Abstract
Preterm birth remains the major cause of neonatal mortality and morbidity, mediated largely by an inflammatory process. The sirtuin (SIRT) family of cellular regulators has been implicated as key inhibitors of inflammation. We have previously reported a role for SIRT1, SIRT2, and SIRT6 in regulating inflammation-induced prolabor mediators. In this study, we determined the effect of term labor and pro-inflammatory cytokines on SIRT3, SIRT4, SIRT5, and SIRT7 expression in human myometrium. Functional studies were also used to investigate the effect of small interfering RNA (siRNA) knockdown of SIRTs in regulating inflammation-induced prolabor mediators. Western blot analysis and qRT-PCR were used to determine SIRT3, SIRT4, SIRT5, and SIRT7 mRNA and protein expression in human myometrium. Small interfering RNA knockdown of SIRT3 in myometrial primary cells determined its role in response to inflammatory stimuli IL1B and TNF. SIRT3 mRNA and protein expression levels were significantly lower in term laboring myometrium compared with term nonlaboring myometrium. There was no effect of labor on SIRT4, SIRT5 or SIRT7 protein expression. The pro-inflammatory cytokines IL1B and TNF significantly decreased levels of SIRT3 mRNA and protein expression. SIRT3 knockdown by siRNA significantly augmented IL1B- and TNF-stimulated IL6, CXCL8, and CCL2 mRNA expression and release; PTGS2 mRNA expression and subsequent PGF2alpha release; the mRNA expression and secretion of the adhesion molecule ICAM1 and the extracellular matrix remodeling enzyme MMP9; and nuclear factor kappa B1 (NFkappaB1) transcriptional activity. In human myometrium, SIRT3 expression decreases with term labor and regulates the mediators involved in the terminal effector pathways of human labor and delivery through the NFkappaB1 pathway.
Keywords: human labor, inflammation, myometrium, SIRT3
INTRODUCTION
Preterm labor is the major cause of perinatal morbidity and mortality worldwide, responsible for approximately 1 million annual neonatal deaths globally [1]. Preterm birth is a costly complication of human pregnancy with significant long-term health impacts on children and their families [2]. Approximately 60% of all preterm births are the result of idiopathic preterm labor (intact membranes) [1]. Thus, an efficacious medical therapy that can stop idiopathic preterm labor would be a major advance. Such a treatment, however, does not exist, and its development is hampered by the fact the molecular mechanisms regulating uterine activity are incompletely understood [3]. A better understanding of the processes that regulate human labor and delivery would increase the likelihood of identifying therapeutic targets to stop or delay preterm labor.
Inflammation is frequently associated with preterm birth and is thought to have a driving role in initiating uterine contractions [4, 5]. It is postulated that up-regulation in the expression of cell adhesion molecules such as ICAM1 and VCAM1 [6] play a role in regulating leukocyte trafficking in the myometrium during labor [7, 8]. Interleukin 1B (IL1B) and tumor necrosis factor (TNF), released by the leukocytes that infiltrate the uterus, can act on gestational tissues to further increase the expression of pro-inflammatory cytokines and adhesion molecules; induce cyclooxygenase (COX)-2 (prostaglandin endoperoxide synthase-2 [PTGS2])-dependent prostaglandin synthesis and increase the expression of prostaglandin F receptor (PTGFR); and activate extracellular matrix (ECM) remodeling enzymes involved in the processes of human labor (such as matrix metalloproteinase [MMP]-9) [5, 9–15]. Numerous studies have shown that nuclear factor κB1 (NFκB1) plays a central role in these processes [16–18].
Mammalian sirtuins (SIRTs) constitute a family of cellular regulators involved in important processes including metabolism, cell division, and aging [19–24]. As such, they have been implicated as potential targets in a number of diseases such as diabetes, cancer, and neurodegenerative disease. The sirtuin family consists of seven enzymes (SIRT1-7) that share a conserved core catalytic domain but differ in their cellular localization and tissue distributions [25, 26]. They possess histone/protein deacetylase activity, which is involved in the repression of gene transcription. In nongestational tissues, SIRTs have been implicated as key inhibitors of inflammation; and SIRT knockouts or in vitro silencing of SIRTs leads to increased inflammation, whereas SIRT activation or overexpression inhibits inflammation [27–35]. Furthermore, SIRTs have inhibitory effects in experimental chronic inflammatory diseases such as chronic obstructive pulmonary disease and colitis [29, 36]. In nongestational tissues, SIRTs can repress inflammation by inhibiting NFκB1 activity [33, 35, 37, 38].
To date, we have begun to unravel the biological function of a number of SIRTs. Our published studies provide the first evidence of functional roles for SIRT1 [39] and SIRT6 [40] in regulating the mediators involved in the terminal effector pathways of human labor and delivery in human fetal membranes. Specifically, the SIRT1 activators resveratrol and SRT1720 significantly decreased lipopolysaccharide-induced cytokine gene expression and release, PTGS2 expression, and prostaglandin release from human gestational tissues [39]. Furthermore, knockdown of SIRT1 by RNA interference diminished the anti-inflammatory effects of resveratrol [39]. In human primary amnion cells, small interfering RNA (siRNA) knockdown of SIRT6 augments IL1B-induced cytokine gene expression and release, PTGS2 expression, and subsequent prostaglandin release, and MMP9 expression and activity [40]. Very little, however, is known about the role of the SIRT family members in human myometrium. Thus, this study aimed to characterize the expression of SIRT1, SIRT3, SIRT4, SIRT5, SIRT6, and SIRT7 in human myometrium and to use siRNA to determine whether SIRTs are important in the downstream pathways involved in labor.
MATERIALS AND METHODS
Tissue Collection
The Research Ethics Committee of Mercy Hospital for Women approved this study. Written, informed consent was obtained from all participating women. All tissues were obtained from women who delivered healthy, singleton infants. All tissues were brought to the research laboratory and processed within 15 min of the cesarean delivery. Women with any underlying medical conditions such as diabetes, asthma, polycystic ovary syndrome, pre-eclampsia, and macrovascular complications were excluded. Additionally, women with a history multiple pregnancies, obese women, and fetuses with chromosomal abnormalities were excluded.
Myometrium was obtained from consenting women at the time of term cesarean section (≥37 wk gestation). Myometrial biopsies were performed and samples were collected from pregnant women undergoing elective cesarean sections in the absence of labor (n = 8 patients; mean gestational age, 39.4 ± 0.3 wk) and from pregnant women who delivered during active labor, defined as the presence of regular uterine contractions (every 3–4 min), resulting in cervical effacement and dilation (n = 8 patients; mean gestational age, 39.8 ± 0.2 wk). Indications for cesarean section in the absence of labor were breech presentation or previous cesarean section or both. Indications for cesarean section in the laboring samples were for placenta praevia, fetal distress, and delayed or failure to progress. A myometrial biopsy sample was obtained from the upper margin of the lower uterine segment incision during the cesarean section. There were no differences in maternal age and body mass index, parity, or gestational age of the patients recruited. None of these patients received any medications to augment or induce labor, and time of labor before cesarean section was 10 h ± 6 h 40 min (mean ± SEM). Tissue samples were snap frozen in liquid nitrogen and immediately stored at −80°C for analysis by qRT-PCR and Western blotting as detailed below.
Primary Myometrial Cell Culture
Fresh myometrium was obtained from women who delivered healthy, singleton infants at term (37–41 wk gestation) undergoing elective cesarean section in the absence of labor. Cells were isolated and cultured as previously described [41]. Briefly, myometrium was minced and digested for 1 h in Dulbecco modified Eagle Medium/Nutrient Mixture F-12 Ham medium (DMEM/F-12) with 3 mg/ml type I collagenase (Worthington Biochemical) and 80 μg/ml DNase 1 (Roche Diagnostics) at 37°C. Cells were centrifuged at 400 × g for 10 min and grown in DMEM/F-12 enriched with 10% heat-inactivated fetal calf serum (containing 100 U/ml penicillin G and 100 mg/ml streptomycin).
