Abstract
Human Embryonic Stem Cells (hESC) offer an important resource as a limitless supply of any differentiated cell type of the human body. Keratocytes, cells from the corneal stroma, may have the potential for restoration of vision in cell therapy and biomedical engineering applications, but these specialized cells are not readily expanded in vitro. Here we describe a two-part method to produce keratocytes from the H1 hESC cell line. The hESC cells, maintained and expanded in feeder-free culture medium are first differentiated to neural crest cells using the stromal-derived inducing activity (SDIA) of the PA6 mouse embryonic fibroblast cell line. The resulting neural crest cells are selected by their expression of cell-surface CD271 and subsequently cultured as 3D pellets in a defined differentiation medium to induce a keratocyte phenotype.
Keywords: Cornea, Human embryonic stem cells, hESC, SDIA, Neural crest, Keratocyte, Stem cells
1 Introduction
1.1 The Need for Corneal Progenitor Cells
Vision loss and impairment due to corneal scarring affects millions of people worldwide. Currently, treatment is limited to corneal transplant, a procedure that requires donor tissue availability and which can be complicated by immunological rejection and graft failure. As a result, there is increased interest in therapies that decrease dependency on donor tissue such as cell-based therapies, and in bioengineering of corneal tissue. Corneal scarring occurs in the stroma, the transparent connective tissue which constitutes >90 % of corneal thickness. Cells of the stroma, the keratocytes, are a quiescent population of neural crest-derived mesenchymal cells responsible for secretion of the highly specialized transparent tissue of the stroma. Keratocytes are limited in abundance and change phenotype when expanded in culture to fibroblasts and myofibroblasts, cells that secrete non-transparent corneal scar tissue (1–5). Thus, access to these cells for regenerative approaches is only possible using cells with the potential to differentiate into keratocytes, i.e., stem or progenitor cells.
1.2 Generating Keratocytes from Stem Cells
Directed differentiation of hESC to any cell fate within the body may lead to remarkable advances in tissue regeneration via cell therapy. To realize this goal, conditions for directed differentiation must be established to generate pure, high quality, functional populations of the cell type of interest. We have developed a method for generating corneal keratocytes, the functional cell type of the corneal stroma (6).
During embryonic development keratocytes are derived from neural crest stem cells (NCSC) that migrate from the neural tube. To recapitulate this in vitro, hESC are differentiated to NCSC via stromal-derived inducing activity (SDIA) by coculture with the murine PA6 cell line (7, 8). NCSC are then sorted to select for cells expressing the NCSC cell surface marker CD271 (NGFR, nerve growth factor receptor; p75NTR). CD271+ cells are subsequently cultured and expanded in a serum-free medium that preserves the neural crest phenotype. When the desired cell number is reached, CD271+ cells are cultured for 2 weeks as pellets in keratocyte differentiation medium (KDM). Upregulation of genes present in keratocytes such as CHST6, PTGDS, and the keratocyte-specific proteoglycan keratocan can be used to confirm differentiation of hESC to corneal keratocytes.
2 Materials
hESC line WA01 (H1) (WiCell, Madison, WI) (We expect the same approach to be applicable to other lines such asH9.) (see Note 1).
Matrigel (BD Biosciences).
Thaw at 4 °C and aliquoted into individual samples containing 270–350 µL (based on the Product Certificate of Analysis). The samples are stored at 80 °C and thawed at 4 °C before dilution to working strength.
mTeSR-1 (StemCell Technologies).
StemPro EZPassage Tool (Life Technologies).
Tissue culture dish 100 × 15 mm TC treated.
PA6 Cell Line (Riken Bioresource Center Cell Bank, Japan).
Gelatin.
TrypLE Express.
PA6 Culture Medium; 90 % MEM-alpha, 10 % fetal bovine serum.
Induction Medium; 90 % BHK21-medium/Glasgow modified Eagle’s medium, 2 mM glutamine, 10 % knockout serum replacement, 1 mM sodium pyruvate, 0.1 mM nonessential amino acid solution, 0.1 mM β-mercaptoethanol, 100 IU/mL penicillin, 100 µg/mL streptomycin.
Accutase.
Cell strainer, 70 µm.
Wash Buffer: phosphate-buffered saline (PBS), 0.5 % fetal bovine serum (FBS), 0.1 mM ethylenediaminetetraacetic acid (EDTA).
