Abstract
Dopaminergic systems in the brain adapt in response to a variety of stimuli from the internal and external world, but the mechanisms underlying this process are incompletely understood. In recent years, evidence has emerged that certain types of transcription factor of the nuclear receptor family, specifically Nur77 and retinoid X receptors, play an important role in the Nuradaptation, and importantly, in the homeostatic regulation, of dopaminergic systems. These findings call for a reassessment of our fundamental understanding of the molecular and cellular basis of dopaminergic transmission. Given that diseases such as Parkinson’s disease and schizophrenia are thought to involve maladaptation of dopamine signalling, these findings might lead new insight into these pathologies and offer new avenues for drug development.
Introduction
Dopamine neurotransmission plays an important role in a large series of physiological functions such as control of motor behaviour, learning, cognition, motivated behaviours and hormone production. Clinical evidences suggest that dopaminergic pathways are involved in several neurological and psychiatric disorders [1]. For example, a gradual loss of midbrain dopamine producing cells result in extensive dopamine depletion in the striatum and the characteristic motor symptoms observed in Parkinson’s disease; i.e. bradykinesia, rigidity, resting tremors and postural instability. The main treatment for Parkinson’s disease is based on dopamine replacement using the precursor L-DOPA for the biosynthesis of endogenous dopamine. At the other end of the spectrum, a hyperactivity of the mesolimbic dopamine system is thought to be a prominent driving force in the pathophysiology of schizophrenia. This hypothesis is based on the fact that the antagonism of the D2 class of dopamine receptors is an essential prerequisite for the therapeutic efficacy of antipsychotic medications [2]. Dopamine neurotransmission is also deeply involved in drug addiction. The mesolimbic dopamine system, comprised of dopamine neurons in the ventral tegmental area (VTA) of the midbrain and their projections to the nucleus accumbens and prefrontal cortex, is the most important mediator of drug reward [3]. It is generally believed that drugs of abuse usurp the normal functioning of this neural circuitry, which normally controls responses to natural rewards, such as food, sex and social interactions.
The effects of dopamine are mediated through its interaction with G-protein-coupled membrane receptors. The dopamine receptor family contains five members distributed in two subfamilies; the D1-like family, which includes D1 and D5 receptors, and the D2-like family, which includes D2, D3 and D4 receptors (reviewed in [4]). However, changes in dopamine neurotransmission are short-lived and therefore unlikely by themselves, to account for behavioural changes that are long lasting. Transcription factors are key elements that convert transient and reversible modulation of signal transduction molecules such as kinases (triggered by neurotransmitter receptor activation) to long lasting changes at the molecular and cellular levels. They represent a vast family of genes that encode regulatory factors, which modulate the expression of target genes. They play an important role during brain development and actively participate in adaptive responses following changes in the environment of neuronal cells, such as after ischemia, lesion or denervation and following exposure to drugs that affect neurotransmitter systems in mature brain [5]. The activation of immediate-early genes (IEGs) constitutes one of the initial steps of the mechanisms by which stimuli at the cell membrane are transduced into short and long-term neuronal responses [6]. In recent years, data supporting an important role for a class of transcription factors, namely Nurs and retinoid X receptor (RXR) subgroups, in dopamine-mediated effects have emerged. In this review, we will describe recent literature indicating that Nur77 and RXR work in concert as adaptive and homeostatic regulators of dopamine functions in the context of dopamine-related neuropsychiatric and neurological disorders. Based on current knowledge, we would like to propose a model in which Nur77 plays a key role as a molecular switch in dopamine-related neuroadaptation processes.
Nurs are versatile transcription factors that exert pleiotropic functions in periphery
Nurs are part of the nuclear hormone receptor superfamily and are defined as the orphan NR4A subgroup, which included Nur77 (NR4A1 (also known as Nerve Growth Factor-Inducible gene B (NGFI-B))), Nurr1 (NR4A2) and Nor-1 (NR4A3). Nurs are classified as early response genes and are induced by a diverse range of signals, including growth factors, cytokines, peptide hormones, neurotransmitters and stress. Their ability to sense and rapidly respond to changes in the cellular environment appears to be a hallmark of this subgroup. These receptors bind as monomers, homodimers and heterodimers with RXR to different permutations of the canonical nuclear receptor binding motif (Fig. 1). In line with the pleiotropic physiological stimuli that induce the expression of members of the Nurs subgroup, they have been implicated in cell cycle regulation (and apoptosis), steroidogenesis, inflammation, carcinogenesis and atherogenesis (for review see [7]). For example, Nur77 represents a key regulator of the negative selection of thymocytes. Nuclear export of Nur77 plays an important role in modulation of retinoid signalling in PC12 phaeochromcytoma cells and in a Bcl-2-dependent apoptotic cascade in specific cancer cell contexts [8]. In the cardiovascular system, Nur77 is involved in vascular cell functions by regulating endothelial cell activation and vascular smooth muscle cell proliferation [9]. In the neuroendocrine system, Nur77 is associated with the control of proopiomelanocortin expression and corticotropin-releasing hormone activity [10]. However, its role in the central nervous system (CNS) remains mostly unexplored.
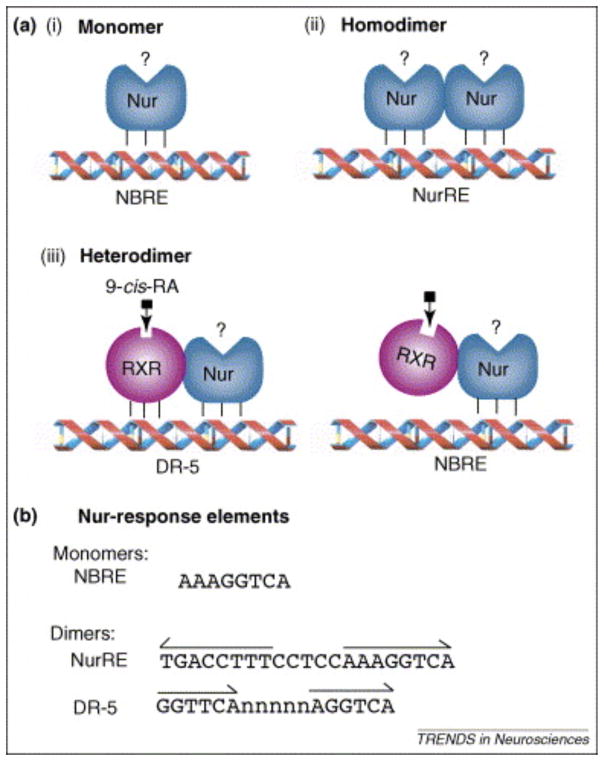
Figure 1. Transcriptional mechanisms of action of Nurs.
Nurs can exert their transcriptional activity through multiple mechanisms. They can interact with the Nerve Growth-factor Inducible gene B (NGFI-B) Responsive Element (NBRE) as monomers (A), as homodimers on the Nur Responsive Element (NurRE) (B) or as heterodimers with retinoid X receptor (RXR) as a partner on the retinoic acid direct repeat-5 (DR-5) responsive element or on the NBRE site (C). Note however that Nor-1 cannot form heterodimer with RXR [60]. (D) DNA sequences of the various Nur response elements. The NurRE is composed of two canonical NBRE sites. The NurRE sequence presented corresponds to the previously characterized proopiomelanocortin promoter sequence [70]. The Nur-responsive DR-5 site is composed of two retinoid responsive half sites separated by 5 random nucleotides (n). This figure is adapted from [71] with the permission of Dr Jacques Drouin, IRCM, Montreal, Qc, Canada.
