Abstract
Beta-defensins are innate immune molecules, often described as antimicrobial peptides because of their bactericidal activity and are now known to have diverse additional functions, including cell signaling, chemoattraction, immunoregulation, and reproduction. In humans and primates, beta-defensin 126 has been shown to regulate the ability of sperm to swim through cervical mucus and to protect sperm from attack by the female immune system during transit toward the oviduct. Bovine beta-defensin 126 (BBD126) is the ortholog of human defensin 126, and computational analysis here revealed significant conservation between BBD126 and other mammalian orthologs at the N-terminus, although extensive sequence differences were detected at the C-terminus, implying possible species-specific roles for this beta-defensin in reproduction. We had previously demonstrated preferential expression of this and related beta-defensin genes in the bovine male reproductive tract, but no studies of bovine beta-defensin proteins have been performed to date. Here, we analyzed BBD126 protein using a monoclonal antibody (a-BBD126) generated against a 14 amino acid peptide sequence from the secreted fragment of BBD126. The specificity of a-BBD126 was validated by testing against the native form of the peptide recovered from bovine caudal epididymal fluid and recombinant BBD126 generated using a prokaryotic expression system. Western blot analysis of the native and recombinant forms showed that BBD126 exists as a dimer that was highly resistant to standard methods of dissociation. Immunohistochemical staining using a-BBD126 demonstrated BBD126 protein expression by epithelial cells of the caudal epididymis and vas deferens from both mature and immature bulls. BBD126 could also be seen (by confocal microscopy) to coat caudal sperm, with staining concentrated on the tail of the sperm cells. This study is the first to demonstrate beta-defensin 126 protein expression in the bovine reproductive tract and on bull sperm. Its dissociation-resistant dimeric structure is likely to have important functional implications for the role of BBD126 in bovine reproduction.
Keywords: β-defensin 126, bovine, epididymis
INTRODUCTION
β-Defensins represent a major subfamily of antimicrobial or host defense peptides. Described as pore-forming cationic molecules that aggregate on the surfaces of bacterial cells to cause cell leakage and death, recent studies have revealed additional mechanisms by which these pleiotropic molecules mediate other biological activities, including coat color, chemotaxis, and immune regulation [1, 2]. In addition to these biological capabilities, a role for specific β-defensins in the regulation of fertility in multiple species, including mice [3] and primates [4], has been demonstrated.
One of these β-defensins, β-defensin 126 (BD126), has been ascribed a particularly key role in primate and human reproduction [5–7]. Originally discovered in macaque [8], it was termed epididymal specific protein (ESP13.2) and shown to coat sperm. BD126 has several distinctive structural features, including the cysteine-rich canonical β-defensin core motif and a highly glycosylated, carboxyl terminal tail (C-terminus). In primates and humans, BD126 has been shown to be required for efficient transport of sperm in the female reproductive tract by two distinct mechanisms. First, by extensively coating the sperm, sialyated BD126 confers an increased negative charge, causing mutual repulsion between sperm and mucus and enabling efficient passage [5]. Second, BD126 is a major component of the sperm glycocalyx in primates, a scaffold of surface glycoproteins that is thought to protect sperm from the female immune system [9].
Despite evidence linking β-defensins and fertility in other species [10], and fertility being a central issue for the animal breeding industry worldwide, almost nothing is known about these proteins in cattle. We previously used a bioinformatics approach to identify a cluster of 19 novel genes on bovine chromosome 13 that encoded peptides with the conserved β-defensin cysteine structural signature [11]. Combined with an expansion of β-defensin genes on chromosome 27, the current predicted number of β-defensin genes in the bovine genome is more than 50, clustered in groups on four chromosomes [12]. While characterization in cattle is limited, the orthologs of genes located on bovine chromosomes 8 and 23 have been shown to be expressed in the reproductive tracts of rats and pigs [13, 14]. Interestingly, the expanded bovine chromosome 27 cluster of β-defensins includes multiple potential members of the bovine ortholog of DEFB1, a gene in humans that is also of relevance to male fertility [15]. Interference with DEFB1 function has been shown to decrease both sperm motility and bactericidal activity, whereas treatment with recombinant DEFB1 markedly restored overall and progressive motility as well as egg-penetrating ability and bactericidal activity of sperm from infertile patients.
Previous characterization of the expression of the chromosome 13 gene cluster, including the bovine ortholog of 126 (BBD126), showed site-specific mRNA expression localized to the epididymis of the bull reproductive tract [16]. This led us to suggest that these molecules had important functional roles in bovine reproduction and immunology [17]. However, progress in defining the specific roles of β-defensin proteins in cattle has been slowed by the lack of bovine-specific reagents. Reliable expression systems for the production of β-defensin proteins are also unavailable. Here, we focused on BBD126 and aimed to characterize the localization of this β-defensin protein in the bovine male reproductive tract and on bovine sperm using a novel monoclonal antibody generated against a BBD126-derived peptide.
MATERIALS AND METHODS
Bioinformatic Analysis of BBD126
BBD126 was compared with BD126 sequences from other species using several computational techniques. The peptide sequence of BBD126 was compared to all nonredundant BD126 protein sequences from other mammalian species, including human, dog, mouse, rat, and macaque using BLASTP [18]. A total of 72 orthologs were found, which was reduced to 15 species representative of primates, livestock species, and rodents. Sequences were aligned using Clustal Omega [19, 20]. MEGA software, version 5.2, was then used to generate a phylogenetic tree of the same sequences [21]. Glycosylation sites were predicted in silico using NetOGlyc 3.1 and NetNGlyc 1.0 (available at www.cbs.dtu.dk/services/) [22].
Monoclonal Antibody to BBD126
A custom monoclonal antibody specific for BBD126 (a-BBD126) was commercially generated by Genscript using a standard protocol. Briefly, a 14-amino acid peptide sequence (RNGERVINPPTGMC) from the secreted fragment of BBD126 was selected based on computational modeling to be surface expressed and unglycosylated. After chemical synthesis and conjugation to keyhole limpet hemocyanin (KLH), the epitope was inoculated into five BALB/c mice. An immune response was confirmed by binding of serum to the antigen on an enzyme-linked immunosorbent-type assay. Spleen cells were isolated for cell fusion and hybridoma production. Four hybridoma clones were selected and tested in the enzyme-linked immunosorbent assay against the peptide. Unpurified antibody produced by each of the clones was also tested against BBD126 by Western blot analysis. One clone, 6A11E2, was selected for large-scale production and purification of a-BBD126.