CD271 Microbead Kit (Miltenyi Biotec) containing MACS MS Column and MACS Cell Separator.
PO/L/F (poly-l-ornthine/laminin/fibronectin) Coated Plates; Poly-l-ornithine 15 µg/mL, Laminin 1 µg/mL, Fibronectin 10 µg/mL, to coat P/O/L/F plates, first coat with poly-l-ornithine overnight at 4 °C. Rinse with PBS twice the following-day. Combine and add laminin (1 µg/mL) and fibronectin (10 µg/mL) and incubate for 2 h at 37 °C.
DME/F12.
N2 Supplement (Life Technologies).
10 ng/mL FGF-2.
10 ng/mL EGF.
Advanced DMEM.
0.1 mM ascorbic acid-2-phosphate (Sigma).
10 ng/mL FGF-2.
2.1 Stock Solutions
| Reagent | Concentration | Solvent | Storage |
|---|---|---|---|
| FGF-2 | 10 μg/mL | Sterile water | −80 °C |
| Ascorbic acid-2-phosphate | 500 mM | Sterile water | −80 °C |
| Poly-l-ornithine | 1 mg/mL | Sterile water | −80 °C |
| Laminin | 1 mg/mL | Sterile water | −80 °C |
| Fibronectin | 1 mg/mL | Sterile water | −80 °C |
| EDTA | 500 mM | Sterile water | Ambient |
| Penicillin/streptomycin | Sterile water | −20 °C | |
| Gentamycin | Sterile water | 4 °C | |
| Gelatin | 1 % | Sterile water | 4 °C |
| β-mercaptoethanol | Sterile water | −20 °C |
3 Methods
3.1 Expansion of hESC Cultures
hESC are cultured on Matrigel-coated plates in mTeSR-1 basal medium (9, 10).
To coat a 100 mm culture dish, frozen (see above) aliquots of Matrigel are thawed at 4 °C and diluted in 25 mL DMEM/F12 with mixing by inversion multiple times.
Add ~8 mL diluted Matrigel + DMEM/F12 mixture to a 100 mm dish. Allow the proteins to attach to the plastic at room temperature for at least 1 h (see Note 2).
Undifferentiated hESC either cryopreserved or from passage (below) grow on Matrigel-coated dishes in flattened colorless round colonies, 1–2 mm in diameter, with well-defined edges, phase-bright colony centers, and minimal overt spontaneous differentiation.
mTeSR medium is changed daily.
The cultures are expanded every 5–7 days or when the colonies become so large that they begin to touch one another. Without removing medium, a StemPro EZ Passage Tool in is rolled over the plate twice in an orthogonal pattern to fragment hESC colonies. It is important that cells remain in discreet multicellular aggregates of 80–150 µm. Smaller aggregates (<60 µm) may exhibit decrease viability and induce spontaneous differentiation (see Note 3).
The colony fragments are centrifuged 200 × g for 2 min and the pelleted fragments are resuspended in enough medium for a 4× expansion.
hESC are cultured in a humidified incubator at 37 °C in 5 % CO2. Replace the medium daily.
To make a 0.1 % gelatin solution, first make a stock solution of 1 % gelatin by dissolving 1 g gelatin in 100 mL 1 × PBS. Autoclave to dissolve and sterilize the solution.
Dilute the 1 % gelatin stock solution 1:10 in sterile 1 × PBS to make a 0.1 % solution.
3.2 Preparation of PA6 Cells for SDIA
Add 10 mL of a 0.1 % gelatin solution to each 100 mm dish. Allow the protein to attach to the plates for 2 h at room temperature.
Rinse the plates twice with 1× sterile PBS.
Plate PA6 cells at approximately 5 × 104 cells/cm2 on 0.1 % gelatin coated plates.
PA6 cells are grown in a humidified incubator at 37 °C in 5 % CO2. Passage the cultures 1:5 by trypsinization when they become 80 % confluent.
3.3 Induction of NCSC Differentiation by PA6/hESC Coculture
Overgrown and differentiated hESC colonies are first manually removed from culture plates using a glass pipette. Overgrown colonies are identified as pigmented (usually a brown coloration visible without a microscope) and raised. They are removed by scraping with the tip of a Pasteur pipette or similar sterile sharp implement. see Note 2.
Undifferentiated hESC colonies are then sectioned and collected using the StemPro EZPassage tool and collected in a 50 mL conical tube.
