Abstract
Pulmonary hypertension (PH) is a multifaceted vascular disease where development and severity are determined by both genetic and environmental factors. Over the past decade, there has been an acceleration of the discovery of molecular effectors that mediate PH pathogenesis, including large numbers of microRNA molecules that are expressed in pulmonary vascular cell types and exert system-wide regulatory functions in all aspects of vascular health and disease. Due to the inherent pleiotropy, overlap, and redundancy of these molecules, it has been challenging to define their integrated effects on overall disease manifestation. In this review, we summarize our current understanding of the roles of microRNAs in PH with an emphasis on potential methods to discern the hierarchical motifs governing their multifunctional and interconnected activities. Deciphering this higher order of regulatory structure will be crucial for overcoming the challenges of developing these molecules as biomarkers or therapeutic targets, in isolation or combination.
This review summarizes our understanding of non-coding RNAs in pulmonary hypertension, with an emphasis on methods to understand the overarching framework of non-coding RNA activities.
Introduction
Pulmonary hypertension (PH) is a complex and enigmatic vascular disease characterized by pulmonary vascular remodeling and dysfunction, right ventricular hypertrophy and failure, and often death. Exogenous injury (i.e., hypoxia, inflammation, infection) and various other illnesses are linked to PH development, and gene mutations (BMPR2, SMAD9, etc.) predispose to hereditary forms of PH (1). WHO categorizes PH into five main groups (2) based on these observations, but variation in disease manifestation suggests that multiple factors dictate individualized disease phenotypes. Current medications used for PH are limited to three classes of drugs that all affect pulmonary vasodilation (relaxation) and for the most part are only approved for use in a particularly severe form of PH, pulmonary arterial hypertension (PAH; WHO Group 1), as well as chronic thromboembolic PH (CTEPH; WHO Group 4) (3). These medications do not regress, prevent, or cure the disease.
Attempts to identify the underlying pathobiology of this disease, as well as effective targets for the next generation of PH drugs, has led to the study of an evolutionarily ancient, but only recently appreciated, class of molecules called noncoding RNAs (ncRNAs), of which the most widely studied class includes microRNAs (miRNAs). Embedded throughout the human genome, ncRNAs and miRNAs carry pervasive transcriptional and posttranscriptional regulatory actions relevant to human health and disease, including PH. As our appreciation of the actions of these molecules has advanced, our conception of the complexity of PH has also grown, often causing more confusion, particularly about the varied actions of miRNAs. At the same time, there has been growing evidence of overarching programs of regulation of miRNAs that may allow for mapping of an interconnected hierarchy of miRNA functions. In this review, we define the current state of knowledge of these ncRNAs, with an emphasis on miRNAs, in the pulmonary vasculature. We examine our current understanding of the paradigms of hierarchy, overlap, and redundancy of miRNAs in regulating PH development and progression. Understanding these regulatory motifs will not only facilitate new discoveries in this increasingly complicated disease, but also will be useful for overcoming the challenges of developing these molecules for next-generation clinical diagnostics and therapeutics.
Functional classification of regulatory ncRNAs
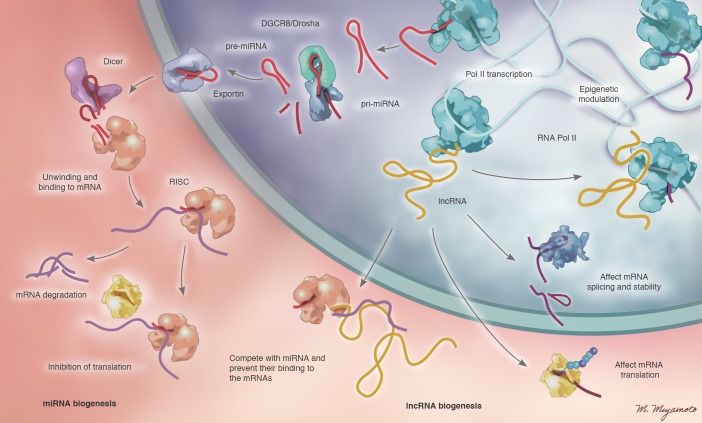
Tens of thousands of regulatory ncRNAs are predicted to be expressed in human cells. These classes include miRNAs, long noncoding RNAs (lncRNAs), circular RNAs (circRNAs), and piwi-interacting RNAs (piRNAs), among others (4), and their complex biology is rapidly being elucidated. For example, lncRNAs are single-stranded RNAs that are dynamically regulated in the development of PH (5). While some have been shown to have alternative protein-coding functions (6), most lncRNAs affect cellular function by complexing with chromosomal DNA, RNAs, or proteins. lncRNAs often epigenetically regulate gene expression via alteration of chromosomal packaging (7, 8), while others have been described to prevent miRNA binding to target mRNAs (9). Recently, a specific lncRNA, MALAT1, was found to be associated with PH in humans (10) and acts as a competitive inhibitor of miRNA-214, thereby affecting endothelial cell functions (5), including proliferation and migration (11). The biogenesis and emerging functions of lncRNAs are summarized in Figure 1 (12, 13), yet the most advanced insights regarding ncRNAs in the pulmonary vasculature have focused on miRNAs.
Figure 1. Biogenesis and functions of miRNAs and lncRNAs in PH.
(Left) miRNAs are transcribed from the genome in the form of long primary miRNAs (pri-miRNAs), which are then processed by the Drosha/DGCR8 enzyme complex into smaller precursor miRNAs (pre-miRNAs). These small pre-miRNAs are exported into the cytoplasm in an energy-dependent process. In the cytoplasm, pre-miRNAs are acted upon by Dicer to form mature miRNAs. Mature, active miRNAs interact with the RISC (RNA-induced silencing complex), leading to unwinding of duplex miRNAs and binding to the complementary sequence in the 3′ untranslated region. Binding of miRNAs to mRNAs can lead to inhibition of translation or mRNA degradation. (Right) Alternatively, after transcription, lncRNAs can interact with DNA or protein molecules directly, often affecting chromatin structure. lncRNAs have also been described to complex with RNAs, such as mRNAs, to influence posttranscriptional splicing and translation or miRNAs to influence target transcript engagement. Illustrated by Mao Miyamoto.
miRNAs.
miRNAs are small, evolutionarily conserved RNAs that negatively regulate gene expression at the posttranscriptional level. As summarized in Figure 1 (14, 15), miRNAs are transcribed either as a part of an miRNA cluster or alone and can be embedded within introns of protein-coding genes or at entirely separate genomic locations. miRNAs are transcribed as longer primary miRNAs (pri-miRNAs), which are then processed in the nucleus into shorter precursor miRNAs (pre-miRNAs). Pre-miRNAs are then exported into the cytoplasm, where they are cleaved by the protein Dicer into mature miRNAs. Mature duplex miRNAs then interact with the Argonaute (Ago) protein complex, leading to binding of either of the two miRNA strands to specific sequences in target mRNAs, typically in the 3′ untranslated regions (3′ UTRs). Engagement of miRNAs with target mRNAs can either degrade mRNAs or inhibit their translation. A number of validated online algorithms exist to predict target mRNAs for individual miRNAs (such as refs. 16, 17), and it has been reported that hundreds of target mRNAs may be engaged by a single miRNA (18).
Over the past five years, our appreciation of the pervasive yet complex activity of miRNAs in the pulmonary vasculature has accelerated. We and others have catalogued lists of these miRNAs that have been studied in cultured pulmonary vascular cells, in various animal models of PH, and in human explanted tissues and cells (including refs. 19–22, among others). As these lists have expanded, the interconnected relationships among these miRNAs and their downstream targets and pathways have been challenging to decipher. In this review, we present a conceptual framework for considering groups of these miRNAs and defining potential hierarchical motifs of these molecules.
Cataloguing miRNAs based on their convergent/divergent functions
Convergence on single molecular pathways and cellular phenotypes
As the numbers of miRNAs relevant to PH pathobiology have grown, a predominant hierarchical motif that has emerged includes the functional convergence of discrete cohorts of miRNAs on single molecular pathways relevant to pulmonary vascular pathogenesis. Below are examples of such a convergence on PH-specific pathways and cellular phenotypes. In some cases, a wide range of redundancy appears to be shared among many miRNAs, emphasizing the actions of particular individual miRNAs more as “fine tuners” rather than binary “on/off” biologic switches. In other cases, these analyses have emphasized the context-specific nature of these miRNAs and their targets, suggesting their role in defining the individualized nature of PH manifestations.
BMPR2 signaling.
Substantial causative links, both genetic and environmental, exist between PH and dysregulated bone morphogenetic protein receptor 2 (BMPR2) signaling (as reviewed by ref. 23). Loss of function of BMPR2 has been linked to cellular pathophenotypes, including proliferation, cell survival (23), repression of mitochondrial metabolism (24), and endothelial-to-mesenchymal transition (25), among others. However, the comprehensive mechanisms of regulation of BMPR2 that therefore control the specific and often individualized cadence of disease manifestations have yet to be elucidated.
