Abstract
Background
We evaluated the influence of prenatal exposure to widespread urban air pollutants on the development of self-regulation and social competence in a longitudinal prospective cohort of children born to nonsmoking minority women in New York City.
Methods
Air pollutant exposure was estimated categorically by level of polycyclic aromatic hydrocarbon (PAH)-DNA adducts in maternal blood collected at delivery, providing a biomarker of maternal exposure to PAH over a 2–3 month period. DESR was defined as moderate elevations on three specific scales of the Child Behavior Checklist (Anxious/Depressed, Aggressive Behavior, and Attention Problems). We used Generalized Estimating Equations to assess the influence of prenatal exposure to PAH on DESR in children at 3–5, 7, 9 and 11 years of age, adjusted for gender, and race/ethnicity. Next, we assessed the association of prenatal exposure to PAH with social competence, as measured by the Social Responsiveness Scale (SRS), the association of impaired self-regulation with social competence, and whether impairment in self-regulation mediated the association of prenatal exposure to PAH with social competence.
Results
We detected a significant interaction (at p=.05) of exposure with time, in which the developmental trajectory of self-regulatory capacity was delayed in the exposed children. Multiple linear regression revealed a positive association between presence of PAH-DNA adducts and problems with social competence (p<0.04), level of dysregulation and problems with social competence (p<0.0001), and evidence that self-regulation mediates the association of prenatal exposure to PAH with SRS (p<0.0007).
Conclusions
These data suggest that prenatal exposure to PAH produces long-lasting effects on self-regulatory capacities across early and middle childhood, and that these deficits point to emerging social problems with real-world consequences for high-risk adolescent behaviors in this minority urban cohort.
Keywords: Polycyclic aromatic hydrocarbons, self-regulation, prenatal exposure, social competence
Introduction
Polycyclic aromatic hydrocarbons (PAH), including benzo[a]pyrene (B[a]P), are neurotoxicant pollutants released during incomplete combustion of fossil fuel, tobacco, and other organic material (Bostrom et al., 2002), found in air and dietary sources. Human exposure to PAH is ubiquitous. Differential siting of outdoor pollution sources in low-income, urban, and minority communities produces striking disparities in exposure levels (Heritage, 1992; Metzer, Delgado, & Herrell, 1995; Olden & Poje, 1995; Pirkle et al., 1996; Wagenknecht, Manolio, Sidney, Burke, & Haley, 1993; Wernette & Nieves, 1992). Exposures to PAH and other environmental pollutants during the prenatal and early postnatal stages are of particular concern for child health and development (Grandjean and Landrigan 2006; National Research Council 1993; Perera et al. 2004). Early exposure may add risk because of the heightened susceptibility of the developing brain to these exposures (Nijland, Ford, & Nathanielsz, 2008; Rodier, 2004). During the fetal period and early childhood years, the brain is rapidly developing and vulnerable to neurotoxic insults that may manifest as adverse outcomes in childhood and adulthood (Shonkoff et al., 2012; Stein, Schettler, Wallinga, & Valenti, 2002). Animal studies of PAH exposure during the prenatal, neonatal, and adult periods have reported a range of neurodevelopmental and behavioral effects (Brown et al., 2007; Wormley, Ramesh, & Hood, 2004), including hyperactivity (Grova et al., 2007; Schellenberger et al., 2013). In humans, a significant dose-response relationship is detected between prenatal PAH exposure and significant reductions in white matter surface in middle childhood (Peterson et al., 2015). Prenatal exposure to PAH is associated with autism (Becerra, Wilhelm, Olsen, Cockburn, & Ritz, 2013; Volk, Hertz-Picciotto, Delwiche, Lurmann, & McConnell, 2011; Volk, Lurmann, Penfold, Hertz-Picciotto, & McConnell, 2013; von Ehrenstein, Aralis, Cockburn, & Ritz, 2014), ADHD (Perera et al., 2014; Perera et al., 2012; Peterson et al., 2015), symptoms of anxiety, depression and inattention (Perera et al., 2014), and externalizing behaviors (Peterson et al., 2015). These disorders all derive at least in part from deficits in self-regulation.
Self-regulation is an important transdiagnostic dimension of behavior. Deficits in self-regulation as measured by cognitive control tasks and alterations in frontostriatal control systems are implicated in many childhood psychopathologies including Attention Deficit Hyperactivity Disorder(Casey et al., 2007; Nigg & Casey, 2005), Obsessive Compulsive Disorder, Tourette’s Syndrome, and Eating Disorders (Marsh, Maia, & Peterson, 2009). The development of these circuits in the perinatal period is vulnerable to the effects of neurtoxicants in the environment, however few studies have examined the long-term effects of neurotoxicants on self-regulation. Little is known about the longer-term effects of prenatal and early life exposure to PAH on self-regulatory behavior and social function in early adolescence. The study presented in this paper examines the effects of prenatal exposure to air pollution (PAH) on the developmental trajectory of self-regulatory capacities across childhood from age 3 to 11 years old.
We undertook the current study to determine whether prenatal exposure to PAH is associated with deficits in self-regulation in late childhood and early adolescence, which could in turn predispose children to negative outcomes in social functioning. Self-regulation develops throughout early and middle childhood. Failure to develop this capacity is associated with behavior problems and psychopathology.
We conceptualize self-regulation as successfully achieving control in cognitive, behavioral, and emotional domains. Cognitive control refers to a set of mental processes that are responsible for executing, guiding, and monitoring these desired behaviors, while inhibiting inappropriate or disadvantageous responses (Dubin, Maia, & Peterson, 2010). Such processes may be measured with survey data about a person’s attention. Behavioral control refers to a set of motor processes that are responsible for inhibiting the urge to perform acts without considering the consequences of having performed such acts. Such behaviors may be measured with survey data reflecting aggressive behavior. Emotional control refers to a set of mental processes that maintain a midrange emotional state, neither too excited nor too dampened. Such behaviors may be measured with survey data reflecting problems in mood.
Given this theoretical model, we operationalize self-regulation with the the Deficient Emotional Self-Regulation (DESR) profile score from the Children’s Behavior Check List (CBCL) (Achenbach, 1992). The CBCL is a well validated survey of children’s behavior with excellent psychometric properties. The DESR (Biederman, Spencer, et al., 2012) score is composed of the Anxiety/Depression, Attention, and Aggressive Behavior scales of the CBCL. These scales map to the domains identified in our model of self-regulation: cognition (Attention Problems Scale), behavior (Aggressive Behavior Scale), and emotion (Anxious/Depressed). Prior studies demonstrate that elevated scores on the DESR scale are associated with increasing levels of social and conduct problems. Compared to children with ADHD who do not have elevated DESR scores, children with ADHD who have moderately elevated DESR scores are more likely to have oppositional defiant disorder and social problems (Biederman, Petty, et al., 2012). Children with ADHD and highly elevated DESR scores are likely to have conduct disorder and bipolar disorder (Biederman, Petty, et al., 2012). Thus, level of dysregulation, as measured by the DESR, is associated with varying levels of poor behavioral outcomes, social difficulties, and psychopathology. These studies are consistent with many reports that self-regulation is associated with social competence (Belsky, Pasco Fearon, & Bell, 2007; Eisenberg et al., 2000; Eisenberg et al., 2005; Eisenberg, Spinrad, & Eggum, 2010; Fabes et al., 1999; Kochanska, Murray, & Harlan, 2000; Krueger, Caspi, Moffitt, White, & Stouthamer-Loeber, 1996; Martel et al., 2007; Melnick & Hinshaw, 2000; Mischel, Shoda, & Rodriguez, 1989; Murphy & Eisenberg, 1997; Nigg, Quamma, Greenberg, & Kusche, 1999; Shoda, Mischel, & Peake, 1990; Spinrad et al., 2006).
