Abstract
Several pharmacophore models have been proposed for 5-HT2A serotonin receptor antagonists. These typically consist of two aromatic/hydrophobic moieties separated by a given distance from each other, and from a basic amine. Although specified distances might vary, the models are relatively similar in their general construction. Because our preliminary data indicated that two aromatic (hydrophobic) moieties might not be required for such action, we deconstructed the serotonin-dopamine antipsychotic agent risperidone (1) into four smaller structural fragments that were thoroughly examined in 5-HT2A receptor binding and functional (i.e., two-electrode voltage clamp – TEVC – and intracellular calcium release) assays. It was apparent that truncated risperidone analogs behaved as antagonists. In particular, 6-fluoro-3-(1-methylpiperidin-4-yl)benzisoxazole (4) displayed high affinity for 5-HT2A receptors (Ki ca 12 nM) relative to risperidone (Ki ca 5 nM) and behaved as a potent 5-HT2A serotonin receptor antagonist. These results suggest that multiple aromatic (hydrophobic) moieties are not essential for high-affinity 5-HT2A receptor binding and antagonist activity and that current pharmacophore models for such agents are very much in need of revision.
Keywords: Risperidone, Antipsychotics, TEVC (two-electrode voltage clamp), Calcium release, 3-(4-Piperidinyl)benzisoxazoles
Graphical Abstract
Introduction
Over the past 25 years, several pharmacophore models have been described for 5-HT2A serotonin receptor antagonists.1–7 To some extent, the various models might reflect the nature of the antagonists examined in the individual studies. Nevertheless, there now seems agreement that multiple binding modes are possible for competitive 5-HT2A receptor antagonists,2,4–8 and this could account for the multiple models. Typically, the pharmacophore models consist of two aromatic (hydrophobic) moieties separated by a given distance from each other, and from a basic amine. Although the distances might vary from model to model,2–5 these three features are common to nearly all the agents examined. When the two aromatic features flank a third (i.e., central) ring, as in a tricyclic ring system, even the fold-angle of the central ring is thought to play a role.9
Two of the few 5-HT2A receptor antagonists that have found clinical application are the SDA (serotonin-dopamine antagonist) antipsychotic agents risperidone (1; Figure 1) and its 9-hydroxy metabolite, paliperidone. Risperidone (1) has been considered only in a few pharmacophore studies.10,11 In one study, the authors noted that their model did not necessarily predict structural features required for binding at 5-HT2A or dopamine D2 receptors, but simply identified features important for 5-HT2A/D2-associated antipsychotic action.10 In another study, multiple pharmacophoric features for 5-HT2A antagonist action were identified including the two aromatic (hydrophobic) moieties.11
Figure 1.
The antipsychotic agent risperidone (1) and its ring numbering system.
Some pharmacophore studies might have been limited by the nature of the agents examined. Indeed, most 5-HT2A receptor pharmacophore studies have examined fairly large molecules and, as already mentioned, nearly all contained at least two aromatic (hydrophobic) centers. The structural requirements for risperidone (1) to act as a 5-HT2A receptor antagonist have not been extensively examined. Hence, it was of interest here to determine how much of the risperidone (1) structure is actually required in order to retain this action. Consequently, four deconstructed analogs (i.e., partial structures) of risperidone (1), 2–5 (Figure 2), were prepared and investigated. Compound 2 retains the amide terminus of risperidone (1) whereas 3 possesses an amine terminus. The terminal chain of 2/3 was truncated to the N-methylpiperidine 4 and piperidine analog 5.
Figure 2.
Deconstructed risperidone analogs examined in the current investigation: tertiary amide 2, diamine 3, N-methylpiperidine 4, and piperidine 5.
Preliminary evaluation of 2–5 (at a single concentration of 10 µM) revealed 5-HT2A antagonist properties. To determine if the effect involved the orthosteric binding site of 5-HT2A receptors, their binding affinity was measured. Their functional activity was then compared with that of risperidone (1) using a two-electrode voltage clamp (TEVC) assay as well as an intracellular calcium release assay.
Results and Discussion
Synthesis
The deconstructed analogs of risperidone, 2–5, were synthesized as outlined in Scheme 1. Compound 5 was prepared according to a literature procedure12 and served as a key intermediate in the preparation of 2–4. N-Alkylation of 5 by 4-chloro-1-(piperidin-1-yl)butan-1-one (12) utilizing a Finkelstein reaction yielded the desired amide 2 (Scheme 1). Reduction of 2 with diborane·THF complex resulted in 3. The N-methyl analog of 5, 4, was obtained using an Eschweiler-Clarke N-methylation reaction. All compounds were prepared as water soluble hydrochloride salts.
Scheme 1.
