Abstract
Background
Reducing inhibitory neurotransmission with pharmacological agents is a potential approach for augmenting plasticity after stroke. Previous work in healthy subjects showed diminished intracortical inhibition after administration of theophylline.
Objective
We assessed the effect of single-dose theophylline on intracortical and interhemispheric inhibition in patients with chronic stroke, in a double-blind, placebo-controlled, cross-over study.
Methods
Eighteen subjects were randomly administered 300 mg of extended-release theophylline or placebo. Immediately and 5 hours following administration, transcranial magnetic stimulation was used to assess bihemispheric resting motor threshold, short-interval intracortical inhibition, long-interval intracortical inhibition, and interhemispheric inhibition. Adverse effects on cardiovascular, neurological, and motor performance outcomes were also surveilled. Change between morning and afternoon sessions were compared across conditions. One week later, patients underwent the same assessments after crossing over to the opposite experimental condition. Subjects and investigators were blinded to the experimental condition during data acquisition and analysis.
Results
For both hemispheres, changes in intracortical or interhemispheric neurophysiology were comparable under theophylline and placebo conditions. Theophylline induced no adverse neurological, cardiovascular, or motor performance effects. For both conditions and hemipsheres, the baseline level of inhibition inversely correlated with its change between sessions: less baseline inhibition (i.e. disinhibition) was associated with a strengthening in inhibition over the day, and vice versa.
Conclusion
A single dose of theophylline is well-tolerated by patients with chronic stroke, but does not alter cortical excitability. The inverse relationship between baseline inhibition and its change suggests the existence of a homeostatic process. The lack of effect on cortical inhibition may be related to an insufficiently long exposure to theophylline, or to differential responsiveness of disinhibited neural circuitry in patients with stroke.
Keywords: Recovery of function, transcranial magnetic stimulation, cerebral infarction, motor cortex, homeostasis
1. Introduction
For the nearly million people annually having a stroke in the US, more than half have motor impairment resulting in long-term disability (Amadi et al., 2013; Kelly-Hayes et al., 2003). During recovery, activity-dependent plasticity induced by physical training is believed to engage the transient injury-induced plasticity triggered by the stroke itself (Overman & Carmichael, 2014; Zeiler & Krakauer, 2013). Clinical interventions are being investigated to augment this plasticity and thereby maximize recovery.
One potential approach is to pharmacologically encourage plasticity by enhancing long-term potentiation (LTP), the long-lasting increase in signal transmission between two neurons (Cooke & Bliss, 2006). LTP is modulated by γ-aminobutyric acid (GABA), the major inhibitory neurotransmitter of the motor cortex. In rodents, antagonism of GABAA receptors (GABAAR) is required for neocortical LTP expression, whereas activation of GABAAR impairs LTP induction (Hess, Aizenman, & Donoghue, 1996; Komaki et al., 2007; Rodgers et al., 2015; Trepel & Racine, 2000).
Following stroke in rodents, GABAAR are down-regulated and GABABR are up-regulated in the perilesional and contralesional cortex (Que et al., 1999). These cellular changes are accompanied by reduced bi-hemispheric inhibition and facilitation of perilesional LTP (Hagemann et al., 1998; M.S. Qu et al., 1998). Similarly, in humans, MRI spectroscopy shows reduced GABA signal in the perilesional motor cortex following stroke (Blicher et al., 2015). In animal models of recovery, activation of synaptic GABAAR reduces motor recovery (Schallert, Hernandez, & Barth, 1986; Watson & Kennard, 1945), whereas blockade of extrasynaptic GABAAR facilitates motor recovery (Clarkson et al., 2010). These findings collectively suggest that additional reduction of GABAergic neurotransmission may be an approach to promote plasticity and enhance recovery.
One potential pharmacological candidate is theophylline, a methylxanthine drug that strongly antagonizes adenosine receptors and weakly antagonizes GABAAR (Fredholm, 1979). Theophylline is used clinically for the treatment of asthma and chronic obstructive pulmonary disease, with a standard maintenance dose of 300 mg administered twice daily. In a recent clinical trial finding respiratory benefit of theophylline 300 mg, there were so few side effects (e.g. nausea, headache, tachyarrythmia, seizure) that it was suggested that serum monitoring no longer be required. (American Lung Association, 2007). In healthy subjects assessed with transcranial magnetic stimulation (TMS), a week-long course of 200 mg theophylline given daily reduced short-interval intracortical inhibition (SICI) (Nardone et al., 2004). SICI primarily reflects activity of GABAA cortical interneurons, although it is also modulated by other neurotransmitter systems (reviewed in (Paulus et al., 2008)). This finding of reduced GABAA neurotransmission in healthy subjects raises the intriguing possibility of using theophylline to promote plasticity in the recovery period.
Patients generally have diminished intracortical inhibition (i.e., “disinhibition”) in both hemispheres following stroke (Hummel et al., 2009; Liepert, 2006; Manganotti et al., 2008; Manganotti et al., 2002; Swayne et al., 2008). It is unknown whether theophylline could induce a further reduction in cortical inhibition, or if disinhibition is effectively at a ceiling in these patients. Thus, our primary aim was to evaluate the effect of theophylline on intracortical and interhemispheric inhibition in patients with chronic stroke. As secondary aims, we evaluated the safety of theophylline in these patients, assessing effects on cardiovascular, neurological, and motor performance outcomes. Because this is the first study investigating theophylline in patients with stroke, we used a single dose of 300 mg theophylline as a conservative first step.
2. Methods
2.1. Design
Subjects were studied in a cross-over design, on 2 separate days separated by at least one week (>21 theophylline half-lives). Subjects were randomized to receive either 300 mg extended-release theophylline or placebo on the first day and the opposite on the second day. TMS neurophysiology and motor behavioral assessments occurred in morning and afternoon sessions on each day. Patients, caretakers, and investigators were blinded to the condition order. Subjects were tested in the Motor Performance Laboratory at Columbia University. They gave written informed consent to participate in this study, which was approved by the CUMC Institutional Review Board.
2.2. Subjects
Twenty subjects were enrolled, but two subjects dropped out midway due to an unrelated medical or transportation issue. The results of 18 subjects are thus reported. Subjects were included if they were ≥ 40 years old and had a single ischemic or hemorrhagic stroke with resulting hemiparesis ≥ 6 months previously. Brain MRI or CT radiology reports were reviewed to confirm the presence of an infarction. Because we sought to examine bilateral neurophysiology, subjects who could at least minimally abduct their paretic index finger (MRC ≥ 1) and who had a recordable TMS-evoked response were included. Subjects were excluded for: multiple stroke events or bilateral paresis; history of seizure, preexisting CNS pathology, major psychiatric disorder, active substance abuse, respiratory disease, congestive heart failure, or peptic ulcer disease; inability to comprehend study requirements due to language abnormalities; thoracic or intracranial metal objects, implants, or devices, except for dental work; active use of any pharmacological agents metabolized through the cytochrome P450 1A2 pathway; or active participation in other trials.
2.3. Medication preparation and administration
The medications were prepared and dispensed by the Columbia University Medical Center Research Pharmacy. Patients received a single 300 mg dose of extended-release theophylline (Heritage Pharmaceuticals, NDC# 23155-062-01) or placebo, identical to theophylline in appearance and packaging. A single dose, rather than a week-long course, was chosen for this initial investigation because of the lack of experience with this medication in patients with stroke. Because many patients with chronic stroke are intracortically disinhibited at baseline (Schambra et al., 2015), we were initially concerned that longer drug exposures could overshoot the intended effect.
The standard dose of theophylline for asthma monotherapy is 300 mg twice daily, with a target serum level of 10–20 µg/ml. In its extended-release formulation, a mean peak serum theophylline concentration of ~4.4 µg/ml is expected 4–8 h after intake (Heritage, 2014). Serologic theophylline levels were not drawn.
Subjects were not required to fast, but were instructed not to take any form of caffeine on testing days. After capsule intake in the morning session, neurophysiological and clinical measures were immediately assessed. These were repeated 5 hours later in the afternoon session. Between sessions, subjects quietly read or napped.
2.4. TMS set-up
Patients were comfortably seated with the forearm resting on a pillow in ~90° elbow flexion. Frameless stereotaxic equipment (Brainsight, Rogue Research, Canada), used to co-register the subject’s scalp positions with a phantom MRI brain image, ensured stimulation accuracy during and across sessions. Co-registration errors to the phantom’s surface landmarks were matched to ≤3 mm at each session.
Surface EMG was obtained from bilateral first dorsal interosseous (FDI) muscles, with electrodes taped in a belly-tendon orientation (SX230-100 and K800; Biometrics Ltd, UK). Electrodes were outlined with permanent ink on the skin to ensure consistency of electrode placement within day. The EMG signal was sampled at 1000 Hz, amplified 1000x, band-pass filtered at 15–450 Hz, and saved for offline analysis. All TMS assessments were taken at rest, and EMG activity was monitored online to ensure muscle relaxation.
TMS always began in the nonlesioned hemisphere. Stimuli were delivered in BiStim mode to the cortical hand representation of the motor cortex using Magstim BiStim2. A 70-mm figure-of-eight remote control coil (Magstim Company Ltd, UK) was used for most measures, with the addition of a 50-mm figure-of-eight coil (Magstim Company Ltd, UK) for the measurement of interhemispheric inhibition. Pulses were generated using specialized software (Signal; Cambridge Electronic Devices, UK) and a 1401 microprocessor (Cambridge Electronic Devices, UK). The TMS coil was held tangentially to the skull with the coil handle pointed 45° posterior-laterally. The scalp location (“hotspot”) producing the largest amplitude motor evoked potential (MEP) for the contralateral first dorsal interosseous (FDI) muscle was identified and marked virtually on the phantom MRI.