We were concerned with two research questions: Is PAH exposure (as measured by the presence of PAH-DNA adducts in maternal blood at delivery) associated with a pattern of dysregulation, and does this pattern persist over time? Does this PAH exposed phenotype contribute to social impairment in late childhood/early adolescence? We hypothesized that 1) Compared to children whose mothers had no detectable PAH-DNA adducts, children whose mothers had detectable PAH-DNA adducts would be more likely to manifest signs of poor self-regulation across childhood, resulting in a persistent pattern of deficient self-regulation over time. 2) In these same children, a persistent pattern of deficient self-regulation would increase the likelihood of social impairment in late childhood/early adolescence. 3) Deficient self-regulation would mediate the association between prenatal exposure to PAH and subsequent social impairment in early adolescence. Self-regulation begins to emerge at age 3 (Rothbart & Rueda, 2005), when children still engage in parallel and independent play. By the time children engage consistently in cooperative play at age 5 (Parten, 1932), many self-regulatory capacities are developed (Gerstadt, Hong, & Diamond, 1994; Mischel et al., 1989). Thus we built the mediation model to match this developmental progression.
Methods
Description of the sample
A complete description of the NYC cohort appears elsewhere (Perera et al., 2006). African-American and Dominican women who resided in Washington Heights, Harlem, or the South Bronx in NYC, U.S. were recruited between 1998 and 2006 through local prenatal care clinics. Enrollment was restricted to women who were non-active cigarette smokers; ages 18–35; non-users of other tobacco products or illicit drugs; free of diabetes, hypertension, or known HIV; and who had initiated prenatal care by the 20th week of pregnancy. The Institutional Review Board of the Columbia University Medical Center approved the study. Mothers signed a consent form, approved by the IRB, for themselves and their children at the time of enrollment and at every subsequent visit. The children signed an IRB-approved assent form beginning at age 7. The consent and assent forms are available in English and Spanish and clearly explain the study goals and procedures.
Measures
Exposure
DNA adducts integrate PAH exposure over a period of months (estimated half-life of 3–4 months in blood) (Mooney et al., 1995), reflecting individual variation in exposure, absorption, metabolic activation, and DNA repair. They thus provide a biological dosimeter that incorporates both exposure and biological susceptibility. Adducts reflect multiple possible biological pathways for the pathogenic effects of PAH, representing DNA damage (genotoxicity), detoxification, DNA repair (Godschalk, Van Schooten, & Bartsch, 2003; Veglia et al., 2008). They may also play a role in epigenetic alterations (DNA methylation) (Herbstman et al., 2012). The assay specifically measures the adducts formed by Benzo[a] Pyrene as a proxy for PAH-DNA because it is considered a representative PAH and is highly correlated with other PAH class members (Perera et al., 2006). The method used to measure the adducts has been described previously (Perera et al., 2006). Briefly, a total amount of 100 ug DNA was used for each analysis. DNA samples were dissolved in 0.1 N HCl and acid hydrolysis carried out at 90°C for 6 hours. The resulting solution was analyzed in a Shimadzu HPLC system with an automatic sample injector and RF-10Axl spectrofluorometric detector. The tetrol concentrations were calculated by comparing the areas of samples to be analyzed with an external calibration curve, generated from the fluorescence peak of an authentic BPDE tetrol standard, every time a set of samples was analyzed. Calibration was conducted using DNA from calf thymus, alone (background) and added to 2, 4, and 8 pg anti-BPDE tetrol. These standard solutions were then treated in the same way as the tested samples (hydrolyzed in 0.1 N HCl at 90°C for 6 hours). The minimum correlation coefficient was 0.98 and the mean coefficient of variation for analyses repeated on different days was 12%. This assay can measure 0.25 adducts/10−8 nucleotides. PAH exposure (DNA adducts) was used as a dichotomous variable because sixty percent of the mothers in the sample had DNA adduct levels in the non-detectable range and therefore the variable is not normally distributed.
Deficient Emotional Self-Regulation (DESR)
DESR was operationalized by summing the standardized T scores for each child on three of the subscales from the CBCL (T. M. Achenbach & Rescorla): Anxious/Depressed, Aggressive Behavior, and Attention Problems Scales (Biederman, Petty, et al., 2012), which measure intense emotions, aggression, and impulsiviity, respectively. The CBCL was selected because of its excellent psychometric properties and ease of administration. Mothers completed the age appropriate version of the CBCL when their children were 3–5 (Achenbach & Rescorla, 2000), 7, 9, and 11 (Achenbach & Rescorla, 2001) years of age. The DESR score was used as a continuous variable. T-scores have a mean of 50 and a standard deviation of 10 points; the children in our study performed largely in the average range, as expected because they are drawn from a community sample. Higher T-scores indicate more dysfunction. The three scales were intercorrelated. At age 9, anxious/depressed scale correlated with attention r = .523 (p < 0.0001) and aggressive r = .626 (p < 0.0001); attention correlated with aggressive r = .628 (p < 0.0001).
Social impairment
The Social Responsiveness Scale (SRS) assesses social impairment. Mothers completed the SRS when their children were 11 years old. The SRS is a continuous, quantitative measure of social ability, yielding scores that range from significant impairment in communication and social behavior (as in autism spectrum disorders) to above average ability, determined by maternal report (Constantino & Gruber, 2005). The scale contains 5 subscales: Social Awareness, Social Cognition, Social Communication, Social Motivation, and Autistic Mannerisms, which respectively measure the ability to recognize social cues, the ability to interpret social cues, the ability to use expressive verbal and non-verbal language skills, the ability to engage in social-interpersonal behaviors, and the tendency to display stereotypical behaviors and restricted interests characteristic of autism. The SRS Total T-score reflects the sum of the T-scores for the five scales.
Statistical analysis
We examined the effect of prenatal exposure to PAH (presence vs. absence of maternal PAH-DNA adducts) on the development of self-regulatory capacity, as measured by the CBCL DESR score. To evaluate the effect of prenatal exposure to PAH on DESR score (continuous) at ages 3–5, 7, 9 and 11 years, we used generalized estimating equations (GEE) (Zeger & Liang, 1986). We used negative binomial regression to model DESR scores, which are considered over-dispersed count data and used an unstructured correlation structure to estimate the PAH-by-age at DESR assessment interaction parameter. We included gender and ethnicity as covariates in the model to account for differences between the normative sample and our cohort. We also considered the following potential confounders and tested whether they differed between exposed and non-exposed groups: maternal education as a measure of SES, marital status which includes presence of a partner in the home, and maternal psychological distress, anxiety and depression (Dohrenwend, Shrout, Egri, & Mendelsohn, 1980) as a measure of family history of psychological problems (Table 1). Variables not associated with PAH exposure were dropped from the final model; variables associated with PAH exposure were tested in the GEE analysis. If a variable was not associated with outcomes in the GEE model it was dropped from the final analysis. Previous work in this cohort has demonstrated that mean PAH levels were not associated with neighborhood socioeconomic status and adjusting for street traffic density near the home did not alter outcomes in models predicting childhood obesity as a result of prenatal exposure to PAH (Rundle et al., 2012). Therefore we did not include neighborhood socioeconomic status in our consideration of potential confounding factors.