Synthesis of compounds 2–5.
a Reagents and conditions: (i) (a) HCOOH, Ac2O, 65 °C, 1 h; (b) room temperature, 16 h; (ii) (a) SOCl2, DMF, room temperature, 6 h; (b) 1,3-difluorobenzene, AlCl3, reflux, 45 h; (iii) NH2OH·HCl, NaOH/H2O, EtOH, reflux, 96 h; (iv) (a) NaH, DMF, room temperature 48 h; (v) (a) conc. HCl, EtOH, reflux, 3 h; (b) room temperature, 48 h; (vi) (a) HCOOH, HCHO, reflux, 10 h; (b) HCl/Et2O (vii) 4-chlorobutyryl chloride, Et3N, CH2Cl2, room temperature, 75 h; (viii) K2CO3, KI, MeCN, 88 °C, 16 h; (ix) (a) BH3·THF, reflux, 2 h; (b) 6N HCl, reflux, 1 h.
Binding
Competition binding assays were performed in plasma membrane preparations of human embryonic kidney (HEK293) cells transiently transfected with a construct encoding 5-HT2A receptors for determining the affinity of risperidone. Risperidone displaced [3H]ketanserin binding (Supporting Information, Figure SI-1) with a Ki value of 5.29 nM, consistent with its previously reported high affinity for 5-HT2A receptors.13
We examined the binding affinity of the risperidone derivatives much in the same way we did for risperidone. Compounds 2 (Ki = 39.81 nM) and 3 (Ki = 34.83 nM) retained high binding affinity to 5-HT2A receptors, even though they exhibited lower affinity (7.4- and 6.6-fold lower affinity, respectively; Figure 3) than risperidone (1). The N-methylpiperidine derivative (4; Ki = 12.27 nM) showed the highest binding affinity (half that of risperidone), whereas the piperidine derivative (5; Ki = 71.41 nM) showed the lowest binding affinity of all derivatives tested (13.5-fold lower than risperidone). Data for the binding of risperidone (Supporting Information, Figure SI-1) and 2–5 are shown in Figure 3 and are summarized in Table 1. These results suggested that all derivatives tested bind competitively at 5-HT2A receptors with relatively high affinity.
Figure 3.
Deconstructed risperidone analogs bind 5-HT2A receptors with comparable high affinities. [3H]Ketanserin binding competition curves by compounds 2–5 in HEK293 cell membrane preparations. Data are means ± SEM (n = 4).
Table 1.
Radioligand binding assays and Ca2+ imaging results for risperidone (1) and its four deconstructed analogs 2–5.
| 5-HT2A receptor binding affinity | Ca2+ imaging | ||
|---|---|---|---|
| log Ki ± SEM | Ki (nM) | IC50 ±SEM (µM) | |
| 1 | −8.27 ± 0.06 | 5.29 | 5.59 ± 1.41 |
| 2 | −7.40 ± 0.11 | 39.81 | 16.65 ± 1.39 |
| 3 | −7.45 ± 0.12 | 34.83 | 43.88 ± 1.47 |
| 4 | −7.91 ± 0.10 | 12.27 | 7.40 ± 1.45 |
| 5 | −7.14 ± 0.09 | 71.41 | 20.12 ± 2.24 |
Functional assays
Ion channels can serve as sensitive reporters for G protein-coupled receptor (GPCR) activity.14,15 We utilized the Xenopus laevis oocyte system to heterologously express 5-HT2A receptors and the G protein-gated inwardly rectifying K+ (GIRK4-S143T or GIRK4*) reporter, a channel activated by Gβγ associated with PTX-sensitive Gα subunits.16,17 When 1 µM serotonin (5-HT) was perfused in the bath in a two-electrode voltage clamp (TEVC) experiment, two effects became apparent: activation of a transient outwardly rectifying (larger outward than inward) current, followed by inhibition of the inwardly rectifying (larger inward than outward) GIRK4* current (Figure 4A). The transient current reflects activation of a calcium-activated chloride channel (ICa-Cl) endogenous to Xenopus oocytes, providing functional evidence that 5-HT2A receptor signaling occurred (i.e. Gq activation → PLCβ1 activation → hydrolysis of PIP2 to DAG and IP3 generation → release of Ca2+ from ER stores).e.g. 18 The ensuing inhibition of the GIRK4* current is due to phosphoinositide hydrolysis and, thus, a decrease in the plasma membrane concentration of PIP2, as interactions of this and most ion channels with PI(4,5)P2 are essential to keep the channel gates open.19 In the presence of 3 µM risperidone (1), 5-HT-mediated current inhibition was greatly attenuated. Some ICa-Cl could be seen only in the outward direction, while the inhibition of the GIRK4* current was abolished (Figure 4B).
Figure 4.
Risperidone acts as an antagonist at 5-HT2A receptors. (A) Representative barium-sensitive GIRK4* inward and outward current traces obtained in response to 1 µM serotonin (5-HT) applied to oocytes expressing 5-HT2A receptors. (B) Representative barium-sensitive GIRK4* inward and outward current traces obtained in response to 1 µM serotonin (5-HT) and 3 µM risperidone concurrently applied to oocytes expressing 5-HT2A receptors. (C) Summary bar graph or (D) concentration response curve of Gq/11 activity in response to 1 µM 5-HT with or without increasing concentrations of risperidone measured in oocytes (n = 7–15/condition. Data are mean ± SEM, **p<0.01, ***p<0.001, significance compared to response to 1 µM 5-HT, Dunnett’s post-hoc test of one-way ANOVA, experiments were performed in ≥2 batches of oocytes).
