Abstract
Most patients with end-stage kidney disease value their health related quality of life (HRQoL) and want to know how it will be affected by their dialysis modality. We extended the findings of two prior clinical trial reports to estimate the effects of frequent compared to conventional hemodialysis on additional measures of HRQoL. The Daily Trial randomly assigned 245 patients to receive frequent (six times per week) or conventional (three times per week) in-center hemodialysis. The Nocturnal Trial randomly assigned 87 patients to receive frequent nocturnal (six times per week) or conventional (three times per week) home hemodialysis. All patients were on conventional hemodialysis prior to randomization, with an average feeling thermometer score of 70 to 75 (a visual analog scale from 0 to 100 where 100 is perfect health), an average general health scale score of 40 to 47 (a score from 0 to 100 where 100 is perfect health), and an average dialysis session recovery time of 2 to 3 hours. Outcomes are reported as the between-treatment group differences in one-year change in HRQoL measures and analyzed using linear mixed effects models. After one year in the Daily Trial, patients assigned to frequent in-center hemodialysis reported a higher feeling thermometer score, better general health, and a shorter recovery time after a dialysis session compared to standard thrice weekly dialysis. After one year in the Nocturnal Trial, patients assigned to frequent home hemodialysis also reported a shorter recovery time after a dialysis session, but no statistical difference in their feeling thermometer or general health scores compared to standard home dialysis schedules. Thus, patients receiving day or nocturnal hemodialysis on average recovered approximately one hour earlier from a frequent compared to conventional hemodialysis session. Patients treated in an in center dialysis facility reported better HRQoL with frequent compared to conventional hemodialysis.
Keywords: daily hemodialysis, nocturnal hemodialysis, health-related quality of life, clinical trial
INTRODUCTION
There is a growing effort to make healthcare and health policies more patient-centered. This requires greater knowledge of how a treatment affects patient-reported outcomes, where information comes directly from a patient without interpretation of their treatment response by a clinician.1 Most patients with end-stage kidney disease place enormous value on their health related quality of life (HRQoL), even over survival, and want to know how their HRQoL will be affected by their choice of dialysis modality.2 Over two million people worldwide receive conventional hemodialysis three sessions per week to sustain life.3 More frequent hemodialysis (five or six sessions per week) results in greater weekly solute and fluid removal, and may be associated with better HRQoL (as observed in small studies 4;5). We previously reported the primary results of two parallel 12 month-follow-up randomized controlled trials of frequent hemodialysis (Frequent Hemodialysis Network (FHN) Daily and Nocturnal Trials).6;7 One of two co-primary outcomes reported in the primary results of these trials was the baseline to 12 month change in a commonly employed patient-reported physical health survey score (the Physical Health Composite (PHC) from the Short-Form RAND 36-item survey 8). After one year in the Daily Trial, patients assigned to frequent versus conventional in-center hemodialysis reported a better score.6 After one year in the Nocturnal Trial, we could not reliably determine whether patients assigned to frequent nocturnal versus conventional hemodialysis truly differed in this score, as the trial did not meet its recruitment target resulting in estimates with wide confidence intervals.7 Several additional patient-reported measures of general well-being were collected in the FHN trials to better assess the multi-dimensional concept of HRQoL. These measures have not been reported elsewhere. In this article we investigated whether frequent hemodialysis compared to conventional hemodialysis affects four measures of HRQoL collected in the two FHN trials.
RESULTS
Patient selection from both trials is presented in Figure 1a and Figure 1b. Baseline characteristics of patients in each trial are summarized in Tables 1 and 2. As confirmed in previous reports, the baseline characteristics of patients assigned to frequent versus conventional hemodialysis were similar in each trial.
Figure 1.
a. Flow diagram for Daily Trial showing the number of patients enrolled and assigned to each study am (intervention/control), and number of patients who completed the baseline and 12-month HRQoL measures, including reasons for drop-out.
*Two patients received transplants late during follow-up and were included in the analyses for 12-month changes for the HUI3, General Health subscore and the Health Thermometer. One of the two transplanted patients also contributed to the analysis for 12-month changes in post-dialysis recovery time.
b. Flow diagram for Nocturnal Trial showing the number of patients enrolled and assigned to each study am (intervention/control), and number of patients who completed the baseline and 12-month HRQoL measures, including reasons for drop-out.
*One patient received a transplant late during follow-up and was included in the analyses for 12-month changes for the HUI3, General Health subscore and post-dialysis recovery time.
Table 1.