Bovine Male Reproductive Tract Collection and Processing
Male reproductive tracts tissues were collected from intact mature (n = 3) and prepubertal (3 mo old, n = 3) Holstein-Friesian bulls within 30 min of slaughter at a local abattoir. After dissection of the testes and attached epididymis, 3 mm3 sized samples from the rete testis, caput, corpus, and cauda of the epididymis and vas deferens were collected and stored in 10% formalin solution (Sigma Aldrich Ltd.). Sperm cells and epididymal fluid were also obtained from testes (n > 60) collected postmortem by retrograde flushing with PBS [23]. To recover sperm from the caudal epididymis, a small incision was made in the cauda, and the lumen of the deferent duct was cannulated with a blunted 22 gauge needle. Sperm cells were then gently flushed through the cauda with a 5 ml syringe containing PBS at 37°C. Separately, the corpus and caput epididymal sections were incised and the sperm isolated by washing with PBS at 37°C [24]. Sperm from the different regions were washed in PBS by centrifugation at 100 × g for 5 min. Seminal plasma was also collected from surgically vasectomized bulls (n = 3) by electroejaculation for comparative analysis of β-defensin expression levels.
Capture of Native BBD126 from Bull Caudal Epididymal Fluid
A HiTrap NHS-Activated high-performance 1-ml chromatography column (GE Healthcare) was loaded with 1 mg of a-BBD126 following the manufacturer's recommended protocol. The loaded column was used to isolate native BBD126 from caudal epididymal fluid. The mobile phase was 50 mM Tris HCl, 150 mM NaCl, pH 7.5, with a flow of 0.5 ml/min and the elution buffer was 1 M glycine, pH 3. PBS (40 ml) used to flush epididymal cauda was centrifuged to remove cells and other debris and aliquoted into 1 ml fractions. These fractions were injected into the gel filtration column (40 runs of individual 1-ml aliquots). An automatic fraction collector collected 0.4 ml fractions; 20 μl of all fractions with protein content, as detected spectrophotometrically, were eletrophoresed on 4%–12% SDS-PAGE gels and stained with Coomassie Blue. Protein containing fractions were pooled, and total protein content using BSA as the standard (Thermo Scientific). This method yielded a total of 175 μg from 40 ml of epididymal flush fluid.
Prokaryotic Expression of Recombinant Bovine β-Defensins
The coding sequence of BBD117 and BBD126 were amplified by PCR using the following primers (BBD117: 5′-GGCCGAAAATCTTGTTGGAT-3′, reverse primer 5′-TTGGAAGATTACTGGTATTT-3′), (BBD126: 5′-GGTAATTGGTATGTGAGAAA-3′, reverse primer 5′- AGCAATGCCTGTTGTAGATC-3′), and Platinum Taq DNA polymerase (Life Technologies). The PCR product was cloned into the pBAD/TOPO ThioFusion Expression Kit (Life Technologies) following the recommended protocol. The resulting fusion protein was formed by an N-terminus thioredoxin (Trx) Tag, followed by an enterokinase Tag, the coding sequence of BBD126, and a C-terminus His-Tag. Sequence specificity was confirmed by Sanger sequencing of the resulting plasmid (GATC Biotech). Expression of the protein was carried out by transforming the resulting plasmid into One Shot TOP10 chemically competent Escherichia coli (Life Technologies) following the protocol suggested by the manufacturer. Two liters of bacterial culture were harvested by centrifugation after overnight culture at 28°C. The bacterial pellet was suspended in 30 ml Ni-NTA washing buffer (50 mM Tris, 200 mM NaCl, 20 mM imidazole, pH 7.5). Cells were lysed by sonication, and cell membranes were pelleted by centrifugation for 20 min at 15 000 × g. Supernatant was incubated with Ni-NTA resin (Qiagen Ltd.), and protein was purified following the manufacturer's protocol. Pooled fractions of the Ni-NTA purification were injected into a Hiprep 16/60 Sephacryl S100HR column (GE Healthcare), and the resulting trace showed a peak of 280 nm absorbance, proportional to protein concentration. Fractions were tested by Western blot analysis using a-BBD126. The resulting fusion protein was treated with enterokinase protease (New England Biolabs) to cleave the N-terminus Trx tag following the manufacturer's protocol. The average yield of recombinant protein was 3 mg/L of bacterial culture.
Immunohistochemical Detection of BBD126 in Bull Reproductive Tissues
Following fixation, coronal sections (3 mm3) of testis, epididymis, and vas deferens were embedded in paraffin wax, sectioned at 4 μm, mounted onto treated glass slides (Superfrost Plus; Fisher Scientific), and dried in a hot air oven (60°C) for 2 h. Preheated dewaxed slides were washed in PBS, treated for antigen retrieval in citrate buffer pH 6.0 in the microwave for 10 min and blocked with 3% H2O2 in 80% methanol for 30 min. The slides were incubated for 1.5 h at 37°C with a-BBD126 primary antibody at a dilution of 1:250. The avidin-biotin detection method was used, incubating slides with Vectastain ABC-kit containing anti-mouse secondary antibody (Vector laboratories) at room temperature (RT) for 30 min. Slides were washed three times in PBS for 5 min, then 3,3′ diaminobenzidine (Sigma) was applied for 30 sec. Harris hematoxylin (Fisher Scientific) was used as a counterstain. Negative controls were subjected to the same procedure except that no primary antibody was added to one set and a cocktail of mouse IgM and the four subclasses of IgG (Universal Negative Control–Mouse; DAKO) in a dilution 1:50 was added with the primary antibody to a second set of slides.
Detection of BBD126 on Bull Sperm by Confocal Microscopy
Sperm cells were seeded onto poly-D-lysine slides for 12 h. Cells were fixed for 30 min on ice with 4% paraformaldehyde, washed in PBS, and permeabilized using 0.2% Triton X-100 (Sigma) for 15 min at RT. Slides were washed in PBS and blocked with blocking buffer (4% BSA and 0.02% Triton X-100 in PBS) for 1 h at RT, then incubated with primary antibody (1:250) in PBS containing 4% BSA overnight at 4°C. Samples were then incubated with goat anti-mouse IgG-biotin antibody (1:500; Life Technologies) in 4% BSA for 1 h at RT, followed by an additional incubation for 1 h with Alexa Fluor 488-streptavidin conjugate (1:500; Life Technologies) in 4% BSA. After further washing with PBS, the samples were incubated with Hoechst 33342 for 10 min, and the slides were mounted with Mowiol. Sperm were then observed under an Olympus FluoView FV1000 confocal microscope equipped with a 60×/1.35NA oil immersion objective. Images were acquired at a resolution of 1024 × 1024 pixels, and a pixel dwell time of 12.5 μsec. Sequential acquisition mode was used in all cases. SPAM1 antibody (1:500 in PBS containing 4% BSA; Santa Cruz) was used as a positive control.
