenesis (mgat3, nqo1, hes2, rgs4, cxcl2, alcam, agmat), steroidogenesis (star, tkt, gipc3), cell proliferation (eef1a2, btg3, pif1, myo16, grik3, trim39, plbd1), cytoskeletal organization (espn, wipf3, cd276), sperm development (klhl10, mast1, hspa1a, slc6a1, ros1, foxj1, hipk1), and sperm transport and motility (hint1, muc13). Analysis of functional annotation and differential expression of testicular transcripts depending on reproductive phases of C. punctatus helped in developing a comprehensive understanding on genetic regulation of spermatogenic and steroidogenic events in seasonally breeding teleosts. Our findings provide the basis for future investigation on the precise role of testicular genes in regulation of seasonal reproduction in male teleosts.
Introduction
Spermatogenesis is an exquisitely orchestrated developmental process during which temporal expression of various testicular genes regulate proliferation and differentiation of diploid spermatogonia to give rise to haploid spermatozoa [1–4]. Nonetheless, reports on molecular control of testicular functions are largely confined to continuous breeders and little attention has been paid to discontinuous breeders in which testis undergoes cyclical changes from inactive to active state depending on season. Among seasonally breeding vertebrates, fishes comprise the largest and most economically important group contributing fifteen percent of average animal protein intake per person for more than 4.3 billion people in the world [5]. In spite of that most of the studies in fishes to comprehend genetic basis of spermatogenesis are restricted to identifying testicular genes [6–18] and only a few reports are focused on differential expression of genes along the testicular cycle [19,20]. The various techniques adopted in these studies were cDNA microarray, EST sequencing, subtractive and suppressive hybridization (SSH), and RNA sequencing (RNA-Seq). Among these, RNA-Seq is the most efficient and cost-effective technique enabling high-throughput sequencing of the entire transcriptome at single-base resolution and accurate quantification of gene expression [21].
In the present study, RNA-Seq using Illumina platform was employed to obtain testicular transcriptome of different reproductive phases from a seasonally breeding freshwater teleost spotted snakehead Channa punctatus belonging to family Channidae and order Perciformes. Fishes of this family constitute one of the major component of pond fishery in the Indian subcontinent and are economically important due to their high nutritional and medicinal value [22]. This species of Channa has been enlisted under the Lower Risk near threatened category due to its declining population [23] and hence, it is important to gain an insight on the genetic regulation of reproduction in this fish. Efforts have been made in the current study to obtain de novo testicular transcriptome from different reproductive phases and develop a comprehensive understanding of temporal expression of genes implicated in regulation of spermatogenesis in C. punctatus.
Methods
Ethics statement
As per guidelines of the Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA), Government of India, the detailed protocol of this study was approved by the Institutional Animal Ethics Committee, Department of Zoology (DUZOOL/IAEC-R/2012/20), University of Delhi, India. To euthanize fishes, 2-phenoxyethanol was added in the water (5 ml per litre).
Animals and tissue collection
The testicular cycle of C. punctatus obtained from Delhi and its vicinity has been delineated into four phases: resting (December to March), preparatory (April to June), spawning (July and August) and postspawning (September to November) [24]. During resting phase, fishes are spermatogenically inactive and their seminiferous lobules consist largely of spermatogonia and a few spermatogonial stem cells. Spermatogenesis commences during preparatory phase which is characterized by the presence of different stages of germ cells from spermatogonia to spermatozoa. Thereafter, during spawning phase, lumen of seminiferous lobules is packed with spermatozoa. Due to release of spermatozoa into the external environment, a few lobules with empty lumen are also seen in spawning phase. Subsequently, remnant germ cells undergo cell death and proliferation of spermatogonia is resumed in order to repopulate the seminiferous lobules during postspawning phase. In the present study, adult male spotted snakehead (100–120 g) captured from freshwater bodies of Delhi (latitude 28.38’N, longitude 77.20’E) and its vicinity were supplied during the mid of January (resting phase), May (preparatory phase), August (spawning phase) and October (postspawning phase) by a local vendor (Kalyanpuri, Delhi, India). Fishes were euthanized by overexposure to 2-phenoxyethanol (Loba Chemie, Mumbai, India) added in water (5 ml per litre). Testes dissected out from ten fishes were pooled to make one sample of 100 mg tissue weight and thus, two such samples were prepared for each reproductive phase. The samples from resting (sample R), preparatory (sample P), spawning (sample S) and postspawning (sample Ps) phases were frozen in liquid nitrogen and stored at -80°C prior to transcriptome sequencing carried out by Genotypic Technology Pvt. Ltd., Bengaluru, India.
RNA extraction, cDNA library preparation and sequencing
One sample from each phase was processed for total RNA extraction with Trizol reagent (Thermo Fisher Scientific, Waltham, Massachusetts, USA) and RNeasy Mini Kit (Qiagen, Valencia, California, USA). RNA concentration and integrity were examined with Bioanalyzer. Samples having A260/A280 absorption ratios within range of 1.8–2.1 and RNA integrity number (RIN) 7 or above were selected for cDNA library preparation. The duplicate tissue sample was processed for RNA extraction only when its corresponding replicate failed to qualify the quality criteria. The protocol outlined in TruSeq RNA sample preparation guide (Illumina, Inc., San Diego, California, USA) was followed for constructing the library. Briefly, mRNA purified from 1 μg of total RNA using oligodT beads (TruSeq RNA Sample Preparation Kit, Illumina) was fragmented at 94°C for 4 min in the presence of divalent cations. Subsequently, mRNA was primed with random hexamers and reverse transcribed using Superscript II reverse transcriptase (Invitrogen, Waltham, Massachusetts, USA). The second cDNA strand was synthesized with DNA Polymerase I and RnaseH and the double-stranded cDNA was purified using solid phase reverse immobilization (SPRI) beads (AgencourtAMPure XP kit, Beckman Coulter, Brea, California, USA). After end repair and addition of base A, Illumina adapters were ligated to the cDNA followed by SPRI cleanup. Further, the adapter ligated fragments were amplified by 11 PCR cycles. The prepared library was quantified using Nanodrop and validated for quality using High Sensitivity Bioanalyzer Kit (Agilent technologies, Santa Clara, California, USA). Finally, Illumina paired-end transcriptome sequencing of the cDNA library was performed on HiSeq 2000 platform to obtain reads of 100 bp length followed by generation of FASTQ files using Illumina pipeline software.
Data filtering and de novo assembly
Obtained FASTQ reads for each sample were subjected to quality check using Genotypic proprietary tool SeqQC- V2.1. Low quality bases (quality score < 20) were trimmed and adapter sequences were removed using custom perl codes. Thereafter, reads ranging between 50–100 bp were selected for de novo assembly into contigs (minimum length 100 bp) using Velvet (version 1.2.07) followed by generation of transcripts (minimum length 200 bp) using Oases (version 0.2.08) assembler. Also, transcripts generated from testicular samples of four reproductive phases (R, P, S and Ps) were clustered using CD-Hit tool [25] to obtain the total testicular transcriptome.
Functional annotation of transcripts
Transcripts obtained from testicular sample of each reproductive phase were annotated based on the best hit of BLASTX results against reference protein sequences of Rattus norvegicus, Oreochromis niloticus and Takifugu rubripes available at Uniprot. Maximum number of transcripts in each reproductive phase was annotated against Uniprot R. norvegicus reference protein sequences, and hence, the same protein database was used for Gene Ontology (GO) annotation of total testicular transcriptome. Using NCBI-BLAST 2.2.28, the identified transcripts were assigned GO subcategories under biological process (BP), molecular function (MF) and cellular component (CC). Further, variation in transcript numbers of different subcategories depending on reproductive phases was analyzed.
Differential gene expression analysis
For comparative analysis of genes expressed along the testicular cycle, four sets of clustered transcripts were generated from samples of different reproductive phases using CD-Hit at 95% identity/coverage (set 1: samples R and P; set 2: samples P and S; set 3: samples S and Ps; set 4: samples Ps and R). Further, differential gene expression (DGE) data was obtained for each set of clustered transcripts using DESeq software [26] and expression fold change was calculated considering the underlined sample in each set as reference. Transcripts having log2 (fold change) value ≥ 1 and ≤ -1 were considered to be upregulated and downregulated, respectively. Thereafter, up- and down-regulated transcripts along the different reproductive phases were grouped as per their GO subcategories. In addition, transcripts showing significant variation in expression fold change (corrected P value ≤ 0.05) in these sets were identified. Also, the expression level of Sertoli cell (SC), Leydig cell (LC) and peritubular myoid cell (PMC) specific genes during different phases of the testicular cycle were analyzed.
