Abstract
The potential of costimulation blockade to serve as a novel transplant immunosuppression strategy has been explored for over 20 years, culminating in the recent clinical approval of belatacept for renal transplant patients. Despite improving long-term graft function and survival compared with calcineurin inhibitors, clinical acceptance of belatacept has been hindered by elevated rates of acute rejection. We examined the signaling pathways required to activate costimulation blockade–resistant alloreactive T cells and identified the OX40/OX40L secondary costimulatory pathway as a promising target. We next sought to improve the clinical efficacy of traditional costimulation blockade using belatacept by coupling it with anti-OX40L. Using a murine transplant model, we demonstrate that combined blockade enhances the suppression of alloreactive T cell proliferation and effector functions including both cytokine release and cytotoxic degranulation. We also show that anti-OX40L may be particularly useful in targeting alloreactive memory T cell responses that are relatively unaffected by traditional costimulation blockade regimens. Finally, we translated this therapy to a clinically relevant nonhuman primate renal transplant model, validating the efficacy of this regimen in a potentially novel steroid- and calcineurin inhibitor–free immunosuppression regimen.
Combined belatacept and humanized anti-OX40L treatment prolongs renal allograft survival in non-human primates.
Introduction
T cell costimulation blockade (CoB) is a novel immunosuppression strategy that targets the costimulatory receptors required for full T cell activation. The best characterized of these costimulatory signals are those mediated by CD28-CD80/86 and CD40-CD154 interactions. CoB offers a means of transplant immunosuppression that avoids the nephrotoxicity and metabolic side effects (e.g., hypertension, hyperlipidemia, and diabetes) of traditional calcineurin inhibitors. Belatacept, a CD28 pathway antagonist, was the first CoB therapy to earn clinical approval to prevent allograft rejection in kidney transplant recipients (1, 2). Patients treated with belatacept have improved long-term graft function compared with those treated with calcineurin inhibitors, and recent 7-year outcome data even demonstrated a patient survival benefit with belatacept (3, 4). However, widespread clinical adoption of belatacept has been limited by its paradoxical association with higher rates of early acute rejection (2). The etiology of this CoB-resistant transplant rejection remains ill defined, but some evidence implicates alloreactive memory T cells (5–8). Additionally, belatacept may fail to block the full spectrum of costimulatory signals required for alloresponse initiation, as it does not target the so-called secondary costimulatory receptors. Many of these secondary costimulatory receptor interactions have been described, such as OX40/OX40L (CD134/CD252), ICOS/ICOSL (CD278/CD275), CD27/CD70, and 4-1BB/4-1BBL (CD137/CD137L) (9). Supporting this hypothesis, previous studies demonstrated that strong activation of some of these secondary costimulatory pathways could abrogate allograft tolerance induced by disruption of CD40/CD154 signaling in a murine cardiac transplant system (10).
In this work, we demonstrate that OX40/OX40L signaling plays a key role in the activation, proliferation, and effector functions of CoB-resistant T cell subsets. Disruption of this secondary costimulatory pathway with anti-OX40L is especially potent in neutralizing alloreactive memory T cell responses, which are not suppressed by conventional CoB alone. We then translate these findings to a rhesus macaque renal transplant model, and demonstrate that combined belatacept and humanized anti-OX40L therapy significantly prolongs allograft survival in this highly relevant preclinical transplant system.
Results
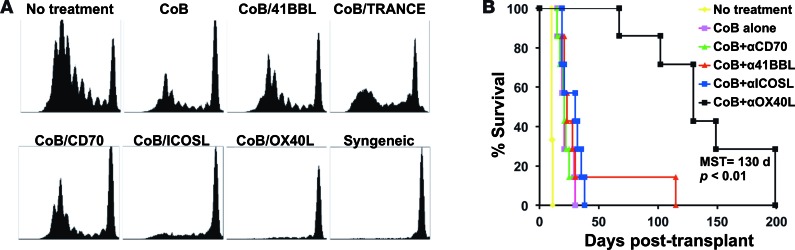
Initially, we sought to determine if secondary costimulatory signaling pathways are required for the development of alloreactive T cell responses that are resistant to traditional CoB. We first performed an in vivo alloreactive T cell activation and proliferation assay. Briefly, responder C57BL/6 splenocytes were labeled with CFSE and then adoptively transferred into sublethally irradiated BALB/c mice, which were then treated with traditional CoB (using anti-CD154 and CTLA-4-Ig) alone or in addition to an antagonist of a secondary costimulatory receptor (i.e., anti-OX40L, anti-ICOSL, anti-CD70, anti–TNF-related activation-induced cytokine [anti-TRANCE], or anti–4-1BBL). Alloresponses were detected by responder cell proliferation, as measured by CFSE dilution 72 hours after transfer. Whereas traditional CoB markedly (although not completely) suppressed T cell proliferation, this inhibition was augmented when alloreactive T cells were deprived of additional signals through either ICOSL or OX40L (Figure 1A). The potency of these combined regimens applied to alloresponses mediated by both CD4+ and CD8+ T cells. We next explored these combinations in a fully allogeneic murine skin graft model (BALB/c to C57BL/6), and found that combined CoB and anti-OX40L (but not anti-ICOSL) substantially improved allograft survival (Figure 1B).
Figure 1. Combined costimulation blockade (CoB) and OX40L blockade inhibits naive alloreactive T cell expansion in vivo and prolongs skin graft survival.
(A) In vivo mixed lymphocyte reaction was performed by adoptively transferring CFSE-labeled C57BL/6 splenocytes into sublethally irradiated BALB/c mice treated with CoB plus a secondary costimulatory receptor antagonist. Splenocytes were harvested after 72 hours and were assessed for CFSE-labeled cell division. Treatment with either CoB + anti-ICOSL or CoB + anti-OX40L suppressed alloproliferation (n = 5 per group, representative of 2 independent experiments). (B) Only CoB + anti-OX40L prolongs survival of BALB/c to C57BL/6 skin grafts (n = 7 per group, P < 0.01 by log-rank test). MST, median survival time. TRANCE, TNF-related activation-induced cytokine.
