Abstract
Endometriosis causes severe chronic pelvic pain and infertility. Because the standard medication and surgical treatments of endometriosis show high recurrence of symptoms, it is necessary to improve the current treatment options. In the initial study, we examined whether niclosamide can be a useful drug for treating endometriosis in a preclinical setting. Endometriotic implants were induced using an established mouse model involving transimplantation of mouse endometrial fragments to the peritoneal wall of recipient mice. When the recipient mice were treated with niclosamide for 3 wk, niclosamide reduced the size of endometriotic implants with inhibition of cell proliferation and inflammatory signaling, including RELA (NFKB) and STAT3 activation, but did not alter expression of steroid hormone receptors. To identify genes whose expression is regulated by niclosamide in endometriotic implants, RNA-sequencing was performed, and several genes downregulated by niclosamide were related to inflammatory responses, WNT, and MAPK signaling. In a second study designed to assess whether niclosamide affects reproductive function, the recipient mice started receiving niclosamide after the induction of endometriosis. Then, the recipient mice were mated with wild-type males, and treatments continued until the pups were born. Niclosamide-treated recipient mice became pregnant and produced normal size and number of pups. These results suggest that niclosamide could be an effective therapeutic drug and acts as an inhibitor of inflammatory signaling without disrupting normal reproductive function.
Keywords: endometriosis, inflammation, mouse model, niclosamide, reproductive function
INTRODUCTION
Endometriosis affects 6%–10% of women of reproductive age [1]. Although endometriosis is a benign disorder, approximately 50% of affected women experience severe chronic pelvic pain and/or infertility [2, 3]. Long-term treatment of patients with chronic pelvic pain associated with endometriosis involves repeated courses of therapy: surgical, medical, or both [4]. However, current strategies for the treatment of endometriosis only temporarily relieve the symptoms of the disease. Laparoscopic surgery provides temporary pain relief, but the recurrence rate is conservatively estimated to be 50% after 5 yr [5, 6]. The most widely used medical drugs are oral contraceptives, GnRH agonists, and progestins, which suppress ovarian function and reduce pelvic disease and associated pain [1, 7]. However, GnRH agonist therapy carries a significant risk of bone loss due to the induced hypoestrogenic state [8] and has a 50% or higher rate of recurrence of symptoms [9, 10]. Progesterone resistance commonly arises as a major complication to progestin therapy, leading to escalation of estrogen function [11]. Although hysterectomy with oophorectomy may be the best treatment, it elicits irreversible fertility loss. Therefore, it is important to identify therapeutic targets and efficient drugs that are improvements over current treatment options.
Endometriosis is defined as the presence of endometrium-like tissue, consisting of proliferating endometrial glands and stroma outside the uterine cavity, primarily on the pelvic peritoneum and ovaries [7, 12]. Major molecular distinctions in endometriotic lesions are overproduction of estrogen, prostaglandins, and cytokines [13–16]. Estrogen enhances the survival and persistence of endometriotic lesions, whereas prostaglandins and cytokines mediate pain, inflammation, and infertility [7, 12–16]. Remarkably, increased macrophage, prostaglandin, cytokine, and chemokine contents have been found in the peritoneal fluid from endometriosis patients [16–24]. This milieu of cytokines and growth factors creates a microenvironment that encourages endometrial cell attachment, invasion, and vasculogenesis [16, 22–25]. Chemokines play a major role in the recruitment of macrophages to the site of endometrial tissue engraftment in the peritoneal cavity, a critical step for endometriotic growth and progression [26, 27]. Thus, the inflammatory environment further enhances inflammation and consequently promotes endometriotic cell survival and growth [26–28].
Niclosamide is an efficacious, minimally toxic and Food and Drug Administration (FDA)-approved antihelminth drug that has been used in patients for decades [29, 30]. The antiparasitic activity of niclosamide was originally reported to be mediated by inhibition of mitochondrial oxidative phosphorylation and anaerobic ATP production [31]. Recently, several groups, including ours, have reported that niclosamide disrupts multiple signaling pathways, including NFĸB, STAT3, and WNT signaling in a variety of cancer models [32–41]. Thus, we hypothesized that niclosamide could be an inhibitor of endometriosis progression by blocking these signaling pathways.
In the present study, we report that niclosamide reduces the size of endometriotic implants in a mouse model of endometriosis by targeting inflammatory mechanisms. Furthermore, we demonstrate that the treatment with niclosamide does not disrupt reproductive function in female mice.
MATERIALS AND METHODS
Animals
Mice were maintained in the vivarium at Southern Illinois University according to the National Institutes of Health guidelines for the care and use of laboratory animals (Assurance A3078-01). Tg (UBC-GFP)30Scha (Strain of Origin: C57BL/6, also known as B6-GFP, Jax 004353) and C57BL/6 (also known as B6, Jax 000664) mice were obtained from the Jackson Laboratory. The genotypes of B6-GFP mice were determined by PCR analysis of tail genomic DNA as previously described [42].
Mouse Model of Endometriosis and Experimental Design
An experimental mouse model of endometriosis was established adopting procedures described previously with some modification [43]. Briefly, the uterine horns were removed from 8-wk-old female B6-GFP mice (donor) during the diestrus stage of the reproductive cycle. Both horns were opened longitudinally and cut into a total of four tissue pieces (two sets of each diameter punch) using 2-mm and 3-mm dermal biopsy punches (15111-20 for 2 mm and 15111-30 for 3 mm, the initial average implant volume with four tissue pieces being 4.95 ± 0.37 mm3; Ted Pella, Inc.). Then, the uterine pieces were maintained in warmed DMEM/F12 (10-090; Corning). As recipient mice, B6 mice (8 wk old) were selected during the diestrus stage and anesthetized. A longitudinal abdominal incision was made, and uterine pieces from donor mice were sutured to the right or left side of peritoneal walls (each 2-mm and 3-mm piece/one side) using a 6-0 braided silk suture (SUT-1073-11; Roboz). Then, the abdominal incision was closed with a 4-0 braided silk suture (SUT-1073-31; Roboz).
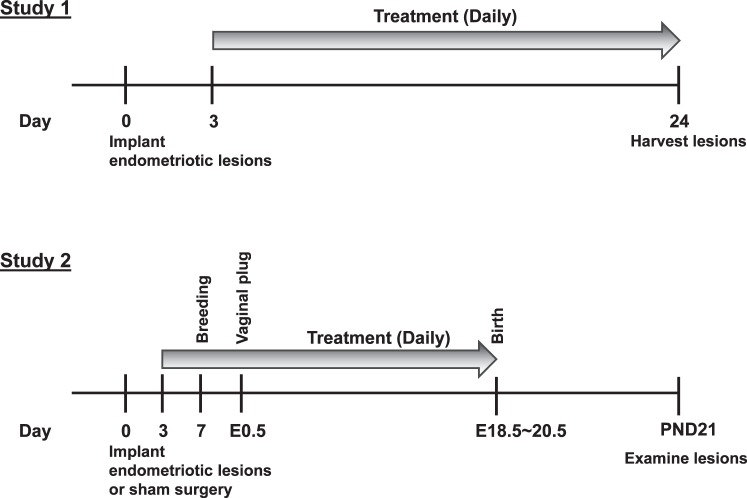
In study 1, we first examined inhibitory effects of niclosamide on endometriotic implants. After 3 days recovery from the transimplantation surgery, recipient mice orally received niclosamide (N3510; Sigma-Aldrich) at a dose of 0 (n = 8), 100 (n = 5), or 200 (n = 10) mg/kg b.w./day (where b.w. is body weight) for 3 wk (Fig. 1). For oral administration, niclosamide was mixed in gelatin (Knox) with artificial flavors (Sweetener, Splenda, and Berry Pomegranate). Note that the mice were generally housed four per cage, but during the treatment, the mice were placed individually in a cage devoid of bedding and provided the gelatin mix. After a few days of training, more than 95% of mice ate their complete dosage of niclosamide (∼150 mm3 gelatin) within 30 min. The mice that did not completely eat the gelatin were eliminated from the studies. After 3 wk of treatment, the recipient mice in the diestrus stage were necropsied, and the endometriotic implants were distinguished under the Fluorescence Stereo Microscope (Leica) and collected for further analysis. The total weight and growth including four implants were recorded. The implant volume was calculated according to the formula (L X W2), where L is length and W is width measured by a digital caliper (VWR) [44, 45].
FIG. 1.
Experimental design of studies 1 and 2. Legend: E, embryonic day; PND, postnatal day.
