Abstract
Vitamins are micronutrients which are essential for the maintenance of biological responses including immune system. Hence, vitamin deficiency increases a risk of infectious, allergic, and inflammatory diseases. Accumulating evidence has recently revealed the molecular and cellular mechanisms of vitamin-mediated regulation in the active and quiescent immune responses. In this review, we focus on the immunologic roles of vitamins in the regulation of homeostasis and surveillance in the gut.
Keywords: Vitamin, IgA, Inflammation, Regulatory T cell, Energy metabolism
INTRODUCTION
Gastrointestinal tracts are continuously exposed to environmental factors including pathogenic microorganisms (e.g., bacteria, virus, toxins, etc.). The invasion of pathogens is primarily prevented by the physical barrier mediated by epithelial cells such as tight junction and mucus (1,2). In the epithelium, Paneth cells produce antimicrobial peptides such as defensins, which provide additional barrier (1,2). In addition to these physical barriers, immunologic barrier is established in the gut (1,2). Among various immunologic factors, secretory immunoglobulin A (IgA) is recognized as a major important factor to prevent the infection in intestinal lumen and epithelium by inhibiting adherence of pathogens to the epithelium and also neutralizing toxins (3).
Peyer's patches (PPs) are major gut-associated lymphoid tissues and are known to be an important tissue for induction and initiation of acquired immunity inclu ding antigen-specific IgA production (4,5). PPs contain T- and B-cell region like conventional lymph nodes and, unlike conventional lymph nodes, dendritic cells (DCs) are present under the epithelium. After DCs take luminal antigens, they bring them into the T cell region and subsequently germinal centers in B cell region for the presentation of antigen and consequent induction of antigen-specific T and B cell responses. Unique immunologic environments (e.g., IL-4, TGF-β, BAFF, and APRIL) in the PPs allow the preferential differentiation of naive B cells into IgA+ B cells. After emigration of IgA+ B cells from PPs, they traffic into the intestinal lamina propria and differentiate into IgA-producing plasma cells (IgA-PCs) (4,5).
In addition to immunosurveillance, gut immune system plays an ingenious role in the keeping immunologic homeostasis (6,7,8). Because intestine is exposed not only to pathogens but also to diets and commensal bacteria, gut immune system simultaneously shows both active and quiescent immune responses against pathogens and non-pathogenic factors, respectively. Indeed, regulatory-type cells such as Foxp3+ regulatory T (Treg) cells, IL-10-producing Tr1 cells, IL-10-producing regulatory macrophages are abundantly present in the intestine (7,8). Accumulating evidence has shown that impaired regulatory functions are associated with induction of allergic (e.g., food allergy) and inflammatory (e.g., Crohn's disease and ulcerative colitis) diseases in the gut (9).
Nutrients are essential for the development, maintenance, and regulation of host immune system (10,11). Indeed, deficient or inappropriate intake of nutrients frequently associates with the increased risk of infectious, allergy, and inflammatory diseases. Among many nutrients, essential nutrients are not generated in the body and thus must be obtained exogenously. Therefore, these are directly reflected by the composition of diets. For example, omega-3 and -6 fatty acids are essential fatty acids and thus fatty acid compositions in the dietary oils directly affect the fatty acid composition in the gut and subsequent generation of lipid mediators and its regulation in the gut immune responses including allergic responses (10,12).
Vitamins are also essential nutrients which are synthesized by many bacteria, yeast and plants, but not in mammalians including humans (11). Therefore, vitamins need to be acquired from the diets and/or commensal bacteria (13). Some of these vitamins are water soluble (e.g., vitamin B family and vitamin C) and the others are hydrophobic (e.g., vitamin A, D, E, and K), which have diverse functions in the metabolic pathways and transcription in all living organisms. These functions are coincident with the immunological regulation and hence vitamin-deficiency results in high susceptibility to infectious and immune diseases. In this review, we describe recent findings on the specific functions of vitamins in the maintenance of immunologic homeostasis and the regulation of immunosurveillance, especially in the gut.