Validation of differential gene expression
To validate the RNA-Seq data of differentially expressed testicular genes, expression of some of these genes enlisted in Table 1 was estimated during different reproductive phases by quantitative polymerase chain reaction (qPCR).
Table 1. Enlisting the selected genes and their primers for quantitative PCR.
| Gene name | Gene accession number | Primer sequences |
|---|---|---|
| transketolase (tkt) | GEMA01054854.1 under TSA accession GEMA00000000 | FP: 5’-GACCACTACCACGAAGG-3’ |
| RP: 5’-AGGAACGTGGGACACAG-3’ | ||
| Mannosyl (beta-1,4-)-glycoprotein beta-1,4-N-acetylglucosaminyltransferase (mgat3) | GEMA01063365.1 under TSA accession GEMA00000000 | FP: 5’-CTGGTAAAGTGTGTGTGCCG-3’ |
| RP: 5’-TTAGTGGGCAGGTTGGAGTGG-3’ | ||
| Activated leukocyte cell adhesion molecule (alcam) | GEKZ01011742.1 under TSA accession GEKZ00000000 | FP: 5’-CATGAAGAAGTCCAAACAAGG-3’ |
| RP: 5’-TTTTTGACTGTTCTCCTCCAC-3’ | ||
| GIPC PDZ domain containing family member 3 (gipc3) | GEKY01111158.1 under TSA accession GEKY00000000 | FP: 5’-TGACCAGAGCATTGTAGG-3’ |
| RP: 5’-CTAGGCGAAGAGTGAAG-3’ | ||
| syntaxin 1B (stx1b) | GEKY01043631.1 under TSA accession GEKY00000000 | FP: 5’-AATCGAACAGCGGCACAAGG-3’ |
| RP: 5’-CTCCTTGTTCTTCGACCAGC-3’ |
In brief, both side testes of a fish were used to make a sample for total RNA extraction and three such samples were made for each reproductive phase. Total RNA was extracted using TRI reagent (Sigma-Aldrich, USA), RNA integrity was estimated by Bioanalyzer (Agilent Technologies, USA) and concentration was measured using NanoDrop (ND-1000, NanoDrop Technologies, USA). Samples with RIN 5 or above were considered for cDNA preparation. Two microgram RNA of each sample was treated with DNase I (Thermo Scientific, USA) for 30 min to remove DNA contamination. DNase I was inactivated by heat denaturation at 70°C for 10 min in the presence of EDTA. Further, single-stranded cDNAs were synthesized using Avian Myeloblastosis Virus Reverse Transcription kit (Cat# K1622, Thermo Scientific, USA) following the manufacturer’s protocol. For qPCR, gene-specific primers were designed from their respective nucleotide sequences using Primer3 Input and Gene runner (Table 1). The efficiency of individual primer was checked using serial dilutions of testicular cDNA and amplification of single specific product was confirmed based on melt curve analysis. The qPCR reactions in samples run in triplicate were carried out in Real-time system CFX96 (Bio-Rad laboratories, USA) using SYBR Green Master Mix (Cat# 4367659, Applied Biosystems, USA). The reaction cycle consisted of the following steps: initial denaturation at 95°C for 10 min, 40 cycles of denaturation at 95°C for 30 s, annealing and extension at gene-specific temperature for 1 min, and a final dissociation step for melt curve analysis. Considering resting phase as reference, 2-ΔΔct method was used to calculate relative fold change in expression of selected genes during preparatory, spawning and postspawning. Ribosomal 18s RNA was used as house-keeping gene for normalizing the expression values of target genes in each testicular sample.
Statistical analysis
One-way analysis of variance (ANOVA) was applied to analyze significant variation in relative fold change of testicular mRNA expression for each gene during different reproductive phases. Newman-Keuls multiple range test was used to compare the means. Data are expressed as mean ± S.E.M (P< 0.05).
Results
Illumina paired-end sequencing and de novo assembly
RNA extracted from testicular samples of four reproductive phases R, P, S and Ps had A260/A280 ratios ranging from 1.8–2.1 and RIN values of 7, 7.4, 8.5 and 8.2, respectively (S1 Table). RNA sequencing on Illumina Hiseq 2000 platform yielded 100 bp reads from both ends of each cDNA fragment. The data generated 41.94 million reads (10.2 GB) for sample R, 47.51 million (11.55 GB) for sample P, 61.81 million (15.03 GB) for sample S and 44.45 million (10.81 GB) for sample Ps (S1 Table). After trimming the adapters and removing low quality bases, processed reads of 40.03 (9.61 GB), 45.01 (10.79 GB), 58.6 (14.05 GB) and 42.19 (10.12 GB) million were obtained for samples R, P, S and Ps, respectively (S2 Table). This reduced the percentage of non-ATGC characters (0.03–0.3%) in the processed reads. Thereafter, transcriptome assembly for samples R, P, S and Ps generated 154557, 158650, 204966 and 178121 contigs and 105526, 105169, 122964 and 106544 transcripts, respectively (S3 Table). The maximum contig length and transcript length for sample of each reproductive phase were 33888 and 43967 bp (sample R), 31822 and 66285 bp (sample P), 13462 and 36569 bp (sample S), and 17866 and 40661 bp (sample Ps), respectively. Length distribution of the assembled transcripts revealed that 789 transcripts for sample R, 1895 for sample P, 1928 for sample S and 1729 for sample Ps were ≥ 10 Kb in size. The total testicular transcriptome generated by clustering of transcripts from the four reproductive phases provided 210833 transcripts.
Functional annotation of transcripts
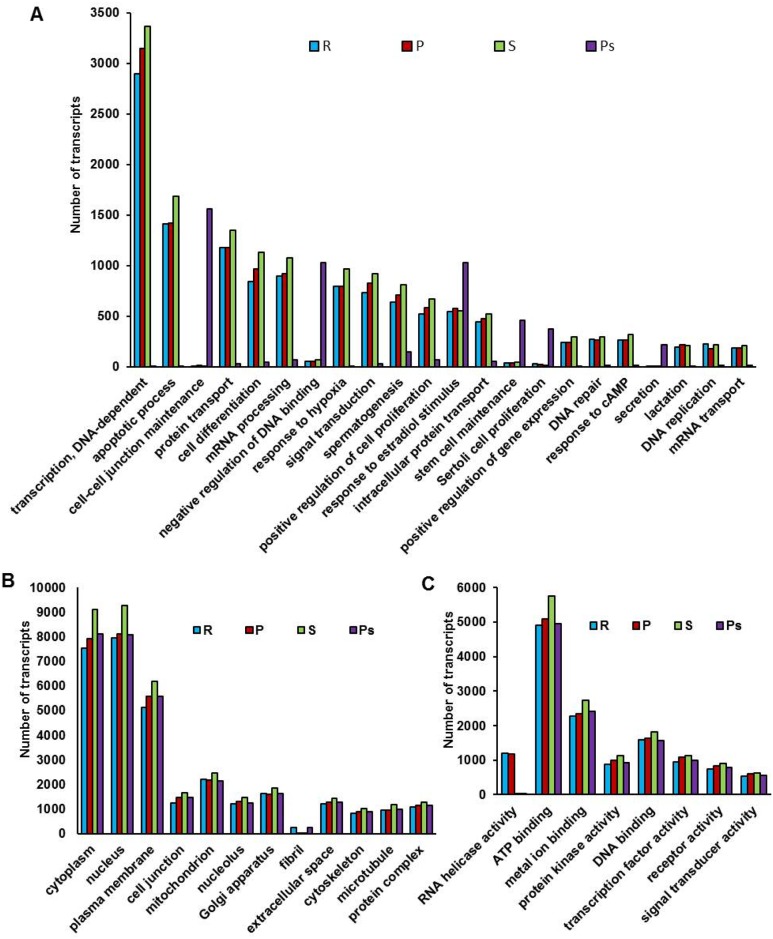
GO classification based on R. norvegicus protein database assigned the transcripts of four testicular samples to 5448, 1910 and 845 subcategories under BP, MF and CC, respectively. Majority of the subcategories under BP had lowest transcript number during postspawning phase that increased considerably in resting and reached the highest during spawning phase (Fig 1A). However, transcript number for some of the BP subcategories such as “cell-cell junction maintenance”, “negative regulation of DNA binding”, “response to estradiol stimulus”, “stem cell maintenance”, “Sertoli cell proliferation”, and “secretion” were appreciably high in postspawning as compared to other reproductive phases. Under MF, all the subcategories except “RNA helicase activity” showed least number of transcripts during resting, subsequent rise in preparatory and maximum during spawning phase (Fig 1C). A similar trend with highest transcript number in spawning and lowest in resting phase was found in most of the subcategories under CC (Fig 1B). Interestingly, transcript number for the subcategory “fibril” was high in postspawning and resting while extremely low in preparatory and spawning (Fig 1B).