A concentration-response of risperidone antagonizing the action of 5-HT (1 µM) was performed and the results showed significant effects at concentrations of 100 nM or greater (Figures 4C and D). The apparent risperidone IC50 value was estimated by this assay at 55.7 nM, ~10-fold lower than its binding affinity (see Supporting Information, Figure SI-1).
Before proceeding with similar functional characterization of antagonist action of the deconstructed risperidone analogs, we examined their possible agonist effects. All compounds, except compound 5, yielded significant current inhibition at concentrations of 50 µM or higher (Supporting Information, Figure SI-2A-D). Compound 3 seemed to cause significant current inhibition at concentrations as low as 5 µM. When we compared the effects of the risperidone derivatives at 50 µM or higher in oocytes expressing GIRK4* alone, versus GIRK4* and 5-HT2A receptors together, we found that the effects of risperidone and simplified derivatives, 2 and 3, showed no significant differences between the two groups, suggesting that high concentrations of risperidone and 2 and 3 inhibited the GIRK4* channel directly (Supporting Information, Figures SI-3A–C). Unlike compounds 1–4, compound 5 did not exhibit significant agonism at the concentrations tested up to 50 µM (data not shown). Overall, these data revealed a limitation as to how high in concentration we could test the effects of risperidone derivatives using GIRK4* as a reporter for 5-HT2A receptor activity.
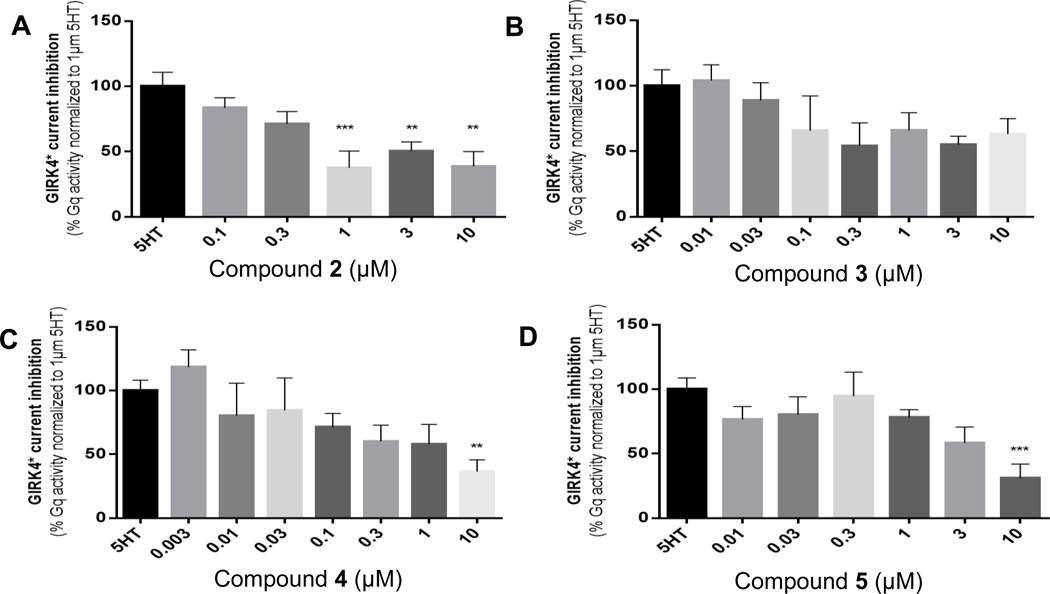
Concentration-response experiments aiming to antagonize 5-HT responses revealed that significant inhibition could be achieved at the highest concentrations tested (10 µM) for compounds 2, 4, and 5, while for compound 3 no significant effects could be achieved, possibly because at as low as 5 µM this compound inhibited the GIRK4* channel directly (Figure 5B and Supporting Information, Figures SI-2C and 3C).
Figure 5.
The deconstructed analogs of risperidone act as antagonists at 5-HT2A receptors as assessed by a TEVC assay. Summary bar graphs of Gq/11 activity in response to 1 µM 5-HT with or without increasing concentrations of (A) compound 2 (n = 7–12/condition), (B) compound 3 (n = 7–9/condition), (C) compound 4 (n = 6–18/per condition), or (D) compound 5 (n = 7–15/condition) measured in oocytes. Data are mean ± SEM, **p<0.01, ***p<0.001, significance compared to response to 1 µM 5-HT, Dunnett’s post-hoc test of one-way ANOVA, experiments were performed in ≥2 batches of oocytes.
The concentration-response experiments in the oocyte system using GIRK4* channel currents as the reporter of the action of risperidone analogs suggested that at least three of the four analogs functioned as antagonists of 5-HT responses. Yet, the direct action of compounds 1–4 on the reporter channel itself limited full characterization of these compounds.