SIRT siRNA Transfection
Transfection of primary myometrial cells was performed as we have previously described [41]. Briefly, cells at approximately 50% confluence were transfected using Lipofectamine 3000 according to manufacturer's guidelines (Life Technologies). SIRT3 siRNA (siSIRT3), SIRT4 siRNA (siSIRT4), SIRT5 siRNA (siSIRT5), SIRT7 siRNA (siSIRT7), and negative control siRNA (siCONT) were obtained from Ambion (Thermo Fisher Scientific). Cells were transfected with 50 nM siRNA in DMEM/F-12 for 48 h, followed by treatment with or without 1 ng/ml IL1B or 10 ng/ml TNF for 24 h. Cells were collected and stored at −80°C until assayed for mRNA expression by qRT-PCR, as detailed below. Medium was collected and stored at −80°C until assayed for cytokine and prostaglandin release, as detailed below. Cell viability was assessed by the 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide (MTT) proliferation assay. Responses to IL1B and TNF among patients varied greatly, as we have previously reported [41]. Thus, data are presented as fold change in expression relative to the expression level in the IL1B- or TNF-stimulated siCONT-transfected cells, which was set at 1. Experiments were performed from myometrium obtained from six patients.
Luciferase Assay
A luciferase assay was also used to determine the possible interactions between SIRT3 and NFκB1 as previously described [41]. Primary myometrial cells were transfected with 300 ng/ml NFκB1 reporter construct (Qiagen), using FuGENE HD transfection reagent (Promega). After 6 h, cells were transfected with 50 nM of siSIRT3 or siCONT (as detailed above) for 48 h. The medium was then replaced with DMEM/F-12 (containing 0.5% bovine serum albumin), with or without 1 ng/ml IL1B or 10 ng/ml TNF, and the cells incubated at 37°C for an additional 20 h. After final incubation, cells were harvested in lysis buffer, and luminescence activity was measured using a Luciferase Reporter Assay Kit (Life Research) and Renilla Luciferase Flash Assay kit (Thermo Fisher Scientific) as instructed. The ratio of the firefly luciferase level to the Renilla luciferase level was determined and the results are expressed as a ratio of normalized luciferase activity. The experiments were performed from myometrium obtained from five patients.
RNA Extraction and qRT-PCR
RNA extractions and qRT-PCR were performed as previously described [41]. RNA quality and integrity were measured using an ND1000 (NanoDrop) and determined by using the absorbance (A) ratio A260:A280. RNA (0.5 μg for tissues and 0.2 μg for cells) was converted to cDNA by using the high-capacity cDNA reverse transcription kit according to the manufacturer's instructions (Applied Biosystems). RT-PCR was performed using the CFX384 real-time PCR detection system (Bio-Rad Laboratories), using 100 nM of predesigned and validated QuantiTect primers (primer sequences not available; Qiagen). Average gene cycle threshold (Ct) values were normalized relative to those of two housekeeping genes (β2-microglobulin [B2M] and succinate dehydrogenase complex subunit A [SDHA]). Of note, there was no effect of experimental treatment on B2M or SDHA mRNA expression. Fold differences were determined using the comparative Ct method.
Western Blotting
Western blotting was performed as previously described [41]. Blots were incubated in a 1/1000 dilution of rabbit monoclonal anti-SIRT3 (2627; Cell Signaling Technology); 1 μg/ml mouse monoclonal anti-SIRT4 (SAB1403134; Sigma-Aldrich), 1 μg/ml rabbit polyclonal anti-SIRT5 (HPA022002; Sigma-Aldrich) or 1/1000 dilution of MaxPab rabbit polyclonal anti-SIRT7 (H00051547-D01; Abnova) prepared in blocking buffer (5% skim milk in TBS [50 mM Tris-Cl, pH 7.5, 150 mM NaCl] with 0.05% Tween-20) for 16 h at 4°C. Semiquantitative analysis of the relative density of the bands in Western blots was performed using Quantity One 4.2.1 image analysis software (Bio-Rad Laboratories). Protein normalization was performed as previously described [41]. For siRNA studies, SIRT expression was normalized to the levels of β-actin (ACTB; Sigma-Aldrich). For all other figures, SIRT protein expression was normalized to Ponceau S stain; a section of the Ponceau S-stained membrane was chosen which did not show variation with labor status [41].
Enzyme Immunoassays
Assessment of cytokine and chemokine release of IL6 and CXCL8 was performed using the CytoSet sandwich ELISA according to the manufacturer's instructions (Life Technologies). The release of CCL2, sICAM1, and sVCAM1 was performed by sandwich ELISA from R&D Systems according to the manufacturer's instructions. The release of PGF2α into the incubation medium was assayed using a commercially available competitive enzyme immunoassay kit according to the manufacturer's specifications (Kookaburra kits; Sapphire Bioscience). The inter- and intraassay coefficients of variation for all assays were less than 10%.
Gelatin Zymography
Incubation medium was also collected, and assessment of MMP9 was performed by gelatin zymography as previously described [42]. Proteolytic activity was visualized as clear zones of lysis on a blue background of undigested gelatin. Gels were scanned using a ChemiDoc XRS system (Bio-Rad Laboratories) and then inverted, and densitometry was performed using Quantity One image analysis software (Bio-Rad Laboratories). Fold change was calculated relative to that of TNF activity, which was set at 1.
Statistical Analysis
All statistical analyses were undertaken using Prism software (GraphPad). For two sample comparisons, either a paired or unpaired Student t-test result was used to assess statistical significance between normally distributed data; otherwise, the nonparametric Mann-Whitney U (unpaired) or the Wilcoxon (matched pairs) test was used. For all other comparisons, the homogeneity of data was assessed by using the Bartlett test with one-way ANOVA (with least significant differences post-hoc testing to discriminate among the means). Statistical significance was ascribed to a P value of <0.05. Data are mean ± SEM.
RESULTS
Expression of SIRT3 in Human Myometrium
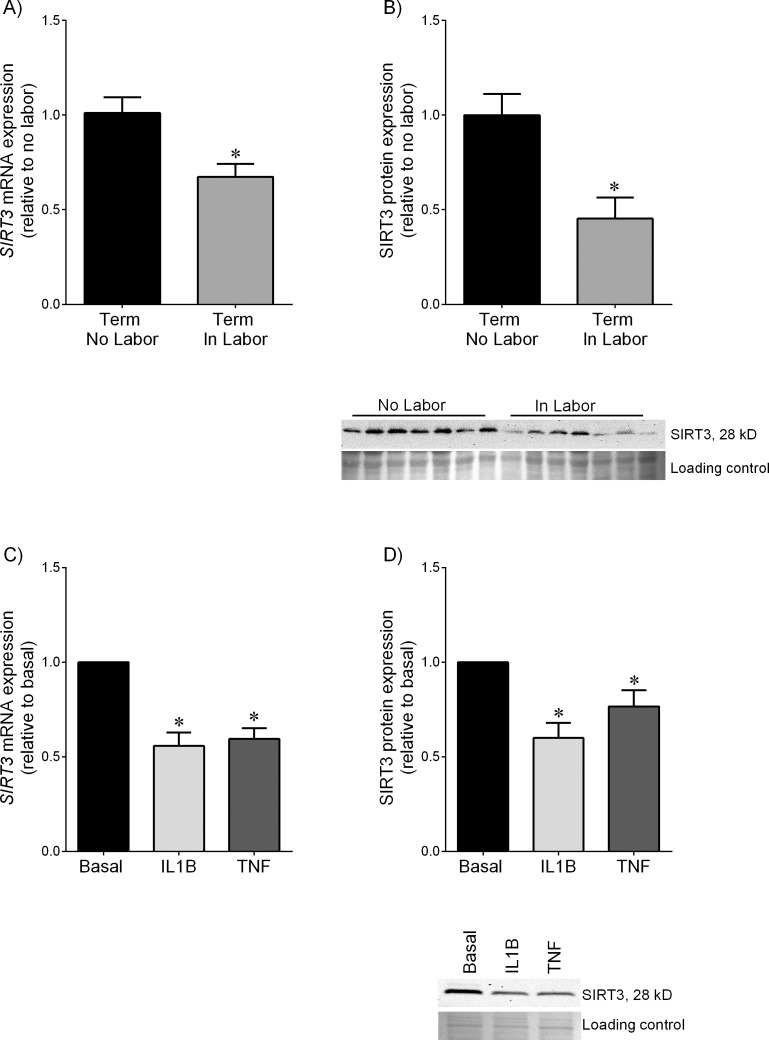
Human myometrium was obtained at term cesarean section in the absence of labor (term, no labor) and at term cesarean section during spontaneous labor onset (term, in labor). The mRNA abundance (Fig. 1A) and protein expression (Fig. 1B) of SIRT3 were significantly lower in laboring myometrium than those in nonlaboring myometrium. However, there were no differences between the expression levels of SIRT4, SIRT5, and SIRT7 in nonlaboring myometrium and those in laboring myometrium (Supplemental Fig. S1; Supplemental Data are available online at www.biolreprod.org).