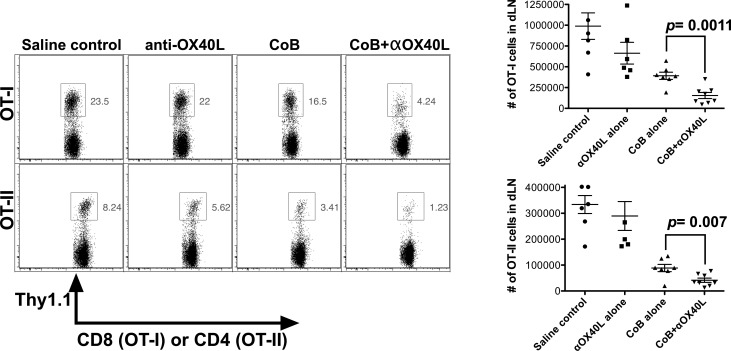
To better characterize the mechanisms responsible for prolonged graft survival with combined CoB and OX40L blockade, we employed a previously described transplant system that allowed us to precisely track donor-specific T cells (8, 11). In this system, C57BL/6 recipients were adoptively transferred with OT-I and OT-II cells, which are CD8+ and CD4+ TCR-transgenic T cells specific for chicken ovalbumin. Two days after cell transfer, the mice were challenged with a skin graft from a transgenic mOVA mouse, which ubiquitously expresses membrane-bound ovalbumin in all tissues. Skin grafts from mOVA mice activate the adoptively transferred OT-I and OT-II cells, which carry the congenic Thy1.1 marker to enable us to easily track and characterize the donor-specific rejection response. These graft recipients were treated with either saline vehicle alone, CoB alone, anti-OX40L alone, or combined CoB + anti-OX40L. Recipients were sacrificed after 10 days and then their spleens and draining lymph nodes (dLNs) were harvested and analyzed via flow cytometry.
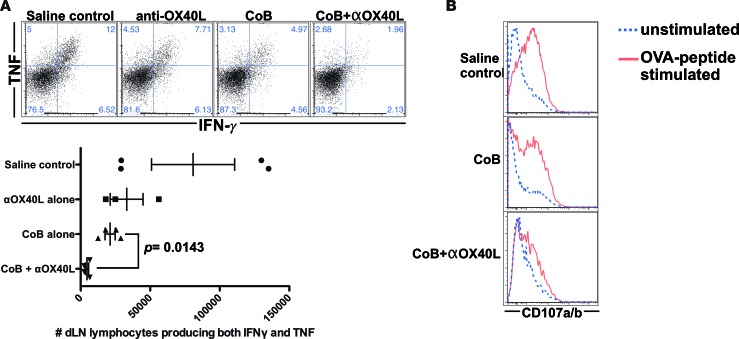
First, we examined the impact of these treatment regimens on donor-specific T cell expansion during a primary alloresponse. We quantified both the number and frequency of donor-specific CD8+ and CD4+ T cells (Thy1.1+ OT-I and Thy1.1+ OT-II cells) in the dLNs, demonstrating that combined therapy significantly suppressed donor-specific T cell expansion compared with either CoB or anti-OX40L alone (Figure 2). Next, intracellular cytokine staining was performed in dLN cells stimulated with the OT-I and OT-II cognate antigen peptides, and treatment with combined CoB and OX40L blockade could significantly suppress production of IFN-γ and TNF compared with the more minimal inhibition by CoB alone (Figure 3A). Finally, compared with CoB alone, the combined regimen also significantly diminished degranulation of toxic mediators as measured by a CD107A/B degranulation assay (Figure 3B).
Figure 2. Combined costimulatory blockade (CoB) and OX40L blockade inhibits naive donor-specific T cell proliferation in vivo.
C57BL/6 recipients were adoptively transferred with ovalbumin-specific OT-I and OT-II T cells and then transplanted with skin grafts from transgenic mouse donors ubiquitously expressing membrane-bound ovalbumin in all tissues (mOVA). Draining lymph nodes (dLNs) were harvested on posttransplant day 10 and analyzed (n = 6–8 mice/group, combined data over 2 independent experiments). Expansion of Thy1.1+ OT-I and OT-II T cells was measured by analyzing the percentage of the total CD8+ and CD4+ T cell population in the dLNs that was also Thy1.1+, demonstrating that treatment of recipients with combined CTLA-4-Ig and OX40L blockade inhibits donor-specific CD4+ and CD8+ T cell expansion. Representative flow plots and dot plots of the calculated total numbers of OT-I and OT-II cells in the dLNs are shown. Dot plots depict means ± SEM. P values determined by Mann-Whitney nonparametric test.
Figure 3. Combined blockade of costimulatory and OX40L pathways inhibits donor-specific T cell effector functions.
C57BL/6 recipients were adoptively transferred with ovalbumin-specific OT-I and OT-II T cells and then transplanted with skin grafts from transgenic mouse donors ubiquitously expressing membrane-bound ovalbumin in all tissues (mOVA). Draining lymph nodes (dLNs) were harvested on posttransplant day 10 and analyzed (n = 3–4 mice/group, representative data of 2 independent experiments shown). (A) Intracellular cytokine staining for IFN-γ and TNF was performed on these dLNs, revealing that combined CTLA-4-Ig and OX40L blockade inhibits cytokine effector responses during the naive alloresponses. (B) Cytotoxic degranulation of donor-specific OT-I T cells was measured using a CD107A/B assay, showing that combined CTLA-4-Ig and OX40L blockade suppresses donor-specific T cell degranulation effector responses. Dot plots depict means ± SEM. P values determined by Mann-Whitney nonparametric test.