PIVOTAL ROLES OF VITAMINS IN THE MAINTENANCE OF IMMUNOLOGIC HOMEOSTASIS IN THE GUT
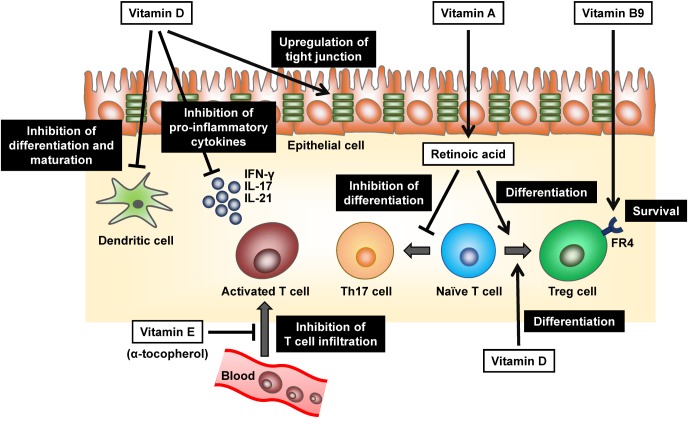
To maintain the immunologic homeostasis in the harsh environment of gut, numerous numbers of Treg cells exist in the gut (7). Previous studies including ours showed that both induction and maintenance of Treg cells were mediated by vitamins (Fig. 1).
Figure 1. Pivotal roles of vitamins in the maintenance of immunologic homeostasis in the gut. Vitamin A-derived retinoic acid promotes the differentiation of naive T cells to Treg cells and simultaneously inhibits the induction of Th17 cells in the steady state. Like retinoic acid, Vitamin D (as an active form1α,25-dihydroxyvitamin D3) inhibits the production of pro-inflammatory cytokines such as IFN-γ, IL-17 and IL-21 from T cells together with the promoted differentiation of Treg cells. It also prevents differentiation and maturation of DCs and increases the expression of tight junction protein such as claudins in the epithelial cells. Upon the differentiation of Treg cells, they express high levels of vitamin B9 receptor (folate receptor 4, FR4), which essential for their survival. α-tocopherol, an isoform of vitamin E, can inhibit T cell infiltration into intestine through the negative regulation of signal transduction from VCAM-1 and ICAM-1 by antagonizing protein kinase C.
Induction of Treg cells is enhanced by vitamin A, especially retinoic acid (RA) (Fig. 1) (14,15,16). RA binds to retinoic acid receptors (RAR), a nuclear receptor, and regulates the gene transcription. RA induces expres sion of Foxp3 in naive T cells through RAR and thus enhances the preferential induction of Treg cells in TGF-β dependent manner. Indeed, treatment of naive T cells with RAR antagonist inhibits the induction of Foxp3 and differentiation to Treg cells. Simultaneously, RA prevents the induction of Th17 cells in the steady state by inhibiting IL-6 receptor expression (Fig. 1) (16,17). Since intestinal DCs predominantly produce RA from vitamin A by the action of retinaldehyde dehydrogenases (18) and thus T cells primed with intestinal DCs tend to differentiate into Treg cells.
Once Treg cells differentiated, they obtain the expression of high levels of vitamin B9 receptor (folate receptor 4, FR4) (19). Administration of FR4 monoclonal antibody specifically reduced Treg cells (19). Consistently, dietary vitamin B9 (also known as folate or folic acid) deficiency resulted in the reduction of Treg cells in the intestine in mice (20,21). In the same line, in vitamin B9-reducing condition in vitro, Treg cells could differentiate from naive T cells but failed to survive due to the decreased expression of anti-apoptotic Bcl2 molecules (20). Due to the impaired maintenance of gut homeostasis, mice fed with a vitamin B9-deficient diet exhibited higher susceptibility to intestinal inflammation (21). Thus, vitamin B9 is a survival factor for Treg cells expressing high levels of FR4, which plays an important role to maintain the immunological homeostasis in the intestine (Fig. 1).
Vitamin D is another vitamin, which is crucial for the maintenance of immunologic homeostasis. Generally, vitamin D is synthesized from cholesterol in skin in sun exposure condition and facilitates intestinal absorption of calcium, which is responsible for the appropriate remodeling of bone (22). It is well known that vitamin D enhances the production of antibacterial peptides from intestinal Paneth cells and macrophages, which provide innate type anti-microbial immunity (Fig. 2) (23,24).