2.5. TMS outcomes
2.5.1. Resting motor threshold
Resting motor threshold (RMT) was defined as the stimulation intensity eliciting at least 5/10 MEPs ≥ 50 µV at the hotspot. RMT is believed to reflect the membrane excitability of cortico-cortical axons that propagate the TMS-induced action potential (Di Lazzaro, 2008).
2.5.2. Paired-pulse measures
For each subject, the test stimulus (TS) intensity evokinganMEPamplitudeof0.5–1mVwasobtained from each hotspot. If this size MEP could not be achieved, particularly in the lesioned hemisphere, the TS was set to a stimulation intensity above which no further increases in amplitude could be found (Schambra et al., 2015; Swayne et al., 2008).
Short-interval intracortical inhibition (SICI) was assessed by pairing a TS with a subthreshold conditioning stimulus (CS) set to a stimulation intensity of 80% RMT. The CS preceded the TS at an interstimulus interval (ISI) of 1 ms (SICI1ms) or 2 ms (SICI2ms). SICI2ms is believed to primarily reflect activity of intracortical GABAA neurotransmission (Hanajima et al., 1998; Werhahn et al., 1999; Ziemann, Lonnecker, Steinhoff, & Paulus, 1996). The neural elements mediating SICI1ms are more controversial. While many believe that SICI1ms probes activity at synaptic GABAAR (see review in (Ziemann et al., 2015), others have speculated that it reflects inhibitory tone mediated by extrasynaptic GABAAR (Stagg et al., 2011). We chose to evaluate both SICI1ms and SICI2ms, to assess for differential activity under the influence of theophylline.
Long-interval intracortical inhibition (LICI) was assessed by pairing a conditioning TS with another TS 100 ms later (Nakamura et al., 1997). LICI is believed to be primarily reflect activity in intracortical GABAB neurotransmission (Werhahn et al., 1999; Ziemann et al., 1996).
Interhemispheric inhibition (IHI) was assessed by delivering a conditioning TS to one hemisphere and another TS to the opposite, receiving hemisphere, 10 ms later (Ferbert et al., 1992). The order of pulse delivery alternated to generate IHI in both directions. In all subjects, the 70-mm coil probed the lesioned hemisphere and the 50-mm coil probed the nonlesioned hemisphere. IHI at this short ISI is believed to be mediated by long-range glutamatergic neurons projecting transcallosally onto local GABAB circuitry (Daskalakis et al., 2002). Because the inhibitory component of IHI is in the receiving hemisphere, here we specify IHI by the receiving hemisphere; for example, “lesioned IHI” means that the conditioning TS was delivered to the nonlesioned hemisphere and the TS was delivered to the lesioned hemisphere.
In all paired-pulse outcomes, 15 trials each of (a) TS alone and (b) CS+TS were recorded in a pseudo-random order. Peak-to-peak MEP amplitudes were measured offline using a custom-made script (Gray, 2015). Trials were discarded if EMG activity exceeded 100 µV in the 250 ms prior to TMS stimulus delivery. The average amplitude of conditioned TS was normalized to the average amplitude of the unconditioned TS, i.e. (CS+TS)/TS, and paired-pulse data are reported as a decimal fraction of the TS amplitude. For all cortical inhibitory measures, an increase in the decimal fraction of the TS amplitude is discussed as “reduced inhibition” or “disinhibition.”
2.6. Safety assessments
2.6.1. Motor performance measures
During motor training, SICI may focally increase or decrease for specific muscles, depending on the specific action that muscle is to take (Liepert et al., 1998). We felt it important to ensure that theophylline, if inducing global SICI reduction, would not lead to abnormal cortical regulation and impaired motor output. Finding aberrant motor function with theophylline would preclude application in motor rehabilitation. Thus, immediately following bilateral TMS assessments, finger strength and dexterity were assessed in both hands.
Pinch force dynamometry was performed first on the paretic side. Subjects sat with shoulder adducted, elbow flexed at 90°, forearm midway between pronation and supination, and wrist in ~15° extension. Subjects held the force transducer (P200; Biometrics, UK) between the pad of thumb and radial side of the flexed index finger (i.e., a lateral or key pinch). Three maximal voluntary contractions (MVC) were held for 3 seconds each, with 10–20 seconds rest between, and stored offline. The maximum voltage of the force was extracted with a custom-made Signal script (S. Gray, CED, UK), and a conversion of 11.34 kg/V was applied. The three trials were averaged for each session.
The Nine-Hole Peg Test (9HPT) was used to evaluate finger dexterity (Beebe & Lang, 2009), and performed first on the paretic side. It has excellent intra-rater reliability in patients with stroke (Hanajima, Ugawa et al., 1998) and is sensitive to functional change (Hanajima et al., 1998). Using one hand, the subject picked up single plastic pegs from a shallow well, placed each into a board with 9 holes, and then replaced the pegs back in the well (Mathiowetz et al., 1985). Time to complete the board and number drops were recorded. Timeout occurred if no more than 2 pegs had been placed in the board by 120 seconds, and time was recorded as 120 seconds (3 subjects).
2.6.2. Cardiovascular and neurological measures
Before each session, heart rate and blood pressure were documented. Following each session, subjects reported levels of alertness during the session and excitement to participate, using a visual analog scale of 1–10 (10 as maximum). They also reported amount of pre-testing sleep and exercise, and occurrence of seizure, nausea, headache, or jitteriness.
Immediately following testing on a morning session, a neurologist (HMS) documented demographics, medical history, handedness (Edinburgh Handedness Inventory; +1 and –1 indicate dominance for right and left hand, respectively (Oldfield, 1971)) and bilateral upper extremity strength indexed by the Medical Research Council (MRC) scale (Kingdom, 1978). After the other morning session, a trained assessor (IMH) evaluated upper extremity impairment using the upper extremity Fugl-Meyer scale (Fugl-Meyer et al., 1975).
2.7. Blinding assessment
Following the second day of testing, patients and the main assessor (HMS) guessed which days they believed theophylline and placebo were administered. Reasons for their speculation were recorded.
2.8. Data analysis
The average change in neurophysiological and behavioral outcomes between morning and afternoon sessions was calculated for each condition. A paired nonparametric regression model was used to estimate differences in change scores associated with theophylline relative to placebo. With this hierarchical modeling approach, we accounted for repeated measurements within each individual. Considering that hemispheres may differentially respond to an intervention, we assessed outcomes in lesioned and nonlesioned hemispheres separately.
To evaluate differences in ordinal data, a non-parametric Wilcoxon Rank Sum was used. To evaluate differences in rate of categorical side effects, Fisher’s Exact Test was used. Inter-rater reliability of guessing with assessed with a kappa statistic. To examine the possibility of a ceiling effect in neurophysiological outcomes, correlations between the baseline (morning) value and the delta between morning and afternoon sessions (PM minus AM) were tested using linear regression analyses. Bonferroni adjustments were made for the assessment of multiple outcome measures. Significance was set at an alpha of 0.05.
2.9. Power calculation
We assumed that theophylline in patients with stroke would decrease SICI by 0.21 decimal percentage points with a standard deviation of 0.23 points, based on previously published results in normal subjects (Nardone et al., 2004). Further assuming zero correlation of the two changes within-subject, the paired regression model with n = 18 and 5% Type I error rate had 78% power to detect this magnitude of change in SICI.
3. Results
3.1. Clinical characteristics
Age, gender, handedness, stroke type, lesion location, lesioned hemisphere, and time since stroke are given in Table 1. Subjects were tested at a median interval of 7.5 days (range 7–38 days) between the placebo and theophylline administrations. No subjects had active tobacco use.
Table 1.
