Abstract
The prefrontal cortex (PFC) is critical for the ability to flexibly adapt established patterns of behavior in response to a change in environmental contingencies. Impaired behavioral flexibility results in maladaptive strategies such as perseveration on response options that no longer produce a desired outcome. Pharmacological manipulations of prefrontal cortical GABAergic signaling modulate behavioral flexibility in animal models, and prefrontal cortical interneuron dysfunction is implicated in impaired behavioral flexibility that accompanies neuropsychiatric disease. As deficits in behavioral flexibility also emerge during the normal aging process, the goal of this study was to determine the role of GABAergic signaling, specifically via prefrontal cortical GABA(B) receptors, in such age-related deficits. Young and aged rats were trained in a set shifting task performed in operant chambers. First, rats learned to discriminate between two response levers to obtain a food reward on the basis of a cue light illuminated above the correct lever. Upon acquisition of this initial discrimination, the contingencies were shifted such that rats had to ignore the cue light and respond on the levers according to their left/right positions. Both young and aged rats acquired the initial discrimination similarly; however, aged rats were impaired relative to young following the set shift. Among aged rats, GABA(B) receptor expression in the medial prefrontal cortex (mPFC) was strongly correlated with set shifting, such that lower expression was associated with worse performance. Subsequent experiments showed that intra-mPFC administration of the GABA(B) receptor agonist baclofen enhanced set shifting performance in aged rats. These data directly link GABAergic signaling via GABA(B) receptors to impaired behavioral flexibility associated with normal aging.
Keywords: aging, prefrontal cortex, behavioral flexibility, GABA(B) receptors, baclofen, rat
INTRODUCTION
Aging results in a decline in prefrontal cortical-dependent cognitive capacities, which include behavioral flexibility or the ability to modify behavior in accord with changes in environmental contingencies. A loss of flexibility at advanced ages can result in perseverative behavioral strategies that can interfere with an individual’s ability to complete activities of daily living and contribute to a loss of personal independence. Understanding the neurobiological mechanisms that underlie age-related behavioral flexibility deficits could provide insight into potential therapeutic targets to promote healthy cognitive aging.
Across species, behavioral flexibility can be assessed using “set shifting” tasks. Such tasks vary in design but typically involve subjects learning an initial response strategy or rule that is then followed by an unsignaled “shift” to a second response rule. The primary measure of behavioral flexibility is how adeptly the subject is able to inhibit responses to the initial rule and behave in accordance with the second rule. Extensive evidence demonstrates that set shifting performance is critically dependent on the dorsolateral prefrontal cortex (PFC) in primates, or the rodent homolog, medial prefrontal cortex (mPFC; Owen et al., 1991; Dias et al., 1996; Birrell and Brown, 2000; Demakis, 2003; Uylings et al., 2003; Ragozzino, 2007; Darrah et al., 2008; Floresco et al., 2008; Bissonette and Powell, 2012). In particular, behavioral flexibility appears sensitive to changes in prefrontal cortical GABAergic signaling and shifts in the normal balance of excitation and inhibition in this brain region. For example, intra-mPFC administration of the GABA(A) receptor antagonist bicuculline can impair set shifting performance in young rats (Enomoto et al., 2011). In addition, experimental disruption of prefrontal cortical GABAergic interneurons impairs set shifting as well as other forms of behavioral flexibility in mice (Jacobson et al., 2006; Bissonette et al., 2012, 2014; Cho et al., 2015). These data are consistent with those from neuropsychiatric diseases such as schizophrenia, which are accompanied by both interneuron dysfunction and inflexible behavior (Everett et al., 2001; Hashimoto et al., 2008; Maldonado-Aviles et al., 2009; Gonzalez-Burgos et al., 2011; Lewis et al., 2012). Indeed, set shifting impairments are prominent in rodent models of schizophrenia that produce cortical hyperexcitability, including developmental exposure to NMDA receptor antagonists and neonatal ventral hippocampal lesions (Stefani and Moghaddam, 2005; Homayoun and Moghaddam, 2007; Brady, 2009; Gruber et al., 2010; O’Donnell, 2012; Placek et al., 2013; Ryan et al., 2013).
It is becoming increasingly clear that prefrontal cortical GABAergic signaling is dysregulated in advanced aging and that altered GABA signaling contributes to age-related cognitive decline (Bories et al., 2013; Baņuelos et al., 2014; McQuail et al., 2015). GABAergic interneurons decline in number in the rat mPFC (Stranahan et al., 2012), and the density of inhibitory synapses (i.e., symmetric) is reduced in the aged cortex (Brunso-Bechtold et al., 2000; Poe et al., 2001; Peters et al., 2008). Despite these structural changes, electrophysiological studies conducted in both rodents and nonhuman primates indicate that some aged PFC pyramidal neurons are subject to increased inhibition (Luebke et al., 2004; Bories et al., 2013). Notably, age-related alterations in metabotropic GABA(B) receptors may mediate shifts in the tonic inhibition of PFC pyramidal neurons. GABA(B) receptors are localized presynaptically on GABAergic and glutamatergic terminals where they regulate neurotransmitter release, as well as postsynaptically on dendrites of pyramidal neurons where they have been implicated in tonic inhibition (Wang et al., 2010). GABA (B) receptor expression is markedly reduced in the aged PFC (McQuail et al., 2012); however it remains unclear how such reductions impact behavioral flexibility. The first goal of the current study was to determine how age-related changes in the expression of mPFC GABA(B) receptor subunits relate to performance on a mPFC-dependent set shifting task. The second goal of this study was to determine if pharmacological activation of GABA (B) receptors in the mPFC could improve set shifting performance in aged rats.
EXPERIMENTAL PROCEDURES
Subjects
Young (n=7) and aged (n=40) male Fischer 344 rats were used in Experiments 1 and 2. Rats were obtained from the National Institute on Aging colony (Charles River Laboratories, Raleigh, NC, USA) and housed in an AAALAC-accredited vivarium in the McKnight Brain Institute Building at University of Florida in accordance with the rules and regulations of the University of Florida Institutional Animal Care and Use Committee and NIH guidelines. The facility was maintained at a consistent 25 °C with a 12-h light/dark cycle (lights on at 0800 h) with free access to food and water except as noted below.
Experiment 1. Relationships between GABA(B) receptor expression and set shifting performance in aged rats
Prior to the start of behavioral testing, young and aged male Fischer 344 rats (n=7 young, 6 months, and n=15 aged, 22 months) were food restricted to 85% of their free feeding weights over the course of five days. Rats were maintained at this weight for the duration behavioral testing.
Apparatus
The set shifting task was conducted in eight standard rat behavioral test chambers (30.5 × 25.4 × 30.5 cm, Coulbourn Instruments, Whitehall, PA, USA) with metal front and back walls, transparent Plexiglas side walls, and a floor made of steel rods (0.4 cm diameter) spaced 1.1 cm apart. Each test chamber was housed in a sound-attenuating cubicle, and contained a recessed food pellet delivery trough located 2 cm above the floor in the center of the front wall. The food trough contained a photobeam to detect head entries and a 1.12 W lamp for illumination. Food rewards in the task consisted of deliveries of a single 45-mg grain-based food pellet (PJAI, Test Diet, Richmond, IN, USA) into the trough for each correct response. Two retractable levers were located to the left and right of the food trough (11 cm above the floor), and a 1.12 W cue lamp was located 3.8 cm above each lever. An additional 1.12 W house light was mounted on the rear wall of the sound-attenuating cubicle. To monitor locomotor activity during task performance, an infrared activity monitor was positioned above each test chamber. The monitor contained an array of infrared (body heat) detectors focused on the chamber. Movement in the chamber was defined as a relative change in the infrared energy falling on the detectors. A computer interfaced with the test chambers and equipped with Graphic State 3.01 software (Coulbourn Instruments) was used for experiment control and data collection.
Shaping
The design of the set shifting task was based on that used by (Floresco et al., 2008; Beas et al., 2013). Prior to training, each rat was given five 45-mg food pellets in its home cage to reduce neophobia to the food reward. Training began with four stages of shaping, with each new stage beginning the day following completion of the prior stage. In Shaping Stage 1, rats received a 64-min session of magazine training, involving 38 deliveries of a single food pellet with an inter-trial interval (ITI) of 100±40 s. In Shaping Stage 2, rats were trained to press each of the response levers. A single lever (left or right, counterbalanced across groups) was extended into the test chamber and a press resulted in delivery of a single food pellet. After reaching a criterion of 50 lever presses in 30 min, rats were then trained on the opposite lever using the same procedures.
In Shaping Stage 3, rats received 90 trials designed to train them to press the levers immediately after their insertion into the test chamber. Each 20-s trial began with the house light being illuminated and insertion of a single lever (either left or right, randomly selected within each pair of trials) into the test chamber where it remained for a maximum of 10 s. A lever press in this time window caused the lever to retract, a single food pellet to be delivered, and the house light to remain on for an additional 4 s. If a rat failed to press the lever within 10 s, the lever was retracted and the house light turned off, and the trial was scored as an omission. Rats received at least four daily sessions in this stage, and training proceeded until rats reached a criterion of fewer than 10 omissions out of the 90 trials in a single session.
In Shaping Stage 4 each rat’s side bias (i.e., preference for one lever over the other) was determined. Trials consisted of multiple phases. In the first phase of a trial, the house light was illuminated and both levers were inserted into the test chamber. A press on either lever caused both levers to retract and a single food pellet to be delivered. In the second phase of a trial, both levers were again inserted, but only a press on the lever opposite to that pressed in the first phase resulted in food delivery. A press on the same lever chosen in the first phase caused the levers to be retracted and the house light to be extinguished. After a “correct” response in this second phase of a trial, a new trial was initiated, whereas after an “incorrect” response, the second phase was repeated until rats made a “correct” response. The session ended after a total of 45 completed trials. The side associated with the greatest number of total responses across this phase of testing was considered a rat’s biased side.