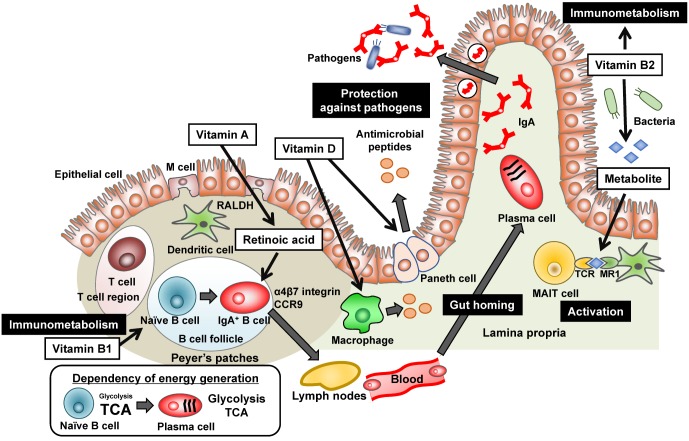
Figure 2. Various roles of vitamins in the regulation of gut immunity. Vitamin A is converted to retinoic acid by retinal dehydrogenases (RALDH) expressing dendritic cells in the Peyer's patches, which induces the expression of gut homing molecules (α4β7 integrin and CCR9) on antigenprimed cells (e.g., IgA+ B cells) and allows them to traffic into the intestinal lamina propria. In the lamina propria, IgA+ B cells differentiate into IgA-producing plasma cells. IgA is then transported into the intestinal lumen, where it binds to pathogens to inhibit their invasion and function. Vitamin B1 is essential for energy metabolism, especially maintenance of TCA cycle, and therefore associates with maintenance of naive B cells which utilize predominantly TCA cycle for energy generation. Vitamin B2 also involves in the energy metabolism of immune cells. In addition, bacterial metabolite of vitamin B2 activates mucosal associated invariant T (MAIT) cells via the presentation by major histocompatibility complex (MHC) related protein MR1. Vitamin D enhances production of antimicrobial peptides from Paneth cells and macrophages via vitamin D receptor, which provides an additional immunosurveillance system.
Additionally, vitamin D (as an active form 1α,25-dihydroxyvitamin D3)-vitamin D receptor axis strengthens the epithelial tight junction in the gut by upregulating tight junction proteins such as claudins (Fig. 1) (25). Furthermore, immune cells such as activated T cells, DCs, and macrophages express vitamin D receptor. 1α,25-dihydroxyvitamin D3 inhibits production of proinflammatory cytokines including IFN-γ, IL-17 and IL-21 from T cells and promote generation of Treg cells (Fig. 1) (26). DCs are also targets of 1α,25-dihydroxyvitamin D3, which prevents differentiation and maturation of DCs (Fig. 1) (27). Indeed, it has been reported that vitamin D deficiency associates with inflammatory diseases such as Crohn's disease (CD), ulcerative colitis (UC), and atopic dermatitis (AD) in human and vitamin D supplementation decreased severity and symptoms of AD (28,29). Collectively, these findings suggest that vitamin D exerts anti-inflammatory properties by modulating immune and epithelial cells (Fig. 1).
Vitamin E (also known as Tocopherol) generally acts as hydrophobic antioxidant and protects cell membranes from free radicals and scavenges reactive oxygen species (ROS) (30). Vitamin E is composed of at least four natural forms, α-, β-, γ-, and δ-tocopherol (30). Intriguingly, they show opposing immune regulatory functions (31,32). α-tocopherol inhibits inflammatory responses whereas γ-tocopherol accelerates them (Fig. 1) (32). The opposite effects of vitamin E is explained at least partly by regulation of adhesion molecules, vascular cell adhesion molecule-1 (VCAM-1) and intercellular adhesion molecule-1 (ICAM-1), both are important factors for cell recruitment during inflammation (Fig. 1) (32,33). α-tocopherol negatively regulates the signal transduction from VCAM-1 and ICAM-1 by antagonizing protein kinase C (PKC), whereas γ-tocopherol act as an agonist of PKC and elevates the signal transduction (32,34). Vitamin E is an essential vitamin and obtained from the diets such as plant oils because plants mainly synthesize it. Of note, sunflower seeds, olive oil, and almonds are rich sources of α-tocopherol but other seeds and seed oils generally contain more γ-tocopherol than α-tocopherol (35). Therefore, dietary habits may influence immunological regulation of vitamin E.
