Abstract
A pigmented yeast R1 with strong tolerance to Hg2+ was isolated. Phylogenetic identification based on the analysis of 26S rDNA and ITS revealed R1 is a Rhodotorula mucilaginosa species. R1 was able to grow in the presence of 80 mg/L Hg2+, but the lag phase was much prolonged compared to its growth in the absence of Hg2+. The maximum Hg2+ binding capacity of R1 was 69.9 mg/g, and dead cells could bind 15% more Hg2+ than living cells. Presence of organic substances drastically reduced bioavailability of Hg2+ and subsequently decreased Hg2+ removal ratio from aqueous solution, but this adverse effect could be remarkably alleviated by the simultaneous process of cell propagation and Hg2+ biouptake with actively growing R1. Furthermore, among the functional groups involved in Hg2+ binding, carboxyl group contributed the most, followed by amino & hydroxyl group and phosphate group. XPS analysis disclosed the mercury species bound on yeast cells was HgCl2 rather than HgO or Hg0.
Introduction
Heavy metal pollutants are generated through a wide range of industrial activities and continue to be released into the environment at harmful quantities[1]. Pitfalls of non-biological approaches to heavy metal removal make a microbial-based technology for the detoxification of heavy metal in polluted systems a cost-effective and more environmentally friendly remediation option[2]. Although typically bacteria are commonly used in bioremediation studies, there are more fungal studies now than ever[3]. As with bacteria, fungi can naturally develop modified metabolism to deal with environmental contaminants and then be used in bioremediation[4]. Yeasts are good examples of fungi having a body size larger than bacteria. Like other eukaryotic organisms, yeasts have a nucleus and associated cytoplasmic organelles. The cytoplasm in living cells is responsible for the interactions with metal ions. Although yeast cells are generally known to be mediocre in terms of metal biouptake[5], recently the use of yeasts cells for accumulating heavy metals has gained more ground[6–8].
Rhodotorula species, which belong to the phylum Basidiomycota, are found to be common in natural environment and have been isolated from industrial and municipal wastes as well as polluted areas. A number of Rhodotorula species have been confirmed to be able to remediate some specific contaminants. For example, R.glutinis and R.rubra have both been found to have a high ability to degrade phenanthrene, while R.minuta was able to degrade benzo(a)anthracene. In a mixed fungal community Rhodotorula species contributed to effective degradation of low molecular weight PAHs, and although bacterial communities alone were not able to, the fungal communities also degraded high molecular weight PAHs (more than 3 benzene rings) such as chrysene and benzo(a)pyrene [9–11]. These results exhibit the promising potential of Rhodotorula species in the field of bioremediation[11]. For heavy metal bioremediation, despite previous studies on Cu tolerance and detoxification by Rhodotorula sp[12–13], few investigations on Hg tolerance and biouptake of these species were performed to our knowledge. Therefore, the biouptake of Hg could be a new use for this species.
In the present study, Rhodotorula mucilaginosa R1, a Hg-tolerant yeast was isolated and identified. The analysis of its tolerance to Hg and uptake of this metal was performed. Furthermore, the effect of coexisting organic substances on Hg uptake of R1 was evaluated, and the strategy to remove Hg from multi-component aqueous environment was developed. Finally, the mechanism of Hg biouptake by the yeast cells was explored based on chemical modification of functional groups and XPS analysis.
Materials and methods
Yeast isolation
Sediment samples were separately collected from Shenzhen Xixiang river, China. No specific permissions are required for these activities because it is a public area andthe field studies do not involve endangered or protected species. The samples were serially diluted and plated onto solid LB culturing medium (peptone, 10 g/L; yeast extract, 5 g/L; NaCl, 10 g/L; agar, 15 g/L) amended with 20 mg/L Hg2+ in the form of HgCl2 and incubated at 30°C for 3 d to allow the appearance of red colonies. The red colonies were further purified by repeated streaking on YPD (yeast extract 10 g/L, peptone 20 g/L, dextrose 20 g/L, pH 7.5) agar plates supplemented with 20–100 mg/L Hg2+ and incubated at 30°C for 2–7 d.
Phylogenetic identification of the isolate
One mercury-tolerant yeast isolate was selected for further studies and its phylogenetic identity was determined by analysis of rRNA gene sequences. Total genomic DNA from the strain was prepared according to the method used by Tristezza et al.[14]. The D1 and D2 regions of 26S ribosomal DNA (rDNA) and internal transcribed spacer (ITS) regions in the rRNA gene were sequenced directly from PCR products using the primer pairs NL-1 (forward; GCATATCAATAAGCGGAGGAAAAG) and NL-4 (reverse; GGTCCGTGTTTCAAGACGG) and ITS1 (forward, TCCGTAGGTGAACCTGCGG) and ITS4 (reverse, TCCTCCGCTTATTGATATGC), respectively.PCR was performed at 94°C for 30 s; 55°C for 30 s; and 72°C for 1.5 min, for 30 cycles, and then followed by a final extension at 72°C for 10min. After agarose gel analysis, the amplicons were purified and then sequenced. The search for sequence similarity with sequences in the GenBank database was performed by using the BLASTN algorithm. Strains with 99% or more similarity of the D1 and D2 regions of 26S rDNA and the overall ITS sequences were defined as conspecific [15]. A phylogenetic tree based on 26S rDNA was constructed using the neighbour-joining method. Furthermore, bootstrap analysis was performed to assess the confidence limits of the branching. The nucleotide sequences of 26S rDNA and ITS determined in this study have been deposited in GenBank under the accession number KU094060 and KU094061, respectively.