Clinical characteristics. Age, Edinburgh handedness score, time since stroke, Upper Extremity (UE) Fugl-Meyer scores are average ±SD. “Mixed” location implies that the lesion includes cortex and underlying white matter
| Subject | Age (years) |
Gender | Handedness | Stroke Type |
Lesion Location |
Lesioned Hemisphere |
Time since Stroke (days) |
UE Fugl-Meyer score |
|---|---|---|---|---|---|---|---|---|
| 1 | 68 | M | 1 | Ischemic | Subcortical | Left | 663 | 64 |
| 2 | 55 | M | 1 | Ischemic | Subcortical | Left | 1942 | 40 |
| 3 | 50 | M | 1 | Ischemic | Subcortical | Left | 3200 | 53 |
| 4 | 75 | F | 1 | Hemorrhagic | Subcortical | Left | 1074 | 11 |
| 5 | 44 | M | 1 | Ischemic | Subcortical | Left | 12775 | 45 |
| 6 | 63 | M | 1 | Ischemic | Mixed | Left | 3820 | 50 |
| 7 | 65 | F | 1 | Ischemic | Mixed | Right | 1507 | 51 |
| 8 | 67 | M | −0.6 | Ischemic | Mixed | Left | 3213 | 53 |
| 9 | 59 | F | −1 | Ischemic | Mixed | Left | 3455 | 59 |
| 10 | 49 | M | 0.9 | Ischemic | Mixed | Left | 2606 | 43 |
| 11 | 71 | M | 1 | Ischemic | Subcortical | Right | 3145 | 65 |
| 12 | 59 | M | 1 | Ischemic | Mixed | Left | 409 | 30 |
| 13 | 62 | M | 1 | Ischemic | Mixed | Right | 4999 | 58 |
| 14 | 75 | M | 1 | Ischemic | Subcortical | Left | 2667 | 47 |
| 15 | 70 | M | −0.4 | Ischemic | Mixed | Left | 5103 | 58 |
| 16 | 70 | M | 1 | Ischemic | Subcortical | Left | 3486 | 52 |
| 17 | 60 | F | 1 | Ischemic | Subcortical | Left | 322 | 54 |
| 18 | 88 | F | 1 | Ischemic | Subcortical | Left | 490 | 57 |
| average | 63.9± 10.7 | 13 M: 5F | 0.72± 0.64 | 17 I: 1 H | 10 S: 8 M | 15 L: 3 R | 3048.7± 2850.4 | 49.4 ±12.9 |
3.2. TMS outcomes
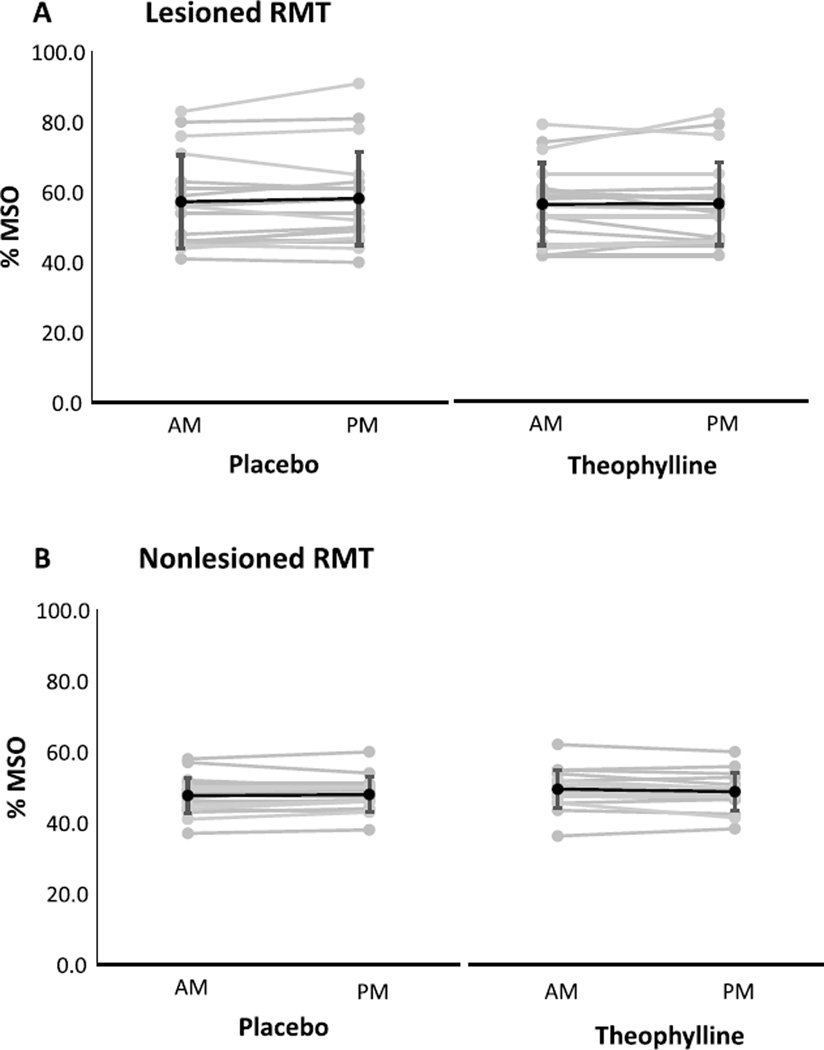
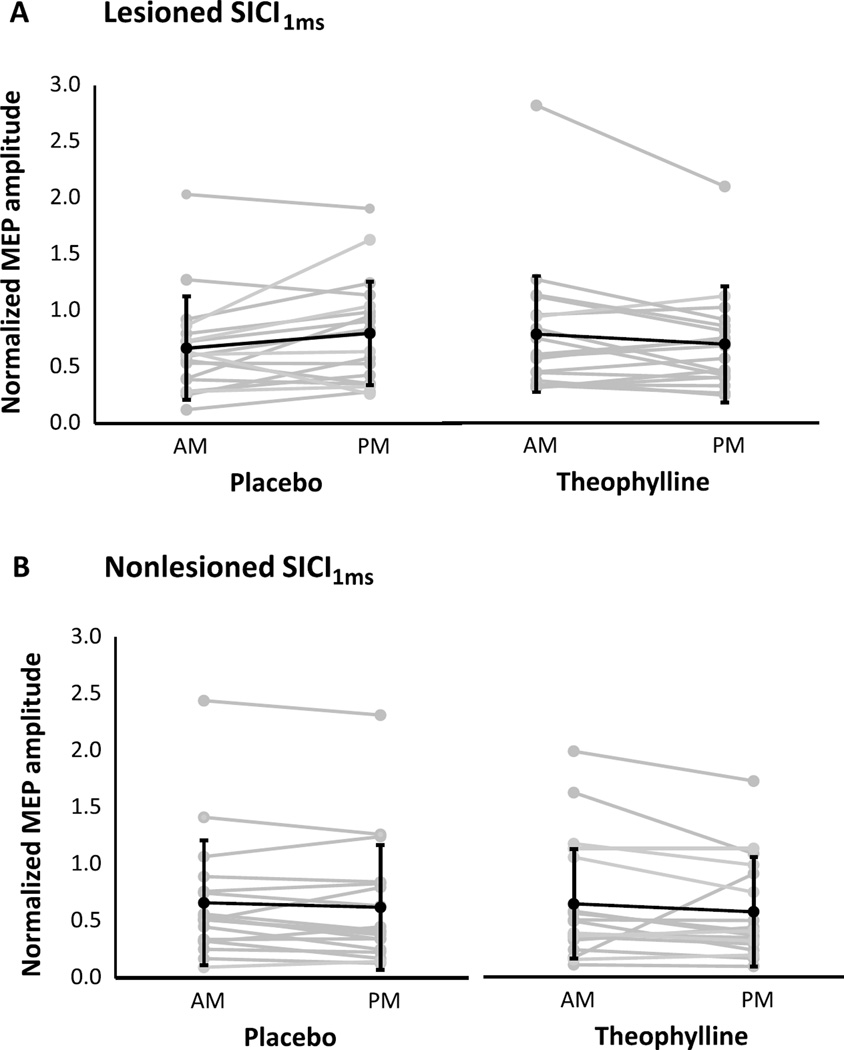
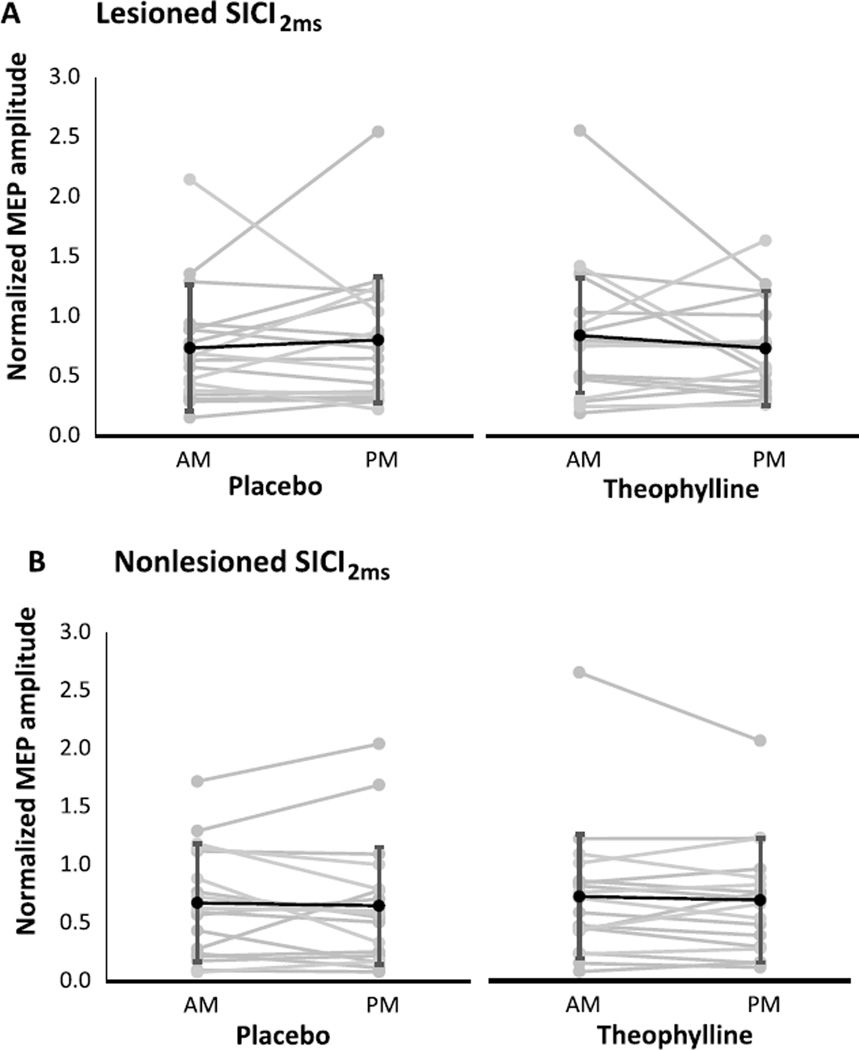
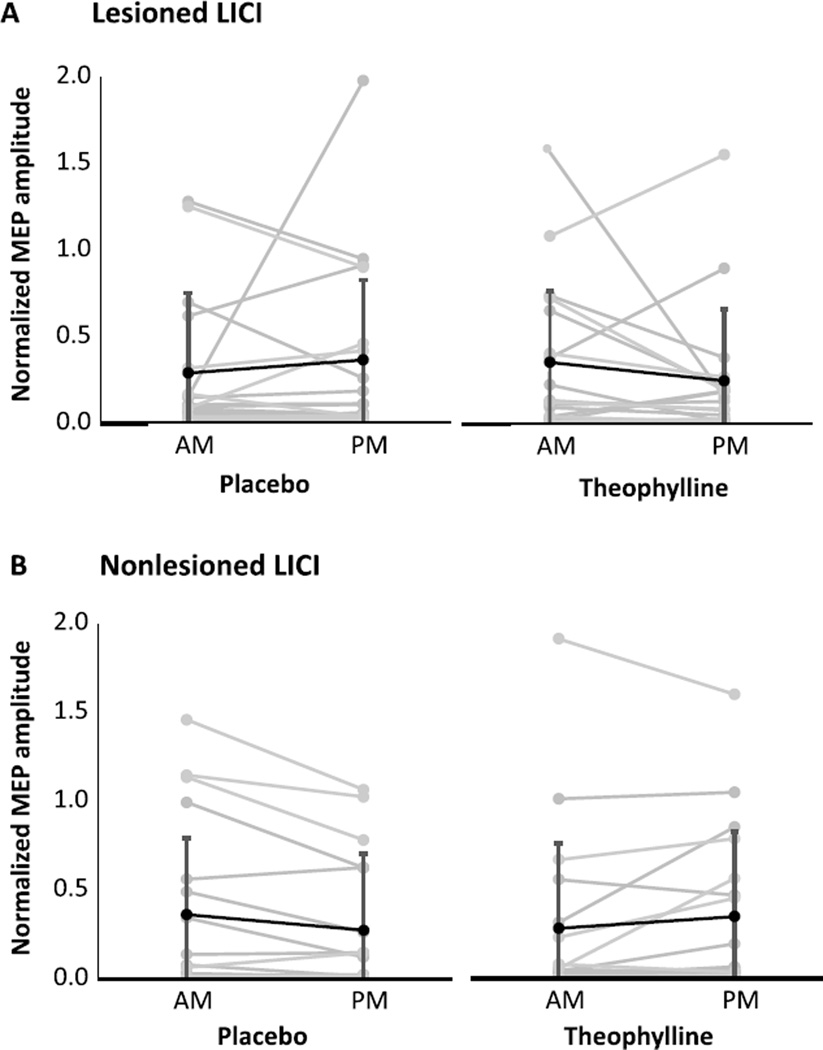
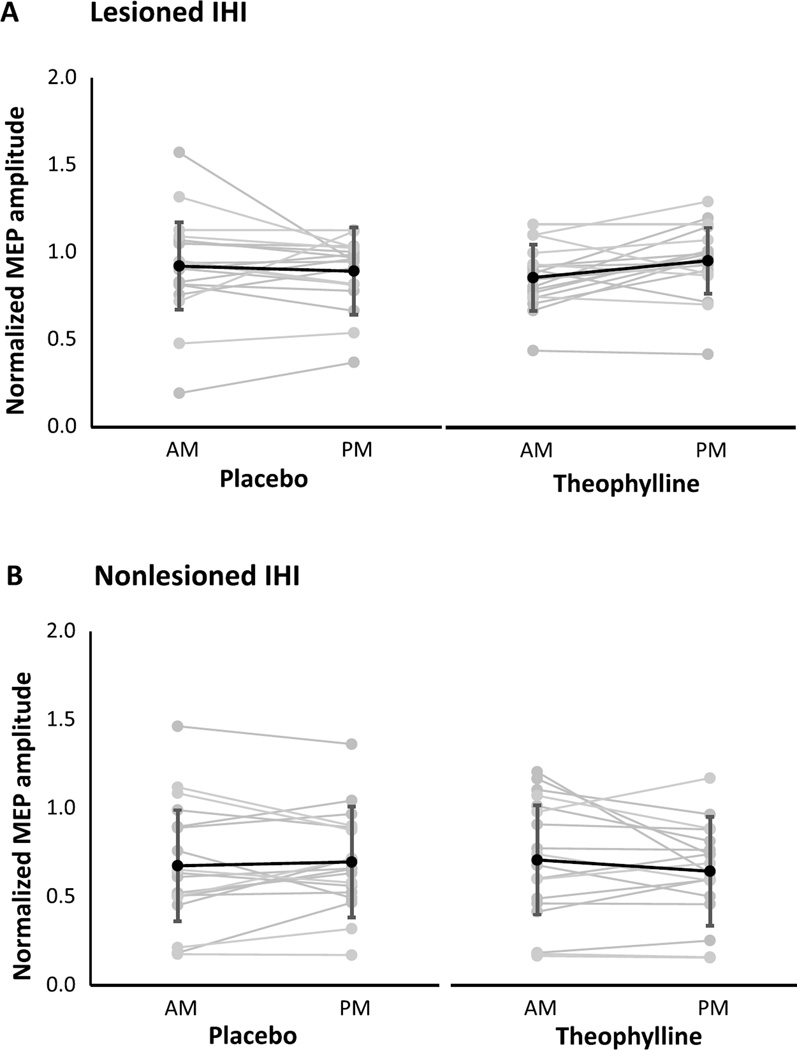
For each condition and session, neurophysiological outcomes are shown for the lesioned and nonlesioned hemispheres (Table 2). For both hemispheres, change scores were not significantly different between theophylline and placebo conditions for RMT (Fig. 1), SICI1ms (Fig. 2), SICI2ms (Fig. 3), LICI (Fig. 4), or IHI (Fig. 5). Subjects with marked disinhibition behaved consistently across days, and removal of their data did not substantively alter comparisons between the conditions.
Table 2.
Cardiovascular and psychometric outcomes, with ranges in parentheses. Heart rate, blood pressure, excitement, and alertness are averages of morning and afternoon sessions, and change scores are deltas between morning and afternoon. Therapy and sleep durations are totals over the day. There were no significant differences between conditions for any outcome
| Placebo |
Theophylline |
|||
|---|---|---|---|---|
| Average | Change | Average | Change | |