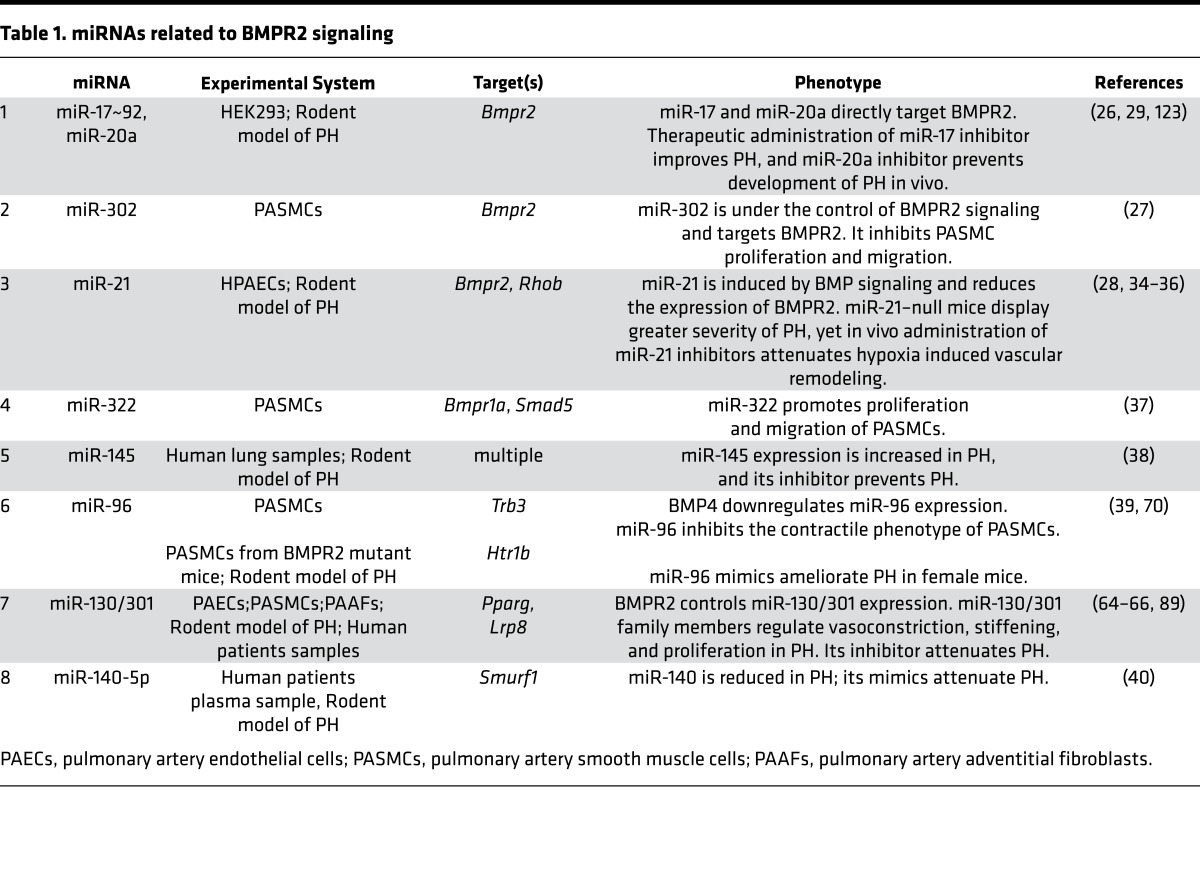
A variety of miRNAs have been causatively linked to BMPR2 expression and signaling in pulmonary vascular cells in vitro and in vivo. Multiple miRNAs directly regulate BMPR2, including the miR-17/92 cluster (26), miR-302-367 cluster (27), and miR-21 (28). In turn, many of these miRNAs were confirmed to modulate PAH in vivo via use of antisense oligonucleotide inhibitor (antagomirs) (29, 30). In addition, miR-21 processing is regulated by BMP signaling globally (31–33) and was shown to control PAH progression in vivo by genetic KO studies (28, 34) and pharmacologic inhibition of miR-21 (35, 36). Despite their modulatory activity in PH, however, actions of these miRNAs do not fully reverse disease manifestations, indicating a level of redundancy and actions as “fine tuners” consistent with their shared targeting of BMPR2. Beyond regulating BMPR2 directly, other miRNAs target other BMP or TGF superfamily signaling components or are themselves modulated by BMP signaling. Under hypoxic conditions, HIF-1α induces miR-322, which targets BMPR1a and SMAD5, thereby downregulating BMPR2 signaling (37). miR-145 is regulated by BMP4 and is overexpressed in PH, and its genetic deletion prevents the development of PH in mice (38). BMP4 also downregulates miR-96, which in turn inhibits TRB3, a BMP signaling effector, thus affecting a cohort of pulmonary vascular smooth muscle–specific genes (39). Similarly, miR–140-5p targets SMURF1, which regulates BMP signaling and PH in vivo (40). Figure 2A and Table 1 summarize the miRNAs identified to date that are involved in BMPR2 signaling and also carry a causative connection to PAH. It has yet to be determined whether targeted alterations of combinations of these related miRNAs may yield more robust and tailored effects on BMP signaling and PH pathogenesis.
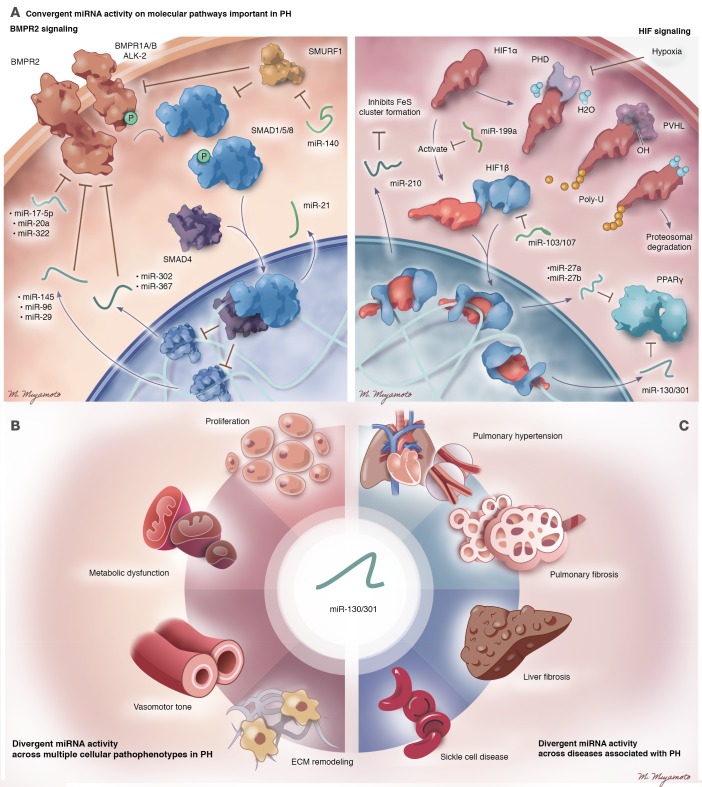
Figure 2. A conceptual schematic to categorize the activities of miRNAs in PH.
Currently identified miRNAs that control PH often congregate into higher-order regulatory motifs consistent with convergent or divergent activity across molecular pathways, cellular pathophenotypes, and associated diseases. Representative examples of each category are shown. Such conceptual and often overlapping annotations may be helpful as roadmaps in deciphering the hierarchies of function among sets of miRNAs, their downstream target pathways, and resultant pulmonary vascular phenotypes. (A) Convergent miRNA activity on single molecular pathways. Related cohorts of miRNAs exist with convergent activity on BMPR2 signaling (left panel) and HIF signaling (right panel). (B) Divergent miRNA activity across multiple cellular pathophenotypes. The miR-130/301 family acts as a system-level regulator of proliferation, vasomotor tone, vascular stiffness, and metabolism across three different pulmonary vascular cell types. (C) Divergent miRNA activity across associated disease. The miR-130/301 family controls manifestations of PH, pulmonary fibrosis, liver fibrosis, and sickle cell disease. Illustrated by Mao Miyamoto.
Table 1. miRNAs related to BMPR2 signaling.
HIF signaling.
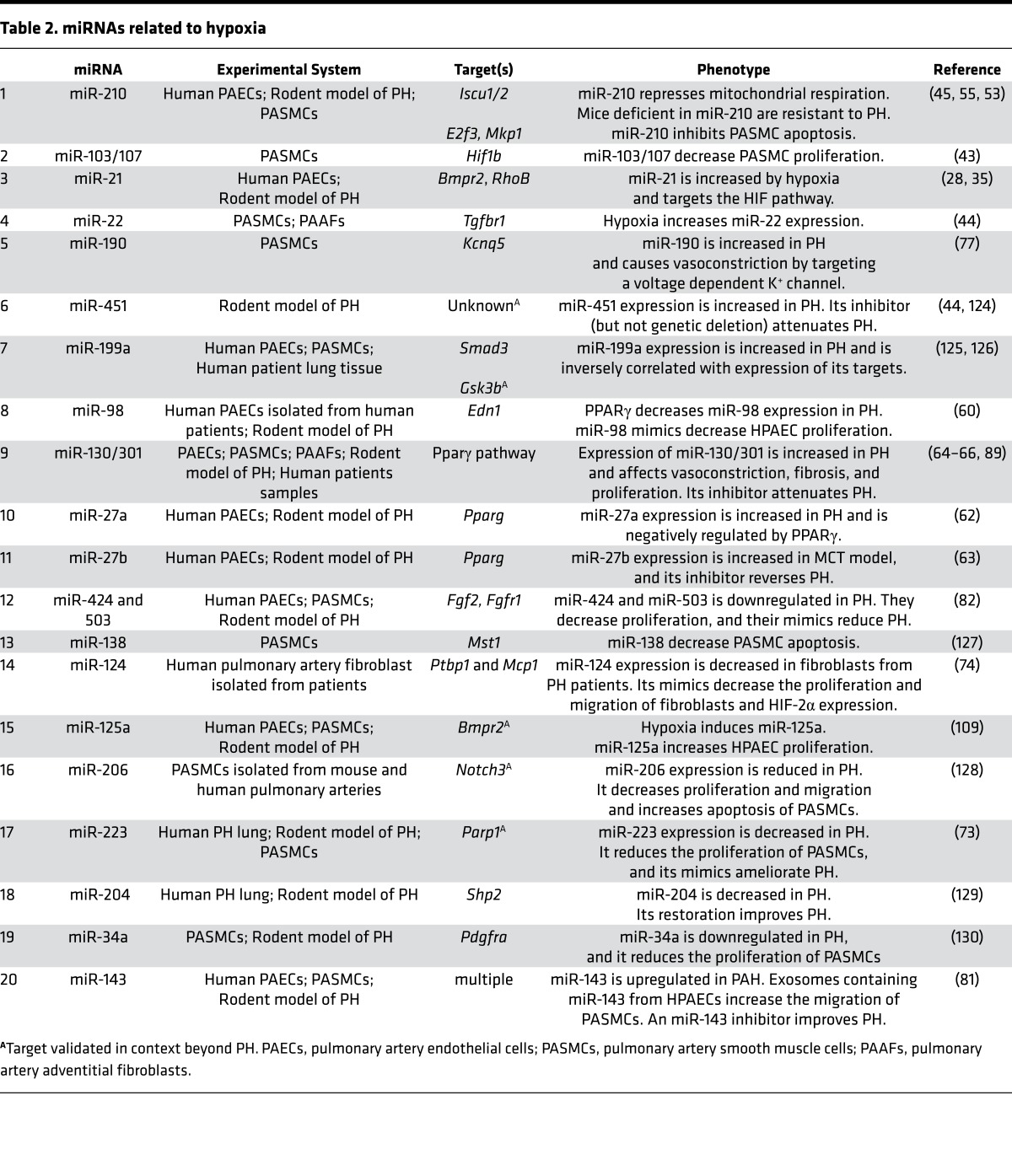
Chronic hypoxia, or low oxygen exposure, is a key trigger for pulmonary vascular remodeling and PH. It is associated with alteration in various PH-relevant pathways leading to vascular proliferation, metabolic shifts from oxidative phosphorylation to glycolysis, inflammation, and vascular stiffness. The transcription factor HIF-1α is a major mediator of these effects, and similar to BMPR2 signaling, HIF-1α also modulates the expression of various miRNAs and reciprocally is regulated by them, as reviewed by ref. 41. For example, miR-424 has been found to stabilize HIF-1α by targeting its negative regulator and thus promoting angiogenesis in vitro and in vivo (42). In another case, the binding partner of HIF-1α, HIF-1β, was reported to be regulated by miR-103/107 (43). Caruso et al. were the first to our knowledge to report substantial numbers of miRNAs dysregulated at various time points in a hypoxic rodent model of PH (44). A number of these miRNAs coincided with prior studies of HIF-dependent miRNAs in cultured pulmonary arterial (45), endothelial (46), and transformed cells (47). While some of these miRNAs, such as miR-210, are direct transcriptional target genes of HIF-1α, the upregulation of others may be due to the global stabilization of Ago complexes in hypoxia, thus augmenting the processing of miRNAs to their mature active forms (48). Additional complexity likely exists, as more recent studies have suggested. For instance, hypoxia decreases Dicer expression, which in turn reduces the overall biogenesis of miRNAs (49–51). In some cases, this process may be promoted by direct miRNA engagement of Dicer (52).