Baseline characteristics of the Daily Trial.
| Characteristics | N (%) Mean ± S.D Median [25th, 75th percentiles] |
||
|---|---|---|---|
| All patients (N=245) |
3X per week (N=120) |
6X per week (N=125) |
|
| Age (years) | 50.4 ± 13.9 50 [42, 59] |
52.0 ± 14.1 52 [43, 60] |
48.9 ± 13.6 47 [41, 58] |
| Male | 151 (61.6%) | 73 (60.8%) | 78 (62.4%) |
| Black Race | 102 (41.6%) | 53 (44.2%) | 49 (39.2%) |
| Education (Completed High School or Less) | 109 (45.1%) | 53 (44.5%) | 56 (45.5%) |
| Primary Language English | 196 (80.0%) | 101 (84.2%) | 95 (76.0%) |
| Duration of ESRD | |||
| < 2yrs | 72 (29.4%) | 35 (29.2%) | 37 (29.6%) |
| 2–5 yrs | 76 (31.0%) | 42 (35.0%) | 34 (27.2%) |
| > 5 yrs | 97 (39.6%) | 43 (35.8%) | 54 (43.2%) |
| Diabetes | 100 (40.8%) | 50 (41.7%) | 50 (40.0%) |
| Charlson Comorbidity Index | 1.82 ± 1.95 2 [0, 3] |
1.88 ± 2.03 2 [0, 3] |
1.76 ± 1.89 2 [0, 3] |
| Predialysis Serum Albumin (g/dL) | 3.94 ± 0.42 | 3.94 ± 0.46 | 3.94 ± 0.37 |
| Predialysis Hemoglobin (mg/dL) | 11.9 ± 1.26 | 12.0 ± 1.24 | 11.9 ± 1.28 |
| Dialysis Std Kt/Vurea | 2.52 ± 0.35 | 2.53 ± 0.39 | 2.50 ± 0.31 |
| Antidepressant | 35 (14.3%) | 15 (12.5%) | 20 (16.0%) |
| Opioid | 42 (17.1%) | 19 (15.8%) | 23 (18.4%) |
| Physical Health Composite (RAND SF36) | 38.1 ± 10.5 | 38.0 ± 9.7 | 38.1 ± 11.2 |
| Mental Health Composite (RAND SF36) | 45.1 ± 11.8 | 46.0 ± 10.3 | 44.3 ± 13.0 |
| Beck Depression Inventory Score | 12.5 ± 9.0 | 12.4 ± 9.5 | 12.6 ± 8.6 |
Table 2.
Baseline Characteristics in the Nocturnal trial
| Characteristics | N (%) Mean ± S.D Median [25th, 75th percentiles] |
||
|---|---|---|---|
| All Patients (N=87) |
3X per week (N=42) |
6X per week (N=45) |
|
| Age (years) | 52.8 ± 13.6 54 [43, 69] |
54.0 ± 12.9 54 [45, 62] |
51.7 ± 14.4 53 [42, 64] |
| Male | 57 (65.5%) | 28 (66.7%) | 29 (64.4%) |
| Black Race | 23 (26.4%) | 11 (26.2%) | 12 (26.7%) |
| Education (Completed High School or Less) | 34 (39.5%) | 14 (33.3%) | 20 (45.5%) |
| Primary Language English | 77 (88.5%) | 36 (85.7%) | 41 (91.1%) |
| ESRD Vintage | |||
| < 2yrs | 58 (66.7%) | 30 71.4%) | 28 (62.2%) |
| 2–5 yrs | 13 (14.9%) | 5 (11.9%) | 8 (17.8%) |
| > 5 yrs | 16 (18.4%) | 7 (16.7%) | 9 (20.0%) |
| Diabetes | 37 (42.5%) | 18 (42.9%) | 19 (42.2%) |
| Charlson Comorbidity Index | 1.72 ± 1.75 2 [0, 3] |
1.88 ± 1.93 2 [0, 3] |
1.58 ± 1.57 2 [0, 2] |
| Predialysis Serum Albumin (g/dL) | 3.91 ± 0.49 | 3.92 ± 0.51 | 3.90 ± 0.48 |
| Predialysis Hemoglobin (mg/dL) | 11.8 ± 1.11 | 11.9 ± 1.09 | 11.6 ± 1.12 |
| Dialysis Std Kt/Vurea | 2.34 ± 0.31 | 2.34 ± 0.34 | 2.35 ± 0.28 |
| Antidepressant | 22 (25.3%) | 10 (23.8%) | 12 (26.7%) |
| Opioid | 18 (20.7%) | 7 (16.7%) | 11 (24.4%) |
| Physical Health Composite (RAND SF36) | 37.8 ± 8.97 | 38.2 ± 8.3 | 37.5 ± 9.6 |
| Mental Health Composite (RAND SF36) | 45.8 ± 11.5 | 45.9 ± 12.6 | 45.6 ± 10.5 |
| Beck Depression Inventory Score | 11.7 ± 8.60 | 12.2 ± 9.2 | 11.2 ± 8.1 |
The baseline and follow-up HRQoL measures for patients assigned to frequent versus conventional hemodialysis in each trial are presented in Table 3. The values of the baseline HRQoL measures were similar in patients assigned to frequent compared to conventional hemodialysis in both trials, with the exception of slight differences in the health utilities index and general health scale scores in the Nocturnal Trial.