Table 1.
Comparison of demographic variables between subjects with non-detectable (N=275) and detectable (N=187) adducts.
| Variable | Non-exposed (n=275) | Exposed (n=187) | P- value |
|---|---|---|---|
| Percent female | 54.18 | 52.41 | 0.71 |
| Percent African American |
41.45 | 35.83 | 0.22 |
| Percent high school education |
67.27 | 53.48 | 0.003 |
| Depress at prenatal |
1.15 ± 0.62 (n= 275) | 1.19 ± 0.67 (n= 187) | 0.43 |
| Percent married/live with same partner |
24.45 | 31.55 | 0.09 |
To evaluate whether the DESR operationalizes self-regulation or simply ADHD-like symptoms, we tested at age 9 the association of prenatal exposure to PAH with each scale that composes the DESR score: attention problems, aggressive behavior, anxious/depressed. To evaluate “real world” outcomes associated with prenatal PAH exposure, we tested the association between maternal adducts and social difficulty as measured by the SRS, and whether emotional dysregulation at age 9 mediated any observed associations between maternal PAH-DNA adducts and social impairment at age 11, using the Sobel test for mediation. All analyses included gender and ethnicity as covariates.
The sample included in the GEE analysis is composed of the children who had available data on maternal PAH-DNA adducts, CBCL data from at least one assessment point (age 3–5, 7, 9 and 11) and all covariates of interest (race/ethnicity and gender) (n=462). The mediation analysis includes children with available data on maternal PAH-DNA adducts, CBCL data at age 9, and SRS data at age 11 (n=262). In the mediation analysis, we substituted 7 year CBCL data for 20 children who were missing 9 year CBCL data.
Results
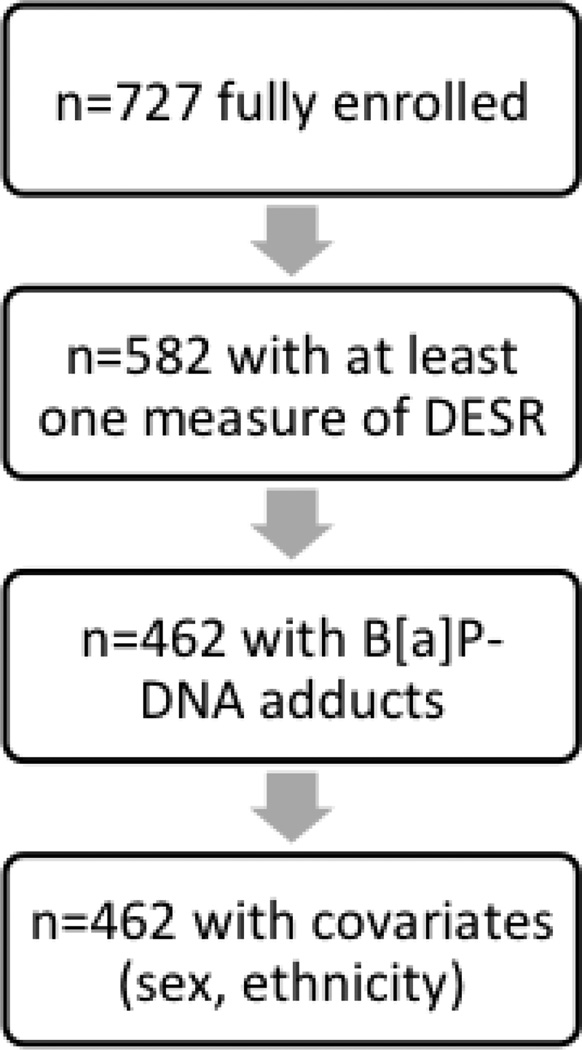
We assessed the association of PAH-DNA adducts in maternal blood with DESR scores at all ages (N=450 at ages 3–5, N=406 at age 7, N=376 at age 9, and N=313 at age 11). Collectively, these measurements included a total of 462 participants selected from the entire CCCEH cohort (see Figure 1).
Figure 1.
Flow chart depicting the sample selection strategy for the current study
Forty percent of mothers (N = 187) in the NYC cohort had detectable levels of PAH-DNA adducts in maternal blood. Table 1 presents a comparison ofdemographic variables between the group of children whose mother had detectable adducts and those who did not.
The DESR scores are within the normal range, as expected since this is a study of a community sample of children, in contrast to the clinic-referred samples reported by Biederman (Biederman, Petty, et al., 2012; Biederman, Spencer, et al., 2012) (Table 2).
Table 2.
Distribution of DESR scores at each age of assessment. DESR, Deficient Emotional Self-Regulation Scale
| Variable | N | Mean | Variance | Minimum | Maximum |
|---|---|---|---|---|---|
| DESR_5 | 450 | 163.598 | 264.651 | 150 | 242 |
| DESR_7 | 406 | 161.441 | 216.983 | 150 | 250 |
| DESR_9 | 376 | 161.904 | 258.508 | 150 | 271 |
| DESR_11 | 313 | 160.166 | 182.312 | 150 | 222 |
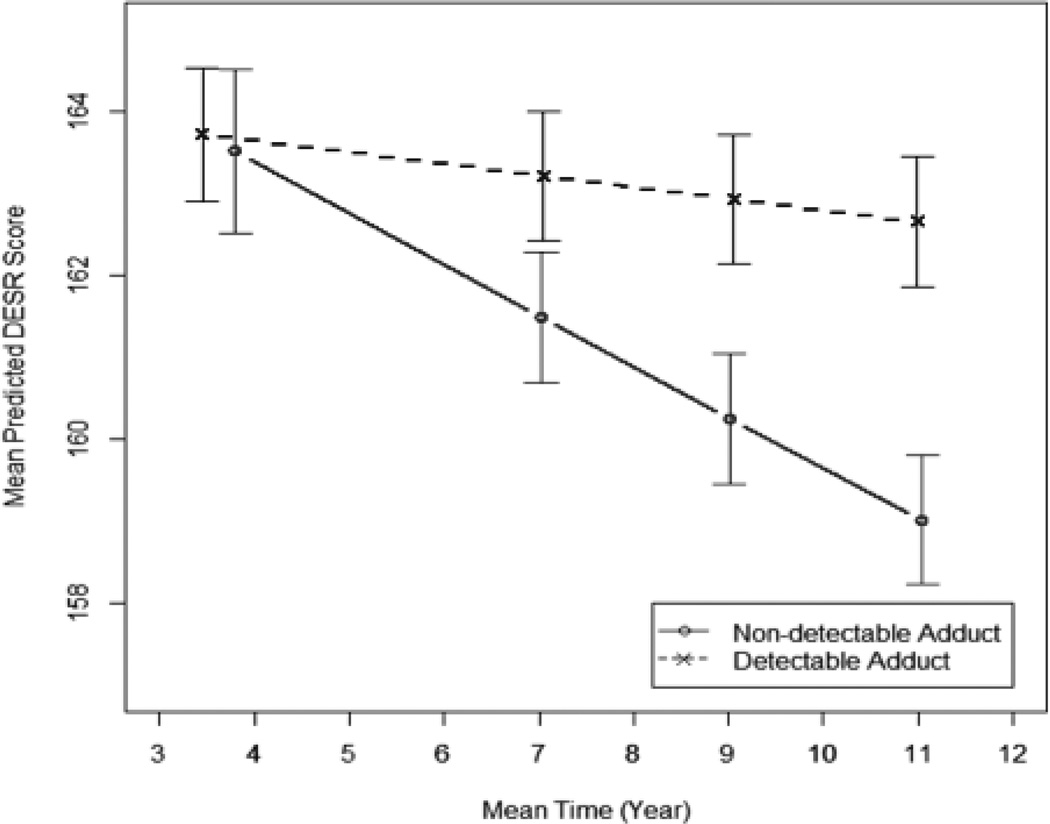
We assessed the effect of prenatal exposure to PAH (presence or absence of detectable PAH-DNA adducts in maternal blood) on children’s self-regulation (DESR score) across childhood from age 3 to 11 years, controlling for gender and race/ethnicity. We detected a significant interaction between prenatal exposure (presence of maternal adducts or not) and age at DESR assessment, p=0.05. The effect of adducts on DESR was not constant over time but increased at a different rate within each exposure group. Among those without detectable adducts, the average DESR score decreased substantially over time (−0.38% per year). Among those with detectable adducts, average DESR score decreases minimally over time (−0.09% per year) (see Figure 2). At age 3, the groups were not appreciably different; children whose mothers had detectable PAH-DNA adducts had average adjusted DESR scores that were the same as those whose mothers did not have detectable adducts (scores of 163.7 and 163.5 among detectable and non-detectable adducts groups, respectively, (p=0.840)). By the last two measurement points at ages 9 and 11, the DESR scores were significantly different between the exposure groups. At age 11, children whose mothers had detectable PAH-DNA adducts had average adjusted DESR scores that were significantly higher than children whose mothers did not have detectable adducts (scores of 162.7 and 159.0 among the detectable and non-detectable adducts groups, respectively, (p=0.020)). This difference at age 11 represents an effect size of approximately one-half of a standard deviation between groups.