Fig 1. Histogram representation of gene ontology classification of transcripts from different reproductive phases.
GO classification of testicular transcripts from different reproductive phases (resting: R, preparatory: P, spawning: S and postspawning: Ps) into various subcategories under Biological process (A), Molecular function (B) and Cellular component (C).
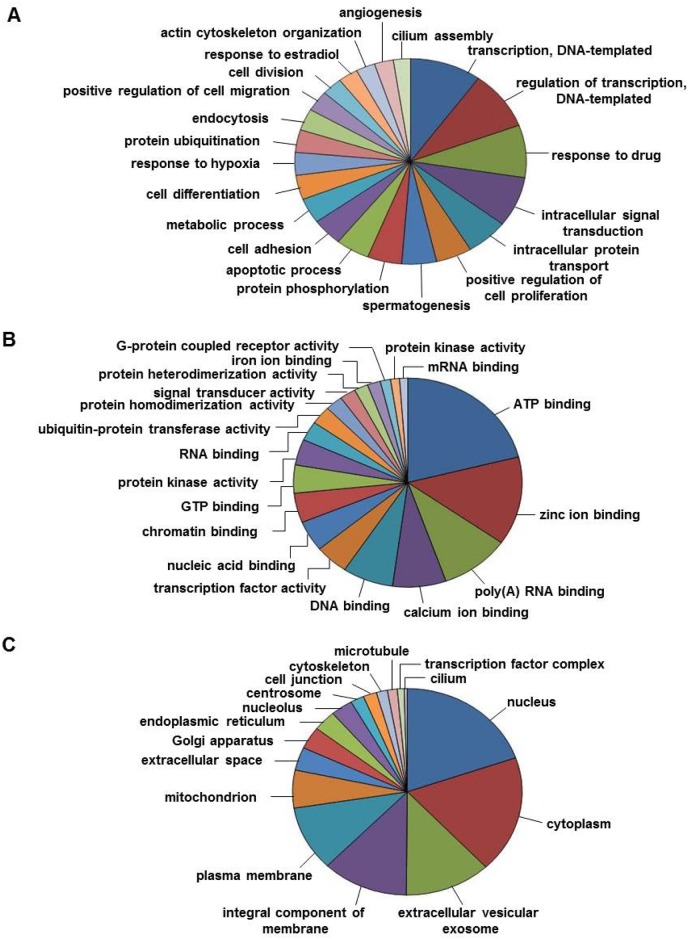
GO classification of the total testicular transcriptome generated by clustering of transcripts from testis of four reproductive phases showed 8838, 2968 and 1187 subcategories under BP, MF and CC, respectively. Under BP category, “transcription”, “regulation of transcription, DNA-dependent”, “intracellular signal transduction” and “intracellular protein transport” were found to be the most represented subcategories (Fig 2A). In addition, significant number of transcripts was assigned to “positive regulation of cell proliferation”, “spermatogenesis”, “apoptotic process”, “cell differentiation” and “response to hypoxia”. In CC category, the subcategories “nucleus” and “cytoplasm” had the highest number of transcripts (Fig 2B). Under category of MF, subcategories related to different types of “binding” were frequently found along with “sequence-specific DNA binding transcription factor activity” and “protein serine/threonine kinase activity” (Fig 2C).
Fig 2. Pie diagram showing gene ontology classification of total testicular transcriptome.
GO classification of total testicular transcriptome into various subcategories under: (A) Biological process (B) Cellular component (C) Molecular function.
Differential gene expression
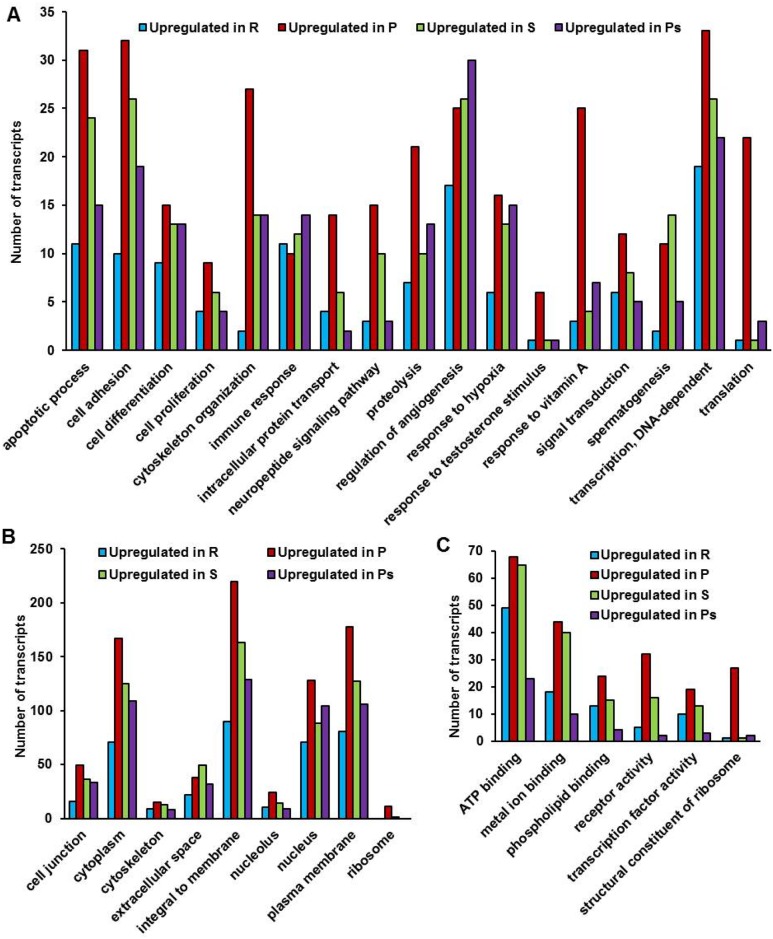
Fig 3 presents a comparative picture of upregulated transcript numbers (sample R vs P, P vs S, S vs Ps and Ps vs R, considering the underlined sample as reference; S4 Table) under various functional subcategories of BP, CC and MF. A substantial increase in number of upregulated transcripts was observed for majority of the subcategories under BP during transition from resting to preparatory phase (Fig 3A) and thereafter transcripts displayed varying trends. The number of upregulated transcripts was maintained for “response to hypoxia” while a decrease was noted for “translation”, “response to testosterone stimulus”, “response to vitamin A”, “apoptotic process”, “cell adhesion” and “DNA-dependent transcription” during spawning. In contrast, an increase in number of upregulated transcripts was observed for “spermatogenesis” and “regulation of angiogenesis” until spawning and postspawning, respectively. Interestingly, upregulated transcript number for “immune response” decreased from resting to preparatory followed by a gradual increase during spawning and postspawning phases. Like BP, majority of the subcategories under CC showed maximum increase in number of upregulated transcripts during preparatory phase which subsequently decreased in spawning and postspawning (Fig 3B). Regarding MF, all the subcategories displayed a similar pattern for upregulated transcripts with highest number in preparatory followed by a decrease in spawning and postspawning (Fig 3C). It was interesting to note that upregulated transcripts for “structural constituent of ribosome” under MF and “ribosome” under CC were essentially present during preparatory phase.
Fig 3. Histogram representation of gene ontology classification of upregulated testicular transcripts from different reproductive phases.
Classification of upregulated testicular transcripts from different reproductive phases (resting: R, preparatory: P, spawning: S and postspawning: Ps) associated with testicular functions under GO subcategories of Biological process (A), Cellular component (B) and Molecular function (C). Upregulated transcripts were obtained from each set of clustered transcripts (set 1: samples R and P; set 2: samples P and S; set 3: samples S and Ps; set 4: samples Ps and R) based on expression fold change that was calculated considering the underlined sample in each set as reference.
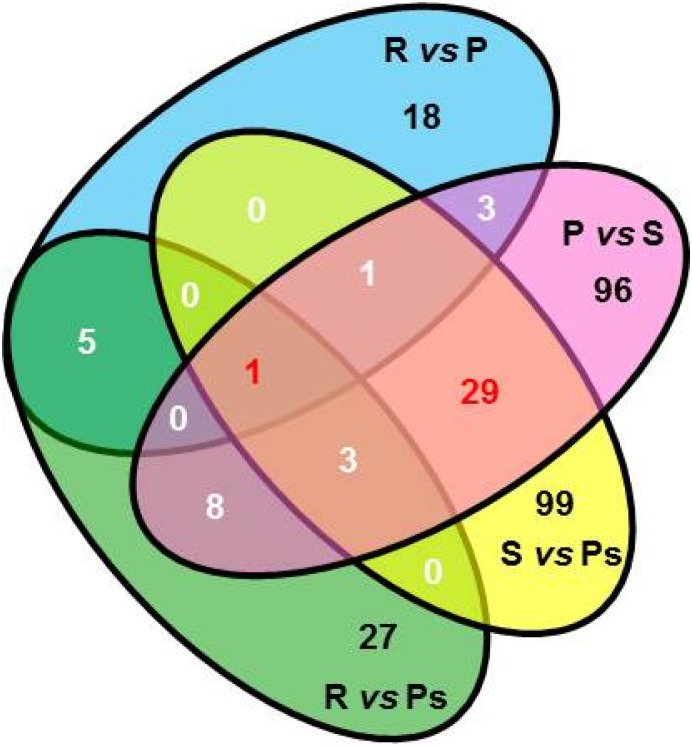
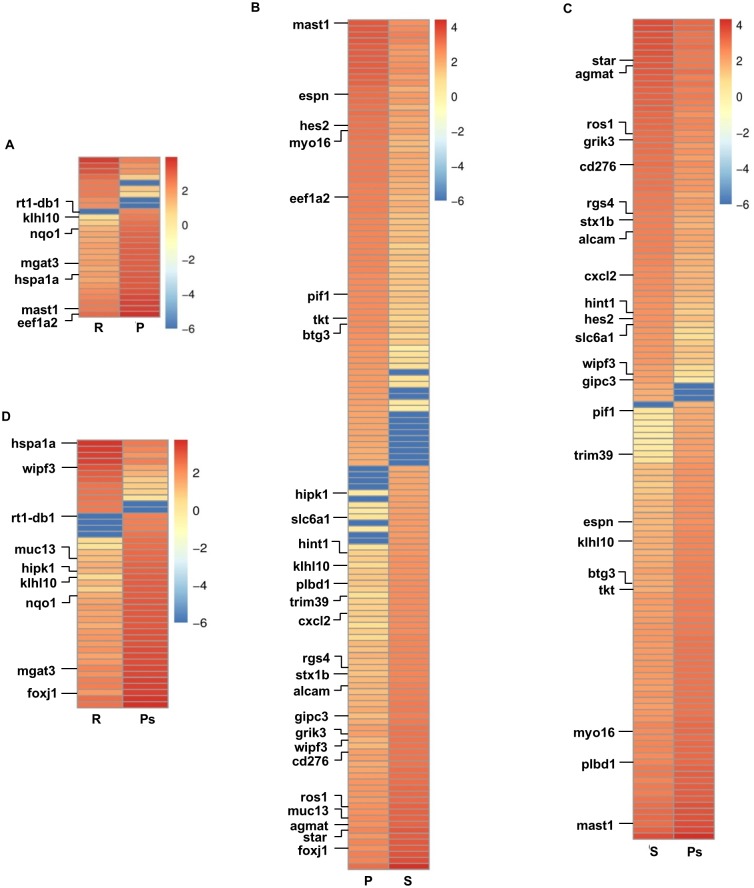
In addition, among transcripts showing significant (corrected P value < 0.05) differential expression, fifty transcripts were differentially expressed in more than two reproductive phases of which thirty were common to preparatory, spawning and postspawning (Fig 4). Throughout the reproductive cycle, the overlapping transcripts showed varying patterns of expression (Fig 5). A significant (corrected P value < 0.05) increase in expression of transcripts for klhl10, rt1-db1, mgat3 and nqo1 was observed in preparatory (R vs P, corrected P value < 0.05) that remained upregulated during spawning and postspawning. Similar upregulation in expression of transcripts for mast1, hspa1a, eef1a2, hes2, btg3, pif1, myo16, espn, and tkt was seen during preparatory phase, though their expression except for hspa1a significantly (P vs S, corrected P value < 0.05) decreased in spawning. Further, a set of transcripts (wipf3, rgs4, star, gipc3, grik3, cd276, cxcl2, alcam, hint1, ros1, slc6a1, stx1b and agmat) were profoundly expressed in spawning (P vs S, corrected P value < 0.05) and thereafter their expression declined during postspawning. On the other hand, upregulated expression of transcripts for muc13, hipk1, foxj1, trim39 and plbd1 during spawning remained high even in postspawning. Further, the analysis of expression levels for SC, LC and PMC specific genes during different phases of the testicular cycle revealed that only amh, a SC specific gene, showed significant upregulation in expression during postspawning phase (S vs Ps, corrected P value < 0.05, S5 Table). The other SC, LC and PMC specific genes, though detected in the testis, did not show any change in their expression along the testicular cycle (S5 Table).
Fig 4. Venn diagram of testicular transcripts showing significant differential expression depending on reproductive phases (corrected P value < 0.05).
Fig 5. Heat map representation of significantly differentially expressed testicular transcripts.
Shows testicular transcripts showing significant (corrected P value < 0.05) differential expression based on comparison between different reproductive phases (resting: R, preparatory: P, spawning: S and postspawning: Ps): (A) R and P, (B) P and S, (C) S and Ps and (D) R and Ps. Transcripts differentially expressed in more than two reproductive phases are labelled.
Validation of differential expression of genes
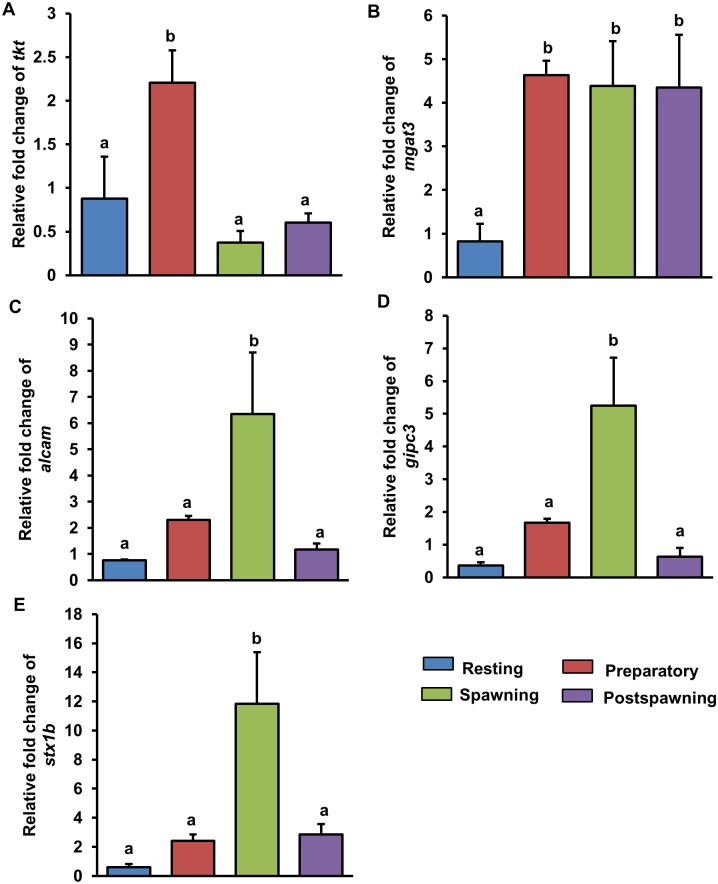
The expression fold change of the selected testicular genes (tkt, mgat3, alcam, gipc3 and stx1b) along the reproductive cycle showed significant (ANOVA, P < 0.05) variation depending on reproductive phases. The level of tkt and mgat3 considerably (P < 0.05) increased during preparatory phase as compared to that of resting phase (Fig 6A and 6B). Thereafter, tkt expression in spawning phase declined (P < 0.05) to the level of resting and remained low until postspawning. Unlike tkt, a steady high expression of mgat3 was recorded from preparatory to postspawning. In case of alcam, gipc3 and stx1b, expression level though did not show any change until preparatory phase, a significant increase was observed during spawning phase (resting/preparatory phase vs spawning phase, P < 0.05; Fig 6C–6E). However, in postspawning, their expression declined to the level of resting/preparatory phase. These qPCR results showed a similar temporal expression pattern as observed following RNA-Seq analysis during different reproductive phases.
Fig 6. Showing expression fold change of some differentially expressed testicular genes tkt (A), mgat3 (B), alcam (C), gipc3 (D) and stx1b (E) along the reproductive cycle.
