Abstract
There is currently no available method to efficiently deliver proteins across the plasma membrane of photoreceptor or retinal pigment epithelium (RPE) cells in vivo. Thus, current clinical application of recombinant proteins in ophthalmology is limited to the use of proteins that perform their biological function extracellularly. The ability to traverse biological membranes would enable the mobilization of a significantly larger number of proteins with previously well characterized properties. Nucleolin is abundantly present on the surface of rapidly dividing cells including cancer cells. Surprisingly, nucleolin is also present on the surface of photoreceptor cell bodies. Here we investigated whether nucleolin can be utilized as a gateway for the delivery of proteins into retinal cells following intravitreal injection. AS1411 is a G-quartet aptamer capable of targeting nucleolin. Subsequent to intravitreal injection, fluorescently labeled AS1411 localized to various retinal cell types including the photoreceptors and RPE. AS1411 linked to streptavidin (a ∼50 kDa protein) via a biotin bridge enabled the uptake of Streptavidin into photoreceptors and RPE. AS1411-Streptavidin conjugate applied topically to the cornea allowed for uptake of the conjugate into the nucleus and cytoplasm of corneal endothelial cells. Clinical relevance of AS1411 as a delivery vehicle was strongly indicated by demonstration of the presence of cell surface nucleolin on the photoreceptors, inner neurons and ganglion cells of human retina. These data support exploration of AS1411 as a means of delivering therapeutic proteins to diseased retina.
Keywords: Aptamer, Retina, Cornea, Intravitreal, Topical, Nucleolin, Shuttle
1. Introduction
Delivery of biologically active proteins into retinal cells is hindered by the presence of multiple barriers. The current standard of care for delivery of proteins to the retina and retinal pigment epithelium (RPE) involves direct injection of the protein into the vitreous cavity (intravitreal injection) (Meyer et al., 2016). Because of the natural barriers associated with the plasma membrane, delivery of biologically active proteins to the retina is largely restricted to the use of molecules that function extracellularly, e.g. antibodies. Should efficient penetration of the plasma membrane by exogenously delivered proteins be possible, it would enable the mobilization of a large library of potentially therapeutic molecules including those that are only biologically active intracellularly. The impermeability of proteins by the plasma membrane can be overcome by the use of viral vectors for the delivery of genes coding for the exogenous protein (Thakur et al., 2014). However, transduction of photoreceptors or the RPE by a virus generally requires the vector to be injected into the subretinal space (Trapani et al., 2014; Carvalho and Vandenberghe, 2015) - a surgical procedure fraught with complications (Kim et al., 2014). Vector capsid modifications are currently being investigated to overcome this limitation and while much progress has been made, intravitreal delivery of viral vectors is generally not as efficient as subretinal delivery in the context of expressing exogenous proteins in photoreceptors and RPE (Trapani et al., 2014). Furthermore, recombinant viral vectors have some limitations including potential immunogenicity, limited transgene capacity and an inability to readily withdraw a therapy if necessitated by toxicity (Carvalho and Vandenberghe, 2015).
The current standard of care for the delivery of molecules to the cornea is via topical application. This procedure results in loss of the majority of the molecule via the lacrimal duct and leakage into systemic circulation (Rawas-Qalaji and Williams, 2012). Again, an efficient method of delivery of potentially therapeutic large molecules such as proteins to the corneal stroma in vivo is a currently unmet clinical need.
In order to address the above limitations, a variety of cell-penetrating proteins (CPPs) have been investigated as a means of delivering therapeutic biomolecules into ocular cells (Cashman et al., 2002, 2003; Kilic et al., 2004; Johnson et al., 2008, 2010; Read et al., 2010). These studies have had some success but efficient delivery of proteins to the photoreceptors or the RPE via intravitreal injection has thus far not been described. Moreover, as CPPs typically enter cells via endocytosis, much of their cargo becomes trapped and degraded in endosomes (Parnaste et al., 2015), resulting in limited bioavailability of the therapeutic molecule.
Nucleolin is an RNA and protein-binding protein involved in numerous cellular activities including rRNA maturation, ribosome assembly, mRNA metabolism, DNA replication and recombination (Tuteja and Tuteja, 1998). Nucleolin has also been implicated in many aspects of cell survival and proliferation (Tuteja and Tuteja, 1998). Nucleolin acts as a shuttle between the plasma membrane and the cytoplasm or the nucleus – a process occurring independently of the endosomes (Borer et al., 1989; Hovanessian et al., 2010). Although primarily a nuclear and cytoplasmic protein, elevated nucleolin has been observed on the cell membrane of mitotic cells, such as cancer cells (Hovanessian et al., 2010) and angiogenic endothelial cells (Hovanessian et al., 2000). Interestingly, cell surface nucleolin has also been observed on photoreceptors of both bovine and murine retina (Hollander et al., 1999; Conley and Naash, 2010), invoking the potential of cell surface nucleolin as a receptor for uptake of therapeutic molecules.
AS1411 is a G-quartet DNA aptamer that targets nucleolin (Bates et al., 2009). We have recently found that topical application of AS1411 can significantly reduce endothelial cell proliferation in the laser-induced model of choroidal neovascularization (Leaderer et al., 2015). In the present study, we investigate the presence of cell surface nucleolin, the target of AS1411, on cells of the murine, non-human primate and human retina. In addition, we describe the development of a platform technology utilizing AS1411 as a mode of delivering molecules, including fluorophore and exogenous protein to cells of the murine retina and cornea. Conjugation of AS1411 to fluorophore or streptavidin was used to determine the ability of AS1411 to deliver varying sizes of cargo to murine ocular cells in vivo. The proof of concept studies described herein indicate that surface nucleolin is present in murine, non-human primate and human retina and that AS1411 can successfully deliver both small and large molecules including protein to the retina and cornea in vivo.
2. Methods
2.1. Materials and reagents
AlexaFluor594 labeled AS1411 (FL-AS1411; 5′-FL-TTTGGTGGTGGTGGTTGTGGTGG TGGTGG-3′) and AlexaFluor594 labeled control oligonucleotide (FL-Control; 5′-FL-TTTATGT-CACCTCTATACGCCCCGGGCTG-3′) were synthesized by Invitrogen (Carlsbad, CA). All oligonucleotides were dissolved in nuclease free water. All other commercially available materials and reagents, including AlexaFluro594 labeled streptavidin (streptavidin594), were purchased from Invitrogen, unless otherwise noted. Ocular cryosections from Cynomolgus monkey were obtained from Covance Laboratories (Dedham, MA). Normal human retina paraffin sections were purchased from Abcam (Cambridge, MA; Ab4364).
2.2. Streptavidin conjugates
4 nmols of biotinylated oligonucleotide was added to 1 nmol of streptavidin594 and allowed to incubate with gentle rocking at room temperature for 1 h. The resulting conjugates were then dialyzed with PBS using 30 K Ultrafree spin columns. The resultant conjugates were visualized on a native polyacrylamide gel stained with SybrGold (S11494).
2.3. Cellular uptake and localization
MCF7a cells were maintained in DMEM/F12 media supplemented with 5% fetal bovine serum (FBS). 24 h following seeding on a 96-well plate, MCF7a cells were washed twice with PBS and treated with 100 ng, 250 ng, 500 ng, or 1000 ng of streptavidin594, control-streptavidin594 or AS1411-streptavidin594 conjugates diluted in DMEM/F12 supplemented with 2% FBS. Following a 1 h incubation at 37 °C, wells were washed 3 times with PBS and subsequently fixed with a 3.7% formaldehyde solution (Alfa Aesar; Ward Hill, MA).
2.4. AS1411-mediated delivery in vivo
Six to eight week old BALB/c males were purchased from Jackson Laboratories (Bar Harbor, ME, USA) and housed under a 12 h light/dark cycle, unless otherwise stated. Mice were cared for in accordance with federal, state and local regulations. Mice were anesthetized by intraperitoneal injection of 0.1 mg/g body weight Ketamine (Phoenix™, St Joseph, MO) and 0.01 mg/g body weight Xylazine (Lloyed, Shenandoah, Iowa), followed by topical application of 0.5% proparacaine hydrochloride (Akorn Inc., Lake Forest, IL, USA) to the cornea. Intravitreal injections were performed using a 32-gauge needle and a 5 µl glass syringe (Hamilton, Reno, NV, USA) using a transcleral approach proximal to the pars plana. A total volume of 1.5 µl containing 0.3 nmol of FL-AS1411 or FL-Control was injected for AS1411 localization experiments. For in vivo protein delivery, streptavidin594, Control-streptavidin594 or AS1411-streptavidin594 conjugate was administered via intravitreal injection (1.5 µg) or topical application (5 µg). At various time-points post-injection/topical application, mice were sacrificed by CO2 inhalation followed by cervical dislocation. Eyes were harvested, fixed in 4% paraformaldehyde, and dehydrated with a sucrose gradient. Frozen sections of retina and cornea were generated by embedding tissue in Optimal Cutting Temperature Compound (Sakura Finetek, Torrance, CA, USA) and sectioning at 12 µm using a Microm 550 Cryostat (Thermo Scientific, Rockford, IL, USA).
2.5. Immunohistochemistry
For nucleolin staining, fixed tissue sections and cell monolayers were incubated in 12% normal goat serum for 1 h followed by incubation with a 1:400 dilution of antibody against nucleolin (Abcam; ab22758) for 2.5 h at room temperature. Subsequent incubation with a Cy3-conjugated goat anti-rabbit antibody (1:200 dilution) for 1.5 h at room temperature was used for detection. Staining with Alexa Fluor488–conjugated Wheat Germ Agglutinin (WGA), a cell surface marker, was performed using a 1:200 dilution in PBS.
2.6. Imaging and statistics
Imaging was performed using an Olympus IX51 microscope equipped with a Retiga 2000r camera. Intensity of fluorescent signal was quantified from images using ImageJ software (National Institutes of Health; Bethesda, MD, USA). Confocal images were captured using a Leica TCS SPE microscope (Leica Microsystems; Wetzlar, Germany).