Initial discrimination (visual cue)
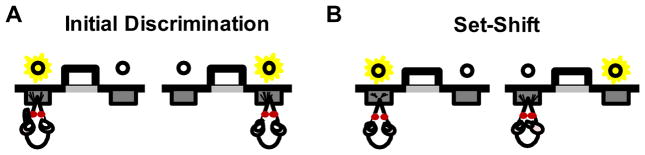
On the day after the side bias determination session, rats were trained on the visual cue discrimination. As shown in Fig. 1A, illumination of a cue light over a lever signaled the correct response, irrespective of its spatial location (left or right). Each 20-s trial began with one of the cue lights being illuminated with the left or right side selected randomly for each pair of trials. The house light was illuminated 3 s later and remained on while both levers were inserted into the chamber. A press on the lever corresponding to the cue light (a correct response) caused the house light to remain on for 4 s, the levers to be retracted, the cue light to be extinguished, and a single food pellet to be delivered. A press on the lever that was not illuminated by the cue light (an incorrect response) or failure to respond within 10 s (omission) caused both levers to be retracted and all lights to be extinguished. The criterion for acquisition of the visual cue discrimination was eight consecutive correct trials (and at least 30 total trials, excluding omissions). Rats performed a maximum of 120 trials per session. Rats not reaching criterion within a single session received additional sessions on subsequent days. The total number of completed trials required to reach criterion (combined across all sessions) was used as the measure of acquisition for the visual cue discrimination.
Fig. 1.
Schematic of the set shifting task. (A) Rats were initially required to discriminate between two response levers on the basis of a cue light illuminated above the correct lever (visual cue discrimination). (B) Upon acquisition of the visual cue discrimination, the response strategy was “shifted”, such that the rats had to ignore the cue light and respond on the basis of a particular lever location (e.g., always press the left lever).
Set shift discrimination (left/right)
The day after achieving criterion performance on the visual cue discrimination, the task contingencies were altered and rats were tested in the set shift condition. As shown in Fig. 1B, in this phase of the task, the trials were identical to those in the visual discrimination but the light illuminated over the levers no longer predicted the correct response. Instead, only responses on one side (left or right, whichever was not the rat’s biased side as calculated in Shaping Stage 4) resulted in food delivery. As such, the rats were required to ignore the light illuminated over the lever (visual cue) and instead “shift” attention toward the spatial location of the lever in order to consistently receive the reward. Rats were considered to have acquired the set shift upon reaching criterion performance of 10 consecutive correct trials (excluding omissions). As with the visual cue discrimination, the maximum number of trials in each session was set at 120. If rats did not acquire the task within a single session, they received additional sessions on subsequent days.
Western blotting procedures
Sample preparation
Following completion of behavioral testing, rats were returned to free feeding for a minimum of one week before being sacrificed by decapitation. The brain was extracted from the skull and the mPFC was dissected from surrounding tissue on an ice cold plate and frozen on dry ice. Tissues were stored at −80 °C until used for the preparation of membrane fractions as in (McQuail et al., 2012; Baņuelos et al., 2014). Frozen tissue was weighed, thawed, and homogenized in ice cold buffer (50 mM HEPES, pH 7.4, 1 mM EDTA and 1 mM EGTA and protease inhibitors (Thermo Fisher Scientific, Waltham, Massachusetts, USA) using a glass-Teflon Dounce homogenizer. Homogenates were centrifuged at 14,000 rpm for 20 min at 4°C. The pellet was resuspended in 20 mL of the same buffer without protease inhibitors and incubated on ice for 30 min followed by centrifugation at 16,500 rpm for 15 min at 4 °C. This pellet was resuspended in seven volumes of 50 mM HEPES, pH 7.4, and aliquots were stored at −80 °C until used for Western blotting assays. Protein concentration was determined using the Pierce BCA Kit according to the manufacturer’s protocol (Rockford, IL, USA).
SDS–PAGE and immunoblotting
All reagents were purchased from Bio-Rad (Hercules, CA, USA), unless noted otherwise. Proteins were denatured and reduced in Laemmli sample buffer with 5% (vol/vol) β-mercaptoethanol (BioWorld, Dublin, OH, USA) and heated at 95 °C for 5 min. In all Western blot experiments, 5 μg of protein per lane were electrophoretically separated on a 4–15% Tris–HCl gel at 200 V for 35 min then transferred to nitrocellulose membranes using a wet transfer apparatus for 40 min at 200 V. Blots were washed three times with Tris-buffered saline (TBS; pH 7.4) then blocked for 1 h in blocking buffer (Rockland, Gilbertsville, PA, USA). Blots were then incubated overnight at 4 °C with anti-GABA(B)R1 (made in rabbit; Cell Signaling Technology, Beverly, MA, USA), anti-vesicular GABA transporter (VGAT; made in rabbit; Millipore, Temecula, CA), and anti-β-tubulin (made in mouse; EnCor Biotechnology, Gainesville, FL, USA) diluted 1:1000 in blocking buffer with 0.1% Tween 20 (Bio-Rad, Hercules, CA, USA). Blots were then washed three times with TBS, incubated with either donkey-anti-rabbit IgG or donkey-anti-mouse IgG conjugated to IRDye 680RD or IRDye 800CW, (LI-COR Biosciences, Lincoln, NE, USA) and diluted 1:15,000 or 1:20,000 in blocking buffer (TBS (1:1) with 0.1% Tween 20) for 1 h. Following 3 additional TBS washes, blots were scanned on an Odyssey imaging system (LI-COR Biosciences). β-Tubulin was probed to verify equality of loading across conditions/experiments. Samples were assayed in triplicate; the loading position of the sample was varied between gels/experiments in a pseudorandom fashion to control for technical variation in the electroblotting procedure.
Statistical analyses
Set shifting statistical analysis
Data were exported from Graphic State 3.0 software as text files, and compiled using a custom macro written for Microsoft Excel (Dr. Jonathan Lifshitz, University of Kentucky). The numbers of trials and errors to reach criterion on the visual cue and left/right (set shift) discriminations were used as the measures of performance. Because the task involved presentation of the same set of stimuli (cue lights and levers) during both the initial discrimination and the set shift, the nature of the errors was also analyzed. Specifically, errors were divided into two subtypes. “Previously reinforced errors” were those in which the cue light was incongruent with the correct lever location and consistent with the previous cue discrimination rule. “Never-reinforced errors” were those in which the cue light and spatial location were congruent and the rat’s choice failed to correspond with either the previous cue discrimination rule or the left/right discrimination rule (Ragozzino et al., 2002; Floresco et al., 2008; Beas et al., 2013). Additional performance measures were also collected: number of omitted trials, response latency (latency to press one of the two levers after they were extended into the chamber), and locomotor activity during ITIs. For each measure, data are presented as mean+standard error of the mean and compared with independent t-tests. All statistical analyses were conducted using SPSS 22.0 (Cary, NC, USA) and GraphPadPrism (La Jolla, California). For all statistical comparisons, values of p<0.05 were considered significant.
Protein expression statistical analysis
For Western blot analyses, integrated protein density was measured for each band using ImageStudio Software (LI-COR) and the individual values of both young and aged samples were normalized to the mean expression of young samples run on the same gel. Age comparisons of protein expression were conducted using independent t-tests. As a negative control, each blot was probed separately for tubulin expression to confirm that age effects were not a result of technical errors in protein quantification or loading (t(20)=.07, p=0.94). Effects of age on protein expression were analyzed using independent t-tests. Relationships between protein expression and behavioral flexibility performance (as measured by total trials to criterion (TTC) on the set shift) were assessed with bivariate correlations performed on data from aged rats.
Experiment 2: Effects of medial prefrontal cortex GABA(B) receptor activation on set shifting in aged rats
Subjects
Aged male Fischer 344 rats (20 months, n=25) were obtained from the National Institute on Aging colony (Charles River Laboratories, Raleigh, NC) and housed as described above for two weeks prior to surgical procedures.
Surgery
Rats were initially anesthetized with isofluorane gas and then placed into a stereotaxic instrument fitted with atraumatic ear bars (Kopf Instruments, Tujunga, CA, USA). The incisor bar was set at −3.3 mm to provide a flat skull position. Bilateral guide cannulae, which consisted of a plastic body holding two 22-gauge stainless steel cannulae 1.4 mm apart (Plastics One, Roanoke, VA, USA), were implanted over the mPFC at the coordinates AP: +2.7 relative to the bregma, ML: ±0.7, DV: −3.8 relative to skull). The guide cannulae were affixed to the skull with dental acrylic and stainless steel screws, and wire stylets were used to occlude them to prevent infection.
Intracerebral microinjections of the GABA(B) receptor agonist baclofen
Two weeks post-surgery, rats were food restricted and trained in the set shifting task as described in Experiment 1. Just prior to testing on the visual cue discrimination, rats received a dummy injection during which the stylets were removed from the guide cannulae and injectors (28 ga. needles which extended 1.0 mm beyond the end of the guide cannulae; Plastics One) were lowered into the mPFC. This dummy injection (in which no fluid was injected) served to acclimate the rats to the handling procedures necessary to administer the drug. Rats were then trained to criterion performance on the visual cue discrimination as in Experiment 1. Prior to the set shift session, rats received bilateral microinjections of the GABA(B)R agonist baclofen (0.5 or 1.5 nmol, Tocris, Ballwin, MO, USA) or vehicle (artificial cerebral spinal fluid, Harvard Apparatus, Holliston, MA, USA). Baclofen doses were chosen based on their efficacy in blocking cocaine-induced locomotor activity (Steketee and Beyer, 2005). Rats were assigned to drug conditions on the basis of their initial (visual cue) discrimination performance, such that all groups had approximately equal performance. Microinjections (0.5 μl per hemisphere over 60 s) were administered 5 min prior to the start of the test sessions and were delivered via 10-μl syringes connected to the injection needles (which extended 1.0 mm beyond the end of the guide cannulae) by a length of PE-20 tubing. The syringes were mounted on a syringe pump (Pump 11 Elite, Harvard Apparatus) and injection needles were left in place for 1 min after injections to allow for drug diffusion.