Retinoic acids (RA) regulate the expression of numerous target genes by activating two specific transcription factors, Retinoic Acid Receptor (RAR) and RXR. These transcription factors are also members of the nuclear receptor superfamily. RARs are specifically involved in retinoid signalling, whereas RXRs serve as heterodimerization partners for other nuclear receptors including vitamin D receptor, thyroid hormone receptors, peroxisome-proliferator-activated receptors, liver X receptors, farnesoid X receptors and orphan members of the nuclear receptor family, such as Nurr1 and Nur77 (Fig. 1) [11–13]. Again, these nuclear receptors interactions have been mainly observed in peripheral tissues.
Relationships between Nurs, retinoids and brain dopamine systems
Recent evidence suggests that orphan members of the Nur subgroup are closely associated with dopamine neurotransmission via their action as transcription factors (Fig. 2). In absence of Nurr1, dopamine midbrain precursors adopt normal localization and neuronal phenotype, but fail to differentiate into dopamine neuron phenotype, as demonstrated by the lack of tyrosine hydroxylase (TH) expression and all other analysed dopamine neuron markers [14]. In normal conditions, Nur77 and Nor-1 are mainly expressed in target areas of dopamine neurons, such as the striatal complex and prefrontal cortex (Fig. 2A,B) [15–17]. Their mRNA levels are extremely low in the substantia nigra (SN) and VTA in basal conditions in the adult brain [15]. However, their expression can be significantly increased in the SN/VTA complex by administration of dopamine D2 antagonists (Fig. 2C) [18] suggesting that their expression in this brain area is tonically repressed in normal conditions.
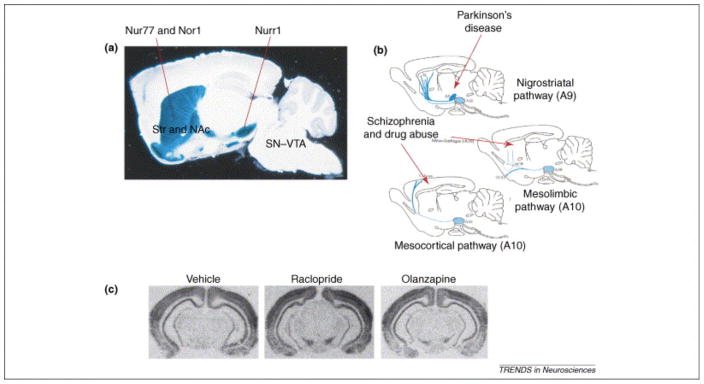
Figure 2. Relationships between Retinoid X Receptor γ1 (RXRγ1) isoform, Nurs and CNS dopamine systems.
(A) RXRγ1 distribution is shown as blue staining on a sagittal section in the adult brain of RXRγ1(+/−) heterozygote mice (adapted with permission from [22]). Nur77 is expressed in dopaminoceptive areas such as the striatum (Str) and nucleus accumbens (Acc), whereas Nurr1 is expressed in dopamine neurons of the substantia nigra/ventral tegmental area (SN/VTA) region. (B) Note the similarities between the distribution of this RXR isoform in A with dopamine nigro-striatal (associated with the control of motor behaviors and Parkinson’s disease), meso-limbic and meso-cortical (associated with associative and limbic functions in schizophrenia and drug abuse) pathways (adapted with permission from [72]). (C) Nur77 is normally not expressed in the SN/VTA region, but typical (raclopride) and atypical (olanzapine) antipsychotic drugs induced high levels of the Nur77 mRNA in midbrain dopamine cells (adapted with permission from [18]).
Several lines of evidence suggest a close relationship between retinoids and dopamine systems as well. First, brain areas receiving dopamine innervations such as the striatum, nucleus accumbens and olfactory tubercle expresses both RARβ and RXRγ isoforms [19–21]. In fact, the distribution of the RXRγ1 isoform is specifically associated with dopamine pathways in the mature CNS (Fig. 2) [22]. Second, genetic manipulations have shown that RARβ/RXRγ–deficient mice displayed impaired locomotion and dopamine signalling and prominent decreases in D1 and D2 receptor mRNA in the striatum [23], whereas RXRγ1–null mice exhibit an altered response to a typical neuroleptic [22]. Third, several lines of evidence support a role of retinoid signalling in schizophrenia [24]. Finally, it has been shown that RXR ligands can promote the survival of dopaminergic cells in a process mediated by Nurr1-RXR heterodimers [25]. Thus, anatomical and genetic data suggest that Nurs and retinoid receptors can be associated with dopamine systems.
A tremendous body of evidence indicates that transcription factors of the Fos family are involved in dopamine-mediated events [26]. For example, the c-fos mRNA and Fos-like proteins (FosB, ΔFosB) levels are strongly modulated by many products that altered dopamine neurotransmission [27]. However, recent data show that orphan nuclear receptors Nur77 and Nor-1 represent other transcription factors whose expression is strongly modulated in response to dopamine transmission manipulation (see Fig. 3 and Table 1).
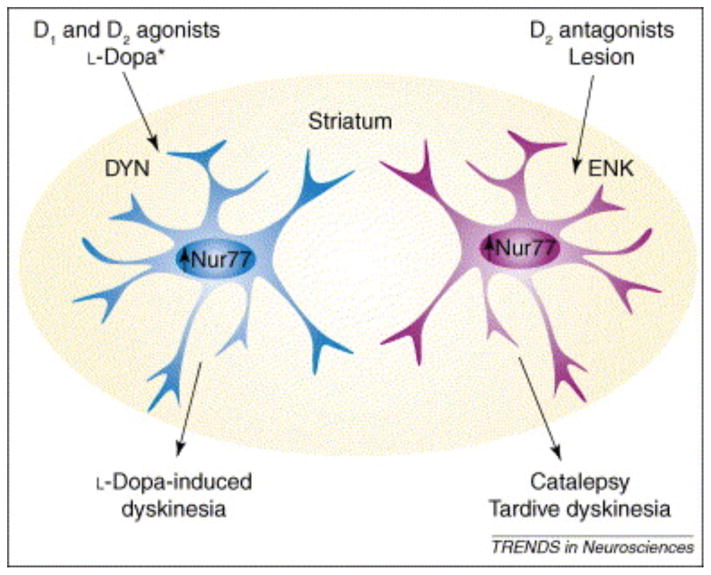
Figure 3. Modulation of Nur77 mRNA levels in selective cell populations of the striatum is associated with specific locomotor effects.
Combined administration of a D1 and D2 dopamine agonists, as well as chronic L-DOPA treatment in 6-OHDA-lesioned animals, modulates Nur77 mRNA levels in dynorphin-containing (DYN) cells of the striatum [42, 44]. In contrast, after D2 antagonists administration or following denervation Nur77 mRNA levels are elevated in enkephalin-containing (ENK) cells of the striatum [16, 42]. Modulation of Nur77 in ENK+-cells of the striatum is associated with hypokinesia, catalepsy and vacuous chewing movements (tardive dyskinesias), whereas modulation of Nur77 in DYN+-cells of the striatum can be associated with L-DOPA-induced dyskinesia and turning behavior in unilaterally lesioned animals. * Note that in unilaterally 6-OHDA-lesioned animals treated with L-DOPA Nur77 is increased in DYN+-cells in the intact side of the striatum, whereas Nur77 mRNA levels are reduced in DYN+-cells in selective areas of the striatum in the lesioned side [42].