| Heart Rate (beats/min) | 73 (46–85) | 0 (–20–10) | 75 (48–100) | 6.5 (–15–16) |
| Systolic Blood Pressure (mmHg) | 133 (113–151) | −1.5 (–22–18) | 134 (116–168) | 0 (–28–16) |
| Diastolic Blood Pressure (mmHg) | 81 (72–87) | 2 (–18–14) | 84 (66–102) | −1 (–20–12) |
| Excitement | 7.5 (3.0–10.0) | 0 (–5.0–2.0) | 8.3 (2.5–10.0) | 0 (–1.0–2.5) |
| Alertness | 7.3 (4.0–9.5) | −1.0 (–6.0–4.0) | 6.8 (4.5–10.0) | 0 (–5.0–7.0) |
| Therapy duration (h) | 0.3 (0–1.5) | 0.2 (0–2.0) | ||
| Sleep duration (h) | 7.5 (4.5–10.0) | 6.8 (4.0–9.2) | ||
Fig. 1.
Resting motor thresholds in the lesioned (A) and nonlesioned (B) hemispheres under placebo and theophylline conditions. Changes from morning (AM) to afternoon (PM) sessions were not significantly different between placebo and theophylline. Values are maximum stimulator output (MSO) mean±SD.
Fig. 2.
Short-interval intracortical inhibition with a 1 ms ISI in the lesioned (A) and nonlesioned (B) hemispheres under placebo and theophylline conditions. Changes from morning (AM) to afternoon (PM) sessions were not significantly different between placebo and theophylline. Normalized MEP amplitudes are mean ± SD.
Fig. 3.
Short-interval intracortical inhibition with a 2 ms ISI in the lesioned (A) and nonlesioned (B) hemispheres under placebo and theophylline conditions. Changes from morning (AM) to afternoon (PM) sessions were not significantly different between placebo and theophylline. Normalized MEP amplitudes are mean ±SD.
Fig. 4.
Long-interval intracortical inhibition under placebo and theophylline conditions in the lesioned (A) and nonlesioned (B) hemispheres. Changes from morning (AM) to afternoon (PM) sessions were not significantly different between placebo and theophylline. Normalized MEP amplitudes are mean ± SD.
Fig. 5.
Interhemispheric inhibition projecting onto the lesioned (A) and nonlesioned (B) hemispheres under placebo and theophylline conditions. Changes from morning (AM) to afternoon (PM) sessions were not significantly different between placebo and theophylline. Normalized MEP amplitudes are mean ± SD.
To evaluate the possibility of reporting a Type II error, we computed the smallest detectable group change (SDCgroup) for two TMS outcome measures, SICI2ms and LICI, using repeated-measures reliability estimates previously attained (Schambra et al., 2015). The SDCgroup is the threshold amount of change necessary to be considered a true change for a group (Beckerman et al., 2001). For a group of 18 patients with chronic stroke, the SDCgroup of SICI2ms is 0.14 and 0.08 for the lesioned and nonlesioned hemisphere, respectively. The SDCgroup of LICI is 1.2 and 1.0 for the lesioned and nonlesioned hemisphere, respectively. Our changes in SICI2ms and LICI did not exceed these SDCsgroup, signifying that the observed deltas occurred within the envelope of measurement noise.