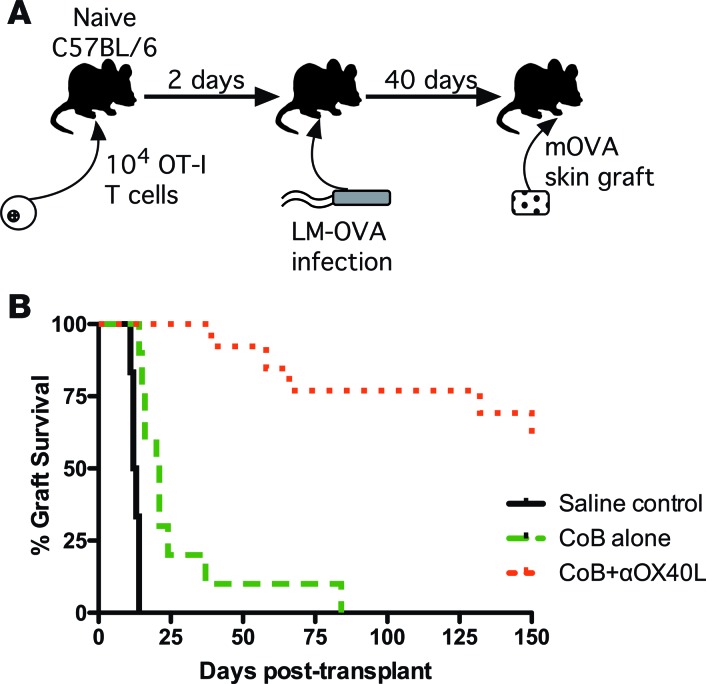
Having demonstrated that combined CoB and OX40L blockade can significantly attenuate the naive T cell alloresponse on both a quantitative and qualitative level, we next sought to determine whether this regimen also impacts memory alloresponses, as some data suggest that alloreactive memory T cells may be dominant mediators of CoB-resistant rejection (5–8). We examined this hypothesis using our previously described donor-specific T cell transplant system (Figure 4A) (8). We again adoptively transferred donor-specific CD8+ T cells into naive C57BL/6 mice, but then infected these mice with LM-OVA, a genetically modified Listeria monocytogenes that expresses chicken ovalbumin, activating the donor-specific T cells and generating ovalbumin-specific memory T cells. Thirty days after LM-OVA infection, we rechallenged the recipients with mOVA skin grafts and then treated them with saline vehicle, CoB alone, or combined CoB + anti-OX40L. Similar to our previously published work with this system (8), recipients treated with CoB alone promptly rejected their skin grafts, demonstrating that memory CD8+ T cells are relatively resistant to the effects of traditional CoB (Figure 4B). In contrast, those recipients treated with combined CoB + anti-OX40L demonstrated prolonged skin graft survival, despite the presence of donor-specific memory CD8+ T cells at the time of transplantation.
Figure 4. Disruption of OX40L signaling pathway prevents costimulation blockade–resistant rejection by memory alloreactive T cells.
(A) Schematic of donor-specific memory alloresponse transplant system. Ovalbumin-specific OT-I cells are adoptively transferred into naive C57BL/6 recipients, which are then infected with modified Listeria monocytogenes that produce the ovalbumin peptide (LM-OVA) to generate memory OT-I cells. Thirty days after infection, the recipients are rechallenged with skin grafts from mice ubiquitously expressing membrane-bound ovalbumin (mOVA). (B) mOVA skin graft recipients with donor-specific memory T cells show improved survival when treated with combined costimulation blockade (CoB) + anti-OX40L compared with treatment with CoB alone (n = 6–13 mice per group, P < 0.0001 by log-rank Mantel-Cox test).
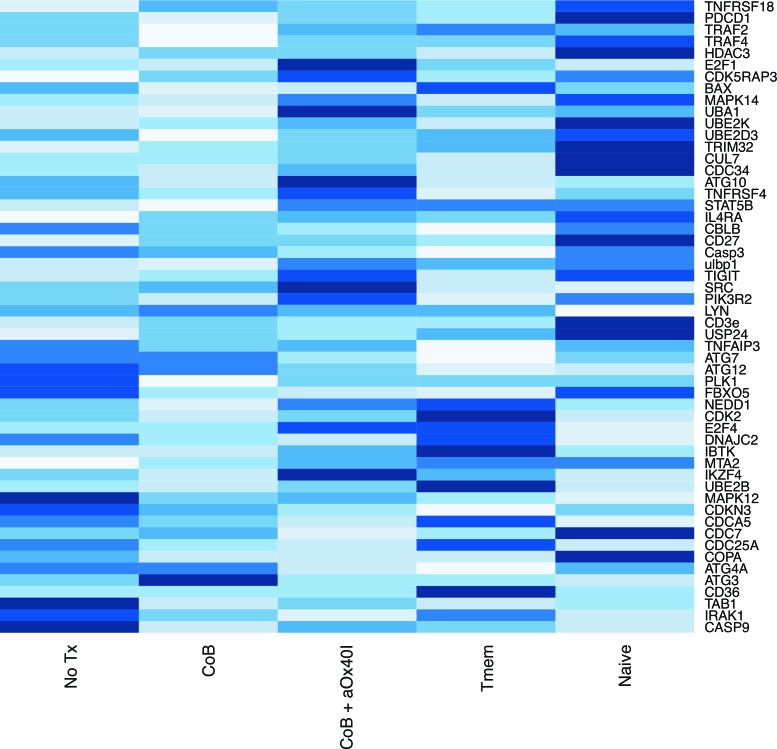
To assess the molecular pathways involved in alloreactive memory T cell suppression with this regimen, we harvested dLNs from mOVA skin graft recipients treated with the different immunosuppression regimens, used magnet-assisted cell separation to purify CD8+Thy1.1+ memory donor-specific T cells, obtained mRNA from these samples, and then performed whole-exome analysis to see which pathways are manipulated (Figure 5). As controls, naive OT-I cells and memory OT-I cells from untransplanted mice were also subjected to gene analysis. In comparison with the other treatment groups, treatment with the combined CoB and OX40L blockade regimen upregulated genes known to negatively regulate T cell activation such as TIGIT (12, 13) and CBLB (14). The dual-blockade regimen also downregulated genes involved in lymphocyte signaling (e.g., CD3e and LYN), genes involved in NF-κB upregulation (e.g., IRAK1), and various genes involved in autophagy (e.g., ATG3, ATG4A, ATG7, and ATG12).