Among upregulated “hypoxamirs”, substantial efforts have been made to delineate the functions of miR-210 in PH, given its direct and robust link to HIF-1α and hypoxia. miR-210 was found to have substantial pleiotropic function in mitochondrial metabolism (45), proliferation (53, 54), and cell survival, thus promoting vascular remodeling and PH in vivo (55). miR-210 is also released into the extracellular space and may function as an endocrine or paracrine messenger of hypoxic stress among anatomically distinct tissues (56). Notably, because miR-210 appears primarily active in hypoxia, its pathogenic actions in PH may be limited to subtypes of HIF-relevant PH and may correlate with individualized differences of PH manifestations in WHO Group 3 PH (stemming from hypoxic lung disease) vs. nonhypoxic disease forms. A multitude of additional HIF-dependent miRNAs have been described (Figure 2A and Table 2), but, in general, their relevance in vivo to PH — particularly in humans — has yet to be defined.
Table 2. miRNAs related to hypoxia.
Peroxisome proliferator-activated receptor-γ (PPARγ) signaling.
PPARγ is a nuclear hormone receptor that interacts with effectors, such as the retinoid receptor (RXR), upon binding its ligand, triggering transcriptional activation of its target genes. PPARγ activates an antiproliferative and proapoptotic program in the pulmonary vasculature, and its reduced expression contributes to PH progression (57, 58). PPARγ regulates various miRNAs, such as miR-199a (59), miR-98 (60), and miR-21 (61), which, in turn, control effectors of hypoxia, including HIF-1α, the vasoconstrictor endothelin-1, and the tumor suppressor PTEN, respectively. Reciprocally, PPARγ is modulated by miRNAs under hypoxic conditions in PH, such as miR-27a (62) and miR-27b (63). Intriguingly, in a computational analysis of miRNAs predicted to target PH-related gene networks, the miR-130/301 family was identified as a master regulator of multiple PH pathways and PH in vivo, by engaging PPARγ as a central mediator (64–66). Other indirect miRNA-mediated links to PPARγ signaling have been noted, including downregulation of miR–193-3p in PH after administration of apolipoprotein A-I mimic peptides that affected the related transcription factor RXRα (67). Thus, although all of these miRNAs converge upon PPARγ, how these miRNAs interact with one another and interface with other signaling pathways in PH remains to be determined.
Estrogen signaling.
PAH is more prevalent in women as compared with men, but PAH tends to manifest greater severity in men (68, 69). The molecular mechanisms for such sex predisposition are not well defined, but historically, emphasis has focused on the differential effects of sex-specific hormones such as estrogen in the pulmonary vasculature. Recently, miRNAs were found to play a role in estrogen signaling in PH. Estrogen was reported to downregulate miR-96 in pulmonary artery smooth muscle cells (PASMCs) isolated from female, but not male, BMPR2 mutant mice. In turn, lower levels of miR-96 directly affected its target serotonin receptor 5-HT1BR, thus promoting PH (70). Another study observed an increase in miR-29 family (miR-29a, -b, -c) expression in female PH patients — an effect amplified by the estrogen metabolite 16α-hydroxyestrone (16αOHE) and with downstream effects on the miR-29 target PPARγ and resultant metabolic vascular phenotypes (71). The role of miRNAs under regulation by other (male or female) sex hormones in PH has yet to be explored in depth. Nonetheless, these initial findings support the notion that miRNA-based tuning of PH differs between males and females and may be essential features of how susceptibility and severity of PAH manifest differently between sexes.
Related cellular phenotypes.
Downstream of these molecular pathways, miRNAs also act in concert to affect various cellular pathophenotypes such as mitochondrial and metabolic dysfunction, vascular stiffness, vasomotor tone, proliferation, and apoptosis among others. For example, HIF-related miRNAs — such as miR-210, which affects electron transport (55) — may functionally interface with miR-138 and miR-25, which target the mitochondrial calcium uniporter complex (MCUC), and ultimately promote aerobic glycolysis in PH (72). Alternatively, the HIF-responsive miR-223 was found to induce a separate process of DNA damage in PASMCs, thus linking hypoxia and chromosomal integrity in PH (73).
There is an increasing appreciation that vascular stiffness and extracellular matrix remodeling are early causative events in PH pathogenesis, with both the miR-130/301 family (64) and miR-124 (74) contributing to this phenotype in adventitial fibroblasts. Other miRNAs have been causatively linked to alterations in pulmonary vasomotor tone. For instance, miR-648, miR-199a2, and miR-27a have been linked to the vasoconstrictor endothelin-1 (59, 62, 75), while miR-328 (76) and miR-190 (77) were found to control downstream calcium and potassium influx in contracting PASMCs. Many other miRNAs have been implicated in the control of the vasodilator nitric oxide (78) but with less emphasis thus far on their roles in the pulmonary vascular compartment. Notably, thrombosis and thrombosis-in-situ have been identified as critical PH pathophenotypes, yet rigorous study of miRNAs in platelet biology and the clotting cascade in relation to the pulmonary vasculature has yet to be pursued. Finally, reflecting the advancing molecular parallels between PAH and cancer (79), numerous miRNAs affecting proliferation and cell survival have been implicated in PH, including those targeting chromatin remodeling, such as miR-204 (80), and endothelial to smooth muscle cellular crosstalk, such as miR-143 (81) and miR-424/503 (82). Thus, when overlaying cellular phenotypes onto molecular pathways, this type of miRNA catalog offers a more expansive hierarchical view of their concerted functions and/or exposes deficiencies in our global understanding of this disease.
Divergence of miRNA function controlling multiple molecular pathways
While the convergence of miRNA networks on distinct target pathways has led to a defining of the overlapping functions of these molecules, a parallel investigation of the divergent actions of specific miRNAs in PH has offered separate insights into pathogenesis. Namely, the sheer but specific pleiotropy of miRNAs has driven the notion that there may exist “master miRNA regulators” of PH that drive pathogenesis via control over numerous, seemingly unrelated, molecular pathways. For example, miR-98 and miR-27a/b have been implicated in the control of overlapping pathways involving both hypoxic adaptation and PPARγ signaling (60, 62, 63). However, utilizing solely traditional reductionist scientific approaches likely limits the search for “master regulators” of PH to only those pathways with already-known molecular links.
For that reason, we have attempted to use both traditional and nontraditional computational approaches in tandem to identify and confirm such miRNA activity in PH. For example, we originally devised a computational method to analyze the architecture of gene networks in order-rank miRNAs with network-wide activity on verified PH-specific genes and their first degree gene interactors (“the PH network, ” mapped using a consolidated set of databases cataloguing all functional interactions reported in the human transcriptome) (28). Our first mathematical iteration identified miR-21 as controlling multiple PH target genes and pathways, including BMPR2 expression and hypoxic reprogramming, which we and others later validated in vitro and in PH rodents in vivo (Tables 1 and 2). Such experimental interrogation also revealed that actions of miR-21 in PH (28, 34–36) and elsewhere (22, 83) are context specific and possibly cell specific. Thus, miR-21, while pleiotropic in its effects in PH, also carries a dynamic and shifting repertoire of actions that makes its functions as a consistent regulator of PH more challenging to define.
Learning from those experiences, we optimized our computational methodology to rank miRNAs based on the number and intercluster spread of their target pools in a larger PH gene network. We identified the miR-130/301 family as the top-ranked miRNAs with actions spanning the entire PH network, and thus highly likely to act as a master regulator of PH (66). We also identified genes in the miR-130/301 target pool in silico by “hubness” and other centrality metrics, thus highlighting the importance of PPARγ as a direct miR-130/301 target. In turn, this led to the discovery linking this mirNA family to two downstream proliferative pathways in the lung, a molecular axis important in fibrosis and extracellular matrix (ECM) remodeling, and vasomotor tone. Specifically, our analyses indicated that miR-130/301 also serves as a proximal regulator of two subordinate miRNAs important in controlling cellular proliferation in the pulmonary vasculature: miR-424/503 and miR-204. We verified these predictions in vivo and in vitro, demonstrating that the miR-130/301 family was upregulated in the pulmonary vasculature of PH patients (driven by hypoxia, inflammatory cytokines, and BMPR2 deficiency). It modulated vascular proliferation, controlled pulmonary vasomotor tone, and drove a mechanosensitive YAP/TAZ-miR-130/301 axis to promote pulmonary vascular stiffness and a metabolic shift in glutamine consumption (64–66, 84) (Figure 2B). Such system-level discovery in PH provides a glimpse of the extent of miRNA pleiotropy and suggests that the divergent activity of specific miRNAs may provide an opportunity for defining effective diagnostic and therapeutic targets based on their far-reaching influences on multiple PH pathways.