Table 1.
Summary of the data on the relationship between Nur77 and dopamine systems.
| Type of data | Effect | References |
|---|---|---|
| Drug treatment | ||
| Dopamine D1 agonists: | No effect | [42, 63] |
| Dopamine D2 agonists: | Decrease Nur77 mRNA levels | [42, 63] |
| Combined D1+D2 agonists: | Increase Nur77 mRNA levels (see Fig. 3 for more details) | [42] |
| Psychostimulants: (amphetamine, cocaine and high dose of caffeine) | Increase Nur77 mRNA levels in the Str and NAcc | [17, 32, 37, 64, 65] |
| Typical antipsychotic drugs: (D2 antagonists) | Strong increase of Nur77 mRNA levels in the Str, NAcc, PFC and SN/VTA (see Fig. 3) | [16, 18, 19, 66] |
| Atypical antipsychotics: | Moderate to small increase of Nur77 mRNA levels in the Str, NAcc, PFC and SN/VTA | [18] |
| Serotonin 5-HT1A agonist: | Decrease Nur77 mRNA levels | [63] |
| Serotonin 5-HT2A/C agonist: | Increase Nur77 mRNA levels | [63] |
| Adenosine A2A antagonist: | Decrease Nur77 mRNA levels | [67] |
| Morphine: | Increase Nur77 mRNA levels | [36, 68] |
| Naloxone: (opioid receptor antagonist) | Decrease Nur77 mRNA levels | [36, 68] |
| Delta(9)-tetrahydrocannabinol: (THC) | Increase Nur77 mRNA levels | [69] |
| Animal models | ||
| Unilateral 6-OHDA lesion in rats (PD model): | Complex modulation of Nur77 mRNA levels in selective striatal cell populations (see Fig. 3 for detail) | [42] |
| 6-OHDA+L-DOPA: | Complex modulation of Nur77 mRNA levels in selective striatal cell populations (see Fig. 3 for detail) | [42, 44] |
| Aphakia mice (PD model): | Increase Nur77 mRNA levels | [43] |
| Aphakia mice + L-DOPA: | Decrease Nur77 mRNA levels (restored to normal) | [43] |
| nVH lesion (schizophrenia): | Decrease Nur77 mRNA levels in PFC | [32] |
| Post-mortem brain tissues in schizophrenics: | Decrease Nur77 mRNA levels in PFC | [33] |
| Nur77 gene knockout | ||
| Behaviors: | Reduce cataleptic response to typical antipsychotics | [29] |
| No effect on the cataleptic response induced by a D1 antagonist | [29] | |
| Generate spontaneous VCM and exacerbate antipsychotic-induced VCM | [30] | |
| Increase spontaneous locomotor activity | [38] | |
| Increase reactivity to D2 autoreceptor stimulation | [38] | |
| Exacerbate L-DOPA-induced turning behavior in unilaterally 6-OHDA-lesioned mice | [45] | |
| Gene expression: | Reduce COMT expression and alter dopamine turnover | [38] |
| Reduce lesion- and antipsychotic-induced ENK and NT expression | [29, 45] | |
| Increase TH and Nurr1 expression | [38] | |
| Increase D2 receptor expression in Str, but not in SN/VTA | [29] | |
Data on Nur77 mRNA level modulations are shown for the striatum (Str), when brain areas are not mentioned. Abbreviations: NAcc, nucleus accumbens; COMT, catechol-O-methyltransferase; ENK, enkephalin; NT, neurotensin; TH, tyrosine hydroxylase; PD, Parkinson’s Disease; SN/VTA, substantia nigra/ventral tegmental area region; 6-OHDA, 6-hydroxydopamine; PFC, prefrontal cortex; AMPH, amphetamine, nVH lesion, neonatal ventral hippocampus lesion; VCM, vacuous chewing movements
Nur77 and RXR in schizophrenia model and antipsychotic drug effects
Haloperidol, a typical neuroleptic, increased Nur77 mRNA levels in a selective striatal cell population expressing the neuropeptide enkephalin (ENK) (Fig. 3 and Table 1) [16]. This striatal subpopulation, which also bears dopamine D2 receptors, is specifically associated with the indirect striatal output pathway. We have recently shown that modulation of Nur77 mRNA levels can be used to calculate an index predictive of the typical vs atypical profile of antipsychotic drugs [18] in a similar fashion to what has been observed with Fos-like protein expression [28]. Inductions of Nurs (Nur77 or Nor-1) are correlated with dopamine D2 and D3 receptor affinities and 5-HT2A/D2 affinity ratios can also be used to predict Nur77 and Nor-1 patterns of expression [18]. Interestingly, Nur77 mRNA up-regulation is maintained upon chronic typical antipsychotic drug treatments without any apparent desensitization, suggesting that Nur77 not only participate in the initiation of a neuroadaptive signalling cascade but also to more prolonged effects [16, 19]. Thus, Nurs represent another important class of transcription factors that react to modification of dopamine neurotransmission. Although the patterns of expression of c-fos and Nur77 are sometime similar, we do not know yet if these two classes of transcription factors act in synergy or if they have distinct subset of transcriptional targets.
Nur77 and RXR are also directly involved in motor side effects produced by typical antipsychotic drugs (Fig. 3 and Table 1). Moreover, the effects of antipsychotic drugs are significantly altered by genetic ablation of Nur77. Nur77(−/−) mice have a blunted cataleptic response to typical antipsychotics and this blunted response is restricted to dopamine D2 antagonists [29]. The effects of haloperidol on the neuropeptide ENK and neurotensin mRNA are also significantly reduced in Nur77(−/−) mice [29], suggesting again a preferential effect on dopamine D2-mediated processes. Mice display vacuous chewing movements (VCMs) following chronic haloperidol treatment. These VCMs share similarities with tardive dyskinesias in man. Interestingly, this response is exacerbated in Nur77(−/−) mice [30]. We have previously showed that Nur77 transcripts are highly co-localized with RXRγ1 isoform in the striatum following haloperidol treatment [29]. This drug-induced co-localization of Nur77-RXRγ1 may have triggered new interactions between Nur77 and RXR in striatal cells. Trying to decipher the mechanisms involved in haloperidol-induced behavioural effects, we have demonstrated that both the cataleptic and VCM responses are altered by retinoid ligands, such as 9-cis retinoic acid (9-cisRA), docosahexaenoic acid (DHA) (a polyunsaturated fatty acid that acts as an endogenous RXR ligand in the CNS [25, 31]) and HX531 (a synthetic RXR antagonist) [29, 30]. Interestingly, HX531 and DHA remain inactive on VCM responses in Nur77(−/−) mice, indicating that Nur77 is needed for the expression of the effect of these compounds [30]. Taken together, these observations indicate that Nur77 and RXR actively participate to antipsychotic drug effects and are associated with the generation of abnormal movements induced by dopamine receptor antagonists.