Figure 5. Combined blockade impacts gene expression of donor-specific memory T cells compared with costimulatory blockade (CoB) alone.
Magnet-assisted sorted OT-I cells recovered from draining lymph nodes (dLNs) of mOVA skin graft recipients administered different immunosuppression regimens were subjected to gene expression analysis after sacrifice on postoperative day 10. For controls, naive OT-I cells and memory OT-I cells (Tmem) from untransplanted mice were also analyzed. The heatmap of gene expression in recipients treated with CoB alone rather than combined CoB + anti-OX40L is shown. RNASeq transcriptome data was expressed in reads per million kilobases (RPKM) for normalization, and within each gene (i.e., row), gene expression within each treatment group is graphically depicted from lowest expression (low RPKM, white bars) to highest expression (high RPKM, dark blue bars).
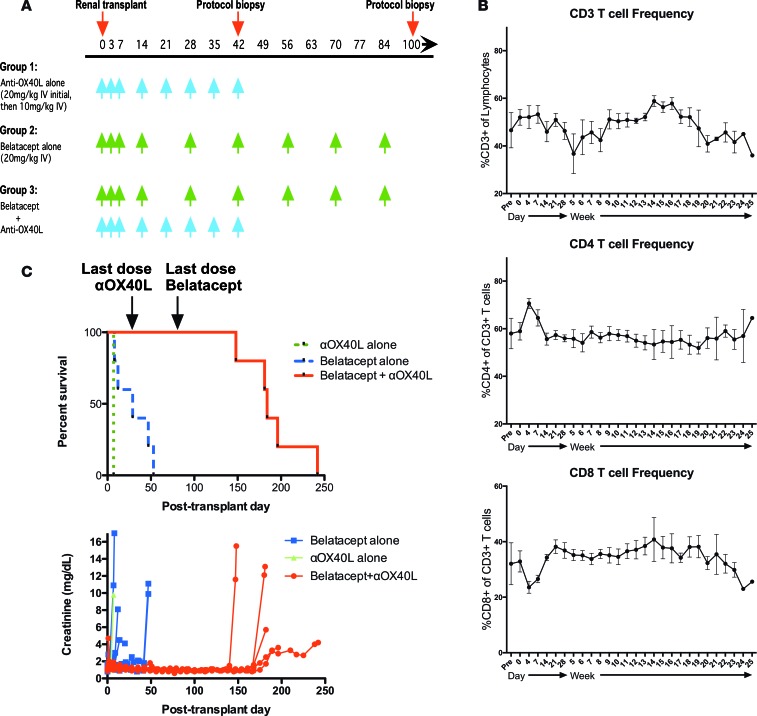
Having demonstrated that the OX40/OX40L signaling pathway plays a critical role in the activation of both naive and memory alloresponses in the setting of CD28 blockade, we next sought to determine if disruption of OX40L signaling could improve the efficacy of traditional CoB therapy in a more clinically relevant nonhuman primate transplant model. We utilized rhesus macaques as both recipients and donors in a preclinical kidney transplant model. All animals were MHC-typed via 454 deep sequencing, and donor-recipient pairings were constructed to maximize MHC disparity. Transplant recipients were treated with either belatacept monotherapy, anti-OX40L monotherapy, or combined belatacept and anti-OX40L (Figure 6A). Unlike some previous immunosuppression regimens that have demonstrated efficacy in nonhuman primate transplant systems, the combination of belatacept and OX40L blockade regimen was nondepleting, as the number of CD3+ cells, CD4+ T cells, and CD8+ T cells in serially obtained peripheral blood samples while on therapy did not change significantly compared with preoperative values (Figure 6B). In addition, there were no significant changes in populations of effector (CD95+CD28–) or central (CD95+CD28+) memory T cells in the peripheral blood over the course of therapy (data not shown). Nonhuman primates treated with anti-OX40L alone experienced severe acute rejection with similar kinetics to untreated controls (Figure 6C). Treatment with belatacept alone minimally prolonged graft survival, with a median graft survival time of 29 days (Figure 6C). In contrast, treatment with combined belatacept and anti-OX40L demonstrated a synergistic response in prolonging allograft survival (median survival time = 184 days, Figure 6C). All rejection episodes in recipients of the combined blockade therapy occurred after cessation of therapy on postoperative day (POD) 84. Planned biopsies of recipients treated with this combined blockade regimen showed minimal cell infiltration and no signs of active rejection (Figure 7). This prolonged graft survival was achieved despite the cessation of all immunosuppression on POD 84. Treatment with regimens containing belatacept suppressed the development of de novo donor-specific antibody in rhesus transplant recipients (Figure 8). Donor-specific antibody only emerged following withdrawal of therapy in the recipients originally treated with combined belatacept and anti-OX40L. Finally, this combined blockade regimen did not profoundly suppress protective immunity, as serial measurements of rhesus cytomegalovirus viral loads failed to show viral reactivation (Supplemental Figure 1; supplemental material available online with this article; https://doi.org/10.1172/jci.insight.90317DS1). Thus, in these experiments we have demonstrated a potential strategy to improve the clinical efficacy of belatacept by simultaneously disrupting signaling through OX40/OX40L interactions.
Figure 6. Combined belatacept and humanized anti-OX40L treatment prolongs renal allograft survival in nonhuman primates.