FIG. 1.
Expression of SIRT3 in myometrium. A and B) Human myometrium was obtained from nonlaboring (term no labor, n = 8 patients) and laboring women at term who underwent cesarean section (term in labor, n = 8 patients). A) SIRT3 mRNA expression was analyzed using qRT-PCR and fold change was calculated relative to term no labor group. Data are mean ± SEM. *P < 0.05 versus term no labor (Student t-test). B) SIRT3 protein expression was analyzed by using Western blotting and fold change was calculated relative to term no labor group. Data are mean ± SEM. *P < 0.05 versus term no labor (Student t-test). Representative Western blots from 4 patients per group are also shown. C and D) Human primary myometrial cells were treated with or without 1 ng/ml IL1B or 10 ng/ml TNF for 20 h (n = 5 patients per treatment). C) SIRT3 mRNA expression was analyzed by using qRT-PCR, and fold change was calculated relative to basal level. Data are mean ± SEM. *P < 0.05 versus basal (paired sample t-test). D) SIRT3 protein expression was analyzed by using Western blotting and the fold change was calculated relative to basal. Data are mean ± SEM. *P < 0.05 versus basal (paired sample t-test). Representative Western blot from 1 patient is also shown.
A range of known mediators of labor were used to determine which could affect SIRT3 expression in human myometrium. We tested the pro-inflammatory cytokines IL1B and TNF, as these have been shown to induce preterm birth in vivo [43]. As shown in Figure 1C, treatment of myometrial cells with IL1B or TNF significantly decreased SIRT3 mRNA (Fig. 1C) and protein (Fig. 1D) expression.
Effect of siSIRT3 on Pro-Inflammatory Cytokines and Chemokines
Next, loss-of-function studies were undertaken to investigate whether SIRT3 was involved in the genesis of prolabor mediators induced by inflammatory cytokines (TNF or IL1B), bacterial infection (using the bacterial product fsl-1), or viral infection (using the double-stranded RNA [dsRNA] analog poly[I:C]). The efficacy of siSIRT3 is demonstrated in Supplemental Figure S2. There was a 70% decrease in SIRT3 mRNA expression and a 75% decrease in SIRT3 protein expression. A MTT cell viability assay showed no differences between absorbance in cells transfected with siCONT and that in siSIRT3 (Supplemental Fig. S2).
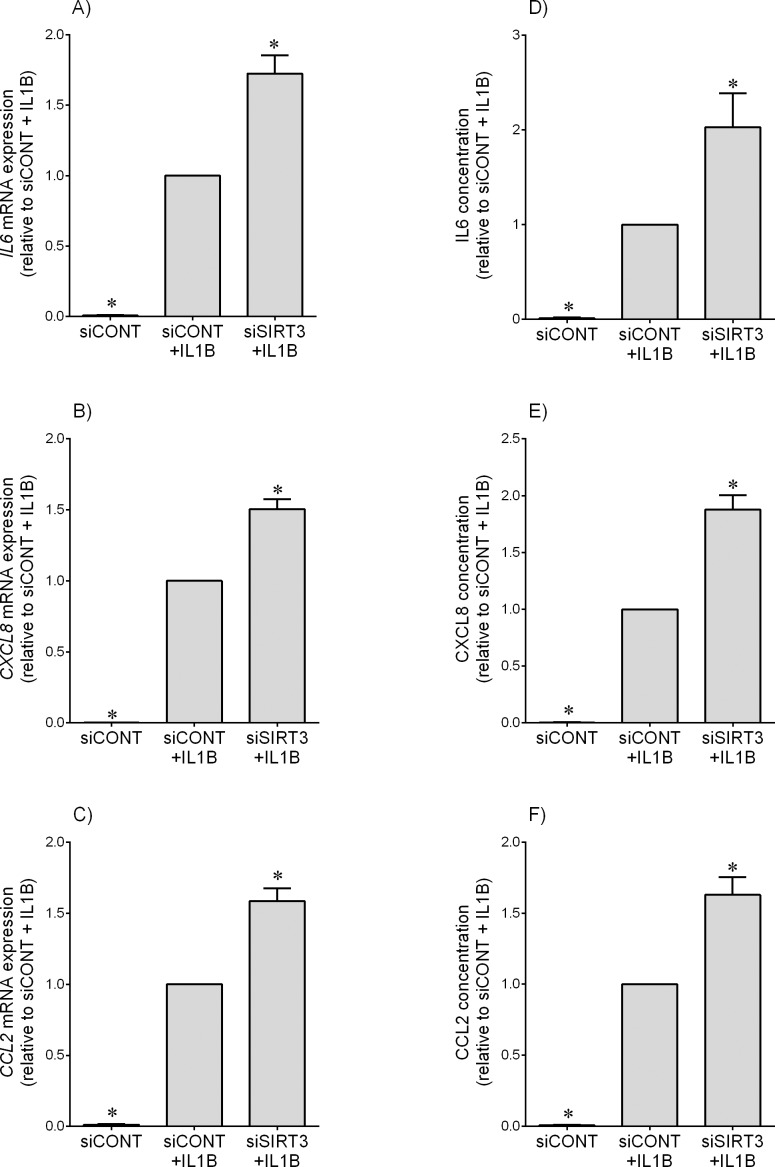
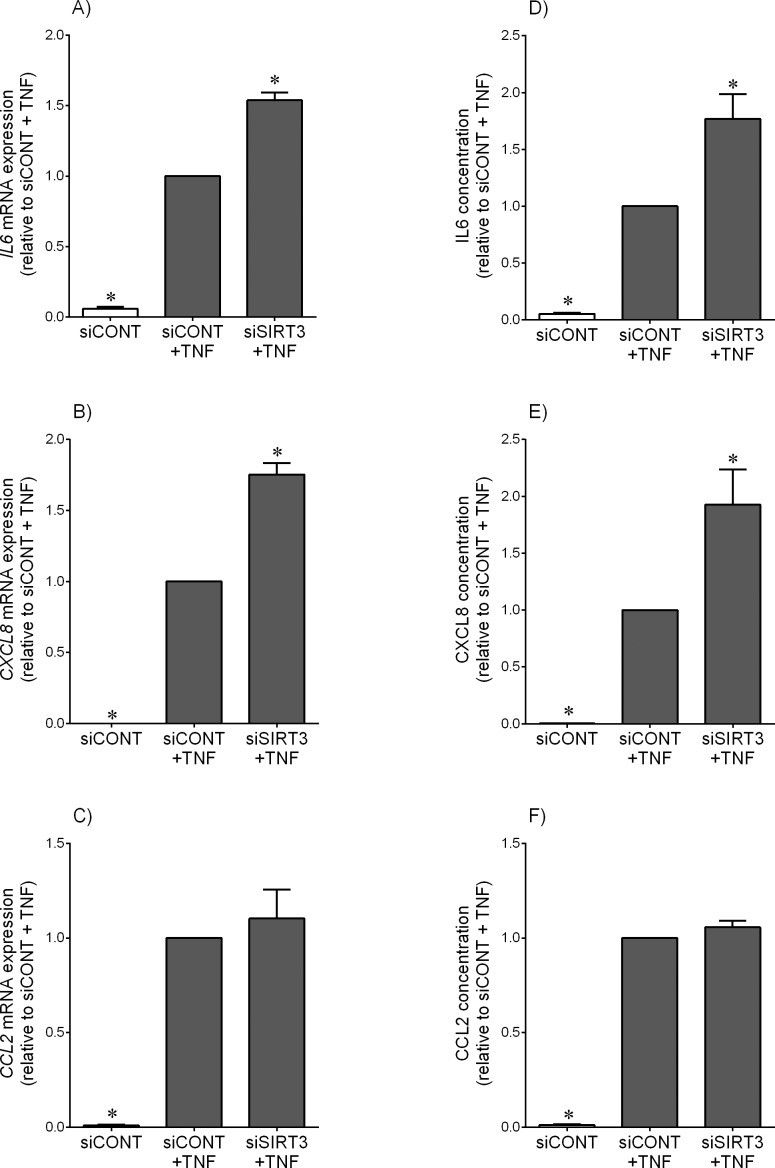
For experiments subsequent to siRNA transfection, cells were treated with IL1B, TNF, fsl-1, or poly(I:C). Compared to siCONT-transfected cells, and as expected, IL1B (Fig. 2) and TNF (Fig. 3) significantly increased IL6, CXCL8, and CCL2 mRNA expression and IL6, CXCL8, and CCL2 secretion. The effect of siSIRT3 was a significant amplification of IL1B-induced IL6, CXCL8, and CCL2 mRNA expression and secretion (Fig. 2) and TNF-induced IL6 and CXCL8 mRNA expression and secretion of IL6, CXCL8, and CCL2 (Fig. 3, A, B, D, and E). There was no effect, however, of siSIRT3 on TNF-induced CCL2 mRNA expression or CCL2 secretion (Fig. 3, C and F). There was no effect of siSIRT3 on inflammation induced by fsl-1, a bacteria-derived Toll-like receptor (TLR) 2/6 agonist or poly(I:C), a viral dsRNA analog and TLR3 ligand (Supplemental Fig. S3 for IL6 and CXCL8 data).