Divergence of PH-relevant miRNA activity among human diseases
Beyond their molecular or cellular landscapes of activity, a cohort of miRNAs is emerging that control shared cellular phenotypes across various diseases. Further identifying miRNAs with shared disease association may offer insights into the relationship of PH to the multitude of its associated secondary diseases or systemic complications beyond the vasculature (2). For example, a number of miRNAs, including let-7 family members (85, 86), are particularly active in both acute pulmonary embolism and CTEPH (WHO Group 4 PH). Additionally, a polymorphism in the 3′ UTR in the fibrinogen-α gene (FGA) that affects the binding of miR-759 has been associated with CTEPH (87) and pulmonary embolism. In the case of PH associated with sickle cell disease, miR-199a was reported to reduce HIF-1α expression (59). In perhaps a related context, placental growth factor (PIGF), which is upregulated in sickle cell anemia and may play a key role in promoting PH, has been associated with alterations in miR-648 (75) and the HIF-dependent and PPARα-dependent miR-301a/454 family (88). Interestingly, in independent work related to chronic lung disease, we demonstrated that the miR-130/301 family can exert pleiotropic control over fibrosis in a network of human diseases, including interstitial lung disease and liver disease (89) — both diseases that carry known clinical associations with PH but with poorly defined molecular underpinnings that link these diseases together. Such insights now highlight the importance of shared miRNA-dependent pathogenic processes across related diseases (Figure 2C).
Beyond the vasculature, alterations of miRNAs have been observed in controlling skeletal muscle abnormalities and right ventricular dysfunction (90) and failure in PH. Via modulation of contractility and angiogenesis, downregulation of miR-208 (91) and miR-126 (92) has been implicated in the transition from a compensated to a decompensated right ventricle (RV). At the same time, downregulation of the endothelial-specific miR-126 was found to impair angiogenic potential in skeletal muscle and was associated with exercise intolerance in PH (93). Further delineation of the shared activity of miRNAs across diseases and in the extra pulmonary compartment may be valuable for establishing molecular links that underlie integral, and potentially surprising, disease associations with PH.
Challenges and opportunities for understanding miRNA biology in PH
The convergent and divergent functions of miRNAs alone offer a wealth of information regarding the pathogenesis of PH, yet because of the sheer number of miRNAs and their target genes, acceleration of these discoveries in the future will necessitate addressing a number of technical and conceptual obstacles (Figure 3).
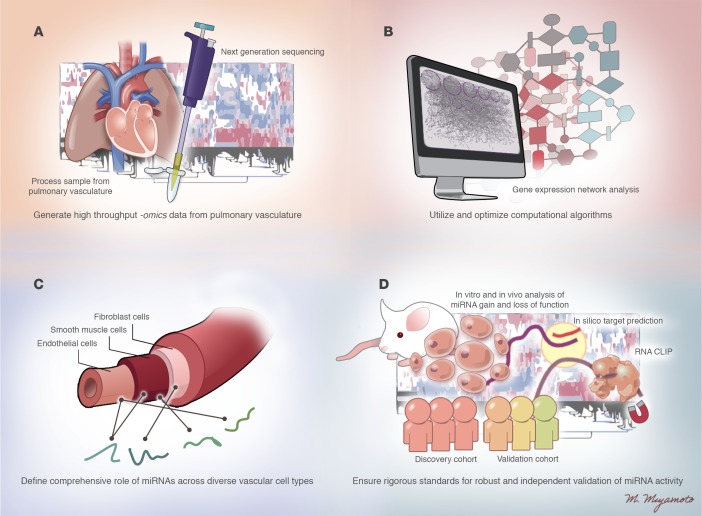
Figure 3. Technical and conceptual challenges for further interrogation of miRNA biology in PH.
Current technical and conceptual challenges are listed for addressing the complexity of miRNA activity in PH and ensuring successful development of miRNA-based diagnostics and therapeutics. (A) Generation of high throughput –omics data from the pulmonary vasculature, accounting for spatial (cell type specific) and temporal (time dependent) events in PH progression. (B) Utilization and optimization of computational algorithms for reliable in silico processing and analysis of forthcoming “big data.” (C) Definition of the comprehensive roles of miRNAs across diverse vascular cell types to explore further the RNA-dependent control of vascular crosstalk. (D) Establishment of rigorous standards for robust and independent validation of miRNA activity in PH. Illustrated by Mao Miyamoto.
First, molecular and phenotypic hierarchies could be further unraveled by generation and analyses of data of high-throughput -omics profiling relevant to pulmonary vascular biology. However, attempts at comprehensive molecular profiling in PH have only begun. The deficiency in these data currently has likely led to bias in the choice of which miRNA and target genes are interrogated further – typically focusing on links to already known pathways relevant to PH. Federal initiatives such as the PVDOMICS program (RFA-HL014-030) are a crucial first step to generating these high-density data sets to convince investigators to take a conceptual leap to study targets entirely unknown to this disease. New methodology to isolate diseased cell types from living PH patients (94) will also ensure a substantial advance toward an era of personalized miRNA medicine in PH.
Second, with a global investment in high-throughput data, our computational techniques will also need to evolve, particularly for ascertaining a comprehensive view of miRNA-target effector network architecture and kinetics. For example, most miRNAs studied to date in PH were identified by their differential disease expression. However, in a disease state such as PH, we now know that a given miRNA can shift its target gene pool and functions, without altering its own expression (i.e., via alterations of target gene stoichiometry, alterations of A-U nucleotide editing, etc.; ref. 95), as in the case of miR-21 (28). Thus, rather than merely attaining differential gene expression lists, the delineation of coexpression networks of miRNAs with their targets could offer otherwise hidden insights embedded in a seemingly endless array of data. Statistical algorithms to generate differential dependency networks such as Evaluation of Dependency DifferentialitY (EDDY) (96) have been implemented effectively in cancer data sets and could reveal insights into the individualized responses of these molecules in PH progression or with therapeutic interventions. To do so, however, a much greater financial and intellectual impetus for collaboration will be necessary to bring together computational scientists, experimentalists, and PH clinicians — groups that have had little previous opportunity for establishing long-lasting working relationships.
Third, few attempts have been made to analyze cell type–specific actions of miRNAs in the pulmonary vasculature and failing RV. This likely stems from the fact that most vascular miRNAs identified as relevant to PH to date are ubiquitously expressed in multiple cell types (22). Thus, many miRNAs have been associated with PH pathophenotypes that are shared among multiple diseased pulmonary vascular cell types (i.e., miR-130/301 affecting proliferation; ref. 66). Alternatively, for some miRNAs, their relevance to PH has thus far only been established in one cell type (i.e., miR-124 in fibroblasts; ref. 74) but may nonetheless be active in other cell types. An interrogation of miRNA actions across cell types may define another layer of interconnectivity. For instance, it may reveal a wide network of direct paracrine or endocrine messaging among cells that rely upon cellular release and uptake of miRNAs. Indeed, recent studies have suggested roles for cell-free miRNAs taken up by pulmonary vascular cell types in response to hypoxia (miR-210) (56) and in remodeled pulmonary vessels (miR-143) (81). Thus, a more comprehensive endeavor to differentiate cell type–specific functions of miRNAs may offer an entirely new appreciation of pulmonary vascular crosstalk.
Fourth, maintaining rigor in miRNA science is especially important, as –omics analyses reach a zenith. Considerations include the ultra-sensitive but differing modalities of quantifying miRNA expression (often leading to false-positive detection of miRNAs in contaminated reagents; ref. 97). In part, these issues may result from the dynamic and context-specific nature of miRNA expression and function coupled with the often modest and “fine tuning” effects on their direct targets. Although there exists no fool-proof solution, rigor can be strengthened at the level of the researcher and peer reviewer by requiring multiple independent modalities of assessing miRNA expression and function. For example, while global study of miRNAs by RNA sequencing continues to become less costly (98), results from this modality still should be verified by a separate method of RNA detection (i.e., RT-PCR, Northern blot). In cases where human tissue or plasma is studied, investigators should strive to include both a discovery and validation cohort, if possible. In screening for miRNA target genes, the use of computational algorithms predicting miRNA targets, which have varying degrees of false-positive and false-negative predictive rates (99), can be strengthened when coupled with biochemical screening assays such as RNA-CLIP (AGO-miRNA immunoprecipitation) (100). Finally, both gain- and loss-of-function assays to overexpress and inhibit miRNA function in vitro and in vivo are extremely valuable when utilized in tandem for assessing the robustness and biologic relevance of a given miRNA. More effective and less cumbersome modalities to modulate cohorts of miRNAs in the same cells or tissues simultaneously will be particularly useful in interrogating the complex interactions of multiple miRNAs. In sum, more comprehensive experimental methodology will be crucial for determining which miRNAs may be developed further for effective diagnostics and therapeutics in PH.
Implementing complex miRNA biology into diagnostics and therapeutics
miRNA diagnostics.