Detection by Western Blot Analysis of BBD126 in Protein Preparations from Bovine Sperm
Sperm cells from three different bulls were harvested from the epididymal fluid by centrifugation with a Ficoll gradient. Cells were lysed using lysis buffer: 50 mM Hepes, 100 mM NaCl, 1 mM ethylenediaminetetraacetic acid, 10% glycerol, 0.5% NP40, aprotinin (5 μg/ml), leupeptin (5 μg/ml), phenylmethane sulfonyl fluoride (1 mM), and Na3VO4 (1 mM). Protein levels of whole cell lysate were measured using a bicinchoninic acid protein assay (Thermo Scientific), and equal amounts of protein were resolved in a 4%–12% SDS-PAGE under reducing conditions (50 mM dithiothreitol [DTT] and 3% SDS), followed by transferring to polyvinylidene difluoride (PVDF) membranes (Merck Millipore) and blocking with PBS containing 0.1% Tween-20 and 5% BSA (PBST). Membranes were blotted with 1:1000 dilution of the a-BBD126 to a final concentration of 1 μg/ml solution in PBST overnight at 4°C. An anti-mouse IgG labeled with IRDye 680RD (1 mg/ml) secondary antibody was used to detect bound antibody in a 1:10 000 dilution in PBST. The membrane was analyzed using the infrared Odyssey imager (LI-COR). To validate the antibody specificity, 30 μg of total protein from cell lysate and media samples of HEK293 cells transfected with pcDNA3.1-Thio-126 BBD126 or pcDNA3.1-LacZ vectors using GeneJuice Transfection Reagent (Millipore) following the manufacturer's recommended protocol were loaded onto 4%–12% SDS-PAGE gels. After transfer into a PVDF membrane, it was blotted using 1:1000 dilution of the a-BBD126 to a final concentration of 1 μg/ml solution in PBST overnight at 4°C. The membrane was then processed as previously described.
Demonstration of a-BBD126 Specificity by Peptide Competition
A total of 30 μg of protein from sperm cell lysate and seminal plasma were loaded in triplicate onto a 4%–12% SDS-PAGE gel and run under reducing conditions (50 mM DTT). After transfer into a PVDF membrane as described, the membrane was divided into three fragments and each was incubated overnight with one of the following:1) 1 μg/ml of a-BBD126, 2) 1 μg/ml of a-BBD126 in 10 ml PBST plus 1 ug/ml of recombinant BBD126 (rBBD126), or 3) 1 μg/ml of a-BBD126 plus 1 μg/ml of rBBD117, another recombinant bovine defensin (i.e., BBD117) produced using the same protocol as described for BBD126. Membranes were then washed three times with PBST and blotted with an anti-mouse IgG labeled with IRDye 680RD (1 mg/ml) secondary antibody as previously described. Membranes were then analyzed using the infrared Odyssey imager (LI-COR).
Characterization of Native and Recombinant BD126 Protein
Based on amino acid sequence of the active BBD126 peptide, a molecular weight of 7.1 kDa was expected. However, Western blot analysis of protein fractions from bovine sperm revealed a significant band at molecular weight ∼14 kDa, suggesting dimerization of the peptides. In order to dissociate possible oligomers, BBD126 was exposed to several denaturing conditions including 8 M urea, 6 M guanidine hydrochloride, a range of increasing concentrations of methanol (from 0% to 80% methanol content), reducing agents (50 mM DTT and 5% 2-mercaptoethanol), and incubation at 95°C for either 5 min or overnight.
RESULTS
Bioinformatic Analysis of BBD126
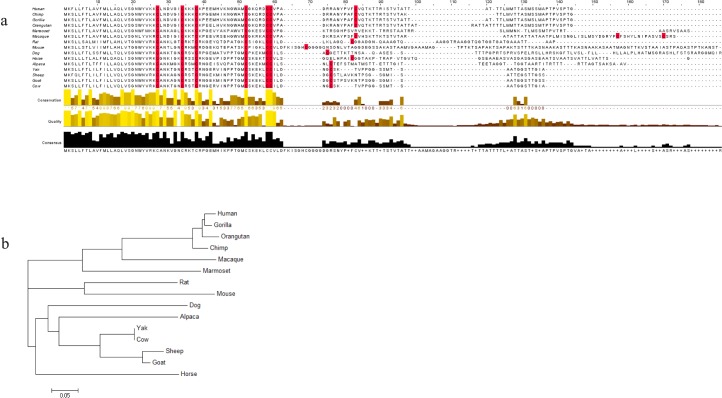
The complete BD126 protein sequences for 15 species were aligned, as shown in Figure 1a. Despite the high conservation of peptide sequence within the signal (N-terminus) region, significant sequence divergence exists within the C-terminus between species. In addition to the characteristic six cysteines detected in all β-defensins, an additional seventh cysteine is evident within all orthologous BD126 sequences. Comparison of the C-terminus amino acid tail length (measured from the last cysteine of the defensin motif to the stop codon) shows significant differences between 126 orthologs. Human BD126 (HBD126) has 52 amino acids in its C-terminus, whereas BBD126 has 30 (only 24 amino acids after the extra seventh cysteine). Given the dissimilarity in the C-terminus of the respective peptide sequences, where glycosylation motifs are predicted to occur, BD126 sequences were also examined for predicted glycosylation sites in silico. One N- and one O-linked glycosylation site was identified in BBD126, whereas no N-linked but nine O-linked glycosylation sites are predicted in the HBD126 sequence. For murine BD126, one N-linked and 14 O-linked glycosylation sites are predicted (data not shown). Phylogenetic analysis of BD126 sequences shows the presence of three distinct clades, reflecting the phylogeny of the primate, rodent, and livestock species included (Fig. 1b).