Mercury tolerance of the isolated yeast
Tolerance to mercury was tested by growth of yeast cells in 0.1YPD (yeast extract 1 g/L, peptone 2 g/L, dextrose 2 g/L, pH7.5) liquid medium supplemented with increasing concentrations of Hg2+ from 20 mg/L to 100 mg/L at 30°C, 150rpm for 2–7 d. In our experiment, 0.1YPD medium was used in an attempt to replicate relatively poor nutrition condition commonly existing in heavy metal wastewaters. A brewing Saccharomyces cerevisiae strain, deposited in our lab, was used as the control. Growth was monitored by optical density measurements at 600 nm. To determine the dry weight of the biomass, 20 mg sample of wet biomass was dried at 110°C to a constant weight.
Mercury biouptake
To obtain biouptake isotherm of the strains, yeast cells were cultured overnight at 30°C, 150 rpm in YPD medium. Then the cells were harvested by centrifugation at 8000×g for 10 min, washed three times with 0.85% NaCl solution, and resuspended in ddH2O solutions with the desired Hg2+ concentrations. After 2 h incubation at 30°C, 150 rpm, the cells were removed and pelleted by centrifugation. Then the supernatants and the pelleted cells were measured for the quantity of Hg2+. For supernatant samples, 5% HNO3-0.05% K2Cr2O7 was added before measurement to minimize Hg2+ loss due to glassware adsorption. For cell samples, the cells were pelleted, dried, and digested overnight with 70% trace-metal grade nitric acid for Hg2+ analysis.
The uptake of mercury by living and dead yeast cells over a period of 2h was examined by separately suspending equivalent dose of viable and dead cells in 10 mg/L Hg2+ solutions. Dead yeast cells were obtained by treating the harvested cells in autoclave at 105°C for 15 min.
To test mercury biouptake behavior in multi-component aqueous solution, equivalent dose of yeast cells were suspended in ddH2O (control), 0.1YPD medium and 0.01YPD medium (peptone, 0.2 g/L; yeast extract, 0.1 g/L; dextrose, 0.2 g/L), respectively supplemented with 10 mg/L Hg2+. After 2 h incubation at 30°C, 150 rpm, the cells were pelleted. Then the supernatants and pelleted cells were measured for Hg2+ as described above.
Mercury uptake by actively growing yeast cells was examined in cultures propagated in 0.1YPD medium with Hg2+added to a final concentration of 10 mg/L at 30°C, 150 rpm for 30 h. During cultivation process the samples were taken out at the different time intervals until the cells reach their stable-state phase. The cell density of samples was first measured as OD600 by a spectrophotometer at 600 nm. Then the samples were centrifuged, and the resultant cell pellets and supernatants were measured for Hg2+ determination as stated above.
All experiments were carried out in triplicate. All glassware was soaked in 20% nitric acid overnight and rinsed three times with ddH2O before complete drying.
Participation of functional groups in Hg2+ uptake
To understand the role of functional groups in metal ion binding, biomasses were chemically treated in different ways. Cells were suspended for 30 min in anhydrous methanol and concentrated hydrochloric acid (1:1, v/v) to esterify carboxylic groups, in formaldehyde and formic acid (1:1, v/v) to methylate amine or hydroxyl groups, and in triethyl phosphate and nitromethane (1:1, v/v) to esterify phosphate groups. After chemical modification, the cells were washed twice with ddH2O, harvested by centrifugation at 8000×g for 10 min and resuspended in10 mg/L Hg2+ solution. The decrease in metal uptake capacity of treated cells was used to evaluate the contribution of blocked functional group in the overall biouptake process [16].
X-ray Photo-electron Spectroscopy (XPS) analysis
XPS analysis of mercury-laden/unladen cells was performed according to the method described before [17–18]. Yeast cell samples were washed with ddH2O, frozen in liquid nitrogen, freeze-drying at 268 K, mounted the obtained powder in a trough and then pressed before being transferred to the analysis chamber of the spectrometer.
Analytical methods
A sequential inductively coupled plasma optical emission spectrometer (ICP-OES, OPTIMA 7000, Perkin Elmer) was employed to determine Hg2+. The dry weight of cells was calculated from the OD600 by using the value of 0.292 g dry weight per liter at an OD600 of 1.0 which was obtained in the experiment. Hg2+ binding capacity was expressed as milligram Hg2+ accumulated per gram dry weight of cells (mg/g). Hg2+ removal ratio was expressed as the percentage of Hg2+ removed from Hg2+ aqueous solution (%). XPS analysis was performed by a X-ray photo-electron spectroscopy (Microlab 350, Thermo VG).
Statistical analysis
Results were expressed as the mean plus or minus the standard deviation. Statistical comparison was performed using one-way analysis of variance. A probability (P) value of less than 0.05 was considered statistically significant.
Results
Strain isolation and identification
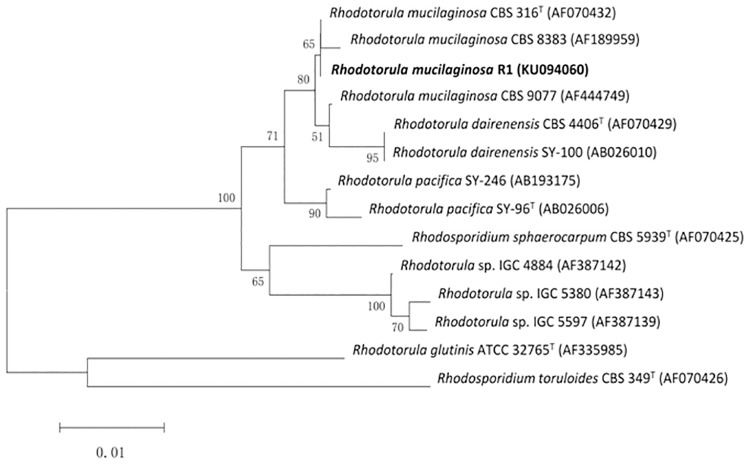
A yeast isolate obtained from sediment enrichment culture, designated as R1, was selected on the basis of its tolerance to Hg2+. The sequence of 26S rRNA gene (D1/D2 region) showed 99% homology with Rhodotorula mucilaginosa (accession no: EU7076.1) in similarity search using BLAST program. Furthermore, ITS1 and ITS2 region showed 99% homology with Rhodotorula mucilaginosa (accession no: AB026003.1). In the phylogenetic tree of the isolated yeast with other closely related strains established in Fig 1, the isolate is near Rhodotorula mucilaginosa CBS8383 (AF189959). Therefore, the isolate R1 is identified as a Rhodotorula mucilaginosa strain.