Histological assessment of cannulae placement
Following completion of testing, rats were euthanized with 100 mg/kg sodium pentobarbital, then perfused with 0.1 M phosphate-buffered solution (PBS) followed by 4% paraformaldehyde (PFA) in 0.1 M PBS. Brains were removed and postfixed in 4% PFA overnight and then cryoprotected in 20% sucrose in PBS. Brains were then flash frozen and sliced coronally at 40 μm on a cryostat (Leica Jung Frigocut 2800E, Richmond, IL, USA). Every other tissue section was stained with thionin using methods described previously by Orsini et al. (2015) and visualized using a microscope under conventional bright-field illumination. Cannula tip placements were verified and mapped onto standardized coronal sections of the rat brain (Paxinos and Watson, 2007).
Statistical analyses
Analysis of performance in the set shifting task was calculated as described above in Experiment 1. Comparisons between drug groups were conducted using one-way analysis of variance (ANOVA) and LSD post-hoc tests when warranted.
RESULTS
Experiment 1: Relationships between GABA(B) receptor expression and set shifting performance in aged rats
Behavioral flexibility is impaired in aged F344 rats
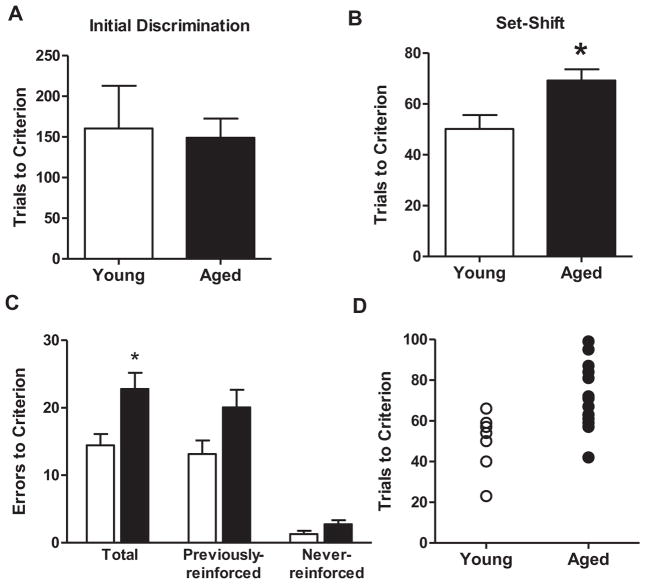
In order to link age-related changes in GABA(B) receptor protein expression to mPFC-dependent behavioral flexibility, young adult and aged rats were characterized on a set shifting task (task schematic shown in Fig. 1). All rats in the young and aged groups reached criterion performance on both the initial (visual cue) and set shift (left/right) discrimination problems. Performance of young and aged rats is shown in Fig. 2. Consistent with previous findings from our lab and others (Barense et al., 2002; Beas et al., 2013), independent samples t-tests indicated that young adult and aged rats required comparable numbers of trials to reach criterion performance on the initial (visual cue) discrimination (t(20)=0.23, p=0.81, Fig. 2A). In contrast, aged rats were impaired relative to young on the set shift (left/right discrimination), requiring significantly more trials and errors to reach criterion performance (t(20)=2.52, p<0.05, Fig. 2B; t(20)=2.24, p<0.05; Fig. 2C). A two-factor repeated measures ANOVA (age X error type) revealed main effects of age (such that aged rats made more errors than young; F(1,20)=5.05, p<0.05) and error type (such that rats made more previously reinforced than never-reinforced errors; F(1,20)=38.29, p<0.05), but no interaction between the two variables (Fig. 2C). Planned t-test comparisons between error types and age groups showed that both young and aged rats made significantly more previously reinforced than never-reinforced errors (paired t-tests; young, t(6)=4.95, p<0.05; aged, t(14)=5.79, p<0.05), but that neither previous-reinforced nor never-reinforced errors differed significantly by age (previously-reinforced, t(20)=1.67, p=0.11; never-reinforced, t(20)=1.50, p=0.14). Fig. 2D shows individual performance (TTC) in the set shifting task. Some aged rats performed within the range of young adults whereas others fell well outside this range, demonstrating impairment. Additional analyses (Table 1) revealed no differences between young and aged rats in the number of omitted trials (t(20)=0.14, p=0.88), mean response latencies (t(20)=1.08, p=0.29), or baseline locomotor activity (t(20)=0.91, p=0.92).
Fig. 2.
Performance of young and aged rats on the set shifting task. (A) Bar graph shows that young and aged rats’ performance was comparable on the initial (visual cue) discrimination, with young and aged rats requiring similar numbers of trials to reach criterion performance. (B) In contrast to initial discrimination learning, aged rats required significantly more trials than young to reach criterion performance on the set shift (left/right) discrimination. (C) Aged rats also made significantly more errors than young before reaching criterion performance on the set shift. In both young and aged rats, errors corresponded primarily to contingencies that had been reinforced previously (during the initial discrimination). Data are expressed as mean+SEM. *p<0.05.
Table 1.
Trial omissions, locomotor activity, and response latencies in the set shifting task for Experiments 1 and 2.
| Trial omissions | Locomotor activity | Response latency | |
|---|---|---|---|
| Experiment 1 | |||
| Young | 1.57 (0.97) | 6.09 (3.99) | 1.36 (0.34) |
| Aged | 1.73 (0.62) | 3.12 (0.48) | 1.39 (0.18) |
| Experiment 2 | |||
| Vehicle | 0.50 (0.26) | 4.33 (1.81) | 1.02 (0.12) |
| Baclofen (0.5 nmol) | 0.17 (0.17) | 6.54 (2.54) | 1.19 (0.17) |
| Baclofen (1.5 nmol) | 1.43 (0.30) | 4.60 (1.41) | 1.29 (0.15) |
Values represent means (SEMs).
GABA(B) receptor protein expression in mPFC and set shifting performance
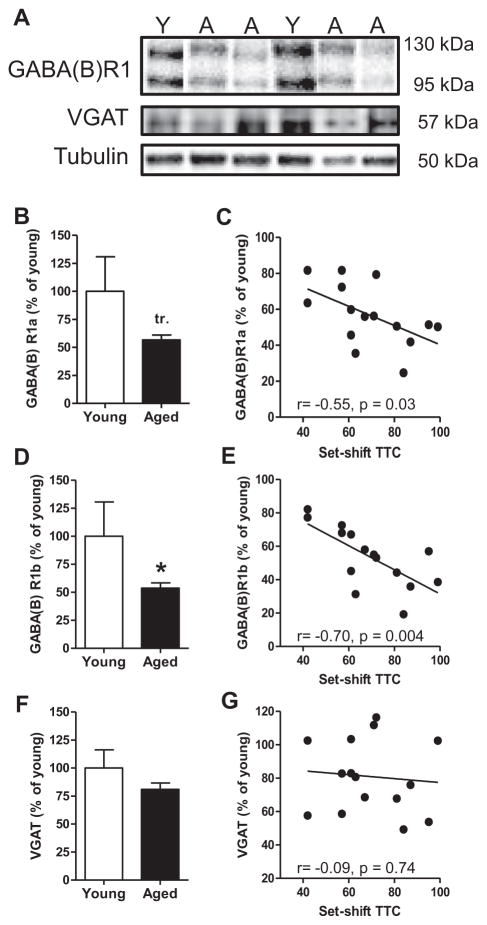
Previous work has shown that performance in the set shifting task critically depends on mPFC (Ragozzino, 2002; Floresco et al., 2008). Fig. 3A shows representative immunoreactive bands from young and aged mPFC samples when incubated with antibodies to the GABA(B)R1 subunit, VGAT, or β-tubulin. In the mPFC, two distinct GABA(B)R1-immunoreactive bands were detected (130 kDa and 95 kDa), corresponding to the two different isoforms of this subunit, GABA(B)R1a and GABA(B)R1b, respectively. The GABA(B)R1a isoform contains a pair of short consensus repeats at the N-terminal that traffic the GABA(B)R1a-containing receptor complex to presynaptic terminals where these receptors modulate neurotransmitter release. GABA(B)R1b lacks this N-terminal extension and is primarily localized to dendrites where it mediates postsynaptic inhibition (Kaupmann et al., 1998; Bettler et al., 2004; Biermann et al., 2010). Given the distinct localization and function of these R1 isoforms, R1a and R1b were analyzed separately. In agreement with previous work from our lab (McQuail et al., 2012; Baņuelos et al., 2014), expression of both R1 isoforms was reduced in aged compared to young mPFC (Figs. 3B, D). Although the magnitude of the age-related reduction was similar for both R1a and R1b, the reduction in R1a did not quite reach statistical significance in this cohort (R1a: −43%, t(20)=2.01, p=.05; R1b: −46%, t(20)=2.15, p<0.05). As shown in Fig. 3 and Table 2, lower expression of both GABA(B) R1a and GABA(B)R1b isoforms was significantly associated with a greater number of trials to reach criterion performance on the set shift phase of the task (R1a: r=−0.55, p=0.03; Fig. 3C; R1b: r=−0.70, p=0.004; Fig. 3E). Expression of R1b also predicted total (r=−0.66, p=0.008) and previously reinforced (r=−0.65, p=0.009) but not never-reinforced (r=0.24, p=0.40) errors to criterion on the set shift. One possibility is that these reductions in GABA(B)R subunits reflect an overt loss of inhibitory synapses in mPFC with aging. To explore this possibility, expression of the vesicular transporter for GABA (VGAT) was evaluated in the same mPFC homogenates from young and aged rats. As shown in Fig. 3F, VGAT expression in mPFC did not differ between young and aged rats (VGAT: t(20)=1.38, p=0.18), nor was there a significant relationship between mPFC VGAT expression and set shifting performance among aged rats (TTC: r=−0.09, p=0.74; total errors: r=0.04, p=0.90; previously reinforced errors: r=0.10, p=0.73; never-reinforced errors: r=−0.28, p=0.32; Table 2).