These results prompted us to consider a complementary functional assay to report on the action of risperidone and its deconstructed analogs. Figure 6 shows epifluorescence experiments in HEK 293 cells stably expressing 5-HT2A receptors and using the Fura 2 dye to report changes in intracellular Ca2+ due to risperidone and its deconstructed analogs. First we applied compounds 1–5 by themselves (up to a concentration of 100 µM) and recorded no changes in intracellular Ca2+ concentration (data not shown). Application of 1 µM 5-HT produced robust Ca2+ transients that could be blocked progressively by increasing concentrations of risperidone and its derivatives (Figure 6). Thus, based on apparent IC50 values in the [Ca2+]i assay the order of potency was as follows: risperidone (5.59 µM) > 4 (7.40 µM) > 2 (16.65 µM) > 5 (20.12 µM) > 3 (43.88 µM) (summarized in Table 1).
Figure 6.
The deconstructed analogs of risperidone act as antagonists at 5-HT2A receptors as assessed by an intracellular Ca2+ release assay A) Sample traces of calcium responses elicited by 1 µM of 5-HT in the presence of 5 µM risperidone. Each line corresponds to the response of an individual cell. B-F) Dose-response curves obtained with 1 µM of 5-HT in the presence of various concentrations of risperidone (B), compound 2 (C), compound 3 (D), compound 4 (E) and compound 5 (F). Data from two independent experiments per compound and a total of 4–6 wells per compound per concentration point are expressed as mean ± SEM. The numbers inside the graphs indicate the total number of points analyzed for each drug.
According to existing pharmacophore models, it might not have been expected that compounds lacking two aromatic (hydrophobic) moieties would be effective 5-HT2A receptor competitive antagonists. Clearly, the entire structure of risperidone (1) is shown here to be unnecessary for this action. For example, compounds 2, 3, and 4 bind with only about 2- to 8-fold lower affinity than 1, and are only about 2- to 3-fold less potent than risperidone as antagonists in the Ca2+ imaging assay.
Although changes in intracellular Ca2+ proved to be a three-orders of magnitude lower sensitivity assay than the GIRK4* channel reporter, results from the two methods were consistent in showing that risperidone (1) was the most potent antagonist, whereas compound 3 was the least potent antagonist of 5-HT responses (compare Figures 4C and 6B versus Figures 5B and 6D). Moreover, the [Ca2+]i assay proved cleaner than the channel reporter assay since risperidone (1) and its derivatives did not change basal [Ca2+]i levels and allowed a complete ranking of these compounds in terms of their functional potency.
In summary, it would appear from the present results that truncated versions of risperidone (1) bind with nanomolar affinity and retain 5-HT2A antagonist character. In fact, we reported some time ago that compound 5 binds only with 16-fold lower affinity than risperidone (1) at [3H]ketanserin-labeled 5-HT2A receptors (rat brain homogenates) and acted as a potent 5-HT2 receptor antagonist in vitro (5-HT-induced inositol phosphate production) and in vivo (rats trained to discriminate the 5-HT2 receptor agonist 1-(2,5-dimethoxy-4-methylphenyl)-2-aminopropane from vehicle) without producing any agonist action when examined alone.13 Taken together with the present findings, future pharmacophore models for 5-HT2A receptor antagonists will need to consider this information. Although most 5-HT2A serotonin receptor antagonists commonly possess more than a single aromatic/hydrophobic feature, this would not appear to be an essential requirement.
Methods
Synthesis
Melting points were taken on a Thomas-Hoover melting point apparatus in glass capillary tubes and are uncorrected. 1H NMR spectra were recorded with a Bruker ARX 400 MHz spectrometer with tetramethylsilane (TMS) as an internal standard. Peak positions are given in parts per million (δ). Infrared spectra were obtained on a Nicolet iS10 FT-IR spectrometer. Elemental analyses were performed by Atlantic Microlab Inc. (Norcross, GA) for the indicated elements and results are within 0.4% of calculated values. Reactions were monitored by thin-layer chromatography (TLC) on silica gel GHLF plates (250 µ, 2.5 × 10 cm; Analtech Inc., Newark, DE).