FIG. 2.
Effect of siSIRT3 on IL1B-induced pro-inflammatory cytokines and chemokines. Human primary myometrial cells were transfected with 50 nM siCONT or 50 nM siSIRT3 for 48 h and then treated with 1 ng/ml IL1B for an additional 24 h (n = 5 patients). A–C) IL6, CXCL8, and CCL2 mRNA expression levels were analyzed by using qRT-PCR. D–F) IL6, CXCL8, and CCL2 concentration in the incubation medium was assayed by ELISA. For all data, the fold change was calculated relative to that of IL1B-stimulated siCONT-transfected cells, and displayed as mean ± SEM. *P < 0.05 versus IL1B-stimulated siCONT-transfected cells (one-way ANOVA).
FIG. 3.
Effect of siSIRT3 on TNF-induced pro-inflammatory cytokines and chemokines. Human primary myometrial cells were transfected with 50 nM siCONT or 50 nM siSIRT3 for 48 h and then treated with 10 ng/ml TNF for an additional 24 h (n = 5 patients). A–C) IL6, CXCL8, and CCL2 mRNA expression levels were analyzed by using qRT-PCR. D–F) IL6, CXCL8, and CCL2 concentrations in the incubation medium were assayed by ELISA. For all data, the fold change was calculated relative to that of TNF-stimulated siCONT-transfected cells and displayed as mean ± SEM. *P < 0.05 versus TNF-stimulated siCONT-transfected cells (one-way ANOVA).
Of note, there was no effect of siSIRT1, siSIRT5, siSIRT6, or siSIRT7 on IL1B- or TNF-induced pro-inflammatory mediators (Supplemental Fig. S4 for IL6 data).
Effect of siSIRT3 on PTGS2-Prostaglandin Pathway
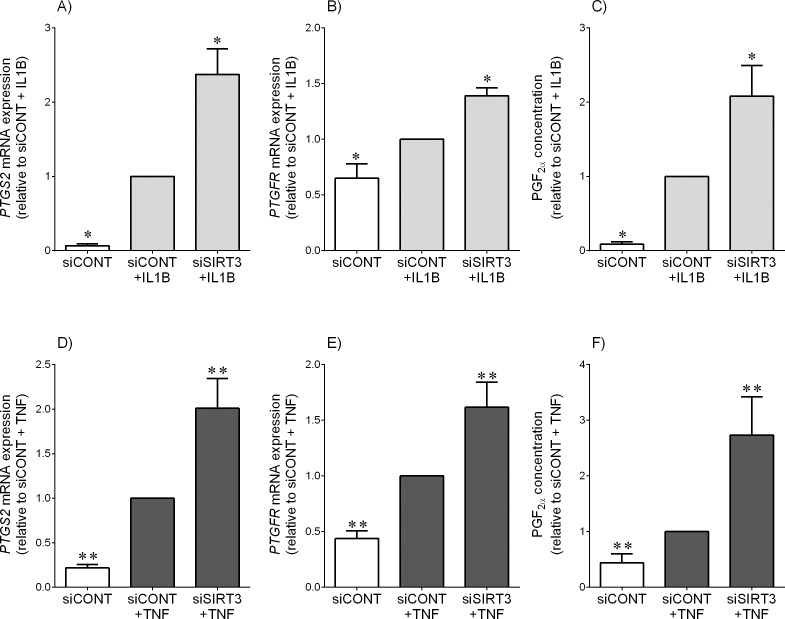
In siCONT-transfected cells, treatment with IL1B (Fig. 4, A–C) and TNF (Fig. 4, D–F) significantly increased PTGS2 and the prostaglandin F receptor PTGFR mRNA expression and subsequent PGF2α release. This increase was further significantly augmented in cells transfected with siSIRT3.
FIG. 4.
Effect of siSIRT3 on the PTGS2-prostaglandin pathway. Human primary myometrial cells were transfected with 50 nM siCONT or 50 nM siSIRT3 for 48 h and then treated with 1 ng/ml IL1B (A–C) or 10 ng/ml TNF (D–F) for an additional 24 h (n = 5 patients). A, B, D, and E) PTGS2 and PTGFR mRNA expression levels were analyzed by using qRT-PCR. C, F) PGF2α concentration in the incubation medium was assayed using ELISA. For all data, the fold change was calculated relative to IL1B-stimulated siCONT-transfected cells and displayed as mean ± SEM. *P < 0.05 versus IL1B-stimulated siCONT-transfected cells (one-way ANOVA); **P < 0.05 versus TNF-stimulated siCONT-transfected cells (one-way ANOVA).
Effect of siSIRT3 on Adhesion Molecules
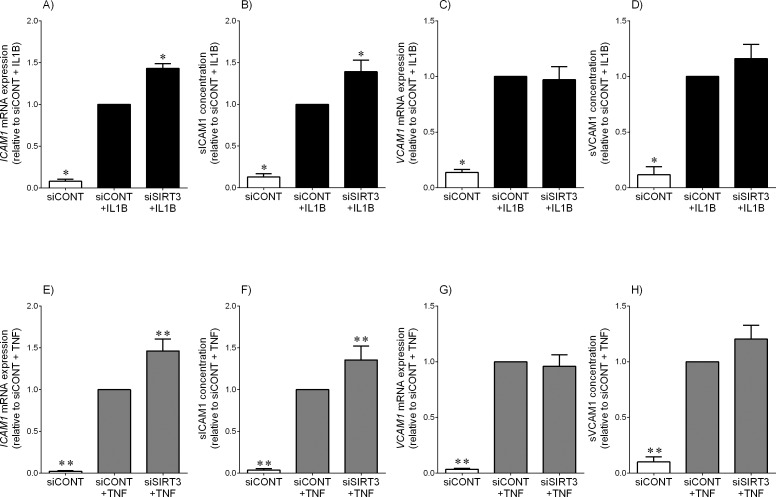
The effect of siSIRT3 on the expression and secretion of adhesion molecules ICAM1 and VCAM1 is shown in Figure 5. In siCONT-transfected cells, both IL1B and TNF significantly increased ICAM1 (Fig. 5, A and E) and VCAM1 (Fig. 5, B and F) mRNA expression. This was associated with a significant increase in sICAM1 (Fig. 5, C and G) and sVCAM1 (Fig. 5, D and H) secretion. The effect of siSIRT3 was further significant increase in ICAM1 mRNA expression and sICAM1 release. There was, however, no effect of siSIRT3 on VCAM1 mRNA expression and sVCAM1 release.
FIG. 5.
Effect of siSIRT3 on the expression and secretion of adhesion molecules. Human primary myometrial cells were transfected with 50 nM siCONT or 50 nM siSIRT3 for 48 h and then treated with 1 ng/ml IL1B (A–D) or 10 ng/ml TNF (E–H) for an additional 24 h (n = 5 patients). A, C, E, and G) ICAM1 and VCAM1 mRNA expression was analyzed by using qRT-PCR. B, D, F, and H) sICAM1 and sVCAM1 concentrations in the incubation medium was assayed using ELISA. The fold change was calculated relative to that of IL1B- or TNF-stimulated siCONT-transfected cells, and data are mean ± SEM. *P < 0.05 versus IL1B-stimulated siCONT-transfected cells (one-way ANOVA); **P < 0.05 versus TNF-stimulated siCONT-transfected cells (one-way ANOVA).