Fig 1. Phylogenetic tree.
Bootstrap values (expressed as percentages of 1000 replications) are shown at the branch points. Bar, 0.01 nucleotide substitution rate (Knuc) units.
Tolerance of R1 to mercury
Fig 2 showed the growth of R1 in 0.1YPD medium supplemented with different concentrations of Hg2+. It was clear that the presence of 20 mg/L Hg2+ didn’t visibly affect R1 growth. However, with the increase of Hg2+ concentration, the inhibition of toxic mercury on the growth of R1 showed up by extending the lag phase and decreasing the maximum OD600 it could reach. As Hg2+ concentration was 80 mg/L, the lag phase was prolonged to 120 h and the maximum OD600 dropped to lower than 3.0, almost 60% decrease compared to that at 20 mg/L Hg2+. When Hg2+ concentration came up to 100 mg/L, no growth of R1 was observed. On the contrary, the brewing S.cerevisiae, which was used as the control in our experiment, was not able to grow in the presence of 20 mg/L Hg2+ (data not shown), possibly suggesting no mercury detoxification strategy existing in brewing S.cerevisiae.
Fig 2. Growth of R1 in 0.1 YPD medium complemented with different concentration of Hg2+.
Biouptake capacity of Hg2+
Our experimental results revealed that Hg2+ bioaccumulated by yeast cells at equilibrium (q, mg/g) as a function of relevant residual Hg2+ concentration (Ce, mg/L) can be expressed by the Langmuir model in a linear form to calculate the maximum Hg2+ accumulation capacity (qm, mg/g). It was found that experimental data were in good agreement with the empirical Langmuir model, and the qm can be calculated to be 69.9 mg/g and 57.5 mg/g for R1 and S.cerevisiae, respectively (Table 1).
Table 1. Equilibrium biouptake of Hg2+.
| Ce(mg/L) | q (mg/g) | Langmuir model | 1/qm | qm(mg/g) | |
|---|---|---|---|---|---|
| R1 | 9.51 | 35.90 | Ce/q = 0.0143×Ce+0.0342 | 0.0143 | 69.90 |
| 19.57 | 55.94 | ||||
| 39.53 | 62.34 | ||||
| 59.67 | 65.00 | ||||
| 80.54 | 68.48 | ||||
| 101.8 | 67.83 | ||||
| S.cerevisiae | 12.29 | 46.06 | Ce/q = 0.0174×Ce+0.0286 | 0.0174 | 57.50 |
| 22.13 | 54.79 | ||||
| 42.46 | 61.85 | ||||
| 63.21 | 58.40 | ||||
| 84.24 | 61.97 | ||||
| 106.1 | 62.93 |
Biouptake in multi-component aqueous solutions
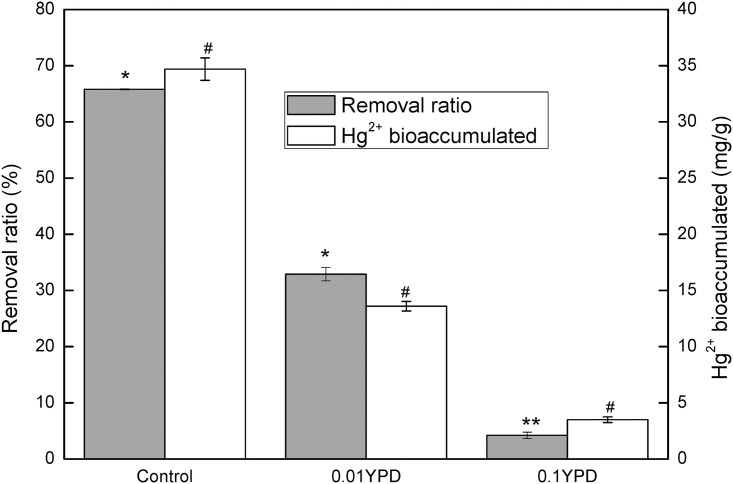
Fig 3 disclosed the remarkable inhibition of organic substances on Hg2+ biouptake. Compared with the control, Hg2+ removal ratio and Hg2+ accumulated by yeast cells decreased 50% and 61%, respectively in 0.01 YPD where the concentration of organic compounds was at a low level. As the concentration of organic compounds increased, only 4.2% Hg2+ could be removed from 0.1 YPD medium, almost 94% less than that of the control. Hg2+ biouptake capacity of R1 dropped to 3.5 mg/g, accounting for only 10% of that obtained in the control.
Fig 3. Comparison of Hg2+ removal ratio and Hg2+ biouptake by R1 from aqueous solutions containing different concentrations of organic substances.
The control was made of ddH2O and contained no organic substance. 0.1YPD and 0.01YPD represented diluted (1:10 and 1:100) YPD media. All solutions were complemented with 10 mg/L Hg2+.