Table 3.
Four general measures of health related quality of life in the Daily and Nocturnal Trials.
The observed baseline data reflects all records for randomized patients. The observed month 4 and 12 data represents patients with data at both baseline and month 4 and at both baseline and month 12, respectively.
Mean changes and treatment effects were adjusted for the baseline value of each variable. In addition, the Daily Trial analyses adjusted for clinical center. For recovery time from dialysis, adjusted mean changes and treatment effects derived from back-transforming percent difference of geometric mean changes based on log-transformed analysis.
The completeness of each HRQoL measure in follow-up is also presented in Table 3. Each measure was completed by over 80% of eligible patients, after patients who died or received a kidney transplant in follow-up were excluded from consideration (14 patients in the Daily Trial and 3 patients in the Nocturnal trial, and 24 patients in the Daily trial and 5 patients in the Nocturnal trial, respectively).
Daily Trial
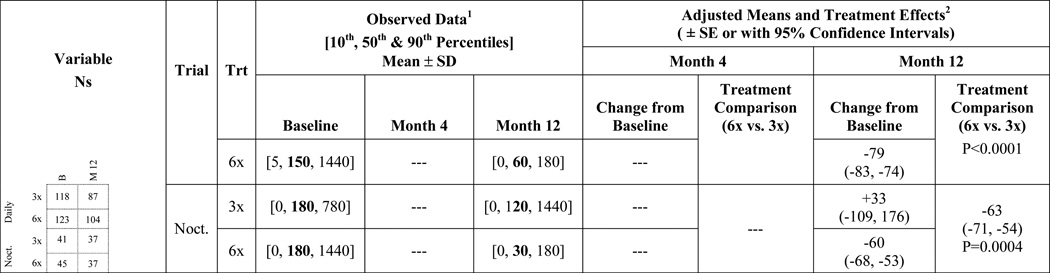
Feeling thermometer: The baseline mean (standard deviation) score was 74 (19) in patients assigned to frequent hemodialysis and 71 (17) in patients assigned to conventional hemodialysis. Patients assigned to frequent hemodialysis demonstrated a significant improvement in their feeling thermometer score over the first 4 months of follow-up, while the score remained similar in patients assigned to conventional hemodialysis (change of 5.6 vs. −0.5 from baseline). These trends persisted to 12 months after randomization (5.8 vs. −0.6 from baseline). There was a significant 6.4 point between-treatment group difference in the change in score over one year (95% CI 1.8 to 11.1). General health scale: The baseline mean (standard deviation) score was 47 (22) in patients assigned to frequent hemodialysis and 44 (22) in patients assigned to conventional hemodialysis. Patients assigned to frequent hemodialysis demonstrated a significant improvement in their general health scale score over the first 4 months of follow-up, while the score remained similar in patients assigned to conventional hemodialysis (change of 6.5 vs. −1.6 from baseline). These trends persisted to 12 months after randomization (6.3 vs. −3.4 from baseline). There was a significant 9.7 point between-treatment group difference in the change in score over one year (95% CI 4.7 to 14.7). Time to recover from a dialysis session: Between baseline and 12 months of follow-up, in patients assigned to frequent hemodialysis the median recovery time (10th, 90th percentile) decreased from 150 minutes (5, 1440) to 60 minutes (0,180), whereas in patients assigned to conventional hemodialysis the median recovery time remained similar from 120 minutes (0,480) to 180 minutes (15, 1440). The mean between-treatment group difference in the change in recovery time over one year was −84 minutes (95% CI: −89, −80; p<0.0001). A recovery time of 60 minutes or more at baseline was observed in 80% of patients assigned to frequent hemodialysis and 79% assigned to conventional hemodialysis. Corresponding numbers one year after randomization were 43% and 63%, respectively. Health utilities index: There was no consistent statistical difference between frequent and conventional hemodialysis in the change in the health utilities index over 4 and 12 months of follow-up, or its eight attributes (Appendix 1).