Gently wash colonies in 1× PBS and spin for 5 min at 200 × g. Be careful to keep colonies as clumps and do not allow cells to become a single-cell suspension.
Resuspend the loose pellet in Induction Medium.
hESC colonies are added dropwise to 95 % confluent PA6 cells at approximately 9,000 colonies per 100 mm dish. The coculture plate is then incubated at 37 °C in 5 % CO2 for 6 days without changing medium (Fig. 1).
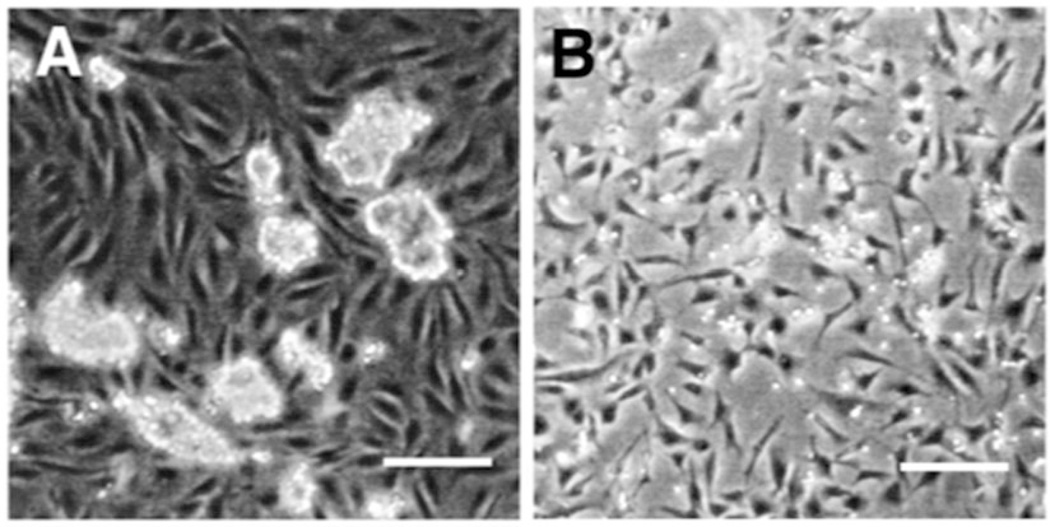
Fig. 1.
hESC coculture and CD271+ cells after isolation. (a) hESC coculture on PA6 cell monolayer. hESC retain colony-like morphology while the underlying PA6 cells remain confluent and spindle-like. (b) CD271+ Cells grown in serum free medium after isolation via MACS cell separator. Scale bars are 200 µm. Figure from ref. (6)
3.3.1 NCSC Isolation
Add 1 mL Accutase per 100 mm plate and rock back and forth to cover entire plate.
Incubate at ambient temperature observing the cultures using an inverted microscope every 5 min to monitor detachment of the hESC colonies from the PA6 monolayer.
Collect the released colonies by washing the plate with 5 mL wash buffer (0.5 % FBS, 0.1 mM EDTA in PBS). Triturate the colonies with a 10 mL pipette to achieve a single cell suspension and remove aggregates with a 70 µm cell strainer.
Count the cells. Each MS column will accommodate up to 2 × 108 cells.
Pellet up to 2 × 108 cells by centrifuging at 500 × g for 10 min.
Resuspend the cells in 80 µL wash buffer and add 10 µL FcR Blocking Reagent (Miltenyi Kit) and 10 µL APC-labeled anti-CD271 antibody (Miltenyi Kit). Incubate for 15 min in the shelf of a 4 °C refrigerator.
Add 1 mL of wash buffer and pellet cells as in step 5.
Resuspend the pellet in 70 µL wash buffer. Add 10 µL FcR blocking reagent followed by 20 µL Anti-APC MicroBeads. Incubate for 15 min in the refrigerator.
Wash the cells as in step 5.
Resuspend the cells in 500 µL wash buffer.
To prepare MS column, place the column in the Miltenyi Cell Separator magnet and rinse the column with 2 × 500 µL portions of wash buffer (see Note 4).
Replace waste tube with a 12 × 75 collection tube labeled “Flow Thru”.
Slowly pipette 500 µL filtered cell suspension to the column, be careful not to introduce bubbles which clog the column.
Wash column 2 × with 500 µL wash buffer.
Remove MS column from magnet and place over a new collection tube labeled “Bound”. Elute bound cells with 500 µL wash to column.
Rinse the column with two more 500 µL portions wash buffer.
Cells in both tubes are centrifuged, resuspended in 1 ml Wash Buffer, and counted. The samples are taken for RNA isolation.
Expression of CD271 in the “Bound” population is confirmed by flow cytometry (Fig. 2).
To improve the proportion of CD271+ cells, the isolation can be repeated immediately by repeating the magnetic column-based isolation.
Neural crest gene mRNA expression is confirmed by qPCR (Fig. 3; Table 1).
The “Bound” cell population is expanded on PO/L/F coated culture dishes at 104 cells per cm2 in N2 medium.
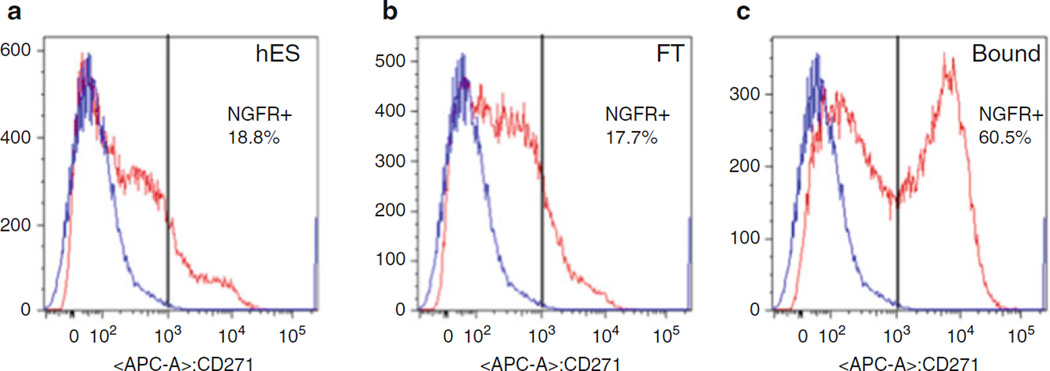
Fig. 2.
Flow cytometry verification of CD271 MACS. MACS separation of CD271 from PA6 cells after 6 days of coculture. (a) CD271 is expressed by 18.8 % of hESC cells before coculture. (b) The flow through (FT) fraction contains 17.7 % CD271+ cells. (c) The bound fraction contains 60.5 % CD271+ cells. The vertical black bar marks the population with <0.1 % nonspecific staining. The blue line shows staining with isotype-matched control antibody. The red line shows CD271-APC stained cells. Percentage of stained cells is shown. Figure from ref. (6)
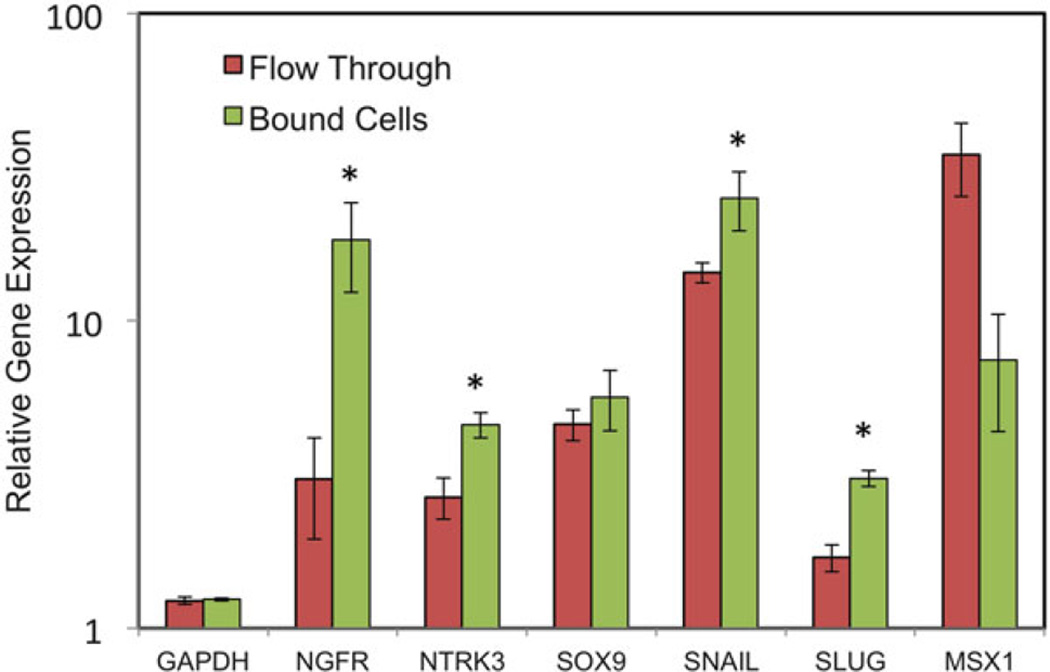
Fig. 3.
Neural Crest Gene Expression of CD271+ Sorted Cells. Neural Crest Genes are Upregulated after PA6 Coculture. Expression of the neural crest genes NGFR, NTRK3, SOX9, SNAI1, SLUG, and MSX1 was measured after 6-days of hES:PA6 coculture. Bound (CD271+) cells were compared to the flow through (CD271+). Error bars represent standard deviation of triplicate reactions. Asterisks show significant (p < 0.05) increase in expression of NGFR, NTRK3, SNAI1, and SLUG by bound cells compared to the flow-through population as determined by Student’s t-test. Figure from ref. (6)
Table 1.
PCR primer sequences used
| Gene | Forward primer | Reverse primer |
|---|---|---|
| GAPDH | TGTTGCCATCAATGACCCCTT | CTCCACGACGTACTCAGCG |
| NGFR | CCTACGGCTACTACCAGGATG | CACACGGTGTTCTGCTTGTC |
| NTRK3 | TCCGTCAGGGACACAACTG | GCACACTCCATAGAACTTGACA |
| SOX9 | GCCAGGTGCTCAAAGGCTA | TCTCGTTCAGAAGTCTCCAGAG |
| SNAI1 | AATCGGAAGCCTAACTACAGCG | GTCCCAGATGAG CATTG GCA |
| SLUG | AAGCATTTCAACGCCTCCAAA | AGGATCTCTGGTTGTGGTATGAC |
| MSX1 | CTCCGCAAACACAAGACGAAC | CACATGGGCCGTGTAGAGTC |
| AQP1 | CTG CACAGGCTTGCTGTATG | TGTTCCTTGGGCTGCAACTA |
| ALDH | CATTGGCACCTGGAACTACC | GGCTTGAGGACCACTGAGTT |
| B3GNT7 | CCTCMGTGGCTGGACATCT | ACGAACAGGTTTTCCTGTGG |
| KERA | ATCTGCAGCACCTTCACCTT | CATTGGAATTGGTGGTTTGA |
| PTGDS | CGGGGTCCCTCGGCTCCTAC | CTGGGGGTCTGGGTTCGGCT |
Table from ref. (6)
3.4 Keratocyte Differentiation
After 1 week culture in N2 Medium NCSC are harvested with TrypLE express.
2 × 105+ cells at are collected by centrifugation at 423 × g for 5 min to form a pellet.
These are cultured for 2 days in DMEM/F12 with 2 % FBS at 37 °C in 5 % CO2.
After 2 days, the medium is changed to KDM, being careful not to disturb the pellet.
50 % of the medium is replaced every 3 days for 2 weeks (11).
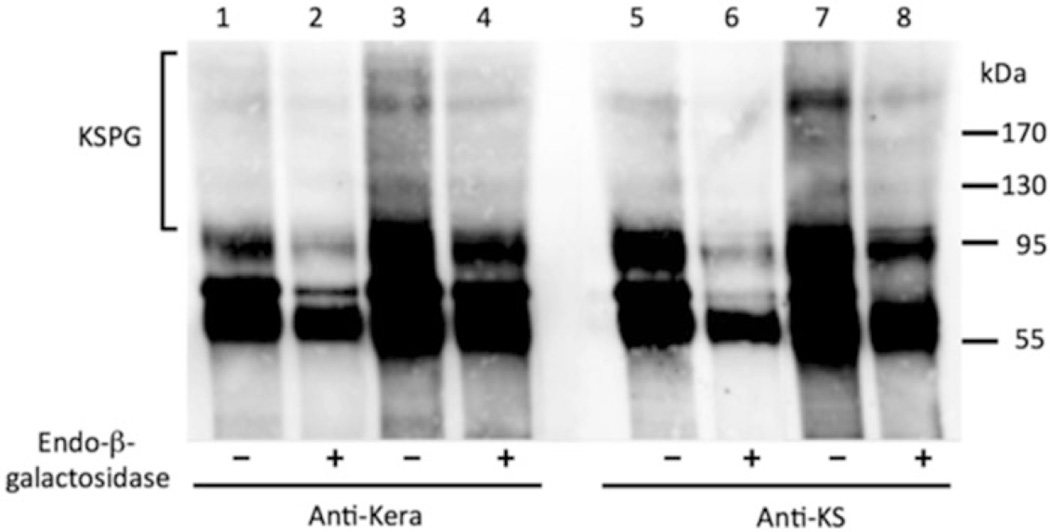
After 2 weeks, keratocyte phenotype can be observed by qPCR (Fig. 4; Table 1) for markers such as Aquaporin 1, Aldehyde Dehydrogenase 3A1, B3GNT7, Keratocan, and Prostaglandin D2 Synthase, or by immunoblotting to detect the stroma-specific keratan sulfate proteoglycans with antibodies to keratocan and keratan sulfate glycosaminoglycan (Fig. 5).
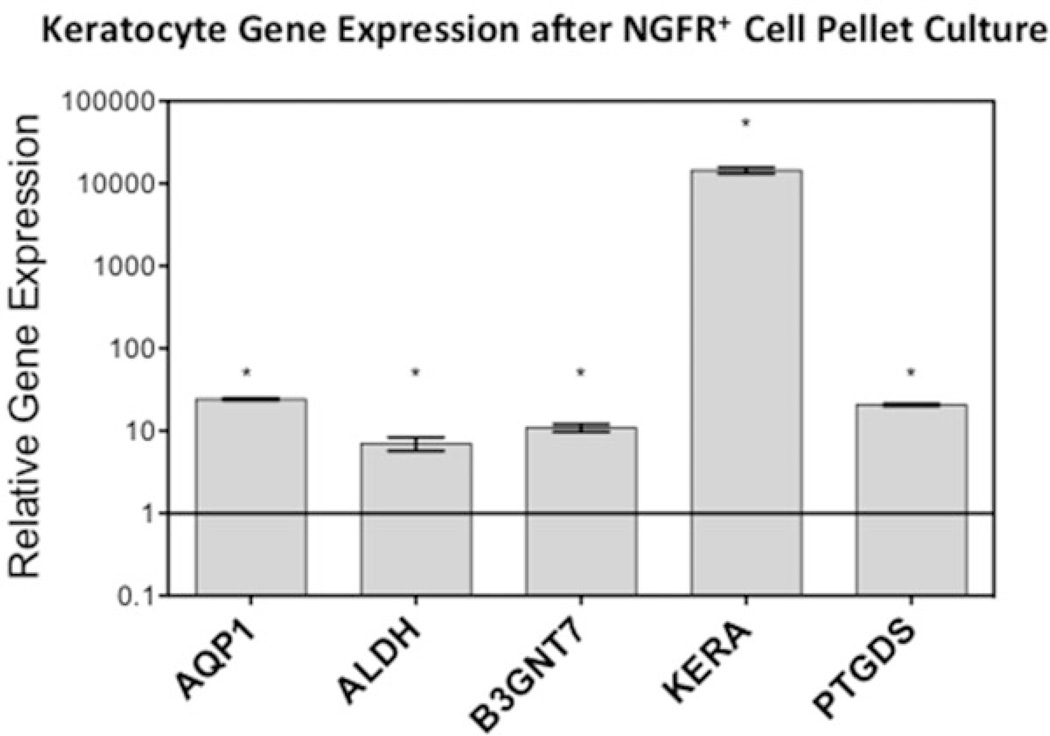
Fig. 4.
Keratocyte gene expression after differentiation. Keratocyte Gene Expression after CD271+ Pellet Culture. After CD271+ cells were expanded as monolayers in N2 medium there were grown as pellets in keratocyte differentiation medium for 2 weeks. Keratocyte gene expression was significantly upregulated 2 weeks after pellet culture compared to CD271+ cells. Asterisks represent significant (p < 0.05) upregulation as determined by Student’s t-test. Error bars represent standard deviation. Figure from ref. (6)
Fig. 5.
Immunoblot of keratocan and keratan sulfate. Keratan sulfate proteoglycan secretion after pellet culture. Differentiated cells secret corneal keratan sulfate proteoglycans after 3 weeks of pellet culture. Medium from cultures was taken before (Lanes 1, 2, 5, 6) or after (Lanes 3, 4, 7, 8) 3 weeks of CD271+ pellet culture in KDM. Proteoglycan fractions were biotin-labeled and immune-precipitated with antibodies against Keratocan (Anti-Kera, Lanes 1–4) or Keratan sulfate glycosaminoglycan (KSPG) (Anti-KS, Lanes 5–8). Both Keratocan and KSPG are present after 3 weeks of pellet culture in KDM. Figure from ref. (6)
Footnotes
Use of hESC cell lines is available via license and is restricted so that only some lines are available for in vivo experimental use. Investigators should consult the NIH Stem Cell Registry http://grants.nih.gov/stem_cells/registry/current.htm to inform themselves about these restrictions.
Matrigel-coated plates can be stored at 4–8 °C for up to 2 weeks if not used immediately after coating by leaving Matrigel solution on the plate and sealing with Parafilm to keep from drying. Before using, let plate sit at room temperature for 1 h.
Manipulations of hESC need to be carried out in BSL2 containment. This will require using an inverted microscope with a low-power objective (typically 2×) in a laminar flow tissue culture hood. Visualization of the colonies is most conveniently effected using a USB eye-piece CCD camera (such Dino-Eye Series AM4023, BigC.com, Torrance, CA) to provide real-time video to a laptop computer outside the sterile field.
Whenever MS columns are washed, it is imperative that no bubbles are introduced as this will cause the column to clog and cell sorting will be ineffective.
References
- 1.Boote C, et al. Quantitative assessment of ultrastructure and light scatter in mouse corneal debridement wounds. Invest Ophthalmol Vis Sci. 2012;53:2786–2795. doi: 10.1167/iovs.11-9305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Connon CJ, et al. Spatial and temporal alterations in the collagen fibrillar array during the onset of transparency in the avian cornea. Exp Eye Res. 2004;78:909–915. doi: 10.1016/j.exer.2004.01.005. [DOI] [PubMed] [Google Scholar]
- 3.Fini ME. Keratocyte and fibroblast phenotypes in the repairing cornea. Prog Retin Eye Res. 1999;18:529–551. doi: 10.1016/s1350-9462(98)00033-0. [DOI] [PubMed] [Google Scholar]
- 4.Fini ME, Stramer BM. How the cornea heals: cornea-specific repair mechanisms affecting surgical outcomes. Cornea. 2005;24:S2–S11. doi: 10.1097/01.ico.0000178743.06340.2c. [DOI] [PubMed] [Google Scholar]
- 5.Stramer BM, et al. Molecular mechanisms controlling the fibrotic repair phenotype in cornea: implications for surgical outcomes. Invest Ophthalmol Vis Sci. 2003;44:4237–4246. doi: 10.1167/iovs.02-1188. [DOI] [PubMed] [Google Scholar]
- 6.Chan AA, et al. Differentiation of human embryonic stem cells into cells with corneal keratocyte phenotype. PLoS One. 2013;8(2):e56831. doi: 10.1371/journal.pone.0056831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pomp O, et al. PA6-induced human embryonic stem cell-derived neurospheres: a new source of human peripheral sensory neurons and neural crest cells. Brain Res. 2008;1230:50–60. doi: 10.1016/j.brainres.2008.07.029. [DOI] [PubMed] [Google Scholar]
- 8.Goldstein RS, et al. Generation of neural crest cells and peripheral sensory neurons from human embryonic stem cells. Methods Mol Biol. 2010;584:283–300. doi: 10.1007/978-1-60761-369-5_15. [DOI] [PubMed] [Google Scholar]
- 9.Ludwig T, Thomson A. Defined, feeder-independent medium for human embryonic stem cell culture. Curr Protoc Stem Cell Biol. 2007 doi: 10.1002/9780470151808.sc01c02s2. Chapter 1:Unit 1C 2. [DOI] [PubMed] [Google Scholar]
- 10.Ludwig TE, et al. Feeder-independent culture of human embryonic stem cells. Nat Methods. 2006;3(8):637–646. doi: 10.1038/nmeth902. [DOI] [PubMed] [Google Scholar]
- 11.Du Y, et al. Secretion and organization of a cornea-like tissue in vitro by stem cells from human corneal stroma. Invest Ophthalmol Vis Sci. 2007;48(11):5038–5045. doi: 10.1167/iovs.07-0587. [DOI] [PMC free article] [PubMed] [Google Scholar]