Additional data suggest that Nur77 might also be involved in the expression of some schizophrenia symptoms (Table 1). Indeed, Nur77 mRNA levels correlate with prefrontal cortex hypo-activity and sub-cortical hypersensitivity to psychostimulants in an animal model of schizophrenia. We have observed that Nur77 mRNA levels are reduced in prefrontal and cingulate cortices of adult rats bearing a neonatal lesion of the ventral hippocampus [32]. Interestingly, a similar reduction of Nur77 expression has been recently observed in prefrontal cortex of schizophrenic patients in a post mortem brain tissue analysis [33]. Neonatal ventral hippocampus lesioned animals also display an increased sensitivity to amphetamine and stronger induction of Nur77 in subcortical brain regions [32]. These effects are reminiscent to the behavioural sensitization induced by psychostimulants in schizophrenic patients [34, 35]. Nur77 and Nor-1 mRNA levels are also correlated with behavioural manifestations associated with cocaine and morphine administration (Table 1) [17, 36, 37].
Finally, Nur77(−/−) mice display alterations of dopamine neuron biochemical activity and disturbance of prefrontal cortex dopamine neurotransmission (Table 1). Nur77(−/−) mice are spontaneously hyperactive and are more sensitive to a low dose of a D2 agonist acting mainly at pre-synaptic autoreceptor sites [38]. This suggests that dopamine neuron activity may be altered in these mice. Indeed, dopamine neuron biochemical activity and dopamine turnover are altered in Nur77(−/−) mice. These changes are accompanied by altered TH and catechol-O-methyltransferase (COMT) expression in Nur77(−/−) mice [38]. Since COMT gene polymorphisms that reduce its activity have consistently been linked with an increased risk of schizophrenia [39, 40], this observation suggests that Nur77 might play a role in the regulation of mesocorticolimbic dopamine pathway and might be associated with generation of psychotic symptoms.
Nur77 and RXR in animal model of Parkinson’s disease and anti-Parkinsonian drug effects
Several lines of evidence suggest the involvement of Nur77 in Parkinson’s disease and its therapeutic treatment (Table 1). We have observed important and complex modulations of striatal Nur77 mRNA expression following denervation and L-DOPA treatment in two different animal models of Parkinson’s disease; unilateral 6-hydroxydopamine (6-OHDA)-lesioned rats and Aphakia mutant mice [41–44]. In normal conditions, Nur77 is expressed equally in both striatal output pathways. In unilateral 6-OHDA rats, denervation induces an up-regulation of Nur77, which takes place selectively in striatal ENK-containing cells (see Fig. 3 and Table 1). On the other hand, the percentage of striatal cells with co-localized Nur77 and dynorphin (DYN), a selective marker of the direct striatal output pathway, is significantly reduced in the lesioned side [42]. However, in the intact side of the striatum, Nur77 mRNA levels are increased in DYN-containing cells following L-DOPA treatment (Fig. 3 and Table 1) [42]. This imbalance between the two striatal output pathways that is first initiated by denervation then further exacerbated by repeated L-DOPA treatment suggests that these molecular changes may contribute the development of behavioural sensitization and long term effects of L-DOPA. This is supported by the fact that Nur77(−/−) mice show impaired behavioural and molecular adaptations to denervation and repeated L-DOPA treatment [45]. It is interesting to note that Nur77 can be selectively up-regulated in the DYN-containing cells by the co-administration of dopamine D1 and D2 agonists in normal rats (Fig. 3 and Table 1), suggesting that the specific expression of Nur77 in the indirect striatal output pathway (ENK+-cells) in 6-OHDA rats might be caused by a change in dopamine signalling cascade associated to the denervation process [46]. Nur77 mRNA levels are also up-regulated in Aphakia mice, which bear a natural deletion of the homeobox Pitx3 gene locus that alters midbrain dopamine neuron development [43]. These mice display hypokinesia that can be restored with L-DOPA treatment and they show striatal biochemical alterations very similar to those observed in 6-OHDA animals [43].
Since RXR in association with Nur77 are involved in haloperidol-induced VCMs and since L-DOPA-induced dyskinesias (LIDs) may share some homologies with neuroleptic-induced tardive dyskinesia, we investigated the effects of DHA, an endogenous activator of RXR, on LIDs in monkeys treated with the neurotoxin 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) [47]. We have shown that DHA can reduce the severity or delay the appearance of LIDs in a non-human primate model of Parkinson’s disease suggesting new targets for the improvement of the quality of life of people suffering from this debilitating disease. Although data obtained in rodent models clearly indicate that DHA activity can be dependent on Nur77 expression [29, 30], additional experiments using more selective RXR ligands need to be performed since DHA may alter brain functions through other mechanisms [48]. Altogether these observations suggest that Nur77 and RXR are associated with abnormal movements observed in Parkinson’s disease and generated after classic dopamine replacement therapy.
Nur77 and RXR in dopamine-mediated neurotransmission
Current knowledge suggests a kind of yin-yang relationship between Nur77 and dopamine; Nur77 levels of expression are modulated by manipulation of dopamine neurotransmission and in turn, Nur77 expression modulates dopamine-mediated effects (Table 1). Based on these observations, we can propose that; 1) Nur77 and RXR are involved in a dopamine-mediated adaptive signalling pathway that tends to reduce the effect of alterations of the dopamine neurotransmission; 2) they are essential for maintaining the homeostasis of striatal dopamine functions by setting the threshold for the adaptive capacity of the striatal dopamine system; and 3) RXR ligands can be used to manipulate this Nur77-dependent pathway.
At first glance, Nur77 can be viewed merely as another transcription factor, along with Fos and others, but its nuclear receptor nature and its putative interaction with RXR in brain dopamine system render it unique amongst other transcription factors involved in dopamine-mediated responses. Indeed, the absence (gene knockout) or reduction (animals bearing a neonatal lesion of the ventral hippocampus) of Nur77 expression generates exacerbated responses of the dopamine system upon challenges. Therefore, Nur77 seems essential for some neuroadaptive properties of the dopamine system. It represents a previously uncharacterized element in those dopamine-mediated responses.
Nurs are characterized by their ability to sense and rapidly respond to changes in the cellular environment. So far, modulation of Nur77 mRNA levels in the CNS has been observed. Figure 4 shows modulation of the Nur77 immunohistochemistry signal following challenge with dopaminergic drugs. Haloperidol increases Nur77 immunostaining in the lateral portion of the striatum, whereas amphetamine had a more medial effect (Fig. 4). These patterns of Nur77 immunostaining induction are similar to previously observed mRNA-induced patterns [16, 29, 32, 42].
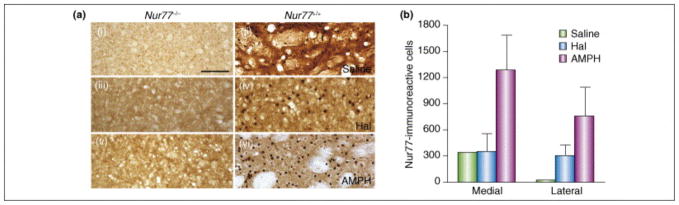
Figure 4. Modulation of Nur77 proteins levels by dopaminergic drugs.