4-(4-(6-Fluorobenzisoxazol-3-yl)piperidin-1-yl)-1-(piperidin-1-yl)butan-1-one Hydrochloride (2)
Compound 12 (0.22 g, 1.14 mmol) and free base of 5 (0.25 g, 1.14 mmol) were added to a stirred solution of K2CO3 (0.31 g, 2.26 mmol) and KI (few crystals) in anhydrous MeCN (5 mL). The reaction mixture was allowed to stir in a screw-cap vial at 88 °C for 16 h. The solvent was evaporated under reduced pressure; the residue was suspended in H2O and extracted with CHCl3 (3 × 15 mL). The combined organic portion was washed with H2O (3 × 10 mL), dried (Na2SO4), and evaporated under reduced pressure to yield an ivory-colored solid. The solid was dissolved in EtOH (5 mL) and the solution was cooled to 0 °C. A saturated solution of gaseous HCl in Et2O (2 mL) was added and the mixture was allowed to stand at room temperature for 3 h. The solvent was evaporated to yield a white solid that was recrystallized from EtOH to yield 0.01 g (3%) of 2 as a tan-colored solid: mp 216–218 °C 1H (DMSO-d6): 1.43–1.69 (m, 4H, CH2), 1.82–2.10 (m, 6H, CH2), 2.21–2.24 (m, 2H, CH2), 2.82–2.88 (m, 2H, CH2), 2.82–2.88 (m, 1H, CH), 3.23–3.26 (m, 2H, CH2), 3.40–3.52 (m, 2H, CH2), 3.63–3.66 (m, 2H, CH2), 3.98–4.02 (m, 2H, CH2), 4.48–4.51 (m, 2H, CH2), 7.29–7.38 (m, 1H, ArH), 7.70–7.76 (m, 1H, ArH), 8.05–8.18 (m, 1H, ArH), 10.17 (br s, 1H, NH+) Anal Calcd for (C21H28FN3O2·HCl·0.5H2O) C, 60.21; H, 7.02; N, 9.77. Found: C, 60.32; H, 7.22; N, 10.03.
6-Fluoro-3-(1-(4-piperidin-1-yl)butyl)piperidin-4-yl)benzisoxazole Hydrochloride (3)
A 1M solution of BH3 in THF (1.41 mL) was added to a stirred solution of 2 (0.13 g, 0.35 mmol) in anhydrous THF (4 mL) at 0 °C (ice-bath). The reaction mixture was allowed to warm to room temperature and stirring was continued for 10 h. The reaction mixture was carefully quenched with 6M aqueous HCl (0.40 mL) and then heated at reflux for 1 h. The mixture was allowed to cool to room temperature and basified with 1N aqueous NaOH (2 mL). Water (10 mL) was added and the aqueous portion was extracted with EtOAc (3 × 15 mL). The organic portions were combined, dried (Na2SO4), and solvent was removed under reduced pressure to give an oily residue that was dissolved in absolute EtOH, and HCl-anhydrous Et2O was added to afford a solid. Recrystallization from absolute EtOH/anhydrous Et2O gave 0.04 g (26%) of 3 as yellow crystals: mp 270–273 °C; 1H-NMR (DMSO-d6: salt) δ 1.37 (m, 1H, CH2), 1.68–1.78 (m, 10H, CH2), 2.17–2.20 (m, 2H, CH2), 2.38–2.53 (m, 2H, CH2), 2.83 (m, 2H, CH2), 3.01–3.10 (m, 7H, CH, CH2), 3.36–3.46 (m, 1H, CH2), 3.58–3.61 (m, 2H, CH2), 7.32 (td, J = 9.1 2.0 Hz, 1H, ArH), 7.71 (dd, J = 9.0, 1.9 Hz, 1H, ArH), 8.25 (dd, J = 8.6, 5.4 Hz, 1H, ArH), 10.33 (br s, 1H, NH+), 11.06 (br s, 1H, NH+). Anal. Calcd for (C21H30FN3O·2HCl· 0.25H2O) C, 57.73; H, 7.50; N, 9.62. Found: C, 57.36; H, 7.21; N, 9.30.
6-Fluoro-3-(1-methylpiperidin-4-yl)benzisoxazole Hydrochloride (4)
The free base of 6-fluoro-3-(piperidin-4-yl)benzisoxazole (5; 0.20 g, 0.90 mmol) was added to a stirred solution of HCOOH (0.2 mL, 5.45 mmol) and HCHO (0.2 mL, 5.45 mmol) in EtOH under an N2 atmosphere. The reaction mixture was heated at reflux for 10 h and the solvent was evaporated to yield a white solid. The solid was dissolved in EtOH (5 mL) and cooled to 0 °C. A saturated solution of gaseous HCl in Et2O (2 mL) was added and the mixture was allowed to stand at room temperature for 3 h. The solvent was evaporated and the crude solid was recrystallized from EtOH to yield 0.01 g (4%) of 4 as a white solid: mp 218–220 °C, 1H (DMSO-d6): 2.20–2.25 (m, 4H, CH2), 2.80 (s, 3H, CH3), 3.09–3.19 (m, 2H, CH2), 3.53–3.56 (m, 2H, CH2), 7.33–7.38 (m, 1H ArH), 7.73–7.75 (dd, J= 2, 8 Hz 1H, ArH), 8.15–8.18 (m, 1H, ArH), 10.64 (br s, 1H, NH+). Anal Calcd for (C13H15FN2O·HCl·0.5H2O) C, 55.82; H, 6.13; N, 10.01. Found: C, 55.50; H, 6.08; N, 9.69.