Statistical analysis was performed using Prism 5 (GraphPad Software Inc, La Jolle, CA). Two-factor analysis of variance (ANOVA) was performed for in vitro streptavidin594 conjugation and dosing studies. Bonferroni’s multiple comparison tests were used for Post hoc analysis. One-way analysis of variance (ANOVA) was performed for AS1411-streptavidin594, Control-streptavidin594 and streptavidin594 topically treated corneas. Bonferroni’s multiple comparison tests were used for Post hoc analysis.
3. Results
3.1. Nucleolin is present on the cell surface of BALB/c photoreceptors
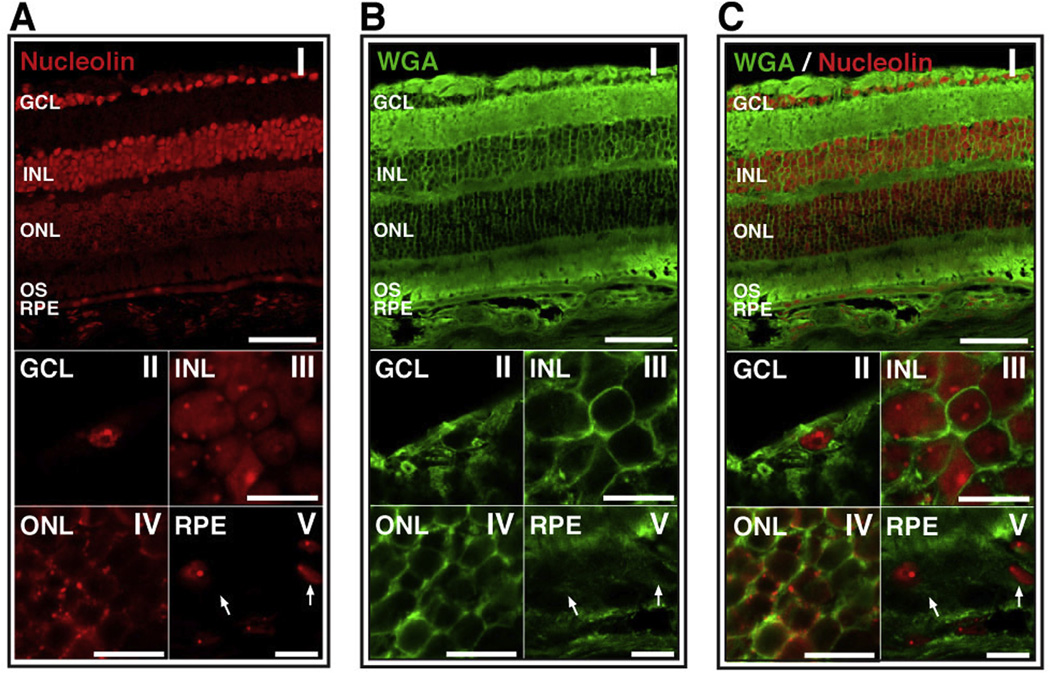
Using an antibody specific for human and mouse nucleolin, retinal sections from BALB/c mice were probed for the presence of nucleolin. We identified nucleolin in the ganglion cell layer (GCL), the inner nuclear layer (INL), the outer nuclear layer (ONL) and the retinal pigment epithelium (RPE) of BALB/c mice (Fig. 1A(I)). The pattern of staining of the cell bodies in the ONL was significantly different to that of the other cell types. Specifically, the pattern of staining in the ONL was consistent with the presence of nucleolin on the cell surface (Fig. 1A(IV)), while that of the GCL, INL and RPE was consistent with cytoplasmic and/or nuclear localization of nucleolin (Fig. 1A(II, III, V)). In order to determine whether the staining of nucleolin in the ONL was consistent with localization at the cell surface, we co-stained the retinal sections with the cell surface marker, wheat germ agglutinin (WGA; Fig. 1B). The WGA-associated signal in the ONL (Fig. 1B(IV)) exhibited a similar pattern to that of nucleolin staining of the ONL (Fig. 1A(IV). An overlay of WGA and nucleolin signal of the ONL exhibited significant co-localization of nucleolin with WGA (Fig. 1C(IV)). However, consistent with previous studies of cell surface nucleolin (Chen et al., 2008a), the cell surface nucleolin signal in the ONL was observed to have a discontinuous, puncate distribution. Presence of cell surface nucleolin on the ONL of BALB/c retina is congruous with nucleolin distribution in the bovine and C57BL/6J murine retina (Hollander et al., 1999; Conley and Naash, 2010). Interestingly, no co-localization was observed between WGA and nucleolin staining in the GCL, INL and RPE of the BALB/c retina (Fig. 1C(II, III, V)), suggesting that the majority of the nucleolin detected in those cells was present in the nucleus and/or cytoplasm. This does not exclude the possibility that cell surface nucleolin is present at undetectable levels in these cells.
Fig. 1.
Nucleolin is present on the surface of cells of the outer nuclear layer in BALB/c retina. (A) Confocal image of BALB/c retinal section stained for nucleolin; (I) whole retina, (II)–(V) increased magnification of GCL, INL, ONL and RPE, respectively. Arrows indicate the nuclei of RPE cells. (B) Confocal image of BALB/c retinal section stained with the cell surface marker, wheat germ agglutinin (WGA); (I) whole retina, (II)–(V) increased magnification of GCL, INL, ONL and RPE, respectively. Arrows indicate the cell surface of RPE cells. (C) Merged image of nucleolin and WGA staining indicates co-localization of these markers only on the ONL (yellow). Top panels scale bar = 60 µm; lower panels scale bar = 10 µm. GCL, ganglion cell layer, INL, inner nuclear layer; ONL, outer nuclear layer; RPE, retinal pigment epithelium. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
3.2. AS1411 localizes to a variety of ocular cell types after intravitreal delivery
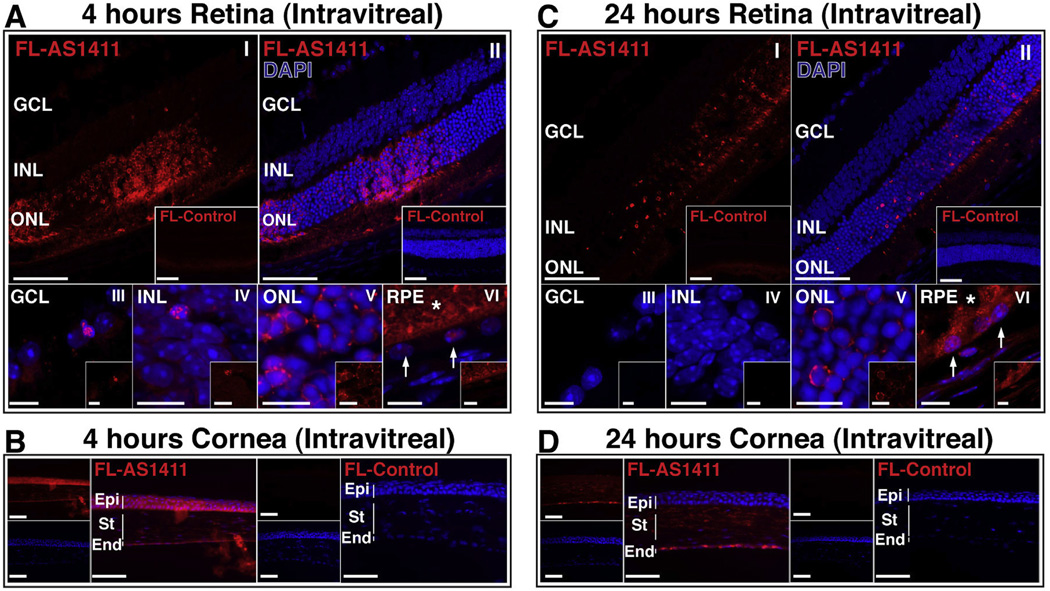
AS1411 is a G-quartet oligonucleotide aptamer that has previously been shown to bind cell surface nucleolin (Bates et al., 2009). In order to determine whether retinal cells can bind and internalize AS1411, we injected 0.3 nmol of a fluorescently labeled AS1411 (FL-AS1411) or a fluorescently labeled control oligonucleotide (FL-Control) into the vitreous of adult BALB/c mice. Eyes were harvested at 4 h or at 24 h post-injection and processed for confocal microscopy.
At 4 h post-injection, confocal microscopy indicated a marked uptake of FL-AS1411 by the ONL, inner and outer segments (Fig. 2A(I, II)). Limited uptake of FL-AS1411 was observed in the GCL and INL (Fig. 2A(I, II)). At higher magnification, uptake of FL-AS1411 by the INL, ONL and RPE (Fig. 2A(IV-VI)) was evident. The increased level of uptake by the ONL relative to the GCL and INL is consistent with the presence of nucleolin on the surface of photoreceptor cell bodies (Fig. 1). Eyes injected intravitreally with FL-Control exhibited significantly less fluorescent signal in all retinal layers at 4 h (Fig. 2A). In addition, at this time-point FL-AS1411 was observed to localize to the endothelial, stromal and epithelial cell layers of the cornea (Fig. 2B). We could not exclude the possibility, that the FL-AS1411 observed in the epithelial cells occurs as a consequence of some leakage of FL-AS1411 during or immediately following ocular injection, rather than by trans-corneal transduction.
Fig. 2.