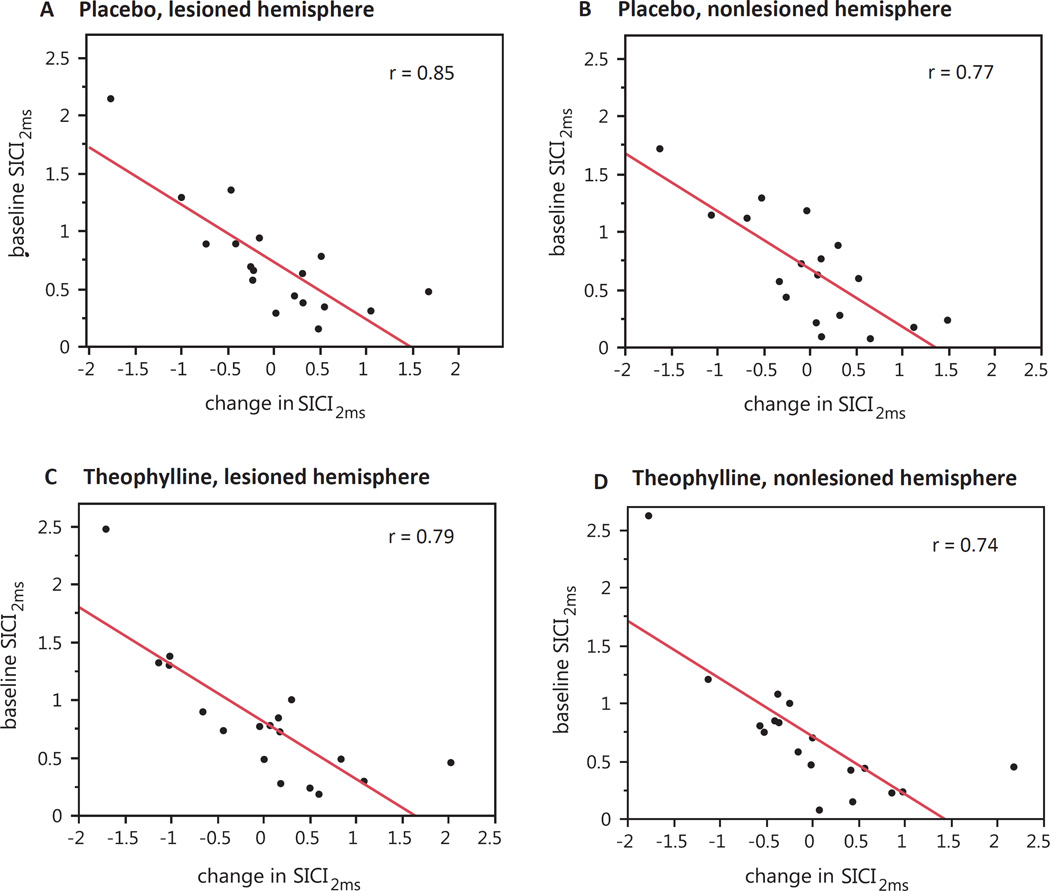
To evaluate for the presence of a ceiling effect in the neurophysiological outcomes, we assessed the correlation between the normalized baseline (morning) value and the delta between morning and afternoon sessions (Table 3). We examined correlations within condition and hemisphere, and corrected for the performance of multiple correlations. We found moderate-to-strong inverse relationships between baseline values and deltas for SICI1ms, SICI2ms, LICI, and IHI, with most being highly significant. These correlations showed that higher baseline values were associated with negative deltas, and lower baseline values were associated with positive deltas (see correlations for SICI2ms in Fig. 6 for example). In other words, less inhibition at baseline was associated with a strengthening of inhibition in that outcome, whereas more inhibition at baseline was associated with a weaknening of inhibition. This relationship was present in both the theophylline and placebo conditions. The theophylline condition did not change the regression line intercept or slope. There was no significant correlation between baseline RMT and its change in either hemisphere or condition.
Table 3.
Relationships between baseline (morning) value and delta between morning and afternoon sessions. Shown are the slope of the regression line, the correlation coefficient (r), and its p-value in parentheses. Signficant correlations are bolded
| Placebo | Theophylline | |||||||
|---|---|---|---|---|---|---|---|---|
| Lesioned |
Nonlesioned |
Lesioned |
Nonlesioned |
|||||
| slope | r (p) | slope | r (p) | slope | r (p) | slope | r (p) | |
| RMT | 0.31 | 0.07 (1) | −1.25 | 0.39 (1) | 0.28 | 0.09 (1) | −0.99 | 0.35 (1) |
| SICI1ms | −0.50 | 0.78 (0.002) | −0.50 | 0.81 (<0.002) | −0.50 | 0.73 (0.01) | −0.50 | 0.80 (<0.002) |
| SICI2ms | −0.49 | 0.85 (<0.002) | −0.50 | 0.77 (0.004) | −0.50 | 0.79 (<0.002) | −0.50 | 0.74 (0.008) |
| LICI | −0.55 | 0.80 (<0.002) | −0.53 | 0.64 (0.07) | −0.63 | 0.85 (<0.002) | −0.49 | 0.60 (0.17) |
| IHI | −0.50 | 0.78 (0.002) | −0.50 | 0.77 (0.004) | −0.50 | 0.61 (0.12) | −0.51 | 0.76 (0.004) |
Fig. 6.
Correlations between morning value of SICI2ms and its delta between morning and afternoon, assessed by condition (placebo and theophylline) and hemisphere (lesioned and nonlesioned). All relationships were strongly inversely correlated and significant (p < 0.001) across conditions and hemispheres. This relationship indicates that subjects with higher morning values (i.e., baseline disinhibition) tend to become more inhibited by the afternoon, whereas subjects with lower morning values (i.e., strong baseline inhibition) tend to become less inhibited by the afternoon. A. Placebo, lesioned hemisphere. B. Placebo, nonlesioned hemisphere. C. Theophylline, lesioned hemisphere. D. Theophylline, nonlesioned hemisphere.
3.3. Behavioral outcomes
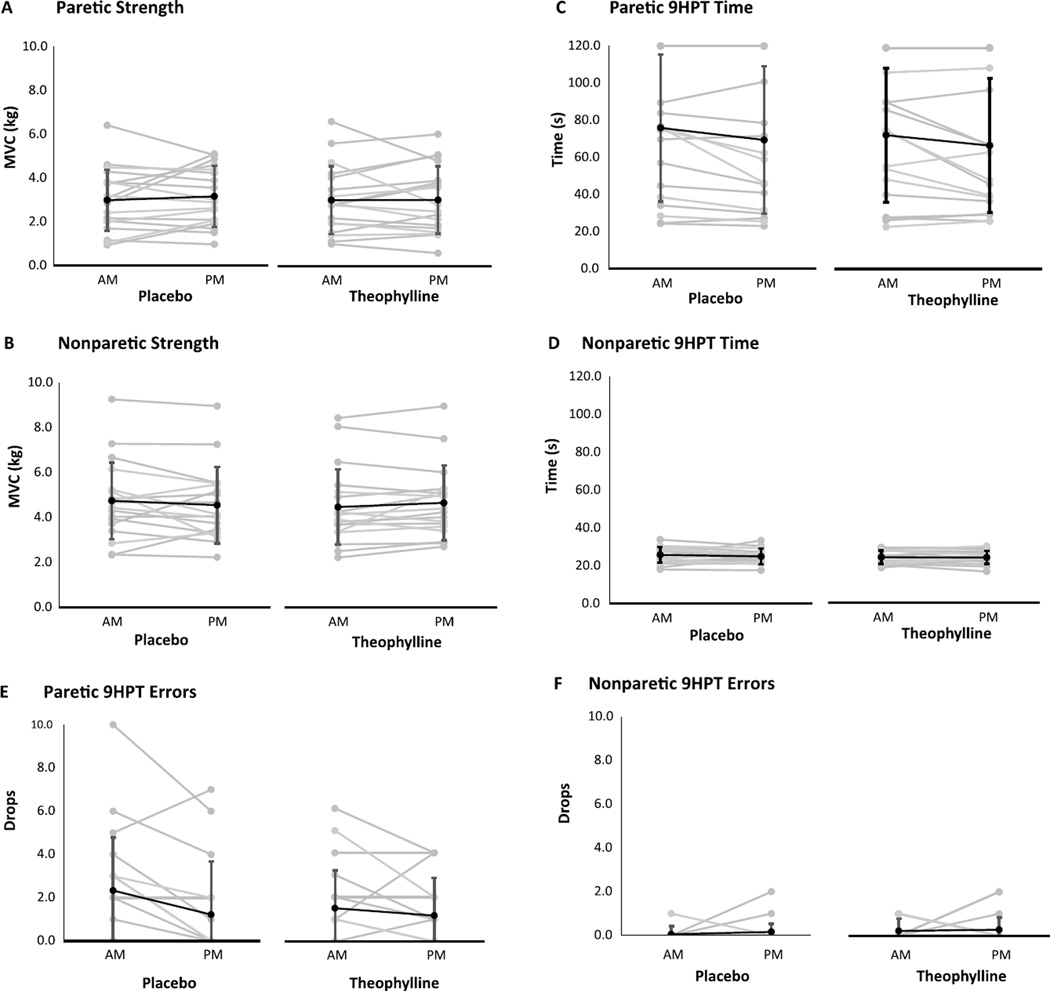
For the paretic and nonparetic hands, change scores were not significantly different between theophylline and placebo conditions for strength, pegboard speed, or pegboard accuracy (Fig. 7).
Fig. 7.
Hand strength and dexterity under placebo and theophylline conditions. Changes from morning (AM) to afternoon (PM) in pinch strength in the paretic (A) and nonparetic (B) hands, time of 9-Hole Peg Test (9HPT) completion in the paretic (C) and nonparetic (D) hands, and errors (peg drops) on the 9HPT in the in the paretic (E) and nonparetic (F) hands did not significantly differ between placebo and theophylline. Values are mean ±SD.
3.4. Cardiovascular and psychometric outcomes
Average heart rate and blood pressure, and change from morning to afternoon sessions, did not differ between conditions (Table 2). Likewise, there were no significant differences in average alertness and excitement, change in alertness and excitement, or total amount of exercise and sleep under theophylline or placebo. The frequency of side effects was low and not significantly different across conditions: 1 headache (placebo condition), 1 episode of jitteriness (theophylline condition), and no nausea or seizures.