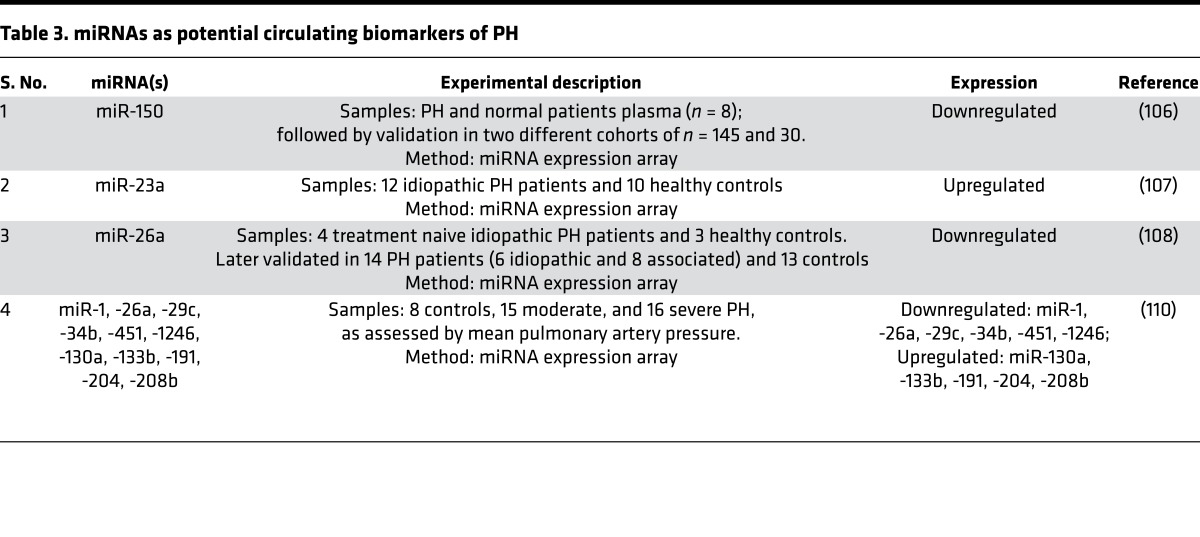
The ability to identify stabilized miRNAs in the plasma and extracellular space has offered the potential to leverage their quantitation for diagnostic possibilities in PH. Cell-free, plasma-based miRNAs are packaged either with AGO2 in microvesicles (101) or as free-floating RNA-protein complexes (102), as we reported (56). Some cell-free miRNAs may be released and/or taken up by diseased vascular or cardiac tissue and may be handled distinctly from endogenous miRNAs (103). This may allow for effective communication among immune cells and vascular endothelium (104, 105) and enable pulmonary vascular crosstalk in diseased pulmonary vessels (miR-143) (81). Other miRNAs have been reported as differentially expressed in the circulating plasma of PH patients, including miR-150 (106), miR-23a (107), miR-26a (108), and miR-125a (109), but their biology is less understood. Alteration of entire cohorts of plasma miRNAs has been reported in PH (107, 110), as well as CTEPH (86). While these and other miRNAs are considered as prospective biomarkers in PH (Table 3), numerous pitfalls remain for their development as robust diagnostic tests, including issues with interindividual variability, reproducibility among patient cohorts, the tissue source of circulating RNAs, appropriate modality of testing, and the complexity of validating a true signature of miRNAs rather than a single biomarker.
Table 3. miRNAs as potential circulating biomarkers of PH.
Beyond plasma, quantification of intracellular miRNA content in blood cells may mitigate some of the variability seen in plasma sampling. While such strategies clearly are not reflective of miRNA-based processes occurring in vascular tissue directly, our advancing understanding of inflammatory activation in PH (111, 112) indicates the relevance of tracking blood-borne inflammatory cells and the miRNAs that control their function.
Finally, rather than relying solely on miRNA expression, diagnostic miRNA testing could be enhanced by further definition of genomic polymorphisms or variants that contribute to PH. Nucleotide variants may localize to a miRNA sequence, the miRNA biogenesis machinery, or to the binding sites of target mRNAs. Such studies have revealed interesting connections of PH to polymorphisms affecting miR-146a processing (113), the 3′ UTR of the epithelial growth factor receptor (114), and the interaction between miR-759 and the fibrinogen α gene (87). As genomic sequencing costs continue to decrease, it may be possible to overlap genomic miRNA-relevant polymorphisms with miRNA expression levels in order to provide more specificity for these diagnostic and prognostic endeavors.
miRNA therapeutics.
Mirroring the emerging trends of RNA-based diagnostics, the prospects of miRNA-specific therapies to augment or knockdown miRNAs are at their inception. Although some miRNAs have been proposed as potential therapeutic targets in PH (19), their clinical potential remains an open question. Primarily, miRNA-based therapeutic strategies have concentrated on the development of stabilized oligonucleotide mimics or inhibitors, but these have yet to be explored in clinical trials for PH. Oligonucleotide therapies have entered the clinical stage for a number of diseases beyond PH (115–117). Regarding miRNAs, currently, only one antisense oligonucleotide miRNA inhibitor, targeting miR-122 in the liver for hepatitis C infection, has undergone a successful phase 2a clinical trial (117). However, a phase 1 clinical trial using miR-34a mimic oligonucleotides for liver cancer (http://clinicaltrials.gov/ct2/show/NCT01829971) was recently halted for several immune-related adverse events.
From a technical perspective, delivery and packaging of oligonucleotides to ensure delivery to the pulmonary vasculature create challenges. Multiple effective routes of administration have been used in PH rodents, including i.v., inhalational, s.c., and i.p. injections. Historically, adenoviral or lentiviral vectors have been proposed for consistent and long-lasting expression in vivo (118, 119), but this approach would require transgenic rather than oligonucleotide-based delivery. For oligonucleotides, conjugation with various agents such as cholesterol, TLR ligands, integrin-specific antibodies, or antibody Fab fragment conjugation have been tested to assist in targeted uptake (120). RNA or DNA binding peptides have also been used in place of protein for linking oligonucleotides to antibodies (120). More recently, the use of polymer-based nanoparticles (55, 121) or packaging in microvesicles (122) has been explored in pharmacologic manipulations of miRNAs in PH. While some tissue specificity was observed, delivery only to the diseased pulmonary vasculature has yet to be achieved in a robust manner. Moreover, targeted delivery may come with a price of unwanted activation of the immune system. Thus, particularly for PH, the desired combinations of oligonucleotide packaging, modifications, and specificity of delivery have been elusive to achieve maximal stability, uptake, and targeting, while minimizing toxicity, immunological activation, and clearance from the system.
Perhaps more importantly, a primary conceptual obstacle includes the choice(s) of which miRNAs to target in PH. While the pleiotropy of several PH-relevant miRNAs has been touted as an advantage for developing broadly acting therapies, disadvantages have emerged regarding the toxic side effects of any miRNA-specific drug and the context-specific activities of these miRNAs across different tissue or cell types. Furthermore, given the overlapping and sometimes redundant biology of miRNAs in PH, it is becoming increasingly likely that a single miRNA target may be insufficient in preventing, improving, or regressing the overall manifestations of PH. Rather, a targeting of a combination of convergent miRNAs, as well as their downstream target genes, may be much more effective. Realization of this strategy, however, would require a systematic mechanism to screen, either computationally or experimentally, such combinations of miRNA-target genes — technology that often is not available in purely academic settings. Thus, successful development of miRNA-based therapeutics in PH will necessitate combining an understanding of the higher-order regulatory hierarchy of miRNA biology with a deeper collaboration of academic investigators with federal, pharma, and biotechnology partners.
Conclusions
The past 15 years have yielded a wealth of scientific insights into the complex biology of ncRNAs and miRNAs in PH and beyond. This growth of knowledge has molded our collective appreciation of the daunting complexity of RNA-based regulation of gene expression in this disease. Thus, in some ways, this complexity has brought more confusion to the precise organized structure of miRNA-based mechanisms that drive disease. To overcome those deficiencies, the next phase of research and discovery will necessitate a pipeline of systematic endeavors designed to catalog and identify the hierarchy of activity inherent in these molecular networks. If successful, that next level of insight should further invigorate interest from academia, federal, and industry partners to pursue the collaborative development of more effective RNA-based diagnostics and therapeutics, based on such systems-level understanding of this disease.
Acknowledgments
This work was supported by NIH grants HL096834 and HL124021 and the American Heart Association (SYC).
Footnotes
Conflict of interest: The authors have declared that no conflict of interest exists.
Published: March 9, 2017
Reference information:JCI Insight. 2017;2(5):e91327. https://doi.org/10.1172/jci.insight.91327.
