Abstract
Background
Changes in left ventricular (LV) systolic function in response to coronary artery bypass grafting (CABG) have not been fully assessed.
Methods
Between January 2001 and December 2014, 2,838 consecutive patients underwent isolated CABG at the Minneapolis Veterans Affairs Health Care System. Of these, 375 had echocardiographic assessment of LV function before (within 6 months) and after (3 to 24 months) CABG and were included in this analysis.
Results
While the mean LV ejection fraction (LVEF) did not change following CABG [(49±13)% vs. (49±12)%, P=0.51], LVEF decreased in the subgroup with normal (≥50%) pre-operative LVEF [from (59±5)% to (56±9)%, P<0.001] and improved in those with decreased (<50%) pre-operative LVEF [from (36±9)% to (41±12)%, P<0.001]. There was a significant reduction in LV internal diameter during end-diastole (LVIDd) (5.4±0.8 vs. 5.3±0.9, P=0.002) and an increase in left atrial diameter (LAD) (4.4±0.7 vs. 4.6±0.7, P<0.001). There were no perioperative changes in LV internal diameter during end-systole, LV mass, posterior wall thickness, or septal wall thickness. LVEF improved by >5% in 24% of the study population, did not change (+/− 5%) in 55%, and worsened by >5% in 21%. Patients with improved EF were less often diabetic and had lower pre-operative LVEF, and greater LV dimensions at baseline.
Conclusions
After CABG, there was a decrease in LVIDd and an increase in LAD. Also, a decrease in LV systolic function with CABG was observed in patients with normal pre-operative LVEF and an improvement in LV systolic function was observed in patients with decreased pre-operative LVEF.
Keywords: Coronary artery bypass graft surgery, echocardiography, left ventricular function
Introduction
Coronary artery revascularization via coronary artery bypass grafting (CABG) is indicated for patients with angina and suitable coronary anatomy, especially those with stenosis of the left main coronary artery or patients with left main equivalent disease (1-5). CABG has been shown to improve survival in left main disease and in certain subgroups with multi-vessel disease (5). With regard to the latter, pivotal studies that assessed survival with CABG versus medical therapy included the Coronary Artery Surgery Study (CASS), the Veterans Administration (VA) Cooperative study, and the European Coronary Surgery Study (ECSS) (5-7). The Surgical Treatment for Ischemic Heart Failure (STICH) trial later assessed survival with CABG in patients with severe LV dysfunction (EF ≤35%), demonstrating a significant survival benefit in the extended-follow-up results (8,9).
The effect of CABG on LV systolic function remains to be elucidated. While the STICH trial evaluated the effect of CABG in patients with severe LV dysfunction (EF ≤35%), to our knowledge, no large studies have assessed the effect of CABG on LV parameters in patients with normal baseline EF. The STICH trial showed a significant decrease in end-systolic volume index (ESVI) in patients with a baseline LV ESVI >90 mL/m2 (10), while no significant change in LV ESVI was observed in the subgroups of patients with smaller LV cavity size (10). While LVEF significantly improved in patients with a baseline LV ESVI ≥60 mL/m2, no significant improvement in LVEF was seen in those with a baseline LV ESVI <60 mL/m2.
The objectives of this study were to evaluate the effect of CABG on LV systolic function in patients with both normal and abnormal pre-operative systolic function.
Methods
Data source
The cardiac surgery database at the Minneapolis Veterans Affairs (VA) Health Care System is part of an ongoing multicenter database of prospectively collected data on all patients undergoing cardiac surgery at VA Medical Centers in the United States. The database includes information regarding patient demographics, clinical and laboratory variables, surgical details, and post-operative outcomes, including long-term survival (11,12). Additional clinical, pharmacologic, and echocardiographic variables, that were not included in the database, were extracted from electronic medical records (13). This study was approved by the Institutional Review Board at the Minneapolis VA Health Care System (No. 3949-B).
Study patients
Adult patients (≥18 years) who underwent isolated CABG at the Minneapolis VA Health Care System between January 1, 2001 and December 31, 2014 were identified from the cardiac surgery database. Patients who had a paired echocardiographic assessment of LV function within 6 months of surgery and between 3 to 24 months post-operatively were included in this retrospective analysis.