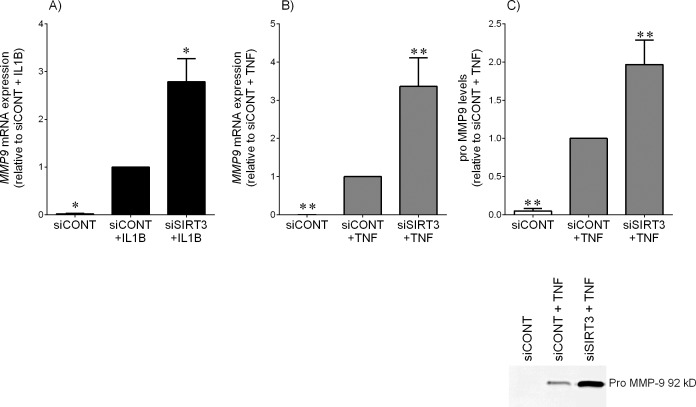
Effect of siSIRT3 on MMP9
Figure 6 demonstrates the effect of siSIRT3 on the expression of the ECM degrading and remodeling enzyme MMP9. In siCONT-transfected cells, IL1B (Fig. 6A) or TNF (Fig. 6B) significantly increased MMP9 mRNA expression. In addition, TNF treatment also significantly increased pro-MMP9 secretion (Fig. 6C). This increase was further significantly augmented in siSIRT3-transfected cells. Of note, the release of pro-MMP9 in the presence of IL1B was not detectable by zymography.
FIG. 6.
Effect of siSIRT3 on the expression and secretion of MMP9 expression. Human primary myometrial cells were transfected with 50 nM siCONT or 50 nM siSIRT3 for 48 h and then treated with 1 ng/ml IL1B (A) or 10 ng/ml TNF (B and C) for an additional 24 h (n = 5 patients). A and B) MMP9 mRNA expression was analyzed by using qRT-PCR. The fold change was calculated relative to that of IL1B- or TNF-stimulated siCONT-transfected cells. C) The incubation medium was assayed for pro-MMP9 expression by using gelatin zymography, and the fold change was calculated relative to that of TNF-stimulated siCONT-transfected cells. Representative gelatin zymographic results from one patient are also shown. All data are mean ± SEM. *P < 0.05 versus IL1B-stimulated siCONT-transfected cells (one-way ANOVA); **P < 0.05 versus TNF-stimulated siCONT-transfected cells (one-way ANOVA).
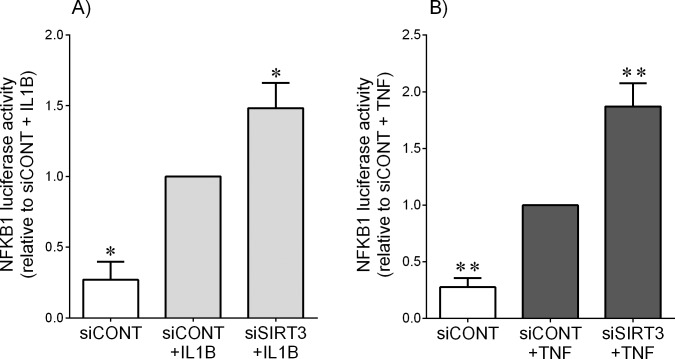
Effect of siSIRT3 on NFkappaB1 Activation
In nongestational tissues, SIRT3 can repress inflammation by inhibiting NFKB1 activity [33, 35, 37]. Given the central role of NFKB1 in the regulation of pro-inflammatory and prolabor mediators in fetal membranes [16–18, 39], we hypothesized that SIRT3 may also regulate prolabor mediators in primary myometrial cells through NFκB1. We addressed this by determining the effect of siSIRT3 on NFκB1 transcriptional activity using a luciferase assay. As shown in Figure 7, treatment of primary myometrial cells with IL1B (Fig. 7A) or TNF (Fig. 7B) significantly increased NFκB1 activity. The result of siSIRT3 was a significant increase in IL1B- and TNF-induced NFκB1 activation.
FIG. 7.
Effect of siSIRT3 on NFκB1 activation. Human myometrial cells were transfected with 300 ng/ml NFκB1 reporter construct. After 6 h, cells were transfected with 50 nM siCONT or 50 nM siSIRT3 for 48 h and then treated with 1 ng/ml IL1B (A) or 10 ng/ml TNF (B) for an additional 20 h (n = 5 patients). The fold change was calculated relative to that of IL1B- or TNF-stimulated siCONT-transfected cells. All data are mean ± SEM. *P < 0.05 versus IL1B-stimulated siCONT-transfected cells (one-way ANOVA); **P < 0.05 versus TNF-stimulated siCONT-transfected cells (one-way ANOVA).
DISCUSSION
For the first time, we report that SIRT3 plays a role in regulating the mediators involved in the terminal processes of human labor and delivery in human myometrium. Specifically, SIRT3 expression was decreased in term laboring myometrium compared to nonlaboring myometrium. The pro-inflammatory cytokines IL1B and TNF, capable of inducing uterine contractions [44–46], significantly decreased SIRT3 expression in myometrium. Furthermore, siRNA knockdown of SIRT3 amplified IL1B- and/or TNF-induced expression and secretion of pro-inflammatory cytokines and chemokines, PTGS2 expression, and ensuing prostaglandin secretion, the expression and secretion of the adhesion molecule sICAM1, and the expression of the ECM remodeling enzyme MMP9. SIRT3 knockdown by siRNA also increased NFκB1 activity.
Whether the decrease in SIRT3 expression in human term laboring myometrium is a cause or consequence of human labor is not known. Treatment, however, of human primary myometrial cells with the pro-inflammatory cytokine IL1B or TNF, both of which are significantly higher in gestational tissues and biological fluids at the time of labor onset [7, 11, 47, 48], significantly decreased SIRT3 expression. These findings suggest that increased inflammation associated with term labor onset may be responsible for the decrease in SIRT3 expression observed in laboring myometrium.
The pro-inflammatory cytokines IL1B and TNF play central roles in the processes of human labor and delivery. Sterile inflammation refers to an inflammatory process where microorganisms cannot be detected in patients with preterm labor and intact membranes [49]. Furthermore, intra-amniotic and/or systemic administration of IL1B or TNF to mice and monkeys induces preterm labor [43, 50, 51]. IL1B and TNF, which are released from infiltrating leukocytes in the uterus and myometrium during labor [7, 8], can further increase the expression of cell adhesion molecules [6] to promote further neutrophil infiltration and activation [52], stimulate PTGS2 activity to produce prostaglandins such as PGF2α, which acts on the PTGFR to increase uterine contractility, and so promote the onset and progression of labor [44–46]. IL1B and TNF also induce the expression of MMP9, which is involved in tissue remodeling of human myometrium during labor [14]. In light of this evidence, IL1B and TNF were thus used in term myometrium as our model of preterm birth. A number of studies have suggested that SIRT3 plays an important role in regulating inflammation [32, 33, 35, 53]; thus, we sought to determine whether SIRT3 also regulates IL1B or TNF induced pro-inflammatory cytokines and pro-labor mediators. In support of the studies in nongestational tissues, targeted siRNA knockdown of SIRT3 has revealed that its decline is required for pro-inflammatory cytokine-stimulated IL6, CXCL8, and CCL2 mRNA expression and IL6, CXCL8, and CCL2 release; PTGS2 and PTGFR mRNA expression and PGF2α release; and expression and secretion of ICAM1 and MMP9.