VITAMINS AND HOST IMMUNE RESPONSES
To achieve the functional immune response, immune cells should traffic appropriately. In this issue, RA plays a pivotal role in the regulation of cell trafficking in addition to the control of T cell differentiation (Fig. 2) (36). The cell trafficking into the gut is mediated by gut homing receptors (e.g., α4β7 integrin and the chemokine receptor CCR9) (37,38). As mentioned above, intestinal DCs produce RA preferentially (18). Therefore, when T and B cells activated by intestinal DCs, they acquire the expression of α4β7 integrin and CCR9, allowing them to traffic preferentially into the gut (Fig. 2) (37,38). Indeed, vitamin A deficiency increases the risk of infectious diseases such as severe diarrhea especially in childhood due to the inappropriate immune cell trafficking and functions in the gut (39,40).
Vitamin B2 (also known as riboflavin) and its active forms (e.g., Flavin adenine dinucleotide [FAD]) are known as cofactors for various enzymatic reactions such as tricarboxylic acid (TCA) cycle and fatty acid oxidation (41). Thus, vitamin B2 has been considered to be involved in the energy metabolism in immune cells (Fig. 2). This area is now recognized as emerging field of immunology as “immunometabolism” (42). Not only energy metabolism, vitamin B2 also stimulates immune cells, especially mucosal associated invariant T (MAIT) cells, an abundant population of innate-like T cells (Fig. 2) (43,44). In addition to dietary sources, bacteria and yeast are able to synthesize vitamin B2 from guanosine triphosphate (GTP) and ribulose 5-phosphate. It is interesting to note that bacterial metabolite of vitamin B2 is recognized by MAIT cells via its presentation by major histocompatibility complex (MHC) related protein MR1 (Fig. 2) (44,45). Activated MAIT cells produce cytokines such as IFN-γ and IL-17 and contribute to exclusion of bacteria as well as the development of inflammatory diseases (45,46).
ASSOCIATION OF VITAMINS WITH IMMUNOMETABOLISM
As vitamin B2, vitamin B1 (also known as thiamine) and its derivatives (e.g., thiamine pyrophosphate) act as a cofactor for several enzymes such as pyruvate dehydrogenase and α-ketoglutarate dehydrogenase involved in TCA cycle (47,48). Although it is well known that vitamin B1 deficiency causes neurological disorders such as Wernicke Korsakoff syndrome, beriberi, increased blood flow, and heart failure (49), vitamin B1 is also immunologically important nutrient. Indeed, mice maintained with vitamin B1-deficient diet showed impaired antigen-specific immune responses against oral vaccine which was associated with the defects of energy metabolism (Fig. 2) (50).
Energy metabolism is determined by the balance between anabolism (ATP generation) and catabolism (ATP consumption). Additionally, cells change the dependency in energy generation on glycolysis, TCA cycle, or both. Generally, quiescent or regulatory-type cells such as naive T cells, Treg cells, M2 macrophages employ anabolic pathway for energy generation from TCA cycle, especially fatty acid oxidation (42). In contrast, activated or inflammatory cells (e.g., Th1, Th2, Th17, and M1 macrophages) show catabolic pathways and shift to glycolysis for energy generation (42). Like these cells, naive B cells in the PPs obtain the energy from TCA cycle and IgA-PCs in the intestinal lamina propria shift to glycolytic pathway (Fig. 2) (50). Consistently, the expression level of the thiamine transporter 1 (THTR1), vitamin B1 transporter, was higher in the naive B cells than in IgA-PCs (50). In agreement with these findings, mice maintained with vitamin B1-deficient diet showed decreased numbers of naïve B cells in the PPs and simultaneous reduction in the size of PPs (50). In contrast, IgA-PCs in the intestinal lamina propria were unchanged in the same condition (50).
CONClUsION
Appropriate balance between active and suppressive immune responses is achieved by intricate and ingenious regulation systems in the gut, which plays an important role in the establishment of both protection against pathogens and prevention of immune diseases. Accumulating evidence has revealed molecular and cellular mechanisms of specific function of vitamins in the control of particular immune responses. Based on the immunologic functions of vitamins, vitamin supplementation is efficient for the reduction of infectious risk, establishment of vaccination, and control of immune diseases. Indeed, world health organization (WHO) recommends vitamin A supplementation with vaccination. Thus, vitamins can be considered to be natural adjuvant for vaccination and immunoregulators. Further studies will extend our understanding about immunologic functions of vitamins and its application to eradicate infectious, allergic, and inflammatory diseases.