Echocardiographic measurements of left ventricular (LV) variables
The 2-D echocardiography studies, including chamber quantification measurements, were performed by experienced cardiac sonographers and interpreted and verified by board-certified cardiologists certified in 2-D echocardiography. Variables of LV performance that were measured included LV internal diameter during end-diastole (LVIDd), LV internal diameter during end-systole (LVIDs), LVEF, left atrial diameter (LAD), LV mass, septal wall thickness and posterior wall thickness. Measurements were performed according to the American Society of Echocardiography chamber quantification guidelines (14).
Definitions
Improvement in LVEF was defined as >5% absolute increase in LVEF in comparison to the pre-operative echocardiogram. Consequently, LVEF that decreased by >5% compared to the pre-operative echocardiogram was categorized as worsened. All other post-operative EF measurements within ±5% of the pre-operative values were categorized as unchanged.
Statistical analysis
Continuous and categorical variables are summarized as mean ± standard deviation (SD) and frequency (%), respectively. We used logistic regression analysis to examine the variables associated with peri-operative improvement in EF. Changes in pre- versus post-operative echocardiographic parameters were compared using paired t-test. All P values were two sided with significance of <0.05. Analyses were performed using SPSS, version 19.0 Armonk, NY: IBM Corp.
Results
Study population
Between January 1, 2001 and December 31, 2014, a total of 2,838 patients underwent isolated CABG at the Minneapolis VA Health Care System. Of these, 375 had a paired echocardiographic assessment of LV function before (within 6 months) and after (3 to 24 months) surgery and were included in this analysis.
Baseline demographic and clinical characteristics in the study population as a whole and in the subgroups of patients with normal and decreased pre-operative LVEF are presented in Table 1. The mean age of the study group was 66±9 years and all patients were men. A substantial proportion of patients had multiple comorbidities. Pre-operative medications included beta blockers in 92% of patients, an angiotensin-converting enzyme inhibitor or an angiotensin receptor blocker in 71%, diuretics in 44%, as well as spironolactone, digoxin, and hydralazine with isosorbide dinitrate combination in a minority of patients (Table 1). Fifty four percent of patients had normal LVEF (≥50%) at baseline. Patients with decreased pre-operative LVEF were more likely to have a prior myocardial infarction and were treated with a more intense heart failure medication regimen (Table 1).
Table 1. Baseline characteristics in the study population as a whole and in the subgroups of patients with normal and decreased pre-operative left ventricular ejection fraction.
| Variables | All patients (n=375) | LVEF ≥50% (n=203) | LVEF <50% (n=172) | P value* |
|---|---|---|---|---|
| Age, years | 66±9 | 67±9 | 66±9 | 0.85 |
| Male sex, % | 100 | 100 | 100 | 1.00 |
| Prior MI, % | 72 | 57 | 90 | <0.001 |