FIG. 1.
a) Multiple sequence alignment of BD126 peptide sequences from species across the animal kingdom. The degree of sequence conservation is shown below the alignment, and the conserved cysteines, including the seventh cysteine, are marked in red. b) Phylogenetic tree showing evolutionary relationship between BD126 peptide sequences for 15 species, including primates, domestic animals, and model species.
Monoclonal Antibody Specific for BBD126
The commercial production of a BBD126 antibody (a-BBD126) resulted in four different hybridoma clones. These clones were tested in Western blots against sperm cell lysate (data not shown). Antibodies from two of the clones showed no cross-reactivity in Western blot and were excluded. The remaining two clones showed binding to a single band, with clone 6A11E2 giving a stronger signal. Clone 6A11E2 was therefore selected for large-scale production and purification of a-BBD126.
Detection of BBD126 Protein in Caudal Epididymis and Vas Deferens
Immunohistochemistry revealed specific brown staining of epithelial cells of the caudal epididymis and the vas deferens of the prepubertal (Fig. 2, B and C) and of the mature bull (Fig. 2, D and E). In the mature bull, the sperm located in the lumen of the cauda epididymis and in the vas deferens also showed intense brown positive staining. Staining of the testis and caput and corpus of the epididymis from both the prepubertal and the mature bull was negative using a-BBD126 (Fig. 2, F and G).
FIG. 2.
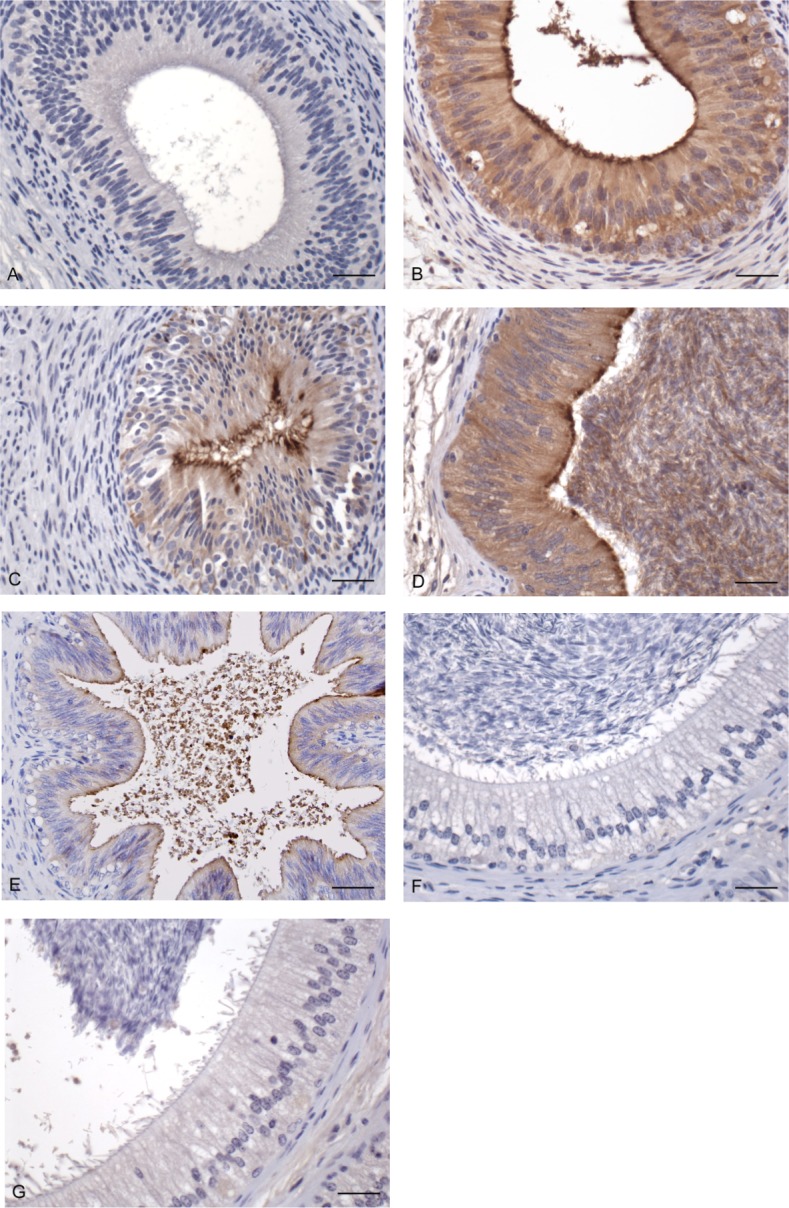
Immunohistochemical localization of BBD126 in the bovine epididymis. Tissue sections from both immature (A–C) and mature (D–G) bulls were stained for the presence of BBD126. Tissue sections were stained with a mouse isotype control (A, cauda) or with anti-BBD126 antibody (B–G). Brown coloration indicative of positive staining was found in the caudal epididymis (B) and vas deferens (C) of the immature bull as well as in the caudal epididymis (D) and vas deferens (E) of the mature bull. Caput and corpal sections of the mature bull epididymis did not stain positive for BBD126 (F and G, respectively). Bar = 25 μm. Representative images of sperm and tissue sections from n = 3 bulls are shown.
Localization of BBD126 on Caudal Sperm Surface
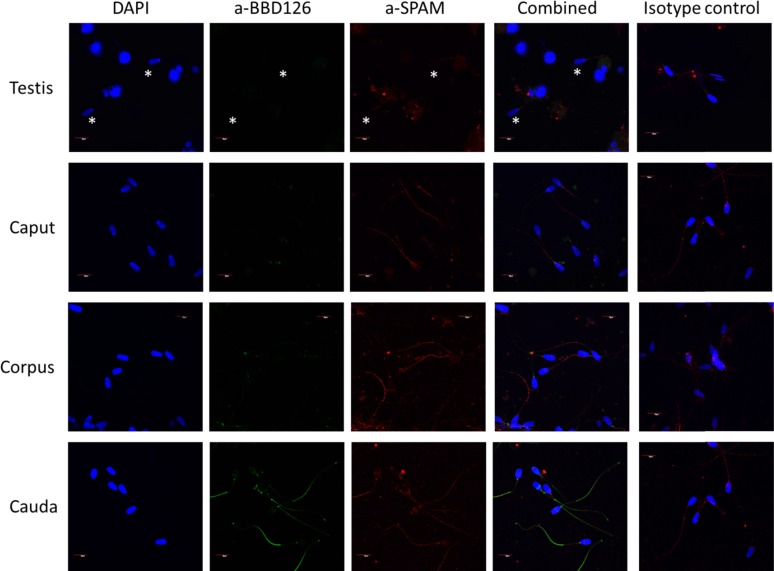
Using a combination of immunofluorescence markers, sperm recovered from the testes and along the epididymis were stained using a-BBD126 and anti-SPAM1 and examined using confocal microscopy. Positive staining for BBD126 was seen on sperm recovered from the caudal epididymis only (Fig. 3). BBD126 staining was localized predominantly to the dorsal section and tail of sperm cells.