AS1411 administered by intravitreal injection localizes to a variety of ocular cells in vivo. (A,C) Representative confocal images of BALB/c retina harvested 4 h and 24 h following intravitreal injection of 0.3 nmol AlexaFluro594 labeled AS1411 (FL-AS1411) or Control oligonucleotide (FL-Control; Insets). Top panels scale bar = 60 µm; lower panels scale bar = 10 µm; (I, II) whole retina, (III–VI) higher magnification of GCL, INL, ONL and RPE. Arrows indicate RPE nuclei; asterisk indicates outer segments. (B,D) Representative images of BALB/c cornea harvested 4 h and 24 h following intravitreal injection of 0.3 nmol FL-AS1411 or FL-Control. Scale bar = 60 µm. DAPI (blue) counterstains nuclei. n = 6 eyes/3 mice per condition/time point. GCL-gcmglion cell layer; INL-inner nuclear layer; ONL-outer nuclear layer; RPE-retinal pigment epithelium; Epi-epithelium; St-stroma; End-endothelium. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
At 24 hours following intravitreal injection, confocal microscopy indicated that FL-AS1411 was taken up by the ONL, inner segments and RPE (Fig. 2C(I,II). Higher magnification confocal images confirmed uptake by the ONL and RPE (Fig. 2C(V,VI)). In contrast, no fluorescence signal was detected in eyes injected with FL-Control at these same time points (Fig. 2C). At 24 h, FL-AS1411 was observed in the endothelial and stromal layers of the cornea, but considerably less fluorescence was observed in the epithelium than at 4 h (Fig. 2D).
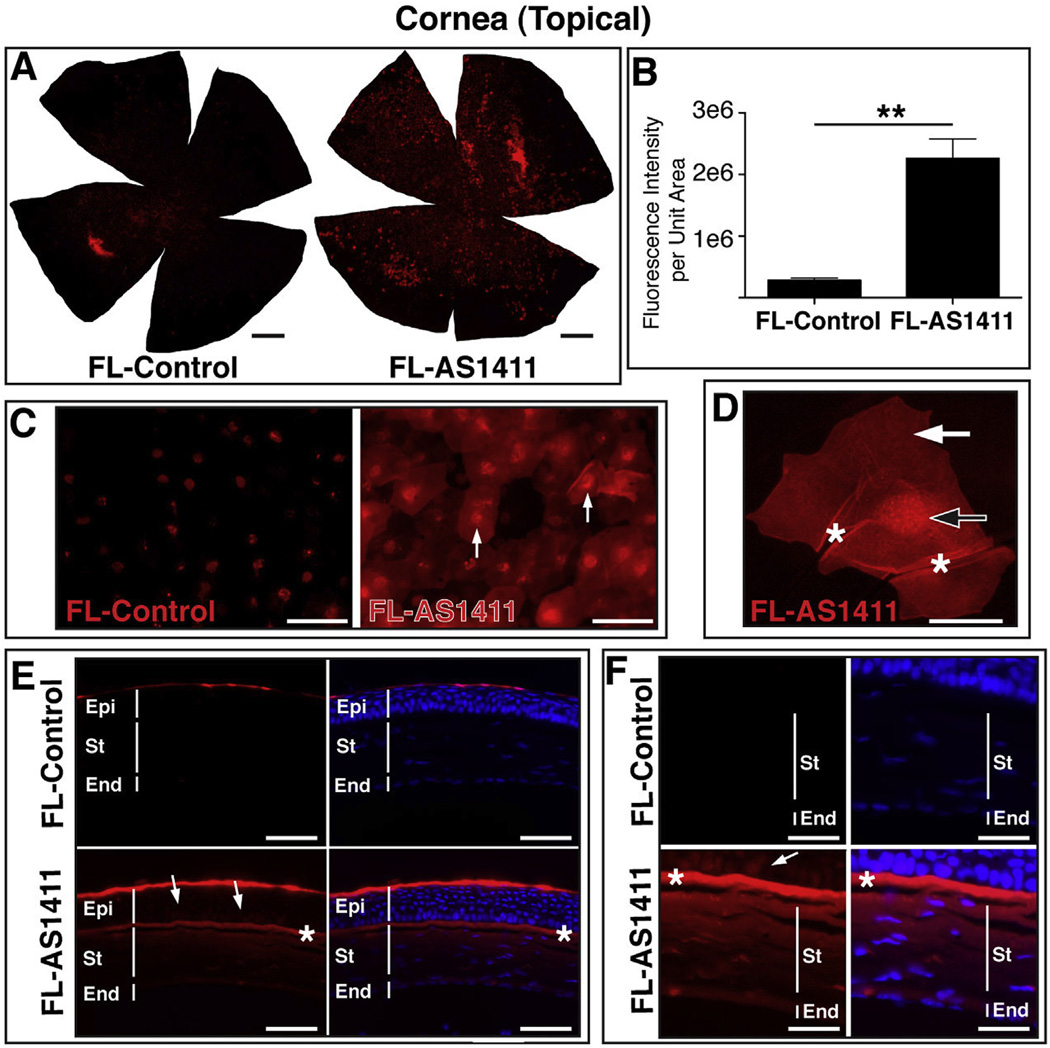
3.3. AS1411 penetrates the cornea following topical delivery in vivo
Delivery of drugs into the cornea via topical application is inefficient, resulting in a considerable loss of drug to the systemic circulation (Rawas-Qalaji and Williams, 2012). In order to examine the potential of FL-AS1411 to act as a transporter of small molecules to the cornea following topical application, 1 nmol of FL-AS1411 or FL-control was administered to the cornea of BALB/c mice. Two hours following application, the corneas were harvested for examination. Fluorescence microscopy of whole corneas indicated considerable binding and possible uptake of FL-AS1411 relative to FL-Control (Fig. 3A). Quantification of the fluorescence intensity of the corneal flatmounts indicated an 8.02 ± 1.15 fold increase (p = 0.002) in signal intensity for FL-AS1411 treated eyes relative to FL-Control treated eyes (Fig. 3B). Images captured at higher magnification revealed a pattern of fluorescence consistent with cytoplasmic and nuclear localization of FL-AS1411 in corneal epithelial cells (Fig. 3C). Binding and possible uptake of FL-Control, although significantly less than that indicated for FL-AS1411, was also observed (Fig. 3A–C). Whether or not this uptake of FL-Control is receptor-mediated or due to a loss of membrane integrity by dying epithelial cells is not known. Some of the fluorescence observed on FL-Control treated corneas was consistent with a nuclear localization in corneal epithelial cells (Fig. 3C), but this occurred with much less efficiency relative to FL-AS1411 treated eyes. Optical sectioning of epithelial cells in the corneal flatmounts using confocal laser scanning microscopy confirmed uptake of FL-AS1411 in the cytoplasm and nucleus of epithelial cells (Fig. 3D). Transverse sections of treated corneas indicates FL-AS1411 to have permeated all layers of the cornea (Fig. 3E). FL-AS1411, although predominantly localized in the superficial layer of the epithelium, most likely squamous cells, was observed in the underlying basal and polygonal epithelial cells as well as the anterior limiting lamina, the stroma and the endothelium (Fig. 3E and F). This result was unexpected, since tight junctions present in the superficial epithelial layer are known to prevent molecules from passing into the cornea. Localization of the FL-Control oligonucleotide within the corneal epithelium, was also observed in transverse sections of control treated corneas, but was limited to the surface (squamous cell) layer (Fig. 3E and F).
Fig. 3.
Topical application of AS1411 results in uptake by corneal cells in vivo. (A) Representative corneal flat mounts harvested 2 h post topical application of 1 nmol FL-AS1411 or FL-Control [scale bar = 0.5 mm]. (B) Quantitation of fluorescent signal per area of topically treated corneas (p = 0.002, n = 6 for each of FL-AS1411, FL-Control). (C) Higher magnification of flat mounts from FL-AS1411 or FL-Control treated cornea. Scale bar = 60 µm. Arrows indicate nuclear localization. (D) A representative image from a series of confocal laser scanned micrographs of a corneal epithelial cell showing FL-AS1411 in the nucleus (black/white arrow) and cytoplasm (white arrow). Asterisks indicate areas of wrinkling, typical of superficial epithelial cells. Scale bar = 20 µm. (E) Transverse sections of corneas topically treated with FL-AS1411 or FL-Control shows FL-AS1411 in all layers of the cornea. Basal and polygonal cells of the epithelium are indicated by arrows. FL-Control was observed only in the superficial squamous cell layer of the epithelium. Asterisks indicate the anterior limiting lamina. Scale bar = 60 µm. (F) Higher magnification of the deeper layers of the epithelium, the stroma and the endothelium show FL-AS1411 permeation of these layers, while FL-Control is undetectable. Scale bar = 30 µm n = 4 eyes/2 mice for each of FL-AS1411, FL-Control. Epi-epithelium; St-stroma; End-endothelium.