Spontaneous preterm birth can also be due to microorganisms such as bacteria or viruses [5]. Pattern recognition receptors like TLRs, which are highly expressed on immune cells at the maternal-fetal interface [54], recognize evolutionarily conserved structures on pathogens termed pathogen-associated molecular patterns (PAMPs). Each TLR1-10 recognizes specific structures and the induction of specific TLR-dependent cytokine responses reflect the different pathogens causing the immune response [55]. In myometrium bacterial ligand activation of TLR2 and TLR5 and viral activation of TLR3 elicits pro-inflammatory and prolabor responses [56, 57]. Additionally, activation of TLR2 and TLR3 can lead to preterm delivery in animal models [58]. It is interesting to note that no effect of SIRT3 knockdown on TLR2- or TLR3-induced prolabor mediators was observed (Supplemental Fig. 3). These findings suggest that in human myometrium, SIRT3 is required for IL1B- and TNF-mediated signaling but dispensable for TLR-induced pro-inflammatory cytokine production. The induction of the inflammatory response through cytokine and TLR signaling is complex and involves different adapter molecules. Our findings suggest that in human myometrium, SIRT3 may be acting on a number of discrete downstream proteins involved in IL1B and TNF signaling but not TLR signaling. Nevertheless, our findings are significant given that over 60% of all preterm births are the result of sterile intra-amniotic inflammation [49].
The anti-inflammatory effects of SIRT3 are a result of its histone deacetylase activity, which thereby represses target gene transcription [33]. Recent studies have highlighted a role for NFκB1 inhibition in the anti-inflammatory actions of SIRT3 [33, 37, 59, 60]. NFκB1 is a pro-inflammatory transcription factor that plays a central role in the terminal processes of human labor and delivery [16–18, 39]. In myometrium, NFκB1 is active in both upper and lower segments prior to the onset of labor at term, where it principally regulates a group of immune/inflammation-associated genes [61]. Similarly, we have previously shown that NFκB1 is required for IL1B and TNF signaling in human myometrium [57, 62, 63]. In this study, using a luciferase assay, IL1B- and TNF-induced NFκB1 transcriptional activity was augmented in cells transfected with siSIRT3. Collectively, our data suggest that in the absence of SIRT3, there is hyperactive NFκB1, leading to increased transcription of pro-inflammatory and prolabor genes.
The exact mechanisms by which SIRT3, which is primarily localized to the mitochondria, regulates NFκB1-mediated nuclear regulation of pro-inflammatory gene expression is not known. Studies in nongestational tissues have shown, however, that SIRT3 can reduce inflammation by decreasing mitochondrial reactive oxygen species production (ROS) [33]. It is well established that pro-inflammatory cytokines increase mitochondrial ROS [64] and we have previously shown that ROS are involved in NFκB1-driven prolabor mediators in human gestational tissues [65]. Thus, we hypothesize that IL1B and TNF decrease SIRT3 expression resulting in increased mitochondrial ROS which can then activate NFκB1 and its target genes.
To our knowledge, this is the first study to explore SIRTs in human myometrium. While the findings of this study show that SIRT3 appears to have a role in human labor in the myometrium, there was no effect of term labor on expression of SIRT1 and SIRT4 to SIRT7 in myometrium. Furthermore, gene silencing of SIRT1 and SIRT4 to SIRT7 did not regulate cytokine signaling in myometrium. In contrast, we have previously reported that SIRT1 exerts anti-inflammatory actions in human placenta and fetal membranes [39] and SIRT6 exerts anti-inflammatory actions in fetal membranes [40]. Although we have not assessed the role or regulation of SIRT3 in human fetal membranes, our findings to date suggest that the SIRT family members may play distinct roles in human gestational tissues. That is, SIRT3 in the myometrium influences uterine contractions, and SIRT1 and SIRT6 having a role in the rupture of fetal membranes.
In summary, decreased SIRT3 expression was found in laboring myometrium and in myometrium treated with IL1B and TNF. In myometrial cells, SIRT3 knockdown by siRNA significantly increased IL1B and TNF-stimulated pro-inflammatory cytokines and chemokines, PTGS2 mRNA and release of prostaglandins, extracellular remodeling enzyme MMP9 and adhesion molecule sICAM1. Collectively, these findings suggest that SIRT3 inhibits the production of pro-inflammatory and prolabor mediators induced by known mediators of preterm birth, IL1B and TNF. Taken together, the data suggests that SIRT3 may be a promising target for the development of therapeutics for preterm birth, as activation of SIRT3 could potentially quench the inflammatory response associated with spontaneous preterm birth.
ACKNOWLEDGMENT
The following are gratefully acknowledged: clinical research midwives Genevieve Christophers, Gabrielle Pell, and Rachel Murdoch for sample collection; and Obstetrics and Midwifery staff, Mercy Hospital for Women, for their cooperation.
Footnotes
Supported by National Health and Medical Research Council (NHMRC) Career Development Fellowship 1047025 to M.L, and NHMRC grant no. 1058786, the Norman Beischer Medical Research Foundation, and the Mercy Research Foundation.
REFERENCES
- Beck S, Wojdyla D, Say L, Betran AP, Merialdi M, Requejo JH, Rubens C, Menon R, Van Look PF. The worldwide incidence of preterm birth: a systematic review of maternal mortality and morbidity. Bull World Health Organ. 2010;88:31–38. doi: 10.2471/BLT.08.062554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saigal S, Doyle LW. An overview of mortality and sequelae of preterm birth from infancy to adulthood. Lancet. 2008;371:261–269. doi: 10.1016/S0140-6736(08)60136-1. [DOI] [PubMed] [Google Scholar]
- Barros FC, Bhutta ZA, Batra M, Hansen TN, Victora CG, Rubens CE. Global report on preterm birth and stillbirth (3 of 7): evidence for effectiveness of interventions. BMC Pregnancy Childbirth. 2010;10((suppl 1)):S3. doi: 10.1186/1471-2393-10-S1-S3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero R, Espinoza J, Goncalves LF, Kusanovic JP, Friel L, Hassan S. The role of inflammation and infection in preterm birth. Semin Reprod Med. 2007;25:21–39. doi: 10.1055/s-2006-956773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christiaens I, Zaragoza DB, Guilbert L, Robertson SA, Mitchell BF, Olson DM. Inflammatory processes in preterm and term parturition. J Reprod Immunol. 2008;79:50–57. doi: 10.1016/j.jri.2008.04.002. [DOI] [PubMed] [Google Scholar]
- Ledingham MA, Thomson AJ, Jordan F, Young A, Crawford M, Norman JE. Cell adhesion molecule expression in the cervix and myometrium during pregnancy and parturition. Obstet Gynecol. 2001;97:235–242. doi: 10.1016/s0029-7844(00)01126-1. [DOI] [PubMed] [Google Scholar]
- Osman I, Young A, Ledingham MA, Thomson AJ, Jordan F, Greer IA, Norman JE. Leukocyte density and pro-inflammatory cytokine expression in human fetal membranes, decidua, cervix and myometrium before and during labour at term. Mol Hum Reprod. 2003;9:41–45. doi: 10.1093/molehr/gag001. [DOI] [PubMed] [Google Scholar]
- Thomson AJ, Telfer JF, Young A, Campbell S, Stewart CJ, Cameron IT, Greer IA, Norman JE. Leukocytes infiltrate the myometrium during human parturition: further evidence that labour is an inflammatory process. Hum Reprod. 1999;14:229–236. [PubMed] [Google Scholar]