References
- 1.Chan SY, Loscalzo J. Pathogenic mechanisms of pulmonary arterial hypertension. J Mol Cell Cardiol. 2008;44(1):14–30. doi: 10.1016/j.yjmcc.2007.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Simonneau G, et al. Updated clinical classification of pulmonary hypertension. J Am Coll Cardiol. 2013;62(25 Suppl):D34–D41. doi: 10.1016/j.jacc.2013.10.029. [DOI] [PubMed] [Google Scholar]
- 3.Perrin S, et al. New pharmacotherapy options for pulmonary arterial hypertension. Expert Opin Pharmacother. 2015;16(14):2113–2131. doi: 10.1517/14656566.2015.1074177. [DOI] [PubMed] [Google Scholar]
- 4.Eddy SR. Non-coding RNA genes and the modern RNA world. Nat Rev Genet. 2001;2(12):919–929. doi: 10.1038/35103511. [DOI] [PubMed] [Google Scholar]
- 5.Wang X, et al. Long noncoding RNA expression profiles of hypoxic pulmonary hypertension rat model. Gene. 2016;579(1):23–28. doi: 10.1016/j.gene.2015.12.044. [DOI] [PubMed] [Google Scholar]
- 6.Wilusz JE, Sunwoo H, Spector DL. Long noncoding RNAs: functional surprises from the RNA world. Genes Dev. 2009;23(13):1494–1504. doi: 10.1101/gad.1800909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kung JT, Colognori D, Lee JT. Long noncoding RNAs: past, present, and future. Genetics. 2013;193(3):651–669. doi: 10.1534/genetics.112.146704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rinn JL, Chang HY. Genome regulation by long noncoding RNAs. Annu Rev Biochem. 2012;81:145–166. doi: 10.1146/annurev-biochem-051410-092902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ballantyne MD, McDonald RA, Baker AH. lncRNA/MicroRNA interactions in the vasculature. Clin Pharmacol Ther. 2016;99(5):494–501. doi: 10.1002/cpt.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhuo Y, Zeng Q, Zhang P, Li G, Xie Q, Cheng Y. Functional polymorphism of lncRNA MALAT1 contributes to pulmonary arterial hypertension susceptibility in Chinese people. Clin Chem Lab Med. 2017;55(1):38–46. doi: 10.1515/cclm-2016-0056. [DOI] [PubMed] [Google Scholar]
- 11.Michalik KM, et al. Long noncoding RNA MALAT1 regulates endothelial cell function and vessel growth. Circ Res. 2014;114(9):1389–1397. doi: 10.1161/CIRCRESAHA.114.303265. [DOI] [PubMed] [Google Scholar]
- 12.Fatica A, Bozzoni I. Long non-coding RNAs: new players in cell differentiation and development. Nat Rev Genet. 2014;15(1):7–21. doi: 10.1038/nri3777. [DOI] [PubMed] [Google Scholar]
- 13.Ponting CP, Oliver PL, Reik W. Evolution and functions of long noncoding RNAs. Cell. 2009;136(4):629–641. doi: 10.1016/j.cell.2009.02.006. [DOI] [PubMed] [Google Scholar]
- 14.Bushati N, Cohen SM. microRNA functions. Annu Rev Cell Dev Biol. 2007;23:175–205. doi: 10.1146/annurev.cellbio.23.090506.123406. [DOI] [PubMed] [Google Scholar]
- 15.Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116(2):281–297. doi: 10.1016/S0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 16.Friedman RC, Farh KK, Burge CB, Bartel DP. Most mammalian mRNAs are conserved targets of microRNAs. Genome Res. 2009;19(1):92–105. doi: 10.1101/gr.082701.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yue D, Liu H, Huang Y. Survey of Computational Algorithms for MicroRNA Target Prediction. Curr Genomics. 2009;10(7):478–492. doi: 10.2174/138920209789208219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lewis BP, Shih IH, Jones-Rhoades MW, Bartel DP, Burge CB. Prediction of mammalian microRNA targets. Cell. 2003;115(7):787–798. doi: 10.1016/S0092-8674(03)01018-3. [DOI] [PubMed] [Google Scholar]
- 19.Chun HJ, Bonnet S, Chan SY. Translational Advances in the Field of Pulmonary Hypertension. Translating MicroRNA Biology in Pulmonary Hypertension. It Will Take More Than “miR” Words. Am J Respir Crit Care Med. 2017;195(2):167–178. doi: 10.1164/rccm.201604-0886PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Boucherat O, Potus F, Bonnet S. microRNA and Pulmonary Hypertension. Adv Exp Med Biol. 2015;888:237–252. doi: 10.1007/978-3-319-22671-2_12. [DOI] [PubMed] [Google Scholar]
- 21.Rothman AM, Chico TJ, Lawrie A. MicroRNA in pulmonary vascular disease. Prog Mol Biol Transl Sci. 2014;124:43–63. doi: 10.1016/B978-0-12-386930-2.00003-3. [DOI] [PubMed] [Google Scholar]
- 22.White K, Loscalzo J, Chan SY. Holding our breath: The emerging and anticipated roles of microRNA in pulmonary hypertension. Pulm Circ. 2012;2(3):278–290. doi: 10.4103/2045-8932.101395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Morrell NW. Pulmonary hypertension due to BMPR2 mutation: a new paradigm for tissue remodeling? Proc Am Thorac Soc. 2006;3(8):680–686. doi: 10.1513/pats.200605-118SF. [DOI] [PubMed] [Google Scholar]
- 24.Diebold I, et al. BMPR2 preserves mitochondrial function and DNA during reoxygenation to promote endothelial cell survival and reverse pulmonary hypertension. Cell Metab. 2015;21(4):596–608. doi: 10.1016/j.cmet.2015.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hopper RK, et al. In Pulmonary Arterial Hypertension, Reduced BMPR2 Promotes Endothelial-to-Mesenchymal Transition via HMGA1 and Its Target Slug. Circulation. 2016;133(18):1783–1794. doi: 10.1161/CIRCULATIONAHA.115.020617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brock M, et al. Interleukin-6 modulates the expression of the bone morphogenic protein receptor type II through a novel STAT3-microRNA cluster 17/92 pathway. Circ Res. 2009;104(10):1184–1191. doi: 10.1161/CIRCRESAHA.109.197491. [DOI] [PubMed] [Google Scholar]
- 27.Kang H, et al. Inhibition of microRNA-302 (miR-302) by bone morphogenetic protein 4 (BMP4) facilitates the BMP signaling pathway. J Biol Chem. 2012;287(46):38656–38664. doi: 10.1074/jbc.M112.390898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Parikh VN, et al. MicroRNA-21 integrates pathogenic signaling to control pulmonary hypertension: results of a network bioinformatics approach. Circulation. 2012;125(12):1520–1532. doi: 10.1161/CIRCULATIONAHA.111.060269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pullamsetti SS, et al. Inhibition of microRNA-17 improves lung and heart function in experimental pulmonary hypertension. Am J Respir Crit Care Med. 2012;185(4):409–419. doi: 10.1164/rccm.201106-1093OC. [DOI] [PubMed] [Google Scholar]
- 30.Brock M, et al. AntagomiR directed against miR-20a restores functional BMPR2 signalling and prevents vascular remodelling in hypoxia-induced pulmonary hypertension. Eur Heart J. 2014;35(45):3203–3211. doi: 10.1093/eurheartj/ehs060. [DOI] [PubMed] [Google Scholar]
- 31.Davis BN, Hilyard AC, Lagna G, Hata A. SMAD proteins control DROSHA-mediated microRNA maturation. Nature. 2008;454(7200):56–61. doi: 10.1038/nature07086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kang H, et al. Bone morphogenetic protein 4 promotes vascular smooth muscle contractility by activating microRNA-21 (miR-21), which down-regulates expression of family of dedicator of cytokinesis (DOCK) proteins. J Biol Chem. 2012;287(6):3976–3986. doi: 10.1074/jbc.M111.303156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Drake KM, et al. Altered MicroRNA processing in heritable pulmonary arterial hypertension: an important role for Smad-8. Am J Respir Crit Care Med. 2011;184(12):1400–1408. doi: 10.1164/rccm.201106-1130OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.White K, et al. Endothelial apoptosis in pulmonary hypertension is controlled by a microRNA/programmed cell death 4/caspase-3 axis. Hypertension. 2014;64(1):185–194. doi: 10.1161/HYPERTENSIONAHA.113.03037. [DOI] [PubMed] [Google Scholar]
- 35.Yang S, et al. miR-21 regulates chronic hypoxia-induced pulmonary vascular remodeling. Am J Physiol Lung Cell Mol Physiol. 2012;302(6):L521–L529. doi: 10.1152/ajplung.00316.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Iannone L, et al. miR-21/DDAH1 pathway regulates pulmonary vascular responses to hypoxia. Biochem J. 2014;462(1):103–112. doi: 10.1042/BJ20140486. [DOI] [PubMed] [Google Scholar]
- 37.Zeng Y, et al. Hypoxia inducible factor-1 mediates expression of miR-322: potential role in proliferation and migration of pulmonary arterial smooth muscle cells. Sci Rep. 2015;5:12098. doi: 10.1038/srep12098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Caruso P, et al. A role for miR-145 in pulmonary arterial hypertension: evidence from mouse models and patient samples. Circ Res. 2012;111(3):290–300. doi: 10.1161/CIRCRESAHA.112.267591. [DOI] [PubMed] [Google Scholar]
- 39.Kim S, Hata A, Kang H. Down-regulation of miR-96 by bone morphogenetic protein signaling is critical for vascular smooth muscle cell phenotype modulation. J Cell Biochem. 2014;115(5):889–895. doi: 10.1002/jcb.24730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rothman AM, et al. MicroRNA-140-5p and SMURF1 regulate pulmonary arterial hypertension. J Clin Invest. 2016;126(7):2495–2508. doi: 10.1172/JCI83361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nallamshetty S, Chan SY, Loscalzo J. Hypoxia: a master regulator of microRNA biogenesis and activity. Free Radic Biol Med. 2013;64:20–30. doi: 10.1016/j.freeradbiomed.2013.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ghosh G, et al. Hypoxia-induced microRNA-424 expression in human endothelial cells regulates HIF-α isoforms and promotes angiogenesis. J Clin Invest. 2010;120(11):4141–4154. doi: 10.1172/JCI42980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Deng B, et al. MicroRNA-103/107 is involved in hypoxia-induced proliferation of pulmonary arterial smooth muscle cells by targeting HIF-1β. Life Sci. 2016;147:117–124. doi: 10.1016/j.lfs.2016.01.043. [DOI] [PubMed] [Google Scholar]
- 44.Caruso P, et al. Dynamic changes in lung microRNA profiles during the development of pulmonary hypertension due to chronic hypoxia and monocrotaline. Arterioscler Thromb Vasc Biol. 2010;30(4):716–723. doi: 10.1161/ATVBAHA.109.202028. [DOI] [PubMed] [Google Scholar]