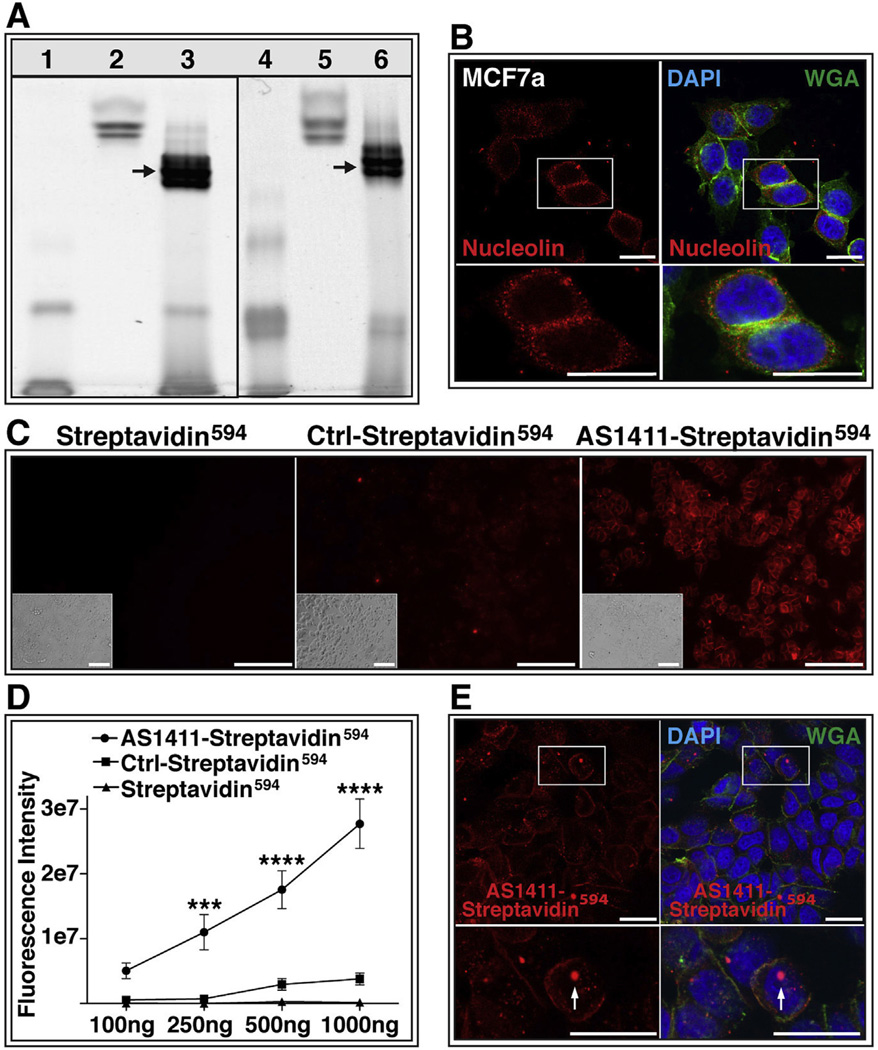
3.4. AS1411 can deliver protein into cells in vitro
In order to determine whether AS1411 can also deliver larger molecules such as proteins across the plasma membrane, we tested the ability of AS1411 to deliver fluorophore-conjugated streptavidin (streptavidin594), an ∼50 kDa protein into MCF7a cells in vitro. We conjugated streptavidin594 to either biotinylated AS1411 (AS1411-streptavidin594) or to biotinylated control oligonucleotide (Control-streptavidin594). In order to examine the conjugate, the reaction products were electrophoresed in parallel with the unconjugated reagents through a native polyacrylamide gel. The AS1411 aptamer was detected in the gel using SybrGold, whereas the streptavidin594 was visualized by its fluorescent label (Fig. 4A). As anticipated with successful conjugation, we observed a shift in migration of the AS1411-conjugated streptavidin594 (AS1411-streptavidin594; Fig. 4A, lane 3) relative to both the unconjugated streptavidin594 (Fig. 4A, lane 2) and the biotinylated AS1411 (Fig. 4A, lane 1). The same shift in migration was observed for the control oligonucleotide conjugated streptavidin594 (Control-streptavidin594; Fig. 4A, lane 6) relative to the unconjugated streptavidin594 (Fig. 4A, lane 5) and the biotinylated control oligonucleotide (Fig. 4A, lane 4). Interestingly, the AS1411-Streptavidin594 and Control-streptavidin594 conjugates (Fig. 4A, lanes 3 and 6) exhibited an accelerated migration through the gel relative to the unconjugated streptavidin594 (lanes 2 and 5). This is possibly due to the conjugation of the DNA that increases the net negative charge of the conjugate. To examine this, the AS1411-Streptavidin594 and Control-streptavidin594 conjugates were incubated with DNAse I and shown to exhibit a migration pattern similar to unconjugated streptavidin594 (data not shown). Collectively, these results suggest efficient conjugation of AS1411 or Control oligonucleotide with streptavidin594.
Fig. 4.
AS1411 delivers protein to cells in vitro. (A) Native polyacrylamide gel confirms conjugation of streptavidin594 with biotinylated AS1411. Lane 1: biotinylated AS1411; Lanes 2 and 5: Streptavidin594; Lane 3: AS1411-Streptavidin594 conjugate; Lane 4: biotinylated control oligonucleotide; Lane 6: Control-Streptavidin594 conjugate;. Arrows indicate conjugation products. (B) MCF7a cells probed with antibody against nucleolin (red) or stained with wheat germ agglutinin (WGA; green) and DAPI (blue). Scale bar = 20 µm (C) MCF7a cells incubated for 1 h with 1000 ng of Streptavidin594, Ctrl-Streptavidin594, or AS1411-Streptavidin594. Bright-field images shown in insert Scale bar = 120 µm. (D) Quantification of raw fluorescence intensity of MCF7a cells incubated with increasing doses of Streptavidin594, Ctrl-Streptavidin594, or AS1411-Streptavidin594 (study performed twice in triplicate) (*** = p < 0.001; **** = p < 0.0001). (E) Confocal microscopy of AS1411-Streptavidin594 (red) incubated cells stained with WGA (green) and DAPI (blue). Arrow indicates nuclear localization of AS1411-Streptavidin594. Scale bar = 20 µm. Ctrl-Control. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
MCF7a is a breast cancer derived cell line previously shown to express cell surface nucleolin (Litchfield et al., 2012). Confocal microscopy of non-permeabilized MCF7a cells stained for both nucleolin and WGA exhibited co-localization of these markers, confirming the presence of nucleolin on the cell surface (Fig. 4B). Incubation of MCF7a cells with 1000 ng AS1411-Streptavidin594 for 1 h resulted in a significantly greater binding and potential uptake of streptavidin594 than did incubation of MCF7a cells with either Control-streptavidin594 or unconjugated streptavidin594 (Fig. 4C). A two-factor analysis of variance of the fluorescence intensity of treated MCF7a cells indicated both a dose dependence and a significant effect of the AS1411 conjugation on streptavidin594 targeting of MCF7a cells, in addition to a significant interaction between the two variables (p < 0.0001; Fig. 4D). A significantly increased fluorescence intensity of AS1411-streptavidin594 incubated cells was observed with 250 ng (p < 0.001), 500 ng (p < 0.0001) and 1000 ng (p < 0.0001) relative to that of Control-streptavidin594 and streptavidin594 incubated cells. No significant difference in fluorescence intensity was observed between Control-streptavidin594 and streptavidin594 incubated cells for any of the doses examined (Fig. 4D). Confocal microscopy performed on AS1411-streptavidin594 incubated MCF7a cells confirmed internalization of the AS1411-streptavidin594 conjugate and demonstrated its localization to both the cytoplasm and the nucleus (Fig. 4E).
3.5. AS1411 mediates delivery of protein to retinal cells in vivo
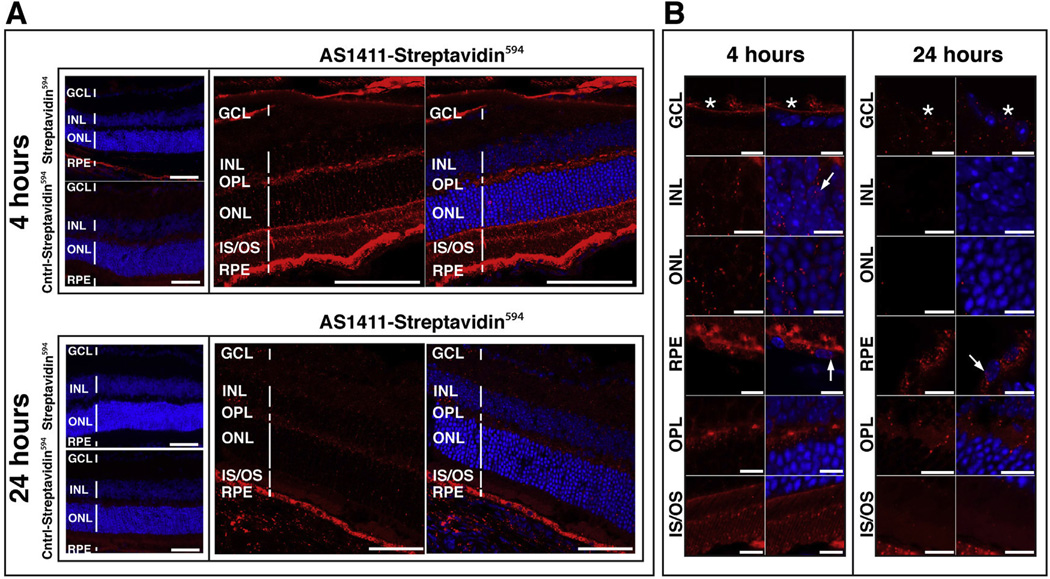
We next examined if AS1411 could deliver streptavidin594 into retinal cells in vivo. Six-week-old BALB/c mice were administered 1.5 µg of either AS1411-streptavidin594, Control-streptavidin594 or streptavidin594 via intravitreal injection. Eyes were harvested at 4 h and 24 h post-injection. At 4 h post-injection, a marked binding of AS1411-streptavidin594 by a variety of retinal tissues was observed (Fig. 5A). At 4 h, the strongest binding of AS1411-streptavidin594 was observed on the RPE, the outer and inner segments (IS/OS) and the outer plexiform layer (OPL), with moderate binding of the ONL and little or no binding of the INL and GCL (Fig. 5A). This pattern of staining was consistent with the binding pattern of fluorophore labeled AS1411 (Fig. 2A). Little or no binding of either Control-streptavidin594 or unconjugated streptavidin594 was noted in the ONL, INL and GCL at this same time point. Some minor binding of the unconjugated streptavidin594 was observed on the RPE – possibly due to the presence of endogenous biotin (Wang and Pevsner, 1999) – but this was considerably less than that observed for the AS1411 -streptavidin594. No binding of the RPE was observed for the Control-streptavidin594. Confocal microscopy of the retinal tissues harvested at 4 h post-injection revealed uptake of AS1411-streptavidin594 by the strongly bound tissues – the RPE, IS/OS and OPL - as well as uptake by both the ONL and INL (Fig. 5B). Despite the limited binding of AS1411 -streptavidin594 by the GCL at 4 h (Fig. 5A), confocal microscopy revealed an intense fluorescent signal in the nerve fiber layer of the GCL (Fig. 5B, asterisks). The majority of AS1411-streptavidin594 taken up by the RPE, ONL and INL was localized to the cytoplasm (Fig. 5B). AS1411-streptavidin594 was detected also in the nuclei of RPE cells and the INL (Fig. 5B, arrows), but at considerably lower levels than in the cytoplasm.
Fig. 5.