(A) Dosage regimen of belatacept and anti-OX40L in the nonhuman primate experimental groups (n = 5 rhesus/group except for only 1 transplant with anti-OX40L alone. (B) Frequency of CD3+, CD4+, and CD8+ in the peripheral blood does not change significantly in the primate renal transplant patients during treatment with combined costimulation blockade (CoB) and anti-OX40L. (C) Compared with treatment with belatacept alone, primate renal transplant recipients treated with combined CoB and OX40L blockade showed significantly prolonged graft survival (median survival time = 29 vs. 184 days, P = 0.0009 by Mantel-Cox test).
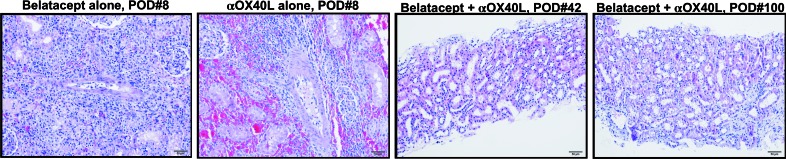
Figure 7. Combined costimulation blockade and OX40L blockade preserves histology of nonhuman primate renal allografts.
Protocol biopsies taken either at time of rejection or at fixed time points after transplant demonstrate that in contrast to the cellular infiltration seen in recipients treated with either belatacept or OX40L alone, those primate recipients treated with combined blockade regimen showed minimal evidence of inflammation or rejection. All histographs shown at ×20 magnification. POD, postoperative day.
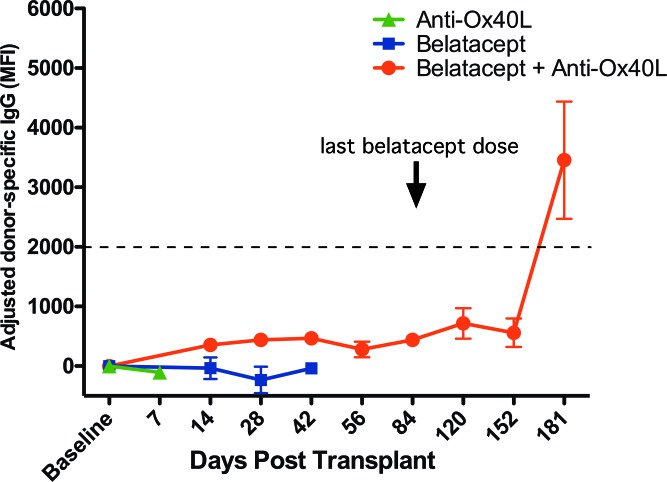
Figure 8. De novo donor-specific antibody is suppressed as long as recipient is treated with belatacept.
Renal allografts were performed in rhesus macaques, and recipients were treated with either anti-OX40L alone, belatacept alone, or combined therapy. Sera from these transplant recipients were collected at routine intervals, and IgG donor-specific antibody was quantified in a flow cytometry–based assay, adjusting for the baseline donor-specific antibody. Recipients treated with anti-OX40L alone rejected the allografts before de novo donor-specific antibody was formed. De novo donor-specific antibody only formed in the recipients treated with combined anti-OX40L and belatacept following cessation of belatacept therapy. Error bars reflect mean ± SEM. MFI, median fluorescence intensity.
Discussion
Long-term outcomes with belatacept in kidney transplant recipients have recently been published, confirming that CoB offers advantages in both allograft function and recipient survival compared with patients treated with conventional calcineurin inhibitor–based immunosuppression (3, 4). Despite these impressive results, widespread clinical adoption of belatacept has been hindered by the relatively high rates of early CoB-resistant transplant rejection. Improving the outcomes of CoB-based immunosuppression requires a thorough characterization of the T cell subsets and signaling pathways implicated in CoB-resistant rejection.
The results of the experiments presented here better define the signals necessary for both naive and memory T cell activation and effector functions required for transplant rejection in the setting of traditional CoB. Blockade of the OX40-OX40L interaction seems to augment traditional CoB by further suppressing the expansion of alloreactive T cells (Figures 1A and 2). Although the in vivo mixed lymphocyte reaction shown in Figure 1A seems to suggest that combined blockade directly impedes allospecific proliferation, we do not exclude the possibility that increased cell death also contributes to the lack of donor-specific T cell expansion evident in Figure 2.
In addition to its impact on alloreactive T cell expansion, blockade of OX40-OX40L interactions also appears to inhibit alloreactive cytokine production and cytotoxic degranulation. Thus, the combination of CoB and anti-OX40L appears to prolong graft survival through both a quantitative and qualitative effect on the T cell alloresponse, impeding donor-specific T cell expansion and by suppressing several effector functions of these cells. These findings suggest that OX40L signaling may be a redundant costimulatory pathway involved in the activation of alloreactive T cell subsets that are resistant to traditional CoB, as optimal suppression of these alloreactive T cells is only achieved when both signaling pathways are simultaneously disrupted.
One of the most important alloreactive T cell subsets involved in CoB-resistant rejection is memory T cells, and blockade of OX40L may be especially useful in targeting the memory alloresponse. Memory T cells are also known to play a critical role in CoB-resistant rejection, as alloreactive memory T cells possess diminished costimulatory requirements for full activation compared with naive T cells (5, 15, 16). Human and primate transplant recipients have a far higher frequency of memory T cells compared with laboratory mice kept in near-sterile conditions, potentially explaining some of the early difficulty of translating CoB regimens from murine transplant models to these more clinically relevant transplant systems (7). While traditional costimulatory pathways (CD28-CD80/86 and CD40/CD154) contribute little to memory T cell activation, OX40/OX40L has previously been shown to play a pivotal role in memory T cell activation (17–20). We therefore speculated that suppression of the alloreactive memory T cell response is a major pathway through which combined CoB and OX40L blockade may circumvent CoB-resistant transplant rejection. Our results in the mOVA transplant system confirm that the addition of anti-OX40L to traditional CoB is an effective effective strategy to target the donor-specific memory alloresponse.