Fig. 3.
Age-related changes in GABA(B) receptor protein expression and relationship to performance in the set shifting task. (A) Representative immunoreactive bands from young and aged mPFC homogenates following incubation with antibodies to GABA(B)R1, VGAT, and loading control β-tubulin. (B) Bar graph shows GABA(B) R1a expression in young and aged mPFC. While expression was numerically reduced in the aged mPFC, this reduction approached but did not reach significance (p=0.05). (C) Among aged rats, expression of GABA(B)R1a was significantly associated with performance in the set shifting task such that that lower expression was associated with worse behavioral flexibility (more TTC). (D) Bar graph shows that GABA(B)R1b expression was significantly reduced in the aged compared to the young mPFC. (E) Among aged rats, expression of GABA(B)R1b was significantly associated with set shifting performance, such that lower expression robustly predicted worse behavioral flexibility. (F) Bar graph shows that VGAT expression in mPFC did not differ between young and aged rats. (G) Among aged rats, expression of VGAT was not associated with performance in the set shifting task. Data are expressed as mean+SEM. tr. p=0.05, *p<0.05.
Table 2.
Relationships between set shifting performance measures and protein expression in Experiment 1.
| GABA(B)R1a | GABA(B)R1b | VGAT | |
|---|---|---|---|
| Trials to criterion | r = −0.55, p = 0.03 | r = −0.70, p = 0.004 | r=−0.09, p=0.74 |
| Total errors to criterion | r=−0.49, p=0.06 | r = −0.66, p = 0.008 | r=0.04, p=0.90 |
| Previously reinforced errors | r=−0.42, p=0.12 | r = −0.65, p = 0.009 | r=0.10, p=0.73 |
| Never-reinforced errors | r=−0.08, p=0.78 | r=0.24, p=0.40 | r=−0.28, p=0.32 |
Significant relationships (p<0.05) are noted in bold and italicized text.
Experiment 2: Effects of medial prefrontal cortex GABA(B) receptor activation on set shifting in aged rats
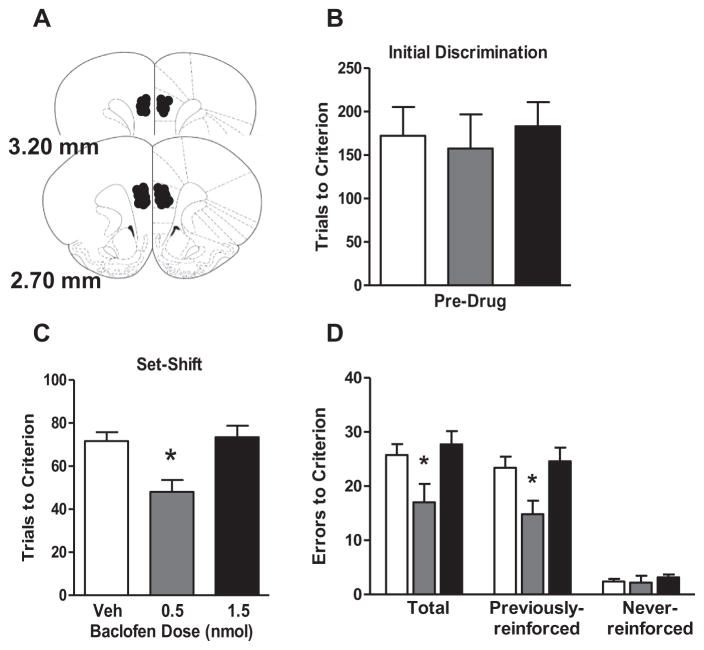
Experiment 1 shows that GABA(B)R expression is reduced in aged mPFC, and that greater age-related reductions in both GABA(B)R1a and GABA(B)R1b expression are significantly associated with worse set shifting performance among aged rats. If this reduction in GABA(B)R expression is critical for set shifting performance deficits, it would be expected that direct stimulation of mPFC GABA(B) receptor activity should facilitate set shifting in aged rats. To test this hypothesis, aged rats were implanted with guide cannulae that permitted direct drug delivery to the mPFC. The cannulae and microinjection volume targeted both prelimbic and infralimbic subregions of the mPFC (Fig. 4A) and n=4 rats were excluded because cannulae were located outside these subregions, leaving a total of n=8 rats in the vehicle group, n=6 rats in the 0.5 nmol group, and n=7 rats in the 1.5 nmol group. The number of trials needed to reach criterion on the visual cue discrimination (performed prior to drug administration) did not differ among these groups (F(2,18)=0.14, p=0.87; Fig. 4B). In contrast, performance on the set shift (left/right discrimination), following vehicle or baclofen administration differed significantly among drug groups on both trials (Fig. 4C; F(2,18)=7.60, p<0.05) and errors (Fig. 4D; F(2,18)=4.49, p<.05) to criterion measures. Post-hoc comparisons showed that the 0.5 nmol dose of baclofen significantly enhanced performance compared to both vehicle and the 1.5 nmol dose (ps<0.05). Error analysis revealed a main effect of drug on previously reinforced errors (F(2,18)=4.60, p<.05), with the 0.5 nmol baclofen group having significantly fewer previously reinforced errors than the vehicle or 1.5 nmol groups (ps<0.05). In contrast, there were no differences between drug groups in the number of never-reinforced errors (F(2,18)=0.42, p=0.65). These results show that stimulation of GABAergic signaling through mPFC GABA(B) receptors can facilitate set shifting performance in aged rats. Analyses of additional task measures (Table 1) revealed that intra-mPFC baclofen did not significantly influence response latencies (F(2,18)=0.89, p=0.43) or locomotor activity (F(2,18)=0.37, p=0.70). There was a main effect of drug condition on trial omissions (F(2,18)=6.04, p=0.01), with the 1.5 nmol group making significantly more omissions than the vehicle control group (p<0.05). The magnitude of this effect was numerically very small, however, amounting to <1 omitted trial difference between the 1.5 nmol and vehicle groups.
Fig. 4.
Intra-mPFC baclofen administration enhanced set shifting performance in aged rats. (A) Schematic shows bilateral cannula placements in mPFC for each rat included in this experiment (illustrations adapted from Paxinos and Watson (2007)). (B) Bar graph shows that aged rats’ performance on the initial (visual cue) discrimination prior to baclofen administration did not differ between drug conditions. (C) Intra-mPFC baclofen significantly enhanced set shifting performance in aged rats, with rats receiving intra-mPFC infusion of 0.5-nmol baclofen requiring significantly fewer trials to reach criterion performance on the set shift in comparison to the rats receiving vehicle. (D) Rats receiving intra-mPFC infusion of 0.5-nmol baclofen also made significantly fewer errors before reaching criterion performance on the set shift compared to those receiving vehicle. Error analysis revealed that rats in the 0–5 nmol condition made fewer previously reinforced errors than rats in the vehicle condition, but did not differ from the vehicle condition in the number of never reinforced errors. Data are expressed as mean+SEM. *p<0.05 compared to vehicle.
DISCUSSION
The ability to flexibly adapt behavior in response to changing environmental contingencies can be compromised in aged individuals. In the current study, a rat model was employed to demonstrate an important role for mPFC GABA(B) receptors in such age-related impairments. GABA(B) receptor expression was significantly reduced in the aged rat mPFC, with greater reductions associated with worse performance on a set shifting task. Consistent with these findings, set shifting performance in aged rats was enhanced by intra-mPFC administration of the GABA(B) receptor agonist baclofen.