Wild type (Nur77(+/+)) and Nur77 knockout (Nur77(−/−)) mice treated with the vehicle (saline) (A,B), haloperidol (Haldol, 0.5 mg/kg, i.p.) (C,D) or d-amphetamine sulfate (AMPH, 2.5 mg/kg, i.p.) (E,F). Immunohistochemistry was performed with the Nur77 antibody (M210, dilution 1:100, Santa Cruz Biotechnology). Note the absence of specific labelling in Nur77(−/−) mice (A,C,E). The immunohistochemistry procedure for Nur77 immunostaining was adapted from [73] with minor modifications. Areas presented correspond to the ventrolateral portion of the striatum. These illustrations are representative of the labelling obtained in 3 animals for each group. Histograms of the right panel represent quantification of the total number of Nur77 positive cells of the medial and lateral portions of the striatum of animals treated with saline (VEH), haloperidol (HAL) or amphetamine (AMPH) (unpublished data).
Nur77 is a direct target of kinases, such as protein kinase A (PKA), that are associated with signalling pathways of G protein-coupled receptors [49, 50]. Additional signalling pathways have been shown to modulate Nur77 expression and/or activity including; Mitogen-Activated Protein (MAP) Kinases (Extracellular signal-Regulated Kinases 1/2 (ERK1/2), c-Jun NH2-terminal Kinase, (JNK) and p38 MAP kinase) [51–53], Mitogen- and Stress-activated Kinase (MSK) [54], Ribosomal S6 Kinase (RSK) [55] and Akt (Protein Kinase B) [56, 57] pathways in different cellular contexts (mostly in lymphocyte T cells). However, the signalling cascade leading to modulation of Nur77 mRNA levels in the brain has never been investigated. The state of phosphorylation of Nur77 is also critical for its function. Indeed, phosphorylation of Nur77 can modulate its transcriptional activity, subcellular localization and heterodimerization with RXR [53, 55–57]. Outside the brain, translocation of Nur77 from the nucleus to the cytoplasm is associated with apoptotic pathways and regulates retinoid-dependent signalling [8, 58]. We have previously shown that antipsychotic drugs increase the cellular co-localization of Nur77 and RXRγ1 isoform in striatal cells [29] and that Nur77 is mandatory for retinoid ligand activities over some dopamine-mediated effects [29, 30]. Thus, signalling events leading to phosphorylation of Nur77 may have an important impact on its activity. Clearly, further studies will be necessary to identify signalling cascades leading to the modulation of Nur77 expression as well on its phosphorylation state in the CNS.
Analysis of the functional role of members of the Nur family in the periphery is complicated by some functional redundancy [7]. For example, Nor-1 can compensate for the genetic deletion of Nur77 in T cell mediated apoptosis [59]. However, functional redundancy of Nur77 and Nor-1 has not been observed so far in dopamine systems. The cataleptic response to dopamine D2 antagonists is not altered in Nor-1 knockout mice [29] and they display behavioural alterations distinct from Nur77(−/−) mice in response to amphetamine (unpublished observations). Interestingly, it has been shown that Nor-1 cannot form heterodimers with RXR [60]. Taken together, these results suggest that Nor-1 and Nur77 may have distinct functions in striatal dopamine-mediated effects. This may confer some brain selectivity for Nur77/RXR-related activities.
The close relationship between Nur77, RXR and dopamine system deduced from anatomical observations and altered responses to RXR ligands in Nur77 knockout mice indicate that Nur77 and RXRγ1 isoform might interact in striatal cells and form a transcriptional complex, which might modulate dopamine-mediated processes. The RARβ isoform is also largely express in the striatum [19, 20, 61] and both RARβ1-3 and RXRγ1 are highly co-expressed (near 100%) in striatum (Fig. 5). Thus, in basal conditions (in which Nur77 expression remains low) transcriptional complexes composed of RAR/RXR might predominate (Fig. 6). RXR is a silent partner in such a complex, i.e. this complex cannot respond to selective RXR ligands [60]. When Nur77 is induced, it might favour the formation of Nur77/RXR heterodimers (Fig. 6). Hence, striatal cells now become responsive to RXR ligands, because RXR is an active partner in such an heterodimer complex [60]. Thus, Nur77 could also be viewed as a molecular switch that allows striatal cells to become responsive to RXR ligands (Fig. 6). This model is consistent with the observations gathered so far in this system, but additional experiments will be required in order to demonstrate the presence of these transcriptional complexes in striatal cells.
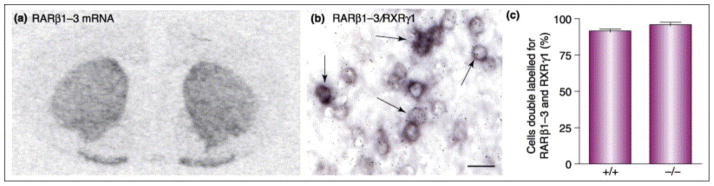
Figure 5. RAR and RXR mRNAs are highly co-localized in the striatum.
(A) In situ hybridization of Retinoic Acid Receptor isoforms β1-3 (RARβ1-3) transcripts in the rat brain. The picture shows a coronal section of the rat brain illustrating RARβ1-3 mRNA levels in the striatum. (B) Representative double in situ hybridization using a radioactive-labeled RXRγ1 probe (sylver grains) in combination with a digoxigenin-labeled RARβ1-3 probe (purple depots) in the dorsolataral portion of the striatum (StDL) in wild type mice. (C) Quantification of RXRγ1 and RARβ1-3 mRNA levels in StDL of wild type (+/+) and Nur77 knockout (−/−) mice. Co-localization of these transcripts is nearly 100% in this part of the brain and co-localization of these transcripts is not affected by Nur77 gene ablation. Histogram bars represent mean ± SEM from 5 animals per groups (unpublished data).
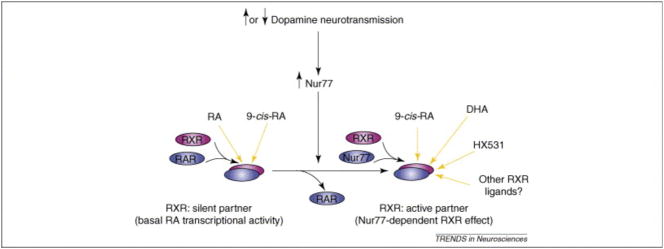
Figure 6. Schematic representation of the molecular switch property of Nur77 in striatal cells.
In basal conditions, co-localization of retinoid X receptors (RXR) along with retinoic acid receptors (RAR) and low expression of Nur77 transcripts in striatal cells suggest that the transcriptional complex composed of RAR/RXR, in which RXR remains silent, is predominant. When dopamine neurotransmission is altered (antipsychotic agents, indirect agonists such as amphetamine or cocaine, denervation or dopamine replacement therapy), Nur77 is induced in specific cell populations of the striatum. Then, competition between partnerships of RXR might occur (RAR/RXR vs Nur77/RXR). This allows the transcriptional complex Nur77/RXR, where RXR is an active partner, to be formed. In this condition, the system becomes responsive to RXR ligands such as docosahexaenoic acid (DHA), HX531 and other selective RXR ligands (to be tested). Note that all-trans retinoic acid (RA) only activates the RAR/RXR complex, whereas the derivative 9-cis retinoic acid (9-cisRA) can activate both complexes.