3.5. Randomization and blinding
Eight (44%) of the first sessions were placebo and 10 (56%) were theophylline. Correct guesses about the administration order occurred for 56% of subject responses and 61% of investigator (HMS) responses, and the subjects and investigator showed low agreement in their guesses (K= 0.33,NS). Patients reported feeling more alert or more physically adept as often on placebo as on theophylline.
4. Discussion
In this double-blind, placebo-controlled, crossover study, we used TMS to evaluate the effects of theophylline on inhibitory cortical circuitry in patients with chronic stroke. Relative to placebo, we found that a single dose of theophylline did not significantly alter neurophysiological outcomes, but importantly had no adverse neurological, cardiovascular, or motor performance effects.
4.1. Neurophysiological effects of single-dose theophylline
Our results are in keeping with a lack of cortical excitability changes found with a single dose of caffeine, another methylxanthine drug with similar pharmacological properties (Orth, Amann, Ratnaraj, Patsalos, & Rothwell, 2005). However, they do not replicate those found in healthy controls receiving theophylline, which resulted in a reduction in SICI2ms relative to placebo (Nardone et al., 2004). There are two reasons why we may have not have observed this effect: methodological differences, or a differential responsiveness of post-stroke neural circuitry.
Our approach differed from that of Nardone and colleagues in two ways. First, Nardone and colleagues administered theophylline daily for 7 days, whereas we administered theophylline in a single dose. Because theophylline had not been investigated before in patients with stroke, we chose a conservative dosing strategy to avoid inducing pathological hyperexcitability in patients who are already disinhibited. However, this single dose resulted in a concentration that was likely too low to modulate neurotransmission: we probed neurophysiology when peak serological concentration was estimated to be ~4 µg/ml, whereas Nardone and colleagues report an effect at 8–21 µg/ml. In addition, a single dose may have resulted in a more variable cortical concentration of theophylline, as the steady state of a drug can be expected after ~5 doses. Thus, a repeated exposure to theophylline may be required to allow for equilibrium across the blood brain barrier, a sufficiently high and constant cortical concentration, and receptor modulation. Second, we based our CS stimulation intensity on 80% RMT, whereas Nardone and colleages used 95% AMT, generating stimulation intensities that equate to 76–81% RMT. However, it is not expected that incremental differences around this CS stimulation intensity would contribute to large differences in inhibition (Kujirai et al., 1993).
We also studied theophylline in patients with stroke, whereas Nardone and colleagues studied healthy subjects. Reduced SICI after stroke, particularly in lesioned hemisphere, is widely observed and may be related to diminished GABAAR expression (M. Qu et al., 1998; Que et al., 1999). In the present sample, SICI2ms in the majority of subjects was more disinhibited at baseline than in the previously sampled healthy controls (Nardone et al., 2004). In these subjects, SICI2ms is nearing the upper limit of dis-inhibition and thus may be at the upper end of its physiologically meaningful range. The addition of an excitatory/disinhibitory perturbation in conjunction with SICI disinhibition may therefore produce nonadditive results. First, responsiveness to further excitatory/disinhibitory interventions may be blunted in patients with stroke, resulting in no substantive change, as observed after motor training (Blicher et al., 2009). Alternatively, disinhibition may be down-regulated through homeostatic metaplasticity mechanisms, returning circuitry to a more physiologically responsive range (Bienenstock, Cooper, & Munro, 1982). This phenomenon has been observed with continuous theta-burst stimulation (Huang et al., 2005; Huang et al., 2008) and anodal transcranial direct current stimulation (Heise et al., 2014).
In our correlation analyses of the neurophysiologic outcomes, we did not find an asymptote centered on zero change, which would suggest a ceiling over some level of baseline disinhibition. We did, however, find strong inverse relationships between the amount of baseline inhibition and its change between morning and afternoon sessions, in both conditions and hemispheres. Subjects with strong baseline inhibition became less inhibited, those with normal inhibition or mild disinhibition had minimal change, and those with strong baseline disinhibition became more inhibited. This observation could be considered a regression toward the mean, where observations that are randomly extreme on the first measurement tend to be closer to the mean on the second. However, the extreme measurements were not random, but rather were consistent within subjects across days, making this phenomenon less likely. This observation therefore would suggest the action of a homeostatic process. Theophylline did not flatten the slopes of these relationships, but also did not increase their intercepts (i.e. right-shift the relationships). These findings suggest that theophylline neither modifies nor usurps this homeostatic process, at least in a single-dose administration.
Of note, we applied a conservative statistical correction for our exploration of multiple outcome measures. Controlling for Type I error when multiple outcomes are evaluated is a practice implemented in other clinical neuroscience methodologies (Bennett, Wolford, & Miller, 2009; Mensen & Khatami, 2013), but uncommonly adopted in multiple-outcome TMS studies. Although it is conceivable that we are reporting Type II errors (false negatives) for our TMS outcomes, the within-condition change scores of SICI2ms and LICI are less than the smallest detectable change expected for their hemisphere and group size. This means that differences between conditions in change scores likely occurred within the envelope of measurement noise (Schambra et al., 2015).
4.2. Safety of single-dose theophylline
We found that theophylline was well-tolerated, with no adverse neurologic or cardiovascular effects. For both upper extremities, theophylline did not worsen finger strength or manual dexterity relative to placebo. These findings imply that, at least for a single dose, theophylline does not interfere with motor performance, allowing the subjects to safely proceed with assessments of motor skill learning.
Unlike a single assay of motor performance, motor skill learning models the activity-dependent plasticity brought about through physical therapy, which engages the robust plasticity milieu triggered by stroke (Zeiler & Krakauer, 2013). While the neurophysiologic effects of any pharmacological intervention are important to establish, justification for its translation to recovering patients requires a beneficial effect on motor skill learning. Given the present study was a single-day drug exposure with a crossover design, we were limited in our ability to assess motor skill learning, a direction for future efforts.
4.3. Theophylline and GABAAR
There is support for the hypothesis that theophylline can modulate neural circuitry to support recovery after stroke. Because Nardone and colleagues found an isolated reduction in SICI2ms after theophylline, antagonism of GABAAR by theophylline was inferred (Nardone et al., 2004; Ziemann et al., 2015). However, the neurophysiological underpinnings of SICI are complex, with contributions by glutaminergic neurotransmission and modulation by dopaminergic, serotonergic, adrenergic, and acetyl-cholinergic systems (reviewed in (Paulus et al., 2008). A decrease in SICI may therefore reflect direct GABAAR antagonism, increased glutamatergic neurotransmission, or modulation of the motor cortex by other neurotransmitters.
Theophylline weakly binds to GABAAR at concentrations within the clinical dosing range (Segev, 1988), but electrophysiological disinhibition only occurs at concentrations far exceeding clinically toxic levels (Scholfield, 1980, 1982). Theophylline is ~100 times more potent at the adenosine than GABAA receptor and nonselectively antagonizes the A1 and A2a subtypes (Fredholm, 1979; Snyder et al., 1981). A1R blockade increases presynaptic neurotransmitter release without affecting basal GABA release, thus increasing post-synaptic excitability (Burke & Nadler, 1988; Dunwiddie & Diao, 1994; Hollins & Stone, 1980; Prince & Stevens, 1992). A2aR antagonism, on the other hand, generally reduces post-synpatic transmission across multiple neurotransmitter systems (reviewed in (Chen & Chern, 2011).
It is thus conceivable that reduced SICI by theophylline could reflect A1R antagonism, resulting in increased glutamatergic, adrenergic, serotoninergic, and acetylcholinergic signaling in/to the motor cortex. Similarly, SICI reduction may reflect A2aR antagonism, resulting in decreased dopamine-mediated action on the motor cortex. Given theophylline’s weak affinity for GABAAR at low concentrations, GABAAR antagonism is less likely to explain SICI reduction. Increasing glutamatergic, adrenergic, and serotoninergic signaling in the motor cortex are approaches currently being explored in preclinical and clinical stroke recovery studies (Cherry, Lenze, & Lang, 2014; Dhawan et al., 2011; Grade et al., 1998; Siepmann et al., 2015).
4.4. Limitations
In the present sample of patients with chronic stroke, we note measurement variability that challenges the detection of group change, irrespective of condition. We were sufficiently powered to detect a significant effect of theophylline on SICI2ms, had the previously reported effect size and variability been borne out in this sample. In our sample, SICI2ms and LICI measurements were more variable than those previously observed in a group of patients with chronic stroke, despite phenotypic similarity, identical methodology, the same TMS operator, and several shared subjects (Schambra et al., 2015). It is possible that the difference lies in the number of trials averaged—15 in the present study versus 40 previously. In the future, increasing the number of trials may help to reduce variability, especially in patients with stroke. In addition, adjusting individual stimulation intensities at baseline may help to standardize the degree of inhibition across individuals, which can then be assessed in the face of an intervention (Florian, Muller-Dahlhaus, Liu, & Ziemann, 2008). We also did not obtain serological levels of theophylline, and therefore can only report that neurophysiological changes and adverse effects did not occur at a single 300 mg dose of theophylline, rather than at a certain serological concentration. Particularly if clinical translation is intended, future investigations may consider delimiting the lower and upper boundaries of drug level that induce a neurophysiological or behavioral learning effect. Finally, we did not evaluate excitatory TMS outcomes given a lack of theophylline effect in healthy subjects (Nardone et al., 2004), although future studies may to wish to investigate differential responses in post-stroke neural circuitry.
5. Conclusion
In summary, in this double-blind, placebo-controlled study, we found no neurophysiological changes in the inhibitory cortical circuitry of patients with chronic stroke receiving a single dose of theophylline. We also found no adverse neurological, cardiovascular, and motor performance effects at this dose. Future investigations using theophylline in stroke may consider investigating a more prolonged drug exposure, effects on motor skill learning, and the relationship between serological levels and neurophysiological and behavioral efficacy.