In study 2, we next determined whether there is an effect of niclosamide treatment on reproductive functions. Endometriotic implants were induced in female mice (total 18 mice) as described in study 1. Then, mice were randomly assigned for control (n = 11) or niclosamide (n = 7) group. Sham surgeries were performed in female mice (n = 5) following the same steps as the endometriosis surgery except that no donor tissues were implanted to the peritoneal walls (only sutures). After 3 days of surgical recovery, recipient mice started receiving niclosamide orally at a dose of 0 or 200 mg/kg b.w./day (Fig. 1). The recipient mice continuously received the treatment throughout pregnancy until the pups were born. Seven days after the surgery, the recipient mice started mating with B6 male mice, and the time of a plug was recorded as Embryonic Day 0.5. Finally, the recipient mice were necropsied when the pups were weaned on Postnatal Day (PND) 21. Gestational length, number of pups, and pup weight at birth and on PND 21, as well as implant volume. were calculated.
Immunohistochemical and Terminal Deoxynucleotidyl Transferase dUTP Nick End Labeling Analyses
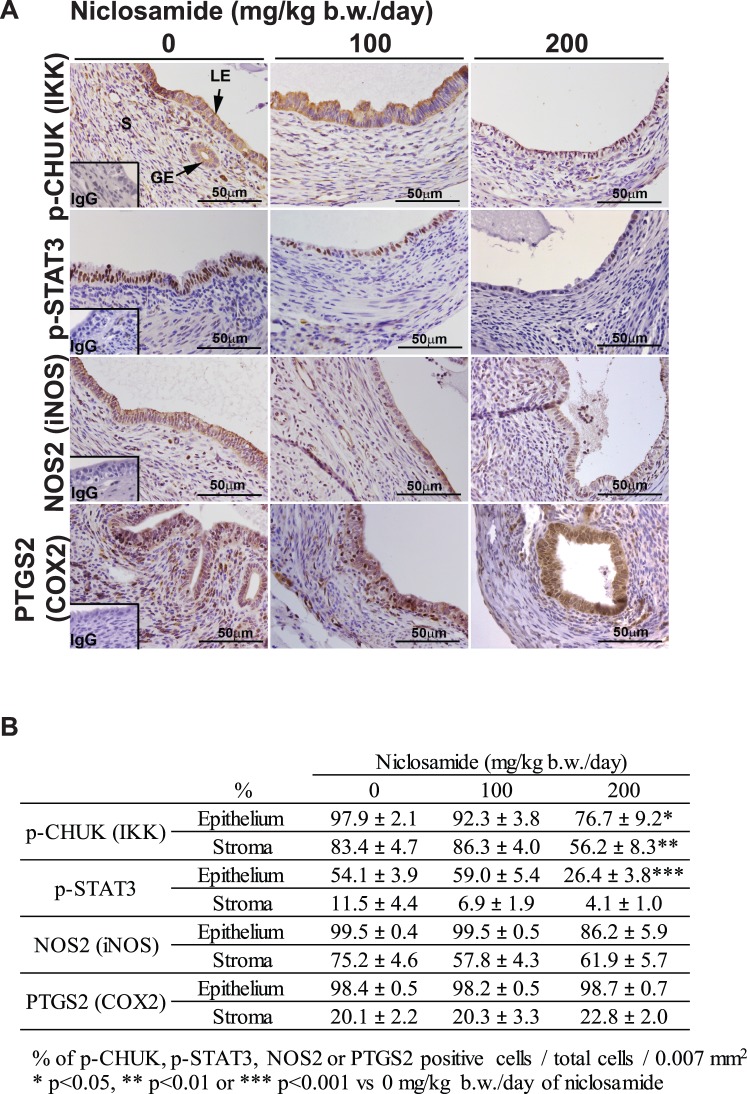
Immunolocalization of MKI67 (Ki67), PECAM1 (CD31), ESR1, PGR, p-CHUK (IKK), p-STAT3, NOS2 (iNOS), and PTGS2 (COX2) was determined in cross sections (5 μm) of paraffin-embedded implant sections using specific primary antibodies and Vectastain Elite ABC Kit (PK-6101), Mouse on Mouse Basic Kit (BMK-2202; Vector laboratories), or DyLight-conjugated secondary antibody (711-516-152; Jackson ImmunoResearch Lab). Antibodies used in these analyses were anti-MKI67 (Ki67, 1.25 μg/ml, 550609; BD Biosciences), anti-PECAM1 (CD31, 1:100 dilution, ab28364; Abcam), anti-ESR1 (1 μg/ml, sc-542; Santa Cruz Biotechnology), anti-PGR (1 μg/ml, RB-9017-P0; Thermo Scientific), anti-p-CHUK (IKK, 1:150 dilution, 2697; Cell Signaling Technology), anti-p-STAT3 (1:50 dilution, 9145; Cell Signaling Technology), anti-NOS2 (iNOS, 5 μg/ml, 610333; BD Biosciences), and anti-PTGS2 (COX2, 1:50 dilution, RM-9121; Thermo Scientific). The terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) assay was performed according to manufacturer's instructions using ApopTag Fluorescein In Situ Apoptosis Detection Kit (S7160; Millipore). Cell-specific MKI67, ESR1, PGR, p-CHUK, p-STAT3, NOS2, and PTGS2 positive and total cell number of either epithelial or stromal cells were counted in an area of 0.007 mm2 (three different areas from each section and four different implants), and the percentage (positive cells/total cells) was semiquantitatively analyzed. Cell-specific TUNEL- and PECAM1-positive cells were counted in the area of 0.02 mm2 (three different areas from each section and four different implants) and semiquantitatively analyzed.
RNA Sequencing and Quantitative PCR Analyses
For RNA sequencing (RNA-seq), total RNA was isolated from the implants collected from the recipient mice in study 1 at a dose of 0 (n = 3) or 200 (n = 3) mg/kg b.w. of niclosamide using the RNeasy mini kit (74104; Qiagen). Note that one of the control samples was pooled from four different recipient mice due to limited amount of RNA, while all the other samples were from a single mouse. RNA quality was assessed, and the samples were sent to the Carver Biotechnology Center at the University of Illinois for sequencing in the High-Throughput Sequencing and Genotyping Unit. Briefly, the stranded RNA-seq libraries were prepared with Illumina's TruSeq Stranded RNA Sample Prep kit (RS-122-2201; Illumina) using 1 μg total RNA. The libraries were pooled in equimolar concentration, and the pool was quantitated by quantitative PCR (qPCR) and sequenced on one lane for 101 cycles on a HiSeq2500 (Illumina) using a HiSeq SBS sequencing kit (FC-401-4002; Illumina). Fastq files were generated and demultiplexed with the bcl2fastq v1.8.4 Conversion Software (Illumina).
The data were then sent to the High-Performance Biological Computing Group of the Carver Biotechnology Center for bioinformatics and statistical analysis. Each sample's fastq file was run through Trimmomatic 0.33 to first remove any remaining standard Illumina TruSeq SE v3 adapters, then to trim bases from both ends with quality scores below 28, and finally to remove reads shorter than 30 bp (parameters ILLUMINACLIP:/home/apps/trimmomatic/trimmomatic-0.33/adapters/TruSeq3-SE.fa:2:15:10 LEADING:28 TRAILING:28 MINLEN:30). Each sample was then aligned to the National Center for Biotechnology Information (NCBI) GRCm38.p3 genome using STAR 2.4.2a with gene models ref_GRCm38.p3_top_level.gff3.gz (NCBI Mus musculus Annotation Release 105) and parameter –sjdbGTFtagExonParentGene gene. Read counts for each gene were generated using featureCounts (from subread v 1.4.6-p4) with parameters -s 2 -g gene -t exon.
The raw counts (27.8–34.8 million per sample) were input into R [46] 3.2.2, and genes that did not have at least one count per million reads in at least three samples, regardless of treatment, were deemed unreliable and filtered out; 15 220 of the 41 786 genes passed this filter and were analyzed using edgeR [47] v 3.12.0. A negative binomial generalized linear model [48] was used, which included tagwise dispersion estimates, trimmed mean of M component normalization factors, and coefficients of treatment effect and body weight as a covariate (the weight of the pooled sample was calculated from a weighted mean of the individuals, based on amount of input RNA). Multiple hypothesis test correction for the treatment effect was done using the false discovery rate (FDR) [49] method.