AS1411-Streptavidin594 transduces mouse retinal cells following intravitreal injection. (A) Sections of BALB/c retina harvested 4 h and 24 h following intravitreal injection of 1.5 µg of either AS1411-Streptavidin594, Control-Streptavidin594, or unconjugated Streptavidin594. Sections were counterstained with DAPI (blue; n = 6 eyes/3 mice per condition/time point). Scale bar = 60 µm. (B) Confocal microscopy of retinal sections at 4 and 24 h following intravitreal delivery of AS1411 -Streptavidin594. Scale bar = 10 µm. Asterisks indicate nerve fiber layer. Arrows indicate red fluorescence signal in the nucleus. Cntrl-Control; GCL-ganglion cell layer; INL-inner nuclear layer; ONL-outer nuclear layer; IS/OS-inner/outer segments; RPE-retinal pigment epithelium; OPL-outer plexiform layer. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
At the 24 h time-point, a considerable reduction of AS1411-streptavidin594 signal was observed on the IS/OS and OPL (Fig. 5A) relative to that observed at 4 h. Although reduced relative to 4 h, an intense AS1411-streptavidin594 signal was still noted in the RPE at 24 h (Fig. 5A). Neither the Control-streptavidin594 or unconjugated streptavidin594 showed detectable fluorescent signal in any retinal tissue at this time point (Fig. 5A). Confocal microscopy of the retinal tissues harvested at 24 h confirmed these findings. AS1411-streptavidin594 was still strongly present in the RPE, in both the cytoplasm and nucleus (Fig. 5B). While the ONL and INL had little or no detectable AS1411-streptavidin594 at this time point, the conjugate was still detectable within the GCL and OPL.
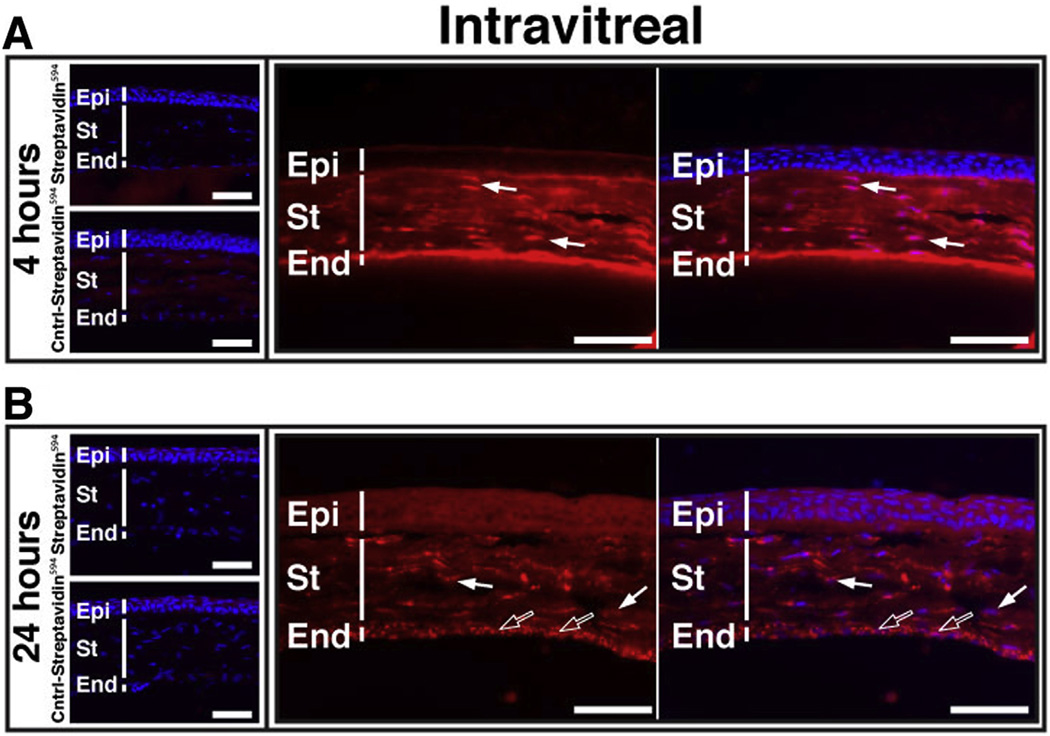
Considering the potential for corneal transduction by AS1411 (Fig. 2B,D), we also analyzed the corneas at 4 and 24 h following intravitreal injection. At 4 h post injection, AS1411-streptavidin594 was observed in both the endothelium and throughout the stroma of the cornea (Fig. 6A). At 24 h, strong fluorescent signal was still observed in both of these corneal layers (Fig. 6B). Counterstain with DAPI suggested uptake of AS1411-streptavidin594 by the nucleus of stromal fibroblasts at 4 h (Fig. 6A) and both stromal fibroblasts and corneal endothelial cells at 24 h (Fig. 6B). There was no detectable transduction of cornea following intravitreal injection of Control-streptavidin594 or Streptavidin594 at either the 4 h (Fig. 6A) or 24 h (Fig. 6B) timepoints.
Fig. 6.
AS1411-Streptavidin594 transduces mouse cornea following intravitreal injection. Corneal sections of BALB/c eyes harvested (A) 4 h and (B) 24 h following intravitreal injection of 1.5 µg of either AS1411-Streptavidin594, Control-Streptavidin594 or Streptavidin594. Scale bar = 60 µm. Arrows indicate nuclear localization (solid arrows, stromal fibroblasts; hollow arrows, endothelial cells). Cntrl-control; Epi-epithelium; St-stroma; End-endothelium.
3.6. Topical application of AS1411-strepatividin594 results in uptake by corneal cells
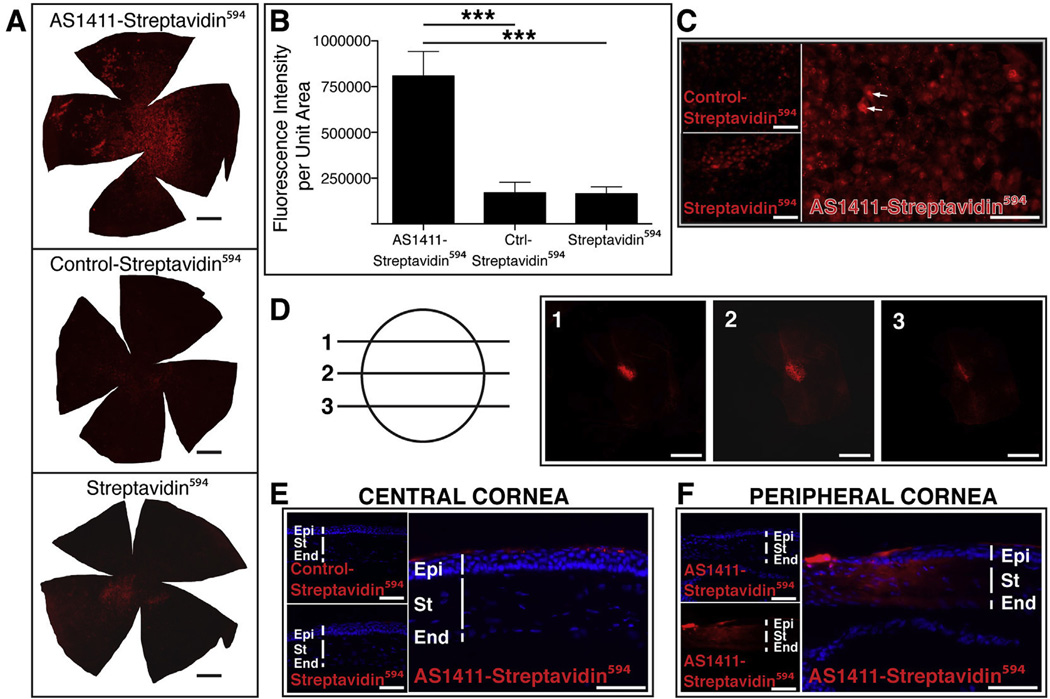
Due to the considerable potential impact of topical delivery of drugs for the treatment of ocular diseases, we next determined whether AS1411 could deliver a 50 kDa protein such as streptavidin into mouse cornea following topical application. We administered 5 µg of either AS1411-streptavidin594, Control-streptavidin594 or streptavidin594 to the cornea of 6 week-old male BALB/c mice. Two hours following topical application, the eyes were harvested and corneas examined for transduction. Fluorescence microscopy of whole cornea flatmounts indicated a markedly enhanced binding with possible uptake by corneas treated with AS1411-streptavidin594 compared to corneas treated with Control-streptavidin594 or streptavidin594 (Fig. 7A). Quantification of the fluorescence intensity of streptavidin594 conjugates on the corneal flatmounts indicated that AS1411-streptavidin594 treated corneas had bound 3.76 ± 0.87 fold and 3.92 ± 0.86 fold (p < 0.001 for both) more strepatividin594 than Control-streptavidin594 and streptavidin594 treated corneas, respectively (Fig. 7B). While some binding and/or uptake of streptavidin594 was observed in both Control-streptavidin594 and streptavidin594 treated corneas (Fig. 7A), consistent with binding of FL-control to cornea following topical delivery (Fig. 3A,B), the amount of streptavidin594 did not differ significantly between these groups.
Fig. 7.
Topical Application of AS1411-streptavidin594 results in uptake by corneal cells and nuclear localization in vivo. (A) Corneal flat mounts harvested 2 h post topical application of 5 µg of AS1411-Streptavidin594, Control-Streptavidin594 or Streptavidin594. Scale bar = 0.5 mm. (B) Quantitation of the intensity of fluorescence of streptavidin594 conjugates per area of corneal flatmount (n = 6 per condition, *** = p < 0.001). (C) Higher magnification images of AS1411-Streptavidin594, Control-Streptavidin594 or Streptavidin594 treated corneal flat mounts. Scale bar =120 µm. Arrows indicate examples of nuclear binding. (D) Confocal series of a flatmount of AS1411-Streptavidin594 treated cornea indicating nuclear localization within a corneal epithelial cell. Scale bar = 20 µm. (E) Transverse sections of corneas treated topically with AS1411-Streptavidin594, Control-Streptavidin594 or Streptavidin594 (n = 4 eyes/2 mice per condition). Scale bar = 60 µm. (F) Transverse section of AS1411-strepatividin594 treated cornea showing transduction of stroma and endothelium at the corneal limbus. Scale bar = 60 µm. Ctrl-Control; Epi-epithelium; St-stroma; End-endothelium.