Concluding remarks
Altogether, these findings call for a reassessment of our fundamental understanding of the molecular and cellular basis of dopaminergic transmission. Given that diseases such as Parkinson’s disease and schizophrenia are thought to involve maladaptation of dopamine signalling, these findings might lead to new insight into these pathologies and offer new avenues for drug development. Indeed, synthetic retinoid ligands (also called rexinoids) or a new class of Nur77 agonists recently identified [62] could eventually be used as new therapeutic targets for dopamine-related disorders. However, many important aspects remain to be further explored. Signalling pathways that trigger Nur77 expression and/or phosphorylation in the brain needs to be documented. Genes targeted by a Nur77-RXR dependent transcriptional activity remain to be identified. Although we have identified some putative candidates, as discussed herein (enkephalin, neurotensin and COMT), a more global understanding of the role of these transcription factors into brain dopamine physiology is essential.
Acknowledgments
We acknowledge support of joint grants from Canadian Institutes for Health Research (D.L.-C.R. and C.R.-D.L.), Parkinson Society of Canada (C.R.-D.L.) and Parkinson’s Disease Foundation of United Stated (D.L.-C.R.), a grant from National Alliance for Research on Schizophrenia and Depression (NARSAD) and a senior scholarship from the “Fonds de la Recherche en Santé du Québec” (FRSQ) to D.L.. We thank Martine Cossette for her help to graphic illustrations.
Abbreviations
- 6-OHDA
6-hydroxydopamine
- CNS
central nervous system
- COMT
catechol-O-methyltransferase
- DHA
docosahexaenoic acid
- DYN
dynorphin
- ENK
enkephalin
- EPS
extrapyramidal symptoms
- IEG
immediate-early gene
- LID
L-DOPA-induced dyskinesias
- NGFI-B
Nerve-Growth Factor Inducible gene B
- RA
retinoic acid
- RAR
retinoic acid receptor
- RXR
retinoid X receptor
- SN
substantia nigra
- VCM
vacuous chewing movement
- VTA
ventral tegmental area
References
- 1.Bozzi Y, Borrelli E. Dopamine in neurotoxicity and neuroprotection: what do D2 receptors have to do with it? Trends Neurosci. 2006;29:167–174. doi: 10.1016/j.tins.2006.01.002. [DOI] [PubMed] [Google Scholar]
- 2.Serretti A, et al. New antipsychotics and schizophrenia: A review on efficacy and side effects. Curr Med Chem. 2004;11:343–358. doi: 10.2174/0929867043456043. [DOI] [PubMed] [Google Scholar]
- 3.Chao J, Nestler EJ. Molecular neurobiology of drug addiction. Annu Rev Med. 2004;55:113–132. doi: 10.1146/annurev.med.55.091902.103730. [DOI] [PubMed] [Google Scholar]
- 4.Missale C, et al. Dopamine receptors: From structure to function. Physiol Rev. 1998;78:189–225. doi: 10.1152/physrev.1998.78.1.189. [DOI] [PubMed] [Google Scholar]
- 5.Gronemeyer H, et al. Principles for modulation of the nuclear receptor superfamily. Nat Rev Drug Discov. 2004;3:950–964. doi: 10.1038/nrd1551. [DOI] [PubMed] [Google Scholar]
- 6.Sheng M, Greenberg ME. The regulation and function of c-fos and other immediate early genes in the nervous system. Neuron. 1990;4:477–485. doi: 10.1016/0896-6273(90)90106-p. [DOI] [PubMed] [Google Scholar]
- 7.Maxwell MA, Muscat GEO. The NR4A subgroup: immediate early response genes with pleiotropic physiological roles. Nucl Recept Signal. 2006;4:e002. doi: 10.1621/nrs.04002. Epub. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lin B, et al. Conversion of Bcl-2 from protector to killer by interaction with nuclear orphan receptor Nur77/TR3. Cell. 2004;116:527–540. doi: 10.1016/s0092-8674(04)00162-x. [DOI] [PubMed] [Google Scholar]
- 9.Martinez-Gonzalez J, Badimon L. The NR4A subfamily of nuclear receptors: new early genes regulated by growth factors in vascular cells. Cardiovascular Research. 2005;65:609–618. doi: 10.1016/j.cardiores.2004.10.002. [DOI] [PubMed] [Google Scholar]
- 10.Martens C, et al. Protein-protein interactions and transcriptional antagonism between the subfamily of NGFI-B/Nur77 orphan nuclear receptors and glucocorticoid receptor. Mol Endocrinol. 2005;19:885–897. doi: 10.1210/me.2004-0333. [DOI] [PubMed] [Google Scholar]
- 11.Shulman AI, Mangelsdorf DJ. Mechanisms of disease: Retinoid X receptor heterodimers in the metabolic syndrome. New Engand J Med. 2005;353:604–615. doi: 10.1056/NEJMra043590. [DOI] [PubMed] [Google Scholar]
- 12.Evans RM. The nuclear receptor superfamily: A Rosetta Stone for physiology. Mol Endocrinol. 2005;19:1429–1438. doi: 10.1210/me.2005-0046. [DOI] [PubMed] [Google Scholar]
- 13.Perlmann T, Jansson L. A novel pathway for vitamin A signaling mediated by RXR heterodimerization with NGFI-B and NURR1. Genes Dev. 1995;9:769–782. doi: 10.1101/gad.9.7.769. [DOI] [PubMed] [Google Scholar]
- 14.Zetterström RH, et al. Dopamine neuron agenesis in Nurr1-deficient mice. Science. 1997;276:248–250. doi: 10.1126/science.276.5310.248. [DOI] [PubMed] [Google Scholar]
- 15.Zetterström RH, et al. Cellular expression of the immediate-early transcription factors Nurr1 and NGFI-B suggests a gene regulatory role in several brain regions including the nigrostriatal dopamine system. Mol Brain Res. 1996;41:111–120. doi: 10.1016/0169-328x(96)00074-5. [DOI] [PubMed] [Google Scholar]
- 16.Beaudry G, et al. Contrasting patterns and cellular specificity of transcriptional regulation of the nuclear receptor Nerve Growth Factor-Inducible B by haloperidol and clozapine in the rat forebrain. J Neurochem. 2000;75:1694–1702. doi: 10.1046/j.1471-4159.2000.0751694.x. [DOI] [PubMed] [Google Scholar]
- 17.Werme M, et al. NGFI-B and Nor-1 mRNAs are upregulated in brain reward pathways by drugs of abuse: different effects in Fischer and Lewis rats. Mol Brain Res. 2000;76:18–24. doi: 10.1016/s0169-328x(99)00327-7. [DOI] [PubMed] [Google Scholar]
- 18.Maheux J, et al. Induction patterns of transcription factors of the Nur family (Nurr1, Nur77 and Nor-1) by typical and atypical antipsychotics in the mouse brain: Implication for their mechanism of action. J Pharmacol Exp Ther. 2005;313:460–473. doi: 10.1124/jpet.104.080184. [DOI] [PubMed] [Google Scholar]
- 19.Langlois MC, et al. Impact of antipsychotic drug administration on the expression of nuclear receptors in the neocortex and striatum of the rat brain. Neuroscience. 2001;106:117–128. doi: 10.1016/s0306-4522(01)00248-2. [DOI] [PubMed] [Google Scholar]