Nocturnal trial
Feeling thermometer
The baseline mean (standard deviation) score was 74 (16) in patients assigned to frequent hemodialysis and 75 (13) in patients assigned to conventional hemodialysis. Patients assigned to frequent hemodialysis demonstrated an improvement in their feeling thermometer score over the first 4 months of follow-up while the score remained similar in patients assigned to conventional hemodialysis (change of 3.7 vs. −0.4 from baseline). This change persisted to 12 months after randomization (4.0 vs. −4.1 from baseline). However, the between-treatment group difference in the change in score by one year was not statistically significant (8.0, 95% CI −0.5 to 16.1).
General health scale
The baseline mean (standard deviation) score was 40 (18) in patients assigned to frequent hemodialysis and 45 (20) in patients assigned to conventional hemodialysis. Patients assigned to frequent hemodialysis demonstrated a greater improvement in their general health scale by 12 months than patients assigned to conventional hemodialysis (change of 8.0 vs. 1.5 from baseline). However, the between-treatment group difference in the change in score by one year was not statistically significant (6.6, 95% CI −1.5 to 16.1).
Time to recover from a dialysis session
Between baseline and 12 months of follow-up, in patients assigned to frequent hemodialysis the median recovery time (10th, 90th percentile) decreased from 180 minutes (0, 1440) to 30 minutes (0,180), whereas in patients assigned to conventional hemodialysis the median recovery time changed from 180 minutes (0,780) to 120 minutes (0, 1440). The between-treatment group difference in the change in score by one year was– 60 minutes (95% CI −68 to −53). A recovery time of 60 minutes or more at baseline was observed in 58% of patients assigned to frequent hemodialysis and 63% assigned to conventional hemodialysis. Corresponding numbers one year after randomization 24% and 59%, respectively.
Health utilities index
There was no consistent statistical difference between frequent and conventional hemodialysis in the change in the health utilities index over 4 and 12 months of follow-up, or its eight attributes (Appendix 1).
Additional Analyses
We performed a post-hoc subgroup analysis of the Daily Trial and confirmed the observed 12 month effects of frequent hemodialysis on our HRQoL measures did not significantly differ in patients who had a daily urine volume less than or greater than 100 mL at the time of randomization (Appendix 2). We performed a post-hoc subgroup analysis of the Daily Trial and found the observed benefit of frequent hemodialysis on our measures of HRQoL did not significantly differ in patients who reported a shorter or longer recovery time after a dialysis session at the time of randomization (Appendix 3). The smaller number of patients in the Nocturnal Trial precluded meaningful subgroup analyses. We performed a post-hoc analysis comparing the two groups on the total weekly dialysis time and post-dialysis session recovery time 12 months after randomization (Appendix 4). As expected, the total weekly time of dialysis treatments was higher with frequent compared to conventional dialysis (Daily Trial median (25th, 75th percentile) 12.2 (10.8, 14.4) versus 10.3 (9.1, 11.4) hours; corresponding numbers for the Nocturnal Trial were 33.1 (20.0, 40.1) versus 12.0 (11.7, 13.5) hours). However, the total weekly recovery time after dialysis sessions was lower with frequent dialysis compared to conventional dialysis (Daily Trial median (25th, 75th percentile) values 5 (0.4, 11) versus 8 (3 to 18) hours; corresponding numbers for the Nocturnal Trial were 2 (0, 5) versus 6 (2, 22) hours (Appendix 4)).
DISCUSSION
Many studies in hemodialysis focus on the interests of researchers, and do not adequately study what a patient would prefer or value most. Most patients with end-stage kidney disease place a high value on their HRQoL, and want to know if it will be affected by their choice of dialysis modality. After a conventional hemodialysis session, many patients feel fatigued and not themselves for the next two to three hours (and sometimes longer). In this analysis of two randomized controlled trials we found patients recovered on average approximately one hour earlier from frequent compared to conventional hemodialysis. This was true whether the dialysis was received in a centre (in the case of the Daily Trial) or at home (in the case of the Nocturnal Trial).
These results extend the findings of a smaller observational study that described marked and persistent reductions in recovery time after patients on conventional hemodialysis initiated frequent hemodialysis.9 Less fluid is removed during a frequent hemodialysis session, and the session time is often shortest with in-centre daily hemodialysis. While there was more time spent on dialysis with frequent compared to conventional hemodialysis each week (in the Daily and Nocturnal Trials 2 hours and 21 more hours respectively), patients reported a sizeable reduction in the total weekly time to recover after their dialysis sessions (Appendix 4). In other words, while a patient receives more hours of hemodialysis, he or she actually experiences more hours during which he or she feels more normal, and less “washed out.” These data can be used to inform patients about a potential benefit of frequent hemodialysis, particularly if they have important responsibilities soon after their hemodialysis session such as childcare or work.