ACKNOWLEDGEMENTS
The author's work featured in this review article was supported by grants from MEXT/JSPS KAKENHI Grant Numbers 16H01373, 15H05790, 26293111; from the The Ministry of Health, Labour and Welfare (MHLW) and the Research on Development of New Drugs, the Japan Agency for Medical Research and Development (AMED); the Science and Technology Research Promotion Program for Agriculture, Forestry, Fisheries, and Food Industry; Astellas Foundation for Research on Metabolic Disorders, Terumo Foundation for Life Sciences and Arts and Suzuken Memorial Foundation.
Abbreviations
- PPs
Peyer's patches
- DCs
Dendritic cells
- BAFF
B cell activating factor
- APRIL
A proliferation inducing ligand
- IgA-PCs
IgA-producing plasma cells
- Treg cells
Foxp3+ regulatory T cells
- IL-10
Interleukin-10
- Tr1 cells
Foxp3- type 1 regulatory T cells
- RA
Retinoic acid
- RAR
Retinoic acid receptors
- FR4
Folate receptor 4
- Bcl2
B cell lymphoma 2
- CD
Crohn's disease
- UC
Ulcerative colitis
- AD
Atopic dermatitis
- ROS
Reactive oxygen species
- PKC
Protein kinase C
- FAD
Flavin adenine dinucleotide
- TCA cycle
Tricarboxylic acid cycle
- MAIT cells
Mucosal associated invariant T cells
- MR1
MHC related molecule 1
- THTR1
Thiamine transporter 1
- WHO
World health organization
Footnotes
CONFLICTS OF INTEREST: The authors declare no conflict of interest associated with this manuscript.
References
- 1.Kunisawa J, Kiyono H. Immune regulation and monitoring at the epithelial surface of the intestine. Drug Discov Today. 2013;18:87–92. doi: 10.1016/j.drudis.2012.08.001. [DOI] [PubMed] [Google Scholar]
- 2.Hooper LV, Macpherson AJ. Immune adaptations that maintain homeostasis with the intestinal microbiota. Nat Rev Immunol. 2010;10:159–169. doi: 10.1038/nri2710. [DOI] [PubMed] [Google Scholar]
- 3.Brandtzaeg P. Secretory IgA: Designed for Anti-Microbial. Defense Front Immunol. 2013;4:222. doi: 10.3389/fimmu.2013.00222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kunisawa J, Kurashima Y, Kiyono H. Gut-associated lymphoid tissues for the development of oral vaccines. Adv Drug Deliv Rev. 2012;64:523–530. doi: 10.1016/j.addr.2011.07.003. [DOI] [PubMed] [Google Scholar]
- 5.Nagatake T, Kunisawa J. Unique functions of mucosa-associated lymphoid tissues as targets of mucosal vaccines. Curr Topics Pharmacol. 2013;17:13–23. [Google Scholar]
- 6.Corthay A. How do regulatory T cells work? Scand J Immunol. 2009;70:326–336. doi: 10.1111/j.1365-3083.2009.02308.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tanoue T, Atarashi K, Honda K. Development and maintenance of intestinal regulatory T cells. Nat Rev Immunol. 2016;16:295–309. doi: 10.1038/nri.2016.36. [DOI] [PubMed] [Google Scholar]
- 8.Bain CC, Mowat AM. Macrophages in intestinal homeostasis and inflammation. Immunol Rev. 2014;260:102–117. doi: 10.1111/imr.12192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kotlyar DS, Shum M, Hsieh J, Blonski W, Greenwald DA. Non-pulmonary allergic diseases and inflammatory bowel disease: a qualitative review. World J Gastroenterol. 2014;20:11023–11032. doi: 10.3748/wjg.v20.i32.11023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lamichhane A, Kiyono H, Kunisawa J. Nutritional components regulate the gut immune system and its association with intestinal immune disease development. J Gastroenterol Hepatol. 2013;28(Suppl 4):18–24. doi: 10.1111/jgh.12259. [DOI] [PubMed] [Google Scholar]
- 11.Suzuki H, Kunisawa J. Vitamin-mediated immune regulation in the development of inflammatory diseases. Endocr Metab Immune Disord Drug Targets. 2015;15:212–215. doi: 10.2174/1871530315666150316122128. [DOI] [PubMed] [Google Scholar]