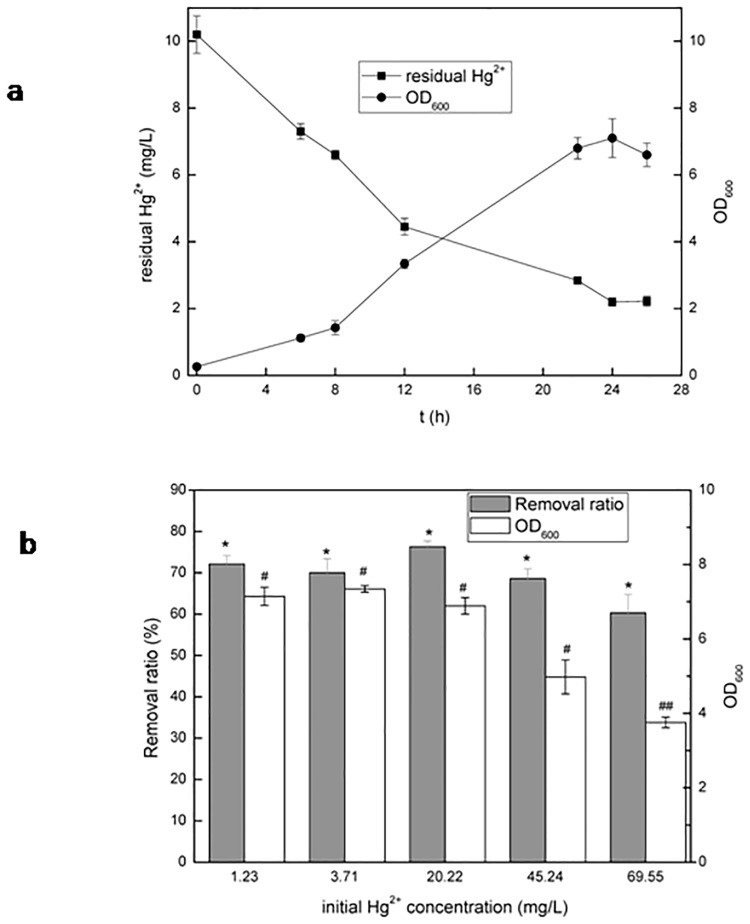
The result in Fig 4a showed that R1 cells could grow well in 0.1 YPD containing 10 mg/L Hg2+. In 24 h OD600 rose up to 6.6. In the meantime, the residual Hg2+ concentration of the medium dropped to 2.2 mg/L, demonstrating that 78% Hg2+ was removed from the solution, which was much higher than 4.2% obtained by resting cells from 0.1 YPD. Fig 4b exhibited Hg2+ removal ratio and OD600 that actively growing R1 was able to achieve at different Hg2+concentrations. The removal ratio of Hg2+ could exceed 60% even when initial Hg2+ concentration was 70 mg/L, suggesting that actively growing R1 cells can effectively remove Hg2+ from multi-component aqueous solutions.
Fig 4. Simultaneous process of cell propagation and Hg2+ removal by actively growing R1 from 0.1YPD media.
a: Time course of the simultaneous process of cell propagation and Hg2+ removal. 0.1YPD medium was complemented with 10 mg/L Hg2+. b: Comparison of Hg2+ removal and cell growth by actively growing R1 under different concentrations of Hg2+ after 30 h cultivation.
Role of functional groups in Hg2+ binding
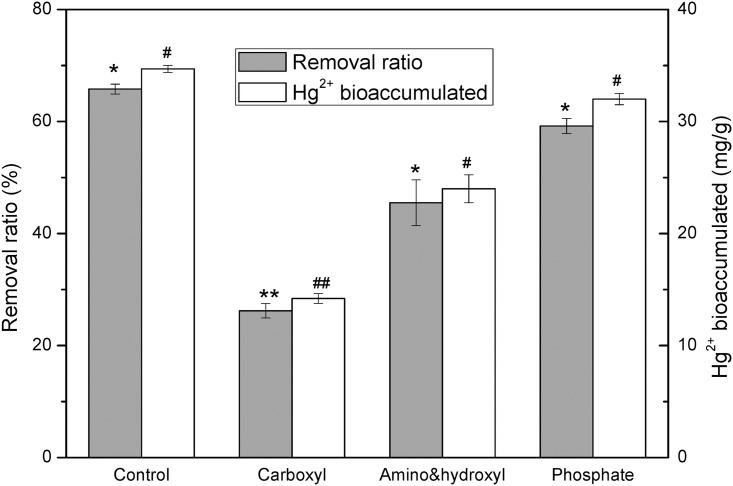
It was clear that carboxyl group was the most important group for Hg2+ uptake (Fig 5). The esterification of carboxyl group resulted in decrease of Hg2+ removal ratio and Hg2+ biouptake capacity from 65.8% and 34.7 mg/g to 26.2% and 14.2 mg/g, respectively in comparison to the control. Blocking of amino & hydroxyl groups did not affect Hg2+ uptake of R1 too much since 45.5% of Hg2+ removal ratio and 24 mg/g Hg2+ uptake remained. On the other hand, phosphate group seemed to slightly contribute to Hg2+ binding. Only 10% decrease for both Hg2+removal ratio and Hg2+ binding capacity occurred after phosphate groups were chemically modified.
Fig 5. Hg2+ removal and Hg2+ biouptake from 10 mg /L Hg2+ solution by unmodified R1 cells (control) and R1 chemically modified to block carboxyl, amino and hydroxyl, and phosphate groups, respectively.
After chemical modification, the cells were washed twice with ddH2O, harvested by centrifugation and resuspended in10 mg/L Hg2+ solution for 2 h biouptake.