Figure 2.
Mean DESR scores at each age of assessment for children whose mothers had detectable B[a]P-DNA adducts (dashed line) and children whose mothers did not have detectable adducts (solid line) at time of delivery. Error bars represent standard deviation.
To evaluate whether the DESR score operationalizes self-regulation and not simply ADHD-like symptoms, we assessed at age 9 the association of each scale composing the DESR (Anxious/Depressed, Aggressive Behavior, and Attention Problems) with prenatal exposure to PAH, controlling for gender and ethnicity. Each of these associations was significant, p<0.02, 0.01, 0.03, respectively. The beta value indicating the strength of the association between the DESR score and PAH was 3 times higher than the beta values estimating the strength of association between each individual scale composing the DESR score and PAH (see Table 3). This suggests that no one individual scale contributed more than another scale to the association of the DESR score and PAH and that the DESR score is not simply a measure of attention capacity, but rather captures self-regulatory behavior that cuts across domains of behavior.
Table 3.
Beta and p-values for association of PAH with the individual CBCL scales composing the DESR score. CBCL, Child Behavior Checklist; DESR, Deficient Emotional Self-Regulation Scale
| Scale | Beta | P-value |
|---|---|---|
| Anxiety/Depression | 1.4 | .02 |
| Aggressive | 1.7 | .01 |
| Attention | 1.5 | .03 |
| DESR | 4.6 | .0004 |
To understand how prenatal PAH exposure might affect behavioral outcomes in early adolescence, we evaluated the association of prenatal exposure to PAH and social competence, as measured by the SRS. We also tested whether self-regulation, as measured by the DESR, mediated this association.
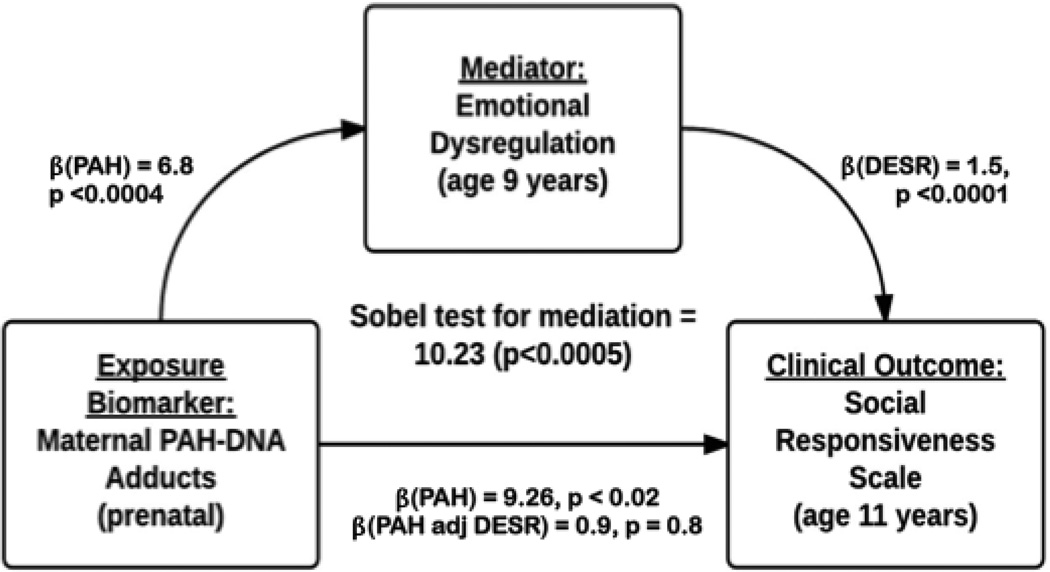
We first tested the association between prenatal exposure to PAH and social competence. Of the entire sample of children with DESR scores (N=462), only 262 children had data available for both maternal PAH-DNA adducts and the SRS. We detected a positive association of prenatal exposure to PAH with SRS Total Score, controlling for gender and ethnicity, p<0.06. Three of the SRS scales drove this association: SRS Cognition, p<0.04; SRS Communication, p<0.06; and SRS Awareness, p<0.06. We then tested the association of self-regulation (DESR) with social competence. The DESR score was positively associated with the SRS Total Score and all SRS subscales, p<0.0001, while controlling for gender and ethnicity. Last, we tested whether the DESR score mediated the association of prenatal exposure to PAH with social competence. When prenatal exposure to PAH and the DESR score were included in the linear regression model predicting SRS, the association of prenatal exposure to PAH with SRS was not significant (SRS Total, p<0.80; Cognition, p<0.60; Communication, p<0.80; Awareness, p<0.50), indicating that the DESR score mediates the association of PAH with SRS. The statistical test of mediation was significant for SRS Total score, p< 0.0004, and for the three SRS subscales, Cognition, p<0.0007; Communication, p<0.0006, and Awareness, p<0.001, while controlling for race/ethnicity and gender. Thus the relationship between prenatal exposure to PAH and social competence appears to be mediated by, and operating through the development of self-regulatory capacities.