4-Chloro-1-(piperidin-1-yl)butan-1-one (12)
Piperidine (11; 1.2 mL, 11.54 mmol) was added in a dropwise manner to a stirred solution of Et3N (1.2 mL, 11.54 mmol) in CH2Cl2 (25 mL) and the reaction mixture was cooled to 0 °C under an N2 atmosphere. A solution of 4-chlorobutyryl chloride (1.5 mL, 11.74 mmol) in CH2Cl2 (5 mL) was added and the reaction mixture was allowed to stir at room temperature for 75 h and washed with H2O (2 × 25 mL). The organic portion was dried (Na2SO4) and evaporated under reduced pressure to yield a yellow-colored oil, which was purified by vacuum distillation to yield 1.00 g (45%) of 12 as a pale yellow-colored oil: bp 103 °C at 0.02 psi. The oil was used without further characterization in preparation of 2.
Radioligand Binding
Transient Transfection of HEK293 cells
Human embryonic kidney (HEK293) cells were maintained in Dulbecco’s modified Eagle’s medium supplemented with 10% (v/v) fetal bovine serum at 37 °C in a 5% CO2-humidified atmosphere. Transfection was performed using Lipofectamine 200 reagent (Invitrogen) according to the manufacturer’s instructions. The N-terminally c-Myc-tagged form of wild type human 5-HT2A receptor (pcDNA3.1-c-Myc-5-HT2A) has been described previously.21
Radioligand binding
Radioligand binding assays were performed as described previously21 with minor modifications. Briefly, HEK293 cell pellets were homogenized using a Teflon-glass grinder (10 up-and-down strokes at 1,500 rpm) in 1 mL of binding buffer (see below), supplemented with 0.25 M sucrose. The crude homogenate was centrifuged at 1,000 × g for 5 min at 4 °C, and the supernatant re-centrifuged at 40,000 × g for 15 min at 4 °C. The resultant pellet (P2 fraction) was washed twice in homogenization buffer and re-centrifuged in similar conditions. Aliquots were stored at −80 °C until assay. Protein concentration was determined using the Bio-Rad protein assay.
[3H]Ketanserin binding was measured at equilibrium in 100-µl aliquots (50 mM Tris-HCl, pH 7.4) of membrane preparations (~ 10 µg of total protein) that were incubated at 37 °C for 60 min. Competition curves were carried out by incubating the tested compound (10−10 – 10−4 M; 14 concentrations) in binding buffer containing 4 nM [3H]ketanserin. Non-specific binding was determined in the presence of 10 µM methysergide. Incubations were terminated by dilution with 200 µl ice-cold incubation buffer and free ligand was separated from bound ligand by rapid filtration under vacuum through GF/C glass fiber filters using a microbeta filtermat-96 harvester (PerkinElmer). These filters were then rinsed twice with 200 µl ice-cold incubation buffer, air dried, and counted for radioactivity by liquid scintillation spectrometry, using a MicroBeta2 detector (PerkinElmer). Radioligand binding data were analyzed using non-linear curve-fitting software (GraphPad Prism).
Functional assays
Expression of Recombinant Proteins in Xenopus oocytes
Oocytes were isolated and microinjected with equal volumes (50 nl) as previously described.14 In all two-electrode voltage-clamp experiments (TEVC), oocytes were injected with 2 ng of 5-HT2A receptor and 2 ng of GIRK4* and were maintained at 18 °C for 1–4 days before recording.
Two-Electrode Voltage-Clamp Recording and Analysis
Whole-cell currents were measured by conventional TEVC with a GeneClamp 500 amplifier (Axon Instruments, Union City, CA), as previously reported.14 A high-potassium (HK) solution was used to superfuse oocytes (96 mM KCl, 1 mM NaCl, 1 mM MgCl2, 5 mM KOH/HEPES; pH 7.4) to obtain a reversal potential for potassium (EK) close to zero. Inwardly rectifying potassium currents through GIRK4* were obtained by clamping the cells at −80 mV. Basal GIRK4* currents were defined as the difference between inward currents obtained at −80 mV in the presence of 3 mM BaCl2 in HK solution and those in the absence of Ba2+ and measured for each trace. Current inhibition to 5-HT was measured and normalized to basal current to compensate for size variability in oocytes. Current inhibition of risperidone derivatives was normalized to 1 µM 5-HT to compensate for variability in basal currents.