- 20.Zetterström RH, et al. Role of retinoids in the CNS: differential expression of retinoid binding proteins and receptors and evidence for presence of retinoic acid. Eur J Neurosci. 1999;11:407–416. doi: 10.1046/j.1460-9568.1999.00444.x. [DOI] [PubMed] [Google Scholar]
- 21.Zetterström RH, et al. Localization of cellular retinoid-binding proteins suggests specific roles for retinoids in the adult central nervous system. Neuroscience. 1994;62:899–918. doi: 10.1016/0306-4522(94)90482-0. [DOI] [PubMed] [Google Scholar]
- 22.Saga Y, et al. Impaired extrapyramidal function caused by the targeted disruption of retinoid X receptor RXRγ1 isoform. Genes to Cells. 1999;4:219–228. doi: 10.1046/j.1365-2443.1999.00253.x. [DOI] [PubMed] [Google Scholar]
- 23.Krezel W, et al. Impaired locomotion and dopamine signaling in retinoid receptor mutant mice. Science. 1998;279:863–867. doi: 10.1126/science.279.5352.863. [DOI] [PubMed] [Google Scholar]
- 24.Goodman AB. Three independent lines of evidence suggest retinoids as causal to schizophrenia. Proc Natl Acad Sci USA. 1998;95:7240–7244. doi: 10.1073/pnas.95.13.7240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wallen-Mackenzie A, et al. Nurr1-RXR heterodimers mediate RXR ligand-induced signaling in neuronal cells. Genes & Development. 2003;17:3036–3047. doi: 10.1101/gad.276003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McClung CA, et al. ΔFosB: a molecular switch for long-term adaptation in the brain. Brain Res Mol Brain Res. 2004;132:146–154. doi: 10.1016/j.molbrainres.2004.05.014. [DOI] [PubMed] [Google Scholar]
- 27.Herrera DG, Robertson HA. Activation of c-fos in the brain. Prog Neurobiol. 1996;50:83–107. doi: 10.1016/s0301-0082(96)00021-4. [DOI] [PubMed] [Google Scholar]
- 28.Robertson GS, et al. Induction patterns of Fos-like immunoreactivity in the forebrain as predictors of atypical antipsychotic activity. J Pharmacol Exp Ther. 1994;271:1058–1066. [PubMed] [Google Scholar]
- 29.Ethier I, et al. The transcription factor NGFI-B (Nur77) and retinoids play a critical role in acute neuroleptic-induced extrapyramidal effect and striatal neuropeptide gene expression. Neuropsychopharmacology. 2004;29:335–346. doi: 10.1038/sj.npp.1300318. [DOI] [PubMed] [Google Scholar]
- 30.Ethier I, et al. Docosahexaenoic acid reduces haloperidol-induced dyskinesias in mice: Involvement of Nur77 and retinoid receptors. Biol Psychiatry. 2004;56:522–526. doi: 10.1016/j.biopsych.2004.06.036. [DOI] [PubMed] [Google Scholar]
- 31.Mata de Urquiza A, et al. Docosahexaenoic acid, a ligand for the retinoid X receptor in mouse brain. Science. 2000;290:2140–2144. doi: 10.1126/science.290.5499.2140. [DOI] [PubMed] [Google Scholar]
- 32.Bhardwaj SK, et al. Neonatal ventral hippocampus lesion leads to reductions in nerve growth factor inducible-B mRNA in the prefrontal cortex and increased amphetamine response in the nucleus accumbens and dorsal striatum. Neuroscience. 2003;122:669–676. doi: 10.1016/j.neuroscience.2003.08.016. [DOI] [PubMed] [Google Scholar]
- 33.Xing GQ, et al. Reduction of dopamine-related transcription factors Nurr1 and NGFI-B in the prefrontal cortex in schizophrenia and bipolar disorders. Schizophrenia Res. 2006;84:36–56. doi: 10.1016/j.schres.2005.11.006. [DOI] [PubMed] [Google Scholar]
- 34.Lipska BK, et al. Effects of reversible inactivation of the neonatal ventral hippocampus on behavior in the adult rat. J Neurosci. 2002;22:2835–2842. doi: 10.1523/JNEUROSCI.22-07-02835.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ujike H. Stimulant-induced psychosis and schizophrenia: the role of sensitization. Curr Psychiatry Rep. 2002;4:177–184. doi: 10.1007/s11920-002-0024-7. [DOI] [PubMed] [Google Scholar]
- 36.Werme M, et al. Addiction-prone Lewis but not Fischer rats develop compulsive running that coincides with downregulation of nerve growth factor inducible-B and neuron-derived orphan receptor 1. J Neurosci. 1999;19:6169–6174. doi: 10.1523/JNEUROSCI.19-14-06169.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.St-Hilaire M, et al. Effects of cocaine on c-fos and NGFI-B mRNA expression in transgenic mice underexpressing glucocorticoid receptors. Neuropsychopharmacology. 2003;28:478–489. doi: 10.1038/sj.npp.1300067. [DOI] [PubMed] [Google Scholar]
- 38.Gilbert F, et al. Nur77 gene knockout alters dopamine neuron biochemical activity and dopamine turnover. Biol Psychiatry. doi: 10.1016/j.biopsych.2006.04.023. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Winterer G, Weinberger DR. Genes, dopamine and cortical signal-to-noise ratio in schizophrenia. Trends Neurosci. 2004;27:683–690. doi: 10.1016/j.tins.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 40.Harrison PJ, Weinberger DR. Schizophrenia genes, gene expression, and neuropathology: on the matter of their convergence. Mol Psychiatry. 2005;10:40–68. doi: 10.1038/sj.mp.4001558. [DOI] [PubMed] [Google Scholar]
- 41.St-Hilaire M, et al. Denervation and repeated L-DOPA induce complex regulatory changes in neurochemical phenotypes of striatal neurons: Implication of a dopamine D1-dependent mechanism. Neurobiol Dis. 2005;20:450–460. doi: 10.1016/j.nbd.2005.04.001. [DOI] [PubMed] [Google Scholar]
- 42.St-Hilaire M, et al. Denervation and repeated L-DOPA induce a coordinate expression of the transcription factor NGFI-B in striatal projection pathways in hemi-parkinsonian rats. Neurobiol Dis. 2003;14:98–109. doi: 10.1016/s0969-9961(03)00081-0. [DOI] [PubMed] [Google Scholar]
- 43.van den Munckhof P, et al. Striatal neuroadaptation and rescue of locomotor deficit by L-dopa in Aphakia mice, a model of Parkinson’s disease. J Neurochem. 2006;96:160–170. doi: 10.1111/j.1471-4159.2005.03522.x. [DOI] [PubMed] [Google Scholar]
- 44.Sgambato-Faure V, et al. Coordinated and spatial upregulation of Arc in striatonigral neurons correlates with L-dopa-induced behavioral sensitization in dyskinetic rats. J Neuropathol Exp Neurol. 2005;64:936–947. doi: 10.1097/01.jnen.0000186922.42592.b7. [DOI] [PubMed] [Google Scholar]
- 45.St-Hilaire M, et al. Impaired behavioural and molecular adaptations to dopamine denervation and repeated L-DOPA treatment in Nur77 knockout mice. Eur J Neurosci. 2006;24:795–805. doi: 10.1111/j.1460-9568.2006.04954.x. [DOI] [PubMed] [Google Scholar]