Studies in rodents, non-human primates, and humans show that performance on set shifting tasks is impaired in aged compared to young subjects (Barense et al., 2002; Bizon et al., 2012; Nieves-Martinez et al., 2012; Beas et al., 2013). Uniformly, such age-associated deficits are evident only when subjects are expected to adapt behavior following a rule shift but not during simple discrimination learning that does not require such flexibility (Robbins et al., 1998; Volkow et al., 1998; Barense et al., 2002; Moore et al., 2003, 2006; Ashendorf and McCaffrey, 2008; Nieves-Martinez et al., 2012; Beas et al., 2013). The present findings are consistent with this prior body of work, in that aged rats performed no differently than young on an initial (visual cue) discrimination, but were impaired in their acquisition of a subsequent left/right discrimination that required a shift in behavioral strategy. A potential limitation of the design employed here is that all rats were shifted from visual cue to left/right discrimination. This design was necessary for several reasons, most notably because even in the absence of a strategy shift, young Fischer 344 rats differ significantly in their ability to acquire visual cue and left/right discriminations (Beas et al., under review). As such, a counterbalanced design results in a non-linear distribution of values for TTC that makes attempts to relate neurobiological markers to behavioral performance challenging. For many reasons, it is likely that the agerelated impairments reported in Experiment 1 are reflective of a response strategy “shift” and not simply an age-related impairment in left/right discrimination. First, a prior study employing a different set shifting task design showed that aged rats were selectively impaired on set shifting (but not initial discrimination learning), regardless of the direction of the shift (Barense et al., 2002). Second, numerous studies (including those using the same task design employed here) have shown that the rodent mPFC plays the same critical role in set shifting, regardless of the direction of the shift (Ragozzino et al., 1999; Birrell and Brown, 2000; Ragozzino, 2007; Floresco et al., 2008; Bissonette and Powell, 2012; Bissonette et al., 2013), and that mPFC manipulations do not influence left/right discrimination performance if this discrimination is conducted in the absence of a shift (Floresco et al., 2008). Third, following the set shift, aged (as well as young) rats committed significantly more errors of the previously reinforced type compared to the never-reinforced type, indicating perseveration on the visual cue discrimination strategy. If the impaired performance in aged rats simply reflected a deficit in left/right discrimination abilities, equivalent numbers of previously- and never-reinforced errors would be expected. Finally, we recently showed that systemic administration of baclofen enhances performance in young rats on the same set shifting task used here, under conditions in which rats were shifted either from the visual cue to left/right discrimination or using the reversed design (from the left/right to the visual cue discrimination; Beas et al., under review). Additional control experiments in that study demonstrated that these enhancing effects of baclofen in young rats were not recapitulated when the left/right discrimination learning was not preceded by a visual cue discrimination (i.e., when no rule shift was required). Together, these findings strongly indicate that the age-related impairments described in the current study reflect an inability to shift behavior as adeptly as young rats, and that baclofen administered into the mPFC facilitates behavioral flexibility.
Findings from both neuropsychiatric disease and rodent models strongly implicate PFC GABAergic signaling in behavioral flexibility (Hashimoto et al., 2008; Brady, 2009; Maldonado-Aviles et al., 2009; Gruber et al., 2010; Enomoto et al., 2011; Bissonette et al., 2014; Cho et al., 2015). Changes in PFC GABAergic signaling in normal aging are evidenced by reductions in both GABAergic interneurons and inhibitory synapses (Grachev and Apkarian, 2001; Smith et al., 2004; Peters et al., 2008; Dumitriu et al., 2010; Soghomonian et al., 2010; Stranahan et al., 2012). Such structural changes suggest a shift in the normal balance of excitation and inhibition in this brain region that could be detrimental to cognitive capacities (Luebke et al., 2004; Stranahan et al., 2012; Bories et al., 2013; Baņuelos et al., 2014; McQuail et al., 2015). Indeed, cortical hyperexcitability has been suggested to underlie cognitive dysfunction across a variety of psychiatric and neurological diseases in which perseverative behaviors are prominent, including schizophrenia, Alzheimer’s disease, and autism (Volk and Lewis, 2002; Lewis et al., 2004; Hashimoto et al., 2008; Maldonado-Aviles et al., 2009; Enticott et al., 2010; Gonzalez-Burgos et al., 2011; Kellner et al., 2014; Silverman et al., 2015; Siwek et al., 2015). Consistent with reduced inhibition in the aged PFC, our laboratory has previously reported a marked decline in GABA(B) receptor expression in the aged mPFC (McQuail et al., 2012; Baņuelos et al., 2014). This age-related decline was not evident in the hippocampus, which is a critical locus for spatial learning that can become compromised in aging (McQuail et al., 2012). The rats in this earlier study were evaluated for spatial learning in the Morris water maze prior to assessment of GABA(B) receptor expression. Consistent with evidence that the PFC is not critical for this behavioral task, there was no relationship between GABA(B) receptor reductions and water maze performance among aged rats.
The current study extends this prior work by demonstrating that reduced mPFC GABA(B) receptor expression is correlated with set shifting performance among aged rats, such that lower GABA(B) receptor expression is associated with worse performance. It is notable that this relationship with set shifting was strongest with the GABA(B)R1b subunit, which is the isoform of the GABA(B) receptor that traffics the receptor to the dendrites of postsynaptic neurons. GABA(B) receptors on pyramidal neuron dendrites are largely localized extrasynaptically (Vigot et al., 2006; Biermann et al., 2010; Pinard et al., 2010) and signaling via these receptors contributes to tonic inhibition of PFC pyramidal neurons (Wang et al., 2010). Thus, the reductions in GABA(B)R1b likely confer increased pyramidal neuron excitability and highlight cortical hyperexcitability in aging as a potentially important mediator of impaired behavioral flexibility. The fact that set shifting performance in aged rats was enhanced by administration of baclofen directly into mPFC (Fig. 4) is consistent with this interpretation. Emerging evidence suggests, however, that the role of PFC GABAergic neurotransmission in behavioral flexibility is not limited to GABA(B) receptor signaling. Intra-mPFC administration of GABA(A) receptor antagonists in rats impairs set shifting, suggesting that signaling through this receptor is critical for flexible behavior (Enomoto et al., 2011; Tse et al., 2015). It will be of interest in future work to determine the extent to which GABA(A) receptors are altered in the aged PFC, and whether targeting these receptors is an effective strategy for reversing flexibility deficits in aged subjects.
The relationship between GABA(B)R1b and set shifting was not mirrored by a second marker of GABAergic synapses, VGAT, the transporter that packages GABA into vesicles in presynaptic inhibitory terminals. This latter finding suggests that the relationships between GABA(B) receptors and set shifting do not simply reflect a robust loss of inhibitory terminals. It should certainly be acknowledged that the biochemical methods employed here are likely insufficiently sensitive for detecting reductions in PFC inhibitory synapse number that have been documented using electron microscopy (Smith et al., 2004; Peters et al., 2008; Dumitriu et al., 2010; Soghomonian et al., 2010). In fact, it is possible that a loss of some inhibitory terminals may elicit compensatory changes to GABA synthesizing enzymes and signaling proteins that facilitate inhibitory neurotransmission at remaining inhibitory terminals in the aged PFC (Luebke et al., 2004; Bories et al., 2013; Baņuelos et al., 2014; McQuail et al., 2015). The degree of compensation may vary among aged individuals, ranging from effective adaptation that preserves cognitive flexibility to insufficient or no compensation that produces cognitive rigidity. One interpretation from the current study is that GABA(B) receptors are a possible mediator of this compensatory process. Future work employing quantitative anatomical methodologies that enable immunolocalization of GABA(B) receptors to young and aged synapses would offer additional insight into this possibility.
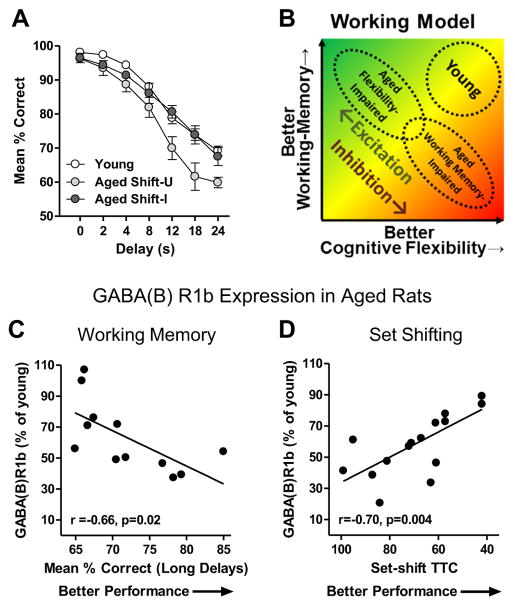
Behavioral flexibility in the context of set shifting is only one aspect of PFC function that is vulnerable to decline in aging (Glisky, 2007; Alexander et al., 2012; Bizon et al., 2012; Beas et al., 2013; Baņuelos et al., 2014). In addition, aged subjects across species (including Fischer 344 rats) show impaired working memory as assessed by delayed response tasks (Arnsten et al., 1994; Robbins et al., 1998; Holtzer et al., 2004; Dumitriu et al., 2010; Beas et al., 2013). In these tasks, subjects are required to remember the location of a response lever over a relatively short delay interval (<30 s) in order to earn a food reward. While performance on both delayed response and set shifting tasks critically depends upon the mPFC (Mishkin, 1957; Goldman and Rosvold, 1970; Freedman and Oscar-Berman, 1986; Floresco et al., 1997; Ragozzino et al., 1998), a previous study examining individual performance of aged rats cross-characterized on both tasks revealed a surprising inverse relationship between the two (Beas et al., 2013). As reproduced in Fig. 5A, this prior study showed that those aged rats with impaired set shifting performance demonstrated delayed response performance on par with young adult subjects. Conversely, aged rats with impaired delayed response performance, particularly at long delay intervals, showed intact behavioral flexibility as assessed on the set shifting task. The inverse relationship between these two aspects of executive function suggests that the neurobiological changes in the mPFC that adversely affect behavioral flexibility may enable preserved working memory, and vice versa.
Fig. 5.
GABAergic signaling may mediate an inverse relationship between behavioral flexibility and working memory in aged rats. (A) Line graph shows performance of young and aged rats cross-characterized on both the set shifting task used in the current study and a delayed response task that assesses working memory (Beas et al., 2013). In this prior study, aged rats that were unimpaired relative to young on the set shifting task (Shift-U) showed impaired performance relative to young on the delayed response task. In contrast, aged rats that were impaired on the set shifting task (Shift-I) showed intact performance on the delayed response task. (B) Working model of potential relationships between PFC excitatory/inhibitory signaling and PFC-supported executive functions that could account for the inverse relationship between set shifting and delayed response performance in aged rats. See text for detailed explanation. (C) Scatter plot reproduced from Baņuelos et al. (2014) showing the negative correlation between performance on a mPFC-dependent delayed response working memory task and mPFC GABA(B)R1b expression in aged rats. (D) Data from Fig. 3E plotted with the X-axis reversed to illustrate the positive correlation between set shifting performance and mPFC GABA(B)R1b expression.