| Hypertension, % | 89 | 92 | 86 | 0.09 |
| Diabetes mellitus, % | 42 | 43 | 41 | 0.69 |
| Atrial fibrillation, % | 11 | 12 | 11 | 0.68 |
| Current smoker,% | 24 | 22 | 25 | 0.52 |
| Body mass index, kg/m2 | 30.2±5.8 | 30.6±6.2 | 29.7±5.4 | 0.12 |
| COPD, % | 33 | 30 | 37 | 0.15 |
| CVA, % | 28 | 31 | 25 | 0.23 |
| PAD, % | 47 | 46 | 48 | 0.71 |
| Prior cardiac surgery, % | 5 | 4 | 6 | 0.54 |
| Permanent pacemaker, % | 7 | 5 | 9 | 0.10 |
| Laboratory values | ||||
| GFR, mL/min/1.73 m2 | 85±36 | 86±38 | 83±34 | 0.40 |
| Hemoglobin, g/dL | 13.4±1.8 | 13.4±1.8 | 13.4±1.9 | 0.34 |
| Medications | ||||
| Beta blocker, % | 92 | 88 | 93 | 0.42 |
| ACEI/ ARB, % | 71 | 67 | 76 | 0.05 |
| Spironolactone, % | 6 | 2 | 12 | <0.001 |
| Hydralazine and ISDN, % | 4 | 2 | 8 | 0.004 |
| Digoxin, % | 8 | 4 | 14 | 0.001 |
| Diuretics, % | 44 | 31 | 59 | <0.001 |
| Operative parameters | ||||
| Number of grafts | 3.0±0.8 | 3.0±0.8 | 3.0±0.8 | 0.98 |
| Bypass time, minutes | 123±39 | 123±40 | 123±38 | 0.74 |
| Ischemic time, minutes | 80±27 | 82±27 | 78±26 | 0.87 |
| # diseased coronary vessels† | 0.35 | |||
| 1 vessel,% | 5 | 0 | 10 | |
| 2 vessels,% | 32 | 25 | 40 | |
| 3 vessels, % | 64 | 75 | 50 | |
| Left main disease, %‡ | 33 | 36 | 30 | 0.30 |
ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; COPD, chronic obstructive pulmonary disease; CVA, cerebrovascular accident; GFR, glomerular filtration rate (calculated using MDRD equation); ISDN, isosorbide dinitrate; LVEF, left ventricular ejection fraction; MI, myocardial infarction; PAD, peripheral arterial disease; *, LVEF ≥50% vs. LVEF <50%; †, defined as having ≥1 lesion(s) with ≥70% stenosis; ‡, defined as having ≥50% stenosis.
Peri-operative changes in echocardiographic LV systolic measurements
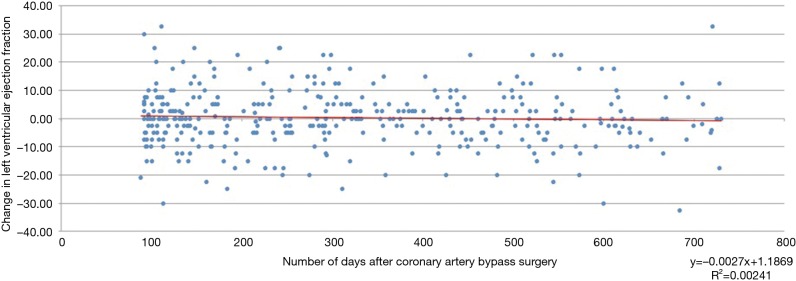
Pre-operative echocardiograms were performed at a median of 15 days [interquartile range (IQR) 4–39 days] prior to CABG. Post-operative follow-up echocardiograms were performed at a median of 291 days (IQR 147–478 days) after CABG. Figure 1 shows the individual ejection fraction changes between pre- and postoperative echocardiograms at the time (days after bypass surgery) of post-operative echocardiogram in all 375 subjects. The time of post-operative echocardiogram did not appear to have any relation to the change in EF.
Figure 1.
Scattergraph showing individual ejection fraction changes between pre- and postoperative echocardiograms at the time (days after bypass surgery) of postoperative echocardiogram.
Echocardiographic measurements before and after CABG in the study population as a whole and in the subgroups of patients with normal and decreased pre-operative LVEF are presented in Table 2. The mean LVEF did not change following CABG in the study cohort as a whole [(49±13)% vs. (49±12)%, P=0.51]. While there was a statistically significant reduction in LVIDd (from 5.4±0.8 to 5.3±0.9 cm, P=0.002) and an increase in LAD (from 4.4±0.7 to 4.6±0.7 cm, P<0.001), there were no peri-operative changes in other LV measurements including LVIDs, posterior wall thickness, septal wall thickness, and LV mass (Table 2).