- Romero R, Espinoza J, Goncalves LF, Kusanovic JP, Friel LA, Nien JK. Inflammation in preterm and term labour and delivery. Semin Fetal Neonatal Med. 2006;11:317–326. doi: 10.1016/j.siny.2006.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keelan JA, Blumenstein M, Helliwell RJ, Sato TA, Marvin KW, Mitchell MD. Cytokines, prostaglandins and parturition–a review. Placenta. 2003;24((suppl A)):S33–46. doi: 10.1053/plac.2002.0948. [DOI] [PubMed] [Google Scholar]
- Bowen JM, Chamley L, Keelan JA, Mitchell MD. Cytokines of the placenta and extra-placental membranes: roles and regulation during human pregnancy and parturition. Placenta. 2002;23:257–273. doi: 10.1053/plac.2001.0782. [DOI] [PubMed] [Google Scholar]
- Olson DM. The role of prostaglandins in the initiation of parturition. Best Pract Res Clin Obstet Gynaecol. 2003;17:717–730. doi: 10.1016/s1521-6934(03)00069-5. [DOI] [PubMed] [Google Scholar]
- Srikhajon K, Shynlova O, Preechapornprasert A, Chanrachakul B, Lye S. A new role for monocytes in modulating myometrial inflammation during human labor. Biol Reprod. 2014;91:10. doi: 10.1095/biolreprod.113.114975. [DOI] [PubMed] [Google Scholar]
- Roh CR, Oh WJ, Yoon BK, Lee JH. Up-regulation of matrix metalloproteinase-9 in human myometrium during labour: a cytokine-mediated process in uterine smooth muscle cells. Mol Hum Reprod. 2000;6:96–102. doi: 10.1093/molehr/6.1.96. [DOI] [PubMed] [Google Scholar]
- Brodt-Eppley J, Myatt L. Prostaglandin receptors in lower segment myometrium during gestation and labor. Obstet Gynecol. 1999;93:89–93. doi: 10.1016/s0029-7844(98)00378-0. [DOI] [PubMed] [Google Scholar]
- Lappas M, Rice GE. The role and regulation of the nuclear factor kappa B signalling pathway in human labour. Placenta. 2007;28:543–556. doi: 10.1016/j.placenta.2006.05.011. [DOI] [PubMed] [Google Scholar]
- Lappas M, Rice GE. Transcriptional regulation of the processes of human labour and delivery. Placenta. 2009;30:S90–S95. doi: 10.1016/j.placenta.2008.10.005. [DOI] [PubMed] [Google Scholar]
- Lindstrom TM, Bennett PR. The role of nuclear factor kappa B in human labour. Reproduction. 2005;130:569–581. doi: 10.1530/rep.1.00197. [DOI] [PubMed] [Google Scholar]
- Canto C, Auwerx J. Targeting Sirtuin 1 to improve metabolism: all you need is NAD(+)? Pharmacol Rev. 2012;64:166–187. doi: 10.1124/pr.110.003905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nogueiras R, Habegger KM, Chaudhary N, Finan B, Banks AS, Dietrich MO, Horvath TL, Sinclair DA, Pfluger PT, Tschoep MH. Sirtuin 1 and sirtuin 3: physiological modulators of metabolism. Physiol Rev. 2012;92:1479–1514. doi: 10.1152/physrev.00022.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porcu M, Chiarugi A. The emerging therapeutic potential of sirtuin-interacting drugs: from cell death to lifespan extension. Trends Pharmacol Sci. 2005;26:94–103. doi: 10.1016/j.tips.2004.12.009. [DOI] [PubMed] [Google Scholar]
- Yamamoto H, Schoonjans K, Auwerx J. Sirtuin functions in health and disease. Mol Endocrinol. 2007;21:1745–1755. doi: 10.1210/me.2007-0079. [DOI] [PubMed] [Google Scholar]
- Sebastian C, Satterstrom FK, Haigis MC, Mostoslavsky R. From sirtuin biology to human diseases: an update. J Biol Chem. 2012;287:42444–42452. doi: 10.1074/jbc.R112.402768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkel T, Deng C-X, Mostoslavsky R. Recent progress in the biology and physiology of sirtuins. Nature. 2009;460:587–591. doi: 10.1038/nature08197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frye RA. Characterization of five human cDNAs with homology to the yeast SIR2 gene: Sir2-like proteins (sirtuins) metabolize NAD and may have protein ADP-ribosyltransferase activity. Biochem Biophys Res Commun. 1999;260:273–279. doi: 10.1006/bbrc.1999.0897. [DOI] [PubMed] [Google Scholar]
- Frye RA. Phylogenetic classification of prokaryotic and eukaryotic Sir2-like proteins. Biochem Biophys Res Commun. 2000;273:793–798. doi: 10.1006/bbrc.2000.3000. [DOI] [PubMed] [Google Scholar]
- Bao JJ, Scott I, Lu ZP, Pang LY, Dimond CC, Gius D, Sack MN. SIRT3 is regulated by nutrient excess and modulates hepatic susceptibility to lipotoxicity. Free Radic Biol Med. 2010;49:1230–1237. doi: 10.1016/j.freeradbiomed.2010.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vakhrusheva O, Smolka C, Gajawada P, Kostin S, Boettger T, Kubin T, Braun T, Bober E. Sirt7 increases stress resistance of cardiomyocytes and prevents apoptosis and inflammatory cardiomyopathy in mice. Circ Res. 2008;102:703–710. doi: 10.1161/CIRCRESAHA.107.164558. [DOI] [PubMed] [Google Scholar]
- Rajendrasozhan S, Yang SR, Kinnula VL, Rahman I. SIRT1, an antiinflammatory and antiaging protein, is decreased in lungs of patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2008;177:861–870. doi: 10.1164/rccm.200708-1269OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang SR, Wright J, Bauter M, Seweryniak K, Kode A, Rahman I. Sirtuin regulates cigarette smoke-induced proinflammatory mediator release via RelA/p65 NF-kappaB in macrophages in vitro and in rat lungs in vivo: implications for chronic inflammation and aging. Am J Physiol Lung Cell Mol Physiol. 2007;292:L567–576. doi: 10.1152/ajplung.00308.2006. [DOI] [PubMed] [Google Scholar]
- Minagawa S, Araya J, Numata T, Nojiri S, Hara H, Yumino Y, Kawaishi M, Odaka M, Morikawa T, Nishimura SL, Nakayama K, Kuwano K. Accelerated epithelial cell senescence in IPF and the inhibitory role of SIRT6 in TGF-beta-induced senescence of human bronchial epithelial cells. Am J Physiol Lung Cell Mol Physiol. 2011;300:L391–401. doi: 10.1152/ajplung.00097.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundaresan NR, Gupta M, Kim G, Rajamohan SB, Isbatan A, Gupta MP. Sirt3 blocks the cardiac hypertrophic response by augmenting Foxo3a-dependent antioxidant defense mechanisms in mice. J Clin Invest. 2009;119:2758–2771. doi: 10.1172/JCI39162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koyama T, Kume S, Koya D, Araki S, Isshiki K, Chin-Kanasaki M, Sugimoto T, Haneda M, Sugaya T, Kashiwagi A, Maegawa H, Uzu T. SIRT3 attenuates palmitate-induced ROS production and inflammation in proximal tubular cells. Free Radic Biol Med. 2011;51:1258–1267. doi: 10.1016/j.freeradbiomed.2011.05.028. [DOI] [PubMed] [Google Scholar]
- Van Gool F, Galli M, Gueydan C, Kruys V, Prevot PP, Bedalov A, Mostoslavsky R, Alt FW, De Smedt T, Leo O. Intracellular NAD levels regulate tumor necrosis factor protein synthesis in a sirtuin-dependent manner. Nat Med. 2009;15:206–210. doi: 10.1038/nm.1906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caton PW, Richardson SJ, Kieswich J, Bugliani M, Holland ML, Marchetti P, Morgan NG, Yaqoob MM, Holness MJ, Sugden MC. Sirtuin 3 regulates mouse pancreatic beta cell function and is suppressed in pancreatic islets isolated from human type 2 diabetic patients. Diabetologia. 2013;56:1068–1077. doi: 10.1007/s00125-013-2851-y. [DOI] [PubMed] [Google Scholar]
- Singh UP, Singh NP, Singh B, Hofseth LJ, Price RL, Nagarkatti M, Nagarkatti PS. Resveratrol (trans-3,5,4′-trihydroxystilbene) induces silent mating type information regulation-1 and down-regulates nuclear transcription factor-kappaB activation to abrogate dextran sulfate sodium-induced colitis. J Pharmacol Exp Ther. 2010;332:829–839. doi: 10.1124/jpet.109.160838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CJ, Fu YC, Yu W, Wang W. SIRT3 protects cardiomyocytes from oxidative stress-mediated cell death by activating NF-kappaB. Biochem Biophys Res Commun. 2013;430:798–803. doi: 10.1016/j.bbrc.2012.11.066. [DOI] [PubMed] [Google Scholar]