Together with prior findings (Baņuelos et al., 2014), the data presented here support this hypothesis. In agreement with the current study, Baņuelos et al. (2014) showed that expression of GABA(B) receptor subunits is reduced in the aged rat mPFC relative to young. Notably, however, aged rats in the Baņuelos et al. study were characterized on the delayed response task, and expression of GABA(B)R1b in the aged mPFC was inversely related to performance such that lower GABA(B)R1b expression was associated with better delayed response performance (data reproduced in Fig. 5C). This relationship is opposite that shown in the current study, in which lower GABA(B)R1b in the aged mPFC was associated with worse set shifting performance (Fig. 5D). Consistent with the opposite directions of the relationships between GABA(B)R1b expression and performance on these two mPFC-dependent tasks in aged rats, intra-mPFC administration of a GABA(B) receptor antagonist enhances accuracy of aged rats on the delayed response task (Baņuelos et al., 2014), whereas intra-mPFC administration of the GABA(B) receptor agonist baclofen enhances aged rats’ performance on the set shifting task. Fig. 5B shows a working model that is based on these collective findings and illustrates how age-associated shifts in the normal balance of excitation and inhibition within the PFC influences distinct executive functions. Young rats are proposed to have a balance between excitation and inhibition in the PFC that allows good performance on tests of both working memory and behavioral flexibility. In aging, GABAergic signaling in PFC becomes dysregulated such that net cortical inhibition is increased relative to young in some aged subjects and decreased in others. We posit that these divergent shifts in cortical inhibition have distinct consequences for executive function. Specifically, reduced cortical inhibition and a shift toward greater excitation (shown in green) contributes to impaired behavioral flexibility (“aged flexibility-impaired”) in aged rats but does not negatively impact working memory. In contrast, increased cortical inhibition (shown in red) contributes to impaired working memory in aged rats (“aged working memory-impaired”), but behavioral flexibility remains relatively preserved.
It is important to note that set shifting is not the only form of behavioral flexibility that can become compromised with age. Aged rats and primates are also impaired when the reward contingencies associated with two stimuli are reversed (e.g., A+, B− is reversed to A−, B+; Bartus et al., 1979; Lai et al., 1995; Dias et al., 1996; Schoenbaum et al., 2002a,b). While reversal learning and set shifting depend upon distinct subregions of the PFC (orbitofrontal and medial PFC, respectively; Dias et al., 1996; Birrell and Brown, 2000; McAlonan and Brown, 2003), a prior study found a nearly significant relationship (p=0.06) between age-associated impairments in the two types of behavioral flexibility (Barense et al., 2002). Moreover, similar to the set shifting impairments produced by GABAergic disruptions in mPFC (Enomoto et al., 2011), manipulations of GABAergic signaling within the orbitofrontal cortex can impair reversal learning (Bissonette et al., 2010). Together, these data suggest that age-associated alterations in GABAergic circuits across PFC subregions could contribute to general deficits in behavioral flexibility, encompassing both set shifting and reversal learning. It will be of significant interest in future studies to determine the status of GABA(B) receptor signaling in aged orbitofrontal cortex and, in particular, whether baclofen can exert broadly enhancing effects across different forms of impaired behavioral flexibility.
The present study replicates previous findings demonstrating reduced GABA(B) receptor expression in mPFC during normal aging. This reduction is strongly related to set shifting abilities such that greater expression is associated with better preservation of behavioral flexibility in aged rats. Moreover, mPFC GABA(B) receptor stimulation enhances set shifting in aged rats. These findings further our understanding of behavioral consequences of altered excitatory–inhibitory dynamics within the aged PFC and highlight the role of cortical inhibition in modulating behavioral flexibility. Lastly, these findings suggest GABA(B) receptors as a potential therapeutic target for improving cognitive functions supported by the PFC.
Acknowledgments
We thank Ms. Kailey Simpson, Ms. Shannon Wall, Ms. Miranda Schwabe, Ms. Bonnie McLaurin, and Ms. Lauren Vetere for assistance with surgical procedures and behavioral testing. This work was supported by NIH grant R01AG029421 (J.L.B.), the McKnight Brain Research Foundation (J.L.B.), the NSF Graduate Research Fellowship Program DGE-0802270 (B.S.B.) and a Diversity Supplement to NIH grant R01AG029421 (B.S.B.).
Abbreviations
- ANOVA
analysis of variance
- EDTA
ethylenediaminetetraacetic acid
- EGTA
ethylene glycol-bis(2-aminoethyle ther)-N,N,N′,N′-tetraacetic acid
- GABA
gamma-aminobutyric acid
- GABA(B)R
GABA(B) receptor
- HEPES
4-(2-hydroxyethyl)piperazine-1-ethanesulfonic-acid,N-(2-hydroxyethyl)piperazine-N′(2ethanesulfonic acid)
- ITI
inter-trial interval
- mPFC
medial prefrontal cortex
- PBS
phosphate-buffered saline
- PFA
paraformaldehyde
- PFC
prefrontal cortex
- TBS
Tris-buffered saline
- TTC
trials to criteria
References
- Alexander GE, Ryan L, Bowers D, Foster TC, Bizon JL, Geldmacher DS, Glisky EL. Characterizing cognitive aging in humans with links to animal models. Front Aging Neurosci. 2012;4:21. doi: 10.3389/fnagi.2012.00021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnsten AF, Cai JX, Murphy BL, Goldman-Rakic PS. Dopamine D1 receptor mechanisms in the cognitive performance of young adult and aged monkeys. Psychopharmacology. 1994;116:143–151. doi: 10.1007/BF02245056. [DOI] [PubMed] [Google Scholar]
- Ashendorf L, McCaffrey RJ. Exploring age-related decline on the Wisconsin Card Sorting Test. Clin Neuropsychol. 2008;22:262–272. doi: 10.1080/13854040701218436. [DOI] [PubMed] [Google Scholar]
- Baņuelos C, Beas BS, McQuail JA, Gilbert RJ, Frazier CJ, Setlow B, Bizon JL. Prefrontal cortical GABAergic dysfunction contributes to age-related working memory impairment. J Neurosci. 2014;34:3457–3466. doi: 10.1523/JNEUROSCI.5192-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barense MD, Fox MT, Baxter MG. Aged rats are impaired on an attentional set-shifting task sensitive to medial frontal cortex damage in young rats. Learn Mem. 2002;9:191–201. doi: 10.1101/lm.48602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartus RT, Dean RL, 3rd, Fleming DL. Aging in the rhesus monkey: effects on visual discrimination learning and reversal learning. J Gerontol. 1979;34:209–219. doi: 10.1093/geronj/34.2.209. [DOI] [PubMed] [Google Scholar]
- Beas BS, Setlow B, Bizon JL. Effects of acute administration of the GABA(B) receptor agonist baclofen on behavioral flexibility in rats. doi: 10.1007/s00213-016-4321-y. (under review) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beas BS, Setlow B, Bizon JL. Distinct manifestations of executive dysfunction in aged rats. Neurobiol Aging. 2013;34:2164–2174. doi: 10.1016/j.neurobiolaging.2013.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bettler B, Kaupmann K, Mosbacher J, Gassmann M. Molecular structure and physiological functions of GABA(B) receptors. Physiol Rev. 2004;84:835–867. doi: 10.1152/physrev.00036.2003. [DOI] [PubMed] [Google Scholar]
- Biermann B, Ivankova-Susankova K, Bradaia A, Abdel Aziz S, Besseyrias V, Kapfhammer JP, Missler M, Gassmann M, Bettler B. The Sushi domains of GABAB receptors function as axonal targeting signals. J Neurosci. 2010;30:1385–1394. doi: 10.1523/JNEUROSCI.3172-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birrell JM, Brown VJ. Medial frontal cortex mediates perceptual attentional set shifting in the rat. J Neurosci. 2000;20:4320–4324. doi: 10.1523/JNEUROSCI.20-11-04320.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bissonette GB, Powell EM. Reversal learning and attentional set-shifting in mice. Neuropharmacology. 2012;62:1168–1174. doi: 10.1016/j.neuropharm.2011.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bissonette GB, Bae MH, Suresh T, Jaffe DE, Powell EM. Astrocyte-mediated hepatocyte growth factor/scatter factor supplementation restores GABAergic interneurons and corrects reversal learning deficits in mice. J Neurosci. 2010;30:2918–2923. doi: 10.1523/JNEUROSCI.5268-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bissonette GB, Lande MD, Martins GJ, Powell EM. Versatility of the mouse reversal/set-shifting test: effects of topiramate and sex. Physiol Behav. 2012;107:781–786. doi: 10.1016/j.physbeh.2012.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bissonette GB, Powell EM, Roesch MR. Neural structures underlying set-shifting: roles of medial prefrontal cortex and anterior cingulate cortex. Behav Brain Res. 2013;250:91–101. doi: 10.1016/j.bbr.2013.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bissonette GB, Bae MH, Suresh T, Jaffe DE, Powell EM. Prefrontal cognitive deficits in mice with altered cerebral cortical GABAergic interneurons. Behav Brain Res. 2014;259:143–151. doi: 10.1016/j.bbr.2013.10.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bizon JL, Foster TC, Alexander GE, Glisky EL. Characterizing cognitive aging of working memory and executive function in animal models. Front Aging Neurosci. 2012;4:19. doi: 10.3389/fnagi.2012.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bories C, Husson Z, Guitton MJ, De Koninck Y. Differential balance of prefrontal synaptic activity in successful versus unsuccessful cognitive aging. J Neurosci. 2013;33:1344–1356. doi: 10.1523/JNEUROSCI.3258-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brady AM. Neonatal ventral hippocampal lesions disrupt set-shifting ability in adult rats. Behav Brain Res. 2009;205:294–298. doi: 10.1016/j.bbr.2009.07.025. [DOI] [PubMed] [Google Scholar]