Bioinformatics analysis was performed using the database for annotation, visualization, and integrated discovery (DAVID) for a total of 199 genes (which exhibited an FDR of less than 0.1), and gene set enrichment analysis (GSEA) to identify additional genetic pathways affected by niclosamide treatment, as we previously performed [50]. Briefly, to examine genomewide expression profiles, the publicly available GSEA software package (www.broad.mit.edu) was used for leading edge analysis to determine whether the members of the identified gene ontology pathways were randomly distributed throughout the ranked gene list or concentrated at the top or bottom. A preranked list of 15 220 unique native RNA-seq features was prepared using the limma (3.10.2) BioConductor package. These sequences collapsed to 10 570 unique gene signatures present within the curated gene sets that were examined. The recommended GSEA metrics for ranking genes were employed, such that significant negative fold changes had the lowest ranks (i.e., they cluster at the na_neg end of the leading edge enrichment plots) and significant, positive fold changes received the highest ranks. Pathway statistics were evaluated by GSEA using the default parameter settings, and gene sets were defined as significantly enriched if the FDR q-value was <0.2 when using Pearson metrics and 1000 permutations of gene sets. Lastly, selected genes identified from the above analyses were tested by qPCR as described previously [51]. Primer sequences were determined using NCBI's design tools and are provided in Supplemental Table S1 (Supplemental Data are available online at www.biolreprod.org).
Statistical Analysis
Data were analyzed using Prism software (version 5.0; GraphPad). All experimental data are presented as mean with standard error of the mean (SEM). For the analyses (lesion growth, fertility assessment, and semiquantitative immunohistochemistry scoring), involving three groups, one-way ANOVA and Tukey multiple-comparison posttest was used to identify differences between individual means. All data met necessary criteria for ANOVA analysis including equal variance as determined by Bartlett test. The qPCR data was analyzed by two-tailed Student t-test (comparing between 0 and 200 mg/kg b.w. niclosamide treatment) with a minimum of eight biological replicates per treatment. F-test was used to determine whether two groups possessed equal variances. Unless otherwise indicated, a P value less than 0.05 was considered to be statistically significant.
RESULTS
Niclosamide Inhibits Growth of Endometriotic Implants
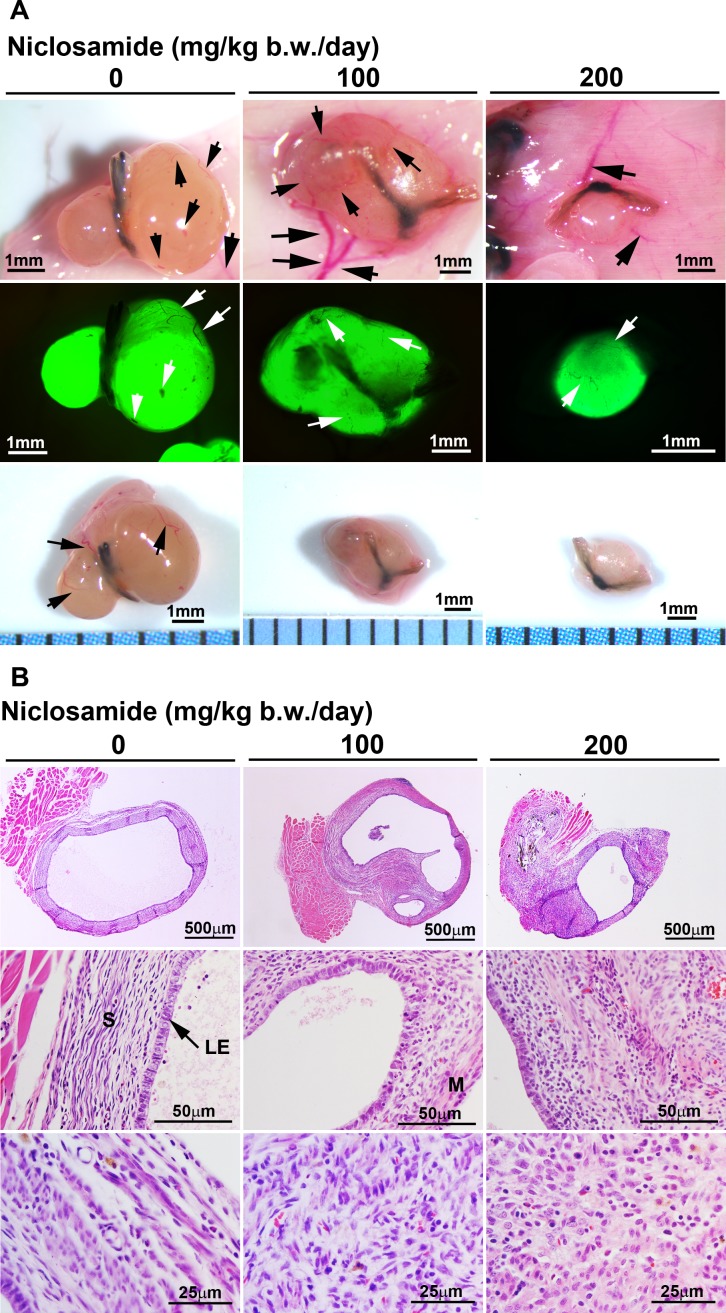
To investigate the effect of niclosamide on the growth of endometriosis, we surgically induced endometrioticlike lesions on the peritoneal wall of mice. We implanted uterine tissue pieces of B6-GFP mice (donor) to the same genetic background of recipient mice (B6) because ectopic GFP-positive implants could be microscopically examined to evaluate the implants for accurate growth. Figure 2A shows peritoneal vessels that surround the endometriotic implants (arrows in Fig. 2A). We were clearly able to distinguish GFP-positive endometriotic implants using the fluorescence microscope. Histological analysis showed that intact epithelial, stromal and some myometrial cells of the endometriotic implants were observed in control and niclosamide-treated mice (Fig. 2B).
FIG. 2.
Assessment of the endometriotic implants. A) Morphology of the endometriotic implants was microscopically examined after 3 wk of treatment at doses of 0 (n = 8), 100 (n = 5), or 200 (n = 10) mg/kg b.w./day of niclosamide. Upper panels: sutured endometriotic implants were vascularized in the recipient mice. Middle panels: GFP-positive implants were observed under the fluorescence light. Bottom panels: the endometriotic implants were isolated from the recipient mice. Black and white arrows show blood vessels. B) Histology of the endometriotic implants is shown. Tissues were stained using hematoxylin and eosin. Legend: LE, luminal epithelium; M, myometrium; S, stroma.
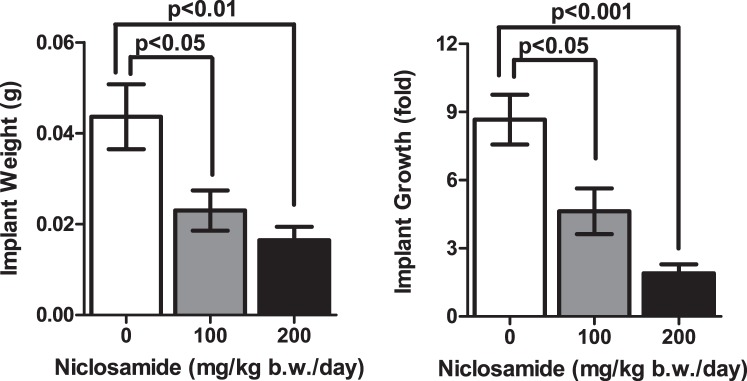
After 3 wk of treatment with niclosamide or control vehicle, all mice appeared healthy with no obvious adverse effects including weight loss (data not shown). Estrous cyclicity was observed in all mice by vaginal cytology, and the samples were collected at the diestrus stage. When the mice were necropsied, we observed a significant difference in the pattern of growth of endometriotic implants (Fig. 3). Niclosamide-treated mice at a dose of 100 mg/kg b.w./day showed a significant reduction of implant weight (0.023 ± 0.004 g) and growth (4.63 ± 1.00 fold from initial implant size) compared to controls (implant weight: 0.044 ± 0.007 g, and growth: 8.66 ± 1.10 fold). Niclosamide-treated mice at a dose of 200 mg/kg b.w./day also had reduced implant weight (0.016 ± 0.003 g) and growth (1.90 ± 0.40 fold) compared to controls. While we did not see any significant differences between 100 and 200 mg/kg b.w. niclosamide treatment, P values were smaller in the 200 mg/kg b.w. treatment.
FIG. 3.
The effect of niclosamide on the growth of endometriotic implants. The implant weight and growth (fold increase over initial implant size) were examined after 3 wk of treatment at doses of 0 (n = 8), 100 (n = 5), or 200 (n = 10) mg/kg b.w./day of niclosamide.