Whole-exome analysis revealed several of the molecular mechanisms by which combined blockade therapy may disrupt this memory alloresponse. Treatment with combined CoB and OX40L blockade not only augmented expression of genes that negatively regulate T cell activation — e.g., TIGIT (12, 13) and CBLB (14) — this treatment also downregulated several mediators of T cell signaling (e.g., CD3 and LYN), several genes involved in NF-κB upregulation (e.g., IRAK1), and genes involved in autophagy (e.g., ATG3, ATG4A, ATG7, and ATG12). Interestingly, the ATG7 gene that is strongly downregulated by the combined blockade regimen was recently described to be essential for the development of CD8+ memory T cell responses (21). However, its impact on memory alloresponses is not necessarily the only mechanism by which combined blockade regimens may prolong allograft survival. An alternative mechanism by which anti-OX40L may improve the efficacy of CoB is through augmentation of regulatory T cell (Treg) suppression of alloresponses, as Li and colleagues have demonstrated that OX40L signaling can inhibit Treg suppression (22, 23). We are actively exploring the impact of combined blockade therapy on Treg numbers and function to assess this hypothesis.
Previous groups have also examined the OX40/OX40L pathway in alloimmune responses. Monotherapy with anti-OX40L did not prolong rat heart or skin graft survival (24), but it did suppress development of cardiac allograft vasculopathy in another murine cardiac transplant system (25). Similar to our findings, the efficacy of OX40L blockade was enhanced when coupled with CD28/CD154 blockade (18). We have substantially extended these earlier findings, examining the molecular mechanisms at play in combined CoB and OX40L blockade and demonstrating the efficacy of this regimen in a rigorous preclinical primate transplant system that spared both calcineurin inhibitors and steroids.
This work highlights the redundancy of costimulatory pathways involved in alloreactive T cell activation, demonstrating that secondary pathways such as OX40/OX40L can assume a more vital role once the primary costimulatory pathways (such as CD28/B7) are blocked. Thus, successful suppression of alloreactivity may require the simultaneous blockade of multiple costimulatory pathways. Relatively high rates of CoB-resistant acute rejection have limited the widespread adoption of belatacept clinically, and strategies that may enhance the efficacy of CoB could offer real clinical benefits. Importantly, the humanized anti-OX40L monoclonal antibody employed in our experiments was utilized in human clinical trials for asthma as a monotherapy treatment and no safety concerns were revealed (NCT00983658). These experiments suggest that humanized anti-OX40L may also merit investigation as a transplant immunosuppressant for use in conjunction with belatacept.
Methods
Mice.
Adult male 6- to 8-week-old C57BL/6 mice (Jackson Laboratories), TCR transgenic OT-I mice (Taconic Farms), μMT mice (Jackson Laboratories), and Act-mOVA mice (gifted by Marc Jenkins, University of Minnesota, Minneapolis, Minnesota, USA) (26) were obtained. To generate mice with memory OT-I cells, 104 OT-I cells were adoptively transferred into naive C57BL/6 mice via tail vein injection, and then 2 days later the recipients were infected with 104 CFU of LM-OVA (27) by i.p. injection, followed by a 30-day waiting period prior to experimental use.
In vivo mixed lymphocyte reaction.
C57BL/6 splenocytes were labeled for 5 minutes with 10 μM CFSE, and 2 × 107 to 3 × 107 of these labeled responders were adoptively transferred i.v. into irradiated BALB/c mice (700 rads), which were treated with the different immunosuppression regimens as described below. Splenocytes were harvested after 72 hours and analyzed by flow cytometry to assess the CFSE dilution and thus proliferation of H-2Kd–negative (responder) T cells.
Skin grafting.
Full-thickness tail skin grafts (~1 cm2) were transplanted onto the dorsal thorax of recipient mice. Where indicated, recipients of skin grafts received treatment with CoB (500 μg each of hamster anti–mouse-CD154 mAb [Bio X Cell, clone MR-1] and human CTLA-4-Ig [Bristol-Meyers Squibb] and/or 250 μg of rat anti–mouse OX40L mAb [Bio X Cell, clone RM134L]). All mAbs were administered i.p. on posttransplant days 0, 2, 4, and 6.
Flow cytometric analyses for frequency and absolute number.
Splenocytes, blood, and/or cells obtained from axillary dLNs in skin graft recipients treated with CTLA-4-Ig and/or anti-OX40L were stained with Thy1.1-PerCP (clone OX-7), CD8A-APC (clone 53-6.7), CD11A-FITC (clone M17/4) and/or CD49D-PE (clone R1-2) (all BD Biosciences) for analysis on an LSRII flow cytometer (BD Biosciences). Absolute numbers of OT-I T cells were determined by TruCount Bead analysis according to the manufacturer’s instructions. Cell sorting for CD8+Thy1.1+ cells was performed using MACS kits from Miltenyi Biotec. Data were analyzed using FlowJo software (Tree Star).
Intracellular cytokine staining.
Splenocyte suspensions were incubated with 10 nM OVA257–264 (SIINFEKL) (Emory University Core Facility) and 10 μg/ml Brefeldin A (BD Biosciences). Replicates without peptide were also performed. After 5 hours in culture, cells were processed using an intracellular staining kit (BD Biosciences) according to the manufacturer’s instructions and stained with anti–TNF-PE (clone MP6-XT22) and anti–IFN-γ-FITC (clone XMG1.2) (both BD Biosciences). The adjusted percentage of dual producers of TNF and IFN-γ for each sample was calculated by subtracting the percentage of dual producers in the nonstimulated samples from the matched SIINFEKL-stimulated samples.
CD107A/B degranulation assay.
As previously described (28), splenocyte suspensions were incubated in R10 media at 37°C in a 96-well plate (4 × 106 cells/well) for 5 hours with monensin and anti–CD107A/B-FITC in the presence or absence of 10 nM SIINFEKL peptide. After incubation, surface staining with anti–Thy1.1-PerCP and anti–CD8A-Pacific Blue was performed. Degranulation was measured as the adjusted median fluorescence intensity (MFI) of CD107A/B (peptide-stimulated – unstimulated).
Immunohistochemistry.