- 46.Gerfen CR, et al. D1 dopamine receptor supersensitivity in the dopamine-depleted striatum results from a switch in the regulation of ERK1/2/MAP kinase. J Neurosci. 2002;22:5042–5054. doi: 10.1523/JNEUROSCI.22-12-05042.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Samadi P, et al. Docosahexaenoic acid (DHA) reduces L-DOPA-induced dyskinesias in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) monkeys. Annals Neurol. 2006;59:282–288. doi: 10.1002/ana.20738. [DOI] [PubMed] [Google Scholar]
- 48.Salem N, Jr, et al. Mechanisms of action of docosahexaenoic acid in the nervous system. Lipids. 2001;36:945–959. doi: 10.1007/s11745-001-0805-6. [DOI] [PubMed] [Google Scholar]
- 49.Kovalovsky D, et al. Activation and Induction of NUR77/NURR1 in Corticotrophs by CRH/cAMP: Involvement of Calcium, Protein Kinase A, and MAPK Pathways. Mol Endocrinol. 2002;16:1638–1651. doi: 10.1210/mend.16.7.0863. [DOI] [PubMed] [Google Scholar]
- 50.Maira M, et al. Dimer-Specific Potentiation of NGFI-B (Nur77) Transcriptional Activity by the Protein Kinase A Pathway and AF-1-Dependent Coactivator Recruitment. Mol Cell Biol. 2003;23:763–776. doi: 10.1128/MCB.23.3.763-776.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jacobs CM, Paulsen RE. Crosstalk between ERK2 and RXR regulates nuclear import of transcription factor NGFI-B. Biochem Biophys Res Commun. 2005;336:646–652. doi: 10.1016/j.bbrc.2005.08.143. [DOI] [PubMed] [Google Scholar]
- 52.Castro-Obregon S, et al. Alternative, nonapoptotic programmed cell death: mediation by arrestin 2, ERK2, and Nur77. J Biol Chem. 2004;279:17543–17553. doi: 10.1074/jbc.M312363200. [DOI] [PubMed] [Google Scholar]
- 53.Han YH, et al. Regulation of Nur77 nuclear export by c-Jun N-terminal kinase and Akt. Oncogene. 2006;25:2974–2986. doi: 10.1038/sj.onc.1209358. [DOI] [PubMed] [Google Scholar]
- 54.Darragh J, et al. MSKs are required for the transcription of the nuclear orphan receptors Nur77, Nurr1 and Nor1 downstream of MAPK signalling. Biochem J. 2005;390:749–759. doi: 10.1042/BJ20050196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wingate AD, et al. Nur77 is phosphorylated in cells by RSK in response to mitogenic stimulation. Biochem J. 2006;393:715–724. doi: 10.1042/BJ20050967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pekarsky Y, et al. Akt phosphorylates and regulates the orphan nuclear receptor Nur77. Proc Natl Acad Sci USA. 2001;98:3690–3694. doi: 10.1073/pnas.051003198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Masuyama N, et al. Akt inhibits the orphan nuclear receptor Nur77 and T-cell apoptosis. J Biol Chem. 2001;276:32799–32805. doi: 10.1074/jbc.M105431200. [DOI] [PubMed] [Google Scholar]
- 58.Cao X, et al. Retinoid X receptor regulates Nur77/thyroid hormone receptor 3-dependent apoptosis by modulating its nuclear export and mitochondrial targeting. Mol Cell Biol. 2004;24:9705–9725. doi: 10.1128/MCB.24.22.9705-9725.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lee SL, et al. Unimpaired thymic and peripheral T cell death in mice lacking the nuclear receptor NGFI-B (Nur77) Science. 1995;269:532–535. doi: 10.1126/science.7624775. [DOI] [PubMed] [Google Scholar]
- 60.Forman BM, et al. Unique response pathways are established by allosteric interactions among nuclear hormone receptors. Cell. 1995;81:541–550. doi: 10.1016/0092-8674(95)90075-6. [DOI] [PubMed] [Google Scholar]
- 61.Krezel W, et al. Differential expression of retinoid receptors in the adult mouse central nervous system. Neuroscience. 1999;89:1291–1300. doi: 10.1016/s0306-4522(98)00342-x. [DOI] [PubMed] [Google Scholar]
- 62.Chintharlapalli S, et al. Activation of Nur77 by selected 1,1-bis(3′-indolyl)-1-(p-substituted phenyl) methanes induces apoptosis through nuclear pathways. J Biol Chem. 2005;280:24903–24914. doi: 10.1074/jbc.M500107200. [DOI] [PubMed] [Google Scholar]
- 63.Gervais J, et al. Dopamine and serotonin interactions in the modulation of the expression of the immediate-early transcription factor, Nerve Growth Factor-Inducible B, in the striatum. Neuroscience. 1999;91:1045–1054. doi: 10.1016/s0306-4522(98)00688-5. [DOI] [PubMed] [Google Scholar]
- 64.Bäckman C, Morales M. Acute methamphetamine administration upregulates NGFI-B mRNA expression in the striatum: Co-localization with c-fos immunoreactivity. Synapse. 2002;44:158–165. doi: 10.1002/syn.10065. [DOI] [PubMed] [Google Scholar]
- 65.Freeman WM, et al. Changes in rat frontal cortex gene expression following chronic cocaine. Mol Brain Res. 2002;104:11–20. doi: 10.1016/s0169-328x(02)00197-3. [DOI] [PubMed] [Google Scholar]
- 66.Werme M, et al. Differential patterns of induction of NGFI-B, Nor1 and c-fos mRNAs in striatal subregions by haloperidol and clozapine. Brain Res. 2000;863:112–119. doi: 10.1016/s0006-8993(00)02109-0. [DOI] [PubMed] [Google Scholar]
- 67.Svenningsson P, et al. Antagonism of adenosine A2A receptors underlies the behavioural activating effect of caffeine and is associated with reduced expression of messenger RNA for NGFI-A and NGFI-B in caudate-putamen and nucleus accumbens. Neuroscience. 1997;79:753–764. doi: 10.1016/s0306-4522(97)00046-8. [DOI] [PubMed] [Google Scholar]
- 68.Werme M, et al. Running and cocaine both upregulate dynorphin mRNA in medial caudate putamen. Eur J Neurosci. 2000;12:2967–2974. doi: 10.1046/j.1460-9568.2000.00147.x. [DOI] [PubMed] [Google Scholar]
- 69.Egerton A, et al. Acute delta9-tetrahydrocannabinol-induced deficits in reversal learning: neural correlates of affective inflexibility. Neuropsychopharmacology. 2005;30:1895–1905. doi: 10.1038/sj.npp.1300715. [DOI] [PubMed] [Google Scholar]
- 70.Philips A, et al. Novel dimeric Nur77 signaling mechanism in endocrine and lymphoid cells. Mol Cell Biol. 1997;17:5946–5951. doi: 10.1128/mcb.17.10.5946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Maira M, et al. Signalisation par le récepteur orphelin Nur77: nouveau mécanisme d’action et antagonisme par les glucocorticoïdes. Médecine/Science. 1998;14:1217–1221. [Google Scholar]
- 72.Epelbaum J. In: Neuropeptides and neuromédiateurs. 2. Epelbaum J, editor. 1995. p. 98. Editions Inserm. [Google Scholar]
- 73.Cossette M, et al. Tyrosine hydroxylase-positive neurons intrinsic to the human striatum express the transcription factor Nurr1. Eur J Neurosci. 2004;20:2089–2095. doi: 10.1111/j.1460-9568.2004.03661.x. [DOI] [PubMed] [Google Scholar]