FIG. 3.
Immunocytofluorescence localization of BBD126 on sperm cells. Live sperm cells extracted from both testis and various regions of the epididymis (caput, corpus, and cauda) stained with a-BBD126 antibody. 4′,6-Diamidino-2-phenylindole (nuclear stain) is shown in column 1, a-BBD126 is shown in column 2, anti-SPAM control antibody is shown in column 3, and merging of both a-BBD126 and anti-SPAM labeled sperm is shown in column 4. An isotype control antibody staining is also shown (column 5). Representative images of sperm from n = 3 bulls are shown.
Detection by Western Blot Analysis of BBD126 in Protein Preparations from Bovine Sperm
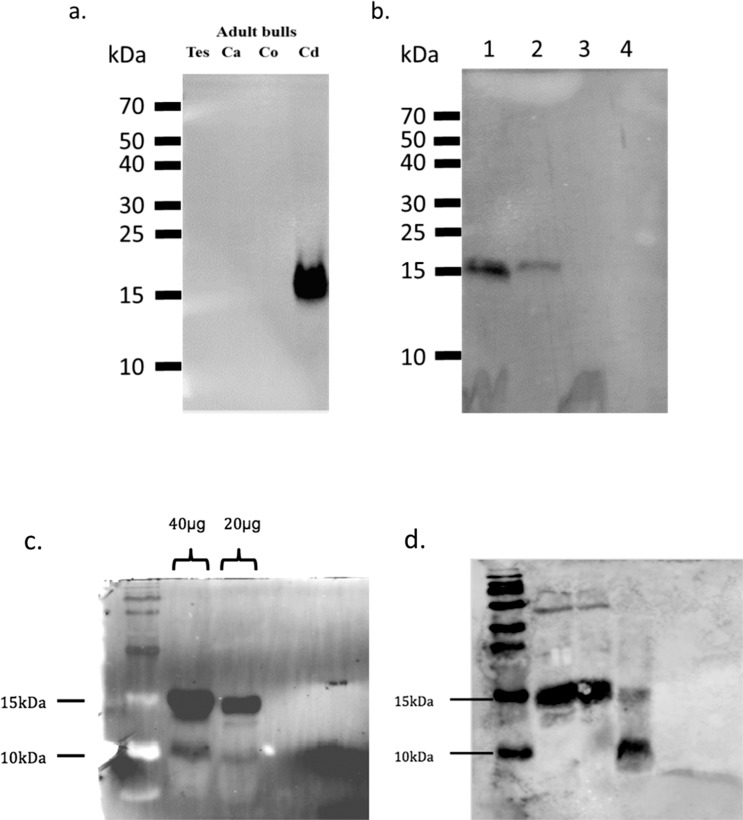
Western blot analysis of protein preparations from sperm recovered from the caudal epididymis revealed a specific band (Fig. 4a), confirming the confocal microscopy results that showed localization of BBD126 to sperm from this region only (Fig. 3). Furthermore, Western blot analysis of seminal plasma collected from surgically vasectomized and electroejaculated bulls did not reveal any BBD126 (Fig. 4b). On the basis of amino acid content, the expected size of the BBD126 is 7.1 kDa. However, the predominant band detected by Western blots with a-BBD126, of protein preparations from caudal sperm (Fig. 4a), and from seminal plasma (Fig. 4b) was ∼14.2 kDa, which suggested that the BBD126 exists in a dimeric form. However, the monomeric form was also detected and is especially visible when higher concentrations of sperm lysate are used (Fig. 4c).
FIG. 4.
Analysis of BBD126 expression on sperm by Western blot analysis. a) Sperm cells lysate extracted from testis (Tes), caput (Ca), corpus (Co), and cauda (Cd) of the epididymis stained with a-BBD126. b) Sperm cell lysate (lane 1) and seminal plasma (lane 2) from a normal bull; centrifuged seminal plasma (lane 3), and seminal plasma (lane 4) from a vasectomized bull. c) The predominant form of BBD126 is the dimer (14.2 kDa) but monomer is also present in sperm lysate, with a stronger band when 40 μg of sperm lysate was used. d) Sperm cell lysate (20 μg of total protein) was incubated at 95°C in sample buffer with 50 mM DTT for 5 min (lanes 1) and with 100 mM DTT (lane 2), but only boiling for 24 h with DTT led to the almost complete dissociation of the BBD126 dimer. Representative images of sperm from n = 3 bulls are shown.
Western Blot Analysis Identifies Dissociation-Resistant Form of BBD126 Dimer
Interestingly, the BBD126 dimer was surprisingly resistant to standard methods for dissociation of the peptide complex (8 M urea, 6 M guanidine hydrochloride, increasing concentrations of methanol (0%–80%), and reducing conditions (50 mM DTT and 5% 2-mercaptoethanol). Extended denaturing conditions of 24-h incubation at 95°C with DTT were required to dissociate the dimer into monomeric BBD126 (Fig. 4d).