Table 2. Echocardiographic measurements before and after coronary artery bypass grafting in the study population as a whole and in the subgroups of patients with normal and decreased pre-operative left ventricular ejection fraction.
| Variables | All patients, n=375 | LVEF ≥50%, n=203 | LVEF <50%, n=172 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Pre-CABG | Post-CABG | P value | Pre-CABG | Post-CABG | P value | Pre-CABG | Post-CABG | P value | |||
| LVEF, % | 49±13 | 49±12 | 0.51 | 59±5 | 56±8 | <0.001 | 36±9 | 41±12 | <0.001 | ||
| LVIDd, cm | 5.4±0.8 | 5.3±0.9 | 0.002 | 5.1±0.7 | 4.9±0.7 | 0.02 | 5.8±0.8 | 5.7±0.9 | 0.03 | ||
| LVIDs, cm | 4.0±1.0 | 3.9±1.0 | 0.43 | 3.4±0.6 | 3.4±0.7 | 0.81 | 4.6±0.9 | 4.6±0.9 | 0.39 | ||
| LAD, cm | 4.4±0.7 | 4.6±0.7 | <0.001 | 4.3±0.7 | 4.6±0.8 | <0.001 | 4.5±0.7 | 4.7±0.7 | <0.001 | ||
| LV mass, g | 273±83 | 265±84 | 0.11 | 249±68 | 240±65 | 0.17 | 300±91 | 293±95 | 0.36 | ||
| PWT, cm | 1.2±0.2 | 1.2±0.2 | 0.58 | 1.2±0.2 | 1.2±0.2 | 0.81 | 1.2±0.2 | 1.2±0.2 | 0.32 | ||
| SWT, cm | 1.2±0.3 | 1.2±0.2 | 0.70 | 1.2±0.2 | 1.2±0.2 | 0.85 | 1.2±0.2 | 1.2±0.3 | 0.72 | ||
CABG, coronary artery bypass grafting; LAD, left atrial diameter; LVEF, left ventricular ejection fraction; LVIDd, left ventricular internal diameter during end-diastole; LVIDs, left ventricular internal diameter during end-systole; PWT, posterior wall thickness; SWT, septal wall thickness. Values presented as mean ± standard deviation.
With CABG, there was a statistically significant decline in LVEF in the subgroup of patients with normal pre-operative LVEF [from (59±5)% to (56±8)%, P<0001], resulting in a mean change in EF of −3% (range −33% to 15%). There was a significant improvement in LVEF in the subgroup of patients with pre-operative LV dysfunction [from (36±9)% to (41±12)%, P<0.001], resulting in a mean change in EF of 5% (range −23% to 33%) (Figure 2). Similar to the overall group, LVIDd decreased and LAD increased significantly, regardless of baseline normal or abnormal LV systolic function.
Figure 2.
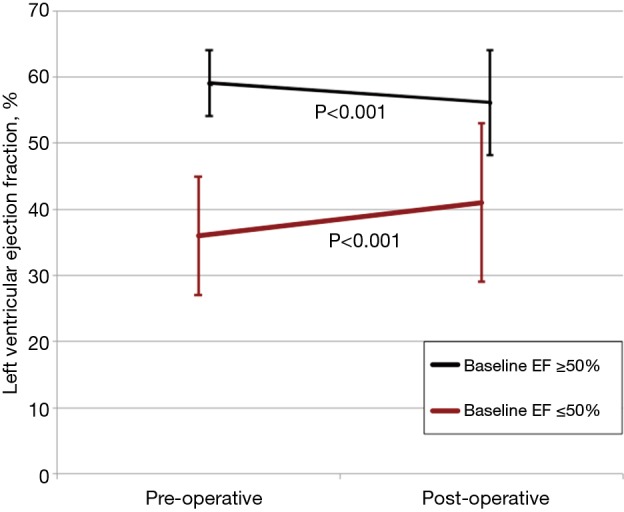
Changes in left ventricular ejection fraction with coronary artery bypass graft surgery in patients with normal and decreased pre-operative left ventricular ejection fraction (EF).
Predictors of changes in LV systolic parameters and function post-CABG surgery
LVEF improved by >5% in 89 patients (24% of the study population), did not change (±5%) in 207 patients (55%), and worsened by >5% in 79 (21%) patients. Pre- and post-operative LVEF was 38%±12% vs. 52%±12%, respectively, (P<0.001) in patients with improved EF and 55%±11% vs. 42%±12%, respectively, (P<0.001) in patients with worsened EF.