Measurement of Intracellular Ca2+
The 5-HT2A receptor cDNA was subcloned into the pcDNA5/FRT/TO plasmid and stable inducible cell lines were produced using the single site recombination T-Rex Flp-In system as previously described.20 5-HT2A receptor expression was induced adding 1 µg/mL doxycycline to the culture media at least two days before the experiment, and cells were plated on 96-well dishes one day before the experiment. The day of the experiment the cells were switched to serum-free medium for about 3–4 h, before being loaded with 5 µm Fura2-AM (Molecular Probes, OR) in Imaging Solution (125 mM NaCl, 5 mM KCl, 2 mM CaCl2, 1 mM MgSO4, 6 mM glucose, and 25 mM Hepes/Tris, pH 7.4). Following incubation for 30 min at 37 °C, cells were washed with Imaging Solution and kept at room temperature for about 15 min before being placed on the stage of an epifluorescence microscope, coupled to an automatic perfusion system and controlled by the Live Acquisition Software from Till Photonics (see reference 20 for details). The Fura-2 signal was acquired at 510 nm by switching the excitation wavelength between 340 nm and 380 nm. Baseline was recorded for 30 s before perfusion of 5-HT (1 µM) in the presence and absence of various concentrations of the drugs studied for another 45 s, followed by perfusion of Imaging Solution for 30 s to wash out the drugs. Intracellular calcium concentration was reported as fluorescence ratio (F 340/F380) and values were normalized to the basal F340/F380 ratio level before perfusion of the drugs. Data obtained for each drug in the presence of 5-HT were further normalized to the mean responses elicited by 5-HT alone in the same experiment. Results are expressed as mean ± SEM. Dose-response curves, curve fitting and IC50 values were obtained by analysis using GraphPad PRISM 6 software.
Supplementary Material
Acknowledgments
The authors would like to thank Mario de la Fuente, Meng Cui, Yu Xu, and Takeharu Kawano for their insightful discussions on the project.
Funding
This work was supported in part by NIH R01 HL59949-19 (DEL) and R01MH084894 (JMG).
Footnotes
Author Contributions
SAG and RHV synthesized and characterized the target compounds and were overseen by MD and RAG, respectively. JLM and KH performed the binding assays under the supervision of JG-M. JWY, AE, SS, and PD performed the TEVC assays under the supervision of DEL. LB and JME produced stable-expressing 5HT2A receptor cell lines and performed the intracellular Ca2+ measurements. RAG, MD, DEL, and JG-M oversaw the entire project and wrote the first draft of the manuscript. All authors had an opportunity to contribute to the preparation of the final manuscript.
Supporting Information Available
The Supporting Information section includes a [3H]ketanserin binding competition curve by risperidone in HEK293 cells transfected with the 5-HT2A receptor, and summary bar graphs of Gq/11 activity in response to 5-HT or test compounds. This information is available free of charge via the Internet at http://pubs.acs.org/.
References
- 1.Glennon RA, Westkaemper R, Bartyzel P. Medicinal chemistry of serotonergic agents. In: Peroutka S, editor. Serotonin Receptor Subtypes. New York: Wiley-Liss; 1991. pp. 19–64. [Google Scholar]
- 2.Andersen K, Liljefors T, Gundertofte K, Perregaard J, Bøgesø KP. Development of a receptor-interaction model for serotonin 5-HT2 receptor antagonists. Predicting selectivity with respect to dopamine D2 receptors. J. Med. Chem. 1994;37:950–962. doi: 10.1021/jm00033a013. [DOI] [PubMed] [Google Scholar]
- 3.Höltje HD, Jendretzki UK. Construction of a detailed serotoninergic 5-HT2a receptor model. Arch. Pharm. (Weinheim) 1995;328:577–584. doi: 10.1002/ardp.19953280704. [DOI] [PubMed] [Google Scholar]
- 4.Mokrosz MJ, Strekowski L, Kozak WX, Duszyńska B, Bojarski AJ, Kłodzinska A, Czarny A, Cegła MT, Dereń-Wesołek A, Chojnacka-Wójcik E, Dove S, Mokrosz JL. Structure-activity relationship studies of CNS agents, Part 25: 4,6-Di(heteroaryl)-2-(N-methylpiperazino)pyrimidines as new, potent 5-HT2A receptor ligands: A verification of the topographic model. Arch. Pharm. (Weinheim) 1995;328:659–666. doi: 10.1002/ardp.19953280906. [DOI] [PubMed] [Google Scholar]
- 5.Westkaemper RB, Glennon RA. Application of ligand SAR, receptor modeling and receptor mutagenesis to the discovery and development of a new class of 5-HT2A ligands. Curr. Top. Med. Chem. 2002;2:575–598. doi: 10.2174/1568026023393741. [DOI] [PubMed] [Google Scholar]