Recombinant BBD126
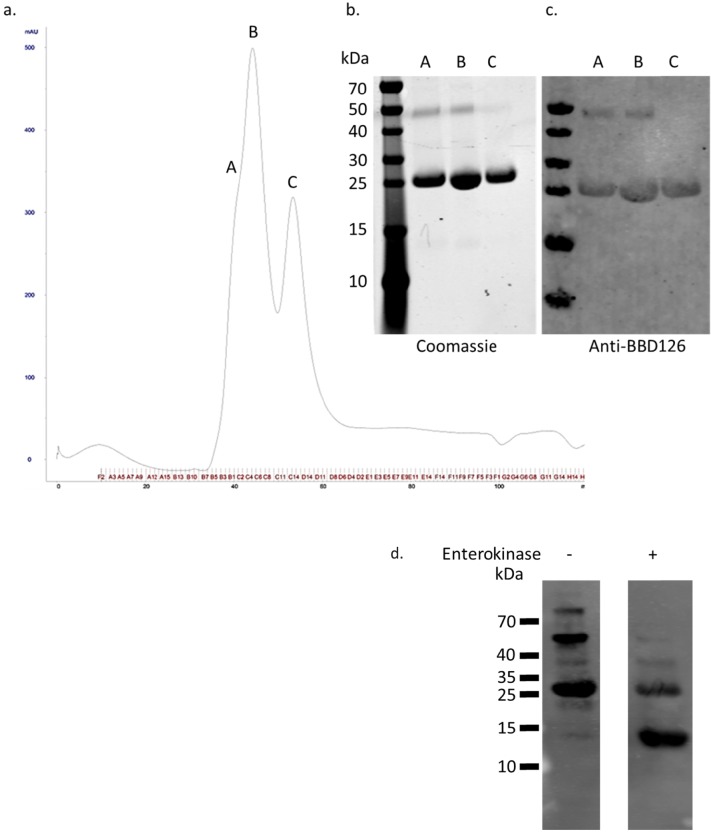
Because of detection of BBD126 at a much higher molecular weight than expected, there was some concern regarding the specificity of the antibody. In order to validate the specificity of a-BBD126, a method was developed to express recombinant β-defensin peptides. For large-scale production, the prokaryotic system, using a Trx fusion protein approach was employed, and 2 L of culture yielded 3 mg of purified Trx-BBD126. The trace provided by fast protein liquid chromatography indicated the presence of two proteins of different size (Fig. 5a). When aliquots of these peaks were analyzed and stained for total protein (Fig. 5b) and a-BBD126 (Fig. 5c), two bands were identified, a 23.4 kDa band that matched the predicted size of the Trx-BBD126 fusion protein, and a 46.8 kDa band that is thought to represent a dimer of the fusion peptide construct. An enterokinase digestion site facilitated the separation of recombinant BBD126 (Fig. 5d) with an expected molecular weight of 10.6 kDa. The size differential between the native and recombinant peptide is due to the presence of a His-Tag and a v5 tag after the enterokinase site in the recombinant peptide, which contributes an additional 3.5 kDa.
FIG. 5.
a) Recombinant BBD126 purification by gel filtration column in a fast protein liquid chromatography system. Two distinctive peaks B and C were observed plus a shoulder on the main peak, A. These were analyzed by SDS-PAGE and analyzed for total protein using Coomassie gel stain (b) or Western blot for BBD126 (c). The composition of peaks A and B is identical where most of the protein is found as the Trx-BBD126 form, 23.4 kDa. However, we can observe the presence of a 46.8 kDa band that seems to correspond to a dimer of the recombinant BBD126 as is detected by a-BBD126 in the Western blot analysis. In contrast C only contains the monomeric Trx-BBD126. Enterokinase digestion of the Trx-BBD126 fusion protein yields both forms of the rBBD126 peptide (d).
Validation of a-BBD126
A peptide competition assay and Western blot analysis validated the monoclonal a-BD126 (Supplemental Fig. S1; all Supplemental Data are available online at www.biolreprod.org). Sperm cell lysate was blotted with a-BBD126 in the presence of rBBD126 or rBBD117 (another bovine β-defensin found in the same gene cluster). The presence of rBBD126 blocked detection of the 14.2 kDa band by a-BBD126. Recombinant BBD117 failed to block a-BBD126 binding, therefore confirming the specificity of the antibody for BBD126.
DISCUSSION
β-Defensins are host defense peptides that have evolved in diverse species across the plant and animal kingdoms. The bovine genome hosts an expanded suite of over 50 β-defensin genes arranged in four genomic clusters across four chromosomes (for detailed review, see [12]). With improved annotation of the bovine genome and accurate characterization of copy-number variation [25], the final count of bovine β-defensin genes may change. The reason underlying the evolutionary expansion of this gene family in cattle is unknown although it has been suggested that the bovid herd structure and polygynous mating system could promote rapid disease transmission and may have been a contributory selective pressure for expansion of protective β-defensin genes in these and related species [12].
Here, in silico analysis demonstrated significant conservation in the N-terminal of the BD126 protein although significant divergence between species was seen in the C-terminus. Immediately apparent was the presence of an additional cysteine in BBD126 in addition to the canonical six cysteines usually found in β-defensins. This extra cysteine is common to all orthologs of BD126, except for the marmoset. Furthermore, macaque BD126 has nine cysteine residues, all of which occur after the last canonical signature cysteine. The functional significance of these additional cysteines remains unknown, although the extended C-termini of some orthologs could imply species-specific differences in functionality. It is also possible that the additional cysteines could affect higher-order structure of these defensin molecules [26], and thereby regulate peptide stability and function.
The extended tail of HBD126 has been shown to encode glycosylation sites, and it is thought that in primates, glycosylated BD126 forms an extensive part of the sperm outer glycocalyx [27]. This adds a negative charge to the sperm to facilitate migration through cervical mucus and also forms a cloak to protect sperm from detection by the female immune system during transit to the site of fertilization [5, 9]. Experimental removal of these glycan structures reduced the human peptide in size from >32 kDa to <10 kDa, illustrating the extent to which HBD126, in particular, is glycosylated [4]. However, in contrast to the nine O-linked glycosylation sites predicted in the HBD126 sequence, only one O-linked glycosylation site was identified in BBD126. Phylogenetic analysis provided additional evidence of divergence between primate, murine, and bovine BD126 sequences, perhaps implying functional differences. This may also reflect neofunctionalization in cattle, where the expansion of the defensin gene family likely has enabled other members to acquire new functions [12]. These differences may infer species-specific BBD126 functionality, although this will require detailed comparative experimental investigation.
In this study, we found extensive staining of the bovine male reproductive tract using the commercial monoclonal antibody against a BBD126-derived peptide. BBD126 protein could be seen along the epididymal epithelium of the bull, recapitulating our earlier mRNA expression data [16] as well supporting previous findings for the orthologous protein in other species. We also show extensive staining for BBD126 on bull sperm, with higher localization to the dorsal and tail section of the head. Western blot analysis also revealed significant staining of protein from seminal plasma. The lack of BBD126 staining in protein preparations from seminal plasma collected from vasectomized bulls rules out the possibility of expression or secretion of BBD126 from any accessory sex glands in the bull. Interestingly, the expression of BBD126 in the reproductive tract of sexually immature bulls suggests that this β-defensin may have a function that is not exclusively related to sperm function, although further functional studies are required to explore that possibility.