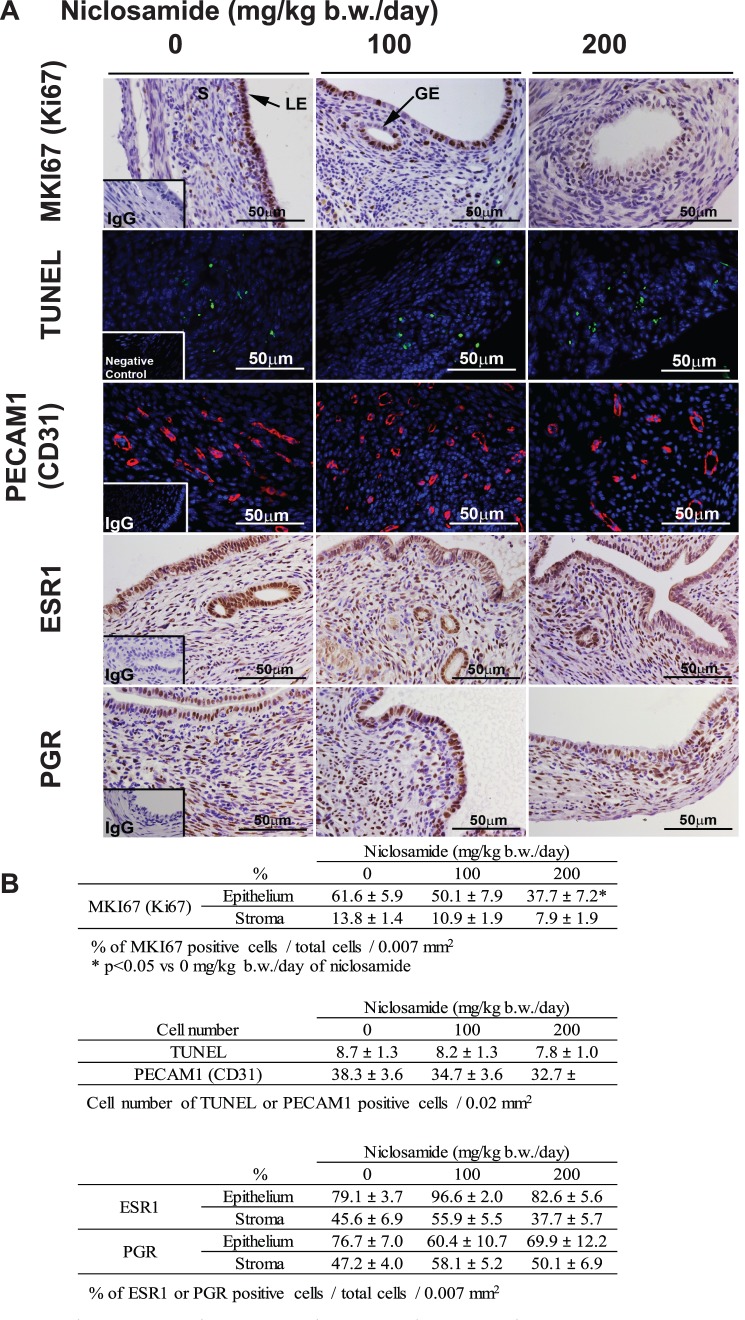
Next, to determine whether reduced implant sizes after treatment with niclosamide resulted from alterations in cell proliferation, angiogenesis, and/or apoptosis, we performed immunohistochemical analysis of MKI67 (Ki67) and PECAM1 (CD31) as well as the TUNEL assay (Fig. 4A). Immunohistochemical and TUNEL analyses were semiquantitatively scored by counting of positively stained cells and/or total cells (Fig. 4B). The majority of the cells, especially epithelial cells, were MKI67 positive in the control implants. However, in agreement with the reduction of growth of endometriotic implants, fewer cells were MKI67 positive in the implants of niclosamide-treated mice (Fig. 4A). Treatment with niclosamide at a dose of 200 mg/kg b.w./day significantly reduced epithelial cell proliferation to 37.7% ± 7.2 % compared with 61.6% ± 5.9 % of control implants, but there was no significant difference by a dose of 100 mg/kg b.w./day niclosamide treatment (50.1% ± 7.9 %). We did not observe any differences in stromal cell proliferation, TUNEL-positive cells, and PECAM1 staining in any endometriotic implants. Furthermore, epithelial and stromal ESR1 and PGR were positive in the endometriotic implants, and there were no differences between control and niclosamide-treated mice (Fig. 4), suggesting that niclosamide does not affect steroid hormone signaling.
FIG. 4.
The effect of niclosamide on proliferation, apoptosis, angiogenesis, and steroid hormone receptors in the endometriotic implants. A) MKI67 (Ki67), ESR1, and PGR were detected by immunohistochemistry. Cellular apoptosis was determined by TUNEL analysis. Endothelial cell marker, PECAM1 (CD31) was detected by immunofluorescence. Legend: GE, glandular epithelium; LE, luminal epithelium; S, stroma. B) Immunoreactive MKI67, ESR1, and PGR were semiquantitatively analyzed by counting of positive and total cell number in the epithelial and stromal cells, and the percentages of positive cells/total cells are shown. Cell number of TUNEL- and PECAM1-positive cells is shown.
Downregulation of Inflammatory Signaling by Niclosamide
The importance of inflammatory signaling in the development of endometriosis has been documented [12, 16]. Niclosamide has been shown to target NFĸB and STAT3 signaling in cancer cells [32–35, 37, 38]. Therefore, activation of CHUK and STAT3 as well as RELA (NFKB) downstream molecules NOS2 (iNOS) and PTGS2 (COX2) were examined (Fig. 5). Immunoreactive p-CHUK was significantly reduced in the epithelial and stromal endometriotic implants by niclosamide treatment at a dose of 200 mg/kg b.w./day. This dose of niclosamide treatment significantly decreased STAT3 activity in the epithelial cells of endometriotic implants. However, NOS2 and PTGS2 were not affected by niclosamide.
FIG. 5.
The effect of niclosamide on inflammatory signaling in the endometriotic implants. A) Immunoreactive p-CHUK (IKK), p-STAT3, NOS2 (iNOS), and PTGS2 (COX2) were detected. Legend: GE, glandular epithelium; LE, luminal epithelium; S, stroma. B) Immunoreactive p-CHUK, p-STAT3, NOS2, and PTGS2 were semiquantitatively analyzed by counting of positive and total cell number in the epithelial and stromal cells, and the percentages of positive cells/total cells are shown.
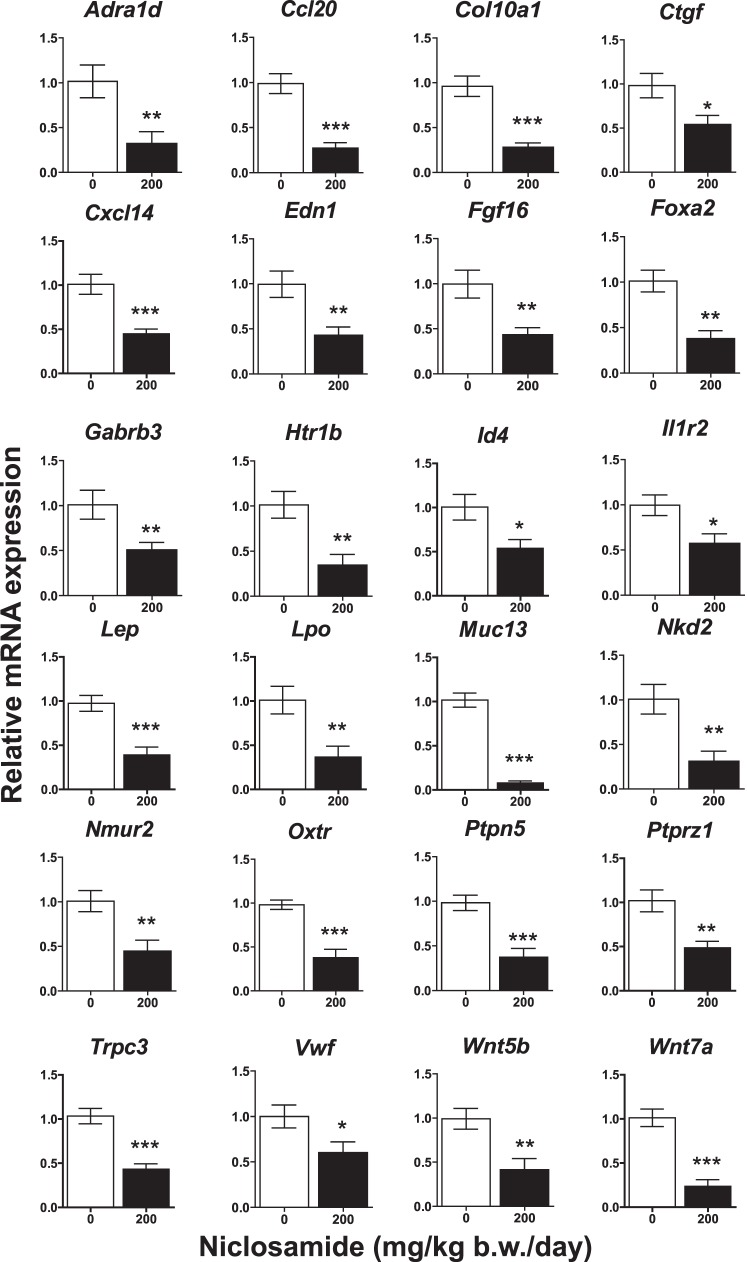
While our results suggest that reduction of growth of endometriotic implants by niclosamide are brought about through inhibition of inflammatory mechanisms, we performed RNA-seq in order to further identify genes whose expression is regulated by niclosamide in endometriotic implants. We have deposited this data in the GEO database (GSE79698). Because a dose of 200 mg/kg b.w./day was more effective at reducing the size of the implants, transcriptional alteration on implants from mice treated with control or a 200 mg/kg b.w./day niclosamide (n = 3 each treatment) for 3 wk were compared. A total of 15 220 genes (>1 count per million reads) were identified by RNA-seq. A total of 199 genes exhibiting differential expression (FDR less than 0.1) were classified by functional annotation using DAVID analysis [52, 53]. These 199 genes were categorized into groups of cell-cell signaling, immune system process, immune response, and extracellular matrix (Supplemental Table S2). Differentially expressed transcripts were further confirmed by qPCR (n = 8), and a summary of representative genes is provided in Figure 6. These results highlighted that genes related to inflammation (Ccl20, Cxcl14, Il1r2, Lep, Lpo, Muc13, Trpc3, and Ptprz1) were significantly decreased in the endometriotic implants after niclosamide treatment. Additionally, Ctgf and Col10a1 (extracellular matrix) as well as Adra1d, Edn1, Foxa2, Fgf16, Gabrb3, Htr1b, Id4, Nkd2, Nmur2, Oxtr, Ptpn5, Vwf, Wnt5b, and Wnt7a were also reduced in treated implants.