- Yang H, Zhang W, Pan H, Feldser HG, Lainez E, Miller C, Leung S, Zhong Z, Zhao H, Sweitzer S, Considine T, Riera T, et al. SIRT1 activators suppress inflammatory responses through promotion of p65 deacetylation and inhibition of NF-kappaB activity. PLoS One. 2012;7:e46364. doi: 10.1371/journal.pone.0046364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lappas M, Mitton A, Lim R, Barker G, Riley C, Permezel M. SIRT1 is a novel regulator of key pathways of human labor. Biol Reprod. 2011;84:167–178. doi: 10.1095/biolreprod.110.086983. [DOI] [PubMed] [Google Scholar]
- Lim R, Barker G, Lappas M. SIRT6 is decreased with preterm labor and regulates key terminal effector pathways of human labor in fetal membranes. Biol Reprod. 2013;88:17. doi: 10.1095/biolreprod.112.105163. [DOI] [PubMed] [Google Scholar]
- Lim R, Tran HT, Liong S, Barker G, Lappas M. The transcription factor interferon regulatory factor-1 (IRF1) plays a key role in the terminal effector pathways of human preterm labor. Biol Reprod. 2016;94:32. doi: 10.1095/biolreprod.115.134726. [DOI] [PubMed] [Google Scholar]
- Lim R, Barker G, Wall CA, Lappas M. Dietary phytophenols curcumin, naringenin and apigenin reduce infection-induced inflammatory and contractile pathways in human placenta, foetal membranes and myometrium. Mol Hum Reprod. 2013;19:451–462. doi: 10.1093/molehr/gat015. [DOI] [PubMed] [Google Scholar]
- Sadowsky DW, Adams KM, Gravett MG, Witkin SS, Novy MJ. Preterm labor is induced by intraamniotic infusions of interleukin-1beta and tumor necrosis factor-alpha but not by interleukin-6 or interleukin-8 in a nonhuman primate model. Am J Obstet Gynecol. 2006;195:1578–1589. doi: 10.1016/j.ajog.2006.06.072. [DOI] [PubMed] [Google Scholar]
- Rauk PN, Chiao JP. Interleukin-1 stimulates human uterine prostaglandin production through induction of cyclooxygenase-2 expression. Am J Reprod Immunol. 2000;43:152–159. doi: 10.1111/j.8755-8920.2000.430304.x. [DOI] [PubMed] [Google Scholar]
- Bartlett SR, Sawdy R, Mann GE. Induction of cyclooxygenase-2 expression in human myometrial smooth muscle cells by interleukin-1beta: involvement of p38 mitogen-activated protein kinase. J Physiol. 1999;2:399–406. doi: 10.1111/j.1469-7793.1999.00399.x. 520 Pt. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erkinheimo TL, Saukkonen K, Narko K, Jalkanen J, Ylikorkala O, Ristimaki A. Expression of cyclooxygenase-2 and prostanoid receptors by human myometrium. J Clin Endocrinol Metab. 2000;85:3468–3475. doi: 10.1210/jcem.85.9.6809. [DOI] [PubMed] [Google Scholar]
- Elliott CL, Loudon JAZ, Brown N, Slater DM, Bennett PR, Sullivan MHF. IL-1β and IL-8 in human fetal membranes: changes with gestational age, labor, and culture conditions. Am J Reprod Immunol. 2001;46:260–267. doi: 10.1034/j.1600-0897.2001.d01-11.x. [DOI] [PubMed] [Google Scholar]
- Tattersall M, Engineer N, Khanjani S, Sooranna SR, Roberts VH, Grigsby PL, Liang Z, Myatt L, Johnson MR. Pro-labour myometrial gene expression: are preterm labour and term labour the same? Reproduction. 2008;135:569–579. doi: 10.1530/REP-07-0461. [DOI] [PubMed] [Google Scholar]
- Romero R, Miranda J, Chaiworapongsa T, Korzeniewski SJ, Chaemsaithong P, Gotsch F, Dong Z, Ahmed AI, Yoon BH, Hassan SS, Kim CJ, Yeo L. Prevalence and clinical significance of sterile intra-amniotic inflammation in patients with preterm labor and intact membranes. Am J Reprod Immunol. 2014;72:458–474. doi: 10.1111/aji.12296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero R, Mazor M, Tartakovsky B. Systemic administration of interleukin-1 induces preterm parturition in mice. Am J Obstet Gynecol. 1991;165:969–971. doi: 10.1016/0002-9378(91)90450-6. [DOI] [PubMed] [Google Scholar]
- Romero R, Tartakovsky B. The natural interleukin-1 receptor antagonist prevents interleukin-1-induced preterm delivery in mice. Am J Obstet Gynecol. 1992;167:1041–1045. doi: 10.1016/s0002-9378(12)80035-4. [DOI] [PubMed] [Google Scholar]
- Bevilacqua MP, Pober JS, Mendrick DL, Cotran RS, Gimbrone MA., Jr. Identification of an inducible endothelial-leukocyte adhesion molecule. Proc Natl Acad Sci U S A. 1987;84:9238–9242. doi: 10.1073/pnas.84.24.9238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H, Hertzel AV, Steen KA, Bernlohr DA. Loss of fatty acid binding protein 4/aP2 reduces macrophage inflammation through activation of SIRT3. Mol Endocrinol. 2016;30:325–334. doi: 10.1210/me.2015-1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patni S, Flynn P, Wynen LP, Seager AL, Morgan G, White JO, Thornton CA. An introduction to Toll-like receptors and their possible role in the initiation of labour. BJOG. 2007;114:1326–1334. doi: 10.1111/j.1471-0528.2007.01488.x. [DOI] [PubMed] [Google Scholar]
- Bauer S, Muller T, Hamm S. Pattern recognition by Toll-like receptors. Adv Exp Med Biol. 2009;653:15–34. doi: 10.1007/978-1-4419-0901-5_2. [DOI] [PubMed] [Google Scholar]
- Lim R, Barker G, Lappas M. The TLR2 ligand FSL-1 and the TLR5 ligand flagellin mediate pro-inflammatory and pro-labour response via MyD88/TRAF6/NF-kappaB-dependent signalling. Am J Reprod Immunol. 2014;71:401–417. doi: 10.1111/aji.12229. [DOI] [PubMed] [Google Scholar]
- Liong S, Lappas M. The stress-responsive heme oxygenase (HO)-1 isoenzyme is increased in labouring myometrium where it regulates contraction-associated proteins. Am J Reprod Immunol. 2015;74:62–76. doi: 10.1111/aji.12366. [DOI] [PubMed] [Google Scholar]
- Ilievski V, Lu SJ, Hirsch E. Activation of toll-like receptors 2 or 3 and preterm delivery in the mouse. Reprod Sci. 2007;14:315–320. doi: 10.1177/1933719107302959. [DOI] [PubMed] [Google Scholar]
- Storka A, Fuhrlinger G, Seper M, Wang L, Jew M, Leisser A, Wolzt M. E. coli endotoxin modulates the expression of Sirtuin proteins in PBMC in humans. Mediators Inflamm. 2013;2013:876943. doi: 10.1155/2013/876943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song Y, Shi J, Wu Y, Han C, Zou J, Shi Y, Liu Z. Metformin ameliorates insulin resistance in L6 rat skeletal muscle cells through upregulation of SIRT3. Chin Med J (Engl) 2014;127:1523–1529. [PubMed] [Google Scholar]
- Khanjani S, Kandola MK, Lindstrom TM, Sooranna SR, Melchionda M, Lee YS, Terzidou V, Johnson MR, Bennett PR. NF-κB regulates a cassette of immune/inflammatory genes in human pregnant myometrium at term. J Cell Mol Med. 2011;15:809–824. doi: 10.1111/j.1582-4934.2010.01069.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lappas M. Cellular inhibitors of apoptosis proteins cIAP1 and cIAP2 are increased after labour in foetal membranes and myometrium and are essential for TNF-alpha-induced expression of pro-labour mediators. Am J Reprod Immunol. 2015;73:313–329. doi: 10.1111/aji.12295. [DOI] [PubMed] [Google Scholar]
- Lappas M. RAF1 is increased in labouring myometrium and modulates inflammation-induced pro-labour mediators. Reproduction. 2016;151:411–420. doi: 10.1530/REP-15-0607. [DOI] [PubMed] [Google Scholar]
- Yang D, Elner SG, Bian ZM, Till GO, Petty HR, Elner VA. Pro-inflammatory cytokines increase reactive oxygen species through mitochondria and NADPH oxidase in cultured RPE cells. Exp Eye Res. 2007;85:462–472. doi: 10.1016/j.exer.2007.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lappas M, Permezel M, Rice GE. N-Acetyl-cysteine inhibits phospholipid metabolism, proinflammatory cytokine release, protease activity, and nuclear factor-kappaB deoxyribonucleic acid-binding activity in human fetal membranes in vitro. J Clin Endocrinol Metab. 2003;88:1723–1729. doi: 10.1210/jc.2002-021677. [DOI] [PubMed] [Google Scholar]