Patients with improved EF were less often diabetic, had lower pre-operative LVEF, and had greater LV dimensions at baseline (Tables 3 and 4). The improved EF group had a significant reduction in mean LVIDd (5.7 vs. 5.3 mm, P=0.03) and mean LVIDs (4.3 vs. 4.0 mm, P=0.09) pre- and post-CABG (data not shown). The use of angiotensin converting enzyme inhibitors or angiotensin II receptor blocking medication use did not have a statistically significant influence on peri-operative EF change (P=0.46).
Table 3. Baseline characteristics of all patients in relation to perioperative change in left ventricular ejection fraction.
| Variables | All patients (n=375) | Improved LVEF (n=89) | Unchanged LVEF (n=207) | Worsened LVEF (n=79) | P value* |
|---|---|---|---|---|---|
| Age, years | 66±9 | 66±8 | 66±9 | 67±9 | 0.59 |
| Male sex, % | 100 | 100 | 100 | 100 | 1.00 |
| Prior myocardial infarction, % | 72 | 79 | 69 | 73 | 0.20 |
| Hypertension, % | 89 | 85 | 88 | 95 | 0.13 |
| Diabetes mellitus, % | 42 | 30 | 46 | 46 | 0.03 |
| Atrial fibrillation, % | 11 | 9 | 12 | 13 | 0.73 |
| Chronic kidney disease stage ≥3, % | 25 | 25 | 25 | 27 | 0.96 |
| Chronic obstructive pulmonary disease, % | 33 | 40 | 28 | 37 | 0.08 |
| Cerebrovascular disease, % | 28 | 26 | 29 | 29 | 0.87 |
| Peripheral vascular disease, % | 47 | 40 | 50 | 47 | 0.3 |
| Prior cardiac surgery, % | 5 | 8 | 3 | 6 | 0.23 |
| Permanent pacemaker, % | 7 | 9 | 5 | 9 | 0.39 |
| Laboratory values | |||||
| GFR**, mL/min/1.73 m2 | 85±36 | 84±34 | 84±35 | 86±43 | 0.93 |
| Hemoglobin, g/dL | 13.4±1.8 | 13.5±2.0 | 13.3±1.8 | 13.4±1.7 | 0.72 |
| Medications | |||||
| Beta blocker, % | 93 | 93 | 92 | 93 | 0.95 |
| ACEI/ ARB, % | 71 | 76 | 70 | 68 | 0.46 |
| Spironolactone, % | 6 | 7 | 7 | 4 | 0.61 |
| Hydralazine and ISDN, % | 4 | 7 | 4 | 4 | 0.43 |
| Digoxin, % | 8 | 9 | 8 | 10 | 0.73 |
| Diuretics, % | 44 | 51 | 42 | 41 | 0.31 |
| Operative parameters | |||||
| Number of grafts | 3.0±0.8 | 3.0±0.8 | 2.9±0.8 | 3.0±0.9 | 0.73 |
| Bypass time, minutes | 123±39 | 130±41 | 120±36 | 125±42 | 0.09 |
| Ischemic time, minutes | 80±27 | 81±26 | 80±25 | 81±31 | 0.94 |
| Emergent/urgent surgery, % | 25 | 24 | 26 | 23 | 0.81 |
ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; GFR, glomerular filtration rate (**calculated using MDRD equation); ISDN, isosorbide dinitrate; LV, left ventricular; LVEF, left ventricular ejection fraction. *, P value comparing trend between improved, unchanged, and worsened LVEF groups.