- Brunso-Bechtold JK, Linville MC, Sonntag WE. Age-related synaptic changes in sensorimotor cortex of the Brown Norway X fischer 344 rat. Brain Res. 2000;872:125–133. doi: 10.1016/s0006-8993(00)02515-4. [DOI] [PubMed] [Google Scholar]
- Cho KK, Hoch R, Lee AT, Patel T, Rubenstein JL, Sohal VS. Gamma rhythms link prefrontal interneuron dysfunction with cognitive inflexibility in Dlx5/6(+/-) mice. Neuron. 2015;85:1332–1343. doi: 10.1016/j.neuron.2015.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darrah JM, Stefani MR, Moghaddam B. Interaction of N-methyl-D-aspartate and group 5 metabotropic glutamate receptors on behavioral flexibility using a novel operant set-shift paradigm. Behav Pharmacol. 2008;19:225–234. doi: 10.1097/FBP.0b013e3282feb0ac. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demakis GJ. A meta-analytic review of the sensitivity of the Wisconsin Card Sorting Test to frontal and lateralized frontal brain damage. Neuropsychology. 2003;17:255–264. doi: 10.1037/0894-4105.17.2.255. [DOI] [PubMed] [Google Scholar]
- Dias R, Robbins TW, Roberts AC. Dissociation in prefrontal cortex of affective and attentional shifts. Nature. 1996;380:69–72. doi: 10.1038/380069a0. [DOI] [PubMed] [Google Scholar]
- Dumitriu D, Hao J, Hara Y, Kaufmann J, Janssen WG, Lou W, Rapp PR, Morrison JH. Selective changes in thin spine density and morphology in monkey prefrontal cortex correlate with aging-related cognitive impairment. J Neurosci. 2010;30:7507–7515. doi: 10.1523/JNEUROSCI.6410-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enomoto T, Tse MT, Floresco SB. Reducing prefrontal gamma-aminobutyric acid activity induces cognitive, behavioral, and dopaminergic abnormalities that resemble schizophrenia. Biol Psychiatry. 2011;69:432–441. doi: 10.1016/j.biopsych.2010.09.038. [DOI] [PubMed] [Google Scholar]
- Enticott PG, Rinehart NJ, Tonge BJ, Bradshaw JL, Fitzgerald PB. A preliminary transcranial magnetic stimulation study of cortical inhibition and excitability in high-functioning autism and Asperger disorder. Dev Med Child Neurol. 2010;52:e179–e183. doi: 10.1111/j.1469-8749.2010.03665.x. [DOI] [PubMed] [Google Scholar]
- Everett J, Lavoie K, Gagnon JF, Gosselin N. Performance of patients with schizophrenia on the Wisconsin Card Sorting Test (WCST) J Psychiatry Neurosci. 2001;26:123–130. [PMC free article] [PubMed] [Google Scholar]
- Floresco SB, Seamans JK, Phillips AG. Selective roles for hippocampal, prefrontal cortical, and ventral striatal circuits in radial-arm maze tasks with or without a delay. J Neurosci. 1997;17:1880–1890. doi: 10.1523/JNEUROSCI.17-05-01880.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floresco SB, Block AE, Tse MT. Inactivation of the medial prefrontal cortex of the rat impairs strategy set-shifting, but not reversal learning, using a novel, automated procedure. Behav Brain Res. 2008;190:85–96. doi: 10.1016/j.bbr.2008.02.008. [DOI] [PubMed] [Google Scholar]
- Freedman M, Oscar-Berman M. Bilateral frontal lobe disease and selective delayed response deficits in humans. Behav Neurosci. 1986;100:337–342. doi: 10.1037//0735-7044.100.3.337. [DOI] [PubMed] [Google Scholar]
- Glisky EL. Changes in cognitive function in human aging. In: Riddle DR, editor. Brain aging: models, methods, and mechanisms. Boca Raton (FL): 2007. [PubMed] [Google Scholar]
- Goldman PS, Rosvold HE. Localization of function within the dorsolateral prefrontal cortex of the rhesus monkey. Exp Neurol. 1970;27:291–304. doi: 10.1016/0014-4886(70)90222-0. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Burgos G, Fish KN, Lewis DA. GABA neuron alterations, cortical circuit dysfunction and cognitive deficits in schizophrenia. Neural Plast. 2011;2011:723184. doi: 10.1155/2011/723184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grachev ID, Apkarian AV. Aging alters regional multichemical profile of the human brain: an in vivo 1H-MRS study of young versus middle-aged subjects. J Neurochem. 2001;76:582–593. doi: 10.1046/j.1471-4159.2001.00026.x. [DOI] [PubMed] [Google Scholar]
- Gruber AJ, Calhoon GG, Shusterman I, Schoenbaum G, Roesch MR, O’Donnell P. More is less: a disinhibited prefrontal cortex impairs cognitive flexibility. J Neurosci. 2010;30:17102–17110. doi: 10.1523/JNEUROSCI.4623-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto T, Arion D, Unger T, Maldonado-Aviles JG, Morris HM, Volk DW, Mirnics K, Lewis DA. Alterations in GABA-related transcriptome in the dorsolateral prefrontal cortex of subjects with schizophrenia. Mol Psychiatry. 2008;13:147–161. doi: 10.1038/sj.mp.4002011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtzer R, Stern Y, Rakitin BC. Age-related differences in executive control of working memory. Mem Cognit. 2004;32:1333–1345. doi: 10.3758/bf03206324. [DOI] [PubMed] [Google Scholar]
- Homayoun H, Moghaddam B. NMDA receptor hypofunction produces opposite effects on prefrontal cortex interneurons and pyramidal neurons. J Neurosci. 2007;27:11496–11500. doi: 10.1523/JNEUROSCI.2213-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson LH, Kelly PH, Bettler B, Kaupmann K, Cryan JF. GABA(B(1)) receptor isoforms differentially mediate the acquisition and extinction of aversive taste memories. J Neurosci. 2006;26:8800–8803. doi: 10.1523/JNEUROSCI.2076-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaupmann K, Malitschek B, Schuler V, Heid J, Froestl W, Beck P, Mosbacher J, Bischoff S, Kulik A, Shigemoto R, Karschin A, Bettler B. GABA(B)-receptor subtypes assemble into functional heteromeric complexes. Nature. 1998;396:683–687. doi: 10.1038/25360. [DOI] [PubMed] [Google Scholar]
- Kellner V, Menkes-Caspi N, Beker S, Stern EA. Amyloid-beta alters ongoing neuronal activity and excitability in the frontal cortex. Neurobiol Aging. 2014;35:1982–1991. doi: 10.1016/j.neurobiolaging.2014.04.001. [DOI] [PubMed] [Google Scholar]
- Lai ZC, Moss MB, Killiany RJ, Rosene DL, Herndon JG. Executive system dysfunction in the aged monkey: spatial and object reversal learning. Neurobiol Aging. 1995;16:947–954. doi: 10.1016/0197-4580(95)02014-4. [DOI] [PubMed] [Google Scholar]
- Lewis DA, Volk DW, Hashimoto T. Selective alterations in prefrontal cortical GABA neurotransmission in schizophrenia: a novel target for the treatment of working memory dysfunction. Psychopharmacology. 2004;174:143–150. doi: 10.1007/s00213-003-1673-x. [DOI] [PubMed] [Google Scholar]
- Lewis DA, Curley AA, Glausier JR, Volk DW. Cortical parvalbumin interneurons and cognitive dysfunction in schizophrenia. Trends Neurosci. 2012;35:57–67. doi: 10.1016/j.tins.2011.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luebke JI, Chang YM, Moore TL, Rosene DL. Normal aging results in decreased synaptic excitation and increased synaptic inhibition of layer 2/3 pyramidal cells in the monkey prefrontal cortex. Neuroscience. 2004;125:277–288. doi: 10.1016/j.neuroscience.2004.01.035. [DOI] [PubMed] [Google Scholar]
- Maldonado-Aviles JG, Curley AA, Hashimoto T, Morrow AL, Ramsey AJ, O’Donnell P, Volk DW, Lewis DA. Altered markers of tonic inhibition in the dorsolateral prefrontal cortex of subjects with schizophrenia. Am J Psychiatry. 2009;166:450–459. doi: 10.1176/appi.ajp.2008.08101484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAlonan K, Brown VJ. Orbital prefrontal cortex mediates reversal learning and not attentional set shifting in the rat. Behav Brain Res. 2003;146:97–103. doi: 10.1016/j.bbr.2003.09.019. [DOI] [PubMed] [Google Scholar]
- McQuail JA, Baņuelos C, LaSarge CL, Nicolle MM, Bizon JL. GABA(B) receptor GTP-binding is decreased in the prefrontal cortex but not the hippocampus of aged rats. Neurobiol Aging. 2012;33(1124):e1121–1112. doi: 10.1016/j.neurobiolaging.2011.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McQuail JA, Frazier CJ, Bizon JL. Molecular aspects of age-related cognitive decline: the role of GABA signaling. Trends Mol Med. 2015;21:450–460. doi: 10.1016/j.molmed.2015.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishkin M. Effects of small frontal lesions on delayed alternation in monkeys. J Neurophysiol. 1957;20:615–622. doi: 10.1152/jn.1957.20.6.615. [DOI] [PubMed] [Google Scholar]