At higher magnification, the pattern of fluorescence on the AS1411-streptavidin594 treated corneas was observed to be consistent with nuclear localization (Fig. 7C, arrows). Confocal microscopy of AS1411-streptavidin594 treated corneal flatmounts confirmed nuclear uptake of the conjugate (Fig. 7D). Unlike the FL-AS1411, transverse sections of treated corneas revealed that the AS1411-streptavidin594 applied topically to the cornea was delivered almost exclusively to corneal epithelial cells (Fig. 7E), likely due to the presence of tight junctions between the epithelial cells (Eghrari et al., 2015) preventing passage of larger molecules through the cornea. At the corneal limbus (peripheral cornea), however, AS1411-strepatividin594 could be observed throughout the stroma and endothelium (Fig. 7F). Whether this is due to structural differences at the corneal limbus or a non-uniform dispersion of the topically applied “drop” over the corneal surface is not known.
3.7. Cell surface nucleolin is present on neurons of non-human primate retina
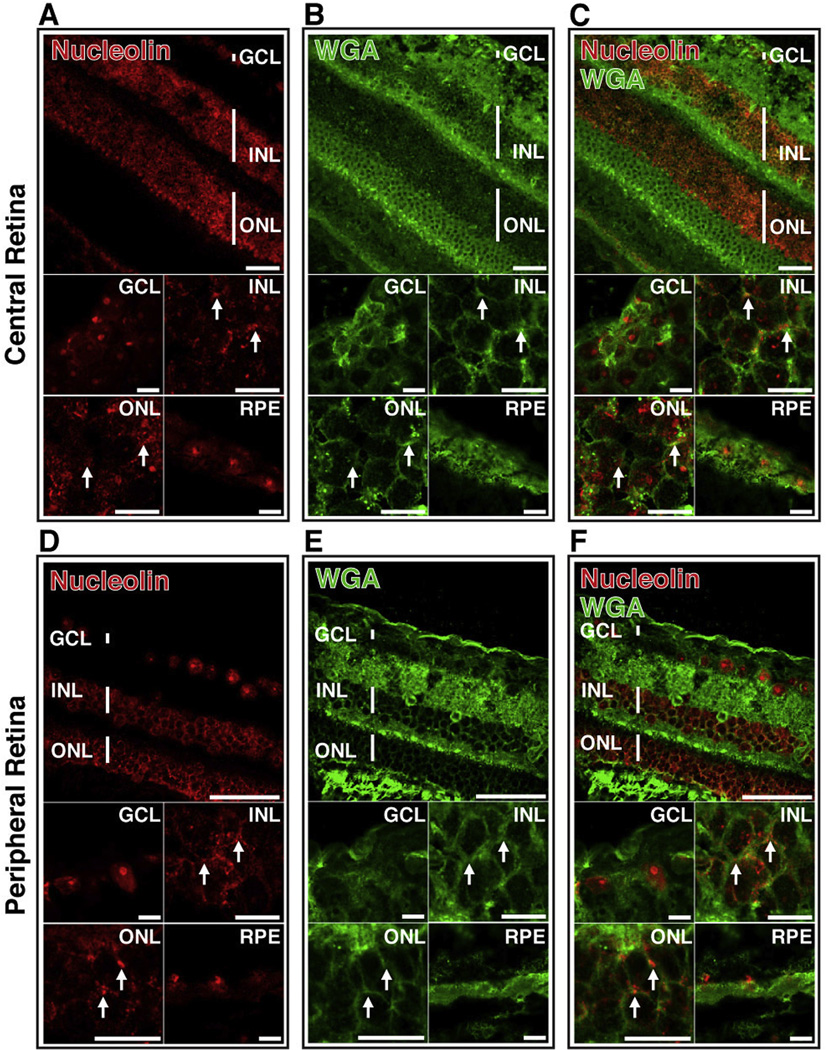
While mammalian retinas share a significant degree of homology, there are significant differences between murine and primate retina, most notably the absence of a macula in the murine retina (Volland et al., 2015). To assess the potential of AS1411 as a delivery vehicle for primate retina, transverse sections of retina of Cynomolgus monkey (Macaca fascicularis) were probed for the presence of nucleolin using a polyclonal anti-nucleolin antibody (Fig. 8). Presence of nucleolin on the cell surface was determined by co-staining with WGA. Non-human primate retinal cells stained strongly positive for nucleolin in the GCL, INL, ONL and RPE of both the central (Fig. 8A) and peripheral (Fig. 8D) retina. To determine localization and make a qualitative assessment of WGA and nucleolin, optical sectioning of retinal sections using confocal microscopy was performed. In the central retina, we observed limited overlap between WGA and nucleolin, indicating an intracellular and predominantly nuclear localization of nucleolin in the GCL and RPE (Fig. 8A–C). In both the INL and ONL of the central retina, some co-localization of WGA and nucleolin was observed (Fig. 8C, arrows), indicating presence of nucleolin on the surface of these cells, but a majority of the nucleolin appeared to occur intracellularly.
Fig. 8.
Cell surface nucleolin is present on neurons of non-human primate retina. Confocal microscopy of retinal sections of Cynomolgus monkey (macaca fascicularis) stained with anti-nucleolin antibody (A,D; red) and WGA (B,E; green). Images from both central and peripheral retina are shown. Overlay of images A and B (C) and D and E (F) indicates co-localization of nucleolin and WGA (yellow). Arrows indicate regions of co-localization. Top panels scale bar = 60 µm; lower panels scale bar = 10 µm. GCL-ganglion cell layer; INL-inner nuclear layer; ONL-outer nuclear layer; RPE-retinal pigment epithelium. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
In the peripheral retina, observation of limited co-localization of WGA and nucleolin indicated a predominantly cytoplasmic and nuclear localization of nucleolin in the GCL and RPE (Fig. 8D), similar to that of the GCL and RPE of the central retina (Fig. 8A). Unlike the observations for the central retina, however, the GCL of the peripheral retina showed strong nucleolin staining in both the cytoplasm and nucleus (Fig. 8F). There was little or no co-localization of nucleolin with WGA in either the GCL or RPE of the peripheral retina (Fig. 8E, F), indicating little surface nucleolin. The INL and ONL of the peripheral retina exhibited a pattern of nucleolin staining (Fig. 8D) very similar to that of WGA in these cells (Fig. 8E), consistent with presence of nucleolin on the surface of these cells (Fig. 8F).
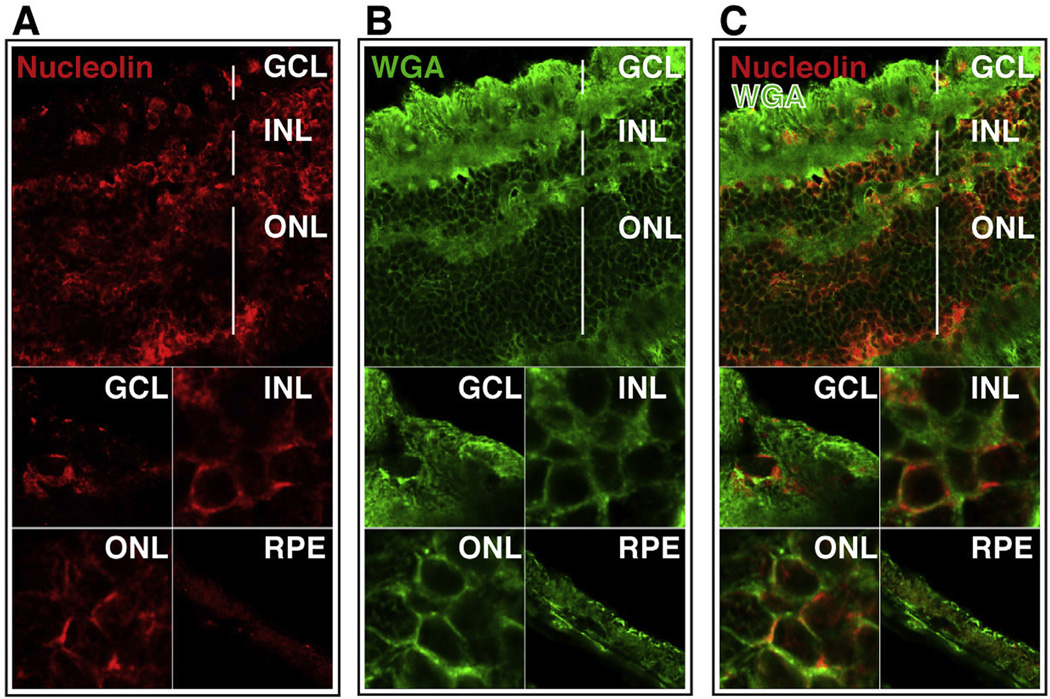
3.8. Cell surface nucleolin is present in all neuronal layers of adult human retina
To further investigate the clinical potential of AS1411 for retinal drug delivery, we determined the distribution of nucleolin in the adult human retina. Human retinal sections stained for nucleolin exhibited the presence of nucleolin in the GCL, INL, ONL and RPE (Fig. 9A). Co-staining of retinal sections with WGA (Fig. 9B), suggested strong co-localization of nucleolin and WGA in the INL, ONL and GCL (Fig. 9C).
Fig. 9.