Table 4. Baseline echocardiographic parameters of all patients in relation to perioperative change in left ventricular ejection fraction.
| Variables | All patients (n=375) | Improved LVEF (n=89) | Unchanged LVEF (n=207) | Worsened LVEF (n=79) | P value* |
|---|---|---|---|---|---|
| LV ejection fraction, % | 49±13 | 38±12 | 51±12 | 55±11 | <0.001 |
| LVIDd, cm | 5.4±0.8 | 5.7±0.9 | 5.4±0.8 | 5.3±0.8 | 0.02 |
| LVIDs, cm | 4.0±1.0 | 4.3±1.1 | 3.9±0.9 | 3.7±0.9 | 0.002 |
| LV mass, g | 275±88 | 291±102 | 273±82 | 261±87 | 0.18 |
| Left atrial diameter, cm | 4.4±0.7 | 4.3±0.7 | 4.4±0.7 | 4.3±0.7 | 0.78 |
| Posterior wall thickness, cm | 1.2±0.2 | 1.2±0.2 | 1.2±0.2 | 1.2±0.2 | 0.93 |
| Septal wall thickness, cm | 1.2±0.3 | 1.2±0.2 | 1.2±0.3 | 1.2±0.2 | 0.50 |
LVIDd, left ventricular internal diameter during end-diastole; LVIDs, left ventricular internal diameter during end-systole; LV, left ventricular; LVEF, left ventricular ejection fraction. *, P value comparing trend between improved, unchanged, and worsened LVEF groups.
In logistic regression analysis, the odds of EF improvement increased by 50% for every 5% absolute decrease in pre-operative EF (odds ratio 1.50, 95% confidence interval 1.35 to 1.66; P<0.0001). As such, patients with severe LV dysfunction (EF ≤35%) were 7 times more likely to have improved EF (odds ratio 7.1, 95% confidence interval 4.1 to 12.4; P<0.0001) than those with a normal pre-operative EF.
Discussion
In this study, a decrease in LV systolic function with CABG was observed in patients with normal pre-operative LVEF and an improvement in LV systolic function was observed in patients with decreased pre-operative LVEF.
The assessment of changes in pre- and post-operative LV systolic indices and function after CABG is limited, perhaps due to the lack of routine echocardiography after CABG. Our study is the largest to assess pre- and post-operative echocardiograms in a population including both normal and reduced pre-operative LV function. While prior studies have similarly found an improvement in LV systolic function in patients with pre-operative LV systolic dysfunction, to our knowledge, this is the first study to show a decrease in LVEF with CABG in patients with normal baseline LV systolic function. While the magnitude of decrease in LVEF was small (mean 3% reduction), which may not have any clinical significance, the change in EF ranged from −33% to 15%, meaning that some patients had a clinically significant decline in EF. A decrease in LV systolic function with CABG surgery may result from intra-operative global ischemia (15) or myocardial stunning (16), or from early post-operative graft failure (17). These results warrant confirmation in prospective studies with unselected patients.
Few studies have compared pre- and post-operative cardiac imaging after CABG. In patients with preserved pre-operative systolic function, Diller et al. prospectively followed 32 patients at 5 days, 6 weeks, and 18 months after CABG, demonstrating an improvement in LV diastolic function. In contrast to our results, this small study did not find a significant reduction in LV systolic function immediately after CABG (18). In patients with reduced LV function, several smaller studies have shown improvement in LV function with CABG in patients with baseline LV systolic dysfunction (19-21). The STICH trial was the only prospective, randomized, controlled trial to specifically investigate the role of CABG in patients with severe LV systolic dysfunction (EF ≤35%). A post hoc subgroup analysis of this trial showed a significant improvement in LV size and function in the subgroup of patients with higher baseline LV end-systolic dimensions (10). Similarly, the results of our study show EF improvement to be associated with greater baseline LV dimensions. Our study is also in agreement with these studies in demonstrating an improvement in LVEF in the subgroup of patients with pre-operative LV systolic dysfunction.
Subject to the limitations of the study, these observations add to our understanding of the effect of CABG on LV systolic performance. The most common etiology of heart failure with decreased EF is ischemic heart disease (22). While ischemic factors and myocardial infarction are believed to be causing this ischemic cardiomyopathy, our study suggests that in patients with normal LV systolic function, factors related to CABG itself may be contributing to the development of LV systolic dysfunction. We believe that these results are worthy of further investigation as they seem to add to our understanding of the factors contributing to LV dysfunction in patients with ischemic heart disease.