XPS analysis
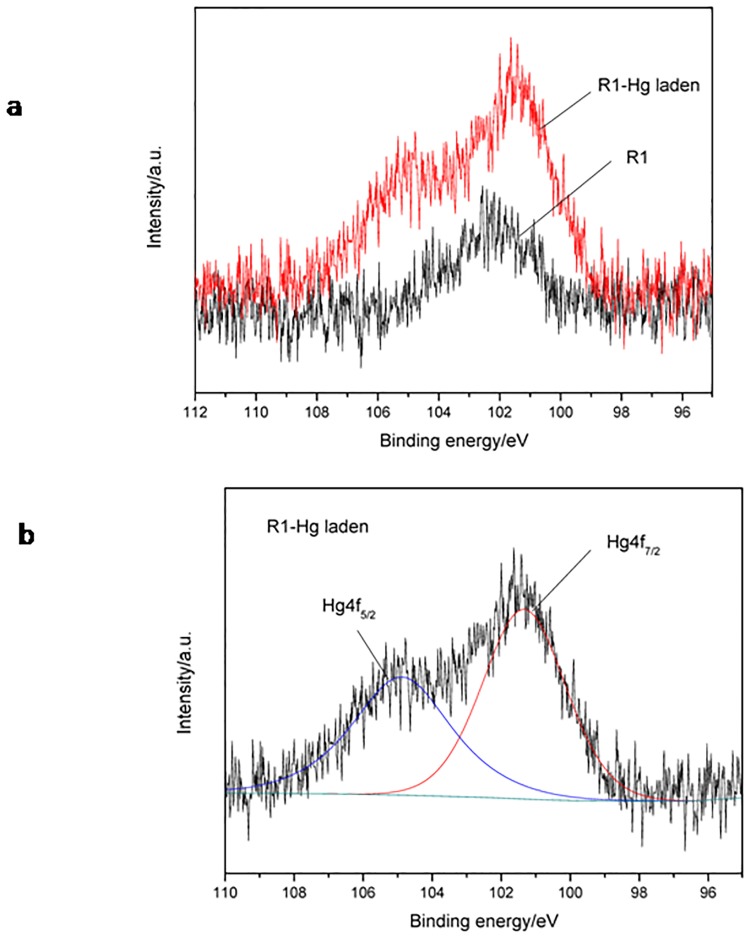
R1 before/after Hg2+ uptake was detected by XPS to analyze mercury bound on the cells. Fig 6a showed after Hg2+ uptake, Hg2+-laden R1 displayed double peaks, whilst original R1 cells had only one peak. In mercury standard spectra, there are supposed to be two peaks in the range of binding energy from 97 to 105 eV, 4f7/2 and 4f5/2, with the ratio of peak area being 4:3[19]. By fitting the peaks via Origin software and calculating the peak area (Fig 6b), it was found that the area of peak 4f7/2 and 4f5/2 was 3802.98 and 3005.59, respectively, accounting for the ratio nearly of 4:3. Therefore, this result confirmed mercury binding on R1 cells. Furthermore, mercury species can be figured out based on the binding energy of peak 4f7/2. It was reported that the binding energies of Hg 4f7/2 of the most appropriate reference compounds are as follows: 101.4 eV(HgCl2); 108.0 eV (HgO) and 99.8 (Hg0)[17]. Fig 6 disclosed the binding energy of 4f7/2 peak was 101.34 eV, clearly shifted beyond the 99.8 eV and 108.0eV reference point for Hg0 and HgO, respectively, suggesting mercury species bound on R1 cells might be HgCl2 rather than Hg0or HgO.
Fig 6. XPS analysis of R1 before/after Hg2+ uptake.
a: XPS spectra of R1 and Hg-laden R1. b: Calculation of peak area based on origin fitting.
Discussion
In some previous studies, Cu tolerance and detoxification by Rhodotorula sp[12–13] were reported. However, few investigations on Hg tolerance and biouptake of these species were performed. Therefore, a Hg-resistant yeast R1, identified to be Rhodotorula mucilaginosa species, was isolated and identified. Our experiment showed that R1 was able to grow in the presence of 80 mg/L Hg2+, much higher than 10 mg/L which is the maximum tolerant Hg2+ concentration for most reported Hg2+-tolerant microorganisms [20], displaying a high tolerance to toxic mercury.
In our experiment, 0.1 YPD medium was used to replicate the relatively poor nutrition condition commonly existing in heavy metal wastewaters. It was reported nutrient condition affects the tolerance of microorganism to heavy metals. Okino et al. [21] found that a Pseudomonas sp. was able to grow in LB medium with 100 mg/L Hg2+, whereas it could not grow in 0.1 LB supplemented with 40 mg/L Hg2+. The tolerance of R1 isolate to Hg2+ might be attributed to the presence of heavy metals in Shenzhen Bay [22]. The Hg resistance of some previously reported microorganisms including bacteria and yeasts is listed in Table 2.
Table 2. Comparison of mercury resistance of reported microorganisms.
| Strain | MIC * (mg/L) | Reference | |
|---|---|---|---|
| Bacteria | Pseudomonas putida | <10 | [20] |
| Pseudomonas sp. | 100 | [21] | |
| Bacillus thruingiensis PW-5 | 50 | [23] | |
| Bacillus cereus MMRF-575 | 100 | [24] | |
| Bacillus sp. AZ-1 | 20 | [25] | |
| Alcaligenes faecalis CH07 | 75 | [26] | |
| Pseudomonas sp. | 120 | [27] | |
| Halobacterial strain | 25 | [28] | |
| Yeasts | Rhodoturula rubra | 6 | [29] |
| Yarrowia sp. | 32 | [30] | |
| R1 | 80 | This study |
* MIC: Minimum inhibitory concentration of Hg2+
Organisms routinely exposed to heavy metal in their ecological niches are subjected to developing tolerance strategies such as bioreduction [23], surface biosorption and biosequestration [24]. Bacterial strains that harbor merA gene are known to reduce Hg2+ to volatile Hg0 and thus show the tolerance[25]. For yeast species, Oyetibo et al [30] found two mercury-resistant yeast strains, identified as Yarrowia species, were able to reduce and vaporize supplemented Hg2+ as metallic mercury (Hg0) and consequently showed tolerance to 32 mg L-1 Hg2+. They postulated Hg2+ reduction to Hg0 and Hg0 volatilization were likely triggered by sulfhydryl compounds present in the biomolecules in response to Hg toxicity. Another mechanism of resistant yeasts responsible for high level tolerance to heavy metal can be the production of some metabolites, such as organic acids, carotenoids and siderophores [13]. On the other hand, yeast cells can sequester heavy metal in vacuoles to prevent toxicity[31].