Patients treated in a dialysis facility reported better HRQoL with frequent shorter hours daily hemodialysis compared to conventional hemodialysis. The feeling thermometer is a single simple measure that embodies both functional and satisfaction-based aspects of HRQoL. Patients allocated to receive shorter hours daily hemodialysis reported a 6.4 point improvement in their feeling thermometer score which meets the accepted minimal important difference of 5 to 8 points.10 They also reported a 9.7 point improvement in their general health scale score which exceeds the accepted minimal important difference of 3 to 5 points.11 For reference, 107 patients with stage 3 and 4 chronic kidney disease randomly assigned 24 exercise sessions over 12 weeks (versus no exercise), demonstrated a between-group 6.1 point improvement in their general health scale score.12
From our clinical experiences we suspect improvements in HRQoL observed with frequent dialysis in the Daily Trial also extend to nocturnal hemodialysis. Numerically many of the effect estimates in the Daily and Nocturnal Trials were similar, but Nocturnal Trial estimates were less precise owing to a much smaller sample with inadequate statistical power to detect clinically important effects. This may be why many between-treatment group comparisons in the Nocturnal Trial were not statistically significant, although it is also possible there is simply no effect. Patients in the Nocturnal Trial had more residual renal function than patients in the Daily Trial.
In both trials there was no apparent effect of frequent hemodialysis on the health utilities index. It is plausible that this index is not responsive to change with frequent hemodialysis, even in the presence of other HRQoL and general health benefits.
The Frequent Hemodialysis Network (FHN) Trials had inadequate statistical power to reliably detect modest differences in mortality or major health events; an adequately powered trial would require at least 1200 to 1800 or more patients depending on whether mortality or a composite endpoint of mortality and major cardiovascular events was considered. Knowledge of an intervention’s effects on HRQoL remains important in determining whether the treatment should be adopted, and if so, whether the treatment is of sufficient value. While the provision of frequent in-center hemodialysis is costly, expanding the capacity of home-based frequent hemodialysis programs might allow some patients to enjoy its benefits at a reasonable cost.13
Randomized trials provide some of the best estimates of treatment effects. However, our analyses do have some limitations which merit consideration. The questionnaires were completed by patients who were aware of the type of hemodialysis they received, and enthusiasm for a novel therapy like frequent hemodialysis could influence responses on HRQoL measures. To improve the rigour of our measurements, three of our four HRQoL surveys were administered by personnel unaware of the dialysis regimen to which a patient was assigned. We also looked for consistency of effects at 12 months, beyond four months when any initial enthusiasm for frequent hemodialysis may wane. However, the best methods to assess the complex domain of patient centric HRQoL remains controversial, and some may disagree with the feasible HRQoL measures employed in the FHN trials. Furthermore, only a fraction of patients receiving conventional hemodialysis agreed to be randomized into the FHN Trials. Patients who agreed to be randomized into each trial had a higher baseline HRQoL than patients who provided consent but were not randomized, and it remains possible that the effects observed in these two trials will not generalize well to the entire hemodialysis population.14 Finally, these HRQoL measures were completed at a time most convenient to each patient and not at a fixed time after a hemodialysis session; it remains possible this influenced the accuracy of the measures.
In conclusion, as compared to conventional thrice weekly hemodialysis, frequent in-center hemodialysis and frequent home-based nocturnal hemodialysis both reduce the time it takes to recover from a hemodialysis session. Frequent in-center hemodialysis also yielded statistically significant and clinically important changes in general measures of HRQoL.