Discussion
To our knowledge, this is the first study to examine the effects of prenatal exposure to PAH on neurodevelopment that incorporates multiple measurement times across childhood. This design allows us to model the effect of exposure on the developmental trajectory of self-regulatory capacities. In this prospective cohort study of 462 children, we detected significant alterations in the developmental trajectory of self-regulatory capacities in children with prenatal exposure to PAH. We operationalized self-regulatory capacity with the DESR score derived from the CBCL. Children whose mothers did not have detectable PAH-DNA adducts demonstrated increased capacity for self-regulation from 3 to 11 years. In contrast, children whose mothers had detectable PAH-DNA adducts at delivery on average failed to improve in their capacity for self-regulation from 3 to 11 years. These children evidenced persistent problems in the domains of attention problems, aggression, and anxiety/depression. The strength of the association between the DESR score at age 9 and prenatal exposure to PAH was three times greater than the strength of association of each individual scale composing the DESR score and prenatal exposure to PAH, indicating that the DESR score captures self-regulatory capacity across domains of behavior and is not simply measuring ADHD-like symptoms. Prenatal exposure to PAH was also associated with level of social competence at age 11. Level of self-regulation at age 9 was associated with level of social competence at age 11, and mediated the association of prenatal exposure to PAH with social competence. These findings suggest the presence of a developmental pathway to dysregulated attention, behavior, and emotion, and impaired socialization in children with prenatal exposure to PAH.
Self-regulatory systems follow a well-known developmental trajectory from age 3 to 11 years. Many experimental tasks tap discrete neuropsychological skills that are associated with self-regulatory control in the behavioral, cognitive, and emotional domains. Performance on these tasks suggests that development of these capacities follows a well-defined temporal sequence. Across domains studies demonstrate behavioral (motor) control emerges first at age 3 (Rothbart & Rueda, 2005), followed by cognitive (inhibitory) control emerging at age 5 (Williams, Ponesse, Schachar, Logan, & Tannock, 1999), and last, emotional (delay of gratification) control begins to emerge at age 4 (Mischel et al., 1989). In the behavioral domain, between 3 and 4 years of age children develop the ability to inhibit a prepotent motor response, as measured by various motor conflict tasks including the Simon Says (Jones, Rothabrt, & Posner, 2003; Reed, Pien, & Rothbart, 1984), Luria Tapping (Diamond & Taylor, 1996), and spatial versus identity conflict task (Gerardi-Caulton, 2000); by age 5 most children have developed this capacity (Rothbart & Rueda, 2005). In the cognitive domain, between 3 and 4 years of age, children have difficulty inhibiting a cognitive response as measured by various inhibitory control tasks including card sorting (Jacques, Zelazo, Kirkham, & Semcesen, 1999), and the ‘day-night’ task (Gerstadt et al., 1994); by age 5 most children can generally perform these tasks. In the emotional domain, beginning at age 4, children begin to be able to delay gratification and continue to develop more efficient delay strategies at least until age 10 (Mischel et al., 1989). In this current study, children with prenatal exposure to PAH appear not to follow this typical developmental trajectory. Children of mothers with PAH-DNA adducts demonstrate an altered developmental trajectory characterized by a failure to develop increased behavior, attention, and emotion regulation.
Self-regulatory capacity is associated with social competence across the range of possible behaviors and across early childhood development into adolescence. Higher levels of self-regulation are strongly associated with higher levels of social competence (i.e. increased levels of emotional control and prosocial behavior) over the course of development, as demonstrated in cross-sectional and longitudinal studies. In preschool children, capacity for effortful control is associated concurrently with ability to control anger and joy (Kochanska et al., 2000) and with less negative emotional arousal and better social competence (Fabes et al., 1999). Good self-regulation in preschool children predicts better social functioning in school age children (Murphy, Eisenberg, Fabes, Shepard, & Guthrie, 1999; Spinrad et al., 2006). Better ability to delay gratification in preschool children is associated in adolescence with better ability to cope with frustration and stress (Shoda et al., 1990) and with better interpersonal and social skills (Mischel et al., 1989). Similarly, higher levels of self-regulatory capacity are associated with lower levels of aggressive behavior in typically developing children (Nigg, Quamma, Greenberg, and Kusche, 1999) and in children with behavior problems (Mischel 1989). Conversely, lower levels of self-regulatory capacity in childhood are associated with higher levels of social impairment (i.e. higher levels of externalizing behaviors, aggression, and peer conflict) over the course of development. Poor attention regulation in 6 month olds predicts aggressive behavior at age 2 (Eisenberg et al., 2010). In preschool children poor inhibitory control is associated with externalizing problems (Utendale & Hastings, 2011), and greater conflict with peers (Eisenberg et al., 2000; Murphy & Eisenberg, 1997). In elementary and junior high school students, poor inhibitory control is associated with higher levels of externalizing problems in typically developing (Belsky et al., 2007; Eisenberg et al., 2005; Eisenberg et al., 2010; Lengua, 2003) and high-risk children (Martel et al., 2007). In early adolescence, a poor ability to delay gratification is associated with externalizing disorders (Krueger et al., 1996; Mischel et al., 1989).. This association between self-regulation and social competence is consistent with our findings in the current study that demonstrate a strong association between level of self-regulatory capacity at age 9 and social competence at age 11. These findings suggest that the effects of impaired self-regulatory capacities extend over time and that impairment in self-regulatory capacities can affect children in discrete domains of social functioning.
In the current study, prenatal exposure to PAH was associated with level of social competence at age 11. Other previous studies report that prenatal exposure to sources of PAH is associated with disorders characterized by social problems. Prenatal exposure to traffic-related air pollution, of which PAHs are a component, is associated with an autism diagnosis (Volk et al., 2011; Volk et al., 2013). Not all social problems derive from autism spectrum dysfunction in social processing or social cognition; some derive from deficits in self-regulation, consistent with the long known finding that children with ADHD have social difficulties. For example, prior studies suggest that children with ADHD who are low in inhibitory control are more poorly regarded by peers than children high in inhibitory control (Bagwell, Molina, Pelham, & Hoza, 2001; Ronk, Hund, & Landau, 2011). In the present study, three SRS subscales drove the association of PAH with social competence: Social Awareness (i.e. the ability to pick up on social cues); Social Cognition (i.e. the ability to interpret social cues); and Social Communication (i.e. expressive verbal and non-verbal language skills). These subscales relate directly to self-regulation. With respect to social awareness, self-regulation is necessary for paying attention to interactions with other people. In terms of social cognition, self-regulation is necessary for identifying other people's emotional states. In terms of social communication, self-regulation is necessary for ensuring that a response is appropriate to the situation. The two subscales that were not associated with PAH seem to have little to do with self-regulation: Social Motivation (i.e. motivation to engage in social-interpersonal relationships); and Autistic Mannerisms (i.e. stereotypical behaviors and restricted interests characteristic of autism). We demonstrated further that the association of PAH with social competence is mediated by self-regulatory capacity. The relationship between prenatal exposure to PAH and the development of later social competence appears to be operating mainly through the development of self-regulatory capacities throughout childhood.
The alterations in the developmental trajectory of self-regulatory capacity that is associated with prenatal exposure to PAH may derive from alterations in brain development in the perinatal or early childhood periods. Prenatal exposure to PAH is associated with reductions in white matter surface of the brain and externalizing psychopathology (Peterson et al., 2015). Postnatal exposure was associated with further alterations in white matter surface in bilateral prefrontal cortices, above and beyond the effects of prenatal exposure to PAH (Peterson et al., 2015). Cortico-striatal-thalamo-cortical (CTSC) circuits comprise frontal, parietal, and motor cortices. These circuits govern subsystems that produce behavior relevant to self-regulation: motor, attentional, and emotional responses. These neural subsystems interact with one another, such that frontal control systems interact with emotional and motor systems to regulate activity through the subcortical circuits. Dysfunction in these circuits is associated with numerous childhood disorders, including Attention Deficit Hyperactivity Disorder (Mills et al., 2012), Obsessive Compulsive Disorder (Pauls, Abramovitch, Rauch, & Geller, 2014), Tourette’s Syndrome (Peterson et al., 1998), Substance Use disorders (Hyman, Malenka, & Nestler, 2006), and Eating Disorders (Marsh et al., 2011). In our current study, prenatal exposure to PAH is strongly associated with an altered developmental trajectory of self-regulatory capacities that is likely associated with altered function of the fronto-parietal control systems. Future studies should examine how functional activation of these circuits during the engagement of cognitive control varies between children with and without prenatal exposure to PAH.