- 45.Chan SY, Zhang YY, Hemann C, Mahoney CE, Zweier JL, Loscalzo J. MicroRNA-210 controls mitochondrial metabolism during hypoxia by repressing the iron-sulfur cluster assembly proteins ISCU1/2. Cell Metab. 2009;10(4):273–284. doi: 10.1016/j.cmet.2009.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fasanaro P, et al. An integrated approach for experimental target identification of hypoxia-induced miR-210. J Biol Chem. 2009;284(50):35134–35143. doi: 10.1074/jbc.M109.052779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kulshreshtha R, et al. A microRNA signature of hypoxia. Mol Cell Biol. 2007;27(5):1859–1867. doi: 10.1128/MCB.01395-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wu C, et al. Hypoxia potentiates microRNA-mediated gene silencing through posttranslational modification of Argonaute2. Mol Cell Biol. 2011;31(23):4760–4774. doi: 10.1128/MCB.05776-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ho JJ, et al. Functional importance of Dicer protein in the adaptive cellular response to hypoxia. J Biol Chem. 2012;287(34):29003–29020. doi: 10.1074/jbc.M112.373365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bandara V, Michael MZ, Gleadle JM. Hypoxia represses microRNA biogenesis proteins in breast cancer cells. BMC Cancer. 2014;14:533. doi: 10.1186/1471-2407-14-533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fan Y, et al. Dicer suppresses the malignant phenotype in VHL-deficient clear cell renal cell carcinoma by inhibiting HIF-2α. Oncotarget. 2016;7(14):18280–18294. doi: 10.18632/oncotarget.7807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rupaimoole R, et al. Hypoxia-upregulated microRNA-630 targets Dicer, leading to increased tumor progression. Oncogene. 2016;35(33):4312–4320. doi: 10.1038/onc.2015.492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gou D, et al. miR-210 has an antiapoptotic effect in pulmonary artery smooth muscle cells during hypoxia. Am J Physiol Lung Cell Mol Physiol. 2012;303(8):L682–L691. doi: 10.1152/ajplung.00344.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jin Y, et al. MKP-1 is a target of miR-210 and mediate the negative regulation of miR-210 inhibitor on hypoxic hPASMC proliferation. Cell Biol Int. 2015;39(1):113–120. doi: 10.1002/cbin.10339. [DOI] [PubMed] [Google Scholar]
- 55.White K, et al. Genetic and hypoxic alterations of the microRNA-210-ISCU1/2 axis promote iron-sulfur deficiency and pulmonary hypertension. EMBO Mol Med. 2015;7(6):695–713. doi: 10.15252/emmm.201404511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hale A, et al. An Argonaute 2 switch regulates circulating miR-210 to coordinate hypoxic adaptation across cells. Biochim Biophys Acta. 2014;1843(11):2528–2542. doi: 10.1016/j.bbamcr.2014.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ameshima S, et al. Peroxisome proliferator-activated receptor gamma (PPARgamma) expression is decreased in pulmonary hypertension and affects endothelial cell growth. Circ Res. 2003;92(10):1162–1169. doi: 10.1161/01.RES.0000073585.50092.14. [DOI] [PubMed] [Google Scholar]
- 58.Rabinovitch M. PPARgamma and the pathobiology of pulmonary arterial hypertension. Adv Exp Med Biol. 2010;661:447–458. doi: 10.1007/978-1-60761-500-2_29. [DOI] [PubMed] [Google Scholar]
- 59.Li C, Mpollo MS, Gonsalves CS, Tahara SM, Malik P, Kalra VK. Peroxisome proliferator-activated receptor-α-mediated transcription of miR-199a2 attenuates endothelin-1 expression via hypoxia-inducible factor-1α. J Biol Chem. 2014;289(52):36031–36047. doi: 10.1074/jbc.M114.600775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kang BY, et al. Peroxisome Proliferator-Activated Receptor γ and microRNA 98 in Hypoxia-Induced Endothelin-1 Signaling. Am J Respir Cell Mol Biol. 2016;54(1):136–146. doi: 10.1165/rcmb.2014-0337OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Green DE, Murphy TC, Kang BY, Searles CD, Hart CM. PPARγ Ligands Attenuate Hypoxia-Induced Proliferation in Human Pulmonary Artery Smooth Muscle Cells through Modulation of MicroRNA-21. PLoS One. 2015;10(7):e0133391. doi: 10.1371/journal.pone.0133391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kang BY, et al. Hypoxia mediates mutual repression between microRNA-27a and PPARγ in the pulmonary vasculature. PLoS One. 2013;8(11):e79503. doi: 10.1371/journal.pone.0079503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bi R, et al. MicroRNA-27b plays a role in pulmonary arterial hypertension by modulating peroxisome proliferator-activated receptor γ dependent Hsp90-eNOS signaling and nitric oxide production. Biochem Biophys Res Commun. 2015;460(2):469–475. doi: 10.1016/j.bbrc.2015.03.057. [DOI] [PubMed] [Google Scholar]
- 64.Bertero T, et al. Matrix Remodeling Promotes Pulmonary Hypertension through Feedback Mechanoactivation of the YAP/TAZ-miR-130/301 Circuit. Cell Rep. 2015;13(5):1016–1032. doi: 10.1016/j.celrep.2015.09.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bertero T, et al. The microRNA-130/301 family controls vasoconstriction in pulmonary hypertension. J Biol Chem. 2015;290(4):2069–2085. doi: 10.1074/jbc.M114.617845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bertero T, et al. Systems-level regulation of microRNA networks by miR-130/301 promotes pulmonary hypertension. J Clin Invest. 2014;124(8):3514–3528. doi: 10.1172/JCI74773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sharma S, et al. Apolipoprotein A-I mimetic peptide 4F rescues pulmonary hypertension by inducing microRNA-193-3p. Circulation. 2014;130(9):776–785. doi: 10.1161/CIRCULATIONAHA.114.007405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lahm T, Tuder RM, Petrache I. Progress in solving the sex hormone paradox in pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol. 2014;307(1):L7–26. doi: 10.1152/ajplung.00337.2013. [DOI] [PubMed] [Google Scholar]
- 69.Martin YN, Pabelick CM. Sex differences in the pulmonary circulation: implications for pulmonary hypertension. Am J Physiol Heart Circ Physiol. 2014;306(9):H1253–H1264. doi: 10.1152/ajpheart.00857.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wallace E, et al. A Sex-Specific MicroRNA-96/5-Hydroxytryptamine 1B Axis Influences Development of Pulmonary Hypertension. Am J Respir Crit Care Med. 2015;191(12):1432–1442. doi: 10.1164/rccm.201412-2148OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Chen X, et al. Estrogen Metabolite 16α-Hydroxyestrone Exacerbates Bone Morphogenetic Protein Receptor Type II-Associated Pulmonary Arterial Hypertension Through MicroRNA-29-Mediated Modulation of Cellular Metabolism. Circulation. 2016;133(1):82–97. doi: 10.1161/CIRCULATIONAHA.115.016133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hong Z, et al. miR-138 miR-25 Downregulate MCU, Causing Pulmonary Arterial Hypertension’s Cancer Phenotype. Am J Respir Crit Care Med [published online ahead of print September 20, 2016] doi: 10.1164/rccm.201604-0814OC. https://doi.org/10.1164/rccm.201604-0814OC. [DOI] [Google Scholar]
- 73.Meloche J, et al. miR-223 reverses experimental pulmonary arterial hypertension. Am J Physiol, Cell Physiol. 2015;309(6):C363–C372. doi: 10.1152/ajpcell.00149.2015. [DOI] [PubMed] [Google Scholar]
- 74.Wang D, et al. MicroRNA-124 controls the proliferative, migratory, and inflammatory phenotype of pulmonary vascular fibroblasts. Circ Res. 2014;114(1):67–78. doi: 10.1161/CIRCRESAHA.114.301633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Li C, Gonsalves CS, Eiymo Mwa Mpollo MS, Malik P, Tahara SM, Kalra VK. MicroRNA 648 Targets ET-1 mRNA and is cotranscriptionally regulated with MICAL3 by PAX5. Mol Cell Biol. 2015;35(3):514–528. doi: 10.1128/MCB.01199-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Guo L, et al. The microRNA-328 regulates hypoxic pulmonary hypertension by targeting at insulin growth factor 1 receptor and L-type calcium channel-α1C. Hypertension. 2012;59(5):1006–1013. doi: 10.1161/HYPERTENSIONAHA.111.185413. [DOI] [PubMed] [Google Scholar]
- 77.Li SS, Ran YJ, Zhang DD, Li SZ, Zhu D. MicroRNA-190 regulates hypoxic pulmonary vasoconstriction by targeting a voltage-gated K+ channel in arterial smooth muscle cells. J Cell Biochem. 2014;115(6):1196–1205. doi: 10.1002/jcb.24771. [DOI] [PubMed] [Google Scholar]
- 78.Tay Y, Rinn J, Pandolfi PP. The multilayered complexity of ceRNA crosstalk and competition. Nature. 2014;505(7483):344–352. doi: 10.1038/nature12986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Guignabert C, et al. Pathogenesis of pulmonary arterial hypertension: lessons from cancer. Eur Respir Rev. 2013;22(130):543–551. doi: 10.1183/09059180.00007513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Meloche J, et al. Bromodomain-Containing Protein 4: The Epigenetic Origin of Pulmonary Arterial Hypertension. Circ Res. 2015;117(6):525–535. doi: 10.1161/CIRCRESAHA.115.307004. [DOI] [PubMed] [Google Scholar]
- 81.Deng L, et al. MicroRNA-143 Activation Regulates Smooth Muscle and Endothelial Cell Crosstalk in Pulmonary Arterial Hypertension. Circ Res. 2015;117(10):870–883. doi: 10.1161/CIRCRESAHA.115.306806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kim J, et al. An endothelial apelin-FGF link mediated by miR-424 and miR-503 is disrupted in pulmonary arterial hypertension. Nat Med. 2013;19(1):74–82. doi: 10.1038/nm.3040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Patrick DM, et al. Stress-dependent cardiac remodeling occurs in the absence of microRNA-21 in mice. J Clin Invest. 2010;120(11):3912–3916. doi: 10.1172/JCI43604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Bertero T, et al. Vascular stiffness mechanoactivates YAP/TAZ-dependent glutaminolysis to drive pulmonary hypertension. J Clin Invest. 2016;126(9):3313–3335. doi: 10.1172/JCI86387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wang L, et al. MicroRNA expression profile of pulmonary artery smooth muscle cells and the effect of let-7d in chronic thromboembolic pulmonary hypertension. Pulm Circ. 2013;3(3):654–664. doi: 10.1086/674310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Guo L, et al. Differentially expressed plasma microRNAs and the potential regulatory function of Let-7b in chronic thromboembolic pulmonary hypertension. PLoS One. 2014;9(6):e101055. doi: 10.1371/journal.pone.0101055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Chen Z, et al. Susceptibility to chronic thromboembolic pulmonary hypertension may be conferred by miR-759 via its targeted interaction with polymorphic fibrinogen alpha gene. Hum Genet. 2010;128(4):443–452. doi: 10.1007/s00439-010-0866-8. [DOI] [PubMed] [Google Scholar]