FIG. 6.
The effect of niclosamide on gene transcripts. Genes identified by RNA-seq were analyzed by qPCR. The results were normalized against Rpl19. *P < 0.05, **P < 0.01, or ***P < 0.001 versus control (0 mg/kg b.w./day of niclosamide).
To extend the bioinformatics analysis beyond these 199 genes, a list of all detected genes was prepared, ranked according to significance and fold of differential expression, and subjected to GSEA (a total of 10 570 genes). The results from GO, KEGG, Biocarta, and Reactome analyses were pooled, and 30 enriched pathways downregulated in response to niclosamide were identified (Supplemental Table S3). Enriched pathways upregulated in response to niclosamide were identified, but none held up to additional scrutiny and were precluded from further analysis (data not shown). In Supplemental Figure S1, these results highlighted downregulation in WNT signaling (Wnt7a, Wnt5b, and Nkd2) as a route for niclosamide action, and by association melanogenesis (Wnt7a, Edn1, and Wnt5b). Interestingly, two novel pathways—MAPK signaling (Ptpn5, Fgf16, and Il1r2) and neuroactive ligand receptor interaction (Htr1b, Lep, Gabrb3, Adra1d, Nmur2, and Oxtr)—were identified as significantly enriched among the misregulated genes. In agreement with DAVID analysis, the extracellular matrix was identified as a niclosamide-responsive pathway as exhibited by downregulation of Col10a1 and Ctgf. Note that gene sets related to inflammation were not flagged in the leading edge analysis because they were evenly distributed across the ranked list and not clustered at the downregulated end of the correlation with respect to changes in transcription.
Niclosamide Does Not Affect Reproductive Function
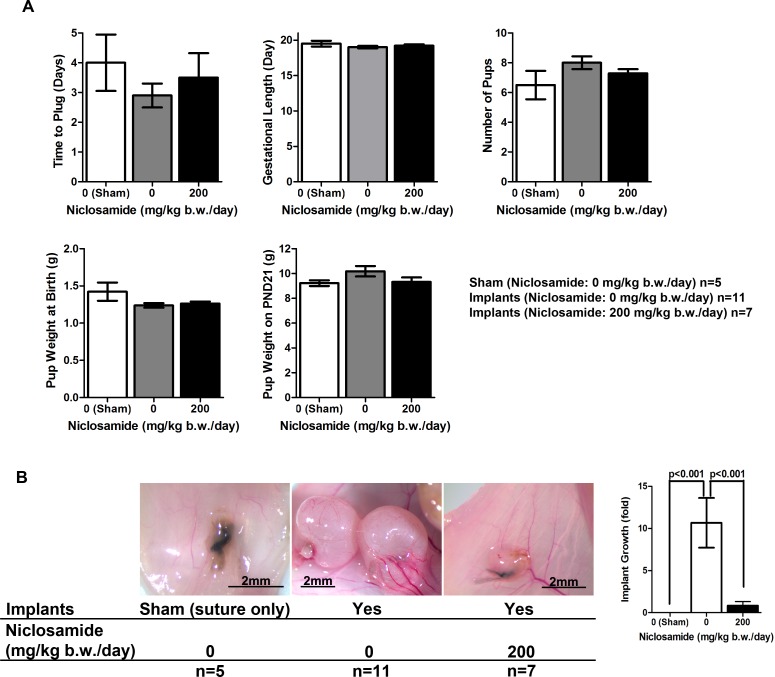
Currently, the most widely used drugs (GnRH agonists and progestins) suppress and/or disrupt normal ovarian function. Therefore, we sought to determine whether niclosamide affected reproductive function in mice (Fig. 7). As described in Figure 1, the mice received control or niclosamide (200 mg/kg b.w./day) beginning 3 days after the either sham or implantation surgeries until pups were born. During the treatment, the mice were bred with wild-type B6 males, and vaginal plugs were determined. Most of the mice had plugs 1–4 days after breeding. No significant differences in the time to receive vaginal plug were observed between the groups. Furthermore, all mice exposed to niclosamide became pregnant and gave birth. Niclosamide treatment did not cause any alterations of gestational length, number of pups, and weight of the pups at birth and on PND 21. These results suggest that niclosamide does not disturb important uterine functions including implantation, maintenance of pregnancy, fetal growth, and parturition. Lastly, when the endometriotic implants were examined on PND 21, the treatment of niclosamide maintained the reduction of implant sizes even though they were examined 3 wk after the final dosage of niclosamide was administered (Fig. 7B).
FIG. 7.
The effect of niclosamide on reproductive functions. Following transimplantation or sham surgeries, mice received daily treatment of either 0 or 200 mg/kg b.w. niclosamide, and were subsequently mated with B6 male mice. The treatments were continued until the pups were born, and the recipient mice were necropsied when the pups were weaned on Postnatal Day (PND) 21. A) Time of plug, gestational length, number of pups, and pup weight at birth and on PND 21 were examined. B) Morphology and volume of the implants were determined on PND 21.
DISCUSSION
Despite the large number of women who suffer from severe chronic pain and infertility related to endometriosis, current treatments temporarily relieve the symptoms of the disease but abolish fertility. In the present study, we report that the FDA-approved small molecule, niclosamide, inhibits the growth of endometriotic implants using an established mouse model [43]. Niclosamide has been orally administered for the treatment of intestinal helminthic infections. One of the features of niclosamide is low toxicity as shown when it was evaluated by World Health Organization (WHO) and Food and Agriculture Organization of the United Nations (FAO) in 1988, and published in Data Sheet on Pesticides, No. 63, Niclosamide (WHO/VBC/DS/88.63) [54]. The toxicity kinetics of niclosamide administered orally in rats for 4 wk elicits no adverse effects up to 2000 mg/kg daily. Similarly, niclosamide treatment in dogs is safe at doses up to 4500–6000 mg/day for 4 wk. No signs of intoxication have been observed in humans treated at 1000 mg/day. However, it has been reported that nausea and abdominal pain occurs in only 10% of human patients following an oral dosage of 2000 mg/day. Acute toxicity in mice is reported as median lethal dose ≥1500 mg/kg b.w. Thus, the dosages (maximum 200 mg/kg b.w.) used in our study, that were effective for implant reduction, were much lower than the reported median lethal dose of acute toxicity in mice. Indeed, daily administration of niclosamide at a dose of 200 mg/kg b.w. for 3 wk in study 1 to 4 wk (average) in study 2 did not cause any adverse reactions, such as weight loss or changes in behavior in the recipient mice. Previous analyses of niclosamide toxicity have ignored the potential impact on reproductive function. We found that mice receiving niclosamide treatment exhibited normal estrous cyclicity, and all mice could successfully conceive. Furthermore, treatment with niclosamide did not induce preterm birth or impact fetal development, and normal postnatal growth curves were observed. These results suggest that niclosamide does not directly cause any toxic effects, and effective doses do not disrupt critical reproductive functions, whereas complete toxicology studies need to be done before any clinical trials. Nevertheless, niclosamide maintained the reduction of size of endometriotic implants. Thus, drug repurposing of niclosamide could be a rapidly distributed, potential therapy without major side effects for the treatment of endometriosis patients.