Acknowledgments
We wish to thank Dr. Ken Cheung for his early statistical assistance. This study was supported by K23NS078052 (HMS), the Levine Research Gift Fund, and an unrestricted gift by John K. Castle. None of the authors have potential conflicts of interest. The study sponsors had no involvement in the execution of the study.
References
- Amadi U, Ilie A, Johansen-Berg H, Stagg CJ. Polarity-specific effects of motor transcranial direct current stimulation on fMRI resting state networks. Neuroimage. 2013;88C:155–161. doi: 10.1016/j.neuroimage.2013.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Lung Association. Clinical trial of low-dose theophylline and montelukast in patients with poorly controlled asthma. American Journal of Respiratory and Critical Care Medicine. 2007;175(3):235–242. doi: 10.1164/rccm.200603-416OC. [DOI] [PubMed] [Google Scholar]
- Beckerman H, Roebroeck ME, Lankhorst GJ, Becher JG, Bezemer PD, Verbeek AL. Smallest real difference, a link between reproducibility and responsiveness. Quality of Life Research. 2001;10(7):571–578. doi: 10.1023/a:1013138911638. [DOI] [PubMed] [Google Scholar]
- Beebe JA, Lang CE. Relationships and responsiveness of six upper extremity function tests during the first six months of recovery after stroke. Journal of Neurologic Physical Therapy. 2009;33(2):96–103. doi: 10.1097/NPT.0b013e3181a33638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett CM, Wolford GL, Miller MB. The principled control of false positives in neuroimaging. Social Cognitive and Affective Neuroscience. 2009;4(4):417–422. doi: 10.1093/scan/nsp053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bienenstock EL, Cooper LN, Munro PW. Theory for the development of neuron selectivity: Orientation specificity and binocular interaction in visual cortex. Journal of Neuroscience. 1982;2(1):32–48. doi: 10.1523/JNEUROSCI.02-01-00032.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blicher JU, Jakobsen J, Andersen G, Nielsen JF. Cortical excitability in chronic stroke and modulation by training: A TMS study. Neurorehabilitation and Neural Repair. 2009;23(5):486–493. doi: 10.1177/1545968308328730. [DOI] [PubMed] [Google Scholar]
- Blicher JU, Near J, Naess-Schmidt E, Stagg CJ, Johansen-Berg H, Nielsen JF, Ho YC. GABA levels are decreased after stroke and GABA changes during rehabilitation correlate with motor improvement. Neurorehabilitation and Neural Repair. 2015;29(3):278–286. doi: 10.1177/1545968314543652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke SP, Nadler JV. Regulation of glutamate and aspartate release from slices of the hippocampal CA1 area: Effects of adenosine and baclofen. Journal of Neurochemistry. 1988;51(5):1541–1551. doi: 10.1111/j.1471-4159.1988.tb01123.x. [DOI] [PubMed] [Google Scholar]
- Chen JF, Chern Y, editors. Methylxanthines. Berlin: Springer Verlag; 2011. [Google Scholar]
- Cherry KM, Lenze EJ, Lang CE. Combining d-cycloserine with motor training does not result in improved general motor learning in neurologically intact people or in people with stroke. Journal of Neurophysiology. 2014;111(12):2516–2524. doi: 10.1152/jn.00882.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarkson AN, Huang BS, Macisaac SE, Mody I, Carmichael ST. Reducing excessive GABA-mediated tonic inhibition promotes functional recovery after stroke. Nature. 2010;468(7321):305–309. doi: 10.1038/nature09511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke SF, Bliss TV. Plasticity in the human central nervous system. Brain. 2006;129(Pt 7):1659–1673. doi: 10.1093/brain/awl082. [DOI] [PubMed] [Google Scholar]
- Daskalakis ZJ, Christensen BK, Fitzgerald PB, Roshan L, Chen R. The mechanisms of interhemispheric inhibition in the human motor cortex. Journal of Physiology. 2002;543(Pt 1):317–326. doi: 10.1113/jphysiol.2002.017673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhawan J, Benveniste H, Luo Z, Nawrocky M, Smith SD, Biegon A. A new look at glutamate and ischemia: NMDA agonist improves long-term functional outcome in a rat model of stroke. Future Neurology. 2011;6(6):823–834. doi: 10.2217/fnl.11.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Lazzaro V. State of the art: Physiology of transcranial motor cortex stimulation. Brain stimulation. 2008;1(4):345–362. doi: 10.1016/j.brs.2008.07.004. [DOI] [PubMed] [Google Scholar]
- Dunwiddie TV, Diao L. Extracellular adenosine concentrations in hippocampal brain slices and the tonic inhibitory modulation of evoked excitatory responses. Journal of Pharmacology and Experimental Therapeutics. 1994;268(2):537–545. [PubMed] [Google Scholar]
- Ferbert A, Priori A, Rothwell JC, Day BL, Colebatch JG, Marsden CD. Interhemispheric inhibition of the human motor cortex. Journal of Physiology. 1992;453:525–546. doi: 10.1113/jphysiol.1992.sp019243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Florian J, Muller-Dahlhaus M, Liu Y, Ziemann U. Inhibitory circuits and the nature of their interactions in the human motor cortex a pharmacological TMS study. Journal of Physiology. 2008;586(2):495–514. doi: 10.1113/jphysiol.2007.142059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fredholm BB. Are methylxanthine effects due to antagonism of endogenous adenosine? Trends in Pharmacological Sciences. 1979;1(1):129–132. doi: http://dx.doi.org/10.1016/0165-6147(79)90046-4. [Google Scholar]
- Fugl-Meyer AR, Jaasko L, Leyman I, Olsson S, Steglind S. The post-stroke hemiplegic patient. 1. a method for evaluation of physical performance. Scandinavian Journal of Rehabilitation Medicine. 1975;7(1):13–31. [PubMed] [Google Scholar]
- Grade C, Redford B, Chrostowski J, Toussaint L, Blackwell B. Methylphenidate in early poststroke recovery: A double-blind, placebo-controlled study. Archives of Physical Medicine and Rehabilitation. 1998;79(9):1047–1050. doi: 10.1016/s0003-9993(98)90169-1. [DOI] [PubMed] [Google Scholar]
- Gray S. PktoPk.zip. 2015 Retrieved from http://ced.co.uk/downloads/signalscripts#analysis.
- Hagemann G, Redecker C, Neumann-Haefelin T, Freund HJ, Witte OW. Increased long-term potentiation in the surround of experimentally induced focal cortical infarction. Annals of Neurology. 1998;44(2):255–258. doi: 10.1002/ana.410440217. [DOI] [PubMed] [Google Scholar]
- Hanajima R, Ugawa Y, Terao Y, Sakai K, Furubayashi T, Machii K, Kanazawa I. Paired-pulse magnetic stimulation of the human motor cortex: Differences among I waves. Journal of Physiology. 1998;509(Pt 2):607–618. doi: 10.1111/j.1469-7793.1998.607bn.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanajima R, Ugawa Y, Terao Y, Sakai K, Furubayashi T, Machii K, Kanazawa I. Cortico-cortical inhibition of the motor cortical area projecting to sternocleidomastoid muscle in normals and patients with spasmodic torticollis or essential tremor. Electroencephalography and Clinical Neurophysiology. 1998;109(5):391–396. doi: 10.1016/s0924-980x(98)00036-8. [DOI] [PubMed] [Google Scholar]
- Heise K-F, Niehoff M, Feldheim JF, Liuzzi G, Gerloff C, Hummel FC. Differential behavioral and physiological effects of anodal transcranial direct current stimulation in healthy adults of younger and older age. Frontiers in Aging Neuroscience. 2014;6:146. doi: 10.3389/fnagi.2014.00146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heritage P. Theophylline Extended-Release Tablets: FDA information. 2014 Retrieved from http://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=09325220-e4c5-4fac-aefd-ee3e42602198.