Explanted skin grafts were fixed in OTC and frozen. Sections were stained with anti-Thy1.1 mAb and developed with horseradish peroxidase to visualize infiltrating OT-I cells. Representative images are shown at an original magnification of ×40.
RNA sequencing.
C57BL/6 mice with memory OT-I cells were generated as above, and these received an mOVA skin graft as well as CoB ± anti-OX40L therapy. Recipients were sacrificed on POD 5, dLNs were harvested and processed into a single-cell suspension, and magnetically sorted with both CD8+ and Thy1.1+ MACS beads (Miltenyi Biotec) to enrich OT-I cells. Fifty thousand cells per mouse were then taken for RNA sequencing at the Yerkes Genomics Core. Data were analyzed using R. A heatmap was constructed containing a subset of genes of interest from whole-genome RNASeq. RNAseq transcriptome data were expressed in reads per million kilobases (RPKM) for normalization, and represented as low to high RPKM by color depth (white = low RPKM, dark blue = high RPKM).
Rhesus kidney transplants.
Rhesus macaque renal transplants were performed in the sterile operating theater at the National Yerkes Primate Research Center using standard techniques. All rhesus subjects were MHC haplotyped at the University of Wisconsin to construct maximally mismatched donor-recipient pairings. Kidney recipients were treated with belatacept (20 mg/kg on POD 0, 3, 7, and 14, and every 2 weeks thereafter until POD 84), humanized anti-OX40L (Roche, 20 mg/kg on POD 0, 10 mg/kg on POD 3, 7, 14, 21, 28, 35, and 42), or combined therapy. Ratios of blood urea nitrogen to creatinine (BUN/Cre) were measured on POD 1, 3, and 7, and weekly thereafter, and weekly peripheral blood samples were analyzed by flow cytometry.
Donor-specific antibody development.
The development of donor-specific antibody was monitored by flow cross-match. Donor peripheral blood mononuclear cells (3 × 105) were isolated and incubated with titrated recipient serum. Cells were subsequently stained with FITC-labeled goat anti–rhesus IgG (KPL, catalog 072-11-021), PE-labeled anti-CD20 (BD Biosciences, catalog 556633), and PerCP-Cy5.5–labeled anti-CD3 (BD Biosciences, catalog 552852). The positive control was determined by utilizing known rhesus-reactive rhesus IgG1 antibody. MFI was normalized against pretransplant MFI and changes in MFI were measured over the course of transplantation.
Study approval.
Both murine and nonhuman primate experimental subjects received humane care and treatment in accordance with Emory University IACUC guidelines, and all experimental protocols utilizing animals were conducted with approval by this institutional review board.
Statistics.
Skin graft experiments and primate renal transplants are presented on Kaplan-Meier survival curves and were compared with log-rank test. All other assays were compared with the 1-tailed Mann-Whitney nonparametric test. Statistical analyses were conducted using GraphPad Prism. A P value less than 0.05 was considered significant.
Author contributions
WK designed many of the experiments, helped conduct many of the murine skin grafts and primate kidney transplants, analyzed the data, and wrote the manuscript. YD helped perform murine skin grafts and conduct some flow cytometric analyses. DM performed the gene expression analysis experiments. CB performed many of the flow cytometric analyses of primate data and the donor-specific antibody experiments. ES was the supervising veterinarian for all primate experiments. MF and CPL provided help with experimental design and manuscript revision. AA conceived of many of the experiments, assisted with analysis, helped perform primate kidney transplants, and assisted with manuscript editing. MEF provided the anti-OX40L monoclonal antibody and contributed to experiment design.
Supplementary Material
Acknowledgments
WK was supported by the fellowship in transplantation from the American Society of Transplant Surgeons. The primate experiments were also supported by the National Institute for Allergy and Infectious Diseases Nonhuman Primate Transplantation Tolerance Cooperative Study Group grant AI051731 from the NIH. Primate experiments were also supported by the Yerkes National Primate Research Center base grant RR00165.
Footnotes
MEF’s present address is: Bristol-Myers Squibb, Pennington, New Jersey, USA.
Conflict of interest: The authors have declared that no conflict of interest exists.
Reference information:JCI Insight. 2017;2(5):e90317. https://doi.org/10.1172/jci.insight.90317.
Contributor Information
Ying Dong, Email: ydong@emory.edu.
Elizabeth Strobert, Email: estrobe@emory.edu.
Andrew B. Adams, Email: Andrew.b.adams@emory.edu.