There are several limitations to point out. The study has the disadvantages of being retrospective and uncontrolled and is thus hypothesis generating rather than definitive. Because echocardiograms were not routinely obtained after CABG surgery, there may be selection bias. Echocardiographic measurements in individual patients were made on only two occasions, so we cannot say whether these changes remained consistent in the longer term in their direction or magnitude. In addition, echocardiography is not the best way to assess cardiac function, with a reported ~6% inter-observer variability in the measurement of EF (23). Finally, the study was carried out at the VA Health Care System and was therefore limited to only men.
Conclusions
In patients undergoing CABG, pre-operative LVEF appears to be an important determinant of change in LV function following surgery. In our study, patients with pre-operative LVEF <50% had an improvement in LV systolic function whereas those with normal pre-operative LVEF had a decline in LV systolic function. The other LV indices that changed after CABG were LVIDd, which decreased, and LAD, which increased. The present study broadens the existing knowledge on peri-operative changes in LV systolic function in patients undergoing CABG, to include a population with both normal and depressed pre-operative LV systolic function.
Acknowledgements
None.
Ethical Statement: This study was approved by the Institutional Review Board at the Minneapolis VA Health Care System (No. 3949-B).
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2013;62:e147-239. 10.1016/j.jacc.2013.05.019 [DOI] [PubMed] [Google Scholar]
- 2.Hillis LD, Smith PK, Anderson JL, et al. 2011 ACCF/AHA Guideline for Coronary Artery Bypass Graft Surgery: executive summary: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation 2011;124:2610-42. 10.1161/CIR.0b013e31823b5fee [DOI] [PubMed] [Google Scholar]
- 3.Levine GN, Bates ER, Blankenship JC, et al. 2011 ACCF/AHA/SCAI Guideline for Percutaneous Coronary Intervention: executive summary: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Society for Cardiovascular Angiography and Interventions. Circulation 2011;124:2574-609. 10.1161/CIR.0b013e31823a5596 [DOI] [PubMed] [Google Scholar]
- 4.Fihn SD, Gardin JM, Abrams J, et al. 2012 ACCF/AHA/ACP/AATS/PCNA/SCAI/STS guideline for the diagnosis and management of patients with stable ischemic heart disease: a report of the American College of Cardiology Foundation/American Heart Association task force on practice guidelines, and the American College of Physicians, American Association for Thoracic Surgery, Preventive Cardiovascular Nurses Association, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. Circulation 2012;126:e354-471. 10.1161/CIR.0b013e318277d6a0 [DOI] [PubMed] [Google Scholar]
- 5.Caracciolo EA, Davis KB, Sopko G, et al. Comparison of surgical and medical group survival in patients with left main equivalent coronary artery disease. Long-term CASS experience. Circulation 1995;91:2335-44. 10.1161/01.CIR.91.9.2335 [DOI] [PubMed] [Google Scholar]
- 6.Varnauskas E. Twelve-year follow-up of survival in the randomized European Coronary Surgery Study. N Engl J Med 1988;319:332-7. 10.1056/NEJM198808113190603 [DOI] [PubMed] [Google Scholar]
- 7.VA Coronary Artery Bypass Surgery Cooperative Study Group . Eighteen-year follow-up in the Veterans Affairs Cooperative Study of Coronary Artery Bypass Surgery for stable angina. Circulation 1992;86:121-30. 10.1161/01.CIR.86.1.121 [DOI] [PubMed] [Google Scholar]