Western blots of protein preparations from seminal plasma and caudal fluid revealed specific bands migrating at 14.2 kDa (twice the predicted molecular weight of the BD126 monomer), leading to concern regarding the specificity of the antibody. Methods were developed for the in vitro expression of recombinant peptide in order to validate the antibody. Furthermore, blocking with rBD126 but not rBD117 blocked binding of the monoclonal a-BD126, confirming antibody specificity.
Western blots that showed consistent staining of bands at twice the expected molecular weight for both native and recombinant BBD126 led us to hypothesize that BBD126 exists as a dimer. Dimer disruption of the bovine peptide was tested using several biochemical tools, including higher concentration of reducing agents (DTT) as well as increasing concentration of methanol and other denaturing agents, including urea and guanidine hydrochloride. However, only prolonged incubation at 95°C could completely disrupt the dimer into BBD126 monomers of the expected size (7.1 kDa). Whereas dimers were detected both in vitro and in vivo, the dimer formed by rBBD126 is perhaps not as strong as the native BD126 dimer because monomers are formed on a reducing gel (unlike native BBD126). This is possibly due to the inability of E. coli to express a similar disulfide-locked protein, although future eukaryotic expression systems are required to resolve this issue. Interestingly, the formation of β-defensin dimers has been described in other species. HBD126 is thought to interact with the lipid membrane on sperm as a covalently linked dimer, and Defb22 in rats also exists as a disulfide-linked homodimer [26, 28, 29]. The phenomenon of dimerization in these peptides with highly conserved cysteines has been described as a disulfide lock [30].
In conclusion, comparative genomic analysis has been facilitated by the recent explosion of more accurately annotated genomes for humans, model organisms, and now livestock species. This has shed light on the evolutionary conservation of immune genes, including BD126, and also on species-specific differences that could underpin divergent responses in traits of economic and agricultural interest, including fertility. Extensive analysis of the ortholog in rodents (Defb22), expression profiling showing consistent expression of this gene in the epididymis [31], and a dinucleotide mutation in the HBD126 gene causes a reading frame shift resulting in significantly reduced sperm function and subfertility in men [7]. This is the first demonstration of BBD126 protein in the reproductive tract of the bull and its localization on caudal sperm. The dissociation-resistant dimeric structure of BBD126 is likely to have important functional implications for its role in bovine reproduction.
ACKNOWLEDGMENT
We are grateful to Michael Carty, PhD, for advice on Western blot analysis. The authors would also like to acknowledge Sheila Worrall, UCD, for her assistance with histopathology.
Footnotes
The work was funded by the Irish Department of Agriculture, Food and the Marine, under the Stimulus Research Initiative (11/S/104).
These authors contributed equally to the direction of this study.
REFERENCES
- Selsted ME, Ouellette AJ. Mammalian defensins in the antimicrobial immune response. Nat Immunol. 2005;6:551–557. doi: 10.1038/ni1206. [DOI] [PubMed] [Google Scholar]
- Semple F. Dorin JR. beta-Defensins: multifunctional modulators of infection, inflammation and more? J Innate Immun. 2012;4:337–348. doi: 10.1159/000336619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou YS, Webb S, Lettice L, Tardif S, Kilanowski F, Tyrrell C, Macpherson H, Semple F, Tennant P, Baker T, Hart A, Devenney P, et al. Partial deletion of chromosome 8 beta-defensin cluster confers sperm dysfunction and infertility in male mice. PLoS Genet. 2013;9:e1003826. doi: 10.1371/journal.pgen.1003826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tollner TL, Bevins CL, Cherr GN. Multifunctional glycoprotein DEFB126–a curious story of defensin-clad spermatozoa. Nat Rev Urol. 2012;9:365–375. doi: 10.1038/nrurol.2012.109. [DOI] [PubMed] [Google Scholar]
- Tollner TL, Yudin AI, Treece CA, Overstreet JW, Cherr GN. Macaque sperm coating protein DEFB126 facilitates sperm penetration of cervical mucus. Hum Reprod. 2008;23:2523–2534. doi: 10.1093/humrep/den276. [DOI] [PubMed] [Google Scholar]
- Tollner TL, Yudin AI, Tarantal AF, Treece CA, Overstreet JW, Cherr GN. Beta-defensin 126 on the surface of macaque sperm mediates attachment of sperm to oviductal epithelia. Biol Reprod. 2008;78:400–412. doi: 10.1095/biolreprod.107.064071. [DOI] [PubMed] [Google Scholar]
- Tollner TL, Venners SA, Hollox EJ, Yudin AI, Liu X, Tang G, Xing H, Kays RJ, Lau T, Overstreet JW, Xu X, Bevins CL, et al. A common mutation in the defensin DEFB126 causes impaired sperm function and subfertility. Sci Transl Med. 2011 doi: 10.1126/scitranslmed.3002289. 3:92ra65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yudin AI, Tollner TL, Li MW, Treece CA, Overstreet JW, Cherr GN. ESP13.2, a member of the beta-defensin family, is a macaque sperm surface-coating protein involved in the capacitation process. Biol Reprod. 2003;69:1118–1128. doi: 10.1095/biolreprod.103.016105. [DOI] [PubMed] [Google Scholar]
- Yudin AI, Generao SE, Tollner TL, Treece CA, Overstreet JW, Cherr GN. Beta-defensin 126 on the cell surface protects sperm from immunorecognition and binding of anti-sperm antibodies. Biol Reprod. 2005;73:1243–1252. doi: 10.1095/biolreprod.105.042432. [DOI] [PubMed] [Google Scholar]