References
- 1.Larsen CP, et al. Rational development of LEA29Y (belatacept), a high-affinity variant of CTLA4-Ig with potent immunosuppressive properties. Am J Transplant. 2005;5(3):443–453. doi: 10.1111/j.1600-6143.2005.00749.x. [DOI] [PubMed] [Google Scholar]
- 2.Vincenti F, et al. A phase III study of belatacept-based immunosuppression regimens versus cyclosporine in renal transplant recipients (BENEFIT study) Am J Transplant. 2010;10(3):535–546. doi: 10.1111/j.1600-6143.2009.03005.x. [DOI] [PubMed] [Google Scholar]
- 3.Vincenti F, et al. Belatacept and long-term outcomes in kidney transplantation. N Engl J Med. 2016;374(4):333–343. doi: 10.1056/NEJMoa1506027. [DOI] [PubMed] [Google Scholar]
- 4.Vincenti F, et al. Five-year safety and efficacy of belatacept in renal transplantation. J Am Soc Nephrol. 2010;21(9):1587–1596. doi: 10.1681/ASN.2009111109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Valujskikh A, Pantenburg B, Heeger PS. Primed allospecific T cells prevent the effects of costimulatory blockade on prolonged cardiac allograft survival in mice. Am J Transplant. 2002;2(6):501–509. doi: 10.1034/j.1600-6143.2002.20603.x. [DOI] [PubMed] [Google Scholar]
- 6.Zhai Y, Meng L, Gao F, Busuttil RW, Kupiec-Weglinski JW. Allograft rejection by primed/memory CD8+ T cells is CD154 blockade resistant: therapeutic implications for sensitized transplant recipients. J Immunol. 2002;169(8):4667–4673. doi: 10.4049/jimmunol.169.8.4667. [DOI] [PubMed] [Google Scholar]
- 7.Brook MO, Wood KJ, Jones ND. The impact of memory T cells on rejection and the induction of tolerance. Transplantation. 2006;82(1):1–9. doi: 10.1097/01.tp.0000226082.17507.da. [DOI] [PubMed] [Google Scholar]
- 8.Kitchens WH, et al. Integrin antagonists prevent costimulatory blockade-resistant transplant rejection by CD8(+) memory T cells. Am J Transplant. 2012;12(1):69–80. doi: 10.1111/j.1600-6143.2011.03762.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kinnear G, Jones ND, Wood KJ. Costimulation blockade: current perspectives and implications for therapy. Transplantation. 2013;95(4):527–535. doi: 10.1097/TP.0b013e31826d4672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Burrell BE, Lu G, Li XC, Bishop DK. OX40 costimulation prevents allograft acceptance induced by CD40-CD40L blockade. J Immunol. 2009;182(1):379–390. doi: 10.4049/jimmunol.182.1.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ford ML, et al. Antigen-specific precursor frequency impacts T cell proliferation, differentiation, and requirement for costimulation. J Exp Med. 2007;204(2):299–309. doi: 10.1084/jem.20062319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chauvin JM, et al. TIGIT and PD-1 impair tumor antigen-specific CD8+ T cells in melanoma patients. J Clin Invest. 2015;125(5):2046–2058. doi: 10.1172/JCI80445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kurtulus S, et al. TIGIT predominantly regulates the immune response via regulatory T cells. J Clin Invest. 2015;125(11):4053–4062. doi: 10.1172/JCI81187. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 14.Bachmaier K, et al. Negative regulation of lymphocyte activation and autoimmunity by the molecular adaptor Cbl-b. Nature. 2000;403(6766):211–216. doi: 10.1038/35003228. [DOI] [PubMed] [Google Scholar]
- 15.Croft M, Bradley LM, Swain SL. Naive versus memory CD4 T cell response to antigen. Memory cells are less dependent on accessory cell costimulation and can respond to many antigen-presenting cell types including resting B cells. J Immunol. 1994;152(6):2675–2685. [PubMed] [Google Scholar]
- 16.Ashokkumar C, et al. Alloreactive CD154-expressing T-cell subsets with differential sensitivity to the immunosuppressant, belatacept: potential targets of novel belatacept-based regimens. Sci Rep. 2015;5:15218. doi: 10.1038/srep15218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Weinberg AD. The role of OX40 (CD134) in T-cell memory generation. Adv Exp Med Biol. 2010;684:57–68. doi: 10.1007/978-1-4419-6451-9_5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vu MD, Clarkson MR, Yagita H, Turka LA, Sayegh MH, Li XC. Critical, but conditional, role of OX40 in memory T cell-mediated rejection. J Immunol. 2006;176(3):1394–1401. doi: 10.4049/jimmunol.176.3.1394. [DOI] [PubMed] [Google Scholar]
- 19.Ruby CE, Redmond WL, Haley D, Weinberg AD. Anti-OX40 stimulation in vivo enhances CD8+ memory T cell survival and significantly increases recall responses. Eur J Immunol. 2007;37(1):157–166. doi: 10.1002/eji.200636428. [DOI] [PubMed] [Google Scholar]
- 20.Mousavi SF, et al. OX40 costimulatory signals potentiate the memory commitment of effector CD8+ T cells. J Immunol. 2008;181(9):5990–6001. doi: 10.4049/jimmunol.181.9.5990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xu X, et al. Autophagy is essential for effector CD8(+) T cell survival and memory formation. Nat Immunol. 2014;15(12):1152–1161. doi: 10.1038/ni.3025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vu MD, et al. OX40 costimulation turns off Foxp3+ Tregs. Blood. 2007;110(7):2501–2510. doi: 10.1182/blood-2007-01-070748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xiao X, Kroemer A, Gao W, Ishii N, Demirci G, Li XC. OX40/OX40L costimulation affects induction of Foxp3+ regulatory T cells in part by expanding memory T cells in vivo. J Immunol. 2008;181(5):3193–3201. doi: 10.4049/jimmunol.181.5.3193. [DOI] [PubMed] [Google Scholar]
- 24.Yuan X, et al. The role of the CD134-CD134 ligand costimulatory pathway in alloimmune responses in vivo. J Immunol. 2003;170(6):2949–2955. doi: 10.4049/jimmunol.170.6.2949. [DOI] [PubMed] [Google Scholar]
- 25.Wang H, et al. Memory T cells mediate cardiac allograft vasculopathy and are inactivated by anti-OX40L monoclonal antibody. Cardiovasc Drugs Ther. 2014;28(2):115–122. doi: 10.1007/s10557-013-6502-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ehst BD, Ingulli E, Jenkins MK. Development of a novel transgenic mouse for the study of interactions between CD4 and CD8 T cells during graft rejection. Am J Transplant. 2003;3(11):1355–1362. doi: 10.1046/j.1600-6135.2003.00246.x. [DOI] [PubMed] [Google Scholar]
- 27.Dudani R, et al. Multiple mechanisms compensate to enhance tumor-protective CD8(+) T cell response in the long-term despite poor CD8(+) T cell priming initially: comparison between an acute versus a chronic intracellular bacterium expressing a model antigen. J Immunol. 2002;168(11):5737–5745. doi: 10.4049/jimmunol.168.11.5737. [DOI] [PubMed] [Google Scholar]
- 28.Betts MR, et al. Sensitive and viable identification of antigen-specific CD8+ T cells by a flow cytometric assay for degranulation. J Immunol Methods. 2003;281(1–2):65–78. doi: 10.1016/s0022-1759(03)00265-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.