- 8.Velazquez EJ, Lee KL, Deja MA, et al. Coronary-artery bypass surgery in patients with left ventricular dysfunction. N Engl J Med 2011;364:1607-16. 10.1056/NEJMoa1100356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Velazquez EJ, Lee KL, Jones RH, et al. Coronary-Artery Bypass Surgery in Patients with Ischemic Cardiomyopathy. N Engl J Med 2016;374:1511-20. 10.1056/NEJMoa1602001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Michler RE, Rouleau JL, Al-Khalidi HR, et al. Insights from the STICH trial: change in left ventricular size after coronary artery bypass grafting with and without surgical ventricular reconstruction. J Thorac Cardiovasc Surg 2013;146:1139-1145.e6. 10.1016/j.jtcvs.2012.09.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grover FL, Shroyer AL, Hammermeister K, et al. A decade's experience with quality improvement in cardiac surgery using the Veterans Affairs and Society of Thoracic Surgeons national databases. Ann Surg 2001;234:464-72; discussion 472-4. 10.1097/00000658-200110000-00006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jain R, Duval S, Adabag S. How accurate is the eyeball test?: a comparison of physician's subjective assessment versus statistical methods in estimating mortality risk after cardiac surgery. Circ Cardiovasc Qual Outcomes 2014;7:151-6. 10.1161/CIRCOUTCOMES.113.000329 [DOI] [PubMed] [Google Scholar]
- 13.Garcia S, Ko B, Adabag S. Contrast-induced nephropathy and risk of acute kidney injury and mortality after cardiac operations. Ann Thorac Surg 2012;94:772-6. 10.1016/j.athoracsur.2012.04.089 [DOI] [PubMed] [Google Scholar]
- 14.Lang RM, Bierig M, Devereux RB, et al. Recommendations for chamber quantification: a report from the American Society of Echocardiography's Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr 2005;18:1440-63. 10.1016/j.echo.2005.10.005 [DOI] [PubMed] [Google Scholar]
- 15.Adabag AS, Rector T, Mithani S, et al. Prognostic significance of elevated cardiac troponin I after heart surgery. Ann Thorac Surg 2007;83:1744-50. 10.1016/j.athoracsur.2006.12.049 [DOI] [PubMed] [Google Scholar]
- 16.Leung JM. Clinical evidence of myocardial stunning in patients undergoing CABG surgery. J Card Surg 1993;8:220-3. 10.1111/j.1540-8191.1993.tb01310.x [DOI] [PubMed] [Google Scholar]
- 17.Al Aloul B, Mbai M, Adabag S, et al. Utility of nuclear stress imaging for detecting coronary artery bypass graft disease. BMC Cardiovasc Disord 2012;12:62. 10.1186/1471-2261-12-62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Diller GP, Wasan BS, Kyriacou A, et al. Effect of coronary artery bypass surgery on myocardial function as assessed by tissue Doppler echocardiography. Eur J Cardiothorac Surg 2008;34:995-9. 10.1016/j.ejcts.2008.08.008 [DOI] [PubMed] [Google Scholar]
- 19.Elefteriades JA, Tolis G, Jr, Levi E, et al. Coronary artery bypass grafting in severe left ventricular dysfunction: excellent survival with improved ejection fraction and functional state. J Am Coll Cardiol 1993;22:1411-7. 10.1016/0735-1097(93)90551-B [DOI] [PubMed] [Google Scholar]
- 20.Cornel JH, Bax JJ, Elhendy A, et al. Biphasic response to dobutamine predicts improvement of global left ventricular function after surgical revascularization in patients with stable coronary artery disease: implications of time course of recovery on diagnostic accuracy. J Am Coll Cardiol 1998;31:1002-10. 10.1016/S0735-1097(98)00067-9 [DOI] [PubMed] [Google Scholar]
- 21.Vakil K, Florea V, Koene R, et al. Effect of Coronary Artery Bypass Grafting on Left Ventricular Ejection Fraction in Men Eligible for Implantable Cardioverter-Defibrillator. Am J Cardiol 2016;117:957-60. 10.1016/j.amjcard.2015.12.029 [DOI] [PubMed] [Google Scholar]
- 22.Gheorghiade M, Sopko G, De Luca L, et al. Navigating the crossroads of coronary artery disease and heart failure. Circulation 2006;114:1202-13. 10.1161/CIRCULATIONAHA.106.623199 [DOI] [PubMed] [Google Scholar]
- 23.Wood PW, Choy JB, Nanda NC, et al. Left ventricular ejection fraction and volumes: it depends on the imaging method. Echocardiography 2014;31:87-100. 10.1111/echo.12331 [DOI] [PMC free article] [PubMed] [Google Scholar]