METHODS
FHN Trials
The Frequent Hemodialysis Network Daily (ClinicalTrials.gov # NCT00264758) and Nocturnal (ClinicalTrials.gov # NCT00271999) Trials are two multicenter, randomized, prospective trials of frequent in-center daily hemodialysis and home nocturnal hemodialysis, respectively, sponsored by the U.S. National Institutes of Health (National Institute of Diabetes and Digestive and Kidney Diseases) and the Center for Medicare and Medicaid Services. The designs, inclusion and exclusion criteria of both the trials are fully described elsewhere, as are the methods of randomization, data collection procedures and baseline clinical characteristics.8 Briefly, patients receiving maintenance hemodialysis with the ability to communicate in English or Spanish were enrolled into the two trials across centers in the United States and Canada between 2006 and 2009 (daily trial 19 centers, nocturnal trial 8 centers). In the Daily Trial we randomly assigned 245 patients to receive frequent (six times per week) or conventional (three times per week) in-center hemodialysis. In the Nocturnal Trial we randomly assigned 87 patients to receive frequent nocturnal (six times per week) or conventional (three times per week) hemodialysis at home. As described elsewhere, the Daily Trial screened 6276 hemodialysis patients on three times weekly hemodialysis in 65 hemodialysis clinics, 3481 (55%) were considered eligible for enrollment, and 3124 (90%) were approached for consent; 378 (12%) provided consent and 245 were randomized (65% of those who provided consent); prospective patients chose not to participate primarily because of the anticipated time required for three extra treatments per week and the difficulties in following the trial protocol.15 Although not reported in detail, a similar pattern of screening and randomization occurred in the Nocturnal Trial. As previously reported, patients randomly assigned into the Daily Trial, compared to patients who provided consent but were not randomly assigned, had higher baseline measures of health related quality of life.14 The same was also true in the Nocturnal Trial.14 As previously described, frequent hemodialysis resulted in more solute and fluid removal per week, with less fluid removal for each hemodialysis session.6;7
Health Related Quality of Life Measurements
As previously reported, a co-primary pre-specified HRQoL measure in each of the FHN trials was the PHC score from the RAND 36-item health survey.8 This informed the target number of 250 patients who were to be randomized into each of the trials (as described elsewhere for adequate statistical power to detect a 4.6 to 5.0 between group difference in the RAND 36).6;8 Four other patient-reported measures (questionnaires) of general health were collected during the FHN trials, and we analyzed these measures for this report. Patients and providers were aware of the frequency of hemodialysis prescribed and delivered. As described elsewhere, HRQoL questionnaires were administered by telephone in English or Spanish through a centralized call center by personnel unaware of group assignment (at baseline, month 4 and month 12).16 The only exception was the feeling thermometer (described below), which was completed with the assistance of a local research coordinator. Both trials were approved by the local Institutional Review Board at each participating site.
The feeling thermometer is a single question that asks patients to rate their own health on a visual analog scale from 0 to 100, where 0 is dead and 100 is perfect health.10 This commonly-used scale has been responsive to various therapies in multiple different conditions.17 A change in the score of 5 to 8 points is considered clinically important.10
A general health scale can be derived from five items in the RAND-36 questionnaire. It is scored from 0 to 100, where a low score is poor health likely to get worse, and a high score is excellent health.18 A change in the score of 3 to 5 points or more is considered clinically important.11
The time to recover from a dialysis session is understood by asking a patient: “How long does it take you to recover from a dialysis session and resume your normal, usual activities?”. In a prior non-randomized comparison of frequent versus conventional hemodialysis, this question was reliable, valid and responsive to change.9 Since then, the question has been further validated in larger studies against concurrent symptoms and clinically meaningful outcomes.19 In addition to examining the question as a continuous measure, we also considered the threshold of requiring 60 minutes or more to recover from a dialysis session.
The health utilities index (The Health Utilities Index Mark 3, HUI-3) is a commonly-used validated survey where participants rate their health on eight attributes: vision, hearing, speech, ambulation, dexterity, emotion, cognition and pain. It is scored to a final result from 0 to 1, with a function derived from community preferences for various health attributes. A final result of 0 represents death and 1 perfect health. A change in the score of 0.03 or more is considered clinically important.20
Statistical analysis
We adhered to the intention-to-treat principle for all analyses and did not adjust for multiple comparisons. We performed parallel analyses stratified by trial. Baseline characteristics of the two trials were stratified by intervention status (frequent versus conventional hemodialysis). We used linear mixed effects models incorporating baseline, month 4 and month 12 scores to assess the between-treatment group differences in the one-year change in the HRQoL measures, with the exception of dialysis recovery time which was only recorded at baseline and month 12. The PROC MIXED procedure was chosen for its flexibility, allowing us to incorporate all available data without restriction on whether it was available or missing at baseline, month 4 or month 12. We applied an unstructured covariance matrix to obviate assumptions of data structure. In accordance with the pre-specified analytic plan, we adjusted for baseline score and, in the case of the Daily Trial, we also adjusted for clinical center. We performed the analyses for dialysis recovery time after log-transforming the values, with the reported results subsequently back-transformed using the delta method. We used SAS software, version 9.2 (SAS Institute) for the analysis.
Supplementary Material
Figure 2.