- Dorin JR, Barratt CL. Importance of beta-defensins in sperm function. Mol Hum Reprod. 2014;20:821–826. doi: 10.1093/molehr/gau050. [DOI] [PubMed] [Google Scholar]
- Cormican P, Meade KG, Cahalane S, Narciandi F, Chapwanya A, Lloyd AT, O'Farrelly C. Evolution, expression and effectiveness in a cluster of novel bovine beta-defensins. Immunogenetics. 2008;60:147–156. doi: 10.1007/s00251-007-0269-8. [DOI] [PubMed] [Google Scholar]
- Meade KG, Cormican P, Narciandi F, Lloyd A, O'Farrelly C. Bovine beta-defensin gene family: opportunities to improve animal health? Physiol Genomics. 2014;46:17–28. doi: 10.1152/physiolgenomics.00085.2013. [DOI] [PubMed] [Google Scholar]
- Patil AA, Cai Y, Sang Y, Blecha F, Zhang G. Cross-species analysis of the mammalian beta-defensin gene family: presence of syntenic gene clusters and preferential expression in the male reproductive tract. Physiol Genomics. 2005;23:5–17. doi: 10.1152/physiolgenomics.00104.2005. [DOI] [PubMed] [Google Scholar]
- Guyonnet B, Marot G, Dacheux JL, Mercat MJ, Schwob S, Jaffrezic F, Gatti JL. The adult boar testicular and epididymal transcriptomes. BMC Genomics. 2009;10:369. doi: 10.1186/1471-2164-10-369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diao R, Fok KL, Chen H, Yu MK, Duan Y, Chung CM, Li Z, Wu H, Li Z, Zhang H, Ji Z, Zhen W, et al. Deficient human beta-defensin 1 underlies male infertility associated with poor sperm motility and genital tract infection. Sci Transl Med. 2014 doi: 10.1126/scitranslmed.3009071. 6:249ra108. [DOI] [PubMed] [Google Scholar]
- Narciandi F, Lloyd AT, Chapwanya A, O'Farrelly C, Meade KG. Reproductive tissue-specific expression profiling and genetic variation across a 19 gene bovine beta-defensin cluster. Immunogenetics. 2011;63:641–651. doi: 10.1007/s00251-011-0551-7. [DOI] [PubMed] [Google Scholar]
- Narciandi F, Lloyd A, Meade KG, O'Farrelly C. A novel subclass of bovine beta-defensins links reproduction and immunology. Reprod Fertil Dev. 2014;26:769–777. doi: 10.1071/RD13153. [DOI] [PubMed] [Google Scholar]
- Altschul SF, Koonin EV. Iterated profile searches with PSI-BLAST–a tool for discovery in protein databases. Trends Biochem Sci. 1998;23:444–447. doi: 10.1016/s0968-0004(98)01298-5. [DOI] [PubMed] [Google Scholar]
- Sievers F, Wilm A, Dineen D, Gibson TJ, Karplus K, Li W, Lopez R, McWilliam H, Remmert M, Soding J, Thompson JD, Higgins DG. Fast, scalable generation of high-quality protein multiple sequence alignments using Clustal Omega. Mol Syst Biol. 2011;7:539. doi: 10.1038/msb.2011.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goujon M, McWilliam H, Li W, Valentin F, Squizzato S, Paern J, Lopez R. A new bioinformatics analysis tools framework at EMBL-EBI. Nucleic Acids Res. 2010;38:W695–W699. doi: 10.1093/nar/gkq313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura K, Dudley J, Nei M, Kumar S. MEGA4: molecular evolutionary genetics analysis (MEGA) software version 4.0. Mol Biol Evol. 2007;24:1596–1599. doi: 10.1093/molbev/msm092. [DOI] [PubMed] [Google Scholar]
- Julenius K, Molgaard A, Gupta R, Brunak S. Prediction, conservation analysis, and structural characterization of mammalian mucin-type O-glycosylation sites. Glycobiology. 2005;15:153–164. doi: 10.1093/glycob/cwh151. [DOI] [PubMed] [Google Scholar]
- Druart X, Gatti JL, Huet S, Dacheux JL, Humblot P. Hypotonic resistance of boar spermatozoa: sperm subpopulations and relationship with epididymal maturation and fertility. Reproduction. 2009;137:205–213. doi: 10.1530/REP-08-0225. [DOI] [PubMed] [Google Scholar]
- Tajik P, Arman A, Taktaz T. Bovine epididymal sperm morphology obtained from caput, corpus and cauda epididymides. Pak J Biol Sci. 2007;10:3936–3939. doi: 10.3923/pjbs.2007.3936.3939. [DOI] [PubMed] [Google Scholar]
- Bickhart DM, Hou Y, Schroeder SG, Alkan C, Cardone MF, Matukumalli LK, Song J, Schnabel RD, Ventura M, Taylor JF, Garcia JF, Van Tassell CP, et al. Copy number variation of individual cattle genomes using next-generation sequencing. Genome Research. 2012;22:778–790. doi: 10.1101/gr.133967.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campopiano DJ, Clarke DJ, Polfer NC, Barran PE, Langley RJ, Govan JR, Maxwell A, Dorin JR. Structure-activity relationships in defensin dimers: a novel link between beta-defensin tertiary structure and antimicrobial activity. J Biol Chem. 2004;279:48671–48679. doi: 10.1074/jbc.M404690200. [DOI] [PubMed] [Google Scholar]
- Yudin AI, Treece CA, Tollner TL, Overstreet JW, Cherr GN. The carbohydrate structure of DEFB126, the major component of the cynomolgus Macaque sperm plasma membrane glycocalyx. J Membr Biol. 2005;207:119–129. doi: 10.1007/s00232-005-0806-z. [DOI] [PubMed] [Google Scholar]
- Song X, Zhang M, Zhou Z, Gong W. Ultra-high resolution crystal structure of a dimeric defensin SPE10. FEBS Lett. 2011;585:300–306. doi: 10.1016/j.febslet.2010.12.039. [DOI] [PubMed] [Google Scholar]
- Zanich A, Pascall JC, Jones R. Secreted epididymal glycoprotein 2D6 that binds to the sperm's plasma membrane is a member of the beta-defensin superfamily of pore-forming glycopeptides. Biol Reprod. 2003;69:1831–1842. doi: 10.1095/biolreprod.103.018606. [DOI] [PubMed] [Google Scholar]
- Wommack AJ, Ziarek JJ, Tomaras J, Chileveru HR, Zhang Y, Wagner G, Nolan EM. Discovery and characterization of a disulfide-locked C(2)-symmetric defensin peptide. J Am Chem Soc. 2014;136:13494–13497. doi: 10.1021/ja505957w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yudin AI, Tollner TL, Treece CA, Kays R, Cherr GN, Overstreet JW, Bevins CL. Beta-defensin 22 is a major component of the mouse sperm glycocalyx. Reproduction. 2008;136:753–765. doi: 10.1530/REP-08-0164. [DOI] [PMC free article] [PubMed] [Google Scholar]