According to Xu’s investigation, Hg2+ concentration of seawater sampled from Shenzhen Bay was in the range of 0.001–0.01 mg/L [22]. Although Hg2+ concentrations in contaminated sites are normally not as high as that used in our study, using high Hg2+ concentration to screen high Hg2+-tolerant microorganisms may facilitate the application of bioremediation of Hg contamination.
The maximum Hg2+ binding capacity of R1 is 69.9mg/g, which is not high in comparison to Hg2+ binding capacity of other microorganisms [27,30]. Although qm of R1 was about 20% higher than that of S.cerevisiae, the difference in Hg2+ binding capacity between two kinds of yeasts was much smaller than the difference in their Hg2+tolerance, suggesting that high tolerance to heavy metal doesn’t necessarily accompany with high binding capacity for microorganisms [32]. For example, bacterial strains with merA gene normally show tolerance to Hg2+, but they may not accumulate much Hg2+ since Hg2+ tends to be reduced to volatile Hg0. As a kind of pigmented yeast, R. mucilaginosa produces carotenoid which has a demonstrated role in protecting the cells from oxidative damage caused by heavy metal [33]. However, Production of carotenoids can be part of a physiological response triggered to avoid cellular accumulation of heavy metals. Irazusta et al.[12] found that there is an inverse relationship between carotenoid production and copper biouptake by a R. mucilaginosa strain. Furthermore, the adverse effect of such antioxidants on Hg2+ accumulation of R1 could partly be confirmed by the difference in Hg2+ uptake between dead cells and living cells. Our experiment result showed that dead R1 cells could bind 40.2 ± 0.7 mg g-1 Hg2+, which is 15% more Hg2+ than 34 ± 0.3 mg g-1 obtained by living cells from 10 mg L-1 Hg2+ solution. Only living cells produce carotenoid and subsequently hinder Hg2+ binding whereas dead cells will not.
In previous studies, biouptake of heavy metal was mostly evaluated in experimental surrogates that only contained target heavy metal ions. However, actual heavy metal wastewater is much more complicated. There are normally other coexisting metal ions. Their effect on biouptake of target metal has been widely studied [27,34]. On the other hand, organic substances, though at relatively low concentrations, may also be contained but their effect has rarely been focused. Therefore, we evaluated biouptake of Hg2+ by R1 in 0.1 YPD and 0.01 YPD media.
The result in Fig 3 showed that the presence of organic substances might remarkably inhibit Hg2+ biouptake. YPD medium contains peptone and yeast extract, which are composed of lots of organic compounds such as amino acids, peptides, saccharides, etc. Some of them are able to form chelates or coordination compounds with transition metals, possibly decreasing bioavailability of heavy metals. From the sharp decrease of Hg2+ removal ratio caused by the presence of organic matters, it could be deduced that the formed mercury chelates or coordination compounds were soluble but not bioavailable and thus remained in the solution. Obviously this may increase the difficulty in bioremoval of heavy metal from wastewater.
However, the living cells used in Fig 3 is a kind of resting cells (metabolically active but not multiplying) since 2 h of incubation time is not long enough for them to grow and reproduce [30]. Indeed, most of organic compounds are nutrients for microorganisms. If microbial cells possess tolerance to heavy metal, they can make use of organic compounds for growth and propagation and thus increase the bioavailability of heavy metal. It was reported that growing cells may show a greater capacity for the removal of metals than non-viable biomass, especially in environments with nutrients [7]. Actually the results in Fig 4 confirmed that actively growing R1 cells can effectively remove Hg2+ from multi-component aqueous solutions.
Binding of heavy metals on different microorganisms may involve different functional groups to varying extents. Since carboxyl group is easier to be deprotonated than other groups [16], it seems to be more competitive in metal binding. Kapoor and Viraraghavan [35] demonstrated that carboxyl and amino groups were important functional groups involved in biosorption of Pb2+, Cd2+ and Cu2+. Carboxyl group was also confirmed to be the most important functional group for a marine Pseudomonas sp to uptake Hg2+ followed by amino & hydroxyl and phosphate groups to a nearly equivalent extent [27]. However, when using E.coli JM109 to accumulate Ni2+, the contribution of phosphate group was found to be the most important, while carboxyl group contributed the least [36]. For R1 yeast cells, carboxyl group was proved to be the most important functional group. Considering that carboxyl is the easiest deprotonation functional group among the studied groups, it was plausible to postulate that ion-exchange might be the principal mechanism for R1 to accumulate Hg2+.
Furthermore, by XPS analysis, mercury binding on R1 cells was confirmed and the species of bound mercury might be mercuric Hg rather than element Hg or HgO. However, since the 4f7/2 binding energy for the most common reference materials range only from 99.9 to 101.4 eV and the ΔeV (the distance between the 4f5/2 and 4f7/2 peaks) is consistently 4.1±0.1 eV [37], the confirmation of mercury species bound on R1 cells may need more evidence.
As remediation of heavy metal pollution from local environment can be achieved by using heavy metal-tolerant microorganisms, the yeast R1 cells isolated in this study may have the potential for in-situ bioremediation of mercury contamination.