- 88.Gonsalves CS, Li C, Malik P, Tahara SM, Kalra VK. Peroxisome proliferator-activated receptor-α-mediated transcription of miR-301a and miR-454 and their host gene SKA2 regulates endothelin-1 and PAI-1 expression in sickle cell disease. Biosci Rep. 2015;35(6):6. doi: 10.1042/BSR20150190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Bertero T, et al. A YAP/TAZ-miR-130/301 molecular circuit exerts systems-level control of fibrosis in a network of human diseases and physiologic conditions. Sci Rep. 2015;5:18277. doi: 10.1038/srep18277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Thum T, Batkai S. MicroRNAs in right ventricular (dys)function (2013 Grover Conference series). Pulm Circ. 2014;4(2):185–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Paulin R, et al. A miR-208-Mef2 axis drives the decompensation of right ventricular function in pulmonary hypertension. Circ Res. 2015;116(1):56–69. doi: 10.1161/CIRCRESAHA.115.303910. [DOI] [PubMed] [Google Scholar]
- 92.Potus F, et al. Downregulation of MicroRNA-126 Contributes to the Failing Right Ventricle in Pulmonary Arterial Hypertension. Circulation. 2015;132(10):932–943. doi: 10.1161/CIRCULATIONAHA.115.016382. [DOI] [PubMed] [Google Scholar]
- 93.Potus F, et al. Impaired angiogenesis and peripheral muscle microcirculation loss contribute to exercise intolerance in pulmonary arterial hypertension. Am J Respir Crit Care Med. 2014;190(3):318–328. doi: 10.1164/rccm.201402-0383OC. [DOI] [PubMed] [Google Scholar]
- 94.Pollett JB, Benza RL, Murali S, Shields KJ, Passineau MJ. Harvest of pulmonary artery endothelial cells from patients undergoing right heart catheterization. J Heart Lung Transplant. 2013;32(7):746–749. doi: 10.1016/j.healun.2013.04.013. [DOI] [PubMed] [Google Scholar]
- 95.Bushati N, Cohen SM. microRNA functions. Annu Rev Cell Dev Biol. 2007;23:175–205. doi: 10.1146/annurev.cellbio.23.090506.123406. [DOI] [PubMed] [Google Scholar]
- 96.Jung S, Kim S. EDDY: a novel statistical gene set test method to detect differential genetic dependencies. Nucleic Acids Res. 2014;42(7):e60. doi: 10.1093/nar/gku099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Tosar JP, Rovira C, Naya H, Cayota A. Mining of public sequencing databases supports a non-dietary origin for putative foreign miRNAs: underestimated effects of contamination in NGS. RNA. 2014;20(6):754–757. doi: 10.1261/rna.044263.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Mardis ER. Next-generation DNA sequencing methods. Annu Rev Genomics Hum Genet. 2008;9:387–402. doi: 10.1146/annurev.genom.9.081307.164359. [DOI] [PubMed] [Google Scholar]
- 99.Esteller M. Non-coding RNAs in human disease. Nat Rev Genet. 2011;12(12):861–874. doi: 10.1038/nrg3074. [DOI] [PubMed] [Google Scholar]
- 100.Hafner M, et al. Transcriptome-wide identification of RNA-binding protein and microRNA target sites by PAR-CLIP. Cell. 2010;141(1):129–141. doi: 10.1016/j.cell.2010.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Valadi H, Ekström K, Bossios A, Sjöstrand M, Lee JJ, Lötvall JO. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol. 2007;9(6):654–659. doi: 10.1038/ncb1596. [DOI] [PubMed] [Google Scholar]
- 102.Arroyo JD, et al. Argonaute2 complexes carry a population of circulating microRNAs independent of vesicles in human plasma. Proc Natl Acad Sci USA. 2011;108(12):5003–5008. doi: 10.1073/pnas.1019055108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Min PK, Chan SY. The biology of circulating microRNAs in cardiovascular disease. Eur J Clin Invest. 2015;45(8):860–874. doi: 10.1111/eci.12475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Zhang Y, et al. Secreted monocytic miR-150 enhances targeted endothelial cell migration. Mol Cell. 2010;39(1):133–144. doi: 10.1016/j.molcel.2010.06.010. [DOI] [PubMed] [Google Scholar]
- 105.Njock MS, et al. Endothelial cells suppress monocyte activation through secretion of extracellular vesicles containing antiinflammatory microRNAs. Blood. 2015;125(20):3202–3212. doi: 10.1182/blood-2014-11-611046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Rhodes CJ, et al. Reduced microRNA-150 is associated with poor survival in pulmonary arterial hypertension. Am J Respir Crit Care Med. 2013;187(3):294–302. doi: 10.1164/rccm.201205-0839OC. [DOI] [PubMed] [Google Scholar]
- 107.Sarrion I, et al. Role of circulating miRNAs as biomarkers in idiopathic pulmonary arterial hypertension: possible relevance of miR-23a. Oxid Med Cell Longev. 2015;2015:792846. doi: 10.1155/2015/792846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Schlosser K, White RJ, Stewart DJ. miR-26a linked to pulmonary hypertension by global assessment of circulating extracellular microRNAs. Am J Respir Crit Care Med. 2013;188(12):1472–1475. doi: 10.1164/rccm.201308-1403LE. [DOI] [PubMed] [Google Scholar]
- 109.Huber LC, et al. Featured Article: microRNA-125a in pulmonary hypertension: Regulator of a proliferative phenotype of endothelial cells. Exp Biol Med (Maywood) 2015;240(12):1580–1589. doi: 10.1177/1535370215579018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Wei C, et al. Circulating miRNAs as potential marker for pulmonary hypertension. PLoS One. 2013;8(5):e64396. doi: 10.1371/journal.pone.0064396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Dorfmüller P, Perros F, Balabanian K, Humbert M. Inflammation in pulmonary arterial hypertension. Eur Respir J. 2003;22(2):358–363. doi: 10.1183/09031936.03.00038903. [DOI] [PubMed] [Google Scholar]
- 112.Kherbeck N, et al. The role of inflammation and autoimmunity in the pathophysiology of pulmonary arterial hypertension. Clin Rev Allergy Immunol. 2013;44(1):31–38. doi: 10.1007/s12016-011-8265-z. [DOI] [PubMed] [Google Scholar]
- 113.Liu H, et al. rs2910164 Polymorphism Confers a Decreased Risk for Pulmonary Hypertension by Compromising the Processing of microRNA-146a. Cell Physiol Biochem. 2015;36(5):1951–1960. doi: 10.1159/000430163. [DOI] [PubMed] [Google Scholar]
- 114.Zhou S, et al. A single nucleotide polymorphism in 3′ untranslated region of epithelial growth factor receptor confers risk for pulmonary hypertension in chronic obstructive pulmonary disease. Cell Physiol Biochem. 2015;36(1):166–178. doi: 10.1159/000374061. [DOI] [PubMed] [Google Scholar]
- 115.Raal FJ, et al. Mipomersen, an apolipoprotein B synthesis inhibitor, for lowering of LDL cholesterol concentrations in patients with homozygous familial hypercholesterolaemia: a randomised, double-blind, placebo-controlled trial. Lancet. 2010;375(9719):998–1006. doi: 10.1016/S0140-6736(10)60284-X. [DOI] [PubMed] [Google Scholar]
- 116.Coelho T, et al. Safety and efficacy of RNAi therapy for transthyretin amyloidosis. N Engl J Med. 2013;369(9):819–829. doi: 10.1056/NEJMoa1208760. [DOI] [PubMed] [Google Scholar]
- 117.Janssen HL, et al. Treatment of HCV infection by targeting microRNA. N Engl J Med. 2013;368(18):1685–1694. doi: 10.1056/NEJMoa1209026. [DOI] [PubMed] [Google Scholar]
- 118.Verma IM, Somia N. Gene therapy -- promises, problems and prospects. Nature. 1997;389(6648):239–242. doi: 10.1038/38410. [DOI] [PubMed] [Google Scholar]
- 119.Kafri T, Blömer U, Peterson DA, Gage FH, Verma IM. Sustained expression of genes delivered directly into liver and muscle by lentiviral vectors. Nat Genet. 1997;17(3):314–317. doi: 10.1038/ng1197-314. [DOI] [PubMed] [Google Scholar]
- 120.Li Z, Rana TM. Therapeutic targeting of microRNAs: current status and future challenges. Nat Rev Drug Discov. 2014;13(8):622–638. doi: 10.1038/nrd4359. [DOI] [PubMed] [Google Scholar]
- 121.McLendon JM, et al. Lipid nanoparticle delivery of a microRNA-145 inhibitor improves experimental pulmonary hypertension. J Control Release. 2015;210:67–75. doi: 10.1016/j.jconrel.2015.05.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Lee C, et al. Exosomes mediate the cytoprotective action of mesenchymal stromal cells on hypoxia-induced pulmonary hypertension. Circulation. 2012;126(22):2601–2611. doi: 10.1161/CIRCULATIONAHA.112.114173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Brock M, et al. AntagomiR directed against miR-20a restores functional BMPR2 signalling and prevents vascular remodelling in hypoxia-induced pulmonary hypertension. Eur Heart J. 2014;35(45):3203–3211. doi: 10.1093/eurheartj/ehs060. [DOI] [PubMed] [Google Scholar]
- 124.Grant JS, Morecroft I, Dempsie Y, van Rooij E, MacLean MR, Baker AH. Transient but not genetic loss of miR-451 is protective in the development of pulmonary arterial hypertension. Pulm Circ. 2013;3(4):840–850. doi: 10.1086/674751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Liu Y, Liu G, Zhang H, Wang J. MiRNA-199a-5p influences pulmonary artery hypertension via downregulating Smad3. Biochem Biophys Res Commun. 2016;473(4):859–866. doi: 10.1016/j.bbrc.2016.03.140. [DOI] [PubMed] [Google Scholar]
- 126.Wu D, et al. Identifying microRNAs targeting Wnt/β-catenin pathway in end-stage idiopathic pulmonary arterial hypertension. J Mol Med. 2016;94(8):875–885. doi: 10.1007/s00109-016-1426-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Li S, Ran Y, Zhang D, Chen J, Li S, Zhu D. MicroRNA-138 plays a role in hypoxic pulmonary vascular remodelling by targeting Mst1. Biochem J. 2013;452(2):281–291. doi: 10.1042/BJ20120680. [DOI] [PubMed] [Google Scholar]
- 128.Jalali S, et al. Mir-206 regulates pulmonary artery smooth muscle cell proliferation and differentiation. PLoS ONE. 2012;7(10):e46808. doi: 10.1371/journal.pone.0046808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Courboulin A, et al. Role for miR-204 in human pulmonary arterial hypertension. J Exp Med. 2011;208(3):535–548. doi: 10.1084/jem.20101812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Wang P, et al. miRNA-34a promotes proliferation of human pulmonary artery smooth muscle cells by targeting PDGFRA. Cell Prolif. 2016;49(4):484–493. doi: 10.1111/cpr.12265. [DOI] [PMC free article] [PubMed] [Google Scholar]