In support of niclosamide's ability to reduce implant growth, niclosamide decreased cell proliferation. One of the key features in endometriosis is the overproduction of estrogen, which can subsequently accelerate the growth of endometriotic lesions [12, 16]. While systemic estrogen can be a player for endometriosis, local estrogen production by aromatase and development of an inflammatory environment in the presence of prostaglandins and cytokines are hallmarks of the progression of endometriosis [7, 12, 16, 55]. The present study showed that niclosamide did not affect steroid hormone receptors in the endometriotic implants, suggesting that niclosamide does not inhibit local estrogen function in the endometriotic implants or systemic estrogen function in the ovary.
Cytokines and growth factors that have been implicated in proinflammatory environment in endometriosis are upregulated by NFĸB signaling [28, 56]. Aberrant STAT3 activation enhances the etiology of endometriosis [57, 58], and its activation is synergistically increased when endometrial stromal cells are cocultured with macrophages while the inflammatory environment is developing [59]. Similarly, NFĸB activation is also increased via modulation of cytokines and growth factors [28, 56]. Niclosamide suppresses abnormal cellular processes by targeting RELA (NFKB) and STAT3 signaling in cancer cells [32–35, 37, 38]. In the present study, we also report that niclosamide is effective at suppressing the activation of RELA (NFKB) and STAT3 signaling in the endometriotic implants. Our transcriptional profiling also indicated that several genes regulated by niclosamide were linked to inflammatory responses. Lep, a proinflammatory cytokine, Ccl20, a chemokine ligand, and Muc13, a transmembrane mucin glycoprotein, were significantly downregulated in the endometriotic implants by niclosamide treatment. It has been reported that abundant LEP (leptin) levels are observed in serum and peritoneal fluid from endometriosis patients and endometriotic tissues [60–64]. LEP promotes proliferation, migration, and invasion in endometriotic cells through JAK2/STAT3 signaling [65, 66]. Ablation of LEP signaling disrupts endometriotic growth in mouse model [67]. MUC13 is also known to promote RELA (NFKB) activity and further enhances epithelial cell inflammation [68]. CCL20 and its receptor CCR6 system stimulated by cytokines are involved in the migration of Th17 cells to endometriotic tissues [69]. On the other hand, niclosamide did not inhibit PTGS2 (COX2) expression in the endometriotic implants. Overproduction of prostaglandins (PG) via PTGS2 is also a feature of endometriosis [16]. High levels of local estrogen and PGE2 are maintained in endometriotic lesions by positive-feedback mechanisms: PGE2 activates steroidogenic proteins and SYP19A1 (aromatase), leading to local estrogen biosynthesis [55], and estrogen induces PTGS2, which in turn stimulates PGE2 production [12]. The inhibitory mechanisms of niclosamide did not target estrogen and prostaglandin production and function. While niclosamide might not directly inhibit hormone action, niclosamide is able to effectively disrupt the inflammatory environment. In addition, niclosamide alters the behavior of endometriotic cells through modulation of the RELA (NFKB) and STAT3 signaling pathways, including expression of their associated downstream target genes. When we extend the bioinformatics analysis using GSEA, we found several interesting pathways including WNT and MAPK signaling and extracellular matrix. Although several downregulated transcripts were identified in endometriotic implants, the precise mechanism of inhibition of growth of endometriosis and the inflammatory environment as well as other signaling pathways by niclosamide remains to be investigated.
Collectively, the results of the present study indicate that niclosamide could be an effective therapeutic drug for endometriosis and acts as an inhibitor of inflammatory signaling without disrupting normal reproductive function. Niclosamide is an FDA-approved drug with a favorable safety profile. If niclosamide continues to hold promise in further preclinical studies, repurposing may ultimately prove to have a tremendous impact on endometriosis patients in the clinical setting. However, our study is not able to confirm whether fertility defects were rescued by niclosamide because surgically induced endometriosis mouse model does not clearly affect murine fertility. Further fertility studies in which the effects of niclosamide on entire reproductive functions remain to be investigated.
ACKNOWLEDGMENT
We thank Dr. Alvaro Hernandez and his team at The Roy J. Carver Biotechnology Center, The University of Illinois at Urbana-Champaign. for RNA-seq and Dr. Jenny Drnevich for its statistical analysis. We also thank Dr. Joe Cheatwood for advice and verification of our statistical analyses.
Footnotes
This work was supported by NIH CA179214 and SIU-SOM Concept Development Award (to K.H.). This study was presented in part at the 7th Annual Illinois Symposium on Reproductive Sciences, October 12, 2015, University of Illinois at Urbana-Champaign, Illinois. RNA-seq data deposited in the GEO database (accession no. GSE79698).
REFERENCES
- Giudice LC. Clinical practice. Endometriosis. N Engl J Med. 2010;362:2389–2398. doi: 10.1056/NEJMcp1000274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eskenazi B, Warner ML. Epidemiology of endometriosis. Obstet Gynecol Clin North Am. 1997;24:235–258. doi: 10.1016/s0889-8545(05)70302-8. [DOI] [PubMed] [Google Scholar]
- Meuleman C, Vandenabeele B, Fieuws S, Spiessens C, Timmerman D, D'Hooghe T. High prevalence of endometriosis in infertile women with normal ovulation and normospermic partners. Fertil Steril. 2009;92:68–74. doi: 10.1016/j.fertnstert.2008.04.056. [DOI] [PubMed] [Google Scholar]
- Kennedy S, Bergqvist A, Chapron C, D'Hooghe T, Dunselman G, Greb R, Hummelshoj L, Prentice A, Saridogan E. ESHRE guideline for the diagnosis and treatment of endometriosis. Hum Reprod. 2005;20:2698–2704. doi: 10.1093/humrep/dei135. [DOI] [PubMed] [Google Scholar]
- DeCherney AH. Endometriosis: recurrence and retreatment. Clin Ther. 1992;14:766–772. [PubMed] [Google Scholar]
- Evers JL, Dunselman GA, Land JA, Bouckaert PX. Is there a solution for recurrent endometriosis? Br J Clin Pract Suppl. 1991;72:45–50. [PubMed] [Google Scholar]
- Giudice LC, Kao LC. Endometriosis. Lancet. 2004;364:1789–1799. doi: 10.1016/S0140-6736(04)17403-5. [DOI] [PubMed] [Google Scholar]
- Agarwal SK. Impact of six months of GnRH agonist therapy for endometriosis. Is there an age-related effect on bone mineral density? J Reprod Med. 2002;47:530–534. [PubMed] [Google Scholar]
- Practice Committee of American Society for Reproductive Medicine. Treatment of pelvic pain associated with endometriosis. Fertil Steril. 2008;90:S260–S269. doi: 10.1016/j.fertnstert.2008.08.057. [DOI] [PubMed] [Google Scholar]
- Waller KG, Shaw RW. Gonadotropin-releasing hormone analogues for the treatment of endometriosis: long-term follow-up. Fertil Steril. 1993;59:511–515. doi: 10.1016/s0015-0282(16)55791-4. [DOI] [PubMed] [Google Scholar]
- Kim JJ, Kurita T, Bulun SE. Progesterone action in endometrial cancer, endometriosis, uterine fibroids, and breast cancer. Endocr Rev. 2013;34:130–162. doi: 10.1210/er.2012-1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulun SE. Endometriosis. N Engl J Med. 2009;360:268–279. doi: 10.1056/NEJMra0804690. [DOI] [PubMed] [Google Scholar]