Acknowledgments
Dr. Amit Garg is supported by the Dr. Adam Linton Chair in Kidney Health Analytics and his work was conducted in the Lilibeth Caberto London Kidney Clinical Research Unit. Dr. Suri is supported by a research scholarship from le Fonds de recherché du Québec – Santé.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Potential conflicts of interest: Dr. Garg was a co-investigator on investigator initiated research project sponsored by Fresenius. Dr. Suri received an investigator initiated research grant from Baxter Inc Extramural Grant Program. Dr. Unruh has received research support from Baxter, Satellite Healthcare, and DCI Inc. Dr. Chertow served on the Board of Directors for Satellite Healthcare. All other authors declare no conflicts of interest.
Clinical trial ID numbers: NCT00264758 and NCT00271999
Reference List
- 1.Anker SD, Agewall S, Borggrefe M, Calvert M, Jaime CJ, Cowie MR, Ford I, Paty JA, Riley JP, Swedberg K, Tavazzi L, Wiklund I, Kirchhof P. The importance of patient-reported outcomes: a call for their comprehensive integration in cardiovascular clinical trials. Eur Heart J. 2014;35:2001–2009. doi: 10.1093/eurheartj/ehu205. [DOI] [PubMed] [Google Scholar]
- 2.Ramkumar N, Beddhu S, Eggers P, Pappas LM, Cheung AK. Patient preferences for in-center intense hemodialysis. Hemodial Int. 2005;9:281–295. doi: 10.1111/j.1492-7535.2005.01143.x. [DOI] [PubMed] [Google Scholar]
- 3.Jain AK, Blake P, Cordy P, Garg AX. Global trends in rates of peritoneal dialysis. J Am Soc Nephrol. 2012;23:533–544. doi: 10.1681/ASN.2011060607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Walsh M, Culleton B, Tonelli M, Manns B. A systematic review of the effect of nocturnal hemodialysis on blood pressure, left ventricular hypertrophy, anemia, mineral metabolism, and health-related quality of life. Kidney Int. 2005;67:1500–1508. doi: 10.1111/j.1523-1755.2005.00228.x. [DOI] [PubMed] [Google Scholar]
- 5.Suri RS, Nesrallah GE, Mainra R, Garg AX, Lindsay RM, Greene T, Daugirdas JT. Daily hemodialysis: a systematic review. Clin J Am Soc Nephrol. 2006;1:33–42. doi: 10.2215/CJN.00340705. [DOI] [PubMed] [Google Scholar]
- 6.Chertow GM, Levin NW, Beck GJ, Depner TA, Eggers PW, Gassman JJ, Gorodetskaya I, Greene T, James S, Larive B, Lindsay RM, Mehta RL, Miller B, Ornt DB, Rajagopalan S, Rastogi A, Rocco MV, Schiller B, Sergeyeva O, Schulman G, Ting GO, Unruh ML, Star RA, Kliger AS. In-center hemodialysis six times per week versus three times per week. N Engl J Med. 2010;363:2287–2300. doi: 10.1056/NEJMoa1001593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rocco MV, Lockridge RS, Jr, Beck GJ, Eggers PW, Gassman JJ, Greene T, Larive B, Chan CT, Chertow GM, Copland M, Hoy CD, Lindsay RM, Levin NW, Ornt DB, Pierratos A, Pipkin MF, Rajagopalan S, Stokes JB, Unruh ML, Star RA, Kliger AS, Kliger A, Eggers P, Briggs J, Hostetter T, Narva A, Star R, Augustine B, Mohr P, Beck G, Fu Z, Gassman J, Greene T, Daugirdas J, Hunsicker L, Larive B, Li M, Mackrell J, Wiggins K, Sherer S, Weiss B, Rajagopalan S, Sanz J, Dellagrottaglie S, Kariisa M, Tran T, West J, Unruh M, Keene R, Schlarb J, Chan C, McGrath-Chong M, Frome R, Higgins H, Ke S, Mandaci O, Owens C, Snell C, Eknoyan G, Appel L, Cheung A, Derse A, Kramer C, Geller N, Grimm R, Henderson L, Prichard S, Roecker E, Rocco M, Miller B, Riley J, Schuessler R, Lockridge R, Pipkin M, Peterson C, Hoy C, Fensterer A, Steigerwald D, Stokes J, Somers D, Hilkin A, Lilli K, Wallace W, Franzwa B, Waterman E, Chan C, McGrath-Chong M, Copland M, Levin A, Sioson L, Cabezon E, Kwan S, Roger D, Lindsay R, Suri R, Champagne J, Bullas R, Garg A, Mazzorato A, Spanner E, Rocco M, Burkart J, Moossavi S, Mauck V, Kaufman T, Pierratos A, Chan W, Regozo K, Kwok S. The effects of frequent nocturnal home hemodialysis: the Frequent Hemodialysis Network Nocturnal Trial. Kidney Int. 2011;80:1080–1091. doi: 10.1038/ki.2011.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Suri RS, Garg AX, Chertow GM, Levin NW, Rocco MV, Greene T, Beck GJ, Gassman JJ, Eggers PW, Star RA, Ornt DB, Kliger AS. Frequent Hemodialysis Network (FHN) randomized trials: study design. Kidney Int. 2007;71:349–359. doi: 10.1038/sj.ki.5002032. [DOI] [PubMed] [Google Scholar]