The expression fold change of genes during preparatory, spawning and postspawning phases were calculated using expression values obtained during resting phase as reference. Ribosomal 18s RNA was used as the house-keeping gene for normalization of expression values. Three testicular samples were used for each reproductive phase (N = 3). Data represented as mean ± SEM were analyzed by one way analysis of variance (ANOVA) and compared by Newman-Keuls multiple range test. Groups with different alphabets (a-b) as superscripts show significant difference (P < 0.05).
Discussion
In the present study, comparative analysis of testicular transcriptome from different reproductive phases of spotted snakehead C. punctatus highlights the activation/repression patterns of several genes and their correlation with various structural and functional aspects of testis depending on its spermatogenic state. This study also provides the complete testicular transcriptome which is of particular importance as genomic resource for any species of Channa is not available.
Comparative analysis of transcripts based on functional annotation
Total transcripts
Functional categorization of testicular transcripts from different reproductive phases showed high number of transcripts for majority of subcategories under BP, CC and MF during preparatory and spawning phases. The augmented spermatogenic activity during these phases might be correlated with marked increase in transcripts for major biological processes such as cell proliferation and differentiation, DNA repair, response to hypoxia, transcription, mRNA transport and processing, spermatogenesis, signal transduction, positive regulation of gene expression and protein transport. Similar correlation has been drawn in Oncorhynchus mykiss and Mus musculus wherein some of these biological processes were prominent during proliferation and differentiation of spermatogonia [19,27]. During postspawning, spermatogenic quiescence might be associated with decrease in transcripts for several biological processes and increase in transcripts for “negative regulation of DNA binding”. Further, enhanced transcript number for biological processes such as cell-cell junction maintenance, stem cell maintenance and SC proliferation could be implicated in restructuring of testis required for initiation of next testicular cycle. The restructuring of testis during postspawning is evident by an increase in transcripts for an extra-cellular matrix (ECM) component “fibril” as ECM components have been suggested to be involved in reorganization of seminiferous tubules [28]. Number of transcripts for “fibril” remained high during resting phase probably due to marked decrease of germ cells as compared to somatic cells (PMC and SC) that are reported to secrete ECM components [28]. In our earlier study in C. punctatus, estradiol-17β has been suggested to be involved in regression of testis, stem cell renewal and spermatogonial proliferation during postspawning phase [24]. Also, estradiol-17β is implicated in initiation and maintenance of spermatogonial stem cell proliferation in Anguilla japonica [29]. These reports substantiate our observation in the present study where number of transcripts for “response to estradiol stimulus” was highest during postspawning. Among molecular functions, high level of RNA helicase activity during preparatory phase in C. punctatus suggests an increase in translational activity as RNA helicases have been reported to play important role in ribosome biogenesis, pre-mRNA splicing and translation [30]. However, the reason for its increase in resting phase is not clear.
Differentially expressed transcripts
An upsurge in differentially expressed testicular genes has been reported in parallel to advancement of spermatogenesis in mammals [2,27]. A similar increase in upregulated transcripts concomitant to heightened spermatogenic activity was observed during preparatory phase in C. punctatus. Among these, an increase in transcripts specific to “proteolysis”, “signal transduction” and “intracellular protein transport” under BP is in accordance to a report in mice in which these transcripts are seen to be preferentially expressed in spermatids [31]. Moreover, an increase in number of upregulated transcripts for “translation”, “ribosome” and “structural constituent of ribosome” under BP, CC and MF, respectively, in preparatory phase suggests the amplification of protein synthesis during this period in C. punctatus. The rise in upregulated transcripts for “response to vitamin A” evidenced during preparatory phase in spotted snakehead indicates the involvement of vitamin A in proliferation and differentiation of spermatogonia. This is in agreement to a report in mammals where positive role of vitamin A in synchronization of spermatogonial differentiation and meiotic entry is documented [32]. An upregulation of transcripts for “response to hypoxia” during preparatory, spawning and postspawning might be the consequence of increased hypoxia due to escalation in cell proliferation. Our assumption relies on the report of Marti and colleagues [33] where direct relationship has been demonstrated between hypoxia and cell proliferation in mice testis. Further, hypoxia has been reported to induce angiogenesis [34], thus supporting our current observation of gradual increase in upregulated transcripts for “regulation of angiogenesis” from preparatory to postspawning phase. The present study also noted an increase in number of upregulated transcripts for “spermatogenesis” during preparatory and spawning phases probably owing to abundance of spermatocytes, spermatids and spermatozoa. A similar observation is reported in O. mykiss where genes grouped under biological process “spermatogenesis” are suggested to be expressed by meiotic/post meiotic germ cells [19].
Upregulated transcripts and spermatogenic events
Preparatory phase
The present endeavor identified several transcripts significantly upregulated during preparatory phase suggesting their involvement in initiation and maintenance of spermatogenesisin C. punctatus. The cell-specific localization of some of these transcripts and their role in testicular development and spermatogenesis has been studied in mammals. klhl10 and mast1 are reported to be expressed in spermatids and associated with spermiogenesis [35,36]. A SC-specific gene espn has been implicated in the formation of blood-testis barrier [37]. nqo1 is shown to be expressed in LCs [38] and its increased level from infancy to adulthood has been associated with testicular development [39]. Expression of heat shock protein hspa1a in PMC, SC as well as spermatogonia [40] and its downregulation in case of azoospermia [41] indicate the involvement of hspa1a in sperm development.
In addition to these transcripts, expression of hes2, eef1a2, rt1-db1, mgat3, and tkt have been detected in mammalian testis [42–46] though their cell-specific localization and definite testicular function is still unexplored. The upregulation of Notch effector gene hes2 during preparatory phase in spotted snakehead C. punctatus indicates the role of Notch signaling in fish spermatogenesis as reported in mammals [47–49]. Further, current observation of increased eef1a2 expression is substantiated by a report where eEF1A has been shown to be indirectly associated with protein synthesis and cell proliferation throughY-encoded testis-specific protein [43]. In addition, we observed upregulated expression of tkt and mgat3 that encode enzymes involved in pentose phosphate pathway (PPP) and biosynthesis of glycoproteins, respectively, during preparatory phase when steroidogenic and spermatogenic activity markedly increases. In mammals, PPP has been found to be active in germ cells [50]. Further, PPP is known to facilitate steroidogenesis and nucleic acid biosynthesis through production of steroidogenic cofactor nicotinamide adenine dinucleotide phosphate and ribose 5-phosphate, respectively [51]. In fishes, the importance of glycoprotein conjugates in testicular cells has been documented where they have been associated with cell cycle, cell adhesion, proliferation, apoptosis and sperm maturation [52]. These facts provide the basis to assume the involvement of tkt and mgat3 in upregulation of spermatogenic and steroidogenic activity during preparatory phase in C. punctatus. In our study, increased expression of rt1-db1 during preparatory phase points towards its involvement in regulation of testicular functions though correlation between this immune response gene [53] and testicular functions is lacking in vertebrates. It is noteworthy that the current study demonstrates the expression of pif1, btg3 and myo16 for the first time in testis of a vertebrate. pif1 and myo16 are reported to be involved in cell cycle progression [54,55] whereas btg3 has anti-proliferative action [56] and their upregulation during preparatory phase in C. punctatus suggests their role in maintaining cell homeostasis.
Spawning and postspawning phase
The identification of several testicular transcripts upregulated during spawning and postspawning phases in spotted snakehead C. punctatus are of vital importance to enrich our understanding of genes regulating spermatogenic and steroidogenic processes occurring during these phases of the testicular cycle. Transcripts known to be associated with sperm maturation (ros1 [57], foxj1 [58]), sperm transport and motility (muc13 [59], hint1 [60]), and acrosome reaction (stx1b [61]) increased significantly during spawning indicating their role in maturation, spawning of spermatozoa and fusion of gametes in C. punctatus. In addition, in the current study, an increase in expression of (a) gipc3 known to promote luteinizing hormone action [62] and consequently production of maturation inducing steroid [63], (b) grik3 reported to be associated with LC proliferation [64] and (c) star essential for steroid biosynthesis in LC and SC [65] suggest the importance of these genes in sex steroid biosynthesis and somatic cell proliferation in testis of C. punctatus during spawning. Among other upregulated genes of spawning phase, rgs4, alcam and cxcl2 have been associated with gonadal stem cells [66–68] while slc6a1 and hipk1 have been reported in spermatids and spermatozoa [69,70]. Nonetheless, it is difficult to decipher the precise role of these genes in regulation of testicular events attributed to spawning phase in C. punctatus. To our knowledge, expression of agmat in testis is not reported in literature though its upregulation was observed during spawning in the present study. agmat encodes enzyme agmatinase that converts agmatine to putrescine [71]. Further, level of putrescine has been shown to increase in elongated spermatids of rooster [72], indirectly indicating the role of agmat in spermatogenesis. During postspawning, restructuring of seminiferous lobules includes apoptosis of remnant germ cells and proliferation of spermatogonial stem cells to repopulate the testis. In the present study, upregulation of (a) cd276 localized in SCs and involved in tissue remodeling [73], (b) wipf3 expressed immensely at Sertoli-spermatogenic cell junctions [74], (c) trim39 identified in testis [75] and shown to promote apoptotic signaling [76], and (d) plbd1 identified as a marker of testicular germ cell tumor precursor [77] provide the basis to assume pivotal role of these genes in remodeling of testis during postspawning in C. punctatus. In addition, amh which is a SC specific gene and reported to be involved in maintaining immature state of testis in mammals [78], was upregulated during postspawning phase in C. punctatus.
Conclusion
The present study for the first time reports de novo testicular transcriptome sequencing in C. punctatus, thus contributing to the genomic information of this fish which is economically important and widely cultured in the Indian subcontinent. The functional annotation of testicular transcripts in C. punctatus highlighted the various biological processes, molecular functions and cellular components that are important for regulation of steroidogenic activity and spermatogenic events ranging from proliferation and differentiation of spermatogonia to release of spermatozoa. In addition, expression profile of testicular transcripts depending on reproductive phases enabled the identification of numerous upregulated transcripts associated with various testicular activities from preparatory to postspawning. The dataset of annotated transcripts and detailed overview of differential testicular transcriptional activity in the current study will provide basis for further investigation on functional genomic research and molecular regulation of testicular cycle in teleosts. Also, findings of this study may help in increasing fish production by manipulating testicular genes found to be important in regulation of spermatogenesis.
Supporting information
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
Acknowledgments
AR is thankful to University Grants Commission and University of Delhi (DU), India for providing financial aid in form of non-NET fellowship and University teaching assistantship, respectively. RB is grateful to Department of Science and Technology (DST), India and University of Delhi (DU), India for providing Inspire Fellowship and University teaching assistantship, respectively, as financial assistance. We acknowledge Genotypic Technology Private Limited, Bengaluru, India for library preparation and sequence data generation.
Data Availability
Raw reads of the four testicular samples have been deposited in Sequence Read Archive (SRA) at NCBI with accession number SRP070741 under Bioproject accession PRJNA304088. The samples R, P, S and Ps have been listed under individual experiment accession number of SRX1598155, SRX1600033, SRX1600041 and SRX1600062, respectively. The assembled transcript sequences of samples R, P, S and Ps have been deposited in the Transcriptome Shotgun Assembly (TSA) at NCBI with accession numbers GEKU00000000, GEMA00000000, GEKY00000000 and GEKZ00000000, respectively.
Funding Statement
UR received financial support from Delhi University (DU)/Department of Science and Technology (DST) PURSE Grant (Dean (R)/2012/1477). AR is thankful to University Grants Commission and University of Delhi (DU), India for providing financial aid in form of Non-National eligibility test (NET) fellowship (2079) and University teaching assistantship (Sch./UTA/2010/56769), respectively. RB is grateful to Department of Science and Technology (DST), India and University of Delhi (DU), India for providing Inspire Fellowship (IF10530) and University teaching assistantship (Sch./UTA/2010/56765), respectively, as financial assistance. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Yu Z, Guo R, Ge Y, Ma J, Guan J, Li S, et al. Gene expression profiles in different stages of mouse spermatogenic cells during spermatogenesis. Biol. Reprod. 2003;69:37–47. 10.1095/biolreprod.102.012609 [DOI] [PubMed] [Google Scholar]
- 2.Shima J, McLean D, McCarrey J, Griswold M. The murine testicular transcriptome: characterizing gene expression in the testis during the progression of spermatogenesis. Biol. Reprod. 2004;71:319–30. 10.1095/biolreprod.103.026880 [DOI] [PubMed] [Google Scholar]
- 3.Wang PJ, Pan J. The role of spermatogonially expressed germ cell-specific genes in mammalian meiosis. Chromosom. Res. 2007;15:623–32. [DOI] [PubMed] [Google Scholar]
- 4.Manku G, Culty M. Mammalian gonocyte and spermatogonia differentiation: recent advances and remaining challenges. Reproduction. 2015;149:R139–57. 10.1530/REP-14-0431 [DOI] [PubMed] [Google Scholar]
- 5.FAO Fisheries and Aquaculture Department. The state of world fisheries and aquaculture: oppurtunities and challenges. Rome; 2014. [Google Scholar]
- 6.Zeng S, Gong Z. Expressed sequence tag analysis of expression profiles of zebrafish testis and ovary. Gene. 2002;294:45–53. [DOI] [PubMed] [Google Scholar]
- 7.Barman HK, Panda RP, Mohapatra C, Swain A, Eknath AE. Identification of genes preferentially expressed in testis and spermatogonial cells of Labeo rohita by subtractive and suppressive hybridization. Aquac. Res. 2011;42:1196–205. [Google Scholar]
- 8.Sahu DK, Panda SP, Panda S, Das P, Meher PK, Hazra RK, et al. Identification of reproduction-related genes and SSR-markers through expressed sequence tags analysis of a monsoon breeding carp rohu, Labeo rohita (Hamilton). Gene. 2013;524:1–14. 10.1016/j.gene.2013.03.111 [DOI] [PubMed] [Google Scholar]
- 9.Hayashi M, Sato M, Iwasaki Y, Terasawa M, Tashiro M, Yokoyama S, et al. Combining next-generation sequencing with microarray for transcriptome analysis in rainbow trout gonads. Mol. Reprod. Dev. 2012;79:870–8. 10.1002/mrd.22127 [DOI] [PubMed] [Google Scholar]
- 10.Lu J, Luan P, Zhang X, Xue S, Peng L, Mahbooband S, et al. Gonadal transcriptomic analysis of yellow catfish (Pelteobagrus fulvidraco): identification of sex-related genes and genetic markers. Physiol. Genomics. 2014;46:798–807. 10.1152/physiolgenomics.00088.2014 [DOI] [PubMed] [Google Scholar]
- 11.Shen H, Gu R, Xu G, Xu P, Nie Z, Hu Y. In-depth transcriptome analysis of Coilia ectenes, an important fish resource in the Yangtze river: de novo assembly, gene annotation. Mar. Genomics. 2015;23:15–7. 10.1016/j.margen.2015.03.002 [DOI] [PubMed] [Google Scholar]
- 12.Zhou YF, Duan JR, Liu K, Xu DP, Zhang MY, Fang DA, et al. Testes transcriptome profiles of the anadromous fish Coilia nasus during the onset of spermatogenesis. Mar. Genomics. 2015;24:241–3. 10.1016/j.margen.2015.06.007 [DOI] [PubMed] [Google Scholar]
- 13.Sun F, Liu S, Gao X, Jiang Y, Perera D, Wang X, et al. Male-biased genes in catfish as revealed by RNA-Seq analysis of the testis transcriptome. PLoS One. 2013;8:e68452 10.1371/journal.pone.0068452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yue H, Li C, Du H, Zhang S, Wei Q. Sequencing and de novo assembly of the gonadal transcriptome of the endangered chinese sturgeon (Acipenser sinensis). PLoS One. 2015;10:e0127332 10.1371/journal.pone.0127332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sahu DK, Panda SP, Meher PK, Das P, Routray P, Sundaray JK, et al. Construction, de-novo assembly and analysis of transcriptome for identification of reproduction-related genes and pathways from rohu, Labeo rohita (Hamilton). PLoS One. 2015;10:e0132450 10.1371/journal.pone.0132450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bar I, Cummins S, Elizur A. Transcriptome analysis reveals differentially expressed genes associated with germ cell and gonad development in the Southern bluefin tuna (Thunnus maccoyii). BMC Genomics. 2016;17:217 10.1186/s12864-016-2397-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xu K, Wen M, Duan W, Ren L, Hu F, Xiao J, et al. Comparative analysis of testis transcriptomes from triploid and fertile diploid cyprinid fish. Biol Reprod. 2015;92(4):95, 1–12. 10.1095/biolreprod.114.125609 [DOI] [PubMed] [Google Scholar]
- 18.Zeng Q, Liu S, Yao J, Zhang Y, Yuan Z, Jiang C, et al. Transcriptome display during testicular differentiation of channel catfish (Ictalurus punctatus) as revealed by RNA-Seq analysis. Biol Reprod. 2016;95(1):19, 1–17. 10.1095/biolreprod.116.138818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rolland AD, Lareyre JJ, Goupil AS, Montfort J, Ricordel M-J, Esquerré D, et al. Expression profiling of rainbow trout testis development identifies evolutionary conserved genes involved in spermatogenesis. BMC Genomics. 2009;10:546 10.1186/1471-2164-10-546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Redon E, Bosseboeuf A, Rocancourt C, Da Silva C, Wincker P, Mazan S, et al. Stage-specific gene expression during spermatogenesis in the dogfish (Scyliorhinus canicua). Reproduction. 2010;140:57–71. 10.1530/REP-10-0021 [DOI] [PubMed] [Google Scholar]
- 21.Wang Z, Gerstein M, Snyder M. RNA-Seq: a revolutionary tool for transcriptomics. Nat. Rev. Genet. 2009;10:57–63. 10.1038/nrg2484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gam L, Leow C, Baie S. Proteomic analysis of snakehead fish (Channa striata) muscle tissue. Malaysian J. Biochem. Mol. Biol. 2006;14:25–32. [Google Scholar]
- 23.Molur S, Walker S. Report of the workshop “Conservation assessment and management plan for freshwater fishes of India”, Zoo outreach organization, conservation breeding specialist group: Freshwater fishes of India. Coimbatore, India; 1998.