- Noble LS, Simpson ER, Johns A, Bulun SE. Aromatase expression in endometriosis. J Clin Endocrinol Metab. 1996;81:174–179. doi: 10.1210/jcem.81.1.8550748. [DOI] [PubMed] [Google Scholar]
- Noble LS, Takayama K, Zeitoun KM, Putman JM, Johns DA, Hinshelwood MM, Agarwal VR, Zhao Y, Carr BR, Bulun SE. Prostaglandin E2 stimulates aromatase expression in endometriosis-derived stromal cells. J Clin Endocrinol Metab. 1997;82:600–606. doi: 10.1210/jcem.82.2.3783. [DOI] [PubMed] [Google Scholar]
- Tseng JF, Ryan IP, Milam TD, Murai JT, Schriock ED, Landers DV, Taylor RN. Interleukin-6 secretion in vitro is up-regulated in ectopic and eutopic endometrial stromal cells from women with endometriosis. J Clin Endocrinol Metab. 1996;81:1118–1122. doi: 10.1210/jcem.81.3.8772585. [DOI] [PubMed] [Google Scholar]
- Burney RO, Giudice LC. Pathogenesis and pathophysiology of endometriosis. Fertil Steril. 2012;98:511–519. doi: 10.1016/j.fertnstert.2012.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunselman GA, Hendrix MG, Bouckaert PX, Evers JL. Functional aspects of peritoneal macrophages in endometriosis of women. J Reprod Fertil. 1988;82:707–710. doi: 10.1530/jrf.0.0820707. [DOI] [PubMed] [Google Scholar]
- Halme J, Becker S, Hammond MG, Raj S. Pelvic macrophages in normal and infertile women: the role of patent tubes. Am J Obstet Gynecol. 1982;142:890–895. doi: 10.1016/s0002-9378(16)32537-6. [DOI] [PubMed] [Google Scholar]
- Halme J, Becker S, Haskill S. Altered maturation and function of peritoneal macrophages: possible role in pathogenesis of endometriosis. Am J Obstet Gynecol. 1987;156:783–789. doi: 10.1016/0002-9378(87)90333-4. [DOI] [PubMed] [Google Scholar]
- Halme J, Becker S, Wing R. Accentuated cyclic activation of peritoneal macrophages in patients with endometriosis. Am J Obstet Gynecol. 1984;148:85–90. doi: 10.1016/s0002-9378(84)80037-x. [DOI] [PubMed] [Google Scholar]
- Haney AF, Muscato JJ, Weinberg JB. Peritoneal fluid cell populations in infertility patients. Fertil Steril. 1981;35:696–698. doi: 10.1016/s0015-0282(16)45567-6. [DOI] [PubMed] [Google Scholar]
- Shifren JL, Tseng JF, Zaloudek CJ, Ryan IP, Meng YG, Ferrara N, Jaffe RB, Taylor RN. Ovarian steroid regulation of vascular endothelial growth factor in the human endometrium: implications for angiogenesis during the menstrual cycle and in the pathogenesis of endometriosis. J Clin Endocrinol Metab. 1996;81:3112–3118. doi: 10.1210/jcem.81.8.8768883. [DOI] [PubMed] [Google Scholar]
- Berkkanoglu M, Arici A. Immunology and endometriosis. Am J Reprod Immunol. 2003;50:48–59. doi: 10.1034/j.1600-0897.2003.00042.x. [DOI] [PubMed] [Google Scholar]
- Rana N, Braun DP, House R, Gebel H, Rotman C, Dmowski WP. Basal and stimulated secretion of cytokines by peritoneal macrophages in women with endometriosis. Fertil Steril. 1996;65:925–930. [PubMed] [Google Scholar]
- Lebovic DI, Mueller MD, Taylor RN. Immunobiology of endometriosis. Fertil Steril. 2001;75:1–10. doi: 10.1016/s0015-0282(00)01630-7. [DOI] [PubMed] [Google Scholar]
- Kaitu'u-Lino TJ, Morison NB, Salamonsen LA. Neutrophil depletion retards endometrial repair in a mouse model. Cell Tissue Res. 2007;328:197–206. doi: 10.1007/s00441-006-0358-2. [DOI] [PubMed] [Google Scholar]
- Capobianco A, Rovere-Querini P. Endometriosis, a disease of the macrophage. Front Immunol. 2013;4:9. doi: 10.3389/fimmu.2013.00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Ramos R, Defrere S, Devoto L. Nuclear factor-kappaB: a main regulator of inflammation and cell survival in endometriosis pathophysiology. Fertil Steril. 2012;98:520–528. doi: 10.1016/j.fertnstert.2012.06.021. [DOI] [PubMed] [Google Scholar]
- Al-Hadiya BM. Niclosamide: comprehensive profile. Profiles Drug Subst Excip Relat Methodol. 2005;32:67–96. doi: 10.1016/S0099-5428(05)32002-8. [DOI] [PubMed] [Google Scholar]
- Andrews P, Thyssen J, Lorke D. The biology and toxicology of molluscicides, Bayluscide. Pharmacol Ther. 1982;19:245–295. doi: 10.1016/0163-7258(82)90064-x. [DOI] [PubMed] [Google Scholar]
- Weinbach EC, Garbus J. Mechanism of action of reagents that uncouple oxidative phosphorylation. Nature. 1969;221:1016–1018. doi: 10.1038/2211016a0. [DOI] [PubMed] [Google Scholar]
- Jin Y, Lu Z, Ding K, Li J, Du X, Chen C, Sun X, Wu Y, Zhou J, Pan J. Antineoplastic mechanisms of niclosamide in acute myelogenous leukemia stem cells: inactivation of the NF-kappaB pathway and generation of reactive oxygen species. Cancer Res. 2010;70:2516–2527. doi: 10.1158/0008-5472.CAN-09-3950. [DOI] [PubMed] [Google Scholar]
- Ketola K, Hilvo M, Hyotylainen T, Vuoristo A, Ruskeepaa AL, Oresic M, Kallioniemi O, Iljin K. Salinomycin inhibits prostate cancer growth and migration via induction of oxidative stress. Br J Cancer. 2012;106:99–106. doi: 10.1038/bjc.2011.530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khanim FL, Merrick BA, Giles HV, Jankute M, Jackson JB, Giles LJ, Birtwistle J, Bunce CM, Drayson MT. Redeployment-based drug screening identifies the anti-helminthic niclosamide as anti-myeloma therapy that also reduces free light chain production. Blood Cancer J. 2011;1:e39. doi: 10.1038/bcj.2011.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SY, Kang JW, Song X, Kim BK, Yoo YD, Kwon YT, Lee YJ. Role of the IL-6-JAK1-STAT3-Oct-4 pathway in the conversion of non-stem cancer cells into cancer stem-like cells. Cell Signal. 2013;25:961–969. doi: 10.1016/j.cellsig.2013.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King ML, Lindberg ME, Stodden GR, Okuda H, Ebers SD, Johnson A, Montag A, Lengyel E, MacLean JA, II, Hayashi K. WNT7A/beta-catenin signaling induces FGF1 and influences sensitivity to niclosamide in ovarian cancer. Oncogene. 2015;34:3452–3462. doi: 10.1038/onc.2014.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li R, Hu Z, Sun SY, Chen ZG, Owonikoko TK, Sica GL, Ramalingam SS, Curran WJ, Khuri FR, Deng X. Niclosamide overcomes acquired resistance to erlotinib through suppression of STAT3 in non-small cell lung cancer. Mol Cancer Ther. 2013;12:2200–2212. doi: 10.1158/1535-7163.MCT-13-0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li R, You S, Hu Z, Chen ZG, Sica GL, Khuri FR, Curran WJ, Shin DM, Deng X. Inhibition of STAT3 by niclosamide synergizes with erlotinib against head and neck cancer. PLoS One. 2013;8:e74670. doi: 10.1371/journal.pone.0074670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osada T, Chen M, Yang XY, Spasojevic I, Vandeusen JB, Hsu D, Clary BM, Clay TM, Chen W, Morse MA, Lyerly HK. Antihelminth compound niclosamide downregulates Wnt signaling and elicits antitumor responses in tumors with activating APC mutations. Cancer Res. 2011;71:4172–4182. doi: 10.1158/0008-5472.CAN-10-3978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sack U, Walther W, Scudiero D, Selby M, Kobelt D, Lemm M, Fichtner I, Schlag PM, Shoemaker RH, Stein U. Novel effect of antihelminthic Niclosamide on S100A4-mediated metastatic progression in colon cancer. J Natl Cancer Inst. 2011;103:1018–1036. doi: 10.1093/jnci/djr190. [DOI] [PubMed] [Google Scholar]
- Wieland A, Trageser D, Gogolok S, Reinartz R, Hofer H, Keller M, Leinhaas A, Schelle R, Normann S, Klaas L, Waha A, Koch P, et al. Anticancer effects of niclosamide in human glioblastoma. Clin Cancer Res. 2013;19:4124–4136. doi: 10.1158/1078-0432.CCR-12-2895. [DOI] [PubMed] [Google Scholar]