Cell surface nucleolin is present in all neuronal layers of adult human retina. Confocal images of sections of human retina probed with anti-nucleolin antibody (A; red) and WGA (B; green). Overlay of images A and B (C) indicates co-localization of nucleolin and WGA (yellow). Top panels scale bar = 60 µm; lower panels scale bar = 10 µm. GCL-ganglion cell layer, INL-inner nuclear layer, ONL-outer nuclear layer, RPE-retinal pigment epithelium. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
4. Discussion
In recent years, there has been a significant interest in the use of aptamers as drug delivery agents (Zhu et al., 2015). Aptamers can be designed to have a strong affinity for targets, extended shelf life, low immunogenicity and cost-efficient identification and synthesis. Pegaptanib, an aptamer targeting VEGF, has been approved by the FDA for the treatment of Age-related Macular Degeneration (Gragoudas et al., 2004). Phase II of a clinical study has just been completed for the nucleolin-targeting aptamer, AS1411, investigating efficacy as a therapy for Acute Myeloid Leukemia (https://clinicaltrials.gov/ct2/show/NCT00512083). We have recently found the potential of AS1411 delivered by either intravitreal injection or by topical application to inhibit choroidal neo-vascularization in a murine model of AMD (Leaderer et al., 2015). To our knowledge this is the first study, however, demonstrating the use of an aptamer as a protein delivery system in the eye.
Previously, cell-penetrating peptides (CPP) have been utilized for ocular delivery of fluorophore and protein (Cashman et al., 2003; Johnson et al., 2010; Binder et al., 2011). HIV-derived TAT and herpes simplex virus-derived VP22 peptides function efficiently in vitro, but have considerably reduced efficiency in vivo (Cashman et al., 2002, 2003; Kilic et al., 2004). Likely due to a paucity of alternatives, the TAT CPP continues to be investigated as a vehicle for delivery of ocular therapies (Zhang et al., 2015; Ozaki et al., 2015). Peptide for ocular delivery (POD) can deliver cargo to murine photoreceptors and RPE following subretinal delivery, but upon intravitreal administration, transduction is almost exclusively of ganglion cells (Johnson et al., 2008, 2010). In contrast, herein we found that AS1411 aptamer injected intravitreally in mice can deliver an ∼50 kDa protein to the cytoplasm and nucleus of photoreceptors and the RPE. Intravitreal administration of AS1411 also enabled uptake of the 50 kDa protein by the endothelium and stroma of the cornea. Furthermore, topical application of the AS1411 conjugate enabled uptake by the nucleus of corneal epithelial cells, as well as delivery to the stroma and endothelium at the corneal limbus. Uptake of AS1411-strepavidin594 in vitro occurs in a dose-dependent manner and results in delivery to the nucleus. Collectively, these results strongly indicate the potential for AS1411 as a transporter of proteins or other cargo to the RPE, retinal neurons, and cells of the cornea.
Aptamers are currently being used as delivery vehicles for therapeutic conjugates in liver, solid tumors, T lymphocytes and bone tissue (Zhu et al., 2015). In addition, the conjugated therapeutics tested have included a wide range of molecules – chemotherapeutics, photosensitizing agents, siRNAs and enzymes (Zhu et al., 2015). The AS1411 aptamer, in addition to the above, has been used to deliver oligonucleotides (Kotula et al., 2012), antibody complexes (Park et al., 2012), as well as polymer- (Aravind et al., 2012; Guo et al., 2011), protein- (Wu et al., 2013), and liposome-based (Kim et al., 2012; Liao et al., 2015) nanoparticles of up to −200 nm to a variety of cell types in vitro and in vivo. AS1411-coated cell membrane capsules of >900 nm efficiently delivered a chemotherapeutic to xenograft tumors following intravenous injection in mice (Peng et al., 2015), resulting in a 2.2-fold reduction in tumor growth relative to a 1.3-fold reduction in tumor size following injection of uncoated capsules of the same agent. Similar results were observed for AS1411 -conjugated liposomes carrying doxorubicin when injected intravenously in tumor grafted mice, in which a significant inhibition of tumor growth, side effects of the drug – specifically, cardiotoxicity – was observed relative to injection of unconjugated liposomes (Liao et al., 2015). This was likely due to increased targeting of the chemotherapeutic agent to the tumor, with reduced accumulation in other tissues. Nanoparticles conjugated with AS1411 and without the addition of chemotherapeutic agent have themselves been shown to slow growth of liver tumors in vivo to a modest degree (Zhang et al., 2014).
While much use has been made, however, of AS1411 as a targeting agent for a variety of molecules in vivo, to our knowledge all of these studies have involved delivery to tumors. Our study is, therefore, the first to demonstrate the use of AS1411 as a delivery agent to terminally-differentiated, non-dividing cells in vivo. The focus on tumors prior to our study is likely due to the significant literature describing the presence of cell surface nucleolin on proliferating rather than quiescent cells (Hovanessian et al., 2000, 2010). However, a number of studies in addition to that presented herein, have demonstrated the presence of nucleolin on the surface of photoreceptor cell bodies of both murine and bovine retina (Hollander et al., 1999; Conley and Naash, 2010). The capacity of AS1411 to deliver molecules differing from streptavidin in size, charge and composition to non-mitotic cells of the retina remains to be demonstrated. However, delivery of streptavidin to cells in vitro by an RNA aptamer, has been shown to be a reliable indicator of the aptamer’s capacity to deliver a functional lysosomal enzyme (Chen et al., 2008b). Unlike the endosomal dependent, transferrin-binding RNA aptamer (Chen et al., 2008b), AS1411 enters cells through a cell surface nucleolin binding pathway (Bates et al., 2009) that has been previously found to be capable of transporting nanoparticles directly to the nuclei of cells in a pathway independent of endosomes (Chen et al., 2008a).
Delivery of AS1411 conjugates to murine photoreceptors in vivo following intravitreal injection is consistent with the presence of nucleolin exclusively on the ONL of murine retina (Fig. 1). In addition, the significant potential of AS1411 for delivery of protein to photoreceptors of human retina is strongly indicated by nucleolin staining of the cell surface of the ONL of both non-human primate and human retina. Interestingly, equivalent staining for nucleolin was also observed on the cell surface of inner neurons in both non-human primate and human retina, extending potential application of AS1411-mediated delivery of protein to inner retinal neurons. These results suggest that while some similarities of nucleolin localization occur between species, the retinal distribution of nucleolin is, somewhat, species-specific. However, the presence of cell surface nucleolin in all neuronal layers of the human retina bodes favorably for the use of AS1411 as a mode of drug delivery for human retinal diseases.
While this mode of treatment may require repeated injections, intravitreal injection is a current standard of clinical care found to be relatively safe and well tolerated (Meyer et al., 2016). For example, many patients being treated for the “wet” form of AMD receive intravitreal injections every 4–6 weeks (Stewart, 2015).
In the murine retina, intravitreal injection of AS1411-streptavidin594 also led to localization of the conjugate in the RPE. This hints at the potential use of AS1411-meditated delivery of therapeutic proteins for the treatment of diseases associated with degeneration of RPE cells, such as in age-related macular degeneration. The uptake of AS1411-streptavidin594 by murine RPE, however, was not consistent with the observation that nucleolin was not detectable on the surface of RPE cells in either the murine or primate retina. Thus, it is possible that the AS1411-streptavidin594 conjugate was phagocytosed by the RPE either directly or as a constituent of photoreceptor outer segments (Kevany and Palczewski, 2010). This hypothesis is supported by the observation that AS1411-mediated uptake of both fluorophore and protein significantly increases at the later (24-h) time-point after injection, a time when the amount of AS1411 cargo in the ONL and outer segment is seen to be reduced (Figs. 2 and 5).
Topical delivery of drugs to the cornea is highly inefficient, resulting in a considerable loss of drug to the systemic circulation (Rawas-Qalaji and Williams, 2012). Topical delivery of AS1411-streptavidin594 resulted in efficient uptake of AS1411 by corneal epithelial cells. Conjugation of AS1411 to existing topical treatments may increase the bioavailability of these compounds and allow for less “off target” effects.
In conclusion, our study suggests that AS1411 can efficiently deliver a prototypical protein of ∼50 kDa to the nuclei and cytoplasm of a variety of retinal and corneal cells, and that AS1411 employs cell surface nucleolin to deliver its cargo. The presence of surface nucleolin on inner and outer neurons of non-human primate and human retina strongly indicates the potential of AS1411 for delivery of conjugates to human retina and favors further exploration of AS1411 as a delivery vehicle for treatments of a variety of ocular diseases.
Acknowledgments
This study was supported by grants to R.K.S from The Department of Defense/TATRC, The Paul and Phyllis Fireman Foundation and The National Institute of Health/NEI (EY021805 and EY013837).