- 24.Basak R, Roy A, Rai U. Seasonality of reproduction in male spotted murrel Channa punctatus: correlation of environmental variables and plasma sex steroids with histological changes in testis. Fish Physiol. Biochem. 2016; [DOI] [PubMed] [Google Scholar]
- 25.Li W, Godzik A. Cd-hit: a fast program for clustering and comparing large sets of protein or nucleotide sequences. Bioinformatics. 2006;22:1658–9. 10.1093/bioinformatics/btl158 [DOI] [PubMed] [Google Scholar]
- 26.Wang L, Feng Z, Wang X, Wang X, Zhang X. DEGseq: An R package for identifying differentially expressed genes from RNA-Seq data. Bioinformatics. 2010;26:136–8. 10.1093/bioinformatics/btp612 [DOI] [PubMed] [Google Scholar]
- 27.Laiho A, Kotaja N, Gyenesei A, Sironen A. Transcriptome profiling of the murine testis during the first wave of spermatogenesis. PLoS One. 2013;8:e61558 10.1371/journal.pone.0061558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tung PS, Fritz IB. Characterization of rat testicular peritubular myoid cells in culture: alpha-smooth muscle isoactin is a specific differentiation marker. Biol. Reprod. 1990;42:351–65. [DOI] [PubMed] [Google Scholar]
- 29.Miura T, Miura C, Ohta T, Nader MR, Todo T, Yamauchi K. Estradiol-17beta stimulates the renewal of spermatogonial stem cells in males. Biochem. Biophys. Res. Commun. 1999;264:230–4. 10.1006/bbrc.1999.1494 [DOI] [PubMed] [Google Scholar]
- 30.Jankowsky E. RNA helicases at work: binding and rearranging. Trends Biochem. 2011;36:19–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pang ALY, Johnson W, Ravindranath N, Dym M, Rennert OM, Chan W-Y. Expression profiling of purified male germ cells: stage-specific expression patterns related to meiosis and postmeiotic development. Physiol. Genomics. 2006;24:75–85. 10.1152/physiolgenomics.00215.2004 [DOI] [PubMed] [Google Scholar]
- 32.Hogarth C, Griswold M. The key role of vitamin A in spermatogenesis. J. Clin. Invest. 2010;120:956–62. 10.1172/JCI41303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Marti HH, Katschinski DM, Wagner KF, Schäffer L, Stier B, Wenger RH. Isoform-specific expression of hypoxia-inducible factor-1alpha during the late stages of mouse spermiogenesis. Mol. Endocrinol. 2002;16:234–43. 10.1210/mend.16.2.0786 [DOI] [PubMed] [Google Scholar]
- 34.Harris AL. Hypoxia- a key regulatory factor in tumour growth. Nat. Rev. Cancer. 2002;2:38–47. 10.1038/nrc704 [DOI] [PubMed] [Google Scholar]
- 35.Yan W, Ma L, Burns KH, Matzuk MM. Haploinsufficiency of kelch-like protein homolog 10 causes infertility in male mice. Proc. Natl. Acad. Sci. U. S. A. 2004;101:7793–8. 10.1073/pnas.0308025101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Walden PD, Cowan NJ. A novel 205-kilodalton testis-specific serine/threonine protein kinase associated with microtubules of the spermatid manchette. Mol. Cell. Biol. 1993;13:7625–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sluka P, O’Donnell L, Bartles JR, Stanton PG. FSH regulates the formation of adherens junctions and ectoplasmic specialisations between rat Sertoli cells in vitro and in vivo. J. Endocrinol. 2006;189:381–95. 10.1677/joe.1.06634 [DOI] [PubMed] [Google Scholar]
- 38.Zappa F, Ward T, Butler J, Pedrinis E, McGown A. Overexpression of NAD(P)H:quinone oxidoreductase 1 in human reproductive system. J. Histochem. Cytochem. 2001;49:1187–8. 10.1177/002215540104900913 [DOI] [PubMed] [Google Scholar]
- 39.Magre S, Rebourcet D, Ishaq M, Wargnier R, Debard C, Meugnier E, et al. Gender differences in transcriptional signature of developing rat testes and ovaries following embryonic exposure to 2,3,7,8-TCDD. PLoS One. 2012;7:e40306 10.1371/journal.pone.0040306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tully DB, Luft JC, Rockett JC, Ren H, Schmid JE, Wood CR, et al. Reproductive and genomic effects in testes from mice exposed to the water disinfectant byproduct bromochloroacetic acid. Reprod. Toxicol. 2005;19:353–66. 10.1016/j.reprotox.2004.06.009 [DOI] [PubMed] [Google Scholar]
- 41.Yang B, Wang H, Gao X-K, Chen B-Q, Zhang Y-Q, Liu H-L, et al. Expression and significance of Rap1A in testes of azoospermic subjects. Asian J. Androl. 2004;6:35–40. [PubMed] [Google Scholar]
- 42.Murta D, Batista M, Silva E, Trindade A, Henrique D, Duarte A, et al. Dynamics of Notch pathway expression during mouse testis post-natal development and along the spermatogenic cycle. PLoS One. 2013;8:e72767 10.1371/journal.pone.0072767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kido T, Lau YFC. The human Y-encoded testis-specific protein interacts functionally with eukaryotic translation elongation factor eEF1A, a putative oncoprotein. Int. J. Cancer. 2008;123:1573–85. 10.1002/ijc.23697 [DOI] [PubMed] [Google Scholar]
- 44.Kim IH, Kim SK, Kim EH, Kim SW, Sohn SH, Lee SC, et al. Korean red ginseng up-regulates C21-steroid hormone metabolism via Cyp11a1 gene in senescent rat testes. J. Ginseng Res. 2011;35:272–82. 10.5142/jgr.2011.35.3.272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nairn A V., York WS, Harris K, Hall EM, Pierce JM, Moremen KW. Regulation of glycan structures in animal tissues: Transcript profiling of glycan-related genes. J. Biol. Chem. 2008;283:17298–313. 10.1074/jbc.M801964200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sax CM, Salamon C, Todd Kays W, Guo J, Yu FX, Andrew Cuthbertson R, et al. Transketolase is a major protein in the mouse cornea. J. Biol. Chem. 1996;271:33568–74. [DOI] [PubMed] [Google Scholar]
- 47.Hayashi T, Kageyama Y, Ishizaka K, Xia G, Kihara K, Oshima H. Requirement of Notch 1 and its ligand jagged 2 expressions for spermatogenesis in rat and human testes. J. Androl. 2001;22:999–1011. [DOI] [PubMed] [Google Scholar]
- 48.Garcia TX, Hofmann MC. Notch signaling in Sertoli cells regulates gonocyte fate. Cell Cycle. 2013;12:2538–45. 10.4161/cc.25627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Murta D, Batista M, Trindade A, Silva E, Henrique D, Duarte A, et al. In vivo Notch signaling blockade induces abnormal spermatogenesis in the mouse. PLoS One. 2014;9:e113365 10.1371/journal.pone.0113365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bajpai M, Gupta G, Setty BS. Changes in carbohydrate metabolism of testicular germ cells during meiosis in the rat. Eur. J. Endocrinol. 1998;138:322–7. [DOI] [PubMed] [Google Scholar]
- 51.Nelson D., Cox M. Glycolysis, Gluconeogenesis, and the Pentose Phosphate Pathway Lehninger Princ. Biochem. 6th ed W. H. Freeman; 2013. p. 521–59. [Google Scholar]