- Moore TL, Killiany RJ, Herndon JG, Rosene DL, Moss MB. Impairment in abstraction and set shifting in aged rhesus monkeys. Neurobiol Aging. 2003;24:125–134. doi: 10.1016/s0197-4580(02)00054-4. [DOI] [PubMed] [Google Scholar]
- Moore TL, Killiany RJ, Herndon JG, Rosene DL, Moss MB. Executive system dysfunction occurs as early as middle-age in the rhesus monkey. Neurobiol Aging. 2006;27:1484–1493. doi: 10.1016/j.neurobiolaging.2005.08.004. [DOI] [PubMed] [Google Scholar]
- Nieves-Martinez E, Haynes K, Childers SR, Sonntag WE, Nicolle MM. Muscarinic receptor/G-protein coupling is reduced in the dorsomedial striatum of cognitively impaired aged rats. Behav Brain Res. 2012;227:258–264. doi: 10.1016/j.bbr.2011.10.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Donnell P. Cortical disinhibition in the neonatal ventral hippocampal lesion model of schizophrenia: new vistas on possible therapeutic approaches. Pharmacol Ther. 2012;133:19–25. doi: 10.1016/j.pharmthera.2011.07.005. [DOI] [PubMed] [Google Scholar]
- Orsini CA, Trotta RT, Bizon JL, Setlow B. Dissociable roles for the basolateral amygdala and orbitofrontal cortex in decision-making under risk of punishment. J Neurosci. 2015;35:1368–1379. doi: 10.1523/JNEUROSCI.3586-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen AM, Roberts AC, Polkey CE, Sahakian BJ, Robbins TW. Extra-dimensional versus intra-dimensional set shifting performance following frontal lobe excisions, temporal lobe excisions or amygdalo-hippocampectomy in man. Neuropsychologia. 1991;29:993–1006. doi: 10.1016/0028-3932(91)90063-e. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. Amsterdam; Boston: Academic Press/Elsevier; 2007. [Google Scholar]
- Peters A, Sethares C, Luebke JI. Synapses are lost during aging in the primate prefrontal cortex. Neuroscience. 2008;152:970–981. doi: 10.1016/j.neuroscience.2007.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinard A, Seddik R, Bettler B. GABAB receptors: physiological functions and mechanisms of diversity. Adv Pharmacol. 2010;58:231–255. doi: 10.1016/S1054-3589(10)58010-4. [DOI] [PubMed] [Google Scholar]
- Placek K, Dippel WC, Jones S, Brady AM. Impairments in set-shifting but not reversal learning in the neonatal ventral hippocampal lesion model of schizophrenia: further evidence for medial prefrontal deficits. Behav Brain Res. 2013;256:405–413. doi: 10.1016/j.bbr.2013.08.034. [DOI] [PubMed] [Google Scholar]
- Poe BH, Linville C, Brunso-Bechtold J. Age-related decline of presumptive inhibitory synapses in the sensorimotor cortex as revealed by the physical disector. J Comp Neurol. 2001;439:65–72. doi: 10.1002/cne.1335. [DOI] [PubMed] [Google Scholar]
- Ragozzino ME. The effects of dopamine D(1) receptor blockade in the prelimbic-infralimbic areas on behavioral flexibility. Learn Mem. 2002;9:18–28. doi: 10.1101/lm.45802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragozzino ME. The contribution of the medial prefrontal cortex, orbitofrontal cortex, and dorsomedial striatum to behavioral flexibility. Ann N Y Acad Sci. 2007;1121:355–375. doi: 10.1196/annals.1401.013. [DOI] [PubMed] [Google Scholar]
- Ragozzino ME, Adams S, Kesner RP. Differential involvement of the dorsal anterior cingulate and prelimbic-infralimbic areas of the rodent prefrontal cortex in spatial working memory. Behav Neurosci. 1998;112:293–303. doi: 10.1037//0735-7044.112.2.293. [DOI] [PubMed] [Google Scholar]
- Ragozzino ME, Detrick S, Kesner RP. Involvement of the prelimbic-infralimbic areas of the rodent prefrontal cortex in behavioral flexibility for place and response learning. J Neurosci. 1999;19:4585–4594. doi: 10.1523/JNEUROSCI.19-11-04585.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragozzino ME, Ragozzino KE, Mizumori SJ, Kesner RP. Role of the dorsomedial striatum in behavioral flexibility for response and visual cue discrimination learning. Behav Neurosci. 2002;116:105–115. doi: 10.1037//0735-7044.116.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins TW, James M, Owen AM, Sahakian BJ, Lawrence AD, McInnes L, Rabbitt PM. A study of performance on tests from the CANTAB battery sensitive to frontal lobe dysfunction in a large sample of normal volunteers: implications for theories of executive functioning and cognitive aging. Cambridge Neuropsychological Test Automated Battery. J Int Neuropsychol Soc. 1998;4:474–490. doi: 10.1017/s1355617798455073. [DOI] [PubMed] [Google Scholar]
- Ryan RT, Bhardwaj SK, Tse YC, Srivastava LK, Wong TP. Opposing alterations in excitation and inhibition of layer 5 medial prefrontal cortex pyramidal neurons following neonatal ventral hippocampal lesion. Cereb Cortex. 2013;23:1198–1207. doi: 10.1093/cercor/bhs111. [DOI] [PubMed] [Google Scholar]
- Schoenbaum G, Nugent S, Saddoris MP, Gallagher M. Teaching old rats new tricks: age-related impairments in olfactory reversal learning. Neurobiol Aging. 2002a;23:555–564. doi: 10.1016/s0197-4580(01)00343-8. [DOI] [PubMed] [Google Scholar]
- Schoenbaum G, Nugent SL, Saddoris MP, Setlow B. Orbitofrontal lesions in rats impair reversal but not acquisition of go, no-go odor discriminations. NeuroReport. 2002b;13:885–890. doi: 10.1097/00001756-200205070-00030. [DOI] [PubMed] [Google Scholar]
- Silverman JL, Pride MC, Hayes JE, Puhger KR, Butler-Struben HM, Baker S, Crawley JN. GABAB receptor agonist r-baclofen reverses social deficits and reduces repetitive behavior in two mouse models of autism. Neuropsychopharmacology. 2015;40:2228–2239. doi: 10.1038/npp.2015.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siwek ME, Muller R, Henseler C, Trog A, Lundt A, Wormuth C, Broich K, Ehninger D, Weiergraber M, Papazoglou A. Altered theta oscillations and aberrant cortical excitatory activity in the 5XFAD model of Alzheimer’s disease. Neural Plast. 2015;2015:781731. doi: 10.1155/2015/781731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith DE, Rapp PR, McKay HM, Roberts JA, Tuszynski MH. Memory impairment in aged primates is associated with focal death of cortical neurons and atrophy of subcortical neurons. J Neurosci. 2004;24:4373–4381. doi: 10.1523/JNEUROSCI.4289-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soghomonian JJ, Sethares C, Peters A. Effects of age on axon terminals forming axosomatic and axodendritic inhibitory synapses in prefrontal cortex. Neuroscience. 2010;168:74–81. doi: 10.1016/j.neuroscience.2010.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefani MR, Moghaddam B. Systemic and prefrontal cortical NMDA receptor blockade differentially affect discrimination learning and set-shift ability in rats. Behav Neurosci. 2005;119:420–428. doi: 10.1037/0735-7044.119.2.420. [DOI] [PubMed] [Google Scholar]
- Steketee JD, Beyer CE. Injections of baclofen into the ventral medial prefrontal cortex block the initiation, but not the expression, of cocaine sensitization in rats. Psychopharmacology. 2005;180:352–358. doi: 10.1007/s00213-005-2149-y. [DOI] [PubMed] [Google Scholar]
- Stranahan AM, Jiam NT, Spiegel AM, Gallagher M. Aging reduces total neuron number in the dorsal component of the rodent prefrontal cortex. J Comp Neurol. 2012;520:1318–1326. doi: 10.1002/cne.22790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tse MT, Piantadosi PT, Floresco SB. Prefrontal cortical gamma-aminobutyric acid transmission and cognitive function: drawing links to schizophrenia from preclinical research. Biol Psychiatry. 2015;77:929–939. doi: 10.1016/j.biopsych.2014.09.007. [DOI] [PubMed] [Google Scholar]
- Uylings HB, Groenewegen HJ, Kolb B. Do rats have a prefrontal cortex? Behav Brain Res. 2003;146:3–17. doi: 10.1016/j.bbr.2003.09.028. [DOI] [PubMed] [Google Scholar]
- Vigot R, Barbieri S, Brauner-Osborne H, Turecek R, Shigemoto R, Zhang YP, Lujan R, Jacobson LH, Biermann B, Fritschy JM, Vacher CM, Muller M, Sansig G, Guetg N, Cryan JF, Kaupmann K, Gassmann M, Oertner TG, Bettler B. Differential compartmentalization and distinct functions of GABAB receptor variants. Neuron. 2006;50:589–601. doi: 10.1016/j.neuron.2006.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volk DW, Lewis DA. Impaired prefrontal inhibition in schizophrenia: relevance for cognitive dysfunction. Physiol Behav. 2002;77:501–505. doi: 10.1016/s0031-9384(02)00936-8. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Gur RC, Wang GJ, Fowler JS, Moberg PJ, Ding YS, Hitzemann R, Smith G, Logan J. Association between decline in brain dopamine activity with age and cognitive and motor impairment in healthy individuals. Am J Psychiatry. 1998;155:344–349. doi: 10.1176/ajp.155.3.344. [DOI] [PubMed] [Google Scholar]
- Wang Y, Neubauer FB, Luscher HR, Thurley K. GABAB receptor-dependent modulation of network activity in the rat prefrontal cortex in vitro. Eur J Neurosci. 2010;31:1582–1594. doi: 10.1111/j.1460-9568.2010.07191.x. [DOI] [PubMed] [Google Scholar]