- 9.Lindsay RM, Heidenheim PA, Nesrallah G, Garg AX, Suri R. Minutes to recovery after a hemodialysis session: a simple health-related quality of life question that is reliable, valid, and sensitive to change. Clin J Am Soc Nephrol. 2006;1:952–959. doi: 10.2215/CJN.00040106. [DOI] [PubMed] [Google Scholar]
- 10.Schunemann HJ, Griffith L, Jaeschke R, Goldstein R, Stubbing D, Guyatt GH. Evaluation of the minimal important difference for the feeling thermometer and the St George’s Respiratory Questionnaire in patients with chronic airflow obstruction. J Clin Epidemiol. 2003;56:1170–1176. doi: 10.1016/s0895-4356(03)00115-x. [DOI] [PubMed] [Google Scholar]
- 11.Hays RD, Morales LS. The RAND-36 measure of health-related quality of life. Ann Med. 2001;33:350–357. doi: 10.3109/07853890109002089. [DOI] [PubMed] [Google Scholar]
- 12.Rossi AP, Burris DD, Lucas FL, Crocker GA, Wasserman JC. Effects of a renal rehabilitation exercise program in patients with CKD: a randomized, controlled trial. Clin J Am Soc Nephrol. 2014;9:2052–2058. doi: 10.2215/CJN.11791113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee CP, Zenios SA, Chertow GM. Cost-effectiveness of frequent in-center hemodialysis. J Am Soc Nephrol. 2008;19:1792–1797. doi: 10.1681/ASN.2008010001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rocco MV, Larive B, Eggers PW, Beck GJ, Chertow GM, Levin NW, Kliger AS. Baseline characteristics of participants in the Frequent Hemodialysis Network (FHN) daily and nocturnal trials. Am J Kidney Dis. 2011;57:90–100. doi: 10.1053/j.ajkd.2010.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sergeyeva O, Gorodetskaya I, Ramos R, Schiller BM, Larive B, Raimann JG, Ting GO, Eggers PW, Chertow GM, Levin NW. Challenges to enrollment and randomization of the Frequent Hemodialysis Network (FHN) Daily Trial. J Nephrol. 2012;25:302–309. doi: 10.5301/jn.5000160. [DOI] [PubMed] [Google Scholar]
- 16.Jhamb M, Tamura MK, Gassman J, Garg AX, Lindsay RM, Suri RS, Ting G, Finkelstein FO, Beach S, Kimmel PL, Unruh M. Design and rationale of health-related quality of life and patient-reported outcomes assessment in the Frequent Hemodialysis Network trials. Blood Purif. 2011;31:151–158. doi: 10.1159/000321855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Baldassarre FG, Arthur HM, Dicenso A, Guyatt G. Effect of coronary artery bypass graft surgery on older women’s health-related quality of life. Heart Lung. 2002;31:421–431. doi: 10.1067/mhl.2002.127940. [DOI] [PubMed] [Google Scholar]
- 18.Ware JE, Jr, Sherbourne CD. The MOS36-item short-form health survey (SF-36). I Conceptual framework and item selection. Med Care. 1992;30:473–483. [PubMed] [Google Scholar]
- 19.Rayner HC, Zepel L, Fuller DS, Morgenstern H, Karaboyas A, Culleton BF, Mapes DL, Lopes AA, Gillespie BW, Hasegawa T, Saran R, Tentori F, Hecking M, Pisoni RL, Robinson BM. Recovery time, quality of life, and mortality in hemodialysis patients: the Dialysis Outcomes and Practice Patterns Study (DOPPS) Am J Kidney Dis. 2014;64:86–94. doi: 10.1053/j.ajkd.2014.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Horsman J, Furlong W, Feeny D, Torrance G. The Health Utilities Index (HUI): concepts, measurement properties and applications. Health Qual Life Outcomes. 2003;1:54. doi: 10.1186/1477-7525-1-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.