Our study has a number of methodological strengths. The study utilizes the Columbia Children’s Center for Environmental Health dataset (Perera et al., 2006), which is a prospective, longitudinal dataset designed and implemented with gold standard epidemiologic methods. The dataset contains measurements of prenatal exposure to air pollution in the form of maternal PAH-DNA adducts as well as independent measurements of self-regulatory capacity at 4 time points across childhood. The dataset is an excellent representation of a well-defined subset of the population, thereby providing excellent information about the cognitive and emotional development of children born to minority women in large urban environments. Our study also has some limitations, however. One is missing data from loss to follow-up. Another is that we have survey, but not direct measures of children’s self-regulatory capacity across ages. We have controlled for a number of potential confounds, including exposure to tobacco smoke, which contains PAH. We therefore excluded smoking mothers from the sample. We also controlled for ethnicity and gender in our analyses.
Future studies should consider 1) the effects of prenatal exposure to PAH with multiple measures of exposure and with multiple direct measures of children’s self-regulatory capacity, 2) the effects of interactions between exposures on the developing brain and trajectories of development, and 3) the effects of gene-by-environment interactions on the developing brain and trajectories of development.
Conclusion
Our findings suggest children with prenatal exposure to PAH do not follow a typical trajectory for developing self-regulatory capacity, an important transdiagnostic dimension of behavior associated with many childhood psychopathologies (Marsh et al., 2009; Nigg & Casey, 2005), and indicate the presence of a developmental pathway to dysregulated attention, behavior, and emotion, and impaired socialization. This potential etiology of childhood neurodevelopmental disorders is grossly underappreciated given the ubiquity of environmental PAH. We suggest that a range of neurotoxicants likely contribute significantly to the etiology of seemingly idiopathic childhood disorders.
Figure 3.
Mediation Model of Effects of PAH on Social Competence. Sobel’s Test of Mediation statistic (10.23) was significant (p<.0005) for both one- and two-tailed tests, suggesting that direct effects of PAH exposure on SRS were mediated by the DESR phenotype.
Key points.
Prior studies demonstrate prenatal exposure to PAH negatively affects fetal brain development and neurodevelopmental outcomes.
In the current study, children with prenatal exposure to PAH failed to develop self-regulatory capacities along the typical trajectory from age 3 to 11.
Prenatal exposure to PAH was associated with deficits in social competence at age 11.
Self-regulatory capacity mediated the association of prenatal exposure to PAH and social competence.
Prenatal exposure to PAH produces long-lasting effects on self-regulatory capacities across early and middle childhood, and these deficits point to emerging social problems with real-world consequences for high-risk adolescent behaviors in this minority urban cohort.
Acknowledgments
The present research was financially supported by NIEHS R01 ES015579, NIDA R01 DA027100, R01 ES015282, NIEHS/EPA P01 ES09600/R82702701, NIEHS/EPA P01 ES09600/RD83214101, NIEHS/EPA P01 ES09600/RD83450901, NIEHS R01ES08977, The New York Community Trust, Trustees of the Blanchette Hooker Rockefeller Fund, John and Wendy Neu Foundation.
Footnotes
The authors declare that they have no competing or potential conflicts of interest.
References
- Achenbach T. Manual for the Child Behavior Checklist/2-3 and 1992 Profile. Burlington, VT: University of Vermont Department of Psychiatry; 1992. [Google Scholar]
- Achenbach TM, Rescorla LA. Manual for the ASEBA School-Age Forms & Profiles. Burlington, VT: University of Vermont, Research Center for Children, Youth, & Families; 2001. [Google Scholar]
- Achenbach TM, Rescorla LA. Manual for the ASEBA Preschool Forms & Profiles. Burlington, VT: University of Vermont, Research Center for Children, Youth, & Families; 2000. [Google Scholar]
- Bagwell CL, Molina BS, Pelham WE, Jr, Hoza B. Attention-deficit hyperactivity disorder and problems in peer relations: predictions from childhood to adolescence. J Am Acad Child Adolesc Psychiatry. 2001;40(11):1285–1292. doi: 10.1097/00004583-200111000-00008. [DOI] [PubMed] [Google Scholar]
- Becerra TA, Wilhelm M, Olsen J, Cockburn M, Ritz B. Ambient air pollution and autism in Los Angeles county, California. Environ Health Perspect. 2013;121(3):380–386. doi: 10.1289/ehp.1205827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belsky J, Pasco Fearon RM, Bell B. Parenting, attention and externalizing problems: testing mediation longitudinally, repeatedly and reciprocally. J Child Psychol Psychiatry. 2007;48(12):1233–1242. doi: 10.1111/j.1469-7610.2007.01807.x. [DOI] [PubMed] [Google Scholar]
- Biederman J, Petty CR, Day H, Goldin RL, Spencer T, Faraone SV, Wozniak J. Severity of the aggression/anxiety-depression/attention child behavior checklist profile discriminates between different levels of deficits in emotional regulation in youth with attention-deficit hyperactivity disorder. J Dev Behav Pediatr. 2012;33(3):236–243. doi: 10.1097/DBP.0b013e3182475267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biederman J, Spencer TJ, Petty C, Hyder LL, O'Connor KB, Surman CB, Faraone SV. Longitudinal course of deficient emotional self-regulation CBCL profile in youth with ADHD: prospective controlled study. Neuropsychiatr Dis Treat. 2012;8:267–276. doi: 10.2147/NDT.S29670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bostrom CE, Gerde P, Hanberg A, Jernstrom B, Johansson C, Kyrklund T, Westerholm R. Cancer risk assessment, indicators, and guidelines for polycyclic aromatic hydrocarbons in the ambient air. Environ Health Perspect. 2002;110(Suppl 3):451–488. doi: 10.1289/ehp.110-1241197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown LA, Khousbouei H, Goodwin JS, Irvin-Wilson CV, Ramesh A, Sheng L, Hood DB. Down-regulation of early ionotrophic glutamate receptor subunit developmental expression as a mechanism for observed plasticity deficits following gestational exposure to benzo(a)pyrene. Neurotoxicology. 2007;28(5):965–978. doi: 10.1016/j.neuro.2007.05.005. doi: S0161-813X(07)00079-4 [pii]10.1016/j.neuro.2007.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey BJ, Epstein JN, Buhle J, Liston C, Davidson MC, Tonev ST, Glover G. Frontostriatal connectivity and its role in cognitive control in parent-child dyads with ADHD. Am J Psychiatry. 2007;164(11):1729–1736. doi: 10.1176/appi.ajp.2007.06101754. [DOI] [PubMed] [Google Scholar]