- 52.Gallo A, Costantini M. Glycobiology of reproductive processes in marine animals: The state of the art. Mar. Drugs. 2012;10:2861–92. 10.3390/md10122861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cheng B-H, Liu Y, Xuei X, Liao C-P, Lu D, Lasbury ME, et al. Microarray studies on effects of Pneumocystis carinii infection on global gene expression in alveolar macrophages. BMC Microbiol. 2010;10:103 10.1186/1471-2180-10-103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cameron RS, Liu C, Pihkala JPS. Myosin 16 levels fluctuate during the cell cycle and are downregulated in response to DNA replication stress. Cytoskeleton. 2013;70:328–48. 10.1002/cm.21109 [DOI] [PubMed] [Google Scholar]
- 55.Bochman ML, Sabouri N, Zakian VA. Unwinding the functions of the Pif1 family helicases. DNA Repair. 2010;9:237–49. 10.1016/j.dnarep.2010.01.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Winkler GS. The mammalian anti-proliferative BTG/Tob protein family. J. Cell. Physiol. 2010;222:66–72. 10.1002/jcp.21919 [DOI] [PubMed] [Google Scholar]
- 57.Yeung CH, Breton S, Setiawan I, Xu Y, Lang F, Cooper TG. Increased luminal pH in the epididymis of infertile c-ros knockout mice and the expression of sodium-hydrogen exchangers and vacuolar proton pump H +-ATPase. Mol. Reprod. Dev. 2004;68:159–68. 10.1002/mrd.20067 [DOI] [PubMed] [Google Scholar]
- 58.Yu X, Ng CP, Habacher H, Roy S. Foxj1 transcription factors are master regulators of the motile ciliogenic program. Nat. Genet. 2008;40:1445–53. 10.1038/ng.263 [DOI] [PubMed] [Google Scholar]
- 59.Seo JT, Lee JS, Jun JH, Yang MH. Expression of mucin genes in the human testis and its relationship to spermatogenesis. Yonsei Med. J. 2005;46:667–72. 10.3349/ymj.2005.46.5.667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bansal SK, Gupta N, Sankhwar SN, Rajender S. Differential genes expression between fertile and infertile spermatozoa revealed by transcriptome analysis. PLoS One. 2015;10:e0127007 10.1371/journal.pone.0127007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tsai PS, Garcia-Gil N, van Haeften T, Gadella BM. How pig sperm prepares to fertilize: stable acrosome docking to the plasma membrane. PLoS One. 2010;5:e11204 10.1371/journal.pone.0011204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hirakawa T, Galet C, Kishi M, Ascoli M. GIPC binds to the human lutropin receptor (hLHR) through an unusual PDZ domain binding motif, and it regulates the sorting of the internalized human choriogonadotropin and the density of cell surface hLHR. J. Biol. Chem. 2003;278:49348–57. 10.1074/jbc.M306557200 [DOI] [PubMed] [Google Scholar]
- 63.Planas J V, Swanson P. Maturation-associated changes in the response of the salmon testis to the steroidogenic actions of gonadotropins (GTH I and GTH II) in vitro. Biol. Reprod. 1995;52:697–704. [DOI] [PubMed] [Google Scholar]
- 64.Ge RS, Dong Q, Sottas CM, Chen H, Zirkin BR, Hardy MP. Gene expression in rat Leydig cells during development from the progenitor to adult stage: a cluster analysis. Biol. Reprod. 2005;72:1405–15. 10.1095/biolreprod.104.037499 [DOI] [PubMed] [Google Scholar]
- 65.Pollack SE, Furth EE, Kallen CB, Arakane F, Kiriakidou M, Kozarsky KF, et al. Localization of the steroidogenic acute regulatory protein in human tissues. J. Clin. Endocrinol. Metab. 1997;82:4243–51. 10.1210/jcem.82.12.4445 [DOI] [PubMed] [Google Scholar]
- 66.Conrad S, Azizi H, Hatami M, Kubista M, Bonin M, Hennenlotter J, et al. Differential gene expression profiling of enriched human spermatogonia after short- and long-term culture. Biomed Res. Int. 2014;2014:138350 10.1155/2014/138350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gonzalez R, Griparic L, Vargas V, Burgee K, SantaCruz P, Anderson R, et al. A putative mesenchymal stem cells population isolated from adult human testes. Biochem. Biophys. Res. Commun. 2009;385:570–5. 10.1016/j.bbrc.2009.05.103 [DOI] [PubMed] [Google Scholar]
- 68.Oatley JM, Oatley MJ, Avarbock MR, Tobias JW, Brinster RL. Colony stimulating factor 1 is an extrinsic stimulator of mouse spermatogonial stem cell self-renewal. Development. 2009;136:1191–9. 10.1242/dev.032243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hua JH, He XB, Yan YC. Identification of gamma-aminobutyric acid transporter (GAT1) on the rat sperm. Cell Res. 2000;10:51–8. 10.1038/sj.cr.7290035 [DOI] [PubMed] [Google Scholar]
- 70.Khan SA, Suryawanshi AR, Ranpura SA, Jadhav S V., Khole V V. Identification of novel immunodominant epididymal sperm proteins using combinatorial approach. Reproduction. 2009;138:81–93. 10.1530/REP-09-0052 [DOI] [PubMed] [Google Scholar]
- 71.Sastre M, Regunathan S, Galea E, Reis D. Agmatinase activity in rat brain: a metabolic pathway for the degradation of agmatine. J. Neurochem. 1996;67:1761–5. [DOI] [PubMed] [Google Scholar]
- 72.Oliva R, Vidal S, Mezquita C. Cellular content and biosynthesis of polyamines during rooster spermatogenesis. Biochem. J. 1982;208:269–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhou W, Wang G, Small CL, Liu Z, Weng CC, Yang L, et al. Gene expression alterations by conditional knockout of androgen receptor in adult Sertoli cells of Utp14b(jsd/jsd) (jsd) mice. Biol. Reprod. 2010;83:759–66. 10.1095/biolreprod.110.085472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Xiang W, Wen Z, Pang W, Hu L, Xiong C, Zhang Y. CR16 forms a complex with N-WASP in human testes. Cell Tissue Res. 2011;344:519–26. 10.1007/s00441-011-1159-9 [DOI] [PubMed] [Google Scholar]
- 75.Orimo A, Yamagishi T, Tominaga N, Yamauchi Y, Hishinuma T, Okada K, et al. Molecular cloning of testis-abundant finger protein/ring finger protein 23 (RNF23), a novel RING-B box-coiled coil-B30.2 protein on the Class I Region of the human MHC. Biochem. Biophys. Res. Commun. 2000;276:45–51. 10.1006/bbrc.2000.3380 [DOI] [PubMed] [Google Scholar]
- 76.Lee SS, Fu NY, Sukumaran SK, Wan KF, Wan Q, Yu VC. TRIM39 is a MOAP-1-binding protein that stabilizes MOAP-1 through inhibition of its poly-ubiquitination process. Exp. Cell Res. 2009;315:1313–25. 10.1016/j.yexcr.2008.11.021 [DOI] [PubMed] [Google Scholar]
- 77.Alagaratnam S, Lind G., Kraggerud S., Lothe R., Skotheim R. The testicular germ cell tumour transcriptome. Int. J. Androl. 2011;34:e133–50. 10.1111/j.1365-2605.2011.01169.x [DOI] [PubMed] [Google Scholar]
- 78.Sharpe RM, McKinnell C, Kivlin C, Fisher JS. Proliferation and functional maturation of Sertoli cells, and their relevance to disorders of testis function in adulthood. Reproduction. 2003;125:769–84. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
Data Availability Statement
Raw reads of the four testicular samples have been deposited in Sequence Read Archive (SRA) at NCBI with accession number SRP070741 under Bioproject accession PRJNA304088. The samples R, P, S and Ps have been listed under individual experiment accession number of SRX1598155, SRX1600033, SRX1600041 and SRX1600062, respectively. The assembled transcript sequences of samples R, P, S and Ps have been deposited in the Transcriptome Shotgun Assembly (TSA) at NCBI with accession numbers GEKU00000000, GEMA00000000, GEKY00000000 and GEKZ00000000, respectively.