- Schaefer BC, Schaefer ML, Kappler JW, Marrack P, Kedl RM. Observation of antigen-dependent CD8+ T-cell/dendritic cell interactions in vivo. Cell Immunol. 2001;214:110–122. doi: 10.1006/cimm.2001.1895. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Li Q, Katzenellenbogen BS, Lau LF, Taylor RN, Bagchi IC, Bagchi MK. Estrogen-induced CCN1 is critical for establishment of endometriosis-like lesions in mice. Mol Endocrinol. 2014;28:1934–1947. doi: 10.1210/me.2014-1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshioka S, King ML, Ran S, Okuda H, MacLean JA, II, McAsey ME, Sugino N, Brard L, Watabe K, Hayashi K. WNT7A regulates tumor growth and progression in ovarian cancer through the WNT/beta-catenin pathway. Mol Cancer Res. 2012;10:469–482. doi: 10.1158/1541-7786.MCR-11-0177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satpathy M, Cao L, Pincheira R, Emerson R, Bigsby R, Nakshatri H, Matei D. Enhanced peritoneal ovarian tumor dissemination by tissue transglutaminase. Cancer Res. 2007;67:7194–7202. doi: 10.1158/0008-5472.CAN-07-0307. [DOI] [PubMed] [Google Scholar]
- R Development Core Team. R: a language and environment for statistical computing. http://www.gbif.org/resource/81287. Accessed November 2015. [Google Scholar]
- Robinson MD, McCarthy DJ, Smyth GK. edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics. 2010;26:139–140. doi: 10.1093/bioinformatics/btp616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy DJ, Chen Y, Smyth GK. Differential expression analysis of multifactor RNA-Seq experiments with respect to biological variation. Nucleic Acids Res. 2012;40:4288–4297. doi: 10.1093/nar/gks042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Ser B. 1995;57:289–300. [Google Scholar]
- Stodden GR, Lindberg ME, King ML, Paquet M, MacLean JA, Mann JL, DeMayo FJ, Lydon JP, Hayashi K. Loss of Cdh1 and Trp53 in the uterus induces chronic inflammation with modification of tumor microenvironment. Oncogene. 2015;34:2471–2482. doi: 10.1038/onc.2014.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reardon SN, King ML, MacLean JA, II, Mann JL, DeMayo FJ, Lydon JP, Hayashi K. CDH1 is essential for endometrial differentiation, gland development, and adult function in the mouse uterus. Biol Reprod. 2012 doi: 10.1095/biolreprod.112.098871. 86:141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis G, Jr, Sherman BT, Hosack DA, Yang J, Gao W, Lane HC, Lempicki RA. david: database for annotation, visualization, and integrated discovery. Genome Biol. 2003;4:P3. [PubMed] [Google Scholar]
- Huang da W, Sherman BT, Lempicki RA. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc. 2009;4:44–57. doi: 10.1038/nprot.2008.211. [DOI] [PubMed] [Google Scholar]
- World Health Organization. WHO specifications and evaluations for public health pesticides: niclosamide 2′,5-dichloro-4′-nitrosalicylanilide. Geneva: World Health Organization; 2002. [Google Scholar]
- Attar E, Tokunaga H, Imir G, Yilmaz MB, Redwine D, Putman M, Gurates B, Attar R, Yaegashi N, Hales DB, Bulun SE. Prostaglandin E2 via steroidogenic factor-1 coordinately regulates transcription of steroidogenic genes necessary for estrogen synthesis in endometriosis. J Clin Endocrinol Metab. 2009;94:623–631. doi: 10.1210/jc.2008-1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Ramos R, Rocco J, Rojas C, Sovino H, Poch A, Kohen P, Alvarado-Diaz C, Devoto L. Physiologic activation of nuclear factor kappa-B in the endometrium during the menstrual cycle is altered in endometriosis patients. Fertil Steril. 2012;97:645–651. doi: 10.1016/j.fertnstert.2011.12.006. [DOI] [PubMed] [Google Scholar]
- Kim BG, Yoo JY, Kim TH, Shin JH, Langenheim JF, Ferguson SD, Fazleabas AT, Young SL, Lessey BA, Jeong JW. Aberrant activation of signal transducer and activator of transcription-3 (STAT3) signaling in endometriosis. Hum Reprod. 2015;30:1069–1078. doi: 10.1093/humrep/dev050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto M, Nasu K, Abe W, Aoyagi Y, Kawano Y, Kai K, Moriyama M, Narahara H. Enhanced miR-210 expression promotes the pathogenesis of endometriosis through activation of signal transducer and activator of transcription 3. Hum Reprod. 2015;30:632–641. doi: 10.1093/humrep/deu332. [DOI] [PubMed] [Google Scholar]
- Itoh F, Komohara Y, Takaishi K, Honda R, Tashiro H, Kyo S, Katabuchi H, Takeya M. Possible involvement of signal transducer and activator of transcription-3 in cell-cell interactions of peritoneal macrophages and endometrial stromal cells in human endometriosis. Fertil Steril. 2013;99:1705–1713. doi: 10.1016/j.fertnstert.2013.01.133. [DOI] [PubMed] [Google Scholar]
- Lima-Couy I, Cervero A, Bonilla-Musoles F, Pellicer A, Simon C. Endometrial leptin and leptin receptor expression in women with severe/moderate endometriosis. Mol Hum Reprod. 2004;10:777–782. doi: 10.1093/molehr/gah115. [DOI] [PubMed] [Google Scholar]
- Matarese G, Alviggi C, Sanna V, Howard JK, Lord GM, Carravetta C, Fontana S, Lechler RI, Bloom SR, De Placido G. Increased leptin levels in serum and peritoneal fluid of patients with pelvic endometriosis. J Clin Endocrinol Metab. 2000;85:2483–2487. doi: 10.1210/jcem.85.7.6703. [DOI] [PubMed] [Google Scholar]
- Vigano P, Somigliana E, Matrone R, Dubini A, Barron C, Vignali M, di Blasio AM. Serum leptin concentrations in endometriosis. J Clin Endocrinol Metab. 2002;87:1085–1087. doi: 10.1210/jcem.87.3.8286. [DOI] [PubMed] [Google Scholar]
- Wu MH, Chuang PC, Chen HM, Lin CC, Tsai SJ. Increased leptin expression in endometriosis cells is associated with endometrial stromal cell proliferation and leptin gene up-regulation. Mol Hum Reprod. 2002;8:456–464. doi: 10.1093/molehr/8.5.456. [DOI] [PubMed] [Google Scholar]
- Wu MH, Huang MF, Chang FM, Tsai SJ. Leptin on peritoneal macrophages of patients with endometriosis. Am J Reprod Immunol. 2010;63:214–221. doi: 10.1111/j.1600-0897.2009.00779.x. [DOI] [PubMed] [Google Scholar]
- Ahn JH, Choi YS, Choi JH. Leptin promotes human endometriotic cell migration and invasion by up-regulating MMP-2 through the JAK2/STAT3 signaling pathway. Mol Hum Reprod. 2015;21:792–802. doi: 10.1093/molehr/gav039. [DOI] [PubMed] [Google Scholar]
- Oh HK, Choi YS, Yang YI, Kim JH, Leung PC, Choi JH. Leptin receptor is induced in endometriosis and leptin stimulates the growth of endometriotic epithelial cells through the JAK2/STAT3 and ERK pathways. Mol Hum Reprod. 2013;19:160–168. doi: 10.1093/molehr/gas055. [DOI] [PubMed] [Google Scholar]
- Styer AK, Sullivan BT, Puder M, Arsenault D, Petrozza JC, Serikawa T, Chang S, Hasan T, Gonzalez RR, Rueda BR. Ablation of leptin signaling disrupts the establishment, development, and maintenance of endometriosis-like lesions in a murine model. Endocrinology. 2008;149:506–514. doi: 10.1210/en.2007-1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheng YH, Triyana S, Wang R, Das I, Gerloff K, Florin TH, Sutton P, McGuckin MA. MUC1 and MUC13 differentially regulate epithelial inflammation in response to inflammatory and infectious stimuli. Mucosal Immunol. 2013;6:557–568. doi: 10.1038/mi.2012.98. [DOI] [PubMed] [Google Scholar]
- Hirata T, Osuga Y, Takamura M, Kodama A, Hirota Y, Koga K, Yoshino O, Harada M, Takemura Y, Yano T, Taketani Y. Recruitment of CCR6-expressing Th17 cells by CCL 20 secreted from IL-1 beta-, TNF-alpha-, and IL-17A-stimulated endometriotic stromal cells. Endocrinology. 2010;151:5468–5476. doi: 10.1210/en.2010-0398. [DOI] [PubMed] [Google Scholar]