- 12.Buckley CD, Gilroy DW, Serhan CN. Proresolving lipid mediators and mechanisms in the resolution of acute inflammation. Immunity. 2014;40:315–327. doi: 10.1016/j.immuni.2014.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kau AL, Ahern PP, Griffin NW, Goodman AL, Gordon JI. Human nutrition, the gut microbiome and the immune system. Nature. 2011;474:327–336. doi: 10.1038/nature10213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Coombes JL, Siddiqui KR, rancibia-Carcamo CV, Hall J, Sun CM, Belkaid Y, Powrie F. A functionally specialized population of mucosal CD103+ DCs induces Foxp3+ regulatory T cells via a TGF-beta and retinoic acid-dependent mechanism. J Exp Med. 2007;204:1757–1764. doi: 10.1084/jem.20070590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sun CM, Hall JA, Blank RB, Bouladoux N, Oukka M, Mora JR, Belkaid Y. Small intestine lamina propria dendritic cells promote de novo generation of Foxp3 T reg cells via retinoic acid. J Exp Med. 2007;204:1775–1785. doi: 10.1084/jem.20070602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mucida D, Park Y, Kim G, Turovskaya O, Scott I, Kronenberg M, Cheroutre H. Reciprocal TH17 and regulatory T cell differentiation mediated by retinoic acid. Science. 2007;317:256–260. doi: 10.1126/science.1145697. [DOI] [PubMed] [Google Scholar]
- 17.Ouyang X, Zhang R, Yang J, Li Q, Qin L, Zhu C, Liu J, Ning H, Shin MS, Gupta M, Qi CF, He JC, Lira SA, Morse HC, 3rd, Ozato K, Mayer L, Xiong H. Transcription factor IRF8 directs a silencing programme for TH17 cell differentiation. Nat Commun. 2011;2:314. doi: 10.1038/ncomms1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jaensson E, Uronen-Hansson H, Pabst O, Eksteen B, Tian J, Coombes JL, Berg PL, Davidsson T, Powrie F, Johansson-Lindbom B, Agace WW. Small intestinal CD103+ dendritic cells display unique functional properties that are conserved between mice and humans. J Exp Med. 2008;205:2139–2149. doi: 10.1084/jem.20080414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yamaguchi T, Hirota K, Nagahama K, Ohkawa K, Takahashi T, Nomura T, Sakaguchi S. Control of immune responses by antigen-specific regulatory T cells expressing the folate receptor. Immunity. 2007;27:145–159. doi: 10.1016/j.immuni.2007.04.017. [DOI] [PubMed] [Google Scholar]
- 20.Kunisawa J, Hashimoto E, Ishikawa I, Kiyono H. A pivotal role of vitamin B9 in the maintenance of regulatory T cells in vitro and in vivo. PLoS One. 2012;7:e32094. doi: 10.1371/journal.pone.0032094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kinoshita M, Kayama H, Kusu T, Yamaguchi T, Kunisawa J, Kiyono H, Sakaguchi S, Takeda K. Dietary folic acid promotes survival of Foxp3+ regulatory T cells in the colon. J Immunol. 2012;189:2869–2878. doi: 10.4049/jimmunol.1200420. [DOI] [PubMed] [Google Scholar]
- 22.Grant WB, Holick MF. Benefits and requirements of vitamin D for optimal health: a review. Altern Med Rev. 2005;10:94–111. [PubMed] [Google Scholar]
- 23.Gombart AF. The vitamin D-antimicrobial peptide pathway and its role in protection against infection. Future Microbiol. 2009;4:1151–1165. doi: 10.2217/fmb.09.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu PT, Stenger S, Li H, Wenzel L, Tan BH, Krutzik SR, Ochoa MT, Schauber J, Wu K, Meinken C, Kamen DL, Wagner M, Bals R, Steinmeyer A, Zugel U, Gallo RL, Eisenberg D, Hewison M, Hollis BW, Adams JS, Bloom BR, Modlin RL. Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science. 2006;311:1770–1773. doi: 10.1126/science.1123933. [DOI] [PubMed] [Google Scholar]