- Hess G, Aizenman CD, Donoghue JP. Conditions for the induction of long-term potentiation in layer II/III horizontal connections of the rat motor cortex. Journal of Neurophysiology. 1996;75(5):1765–1778. doi: 10.1152/jn.1996.75.5.1765. [DOI] [PubMed] [Google Scholar]
- Hollins C, Stone TW. Adenosine inhibition of gamma-aminobutyric acid release from slices of rat cerebral cortex. British Journal of Pharmacology. 1980;69(1):107–112. doi: 10.1111/j.1476-5381.1980.tb10888.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang YZ, Edwards MJ, Rounis E, Bhatia KP, Rothwell JC. Theta burst stimulation of the human motor cortex. Neuron. 2005;45(2):201–206. doi: 10.1016/j.neuron.2004.12.033. [DOI] [PubMed] [Google Scholar]
- Huang YZ, Rothwell JC, Edwards MJ, Chen RS. Effect of physiological activity on an NMDA-dependent form of cortical plasticity in human. Cerebral Cortex. 2008;18(3):563–570. doi: 10.1093/cercor/bhm087. [DOI] [PubMed] [Google Scholar]
- Hummel FC, Steven B, Hoppe J, Heise K, Thomalla G, Cohen LG, Gerloff C. Deficient intracortical inhibition (SICI) during movement preparation after chronic stroke. Neurology. 2009;72(20):1766–1772. doi: 10.1212/WNL.0b013e3181a609c5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly-Hayes M, Beiser A, Kase CS, Scaramucci A, D’Agostino RB, Wolf PA. The influence of gender and age on disability following ischemic stroke: The Framingham study. Journal of Stroke & Cerebrovasc Disease. 2003;12(3):119–126. doi: 10.1016/S1052-3057(03)00042-9. [DOI] [PubMed] [Google Scholar]
- Kingdom MRCOTU. Aids to Examination of the Peripheral Nervous System. Palo Alto, CA: Pendragon House; 1978. Vol. Memorandum No 45. [Google Scholar]
- Komaki A, Shahidi S, Lashgari R, Haghparast A, Malakouti SM, Noorbakhsh SM. Effects of GABAergic inhibition on neocortical long-term potentiation in the chronically prepared rat. Neuroscience Letters. 2007;422(3):181–186. doi: 10.1016/j.neulet.2007.06.017. [DOI] [PubMed] [Google Scholar]
- Kujirai T, Caramia MD, Rothwell JC, Day BL, Thompson PD, Ferbert A, Marsden CD. Corticocortical inhibition in human motor cortex. Journal of Physiology. 1993;471:501–519. doi: 10.1113/jphysiol.1993.sp019912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liepert J. Motor cortex excitability in stroke before and after constraint-induced movement therapy. Cognitive and Behavioral Neurology. 2006;19(1):41–47. doi: 10.1097/00146965-200603000-00005. [DOI] [PubMed] [Google Scholar]
- Liepert J, Classen J, Cohen LG, Hallett M. Task-dependent changes of intracortical inhibition. Experimental Brain Research. 1998;118(3):421–426. doi: 10.1007/s002210050296. [DOI] [PubMed] [Google Scholar]
- Manganotti P, Acler M, Zanette GP, Smania N, Fiaschi A. Motor cortical disinhibition during early and late recovery after stroke. Neurorehabilitation and Neural Repair. 2008;22(4):396–403. doi: 10.1177/1545968307313505. [DOI] [PubMed] [Google Scholar]
- Manganotti P, Patuzzo S, Cortese F, Palermo A, Smania N, Fiaschi A. Motor disinhibition in affected and unaffected hemisphere in the early period of recovery after stroke. Clinical Neurophysiology. 2002;113(6):936–943. doi: 10.1016/s1388-2457(02)00062-7. [DOI] [PubMed] [Google Scholar]
- Mathiowetz V, Weber K, Kashman N, Volland G. Adult norms for the Nine Hole Peg Test of finger dexterity. Occupational Therapy Journal of Research. 1985;5(1):24–38. [Google Scholar]
- Mensen A, Khatami R. Advanced EEG analysis using threshold-free cluster-enhancement and non-parametric statistics. Neuroimage. 2013;67:111–118. doi: 10.1016/j.neuroimage.2012.10.027. doi: http://dx.doi.org/10.1016/j.neuroimage.2012.10.027. [DOI] [PubMed] [Google Scholar]
- Nakamura H, Kitagawa H, Kawaguchi Y, Tsuji H. Intracortical facilitation and inhibition after transcranial magnetic stimulation in conscious humans. Journal of Physiology. 1997;498(Pt 3):817–823. doi: 10.1113/jphysiol.1997.sp021905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nardone R, Buffone E, Covi M, Lochner PG, Tezzon F. Changes in motor cortical excitability in humans following orally administered theophylline. Neuroscience Letters. 2004;355(1–2):65–68. doi: 10.1016/j.neulet.2003.10.055. [DOI] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia. 1971;9(1):97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Orth M, Amann B, Ratnaraj N, Patsalos PN, Rothwell JC. Caffeine has no effect on measures of cortical excitability. Clinical Neurophysiology. 2005;116(2):308–314. doi: 10.1016/j.clinph.2004.08.012. doi: http://dx.doi.org/10.1016/j.clinph.2004.08.012. [DOI] [PubMed] [Google Scholar]
- Overman JJ, Carmichael ST. Plasticity in the injured brain: More than molecules matter. Neuroscientist. 2014;20(1):15–28. doi: 10.1177/1073858413491146. [DOI] [PubMed] [Google Scholar]
- Paulus W, Classen J, Cohen LG, Large CH, Di Lazzaro V, Nitsche M, Ziemann U. State of the art: Pharmacologic effects on cortical excitability measures tested by transcranial magnetic stimulation. Brain Stimul. 2008;1(3):151–163. doi: 10.1016/j.brs.2008.06.002. [DOI] [PubMed] [Google Scholar]
- Prince DA, Stevens CF. Adenosine decreases neurotransmitter release at central synapses. Proceedings of the National Academy of Sciences of the United States of America. 1992;89(18):8586–8590. doi: 10.1073/pnas.89.18.8586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu M, Buchkremer-Ratzmann I, Schiene K, Schroeter M, Witte OW, Zilles K. Bihemispheric reduction of GABAA receptor binding following focal cortical photothrombotic lesions in the rat brain. Brain Research. 1998;813(2):374–380. doi: 10.1016/s0006-8993(98)01063-4. [DOI] [PubMed] [Google Scholar]
- Qu MS, Buchkremer-Ratzmann I, Schiene K, Schroeter M, Witte OW, Zilles K. Bihemispheric reduction of GABA(A) receptor binding following focal cortical photothrombotic lesions in the rat brain. Brain Research. 1998;813(2):374–380. doi: 10.1016/s0006-8993(98)01063-4. [DOI] [PubMed] [Google Scholar]
- Que M, Witte OW, Neumann-Haefelin T, Schiene K, Schroeter M, Zilles K. Changes in GABA(A) and GABA(B) receptor binding following cortical photothrombosis: A quantitative receptor autoradiographic study. Neuroscience. 1999;93(4):1233–1240. doi: 10.1016/s0306-4522(99)00197-9. [DOI] [PubMed] [Google Scholar]
- Rodgers FC, Zarnowska ED, Laha KT, Engin E, Zeller A, Keist R, Pearce RA. Etomidate Impairs Long-Term Potentiation In Vitro by Targeting alpha5-Subunit Containing GABAA Receptors on Nonpyramidal Cells. Journal of Neuroscience. 2015;35(26):9707–9716. doi: 10.1523/JNEUROSCI.0315-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schallert T, Hernandez TD, Barth TM. Recovery of function after brain damage: Severe and chronic disruption by diazepam. Brain Research. 1986;379(1):104–111. doi: 10.1016/0006-8993(86)90261-1. [DOI] [PubMed] [Google Scholar]
- Schambra HM, Ogden RT, Martinez-Hernandez IE, Lin X, Chang YB, Rahman A, Krakauer JW. The reliability of repeated TMS measures in older adults and in patients with subacute and chronic stroke. Frontiers in Cellular Neuroscience. 2015;9:335. doi: 10.3389/fncel.2015.00335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholfield CN. Convulsants antagonise inhibition in the olfactory cortex slice. Naunyn-Schmiedeberg’s Archives of Pharmacology. 1980;314(1):29–36. doi: 10.1007/BF00498428. [DOI] [PubMed] [Google Scholar]
- Scholfield CN. Antagonism of gamma-aminobutyric acid and muscimol by picrotoxin, bicuculline, strychnine, bemegride, leptazol, D-tubocurarine and theophylline in the isolated olfactory cortex. Naunyn-Schmiedebergs Archives of Pharmacology. 1982;318(4):274–280. doi: 10.1007/BF00501165. [DOI] [PubMed] [Google Scholar]
- Segev R, Rubinstein Quinolones, theophylline, and diclofenac interaction with the gamma-aminobutyric acid receptor. Antimicrobial Agents and Chemotherapy. 1988;32:1624–1626. doi: 10.1128/aac.32.11.1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siepmann T, Penzlin AI, Kepplinger J, Illigens BM, Weidner K, Reichmann H, Barlinn K. Selective serotonin reuptake inhibitors to improve outcome in acute ischemic stroke: Possible mechanisms and clinical evidence. Brain Behav. 2015;5(10):e00373. doi: 10.1002/brb3.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder SH, Katims JJ, Annau Z, Bruns RF, Daly JW. Adenosine receptors and behavioral actions of methylxanthines. Proceedings of the National Academy of Sciences of the United States of America. 1981;78(5):3260–3264. doi: 10.1073/pnas.78.5.3260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swayne OB, Rothwell JC, Ward NS, Greenwood RJ. Stages of motor output reorganization after hemispheric stroke suggested by longitudinal studies of cortical physiology. Cerebral Cortex. 2008;18(8):1909–1922. doi: 10.1093/cercor/bhm218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trepel C, Racine RJ. GABAergic modulation of neocortical long-term potentiation in the freely moving rat. Synapse. 2000;35(2):120–128. doi: 10.1002/(SICI)1098-2396(200002)35:2<120::AID-SYN4>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Watson CW, Kennard MA. The effect of anticonvulsant drugs on recovery of function following cerebral cortical lesions. Journal of Neurophysiology. 1945;8(4):221–231. [Google Scholar]
- Werhahn KJ, Kunesch E, Noachtar S, Benecke R, Classen J. Differential effects on motorcortical inhibition induced by blockade of GABA uptake in humans. Journal of Physiology. 1999;517(Pt 2):591–597. doi: 10.1111/j.1469-7793.1999.0591t.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeiler SR, Krakauer JW. The interaction between training and plasticity in the poststroke brain. Current Opinion in Neurology. 2013;26(6):609–616. doi: 10.1097/WCO.0000000000000025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziemann U, Lonnecker S, Steinhoff BJ, Paulus W. The effect of lorazepam on the motor cortical excitability in man. Experimental Brain Research. 1996;109(1):127–135. doi: 10.1007/BF00228633. [DOI] [PubMed] [Google Scholar]
- Ziemann U, Reis J, Schwenkreis P, Rosanova M, Strafella A, Badawy R, Muller-Dahlhaus F. TMS and drugs revisited 2014. Clinical Neurophysiology. 2015;126(10):1847–1868. doi: 10.1016/j.clinph.2014.08.028. [DOI] [PubMed] [Google Scholar]