Conclusions
A Rhodotorula mucilaginosa R1with high tolerance to Hg2+ was isolated and identified. R1 could grow in 80 mg/L Hg2+, but its Hg2+ biouptake capacity was relatively low, suggesting no connection between metal tolerance and accumulation capacity. Organic substances reduced Hg2+ bioavailability and thus decreased Hg2+ removal, but actively growing R1 cells could consume organic matters and consequently accumulate Hg2+ in the solution. Carboxyl group proved to be the most important functional group in Hg2+ uptake, while phosphate group contributed the least. XPS analysis confirmed the binding of mercury on R1 and the mercury species being HgCl2.
Acknowledgments
The authors would like to acknowledge financial support provided by National Natural Science Foundation of China (No. 51578339) and Shenzhen R&D fund (JCYJ20140418193546101)
Data Availability
All relevant data are within the paper.
Funding Statement
This work was supported by the National Natural Science Foundation of China (No. 51578339) (https://www.nsfc.gov.cn/) to XD; Shenzhen R$D Fund (JCYJ20140418193546101), (http://www.szsti.gov.cn/) to XD. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Nimick DA, Caldwell RR, Skaar DR, Selch TM. Fate of geothermal mercury from Yellowstone National Park in the Madison and Missouri Rivers. USA Sci Total Environ. 2013; 443: 40–54 10.1016/j.scitotenv.2012.10.080 [DOI] [PubMed] [Google Scholar]
- 2.Volesky B. Biosorption and me. Wat Res. 2007; 41: 4017–4029 [DOI] [PubMed] [Google Scholar]
- 3.Zhang H, Huang T, Chen S. Ignored sediment fungal populations in water supply reservoirs are revealed by quantitative PCR and 454 pyrosequencing. BMC Microbiol. 2015; 15(44):1–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Heshem AE, Khan S, Tao Y, Li D, Zhang Y, Yang M. Biodegradation of high molecular weight PAHs using isolated yeast mixtures: application of meta-genomic methods for community structure analysis. Environ Sci Pollut Res. 2012; 19 (8): 3568–3578 [DOI] [PubMed] [Google Scholar]
- 5.Volesky B. Advances in biosorption of metals: selection of biomass types. FEMS Microbiol Rev. 1994; 14:291–302 [DOI] [PubMed] [Google Scholar]
- 6.Hai RT,Feng H,Wang WX.Adsorption of mercury(II) by beer yeast immobilized in chitosan/silicone leg. Asian J Chem. 2013; 25(12): 6528–6530 [Google Scholar]
- 7.Li C, Xu Y, Jiang W, Dong X, Wang D, Liu B. Effect of NaCl on the heavy metal tolerance and bioaccumulation of Zygosaccharomyces rouxii and Saccharomyces cerevisiae. Bioresour Technol. 2013; 143: 46–52 10.1016/j.biortech.2013.05.114 [DOI] [PubMed] [Google Scholar]
- 8.Bilal M, Shah J A, Ashfaq T, Gardazi SMH, Tahir A A, Pervez A, Haroon H, Mahmood Q. Waste biomass adsorbents for copper removal from industrial wastewater—A review. J Hazard Mater. 2013;263: 322–333 10.1016/j.jhazmat.2013.07.071 [DOI] [PubMed] [Google Scholar]
- 9.Balsalobre L, De Silóniz MI, Valderrama MJ, Benito T, Larrea MT, Peinado JM. Occurrence of yeast in municipal wastes and their behavior in presence of cadmium, copper and zinc. J Basic Microbiol. 2003; 43: 185–193 10.1002/jobm.200390021 [DOI] [PubMed] [Google Scholar]
- 10.Villegas LB, Amoroso MJ, de Figueroa LIC. Responses of Candida fukuyamaensis RCL-3 and Rhodotorula mucilaginosa RCL-11 to copper stress. J Basic Microbiol. 2009; 49: 1–9 [DOI] [PubMed] [Google Scholar]
- 11.Jarboui R, Baati H, Fetoui F, Gargouri A, Gharsallah N, Ammar E. Yeast performance in wastewater treatment: case study of Rhodotorula mucilaginosa. Environ Technol. 2012; 33(7–9): 951–960 10.1080/09593330.2011.603753 [DOI] [PubMed] [Google Scholar]
- 12.Irazusta V, Nieto-Penalver CG, Cabral ME, Amoroso MJ, de Figueroa LIC. Relationship among carotenoid production, copper bioremediation and oxidative stress in Rhodotorula mucilaginosa RCL-11. Process Biochem. 2013;48: 803–809 [Google Scholar]
- 13.Rajpert L, Skłodowska A, Matlakowska R. Biotransformation of copper from Kupferschiefer black shale (Fore-Sudetic Monocline, Poland) by yeast Rhodotorula mucilaginosa LM9. Chemosphere. 2013;91: 1257–1265 10.1016/j.chemosphere.2013.02.022 [DOI] [PubMed] [Google Scholar]
- 14.Tristezza M, Gerardi C, Logrieco A, Grieco F. An optimized protocol for the production of interdelta markers in Saccharomyces cerevisiae by using capillary electrophoresis. J Microbiol Meth. 2009; 78: 286–291 [DOI] [PubMed] [Google Scholar]
- 15.Saksinchai S, Suzuki M, Chantawannakul P, Ohkuma M, Lumyong S. A novel ascosporogenous yeast species, Zygosaccharomyces siamensis, and the sugar tolerant yeasts associated with raw honey collected in Thailand. Fungal Diversity. 2012; 52: 123–139 [Google Scholar]