- Constantino JN, Gruber CP. The Social Responsiveness Scale Manual. Los Angeles, CA: Western Psychological Services; 2005. [Google Scholar]
- Diamond A, Taylor C. Development of an aspect of executive control: development of the abilities to remember what I said and to "do as I say, not as I do". Dev Psychobiol. 1996;29(4):315–334. doi: 10.1002/(SICI)1098-2302(199605)29:4<315::AID-DEV2>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- Dubin MJ, Maia TV, Peterson BS. Cognitive Control in the Service of Self-Regulation. In: Koob GF, Le Moal M, Thompson RF, editors. Encyclopedia of Behavioral Neuroscience. Vol. 1. Oxford: Academic Press; 2010. pp. 288–293. [Google Scholar]
- Eisenberg N, Guthrie IK, Fabes RA, Shepard S, Losoya S, Murphy BC, Reiser M. Prediction of elementary school children's externalizing problem behaviors from attentional and behavioral regulation and negative emotionality. Child Dev. 2000;71(5):1367–1382. doi: 10.1111/1467-8624.00233. [DOI] [PubMed] [Google Scholar]
- Eisenberg N, Sadovsky A, Spinrad TL, Fabes RA, Losoya SH, Valiente C, Shepard SA. The relations of problem behavior status to children's negative emotionality, effortful control, and impulsivity: concurrent relations and prediction of change. Dev Psychol. 2005;41(1):193–211. doi: 10.1037/0012-1649.41.1.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg N, Spinrad TL, Eggum ND. Emotion-related self-regulation and its relation to children's maladjustment. Annu Rev Clin Psychol. 2010;6:495–525. doi: 10.1146/annurev.clinpsy.121208.131208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabes RA, Eisenberg N, Jones S, Smith M, Guthrie I, Poulin R, Friedman J. Regulation, emotionality, and preschoolers' socially competent peer interactions. Child Dev. 1999;70(2):432–442. doi: 10.1111/1467-8624.00031. [DOI] [PubMed] [Google Scholar]
- Gerardi-Caulton G. Sensitivity to spatial conflict and the development of self-regulation in children 24–36 months of age. Developmental Science. 2000;3(4):397–404. [Google Scholar]
- Gerstadt CL, Hong YJ, Diamond A. The relationship between cognition and action: performance of children 3 1/2–7 years old on a Stroop-like day-night test. Cognition. 1994;53(2):129–153. doi: 10.1016/0010-0277(94)90068-x. [DOI] [PubMed] [Google Scholar]
- Godschalk RW, Van Schooten FJ, Bartsch H. A critical evaluation of DNA adducts as biological markers for human exposure to polycyclic aromatic compounds. J Biochem Mol Biol. 2003;36(1):1–11. doi: 10.5483/bmbrep.2003.36.1.001. [DOI] [PubMed] [Google Scholar]
- Grova N, Valley A, Turner JD, Morel A, Muller CP, Schroeder H. Modulation of behavior and NMDA-R1 gene mRNA expression in adult female mice after sub-acute administration of benzo(a)pyrene. Neurotoxicology. 2007;28(3):630–636. doi: 10.1016/j.neuro.2007.01.010. [DOI] [PubMed] [Google Scholar]
- Herbstman JB, Tang D, Zhu D, Qu L, Sjodin A, Li Z, Perera FP. Prenatal exposure to polycyclic aromatic hydrocarbons, benzo[a]pyrene-DNA adducts, and genomic DNA methylation in cord blood. Environ Health Perspect. 2012;120(5):733–738. doi: 10.1289/ehp.1104056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyman SE, Malenka RC, Nestler EJ. Neural mechanisms of addiction: the role of reward-related learning and memory. Annu Rev Neurosci. 2006;29:565–598. doi: 10.1146/annurev.neuro.29.051605.113009. [DOI] [PubMed] [Google Scholar]
- Jacques S, Zelazo PD, Kirkham NZ, Semcesen TK. Rule selection versus rule execution in preschoolers: an error-detection approach. Dev Psychol. 1999;35(3):770–780. doi: 10.1037//0012-1649.35.3.770. [DOI] [PubMed] [Google Scholar]
- Jones LB, Rothabrt MK, Posner MI. Development of Executive Attention In Preschool Children. Developmental Science. 2003;6(5):498–504. [Google Scholar]
- Kochanska G, Murray KT, Harlan ET. Effortful control in early childhood: continuity and change, antecedents, and implications for social development. Dev Psychol. 2000;36(2):220–232. [PubMed] [Google Scholar]
- Krueger RF, Caspi A, Moffitt TE, White J, Stouthamer-Loeber M. Delay of gratification, psychopathology, and personality: is low self-control specific to externalizing problems? J Pers. 1996;64(1):107–129. doi: 10.1111/j.1467-6494.1996.tb00816.x. [DOI] [PubMed] [Google Scholar]
- Lengua LJ. Associations among emotionality, self-regulation, adjustment problems, and positive adjustment in middle childhood. Applied Developmental Psychology. 2003;24:595–618. [Google Scholar]
- Marsh R, Horga G, Wang Z, Wang P, Klahr KW, Berner LA, Peterson BS. An FMRI study of self-regulatory control and conflict resolution in adolescents with bulimia nervosa. Am J Psychiatry. 2011;168(11):1210–1220. doi: 10.1176/appi.ajp.2011.11010094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh R, Maia TV, Peterson BS. Functional disturbances within frontostriatal circuits across multiple childhood psychopathologies. Am J Psychiatry. 2009;166(6):664–674. doi: 10.1176/appi.ajp.2009.08091354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martel MM, Nigg JT, Wong MM, Fitzgerald HE, Jester JM, Puttler LI, Zucker RA. Childhood and adolescent resiliency, regulation, and executive functioning in relation to adolescent problems and competence in a high-risk sample. Dev Psychopathol. 2007;19(2):541–563. doi: 10.1017/S0954579407070265. [DOI] [PubMed] [Google Scholar]
- Melnick SM, Hinshaw SP. Emotion regulation and parenting in AD/HD and comparison boys: linkages with social behaviors and peer preference. J Abnorm Child Psychol. 2000;28(1):73–86. doi: 10.1023/a:1005174102794. [DOI] [PubMed] [Google Scholar]
- Mills KL, Bathula D, Dias TG, Iyer SP, Fenesy MC, Musser ED, Fair DA. Altered cortico-striatal-thalamic connectivity in relation to spatial working memory capacity in children with ADHD. Front Psychiatry. 2012;3:2. doi: 10.3389/fpsyt.2012.00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mischel W, Shoda Y, Rodriguez MI. Delay of gratification in children. Science. 1989;244(4907):933–938. doi: 10.1126/science.2658056. [DOI] [PubMed] [Google Scholar]
- Mooney LA, Santella RM, Covey L, Jeffrey AM, Bigbee W, Randall MC, et al. Decline of DNA damage and other biomarkers in peripheral blood following smoking cessation. Cancer Epidemiol Biomarkers Prev. 1995;4(6):627–634. [PubMed] [Google Scholar]
- Murphy BC, Eisenberg N. Young Children's Emotionality, Regulation and Social Functioning and Their Responses When They Are Targets of a Peer's Anger. Social Development. 1997;6(1):18–36. [Google Scholar]
- Murphy BC, Eisenberg N, Fabes RA, Shepard S, Guthrie IK. Consistency and Change in Children's Emotionality and Regulation: A Longitudinal Study. Merrill-Palmer Quarterly. 1999;45(3):413–444. [Google Scholar]
- Nigg JT, Casey BJ. An integrative theory of attention-deficit/hyperactivity disorder based on the cognitive and affective neurosciences. Dev Psychopathol. 2005;17(3):785–806. doi: 10.1017/S0954579405050376. [DOI] [PubMed] [Google Scholar]
- Nigg JT, Quamma JP, Greenberg MT, Kusche CA. A two-year longitudinal study of neuropsychological and cognitive performance in relation to behavioral problems and competencies in elementary school children. J Abnorm Child Psychol. 1999;27(1):51–63. doi: 10.1023/a:1022614407893. [DOI] [PubMed] [Google Scholar]
- Nijland MJ, Ford SP, Nathanielsz PW. Prenatal origins of adult disease. Curr Opin Obstet Gynecol. 2008;20(2):132–138. doi: 10.1097/GCO.0b013e3282f76753. [DOI] [PubMed] [Google Scholar]
- Parten MB. Social participation among pre-school children. The Journal of Abnormal and Social Psychology. 1932;27(3):26. [Google Scholar]
- Pauls DL, Abramovitch A, Rauch SL, Geller DA. Obsessive-compulsive disorder: an integrative genetic and neurobiological perspective. Nat Rev Neurosci. 2014;15(6):410–424. doi: 10.1038/nrn3746. [DOI] [PubMed] [Google Scholar]
- Perera FP, Chang HW, Tang D, Roen EL, Herbstman J, Margolis A, Rauh V. Early-life exposure to polycyclic aromatic hydrocarbons and ADHD behavior problems. PLoS One. 2014;9(11):e111670. doi: 10.1371/journal.pone.0111670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perera FP, Rauh V, Whyatt RM, Tsai WY, Tang D, Diaz D, Kinney P. Effect of prenatal exposure to airborne polycyclic aromatic hydrocarbons on neurodevelopment in the first 3 years of life among inner-city children. Environ Health Perspect. 2006;114(8):1287–1292. doi: 10.1289/ehp.9084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perera FP, Tang D, Wang S, Vishnevetsky J, Zhang B, Diaz D, Rauh V. Prenatal polycyclic aromatic hydrocarbon (PAH) exposure and child behavior at age 6–7 years. Environ Health Perspect. 2012;120(6):921–926. doi: 10.1289/ehp.1104315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson BS, Rauh VA, Bansal R, Hao X, Toth Z, Nati G, Perera F. Effects of Prenatal Exposure to Air Pollutants (Polycyclic Aromatic Hydrocarbons) on the Development of Brain White Matter, Cognition, and Behavior in Later Childhood. JAMA Psychiatry. 2015 doi: 10.1001/jamapsychiatry.2015.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson BS, Skudlarski P, Anderson AW, Zhang H, Gatenby JC, Lacadie CM, Gore JC. A functional magnetic resonance imaging study of tic suppression in Tourette syndrome. Arch Gen Psychiatry. 1998;55(4):326–333. doi: 10.1001/archpsyc.55.4.326. [DOI] [PubMed] [Google Scholar]
- Reed M, Pien DL, Rothbart MK. Inhibitory self-control in preschool children. Merrill-Palmer Quarterly. 1984;30:131–147. [Google Scholar]
- Rodier PM. Environmental causes of central nervous system maldevelopment. Pediatrics. 2004;113(4 Suppl):1076–1083. [PubMed] [Google Scholar]
- Ronk MJ, Hund AM, Landau S. Assessment of social competence of boys with attention-deficit/hyperactivity disorder: problematic peer entry, host responses, and evaluations. J Abnorm Child Psychol. 2011;39(6):829–840. doi: 10.1007/s10802-011-9497-3. [DOI] [PubMed] [Google Scholar]
- Rothbart MK, Rueda MR. The development of effortful control. In: Mayr U, Awh E, Keele S, editors. Developing individuality in the human brain: A tribute to Michael I. Posner. Washington, DC: American Psychological Association; 2005. pp. 167–188. [Google Scholar]
- Schellenberger MT, Grova N, Farinelle S, Willieme S, Schroeder H, Muller CP. Modulation of benzo[a]pyrene induced neurotoxicity in female mice actively immunized with a B[a]P-diphtheria toxoid conjugate. Toxicol Appl Pharmacol. 2013;271(2):175–183. doi: 10.1016/j.taap.2013.05.007. [DOI] [PubMed] [Google Scholar]
- Shoda Y, Mischel W, Peake PK. 1990, Vol.26, No. 6, 978–986. Developmental Psychology. 1990;26(6):978–986. [Google Scholar]
- Shonkoff JP, Garner AS Committee on Psychosocial Aspects of, Child, Family, Health, Committee on Early Childhood, Adoption, Dependent, Care, … Behavioral, Pediatrics. The lifelong effects of early childhood adversity and toxic stress. Pediatrics. 2012;129(1):e232–e246. doi: 10.1542/peds.2011-2663. [DOI] [PubMed] [Google Scholar]
- Spinrad TL, Eisenberg N, Cumberland A, Fabes RA, Valiente C, Shepard SA, Guthrie IK. Relation of emotion-related regulation to children's social competence: a longitudinal study. Emotion. 2006;6(3):498–510. doi: 10.1037/1528-3542.6.3.498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein J, Schettler T, Wallinga D, Valenti M. In harm's way: toxic threats to child development. J Dev Behav Pediatr. 2002;23(1 Suppl):S13–S22. doi: 10.1097/00004703-200202001-00004. [DOI] [PubMed] [Google Scholar]
- Utendale WT, Hastings PD. Developmental Changes in the Relations Between Inhibitory Control and Externalizing Problems During Early Childhood. Infant and Child Development. 2011;20(2):181–193. [Google Scholar]
- Veglia F, Loft S, Matullo G, Peluso M, Munnia A, Perera F Genair, Epic Investigators. DNA adducts and cancer risk in prospective studies: a pooled analysis and a meta-analysis. Carcinogenesis. 2008;29(5):932–936. doi: 10.1093/carcin/bgm286. [DOI] [PubMed] [Google Scholar]
- Volk HE, Hertz-Picciotto I, Delwiche L, Lurmann F, McConnell R. Residential proximity to freeways and autism in the CHARGE study. Environ Health Perspect. 2011;119(6):873–877. doi: 10.1289/ehp.1002835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volk HE, Lurmann F, Penfold B, Hertz-Picciotto I, McConnell R. Traffic-related air pollution, particulate matter, and autism. JAMA Psychiatry. 2013;70(1):71–77. doi: 10.1001/jamapsychiatry.2013.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Ehrenstein OS, Aralis H, Cockburn M, Ritz B. In utero exposure to toxic air pollutants and risk of childhood autism. Epidemiology. 2014;25(6):851–858. doi: 10.1097/EDE.0000000000000150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams BR, Ponesse JS, Schachar RJ, Logan GD, Tannock R. Development of inhibitory control across the life span. Dev Psychol. 1999;35(1):205–213. doi: 10.1037//0012-1649.35.1.205. [DOI] [PubMed] [Google Scholar]
- Wormley DD, Ramesh A, Hood DB. Environmental contaminant-mixture effects on CNS development, plasticity, and behavior. Toxicol Appl Pharmacol. 2004;197(1):49–65. doi: 10.1016/j.taap.2004.01.016. [DOI] [PubMed] [Google Scholar]
- Zeger SL, Liang KY. Longitudinal data analysis for discrete and continuous outcomes. Biometrics. 1986;42(1):121–130. [PubMed] [Google Scholar]