- 25.Fujita H, Sugimoto K, Inatomi S, Maeda T, Osanai M, Uchiyama Y, Yamamoto Y, Wada T, Kojima T, Yokozaki H, Yamashita T, Kato S, Sawada N, Chiba H. Tight junction proteins claudin-2 and -12 are critical for vitamin D-dependent Ca2+ absorption between enterocytes. Mol Biol Cell. 2008;19:1912–1921. doi: 10.1091/mbc.E07-09-0973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jeffery LE, Burke F, Mura M, Zheng Y, Qureshi OS, Hewison M, Walker LS, Lammas DA, Raza K, Sansom DM. 1,25-Dihydroxyvitamin D3 and IL-2 combine to inhibit T cell production of inflammatory cytokines and promote development of regulatory T cells expressing CTLA-4 and FoxP3. J Immunol. 2009;183:5458–5467. doi: 10.4049/jimmunol.0803217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Penna G, Adorini L. 1 Alpha,25-dihydroxyvitamin D3 inhibits differentiation, maturation, activation, and survival of dendritic cells leading to impaired alloreactive T cell activation. J Immunol. 2000;164:2405–2411. doi: 10.4049/jimmunol.164.5.2405. [DOI] [PubMed] [Google Scholar]
- 28.Meckel K, Li YC, Lim J, Kocherginsky M, Weber C, Almoghrabi A, Chen X, Kaboff A, Sadiq F, Hanauer SB, Cohen RD, Kwon J, Rubin DT, Hanan I, Sakuraba A, Yen E, Bissonnette M, Pekow J. Serum 25-hydroxyvitamin D concentration is inversely associated with mucosal inflammation in patients with ulcerative colitis. Am J Clin Nutr. 2016;104:113–120. doi: 10.3945/ajcn.115.123786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim MJ, Kim SN, Lee YW, Choe YB, Ahn KJ. Vitamin D status and efficacy of vitamin D supplementation in atopic dermatitis: A systematic review and meta-analysis. Nutrients. 2016;8:E789. doi: 10.3390/nu8120789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Peng C, Wang X, Chen J, Jiao R, Wang L, Li YM, Zuo Y, Liu Y, Lei L, Ma KY, Huang Y, Chen ZY. Biology of ageing and role of dietary antioxidants. Biomed Res Int. 2014;2014:831841. doi: 10.1155/2014/831841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cook-Mills JM. Isoforms of vitamin E differentially regulate PKC alpha and inflammation: A review. J Clin Cell Immunol. 2013;4:1000137. doi: 10.4172/2155-9899.1000137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Berdnikovs S, Abdala-Valencia H, McCary C, Somand M, Cole R, Garcia A, Bryce P, Cook-Mills JM. Isoforms of vitamin E have opposing immunoregulatory functions during inflammation by regulating leukocyte recruitment. J Immunol. 2009;182:4395–4405. doi: 10.4049/jimmunol.0803659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Soriano A, Salas A, Salas A, Sans M, Gironella M, Elena M, Anderson DC, Pique JM, Panes J. VCAM-1, but not ICAM-1 or MAdCAM-1, immunoblockade ameliorates DSS-induced colitis in mice. Lab Invest. 2000;80:1541–1551. doi: 10.1038/labinvest.3780164. [DOI] [PubMed] [Google Scholar]
- 34.Abdala-Valencia H, Berdnikovs S, Cook-Mills JM. Vitamin E isoforms differentially regulate intercellular adhesion molecule-1 activation of PKCα in human microvascular endothelial cells. PLoS One. 2012;7:e41054. doi: 10.1371/journal.pone.0041054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schneider C. Chemistry and biology of vitamin E. Mol Nutr Food Res. 2005;49:7–30. doi: 10.1002/mnfr.200400049. [DOI] [PubMed] [Google Scholar]
- 36.Iwata M. Retinoic acid production by intestinal dendritic cells and its role in T-cell trafficking. Semin Immunol. 2009;21:8–13. doi: 10.1016/j.smim.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 37.Iwata M, Hirakiyama A, Eshima Y, Kagechika H, Kato C, Song SY. Retinoic acid imprints gut-homing specificity on T cells. Immunity. 2004;21:527–538. doi: 10.1016/j.immuni.2004.08.011. [DOI] [PubMed] [Google Scholar]
- 38.Mora JR, Iwata M, Eksteen B, Song SY, Junt T, Senman B, Otipoby KL, Yokota A, Takeuchi H, Ricciardi-Castagnoli P, Rajewsky K, Adams DH, von Andrian UH. Generation of gut-homing IgA-secreting B cells by intestinal dendritic cells. Science. 2006;314:1157–1160. doi: 10.1126/science.1132742. [DOI] [PubMed] [Google Scholar]
- 39.Bhaskaram P. Micronutrient malnutrition, infection, and immunity: an overview. Nutr Rev. 2002;60:S40–S45. doi: 10.1301/00296640260130722. [DOI] [PubMed] [Google Scholar]
- 40.Fisker AB, Bale C, Jorgensen MJ, Balde I, Hornshoj L, Bibby BM, Aaby P, Benn CS. High-dose vitamin A supplementation administered with vaccinations after 6 months of age: sex-differential adverse reactions and morbidity. Vaccine. 2013;31:3191–3198. doi: 10.1016/j.vaccine.2013.04.072. [DOI] [PubMed] [Google Scholar]
- 41.Huskisson E, Maggini S, Ruf M. The role of vitamins and minerals in energy metabolism and well-being. J Int Med Res. 2007;35:277–289. doi: 10.1177/147323000703500301. [DOI] [PubMed] [Google Scholar]
- 42.Pearce EL, Pearce EJ. Metabolic pathways in immune cell activation and quiescence. Immunity. 2013;38:633–643. doi: 10.1016/j.immuni.2013.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tilloy F, Treiner E, Park SH, Garcia C, Lemonnier F, de la SH, Bendelac A, Bonneville M, Lantz O. An invariant T cell receptor alpha chain defines a novel TAP-independent major histocompatibility complex class Ib-restricted alpha/beta T cell subpopulation in mammals. J Exp Med. 1999;189:1907–1921. doi: 10.1084/jem.189.12.1907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Corbett AJ, Eckle SB, Birkinshaw RW, Liu L, Patel O, Mahony J, Chen Z, Reantragoon R, Meehan B, Cao H, Williamson NA, Strugnell RA, Van SD, Mak JY, Fairlie DP, Kjer-Nielsen L, Rossjohn J, McCluskey J. T-cell activation by transitory neo-antigens derived from distinct microbial pathways. Nature. 2014;509:361–365. doi: 10.1038/nature13160. [DOI] [PubMed] [Google Scholar]
- 45.Cowley SC. MAIT cells and pathogen defense. Cell Mol Life Sci. 2014;71:4831–4840. doi: 10.1007/s00018-014-1708-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Serriari NE, Eoche M, Lamotte L, Lion J, Fumery M, Marcelo P, Chatelain D, Barre A, Nguyen-Khac E, Lantz O, Dupas JL, Treiner E. Innate mucosal-associated invariant T (MAIT) cells are activated in inflammatory bowel diseases. Clin Exp Immunol. 2014;176:266–274. doi: 10.1111/cei.12277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Webb ME, Marquet A, Mendel RR, Rebeille F, Smith AG. Elucidating biosynthetic pathways for vitamins and cofactors. Nat Prod Rep. 2007;24:988–1008. doi: 10.1039/b703105j. [DOI] [PubMed] [Google Scholar]
- 48.Frank RA, Leeper FJ, Luisi BF. Structure, mechanism and catalytic duality of thiamine-dependent enzymes. Cell Mol Life Sci. 2007;64:892–905. doi: 10.1007/s00018-007-6423-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Manzetti S, Zhang J, van der SD. Thiamin function, metabolism, uptake, and transport. Biochemistry. 2014;53:821–835. doi: 10.1021/bi401618y. [DOI] [PubMed] [Google Scholar]
- 50.Kunisawa J, Sugiura Y, Wake T, Nagatake T, Suzuki H, Nagasawa R, Shikata S, Honda K, Hashimoto E, Suzuki Y, Setou M, Suematsu M, Kiyono H. Mode of bioenergetic metabolism during B cell differentiation in the intestine determines the distinct requirement for vitamin B1. Cell Rep. 2015;13:122–131. doi: 10.1016/j.celrep.2015.08.063. [DOI] [PubMed] [Google Scholar]