References
- Aravind A, Jeyamohan P, Nair R, et al. AS1411 aptamer tagged PLGA-lecithin-PEG nanoparticles for tumor cell targeting and drug delivery. Biotechnol. Bioeng. 2012;109:2920–2931. doi: 10.1002/bit.24558. [DOI] [PubMed] [Google Scholar]
- Bates PJ, Laber DA, Miller DM, Thomas SD, Trent JO. Discovery and development of the G-rich oligonucleotide AS1411 as a novel treatment for cancer. Exp. Mol. Pathol. 2009;86:151–164. doi: 10.1016/j.yexmp.2009.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder C, Read SP, Cashman SM, Kumar-Singh R. Nuclear targeted delivery of macromolecules to retina and cornea. J. Gene Med. 2011;13:158–170. doi: 10.1002/jgm.1548. [DOI] [PubMed] [Google Scholar]
- Borer RA, Lehner CF, Eppenberger HM, Nigg EA. Major nucleolar proteins shuttle between nucleus and cytoplasm. Cell. 1989;56:379–390. doi: 10.1016/0092-8674(89)90241-9. [DOI] [PubMed] [Google Scholar]
- Carvalho LS, Vandenberghe LH. Promising and delivering gene therapies for vision loss. Vis. Res. 2015;111:124–133. doi: 10.1016/j.visres.2014.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cashman SM, Sadowski SL, Morris DJ, Frederick J, Kumar-Singh R. Intercellular trafficking of adenovirus-delivered HSV VP22 from the retinal pigment epithelium to the photoreceptors-implications for gene therapy. Mol. Ther. 2002;6:813–823. doi: 10.1006/mthe.2002.0806. [DOI] [PubMed] [Google Scholar]
- Cashman SM, Morris DJ, Kumar-Singh R. Evidence of protein transduction but not intercellular transport by proteins fused to HIV tat in retinal cell culture and in vivo. Mol. Ther. 2003;8:130–142. doi: 10.1016/s1525-0016(03)00131-x. [DOI] [PubMed] [Google Scholar]
- Chen X, Kube DM, Cooper MJ, Davis PB. Cell surface nucleolin serves as receptor for DNA nanoparticles composed of pegylated polylysine and DNA. Mol. Ther. 2008;16:333–342. doi: 10.1038/sj.mt.6300365. [DOI] [PubMed] [Google Scholar]
- Chen CH, Dellamaggiore KR, Ouellette CP, et al. Aptamer-based endocytosis of a lysosomal enzyme. Proc. Natl. Acad. Sci. U. S. A. 2008;105:15908–15913. doi: 10.1073/pnas.0808360105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conley SM, Naash MI. Nanoparticles for retinal gene therapy. Prog. Retin. Eye Res. 2010;29:376–397. doi: 10.1016/j.preteyeres.2010.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eghrari AO, Riazuddin SA, Gottsch JD. Overview of the cornea: structure, function, and development. Prog. Mol. Biol. Transl. Sci. 2015;134:7–23. doi: 10.1016/bs.pmbts.2015.04.001. [DOI] [PubMed] [Google Scholar]
- Gragoudas ES, Adamis AP, Cunningham ET, Jr, Feinsod M, Guyer DR. Pegaptanib for neovascular age-related macular degeneration. N. Engl. J. Med. 2004;351:2805–2816. doi: 10.1056/NEJMoa042760. [DOI] [PubMed] [Google Scholar]
- Guo J, Gao X, Su L, et al. Aptamer-functionalized PEG-PLGA nanoparticles for enhanced anti-glioma drug delivery. Biomaterials. 2011;32:8010–8020. doi: 10.1016/j.biomaterials.2011.07.004. [DOI] [PubMed] [Google Scholar]
- Hollander BA, Liang MY, Besharse JC. Linkage of a nucleolin-related protein and casein kinase II with the detergent-stable photoreceptor cytoskeleton. Cell Motil. Cytoskelet. 1999;43:114–127. doi: 10.1002/(SICI)1097-0169(1999)43:2<114::AID-CM3>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- Hovanessian AG, Puvion-Dutilleul F, Nisole S, et al. The cell-surface-expressed nucleolin is associated with the actin cytoskeleton. Exp. Cell Res. 2000;261:312–328. doi: 10.1006/excr.2000.5071. [DOI] [PubMed] [Google Scholar]
- Hovanessian AG, Soundaramourty C, El Khoury D, Nondier I, Svab J, Krust B. Surface expressed nucleolin is constantly induced in tumor cells to mediate calcium-dependent ligand internalization. PLoS One. 2010;5:e15787. doi: 10.1371/journal.pone.0015787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson LN, Cashman SM, Kumar-Singh R. Cell-penetrating peptide for enhanced delivery of nucleic acids and drugs to ocular tissues including retina and cornea. Mol. Ther. 2008;16:107–114. doi: 10.1038/sj.mt.6300324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson LN, Cashman SM, Read SP, Kumar-Singh R. Cell penetrating peptide POD mediates delivery of recombinant proteins to retina, cornea and skin. Vis. Res. 2010;50:686–697. doi: 10.1016/j.visres.2009.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kevany BM, Palczewski K. Phagocytosis of retinal rod and cone photoreceptors. Physiol. (Bethesda) 2010;25:8–15. doi: 10.1152/physiol.00038.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilic U, Kilic E, Dietz GP, Bahr M. The TAT protein transduction domain enhances the neuroprotective effect of glial-cell-line-derived neurotrophic factor after optic nerve transection. Neurodegener. Dis. 2004;1:44–49. doi: 10.1159/000076669. [DOI] [PubMed] [Google Scholar]
- Kim JK, Choi KJ, Lee M, Jo MH, Kim S. Molecular imaging of a cancer-targeting theragnostics probe using a nucleolin aptamer- and microRNA-221 molecular beacon-conjugated nanoparticle. Biomaterials. 2012;33:207–217. doi: 10.1016/j.biomaterials.2011.09.023. [DOI] [PubMed] [Google Scholar]
- Kim YC, Chiang B, Wu X, Prausnitz MR. Ocular delivery of macromolecules. J. Control Release. 2014;190:172–181. doi: 10.1016/j.jconrel.2014.06.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotula JW, Pratico ED, Ming X, Nakagawa O, Juliano RL, Sullenger BA. Aptamer-mediated delivery of splice-switching oligonucleotides to the nuclei of cancer cells. Nucleic Acid Ther. 2012;22:187–195. doi: 10.1089/nat.2012.0347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leaderer D, Cashman SM, Kumar-Singh R. Topical application of a G-Quartet aptamer targeting nucleolin attenuates choroidal neovascularization in a model of age-related macular degeneration. Exp. Eye Res. 2015;140:171–178. doi: 10.1016/j.exer.2015.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao ZX, Chuang EY, Lin CC, et al. An AS1411 aptamer-conjugated liposomal system containing a bubble-generating agent for tumor-specific chemotherapy that overcomes multidrug resistance. J. Control Release. 2015;208:42–51. doi: 10.1016/j.jconrel.2015.01.032. [DOI] [PubMed] [Google Scholar]
- Litchfield LM, Riggs KA, Hockenberry AM, et al. Identification and characterization of nucleolin as a COUP-TFII coactivator of retinoic acid receptor beta transcription in breast cancer cells. PLoS One. 2012;7:e38278. doi: 10.1371/journal.pone.0038278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer CH, Krohne TU, Charbel Issa P, Liu Z, Holz FG. Routes for drug delivery to the eye and retina: intravitreal injections. Dev. Ophthalmol. 2016;55:63–70. doi: 10.1159/000431143. [DOI] [PubMed] [Google Scholar]
- Ozaki T, Nakazawa M, Yamashita T, Ishiguro S. Delivery of topically applied calpain inhibitory peptide to the posterior segment of the rat eye. PLoS One. 2015;10:e0130986. doi: 10.1371/journal.pone.0130986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park S, Hwang D, Chung J. Cotinine-conjugated aptamer/anti-cotinine antibody complexes as a novel affinity unit for use in biological assays. Exp. Mol. Med. 2012;44:554–561. doi: 10.3858/emm.2012.44.9.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parnaste L, Arukuusk P, Zagato E, Braeckmans K, Langel U. Methods to follow intracellular trafficking of cell-penetrating peptides. J. Drug Target. 2015:1–12. doi: 10.3109/1061186X.2015.1095194. [DOI] [PubMed] [Google Scholar]
- Peng LH, Zhang YH, Han LJ, et al. Cell membrane capsules for encapsulation of chemotherapeutic and cancer cell targeting in vivo. ACS Appl. Mater. Interfaces. 2015;7(33):18628–18637. doi: 10.1021/acsami.5b05065. [DOI] [PubMed] [Google Scholar]
- Rawas-Qalaji M, Williams CA. Advances in ocular drug delivery. Curr. Eye Res. 2012;37:345–356. doi: 10.3109/02713683.2011.652286. [DOI] [PubMed] [Google Scholar]
- Read SP, Cashman SM, Kumar-Singh R. POD nanoparticles expressing GDNF provide structural and functional rescue of light-induced retinal degeneration in an adult mouse. Mol. Ther. 2010;18:1917–1926. doi: 10.1038/mt.2010.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart MW. Individualized treatment of neovascular age-related macular degeneration: what are patients gaining? or losing? J. Clin. Med. 2015;4:1079–1101. doi: 10.3390/jcm4051079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thakur SS, Barnett NL, Donaldson MJ, Parekh HS. Intravitreal drug delivery in retinal disease: are we out of our depth? Expert Opin. Drug Deliv. 2014;11:1575–1590. doi: 10.1517/17425247.2014.927864. [DOI] [PubMed] [Google Scholar]
- Trapani I, Puppo A, Auricchio A. Vector platforms for gene therapy of inherited retinopathies. Prog. Retin. Eye Res. 2014;43:108–128. doi: 10.1016/j.preteyeres.2014.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuteja R, Tuteja N. Nucleolin: a multifunctional major nucleolar phospho-protein. Crit. Rev. Biochem. Mol. Biol. 1998;33:407–436. doi: 10.1080/10409239891204260. [DOI] [PubMed] [Google Scholar]
- Volland S, Esteve-Rudd J, Hoo J, Yee C, Williams DS. A comparison of some organizational characteristics of the mouse central retina and the human macula. PLoS One. 2015;10:e0125631. doi: 10.1371/journal.pone.0125631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Pevsner J. Detection of endogenous biotin in various tissues: novel functions in the hippocampus and implications for its use in avidin-biotin technology. Cell Tissue Res. 1999;296:511–516. doi: 10.1007/s004410051311. [DOI] [PubMed] [Google Scholar]
- Wu J, Song C, Jiang C, Shen X, Qiao Q, Hu Y. Nucleolin targeting AS1411 modified protein nanoparticle for antitumor drugs delivery. Mol. Pharm. 2013;10:3555–3563. doi: 10.1021/mp300686g. [DOI] [PubMed] [Google Scholar]
- Zhang B, Luo Z, Liu J, Ding X, Li J, Cai K. Cytochrome c end-capped mesoporous silica nanoparticles as redox-responsive drug delivery vehicles for liver tumor-targeted triplex therapy in vitro and in vivo. J. Control Release. 2014;192:192–201. doi: 10.1016/j.jconrel.2014.06.037. [DOI] [PubMed] [Google Scholar]
- Zhang X, Li Y, Cheng Y, et al. Tat PTD-endostatin: a novel anti-angiogenesis protein with ocular barrier permeability via eye-drops. Biochim. Biophys. Acta. 2015;1850:1140–1149. doi: 10.1016/j.bbagen.2015.01.019. [DOI] [PubMed] [Google Scholar]
- Zhu G, Niu G, Chen X. Aptamer-drug conjugates. Bioconjugate Chem. 2015;26(11):2186–2197. doi: 10.1021/acs.bioconjchem.5b00291. [DOI] [PMC free article] [PubMed] [Google Scholar]