- 6.Runyon SP, Mosier PD, Roth BL, Glennon RA, Westkaemper RB. Potential modes of interaction of 9-aminomethyl-9,10-dihydroanthracene (AMDA) derivatives with the 5-HT2A receptor: A ligand structure-affinity relationship, receptor mutagenesis and receptor modeling investigation. J. Med. Chem. 2008;51:6808–6828. doi: 10.1021/jm800771x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xiong Z, Du P, Li B, Zhen X, Fu U. Discovery of a novel 5-HT2A inhibitor by pharmacophore-based virtual screening. Chem. Res. Chinese Universities. 2011;27:655–660. [Google Scholar]
- 8.Kanagarajadurai K, Malini M, Bhattacharya A, Panicker MM, Sowdhamini R. Molecular modeling and docking studies of human 5-hydroxytryptamine 2A (5-HT2A) receptor for the identification of hotspots for ligand binding. Mol. Biosyst. 2009;5:1877–1888. doi: 10.1039/b906391a. [DOI] [PubMed] [Google Scholar]
- 9.Runyon SP, Peddi S, Savage JE, Roth BL, Glennon RA, Westkaemper RB. Geometry-affinity relationships of the selective serotonin receptor ligand 9-(aminomethyl)-9,10-dihydroanthracene. J. Med. Chem. 2002;45:1656–1664. doi: 10.1021/jm010354g. [DOI] [PubMed] [Google Scholar]
- 10.Sekhar KGVC, Vyas DRK, Nagesh HN, Rao VS. Pharmacophoric hypothesis for atypical antipsychotics. Bull. Korean Chem. Soc. 2012;23:2930–2936. [Google Scholar]
- 11.Awadallah FM. Synthesis, pharmacophoric modelling, and biological evaluation of novel 5H-thiazolo[3,2-a]pyrimidine-5-one derivatives as 5-HT2A receptor antagonists. Sci. Pharm. 2008;76:415–438. [Google Scholar]
- 12.Strupczewski JT. Synthesis and neuroleptic activity of 3-(1-substituted-4-piperidinyl)-1, 2-benzisoxazoles. J. Med. Chem. 1985;28:761–769. doi: 10.1021/jm00383a012. [DOI] [PubMed] [Google Scholar]
- 13.Iwamura T, Casey CT, Young R, Dukat M, Teitler M, Fadden JSP, Glennon RA. 4-(6-Fluorobenzisoxazol-3-yl)piperidine, a risperidone metabolite with serotonergic activity of potential clinical significance. Med. Chem. Res. 1996;6:593–601. [Google Scholar]
- 14.Fribourg M, Moreno JL, Holloway T, Provasi D, Baki L, Mahajan R, Park G, Adney SK, Hatcher C, Eltit JM, Ruta JD, Albizu L, Li Z, Umali A, Shim J, Fabiato A, MacKerell AD, Jr, Brezina V, Sealfon SC, Filizola M, González-Maeso J, Logothetis DE. Decoding the signaling of a GPCR heteromeric complex reveals a unifying mechanism of action of antipsychotic drugs. Cell. 2011;147:1011–1023. doi: 10.1016/j.cell.2011.09.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hatcher-Solis C, Fribourg M, Spyridaki K, Younkin J, Ellaithy A, Xiang G, Liapakis G, Gonzalez-Maeso J, Zhang H, Cui M, Logothetis DE. G protein-coupled receptor signaling to Kir channels in Xenopus oocytes. Curr Pharm. Biotechnol. 2014;15:987–995. doi: 10.2174/1389201015666141031111916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vivaudou M, Chan KW, Sui JL, Jan LY, Reuveny E, Logothetis DE. Probing the G-protein regulation of GIRK1 and GIRK4, the two subunits of the KACh channel, using functional homomeric mutants. J. Biol. Chem. 1997;272:31553–31560. doi: 10.1074/jbc.272.50.31553. [DOI] [PubMed] [Google Scholar]
- 17.Logothetis DE, Kurachi Y, Galper J, Neer EJ, Clapham DE. The beta gamma subunits of GTP-binding proteins activate the muscarinic K+ channel in heart. Nature. 1987;325:321–326. doi: 10.1038/325321a0. [DOI] [PubMed] [Google Scholar]
- 18.Du X, Zhang H, Lopes C, Mirshahi T, Rohacs T, Logothetis DE. Characteristic interactions with phosphatidylinositol 4,5-bisphosphate determine regulation of kir channels by diverse modulators. J. Biol. Chem. 2004;279:37271–37281. doi: 10.1074/jbc.M403413200. Epub 2004 May 20. [DOI] [PubMed] [Google Scholar]
- 19.Logothetis DE, Petrou VI, Zhang M, Mahajan R, Meng XY, Adney SK, Cui M, Baki L. Phosphoinositide control of membrane protein function: a frontier led by studies on ion channels. Annu. Rev. Physiol. 2015;77:81–104. doi: 10.1146/annurev-physiol-021113-170358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ruchala I, Cabra V, Solis E, Jr, Glennon RA, De Felice LJ, Eltit JM. Electrical coupling between the human serotonin transporter and voltage-gated Ca(2+) channels. Cell Calcium. 2014;56:25–33. doi: 10.1016/j.ceca.2014.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moreno JL, Miranda-Azpiazu P, Garcia-Bea A, Younkin J, Cui M, Kozlenkov A, Ben-Ezra A, Voloudakis G, Fakira AK, Baki L, Ge Y, Georgakopoulos A, Moron JA, Milligan G, Lopez-Gimenez JF, Robakis NK, Logothetis DE, Meana JJ, Gonzalez-Maeso J. Allosteric signaling through an mGlu2 and 5-HT2A heteromeric receptor complex and its potential contribution to schizophrenia. Sci. Signal. 2016;9(410):ra5. doi: 10.1126/scisignal.aab0467. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.