- 16.Chojnacka K, Chojnacki A, Gorecka H. Biosorption of Cr3+, Cd2+ and Cu2+ ions by blue-green algae Spirulina sp.: kinetics, equilibrium and the mechanism of the process. Chemosphere. 2005; 59: 75–84 10.1016/j.chemosphere.2004.10.005 [DOI] [PubMed] [Google Scholar]
- 17.Vieira RS, Oliveira MLM, Guibal E, Rodríguez-Castellón E, Beppu MM. Copper, mercury and chromium adsorption on natural and crosslinked chitosan films: An XPS investigation of mechanism. Colloids and Surfaces A: Physicochem Eng Aspects. 2011;374: 108–114 [Google Scholar]
- 18.Dengis PB, Rouxhet PG. Preparation of yeast cells for surface analysis by XPS. J Microbio Meth. 1996; 26: 171–183 [Google Scholar]
- 19.Hyland MM, Jean GE, Bancroft GM. XPS and AES studies of Hg(II) sorption and desorption reactions on sulphide minerals.Geochimica et Cosmochimica Acta. 1990; 54(7): 1957–1967 [Google Scholar]
- 20.Nascimento AMA, Chartone-Souze E. Operon mer: bacterial tolerance to mercury and potential for bioremediation of contaminated environment. Genet Mol Res. 2003; 2: 92–101 [PubMed] [Google Scholar]
- 21.Okino S, Iwasaki K, Yagi O, Tanaka H. Removal of mercuric chloride by a genetically engineered mercury-volatilizing bacterium Pseudomonas putida PpY101/pSR134. Bullet Environ Contamin Toxicol. 2002; 68: 712–719 [DOI] [PubMed] [Google Scholar]
- 22.Xu GC, Xu SJ, Song Y, Niu AY, Yang Q. Evaluation on the seawater’s heavy metals in Shenzhen Mangrove Nature Reserve. J South China Normal Univ. 2015; 47(1):101–108 [Google Scholar]
- 23.Dash HR, Mangwani N, Das S. Characterization and potential application in mercury bioremediation of highly mercury-resistant marine bacterium Bacillus thuringiensis PW-05. Environ Sci Pollut Res. 2014; 21(4):2642–2653 [DOI] [PubMed] [Google Scholar]
- 24.Abdulaziz A,Vijayan V, Pavanan P, Nair S. Unicellular cyanobacteria Synechocystis accommodate heterotrophic bacteria with varied enzymatic and metal resistance properties.J Basic Microbiol. 2016;56(8):845–856 10.1002/jobm.201500693 [DOI] [PubMed] [Google Scholar]
- 25.Aatif A, Zakia L. Screening of mercury-resistant and indole-3-acetic acid producing bacterial-consortium for growth promotion of Cicer arietinum L. J Basic Microbiol. 2016; [DOI] [PubMed] [Google Scholar]
- 26.De J, Ramaiah N. Characterization of marine bacteria highly resistant to mercury exhibiting multiple resistances to toxic chemicals. Ecol Indicators.2007; 7:511–520. [Google Scholar]
- 27.Deng X,Wang PT. Isolation of marine bacteria highly resistant to mercury and their biaccumulation process. Bioresour Technol. 2012; 121: 342–347. 10.1016/j.biortech.2012.07.017 [DOI] [PubMed] [Google Scholar]
- 28.Williams GP, Gnanadesigan M, Ravikumar S. Isolation, identification and metal tolerance of halobacteial strains. Indian J Geomarine Sci. 2013; 42(3):402–408. [Google Scholar]
- 29.Ghosh SK, Ghosh S, Chaudhuri J, Gachhui R, Mandal A. Studies on mercury resistance in yeasts isolated from natural sources. Bull Environ Contam Toxicol. 2004; 72:21–28. 10.1007/s00128-003-0236-5 [DOI] [PubMed] [Google Scholar]
- 30.Oyetibo GO, Ishola S, Ikeda-Ohtsubo W, Miyauchi K, Ilori MO, Endo G. Mercury bioremoval by Yarrowia strains isolated from sediments of mercury-polluted estuarine water. Appl Microbiol Biotechnol. 2015; 99:3651–3657 10.1007/s00253-014-6279-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Diffels JF, Seret ML, Goffeau A, Baret PV. Heavy metal transporters in Hemiascomycete yeasts. Biochimie. 2006; 88:1639–1649 10.1016/j.biochi.2006.08.008 [DOI] [PubMed] [Google Scholar]
- 32.Debelius B, Forja J M, DelValls A, Lubian L M. Toxicity and bioaccumulation of copper and lead in five marine microalgae. Ecotoxicol Environ Safety. 2009; 72: 1503–1513 10.1016/j.ecoenv.2009.04.006 [DOI] [PubMed] [Google Scholar]
- 33.Irazusta V, Estévez C, Amoroso MJ, de Figueroa LIC. Proteomic study of the yeast Rhodothorula mucilaginosaRCL-11 under copper stress. BioMetals. 2012; 25:517–527 10.1007/s10534-012-9531-0 [DOI] [PubMed] [Google Scholar]
- 34.Kefala MI, Zouboulis AI, Matis KA. Biosorption of cadmium ions by Actinomycetes and separation by flotation. Environ Pollut. 1999; 104(2): 283–293 [Google Scholar]
- 35.Kapoor A, Viraraghavan T. Heavy metal biosorption sites in Aspergillus Niger. Bioresour Technol. 1997; 61: 221–227 [DOI] [PubMed] [Google Scholar]
- 36.Deng X,HeJ M, He N. Comparative study on Ni-affinity transport of NiCoTs and the potential of recombinant E.coli for Ni bioaccumulation. Bioresour Technol. 2013; 130:69–74 10.1016/j.biortech.2012.11.133 [DOI] [PubMed] [Google Scholar]
- 37.Huston N, Attwood BC, Scheckel KG. XAS and XPS characterization of mercury binding on brominated activated carbon. Environ Sci Technol. 2007; 41: 1747–1752. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper.